Abstract
Children with post-infectious bronchiolitis obliterans (PIBO) are frequently hospitalized with acute exacerbation, but clinical differentiation of PIBO exacerbation from acute bronchiolitis is often challenging, which may result in treatment delay and chronic lung function impairment. We aimed to examine whether serum YKL-40 and growth factors could be markers for PIBO exacerbation. Thirty-seven children admitted with acute exacerbation of PIBO were enrolled and studied retrospectively. Diagnosis of PIBO was based on clinical history of acute respiratory infection followed by persistent airway obstruction and characteristic findings in high-resolution computed tomography. Serum levels of YKL-40, vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β1, and platelet-derived growth factor (PDGF)-BB were measured on admission. The biomarkers were also examined in children admitted with acute bronchiolitis serving as positive controls (N = 30) and in age-matched controls (N = 20). Only YKL-40 levels were found to be significantly higher in PIBO patients with exacerbation compared with that in bronchiolitis patients and showed a positive correlation with the severity of disease before diagnosis of PIBO.
Conclusion: Our results suggest that measuring serum YKL-40 levels might help distinguish exacerbation of PIBO from acute bronchiolitis in young children.
|
What is Known: • The children with post-infectious BO (PIBO) usually have recurrent wheezing and need frequent hospitalization due to acute exacerbation during the first disease years. • Clinical differentiation of PIBO exacerbation from acute bronchiolitis in young children is often challenging, which may result in treatment delay and chronic lung function impairment. |
|
What is New: • Measuring serum YKL-40 levels might help distinguish exacerbation of PIBO from acute bronchiolitis in young children. |
Keywords: YKL-40, Growth factor, Post-infectious bronchiolitis obliterans, Children
Introduction
Bronchiolitis obliterans (BO) is an uncommon and irreversible form of chronic obstructive lung disease secondary to severe insults to lower respiratory tract [1]. Various kinds of lung injuries due to infection, allogeneic transplantation, and exposure to toxic fumes have been reported to cause BO [1, 2]. It is known that these diverse medical conditions share some common pathways that result in a characteristic histopathology of BO, subepithelial inflammation, and fibrotic narrowing of bronchioles [2]. In young children, BO occurs most often after respiratory infection and common pathogens including adenovirus, influenza virus, and Mycoplasma pneumoniae have been associated with the development of post-infectious BO (PIBO) [3]. Many children diagnosed with PIBO, in contrast to those with post-transplant BO, show clinical improvement during lung development. However, radiological findings and impaired lung function are persistent or sometimes progressive [4–6].
The children with PIBO usually have recurrent wheezing, coughing, and shortness of breath on exertion. They also need frequent hospitalization because of acute exacerbation due to respiratory infection during the first disease years. Clinical differentiation of PIBO exacerbation from acute bronchiolitis in young children is often challenging, which may result in treatment delay and chronic lung function impairment [7].
YKL-40, also called chitinase-3-like1 (CHI3L1) glycoprotein, is shown to have a role in various human diseases associated with infection, inflammation, and/or tissue remodeling [8]. Recently, serum concentration of YKL-40 was reported to be a predictive biomarker for development of BO in lung transplant recipients [9], but it has not been studied in the children with PIBO.
Vascular endothelial growth factor (VEGF) is a key mediator of vascular neogenesis and is known to play a role in airway inflammation and remodeling [10]. Transforming growth factor (TGF)-β1 and platelet-derived growth factor (PDGF) are important fibrogenic cytokines and are known to contribute to lung fibrosis [11, 12]. These growth factors were shown to be involved in the pathogenesis of bronchiolitis obliterans syndrome following transplantation [13–15], but have not been studied yet in the children with PIBO.
In the present study, we measured serum concentrations of YKL-40 and growth factors in children with PIBO admitted with exacerbation. All values measured were compared to those from children with acute bronchiolitis who served as positive controls. We hypothesized that YKL-40 and growth factors may be increased in the patients with PIBO and could be non-invasive biomarkers for distinguishing exacerbation of PIBO from acute bronchiolitis in young children.
Materials and methods
Patients and controls
The patients who were admitted with acute exacerbation of PIBO or acute bronchiolitis between March 2013 and February 2015 were enrolled. We retrospectively reviewed the medical records of the two patient groups and investigated their clinical characteristics.
