Abstract
An outbreak of human metapneumovirus (hMPV) among children in southern Taiwan in 2004 prompted the investigation of the molecular epidemiology of hMPV from September 2003 to August 2005. Respiratory specimens that were culture negative for a panel of respiratory viruses were examined for the presence of hMPV by RT-PCR. The results indicated that 59 out of 546 (10.8%) children were hMPV-positive. The majority of these hMPV-positive children were less than 2 years old (59.4%), females (61%), and inpatients (67.8%). Infections occurred throughout the year, but peaked during the spring and/or summer months. Sequence analysis of the fusion gene from the isolates revealed two phylogenetic groups with five possible lineages (A1, A2a/A2b, B1, and B2). Among these co-circulating strains, A2 strains were most frequently observed and demonstrated the greatest divergence. Deduced amino acid sequence analysis identified several variant amino acids specific to the A2 lineage. Lineage-specific amino acid substitutions were noted at aa233, aa286, aa312, aa348, and aa296. This study indicated that genetically divergent strains of hMPV which caused respiratory disease and hospitalization were circulating among children in Taiwan.
Keywords: Respiratory Syncytial Virus, Deduce Amino Acid Sequence, Respiratory Specimen, Human Respiratory Syncytial Virus, Human Metapneumovirus
Introduction
Human metapneumovirus (hMPV) was first reported in 2001 in the Netherlands [26] and has been assigned to the family Paramyxoviridae, subfamily Pneumovirinae, and genus Metapneumovirus [20, 26]. Since its discovery, hMPV has been identified on several continents including America, Australia, Asia and Europe, and was thought to be prevalent worldwide [8, 16, 19, 25]. hMPV resembles human respiratory syncytial virus (hRSV) with regard to its clinical presentation and ability to infect and cause disease in young children, elderly persons and immunocompromised patients [4, 7, 9, 14, 17, 20, 29]. As reinfection of hMPV may occur frequently throughout a human’s life, the evolution of variant virus, which leads to severe pathologic outcomes, requires extensive attention.
hMPV is an enveloped virus whose genome consists of a single negative strand of RNA of approximately 13 kb [27]. The genome of hMPV, from 3′ to 5′, is organized as follows: N-P-M-F-M2-1/M2-2-SH-G-L [22, 23, 27, 32]. Similar to the avian metapneumovirus (AMPV), hMPV lacks nonstructural proteins NS1 and NS2 and posses three surface glycoproteins, the fusion (F), attachment (G), and small hydrophobic (SH) proteins. The G protein attaches to host cells, whereas the F protein directly mediates membrane fusion [15]. It is known that the greatest genetic diversity among hMPV strains occurs in the structural genes; therefore, both G and F genes have been widely used for phylogenetic analysis in the context of genetic lineages [2, 3, 10, 28].
The hMPV fusion gene (1.6 kb) encodes a protein of 539 amino acid residues, which includes an amino-terminal signal sequence, a hydrophobic transmembrane domain and a cytoplasmic domain. The F protein is synthesized as a biologically inactive precursor (F0) which must be cleaved by host cell proteases into F1 (a.a.103–539) and F2 (a.a.1–102) for fusion activity. The fusion domain (23 a.a. residues in length) is highly hydrophobic and located at the N-terminus of the F1 subunit. Several heptad repeats (HR) in the F protein are also critical for membrane fusion [2, 15, 27]. HRA (a.a.127–169) is located at the carboxyl terminal end of the fusion peptide. HRB (a.a.457–484) is located in the ectodomain adjacent to the transmembrane region.
Genetic and antigenic studies based on the variation of hMPV F gene have demonstrated the presence of two distinct hMPV groups, designated as groups A and B, which can be further divided into five subgroups A1, A2a/A2b, B1, and B2 [10, 28]. However, it is currently unclear whether the variations in the F gene that lead to alterations of F protein’s functional motifs are the main determinants of the epidemic features of hMPV. In addition, F protein has been demonstrated to contain major antigenic determinants that mediate extensive cross-lineage neutralization [3, 23] and cross-protective immunity [11]. Detailed analysis of the sequence variation of F genes among clinical isolates is therefore critical for defining the molecular evolution of the F gene and may provide clues to understanding the epidemic features of the virus.
