Abstract
The pathogenesis of SARS-CoV remains largely unknown. To study the function of the SARS-CoV nucleocapsid protein, we have conducted a yeast two-hybrid screening experiment to identify cellular proteins that may interact with the SARS-CoV nucleocapsid protein. Pyruvate kinase (liver) was found to interact with SARS-CoV nucleocapsid protein in this experiment. The binding domains of these two proteins were also determined using the yeast two-hybrid system. The physical interaction between the SARS-CoV nucleocapsid and cellular pyruvate kinase (liver) proteins was further confirmed by GST pull-down assay, co-immunoprecipitation assay and confocal microscopy. Cellular pyruvate kinase activity in hepatoma cells was repressed by SARS-CoV nucleocapsid protein in either transiently transfected or stably transfected cells. PK deficiency in red blood cells is known to result in human hereditary non-spherocytic hemolytic anemia. It is reasonable to assume that an inhibition of PKL activity due to interaction with SARS-CoV N protein is likely to cause the death of the hepatocytes, which results in the elevation of serum alanine aminotransferase and liver dysfunction noted in most SARS patients. Thus, our results suggest that SARS-CoV could reduce pyruvate kinase activity via its nucleocapsid protein, and this may in turn cause disease.
Keywords: Pyruvate Kinase, HuH7 Cell, Severe Acute Respiratory Syndrome, Pyruvate Kinase Activity, Murine Hepatitis Virus Type
Introduction
The etiology of SARS (severe acute respiratory syndrome) is associated with a newly discovered coronavirus, SARS-associated coronavirus (SARS-CoV) [24, 28, 30]. SARS-CoV is of animal origin, and its precursor is still present in animal populations [8]. To date, no specific treatment exists to treat infection with this virus [48]. SARS-CoV is only distantly related to members of the other coronavirus clades [18, 24]. Coronaviruses are exceptionally large RNA viruses that employ complex regulatory mechanisms to express their genomes [18, 31]. The genomic structure, gene expression pattern and protein profiles of SARS-CoV are similar to those of other coronaviruses. There are fourteen potential open reading frames (ORF) in the genome of SARS-CoV. Nine SARS-CoV-specific mRNAs have been shown to be synthesized in virus-infected cells. These RNAs are predicted to encode four structural proteins (spike, membrane, envelope, and nucleocapsid [N] proteins), sixteen non-structural proteins and eight accessory proteins [31, 36]. As a structural protein, SARS-CoV N protein has been demonstrated to interact with itself and other structural proteins [16, 21, 34, 50]. SARS-CoV M and E proteins are also able to interact with each other and other structural proteins [1, 5, 16].
The pathogenesis of SARS-CoV remains largely unknown [40]. SARS-CoV E protein is thought to interact with PALS1 and alter tight junction formation and epithelial morphogenesis [37]. The N protein of murine hepatitis virus type 3, another member of the family Coronaviridae, is known to cause fulminant hepatitis [26]. SARS-CoV N protein has multiple activities [35]. In addition to its role as a structural protein that packages the viral genome, the SARS-CoV N protein has also been demonstrated to interact with cellular proteins, and it also performs regulatory functions [14, 33, 41, 42, 51–54]. To study the function of the SARS-CoV N protein, a yeast two-hybrid screening experiment was conducted to identify cellular proteins that may interact with the SARS-CoV N protein. Although the lungs and immune system are the organs that sustain the most severe damage during SARS-CoV infection, the tissue tropism of this virus includes not only the lung but also the gastrointestinal tract, kidney and liver [9, 12, 13, 40]. Liver tissues of SARS autopsies have shown hepatocyte mitoses, balloon degeneration of hepatocytes, lymphocytic infiltrates, fatty degeneration, or central lobular necrosis [4, 7, 12, 19, 32]. Abnormal liver function (e.g., elevated serum aminotransferase levels) is often found in SARS patients [6, 43, 47]. Infection of SARS-CoV in liver has also been demonstrated in animals experimentally infected by this virus [11, 45]. Hepatoma cell lines could also be infected by pseudotype viruses with SARS-CoV S proteins [15]. Therefore, the cDNA library used for the yeast two-hybrid screening in this study was derived from liver, a target organ of SARS-CoV [2, 12, 27].