Diagnosis of PIBO was made based on both clinical and radiologic findings according to the previously described criteria [16]: (1) history of acute lower respiratory infection in previously healthy children; (2) unresolved respiratory symptoms associated with airway obstruction (cough, shortness of breath on exertion, and/or abnormal breath sounds) that last for more than 6 weeks after the initial episode despite treatment; (3) mosaic perfusion with air trapping, bronchiectasis, or atelectasis on pulmonary high-resolution computed tomography (HRCT); (4) exclusion of any underlying diseases including other chronic lung diseases. This study included the patients with PIBO whose clinical data including age at onset of persistent respiratory disease, interval between onset of disease and diagnosis, and severity of disease before diagnosis were available.
The patients admitted with acute bronchiolitis served as positive controls. Diagnosis of bronchiolitis was made clinically on the basis of a thorough history and physical examination [17]. The present study included the patients who were age-matched to the patients with PIBO and it was confirmed that they did not develop BO during a 1-year follow-up period after discharge through a retrospective review of the outpatient medical records. The patients who had persistent respiratory symptoms associated with previous respiratory infection were excluded.
Twenty age-matched control subjects, who were admitted with minor surgical problems, were also enrolled. They had no respiratory symptoms on admission and no previous history of recurrent respiratory illnesses.
Review of clinical characteristics and laboratory findings in the patients
The severity of disease before diagnosis in PIBO group was assessed on sum of scores (with maximum severity score 8) based on their medical history before admission, that is, from 1 to 2 for each of the following clinical findings: (1) cough, shortness of breath on exertion, and/or abnormal breath sounds (1 intermittent; 2 daily); (2) limitation of normal activity (1 none; 2 any); (3) frequency of respiratory disease requiring hospitalization or emergency department visits (1 once; 2 ≥twice); (4) frequency of unscheduled outpatient visits (1 once; 2 ≥twice) [18].
The severity of symptom during current admission was assessed on the symptom score from 0 to 4 according to the number of the following clinical findings: (1) fever over 38.5 °C; (2) tachypnea (age-specific) and/or lower chest wall indrawing; (3) oxygen saturation less than 92% breathing room air; (4) more than 7 days hospitalization [19].
Atopic sensitization was defined as having at least one serum-specific IgE >0.35 kU/L (ImmunoCAP, Phadia, Uppsala, Sweden) to common allergens. Common allergens used for our patients were Dermatophagoides farinae, Dermatophagoides pteronyssinus, pollen mixtures (grass pollen mixture, tree pollen mixture, weed pollen mixture), and food allergens (egg, milk, soybean, peanut).
Nasopharyngeal aspirates were obtained from the patients on admission to detect respiratory viral pathogens using multiplex RT-PCR (Seeplex™ RV Detection kit, Seegene, Seoul, Korea). Respiratory syncytial virus, rhinovirus, human bocavirus, human metapneumovirus, influenza virus, adenovirus, corona virus, and parainfluenza virus were studied.
Evaluation of YKL-40, VEGF, PDGF-BB, and TGF-β1
Serum samples from the two patient groups, PIBO and bronchiolitis, and controls were collected immediately after admission and stored at −70 °C until analysis.
Levels of YKL-40, VEGF, PDGF-BB, and TGF-β1 were measured using quantitative colorometric sandwich ELISA kits (R&D, Minneapolis, MN, USA) according to the manufacturer’s instruction. ELISA sensitivity was 1.25 pg/mL for YKL-40, 5.0 pg/mL for VEGF, 15 pg/mL for PDGF-BB, and 1.7 pg/mL for TGF-β1, respectively. All assays were performed in duplicate for each sample, and the mean values were reported.
Serum levels of YKL-40, VEGF, PDGF-BB, and TGF-β1 were evaluated in relation to the clinical characteristics and laboratory findings including peripheral neutrophil and eosinophil counts in the two patient groups.