Here, we present the demographic and epidemic features of hMPV infections in children in southern Taiwan over a period of 2 years (September 2003 to August 2005). Phylogenetic relationships and genetic features of the evolution of F genes derived from these hMPV clinical isolates are explored to identify possible variant amino acids which may be critical to the evolution of the circulating strains.
Materials and methods
Subjects
Respiratory specimens were received at the Clinical Virology Laboratory, National Cheng Kung University Hospital, from September 2003 to August 2005. Demographic analysis was performed by χ 2 tests. Specimens were collected from the emergency department, inpatient wards, and outpatient clinics and transported to the laboratory on wet ice for viral culture. Specimens from individuals less than 5 years of age who had negative culture results for RSV, parainfluenza virus types 1–3, influenza A and B, and adenovirus were tested for hMPV by RT-PCR.
RNA extraction, reverse-transcription polymerase chain reaction (RT-PCR), and sequencing
Five hundred forty-six specimens (about one tenth of the total specimens collected during the study period) were examined for the presence of hMPV by RT-PCR. Primers MPVF1f (5′-CTTTGGACTTAATGACAGATG-3′) and MPVF1r (5′-GTCTTCCTGTGCTAACTTTG-3′) were used to amplify a 450-bp fragment of the F gene [20]. Viral RNA was extracted from 140 μl of respiratory specimens or viral culture fluids using the QiaAmp Viral RNA Mini Kit (QIAGEN), and the fusion gene was amplified by one-step RT-PCR (QIAGEN) following manufacturer-suggested procedures. Briefly, 5 μl of RNA was added to the RT-PCR mixture, which contained 2 μl of QIAGEN OneStep RT-PCR enzyme mixture, 10 μl of 5× QIAGEN OneStep RT-PCR buffer, 400 μM deoxynucleoside triphosphates, and 0.6 μM of each primer in a final volume of 50 μl. Cycling conditions were as follows: 30 min at 50°C for RT reaction, 5 min at 95°C for RT inactivation, followed by 35 cycles of PCR reaction (94°C for 1 min, 54°C for 1 min, and 72°C for 1 min) with an extension step at 72°C for 10 min. The PCR products derived from approximately 40% (23/59) of the hMPV-positive specimens were purified with the QIAquick PCR purification kit (QIAGEN), and both sense and anti-sense strands of the PCR products were sequenced by an ABI 377 sequencer (Applied Biosystems).
Phylogenetic analysis
Nucleotide sequences of amplified F gene products were aligned using GCG software (GCG WISCONSIN PACKAGE Version 10.3). Phylogenetic trees were constructed by using the neighbor-joining method included in the DNADist and Neighbor software package of the Phylip 3.6 (alfa3) program by means of 1,000 bootstraps with random sequence addition. Bootstrap values were computed for consensus trees created with the Consensus package by applying a 75% majority consensus rule. The tree was re-rooted at its midpoint by using the Retree software of Phylip 3.6 and was visualized with the Treeview 1.6.6 program.
Nucleotide sequence accession numbers
Avian metapneumovirus C (GenBank accession no. AY590688) and avian metapneumovirus B (GenBank accession no. Y14294) were included as outgroups, and hMPV 17 strains from the GenBank database (NC_004148, NC_004148; CAN97-83, AY297749; NL/1/94, AY304362; NL/1/99, AY525843; NL/1/00, AF371337; NL/17/00, AY304360; JPY88-12, AY622381; JPS03-240 AY530095; CAN99-81, AY574224; CAN98-77, AY145291; CAN98-78, AY145292; CAN98-79, AY145293; CAN98-74, AY145288; CAN98-75, AY297748; CAN98-76,AY145290; CAN97-82, AY145295) were included for comparison in the phylogenetic analysis [2, 3, 21, 28]. hMPV isolates used in this study have been deposited in the GenBank database under accession no. EF612439 to EF612461.