Materials and methods
Plasmid construction
To isolate the DNA fragment that contains the SARS-CoV N protein coding sequence, PCR reactions were performed using SARS-CoV cDNA [16, 20] as template and the oligomers 5′ CGCGGATCC ATGTCTGATAATGGACC 3′ and 5′ TGCTCTAGA TTATGCCTGAGTTGAATC 3′ as primers. After PCR, the DNA fragment was digested by the restriction enzymes BamHI and XbaI and inserted into the vectors pcDNA3 (Invitrogene, USA) and pcDNA3-myc [23], which had been linearized with BamHI and XbaI, for transient expression. This SARS-CoV N DNA fragment was also amplified using the primers 5′ CGGAATTC ATGTCTGATAATGGACCC 3′ and 5′ TGCTCTAGATGCCTGAGTTGAATC 3′, digested by treatment with the restriction enzymes EcoRI and XbaI, and inserted into the vector pcDNA3.1-V5-HisA (Invitrogen, USA), which had been linearized with EcoRI and XbaI, for stable expression.
To clone the DNA fragment encoding the SARS-CoV N protein for yeast two-hybrid screening, the oligonucleotide primers 5′ CGGAATTCATGTCTGATAATGGACCC 3′ and 5′ TTATTATGCCTGAGTTGAATC 3′ were used to perform PCR. After PCR, the DNA fragment was treated with T4 polynucleotide kinase, digested by treatment with the restriction enzyme EcoRI, and cloned into the pBDGal4 Cam (Stratagene, USA) expression vector, which had been linearized with EcoRI and SmaI. The first 220 codons of this DNA fragment were cloned using the same forward primer and a different reverse primer (5′ CTATTAGAGGGCAGTTTCACCACC 3′). The same reverse primer and another forward primer (5′ CGGAATTCGCGCTATTGCTGCTAGA 3′) were used to clone the DNA fragment without the first 220 codons.
To clone the full-length pyruvate kinase (liver) DNA fragment (Gene ID: 5313; gi: 19343992) for yeast two-hybrid screening, PCR was performed using the oligomers 5′ CCGAATTCGCATGTCGATCCAGGAGAA 3′ and 5′ CCGCTCGAG TCAGGATATGCTTAGCAC 3′ as primers and the cDNA library from HuH7 cells as template. After PCR, the DNA fragment was digested using the restriction enzymes EcoRI and XhoI and cloned into the pACT2 (Clontech, USA) expression vector (linearized by EcoRI/XhoI). The same forward primer and a different reverse primer (5′ CCGCTCGAG TTATGACAGCATGATGCAGTC 3′) were used to clone the DNA fragment that contained the first 405 codons of the PKL sequence for yeast two-hybrid screening. The same forward primer and a different reverse primer (5′ CCGCTCGAG TTACTGCTCGGACAGCCC 3′) were used to clone the DNA fragment that contained the first 267 codons of the PKL sequence for yeast two-hybrid screening. To clone the C-terminal domain (aa 352-530) of the M2PK gene (Gene ID: 5315; NM_002654.3) into the pACT2 expression vector (linearized by EcoRI/XhoI), the primers 5′ CCGAATTCGCGTCCTGGATGGAGCCGACT 3′ and 5′ CCGCTCGAG TCACGGCACAGGAACAAC 3′ and the cDNA library from NIH3T3 cells were used. To clone the full-length PKL for transient expression in mammalian cells, the primers 5′ CCGAATTCGCATGTCGATCCAGGAGAA 3′ and 5′ TGCTCTAGAGGATATGCTTAGCAC 3′ were used to perform PCR. After PCR, the DNA fragment was digested using the restriction enzymes EcoRI and XbaI and inserted into the vector pcDNA3.1-V5-HisA, which had been linearized with EcoRI and XbaI.