Statistical analysis
Analysis of data was done using IBM SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA). For comparison of serum levels of YKL-40, VEGF, PDGF-BB, and TGF-β1 between two patient groups and controls, since not all distributions were normal, Mann-Whitney U test was used. The serum levels are presented as medians with interquartile ranges (IQRs). Fisher’s exact test was used to test for equality of proportions between two groups. Pearson’s or Spearman’s rank correlation analysis was used to evaluate the relationship of biomarker levels with clinical findings. A receiver operating characteristic (ROC) curve was used to assess the cutoff values of YKL-40 which might help distinguish PIBO exacerbation form acute bronchiolitis. A P < 0.05 was considered statistically significant.
Results
Clinical characteristics of the subjects
Thirty-seven patients with PIBO exacerbation and 30 patients with acute bronchiolitis were enrolled in this study. The clinical characteristics of the two patient groups and controls are presented in Table 1. There was no intergroup difference in age distribution and sex ratio. Mean interval between onset of disease and diagnosis of PIBO was 8.1 months (range 2–24 months), and 16 patients were atopic.
Table 1.
Clinical characteristics of two patient groups and controls
| PIBO group (N = 37) |
Bronchiolitis group (N = 30) | Controls (N = 20) |
P | |
|---|---|---|---|---|
| Age, months, mean (range) | 26.2 (5–51) | 22.7 (5–38) | 21.5 (9–45) | 0.4 |
| Sex, male (%) | 15 (41) | 16 (53) | 11 (55) | 0.3 |
| Atopic patients (%) | 16 (44) | 11 (37) | ND | 0.5 |
|
Age at initial episode, months, mean (range) |
16.1 (2–37) | NA | ||
| Interval between initial episode/diagnosis, months, mean (range) | 8.1 (2–24) | NA | ||
| Symptom score during admission, median (range) | 2 (1–4) | 2 (0–4) | 0.9 |
PIBO post-infectious bronchiolitis obliterans, ND not done, NA not available
There was no difference in viral pathogens detected in nasopharyngeal specimens between the two patient groups (data not shown).
Serum levels of YKL-40, VEGF, PDGF-BB, and TGF-β1
Serum YKL-40 levels in the PIBO group were significantly higher than control group levels [1172.2 (IQR 807.7–1569.9) vs. 196.7 (IQR 147.4–570.2) pg/mL, P = 0.0001] and also higher than the bronchiolitis group levels [1172.2 (IQR 807.7–1569.9) vs. 687.7 (IQR 406.9–1231.6) pg/mL, P = 0.01]. Serum YKL-40 levels in the bronchiolitis group were significantly higher compared with those in controls [687.7 (IQR 406.9–1231.6) vs. 196.7 (IQR 147.4–570.2) pg/mL, P = 0.02] (Fig. 1a).
Fig. 1.
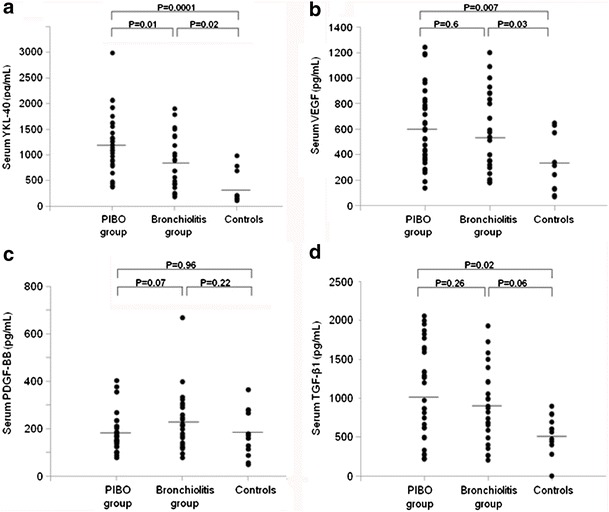
Increased serum YKL-40 levels in both PIBO and bronchiolitis groups compared with those in the controls, which were significantly higher in the PIBO group than in the bronchiolitis group (a). Increased VEGF levels with no difference between two patient groups (b). PDGF-BB levels with no significant difference among the PIBO, bronchiolitis, and control groups (c). Increased TGF-β1 levels in the PIBO group with no difference compared with those in the bronchiolitis group (d)
Serum VEGF levels were significantly increased in both PIBO and bronchiolitis groups compared with control group levels [557.9 (IQR 371.1–793.4) vs. 276.7 (IQR 127.3–511.9) pg/mL, P = 0.007, and 524.7 (IQR 311.1–841.2) vs. 276.7 (IQR 127.3–511.9) pg/mL, P = 0.03, respectively], but showed no difference between those in the PIBO and bronchiolitis groups [557.9 (IQR 371.1–793.4) vs. 524.7 (IQR 311.1–841.2) pg/mL, P = 0.63] (Fig. 1b).