Results
Study population and demographic features of the hMPV-positive children
During the 2-year study period, respiratory specimens from children less than 5 years of age that had negative culture results for RSV, parainfluenza virus types 1–3, influenza A and B, and adenovirus were randomly collected each month to test for the presence of hMPV. There were 546 study cases accumulated in this period, and 59 cases (10.8%) tested hMPV-positive. The demographic characteristics of hMPV-positive and hMPV-negative children were compared (Table 1). The result indicated that the majority of hMPV-positive children, with median age of 23.4 months, were less than 2-years old (59.4%) (P = 0.67), females (61%) (P = 0.004) and inpatients (67.8%) (P = 0.14). Among the hMPV-negative children, males (58.9%) (P = 0.004) were more often observed.
Table 1.
Demographic characteristics of hMPV-positive and hMPV-negative patients
| Parameter | hMPV-positive, n = 59 no. (%) | hMPV-negative, n = 487 no. (%) |
|---|---|---|
| Age | ||
| <6 months | 10 (17.0) | 68 (14.0) |
| 6 months to 1 year old | 10 (17.0) | 112 (23.0) |
| 1 to 2 years old | 15 (25.4) | 131 (26.9) |
| 2 to 5 years old | 24 (40.6) | 176 (36.1) |
| Male or female | ||
| Male | 23 (39.0) | 287 (58.9) |
| Female | 36 (61.0) | 200 (41.1) |
| Inpatients or outpatients | ||
| Inpatients | 40 (67.8) | 281 (57.7) |
| Outpatients | 19 (32.2) | 206 (42.3) |
Peak activities and phylogenic relationship of the circulating hMPV
To investigate the major circulating variants during the study period, the frequency and distribution of co-circulating hMPV strains were evaluated (Table 2). Human metapneumovirus activity was detected throughout the year, with peak activities from March to September 2004 (50/59, 85%), coinciding with a prominent outbreak from March to April (37/59, 63%) 2004, and February to May 2005 (7/59, 12%), suggesting a highly susceptible period around spring and/or summer for hMPV transmission in children in southern Taiwan.
Table 2.
Respiratory specimens tested for the presence and the distribution of circulating hMPV variants from September 2003 to August 2005
| Cases tested | hMPV-positive cases (frequency %) | Subgroup (no.)a | ||
|---|---|---|---|---|
| September 2003 | 19 | 0 | 0 | |
| October | 19 | 0 | 0 | |
| November | 19 | 0 | 0 | |
| December | 19 | 1 | 5.3 | A2 (1) |
| January 2004 | 20 | 0 | 0 | |
| February | 20 | 0 | 0 | |
| March | 76 | 17 | 22.4 | A2 (2) |
| April | 35 | 20 | 57.1 | A2 (2) |
| May | 21 | 2 | 9.5 | A2 (1), B1 (1) |
| June | 20 | 3 | 15.0 | A1 (1), A2 (2) |
| July | 20 | 4 | 20.0 | A2 (2), B1 (1), B2 (1) |
| August | 21 | 1 | 4.8 | A2 (1) |
| September | 20 | 3 | 15.0 | A2 (1) |
| October | 19 | 0 | 0 | |
| November | 19 | 1 | 5.3 | A2 (1) |
| December | 20 | 0 | 0 | |
| January 2005 | 20 | 0 | 0 | |
| February | 20 | 3 | 15.0 | A2 (2) |
| March | 20 | 1 | 5.0 | A1 (1) |
| April | 20 | 2 | 10.0 | A1 (2) |
| May | 20 | 1 | 10.0 | A1 (1) |
| June | 19 | 0 | 0 | |
| July | 20 | 0 | 0 | |
| August | 20 | 0 | 0 | |
| Total | 546 | 59 | 10.8 | |
aThe number in parentheses is the number of cases identified
The major hMPV strains distributed in the peak months were assessed from approximately 40% of hMPV-positive RT-PCR specimens by phylogenetic analysis of their F gene sequences. A total of 23 hMPV-positive RT-PCR samples were sequenced, and the sequences (nucleotide 706–1071 of the F gene) were subjected to phylogenetic analysis in comparison to a Canadian isolate, CAN98-75 (GenBank accession no. AY145289), and several reference strains. The phylogenetic tree (Fig. 1) showed that two distinct hMPV genogroups (A and B) with at least four possible subgroup lineages (A1, A2, B1, and B2) were co-circulating during the study period. Subgroup A2 was further divided into A2a and A2b lineages with the bootstrap values of 81 and 63%, respectively. Since the A2b bootstrap value was less than the 75% limit, the phylogenic relationship between A2a and A2b was re-evaluated by maximum likelihood and parsimony methods (data not shown). The re-evaluation supported the presence of two distinct lineages for the A2 subgroup. hMPV A2 strains were more frequently identified in children during the study period (15 out of 23 strains), and A1 variants were frequently detected in 2005 from March to May (Table 2).