To clone the DNA fragment encoding the full-length SARS-CoV N protein for the expression of a GST-N fusion form of the protein, the primers 5′ CGCGGATCC ATGTCTGATAATGGACC 3′ and 5′ CGGAATTC TTATGCCTGAGTTGAATC 3′ were used to perform the PCR. After PCR, the DNA fragment was digested using restriction enzymes BamHI and EcoRI and cloned into the pGEX2T (Amersham Biosciences, USA) expression vector, which had been linearized by BamHI and EcoRI. All of the expression plasmids were verified by sequencing.
Yeast two-hybrid screening
The yeast two-hybrid system used for screening was purchased from Clontech Laboratories (USA). The screening procedures were conducted following the manufacturer’s instructions [23]. The cDNA library used for this screening was a human fetal liver library, HL4029AH (Clontech, USA).
The GST pull-down assay
Glutathione-S-transferase (GST) and GST-fusion proteins were purified following the manufacturer’s instructions (Pharmacia, USA). Vero E6 cells were maintained in RPMI 1640 medium containing 10% fetal calf serum, 1% glutamine (200 mM, Biological Industries, USA), and 100 μg/ml penicillin/streptomycin (Gibco BRL, USA). A total of 2.5 × 106 cells were plated in a 100-mm dish. After overnight incubation, cells were transfected with 4 μg plasmid DNA (vector pcDNA3.1-V5-His A or pcDNA3.1-PKL expression plasmid) using Effectene transfection reagent (QIAGEN, Germany). At 48 hours after transfection, recombinant proteins in the cells were released by lysis in buffer containing 50 mM Tris-HCl (pH 7.4), 300 mM NaCl, 10 mM NaN3, 0.2 mM DTT, 1% NP-40 and 1x protease inhibitor cocktail (Sigma, USA) and then analyzed by GST pull-down assay. In total, 10% of the cell lysate was loaded directly onto the SDS-PAGE gel to serve as a control and 90% of the cell lysate was incubated at 4°C with about 2 μg purified GST or GST-fusion protein. After overnight incubation, glutathione beads (Pharmacia, USA) were added to the reaction mixture. Two hours later, the samples were centrifuged. After washing, the pellet was resuspended in the protein sample buffer and analyzed by SDS-PAGE and western blotting. In order to detect the PKL protein using western blotting analysis, mouse anti-V5 monoclonal antibody (Invitrogen, USA) was used as the primary antibody.
Co-immunoprecipitation assay
HuH 7 or Vero E6 cells (2 × 106) were harvested 48 hours after transfection with the expression plasmids pcDNA3-myc-N and/or pcDNA3.1-PKL (or using the N-terminus of PKL or the C-terminus of PKL instead of full-length PKL), washed three times in PBS, and lysed in RIPA buffer (150 mM NaCl, 1% NP40, 0.5% deoxychloic acid, 0.1% SDS and 50 mM Tris, pH 7.5). After centrifugation for 5 minutes at full speed in a microcentrifuge, the supernatant was used for further analysis. In each experiment, 10% of the supernatant was used directly for expression analysis by western blotting assay, while 90% of the supernatant was used for the co-immunoprecipitation assay. The supernatant was incubated with homemade rabbit anti-nucleocapsid polyclonal antibody (or anti-myc) at 4°C overnight with shaking. The antigen-antibody complexes were then pulled down using PANSORBIN cells (Merck, USA). The immunoprecipitated pellets were treated at 100°C in the sample buffer (67.5 mM Tris-HCl (pH 6.8), 5% 2-mercaptoethanol, 3% SDS, 0.1% bromophenol blue and 10% glycerol) for 10 minutes, and this was followed by SDS-PAGE and western blotting analysis. In order to detect PKL protein, alkaline-phosphatase-conjugated anti-V5 antibody (Invitrogen, USA) was used, while mouse anti-myc monoclonal antibody (Oncogene, USA) (or homemade rabbit anti-nucleocapsid polyclonal antibody) was used as the primary antibody to detect SARS-CoV N protein.