Serum PDGF-BB levels in the PIBO group did not differ from control group levels [161.1 (IQR 139.2–204.9) vs. 166.4 (IQR 107.8–269.1) pg/mL, P = 0.96] and were slightly lower than those in the bronchiolitis group [161.1 (IQR 139.2–204.9) vs. 214.1 (IQR 138.3–291.6) pg/mL, P = 0.07]. PDGF-BB levels in the bronchiolitis group showed no significant difference compared with control group levels [214.1 (IQR 138.3–291.6) vs. 166.4 (IQR 107.8–269.1) pg/mL, P = 0.22] (Fig. 1c).
Serum levels of TGF-β1 in the PIBO group were significantly higher than those in controls [1087.2 (IQR 589.3–1601.1) vs. 601.3 (IQR 462.7–745.9) pg/mL, P = 0.02]. In the bronchiolitis group, TGF-β1 levels also increased but did not reach statistical significance compared with control group levels [848.9 (IQR 564.1–1210.3) vs. 601.3 (IQR 462.7–745.9) pg/mL, P = 0.06]. TGF-β1 levels showed no difference between those in the PIBO and bronchiolitis groups [1087.2 (IQR 589.3–1601.1) vs. 848.9 (IQR 564.1–1210.3) pg/mL, P = 0.26] (Fig. 1d).
Only YKL-40 levels were found to be significantly higher in the PIBO group compared with those in the bronchiolitis group. ROC curve analysis showed that serum YKL-40 level of 1033.5 pg/mL best differentiated PIBO exacerbation from acute bronchiolitis with an area under the ROC curve of 0.702 (95% confidence interval (CI) 0.604 to 0.829, P < 0.01, Fig. 2), a sensitivity of 62.5%, and a specificity of 70.8%.
Fig. 2.
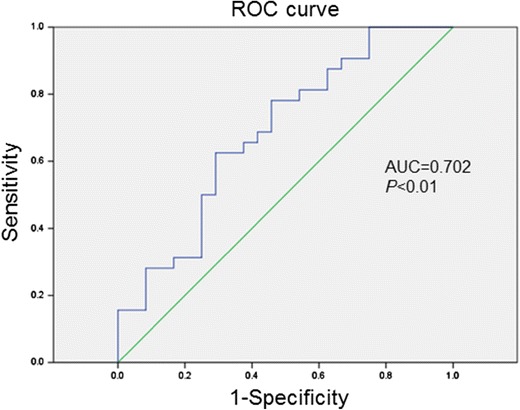
Receiver operating characteristic (ROC) curve for YKL-40 levels to distinguish PIBO exacerbation from acute bronchiolitis. Area under the ROC curve (AUC): 0.702 (95% confidence interval (CI), 0.604 to 0.829)
Relationship between the levels of YKL-40, VEGF, PDGF-BB, and TGF-β1 and clinical or laboratory parameters in the PIBO and bronchiolitis groups
In the PIBO group, serum YKL-40 levels showed a significant correlation with the severity of disease, assessed as sum of scores, before diagnosis of PIBO (r = 0.40, P = 0.03) (Fig. 3). However, severity of acute symptom during current admission showed no significant relationship with YKL-40 levels. Serum YKL-40 levels showed a significant correlation with neutrophil counts in peripheral blood (r = 0.40, P = 0.04). Serum levels of VEGF, PDGF-BB, and TGF-β1 showed no relationship with any clinical or laboratory findings (Table 2).
Fig. 3.