Fig. 1.
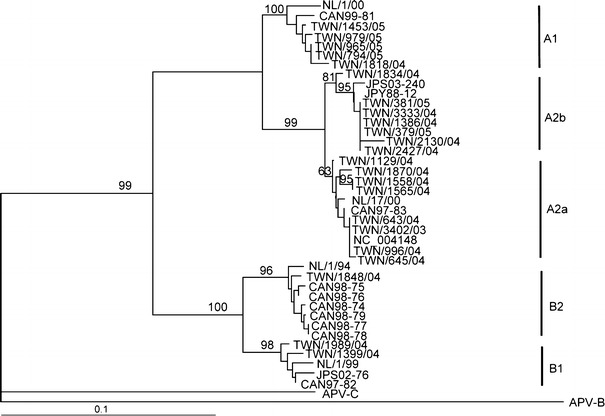
Phylogenetic analysis of F gene of human metapneumovirus during 2003–2005. Dendrogram of 23 hMPV outbreak strains and 19 reference strains from GenBank are generated based on 366 nucleotides (nt 706–1071) of the F gene using the neighbor-joining method with the DNADIST distance measure program (PHYLIP, version 3.6). The percentage bootstrap frequency of each branch of the tree is indicated. Avian metapneumovirus C (GenBank accession no. AY590688) and avian metapneumovirus B (GenBank accession no. Y14294) are included as outgroups
The resemblance of nucleotides and deduced amino acid sequences among the phylogenetic lineages
Sequence diversities among these hMPV isolates and pair-wise nucleotide and deduced amino acid sequences of the 366 bases of the F gene were analyzed. There were 83.6–88% nucleotide similarities between groups A and B, yet a higher similarity was observed either within group A (91.3–100%) or group B (94.3–100%). Similarities of 91.3–99.5% were found between subgroups A1 and A2 and 94.3–94.9% between subgroups B1 and B2. There were greater similarities within subgroups: 98.7–100, 98.4–100, 97.5–100, 98.4 and 100% for subgroups A1, A2a, A2b, B1, and B2, respectively. These phylogenetic relationships were supported by sequence analysis of the deduced amino acids. Overall, the nucleotide and amino acid sequence similarities within the A2 subgroup (96.0–100 and 96.7–100%, respectively) were relatively lower than those within other subgroups, suggesting the presence of more divergent F gene variants in the subgroup A2 during the study period.
Amino acid residues critical in differentiating hMPV lineages
To identify the lineage-specific amino acid substitutions, the deduced amino acid sequences of the 366-nt region of the F gene were compared (data not shown). Variations among aa233, aa286, aa312, and aa348 were noted in the major A and B phylogenetic groups, whereas variations in aa296 were observed in the subgroup lineages (Table 3). It appeared that aa296 was a frequently observed variation, as the consensus Lys (K) residue in position 296 had the tendency to convert to Arg (R) in lineage A2a (TW-04-1565 and TW-05-1558) or A2b (JPS03-240 reference strain). In addition, the substitutions of aa296 by Asn (N) and Asp (D) were distinguishing characteristics of subgroups B1 and B2, respectively.