Western blotting analysis
After electrophoresis, the SDS-PAGE gel was transferred to PVDF paper (Pall Corporation, USA). All procedures were then carried out at room temperature following our previous procedures [20, 22] except that the primary antibodies used in this study were different, namely anti-PK rabbit monoclonal antibody (Abcam, USA), anti-ERK-2 rabbit polyclonal antibody (Santa Cruz, USA), etc., as appropriate.
Confocal analysis
About 2.5 × 105 Huh7 or Vero E6 cells were seeded in 35-mm culture dishes. After overnight incubation, the cells were transfected with various different plasmids (pcDNA3-myc-N and/or pcDNA3.1-PKL) using an Effectene transfection kit (QIAGEN, Germany). At 48 hours after transfection, the recombinant proteins in the cells were analyzed. All procedures were then carried out following our previous procedures [5, 16, 46] except that the antibodies used in this study were different. Specifically, anti-myc mouse monoclonal antibody (Upstate, USA) followed by RITC-conjugated anti-mouse IgG antibody was used to detect SARS-CoV N protein, and FITC-conjugated anti-V5 monoclonal antibody (Invitrogen, USA) was used to detect PKL protein. DAPI (Merck, Germany) was used to stain DNA for localization in the nucleus. Finally, the samples were observed under a confocal microscope (Leica TCS SP2).
Pyruvate kinase activity assay
HuH7 or Vero E6 cells (2 × 106) in 100-mm dishes were harvested 48 hours after transfection with the expression plasmids pcDNA3-myc vector, pcDNA3-myc-N or pcDNA3.1-PKL for transient expression, washed three times in PBS, and then lysed in RIPA buffer. The cellular pyruvate kinase activity was determined following published procedures [10, 39]. For the activity assay in the stable clones, 48 hours after transfection with the pcDNA3.1-N plasmid, the HuH7 cells were treated with 800 μg/ml G418 for selection. After seven days, the transfected cells were harvested and the pyruvate kinase activity assay carried out.
RNAi experiments
RNAi experiments were performed using a lentiviral expression system (http://rnai.genmed.sinica.edu.tw), following the manufacturer’s instructions. RNAi reagents were obtained from the National RNAi Core Facility located at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica.
Results and discussion
Identification of pyruvate kinase (liver) as an interactive protein of SARS-CoV nucleocapsid protein by yeast two-hybrid screening
The SARS-CoV N protein was used as the bait for yeast two-hybrid screening in order to identify cellular proteins that interact with it. Only one cDNA clone, which encoded the C-terminal domain of the pyruvate kinase (liver) (PKL) protein (aa 406-581), was found to interact with the N protein, out of a total of 1 × 106 transformants (Fig. 1). This yeast two-hybrid system was further used to identify the interactive domains of these two proteins. As shown in Fig. 1, the deletion of the C-terminal sequence of PKL downstream of amino acid 406 abolished the interaction between these two proteins in the YTH assay. Similar deletion-mapping experiments were conducted on the SARS-CoV N protein. As shown in Fig. 1, deletion of the C-terminal sequence of the N protein downstream of amino acid 220 had no significant effect on the binding of these two proteins. However, deletion of the N-terminal sequence upstream of amino acid 221 abolished the interaction of these two proteins in the YTH assay. These results indicate that the N-terminal domain of the N protein interacts with the C-terminal domain of the PKL protein.
Fig. 1.