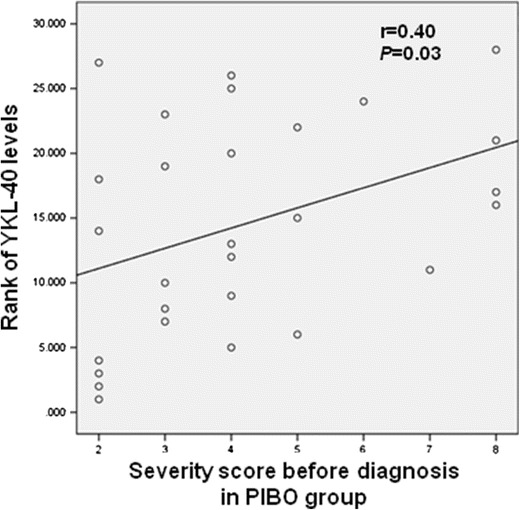
A significant correlation between serum YKL-40 levels and the severity of disease before diagnosis of PIBO
Table 2.
Correlations between levels of YKL-40 and growth factors and clinical parameters in the patients with PIBO
| YKL-40 | VEGF | PDGF-BB | TGF-β1 | |
|---|---|---|---|---|
| Correlation coefficient | r | r | r | r |
| Age |
0.17 P = 0.46 |
−0.12 P = 0.58 |
0.29 P = 0.17 |
0.41 P = 0.07 |
| Interval between initial episode/diagnosis |
0.04 P = 0.86 |
−0.19 P = 0.39 |
0.26 P = 0.24 |
−0.28 P = 0.19 |
|
Severity score before diagnosis |
0.40 P = 0.03* |
0.13 P = 0.53 |
−0.01 P = 0.19 |
0.26 P = 0.98 |
|
Symptom score during admission |
0.10 P = 0.63 |
0.15 P = 0.89 |
0.14 P = 0.16 |
0.07 P = 0.62 |
| Log[serum total IgE] |
0.02 P = 0.95 |
−0.21 P = 0.35 |
0.43 P = 0.05 |
−0.02 P = 0.92 |
| Blood eosinophils |
0.17 P = 0.71 |
−0.18 P = 0.37 |
−0.14 P = 0.59 |
0.11 P = 0.48 |
| Blood neutrophils |
0.40 P = 0.04* |
0.41 P = 0.06 |
0.13 P = 0.45 |
−0.15 P = 0.54 |
PIBO post-infectious bronchiolitis obliterans
*P < 0.05
In the bronchiolitis group, serum levels of YKL-40, VEGF, PDGF-BB, and TGF-β1 showed no relationship with any clinical and laboratory findings (data not shown).
Serum levels of YKL-40, VEGF, PDGF-BB, and TGF-β1 in relation to atopic status in the PIBO group
There was no difference in demographic findings between atopic and non-atopic patients. Interval between onset of disease and diagnosis, severity score before diagnosis, and symptom score during current admission did not show any difference in relation to atopic status of the patients (data not shown).
Serum levels of PDGF-BB and TGF-β1 were significantly higher in atopic patients compared with those in non-atopic patients [174.2 (IQR 149.9–234.0) vs. 143.6 (IQR 108.7–164.5) pg/mL, P = 0.03, and 783.2 (IQR 744.8–1655.6) vs. 743.1 (IQR 275.5–1250.8) pg/mL, P = 0.04, respectively]. YKL-40 and VEGF levels showed no difference between atopic and non-atopic patients [1093.2 (IQR 1093.2–1614.1) vs. 1329.3 (IQR 1066.8–1921.9) pg/mL, P = 0.6, and 495.6 (IQR 344.3–730.4) vs. 590.9 (IQR 362.4–814.0) pg/mL, P = 0.5, respectively].
Discussion
The present study showed that serum YKL-40 levels were increased during exacerbation of pediatric PIBO and were significantly higher compared with those in children with acute bronchiolitis. YKL-40 levels in children with PIBO were positively correlated with the severity of disease before diagnosis.
BO is characterized by peribronchial fibrosis which results in concentric narrowing and obliteration of small airways regardless of the antecedent causes [2]. The children with PIBO are frequently hospitalized with acute exacerbation due to respiratory infection and so were the patients enrolled in this study. However, clinical differentiation of PIBO exacerbation from acute bronchiolitis in young children is often challenging, which might cause treatment delay [7]. The search for non-invasive biomarkers for early diagnosis is needed to prevent chronic lung function impairment associated with PIBO.