Table 3.
Comparison of lineage-specific amino acid substitutions of the five lineages between the reference strains and the Taiwan strains
| Isolate (Sublineage) | aaa233 | aa286 | aa296 | aa312 | aa348 |
|---|---|---|---|---|---|
| Reference strains | |||||
| NL/1/00 (A1) | N | V | K | Q | K |
| NL/17/00 (A2a) | N | V | K | Q | K |
| JPS03-240 (A2b) | N | V | R | Q | K |
| NL/1/99 (B1) | Y | I | N | K | R |
| NL/1/94 (B2) | Y | I | D | K | R |
| Taiwan strains | |||||
| A1 | N | V | K | Q | K |
| A2a | N | V | K or R | Q | K |
| A2b | N | V | K | Q | K |
| B1 | Y | I | N | K | R |
| B2 | Y | I | D | K | R |
aIndicates the position of the amino acid relative to isolate CAN98-75 (GenBank accession no. AY145289)
Discussion
Laboratory diagnosis of hMPV infection is dependent upon RT-PCR of respiratory specimens because the virus is fastidious and difficult to grow in cell culture [5, 12]. We have previously identified an outbreak of hMPV infection from children in southern Taiwan from March to May 2004, and compared the clinical manifestations of hMPV with other virus-associated respiratory tract infections [30]. In this study, we provide genetic evidence identifying the major circulating viral variants that may contribute to the epidemic features of hMPV infection in a longer period from September 2003 to August 2005. Our results revealed all five defined hMPV lineages in southern Taiwan and indicated that the variants in subgroup A2 were more frequently identified in the study period. Seasonality was observed for hMPV infection in southern Taiwan, as hMPV activities were frequently observed during spring and summer months. Overall, the peak of infection in southern Taiwan was similar to that in California in 2004 [6, 31], with less hMPV activity observed in the fall and winter in southern Taiwan. This trend of hMPV infection was observed during both years of the 2-year study period and was similar to high and low incidences in other reports [10, 24].
The detection of group A and B variants in 2004 supported that at least two hMPVs in distinct genetic clusters could co-circulate in a single year during a respiratory season [1, 2, 5, 18, 28]. Furthermore, children were infected more frequently by group A strains, which agrees with Boivin et al. [4] and suggests that age may be a critical fitness factor for group A strains. Also, Boivin et al. [4] have noted a possible shift of infection towards A2 strains in recent years. This was supported by our observation that 75% of the strains from the hMPV-positive specimens belonged to the A2 genotype. It is possible that the co-circulation pattern among children in southern Taiwan is similar throughout the world, and the genetic variations in the A2 subgroup may have certain functional relevance critical for the transmission. On the other hand, a recent report has described a complex circulation pattern based on the sequence variations within the G gene, and certain variations were critical for evading preexisting immunity [13].
The result of our phylogenetic analysis demonstrated that there were five lineage-specific amino acids substitutions (aa233, aa286, aa296, aa312, aa348) within the fusion protein. Others have reported lineage-specific substitutions at aa286, aa296, aa312, aa348 and aa404 [28]. Specific differences between A and B strains were observed among amino acids on the N-terminal side of the fusion protein [4]. The reported signature amino acid positions for differentiating groups A and B were at positions 61, 122, 135, 167, 175, and 233, whereas substitutions at position 185 differentiated the A1 subgroup; substitutions at positions 143 and 179 differentiated the B2 subgroup.
It should be noted that three individuals contributed dual specimens and each tested positive for hMPV; however, they were only counted once in this study. Specimens from two of the individuals were obtained from throat swabs and nasopharyngeal aspirates collected on the same day. The third individual contributed 2 hMPV-positive specimens that were obtained 45 days apart and became hMPV-negative after 231 days. No respiratory specimens were collected from this patient during the 45-day period of the two positive specimens. According to the identical F gene of the two strains, we considered these two consecutive positive specimens to be the result of prolonged shedding of hMPV.