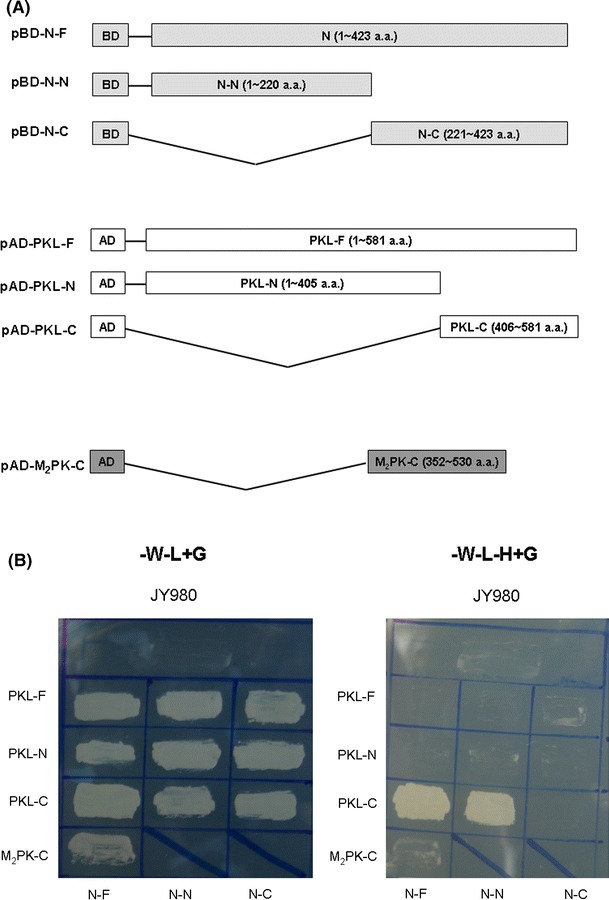
The N-terminal domain of SARS-CoV N protein interacts with the C-terminal domain of the PKL protein in the yeast two-hybrid system. A The various constructs used for the yeast two-hybrid system. B Growth of yeast (JY980), either mock-transfected or transfected with plasmids as indicated on YEPD without tryptophan and leucine (left panel), or on YEPD without tryptophan, leucine and histidine (right panel)
There are four different PK isoenzymes present in mammalian tissues [17]. These are M1 (in skeletal muscle), M2 (in kidney, adipose tissue, and lungs), L (in liver), and R (in red blood cells). The M1 and M2 proteins are the products of alternative splicing of the same mRNA. The other two mammalian PK isoenzymes from the liver and erythrocytes are encoded by the same PKLR gene but by tissue-specific alternate promoters [17, 25]. To determine whether the C-terminal domain of the M2-PK protein, an isoform of the PKL protein, interacts with SARS-CoV N protein, similar experiments were performed (Fig. 1). Unlike the PKL protein, the C-terminal domain of the M2-PK protein does not interact with the SARS-CoV N protein (Fig. 1B). The differences in the C-terminal domains of the PKL and M2-PK proteins therefore appear to affect the interaction with the SARS-CoV N protein [25]. The M2-PK protein has been demonstrated to interact with Rous sarcoma virus [29], human papilloma virus HPV-16 E7 oncoprotein [55], and hepatitis C virus NS5B protein [44]. However, to our knowledge, no other viral protein has been reported to interact with the PKL protein to date.
Physical interaction between SARS-CoV N and PKL proteins in vitro
To confirm that the SARS-CoV N and PKL proteins could indeed bind to each other, we conducted a GST pull-down experiment. To avoid the interaction between endogenous PKL in HuH7 cells and SARS-CoV N protein, the PKL protein was expressed exogenously in Vero cells before lysis. As shown in Fig. 2B, this PKL protein was pulled down by GST-N fusion protein (lane 6) but not by the GST control protein (lane 5). This result confirmed that SARS-CoV N protein is indeed able to bind to PKL in vitro.
Fig. 2.