Increased serum concentrations of YKL-40 are observed in chronic lung diseases such as asthma, pulmonary fibrosis, and chronic obstructive lung disease (COPD) [20–22]. A recent study suggested that serum YKL-40 could be a biomarker for the development of BO after lung transplantation [9]. In the present study, serum YKL-40 levels were significantly increased in the children with PIBO and showed a good correlation with disease severity before diagnosis. Taken together with the previous results, our study seems to support the assumption that YKL-40 might be involved in the development of PIBO and there might be a common pathway through which various insults could lead to similar pathologic and clinical features in the patients with BO as indicated before [2]. Further investigation including histopathological studies will be needed to clarify the active role of YKL-40 in the pathogenesis of PIBO.
YKL-40 is known as an important regulator of oxidant-induced lung injury [8]. Our results seem to be supported by other studies which have shown that oxidative stress is increased in both post-transplant [23] and post-infectious BO [24].
We examined the children who were admitted with acute bronchiolitis during the same period as the positive controls. Serum YKL-40 levels were also increased in bronchiolitis patients compared with those in the controls, but they were significantly lower compared with those in the patients with PIBO exacerbation. ROC curve analysis showed that measuring serum YKL-40 levels might help distinguish PIBO exacerbation from acute bronchiolitis in young children.
Our study showed a significant correlation between serum YKL-40 levels and peripheral blood neutrophil counts in PIBO patients. This result seems to be in line with that in a previous study that reported a good correlation between serum YKL-40 levels and peripheral neutrophils in the children with severe asthma [25]. Although the major source of YKL-40 is known to be alveolar macrophages and epithelial cells [26, 27], it has been reported that YKL-40 can be stored in the specific granules of neutrophils and released upon activation [25].
It has been suggested that VEGF, a potent angiogenic factor, may enhance airway obliteration in post-transplant BO by increasing the proliferation of airway smooth muscle cells through inducing PDGF signaling [14]. TGF-β1, an important mediator of lung fibrosis and remodeling, was reported to be associated with the development of BO after human lung transplantation [15] and overexpressed in the patients with BO caused by toxic-fume inhalation [28]. VEGF and TGF-β1 have also been reported to be increased during acute viral respiratory infections in other studies [29, 30]. In our study, serum levels of VEGF and TGF-β1 were significantly higher in the PIBO group than those in the controls. These results suggest that those growth factors might also be involved in the pathogenesis of PIBO in young children. However, unlike YKL-40, there was no difference in growth factor levels between the patients with PIBO exacerbation and those with acute bronchiolitis. We think more studies including serial measurement might be needed to clarify the role of growth factors as possible biomarkers for PIBO in children.
In our study, TGF-β1 and PDGF-BB levels were significantly higher in atopic than in non-atopic patients. Those growth factors are known to be involved in remodeling of asthmatic airways. Although thickening of reticular basement membrane and subepithelial fibrosis are components of airway remodeling in asthma, similar changes are observed in BO [31]. A previous study reported that the structural changes were observed only in atopic but not in non-atopic subjects with asymptomatic airway hyperresponsiveness [32]. A recent study also showed that atopy was associated with increased airway smooth muscle area in preschool children with severe recurrent wheeze [33]. Although there was no significant difference in clinical findings, taken together with these previous results, our study seems to suggest that the molecular pathogenesis might be different between atopic and non-atopic children with PIBO. We think a future long-term follow-up study is needed to determine the outcome of PIBO in relation to atopic status of the children.
The present study has limitations. YKL-40 and growth factor levels were not measured repeatedly over time and were not studied in the patients without exacerbation, which would be necessary to demonstrate their prolonged effects in the airways of young children and development of PIBO. Although our results showed some relationship between YKL-40 levels and clinical severity of disease, histopathological findings will be needed to clarify the role of YKL-40 in the pathogenesis of PIBO. However, to our knowledge, our study showed for the first time that YKL-40, known to play a role in the development of BO after lung transplantation or other lung injuries, is also increased in the serum of the children who developed BO after respiratory infection.
In conclusion, our study showed that serum YKL-40 levels were significantly increased in the children admitted with acute exacerbation of PIBO and had a positive correlation with the severity of disease before diagnosis of PIBO. It suggests that measuring serum YKL-40 levels might help distinguish exacerbation of PIBO from acute bronchiolitis in young children and seems to support the assumption that YKL-40 might be involved in the pathogenesis of PIBO.