Etiologies of respiratory viral infections commonly include influenza viruses, parainfluenza viruses, adenoviruses, respiratory syncytial viruses, rhinoviruses, and coronaviruses, while hMPV has been documented recently to widely cause seasonal outbreaks of respiratory infections in children. We have previously reported that hMPV also contributes substantially to acute respiratory tract infections in children of Taiwan with respiratory symptoms and signs similar to those of other RV infections, except for the severity of skin rash and duration of fever, together with the presentation of feeding difficulties, tachycardia, and minimal cough [31]. This study further supports the unique epidemic pattern of hMPV in children in southern Taiwan based on the genetic analysis of F gene. These hMPV variants simultaneously circulate with seasonal distribution and may account for many of the undiagnosed infections in Taiwan.
Acknowledgments
This study was supported by National Health Research Institutes grants; and by Department of Health, Taiwan Center for Disease Control grant CDC94-RM-012.
References
- 1.Agapov E, Sumino KC, Gaudreault-Keener M, Storch GA, Holtzman MJ. Genetic variability of human metapneumovirus infection: evidence of a shift in viral genotype without a change in illness. J Infect Dis. 2006;193:396–403. doi: 10.1086/499310. [DOI] [PubMed] [Google Scholar]
- 2.Bastien N, Normand S, Taylor T, Ward D, Peret TC, Boivin G, Anderson LJ, Li Y. Sequence analysis of the N, P, M and F genes of Canadian human metapneumovirus strains. Virus Res. 2003;93:51–62. doi: 10.1016/S0168-1702(03)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biacchesi S, Skiadopoulos MH, Boivin G, Hanson CT, Murphy BR, Collins PL, Buchholz UJ. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315:1–9. doi: 10.1016/S0042-6822(03)00528-2. [DOI] [PubMed] [Google Scholar]
- 4.Boivin G, Mackay I, Sloots TP, Madhi S, Freymuth F, Wolf D, Shemer-Avni Y, Ludewick H, Gray GC, LeBlanc E. Global genetic diversity of human metapneumovirus fusion gene. Emerg Infect Dis. 2004;10:1154–1157. doi: 10.3201/eid1006.031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng MF, Chen BC, Kao CL, Kao CH, Hsieh KS, Liu YC. Human metapneumovirus as a causative agent of lower respiratory tract infection in four patients: the first report of human metapneumovirus infection confirmed by RNA sequences in Taiwan. Scand J Infect Dis. 2006;38:392–396. doi: 10.1080/00365540500388750. [DOI] [PubMed] [Google Scholar]
- 6.Esper F, Martinello RA, Boucher D, Weibel C, Ferguson D, Landry ML, Kahn JS. A 1-year experience with human metapneumovirus in children aged < 5 years. J Infect Dis. 2004;189:1388–1396. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 8.Freymouth F, Vabret A, Legrand L, Eterradossi N, Lafay-Delaire F, Brouard J, Guillois B. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22:92–94. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Greensill J, McNamara PS, Dove W, Flanagan B, Smyth RL, Hart CA. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–375. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huck B, Scharf G, Neumann-Haefelin D, Puppe W, Weigl J, Falcone V. Novel human metapneumovirus sublineage. Emerg Infect Dis. 2006;12:147–150. doi: 10.3201/eid1201.050772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin PY, Lin TY, Huang YC, Tsao KC, Huang YL. Human metapneumovirus and community-acquired pneumonia in children. Chang Gung Med J. 2005;28:683–688. [PubMed] [Google Scholar]