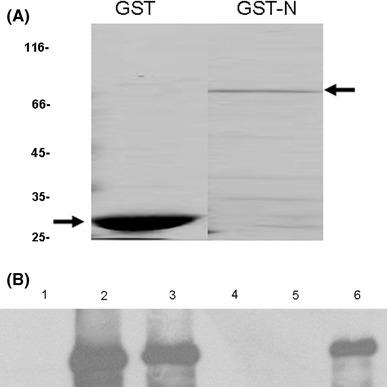
GST-pull down experiment for the analysis of the interaction between SARS-CoV N and cellular PKL proteins. (A) Coomassie blue staining of purified GST (lane 1) and GST-N (lane 2) used in this experiment. (B) Vero E6 cells were transfected with empty vector (lanes 1 and 4) or PKL-V5 (lanes 2, 3, 5 and 6). At 48 h after transfection, cell lysates were extracted. Samples consisting of 10% of each cell lysate were then loaded directly onto an SDS-PAGE gel to serve as controls (lanes 1, 2 and 3). The remaining 90% of the cell lysates were incubated with an equal amount of purified GST (lane 5) and GST-N (lanes 4 and 6). The pulled-down samples were analyzed by western blotting using the anti-V5 antibody
Physical interaction between SARS-CoV N and PKL proteins in cultured cells
To test further whether SARS-CoV N and PKL proteins are able to bind to each other in cells, we performed a co-immunoprecipitation experiment. V5-tagged full-length PKL protein and myc-tagged N protein were co-expressed in HuH 7 cells by transient transfection. Forty-eight hours after transfection, cell lysate was immunoprecipitated with anti-myc antibody followed by western blotting using the anti-V5 antibody. As shown in Fig. 3A, the V5-tagged PKL protein was immunoprecipitated by the anti-myc antibody in the presence (lane 8), but not in the absence (lane 6), of the N protein. This result confirmed that SARS-CoV N and PKL proteins are able to bind to each other in cultured cells. The same experiment was also performed in Vero cells (Fig. 3B). The co-immunoprecipitation of these two proteins in different cells further supports the direct interaction between these two proteins. To avoid the interaction between endogenous PKL in HuH7 cells and SARS-CoV N protein, experiments mapping the binding domain of PKL with SARS-CoV N protein were done in Vero cells. The C-terminus (aa 406-581) but not the N-terminus (aa 1-405), of the PKL protein was found to interact with the SARS-CoV N protein using co-immunoprecipitation experiments (Fig. 3C, D).
Fig. 3.
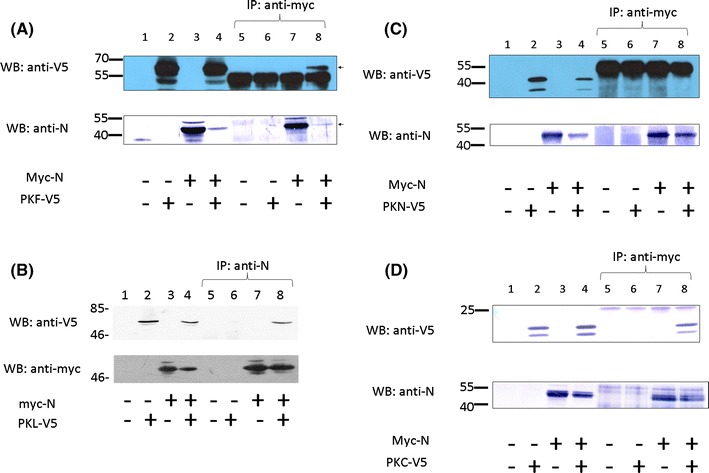
Co-immunoprecipitation experiments on SARS-CoV N protein and PKL protein in HuH7 cells (A) or in Vero E6 cells (B). (A) HuH7 cells were mock transfected (lanes 1 and 5), transfected with a plasmid allowing expression of the PKL protein with the V5 tag (lanes 2 and 6) or the SARS-CoV N protein with the myc tag (lanes 3 and 7), or co-transfected with both these two constructs (lanes 4 and 8). Cell lysates were analyzed directly by western blot (lanes 1-4) or were immunoprecipitated with the anti-myc antibody prior to western blotting (lanes 5-8). Upper panel, western blot analysis carried out using anti-V5 antibody; lower panel, western blot analysis carried out using anti-N antibody. (B) All of the experimental procedures are similar to those described in (A) except Vero cells were used. (C and D) Co-immunoprecipitation experiments on SARS-CoV N protein and the N-terminus (aa 1-405) of the PKL protein (C) or the C-terminus (aa 406-581) of the PKL protein (D) expressed in cells. All of the experimental procedures are the similar to those described in (B) except the N-terminus of PKL (C) or the C-terminus of PKL (D) was used instead of full-length PKL