Abbreviations
- PDGF
Platelet-derived growth factor
- ROC
Receiver operating characteristic
- PIBO
Post-infectious bronchiolitis obliterans
- TGF
Transforming growth factor
- VEGF
Vascular endothelial growth factor
- IQR
Interquartile range
Authors’ contributions
Jang YY, Park HJ, and Chung HL had full access to all of the data in the present study and took responsibility for the integrity of the data and also contributed to the study design, data analysis and interpretation, and writing of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Informed written consents were obtained from all the parents and this study was approved by the Daegu Catholic University Medical Center Institutional Review Board (IRB No. CR-16-015) and was performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Revisions received: 4 January 2017 / 18 May 2017
Contributor Information
Yoon Young Jang, Email: yyjang0117@gmail.com.
Hye Jin Park, Email: grapfarm@daum.net.
Hai Lee Chung, Email: hlchung@cu.ac.kr.
References
- 1.Moonnumakal SP, Fan LL. Bronchiolitis obliterans in children. Curr Opin Pediatr. 2008;20:272–278. doi: 10.1097/MOP.0b013e3282ff62e9. [DOI] [PubMed] [Google Scholar]
- 2.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. doi: 10.1056/NEJMra1204664. [DOI] [PubMed] [Google Scholar]
- 3.Fischer GB, Sarria EE, Mattiello R, Mocelin HT, Castro-Rodriguez JA. Post infectious bronchiolitis obliterans in children. Paediatr Respir Rev. 2010;11:233–239. doi: 10.1016/j.prrv.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Tomikawa SO, Rodrigues JC. Current research on pediatric patients with bronchiolitis obliterans in Brazil. Intractable Rare Dis Res. 2015;4:7–11. doi: 10.5582/irdr.2014.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguerre V, Castaños C, Pena HG, Grenoville M, Murtagh P. Postinfectious bronchiolitis obliterans in children: clinical and pulmonary function findings. Pediatr Pulmonol. 2010;45:1180–1185. doi: 10.1002/ppul.21304. [DOI] [PubMed] [Google Scholar]
- 6.Colom AJ, Maffey A, Garcia Bournissen F, Teper A. Pulmonary function of a paediatric cohort of patients with postinfectious bronchiolitis obliterans. A long term follow-up. Thorax. 2015;70:169–174. doi: 10.1136/thoraxjnl-2014-205328. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Liu C, Wang M, Zhang YI, Li H, Liu G. Clinical features of post-infectious bronchiolitis obliterans in children undergoing long-term azithromycin treatment. Exp Ther Med. 2015;9:2379–2383. doi: 10.3892/etm.2015.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaksch P, Taghavi S, Klepetko W, Salama M. Pretransplant serum human chitinase-like glycoprotein YKL-40 concentrations independently predict bronchiolitis obliterans development in lung transplant recipients. J Thorac Cardiovasc Surg. 2014;148:273–281. doi: 10.1016/j.jtcvs.2014.02.059. [DOI] [PubMed] [Google Scholar]
- 10.Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep. 2013;13:1–9. doi: 10.1007/s11882-012-0317-9. [DOI] [PubMed] [Google Scholar]
- 11.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 12.Nishioka Y, Azuma M, Kishi M, Aono Y. Targeting platelet-derived growth factor as a therapeutic approach in pulmonary fibrosis. J Med Investig. 2013;60:175–183. doi: 10.2152/jmi.60.175. [DOI] [PubMed] [Google Scholar]
- 13.Krebs R, Tikkanen JM, Nykänen AI, et al. Dual role of vascular endothelial growth factor in experimental obliterative bronchiolitis. Am J Respir Crit Care Med. 2005;171:1421–1429. doi: 10.1164/rccm.200408-1001OC. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, Ramachandran S, Gunasekaran M, et al. MicroRNA-144 dysregulates the transforming growth factor-β signaling cascade and contributes to the development of bronchiolitis obliterans syndrome after human lung transplantation. J Heart Lung Transplant. 2015;34:1154–1162. doi: 10.1016/j.healun.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alho HS, Maasilta PK, Vainikka T, Salminen US. Platelet-derived growth factor, transforming growth factor-beta, and connective tissue growth factor in a porcine bronchial model of obliterative bronchiolitis. Exp Lung Res. 2007;33:303–320. doi: 10.1080/01902140701539745. [DOI] [PubMed] [Google Scholar]