- 13.Ludewick HP, Abed Y, van Niekerk N, Boivin G, Klugman KP, Madhi SA. Human metapneumovirus genetic variability, South Africa. Emerg Infect Dis. 2005;11:1074–1078. doi: 10.3201/eid1107.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggi F, Pifferi M, Vatteroni M, Fornai C, Tempestini E, Anzilotti S, Lanini L, Andreoli E, Ragazzo V, Pistello M, Specter S, Bendinelli M. Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J Clin Microbiol. 2003;41:2987–2991. doi: 10.1128/JCM.41.7.2987-2991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison TG. Structure and function of a paramyxovirus fusion protein. Biochim Biophys Acta. 2003;1614:73–84. doi: 10.1016/S0005-2736(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 16.Nissen MD, Siebert DJ, Mackay IM, Sloots TP, Withers SJ. Evidence of human metapneumovirus in Australian children. Med J Aust. 2002;176:188. doi: 10.5694/j.1326-5377.2002.tb04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterhaus A, Fouchier R. Human metapneumovirus in the community. Lancet. 2003;361:890–891. doi: 10.1016/S0140-6736(03)12785-7. [DOI] [PubMed] [Google Scholar]
- 18.Peiris JS, Tang WH, Chan KH, Khong PL, Guan Y, Lau YL, Chiu SS. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelletier G, Dery P, Abed Y, Boivin G. Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg Infect Dis. 2002;8:976–978. doi: 10.3201/eid0809.020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peret TC, Boivin G, Li Y, Couillard M, Humphrey C, Osterhaus AD, Erdman DD, Anderson LJ. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–1663. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham QN, Biacchesi S, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. Chimeric recombinant human metapneumoviruses with the nucleoprotein or phosphoprotein open reading frame replaced by that of avian metapneumovirus exhibit improved growth in vitro and attenuation in vivo. J Virol. 2005;79:15114–15122. doi: 10.1128/JVI.79.24.15114-15122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randhawa JS, Marriott AC, Pringle CR, Easton AJ. Rescue of synthetic minireplicons establishes the absence of the NS1 and NS2 genes from avian pneumovirus. J Virol. 1997;71:9849–9854. doi: 10.1128/jvi.71.12.9849-9854.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skiadopoulos MH, Biacchesi S, Buchholz UJ, Riggs JM, Surman SR, Amaro-Carambot E, McAuliffe JM, Elkins WR, St Claire M, Collins PL, Murphy BR. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J Virol. 2004;78:6927–6937. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22:S21–S32. doi: 10.1097/00006454-200302001-00004. [DOI] [PubMed] [Google Scholar]
- 25.Stockton J, Stephenson I, Fleming D, Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;8:897–901. doi: 10.3201/eid0809.020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Hoogen BG, Bestebroer TM, Osterhaus AD, Fouchier RA. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295:119–132. doi: 10.1006/viro.2001.1355. [DOI] [PubMed] [Google Scholar]
- 28.van den Hoogen BG, Herfst S, Sprong L, Cane PA, Forleo-Neto E, de Swart RL, Osterhaus AD, Fouchier RA. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10:658–666. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viazov S, Ratjen F, Scheidhauer R, Fiedler M, Roggendorf M. High prevalence of human metapneumovirus infection in young children and genetic heterogeneity of the viral isolates. J Clin Microbiol. 2003;41:3043–3045. doi: 10.1128/JCM.41.7.3043-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LC WangSM, Wang HC, Su IJ, Wang JR. Human metapneumovirus infection among children in Taiwan: a comparison of clinical manifestations with other virus-associated respiratory tract infections. Clin Microbiol Infect. 2006;12(12):1221–1224. doi: 10.1111/j.1469-0691.2006.01540.x. [DOI] [PubMed] [Google Scholar]
- 31.Williams JV, Wang CK, Yang CF, Tollefson SJ, House FS, Heck JM, Chu M, Brown JB, Lintao LD, Quinto JD, Chu D, Spaete RR, Edwards KM, Wright PF, Crowe JE., Jr The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Q, Davis PJ, Li J, Cavanagh D. Cloning and sequencing of the matrix protein (M) gene of turkey rhinotracheitis virus reveal a gene order different from that of respiratory syncytial virus. Virology. 1992;186:426–434. doi: 10.1016/0042-6822(92)90007-C. [DOI] [PMC free article] [PubMed] [Google Scholar]