The SARS-CoV N protein is a cytoplasmic protein [16] that has a nuclear localization signal (NLS) [49]. PKL is also a cytosolic protein [25]. If SARS-CoV N and PKL proteins are indeed able to bind to each other in cells, then it is likely that they will be co-localized in cells. For this reason, we performed confocal microscopy to examine the subcellular localization of these two proteins. HuH7 or Vero E6 cells were transfected with DNA plasmids that expressed full-length PKL protein and SARS-CoV N proteins, either individually or together (Fig. 4A, B). When these two proteins were expressed individually, both proteins are localized predominately in the cytoplasm (data not shown). When these two proteins were expressed together, PKL protein was found to be co-localized almost entirely with the SARS-CoV N protein in the cytoplasm (Fig. 4A, B). These results further indicated that SARS-CoV N and PKL proteins are able to physically interact with each other in cultured cells.
Fig. 4.
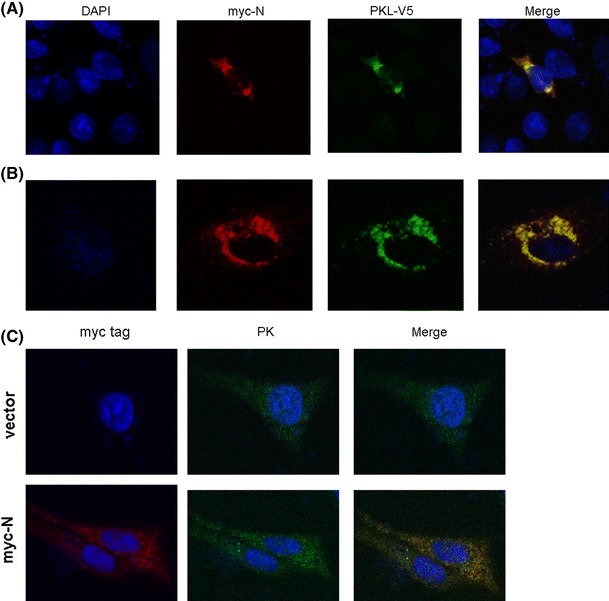
Confocal microscopy analysis of SARS-CoV N and exogenous PKL proteins in cultured cells. HuH7 (A) or Vero E6 (B) cells were transfected with the plasmid that expressed the SARS-CoV N protein with a myc tag and the plasmid that expressed the PKL protein with the V5 tag. Forty-eight hours after transfection, the cells were fixed and stained with mouse anti-myc, followed by RITC-conjugated anti-mouse antibody, and finally with FITC-conjugated anti-V5 antibodies. Blue, nucleus stained with DAPI; red, SARS-CoV N protein; green, PKL protein; yellow, colocalization of SARS-CoV N and PKL proteins. (C) Confocal microscopy analysis of SARS-CoV N and intracellular PK proteins in Vero E6 cells. Cells were transfected either with empty vector (upper panel) or with the plasmid expressing the SARS-CoV N protein with a myc tag (lower panel). Cells were fixed and stained with mouse anti-myc and rabbit anti-PK antibodies, followed by RITC-conjugated anti-mouse and FITC-conjugated anti-rabbit antibodies. Blue, nucleus stained with DAPI; red, SARS-CoV N protein; green, PK protein; yellow, colocalization of SARS-CoV N and PK proteins
The PK activity in HuH7 cells is repressed by SARS-CoV N protein in both transiently transfected and stably transfected systems