- 16.Jones MH, Pitrez PM, Stein RT. Post-infectious bronchiolitis obliterans. Pediatr Pulmonol Suppl. 2004;26:64–65. doi: 10.1002/ppul.70054. [DOI] [PubMed] [Google Scholar]
- 17.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 18.Shin JY, Mi J, Lee KH, et al. Clinical characteristics of post-infectious bronchiolitis obliterans. Pediatr Allergy Respir Dis (Korea) 2011;21:156–164. doi: 10.7581/pard.2011.21.3.156. [DOI] [Google Scholar]
- 19.Chung HL, Lee EJ, Park HJ, et al. Increased epidermal growth factor in nasopharyngeal aspirates from infants with recurrent wheeze. Pediatr Pulmonol. 2015;50:841–847. doi: 10.1002/ppul.23083. [DOI] [PubMed] [Google Scholar]
- 20.Chupp GL, Lee CG, Jarjour N, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 21.Furuhashi K, Suda T, Nakamura Y, et al. Increased expression of YKL-40, a chitinase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis. Respir Med. 2010;104:1204–1210. doi: 10.1016/j.rmed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Gumus A, Kayhan S, Cinarka H, et al. High serum YKL-40 level in patients with COPD is related to hypoxemia and disease severity. Tohoku J Exp Med. 2013;229:163–170. doi: 10.1620/tjem.229.163. [DOI] [PubMed] [Google Scholar]
- 23.Behr J, Maier K, Braun B, Vogelmeier C. Evidence for oxidative stress in bronchiolitis obliterans syndrome after lung and heart-lung transplantation. The Munich Lung Transplant Group. Transplantation. 2000;69:1856–1860. doi: 10.1097/00007890-200005150-00020. [DOI] [PubMed] [Google Scholar]
- 24.Mallol J, Aguirre V, Espinosa V. Increased oxidative stress in children with post infectious bronchiolitis obliterans. Allergol Immunopathol (Madr) 2011;39:253–258. doi: 10.1016/j.aller.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Konradsen JR, James A, Nordlund B, et al. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J Allergy Clin Immunol. 2013;132:328–335. doi: 10.1016/j.jaci.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Létuvé S, Kozhich A, Arouche N, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181:5167–5137. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 27.Park JA, Drazen JM, Tschumperlin DJ. The chitinase-like protein YKL-40 is secreted by airway epithelial cells at base line and in response to compressive mechanical stress. J Biol Chem. 2010;285:29817–29825. doi: 10.1074/jbc.M110.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarin AA, Behmanesh M, Tavallaei M, Shohrati M, Ghanei M. Overexpression of transforming growth factor (TGF)-beta1 and TGF-beta3 genes in lung of toxic-inhaled patients. Exp Lung Res. 2010;36:284–291. doi: 10.3109/01902140903578868. [DOI] [PubMed] [Google Scholar]
- 29.De Silva D, Dagher H, Ghildyal R, et al. Vascular endothelial growth factor induction by rhinovirus infection. J Med Virol. 2006;78:666–672. doi: 10.1002/jmv.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornburg NJ, Shepherd B, Crowe JE., Jr Transforming growth factor beta is a major regulator of human neonatal immune responses following respiratory syncytial virus infection. J Virol. 2010;84:12895–12902. doi: 10.1128/JVI.01273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int. 2008;57:1–10. doi: 10.2332/allergolint.R-07-154. [DOI] [PubMed] [Google Scholar]
- 32.Sohn SW, Chang YS, Lee HS, et al. Atopy may be an important determinant of subepithelial fibrosis in subjects with asymptomatic airway hyperresponsiveness. J Korean Med Sci. 2008;23:390–396. doi: 10.3346/jkms.2008.23.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lezmi G, Gosset P, Deschildre A, et al. Airway remodeling in preschool children with severe recurrent wheeze. Am J Respir Crit Care Med. 2015;192:164–171. doi: 10.1164/rccm.201411-1958OC. [DOI] [PubMed] [Google Scholar]