PK, a key enzyme in the glycolytic pathway, catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate with the synthesis of ATP [25]. PK is a 200-kDa tetramer with four identical subunits, each consisting of four domains, namely domains N (residues 1-42), A, which is subdivided into A1 and A2 (residues 43-115 and 224-387, respectively, of the F. silvestris muscle PK sequence), B (residues 116-223), and C (residues 388-530). The active site lies in a pocket between domains A and B, where there is a high degree of identity among various PK sequences from different organisms [25]. Both erythrocyte and liver isoenzymes are activated by PEP (phosphoenolpyruvate) and F1,6BP (fructose 1,6-bisphosphate). The binding site for F1,6BP involves 16 residues within domain C [25]. To determine whether SARS-CoV N is able to affect the function of the PKL protein, the PK activity in hepatoma cells (HuH7) was analyzed following a published procedure [10, 39]. To confirm the accuracy of this assay, PKL shRNA was used as a loss-of-function control. Comparing the shRNA to the luciferase control, PKL shRNA reduced PK activity significantly (Fig. 5). When overexpressed PKL protein was used as a gain-of-function control in HuH7 cells, as expected, the PK activity measured in these transfected cells was much higher than that of vector-transfected cells (data not shown). To determine whether the presence of SARS-CoV N protein was able to affect PKL activity, since these two proteins interact with each other, HuH7 cells were transiently transfected with a plasmid encoding the SARS-CoV N protein (Fig. 5C, D). It was found that the PK activity in HuH7 cells was repressed by N protein in a dose-dependent manner (Fig. 5D), although expression of PKL was not affected by SARS-CoV N protein (Fig. 5C). A HuH7 cell line showing stable expression of SARS-CoV N protein was also established (Fig. 6A). The PK activity in these cells was repressed by the N protein to 60% compared to the vector-transfected HuH7 cells (Fig. 6B), while PKL expression was not affected (Fig. 6A). These results indicated that the SARS-CoV N protein is able to repress PKL activity in both transiently expressed and stably expressed systems (Figs. 5, 6), possibly through interaction with domain C of PKL, which might result in a reduction of F1,6BP interaction in this region. PK deficiency in red blood cells is known to result in human hereditary non-spherocytic hemolytic anemia [38, 39]. Thus, it is reasonable to assume that an inhibition of PKL activity due to interaction with the SARS-CoV N protein (Figs. 5, 6) is likely to cause the death of the hepatocytes, which results in the elevation of serum alanine aminotransferase and liver dysfunction noted in most SARS patients [3, 6, 43, 47]. Whether PKL is able to affect the assembly of SARS-CoV in some way due to its interaction with the N protein needs further investigation.
Fig. 5.
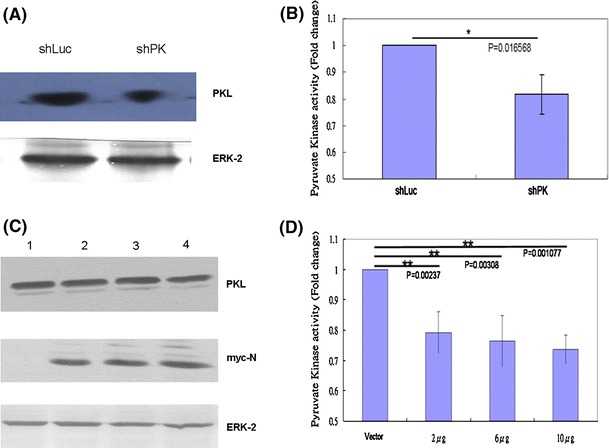
A and B The pyruvate kinase activity was reduced when the PKL gene was knocked down in the cells. (A) Western blotting analysis of PKL expression in HuH7 cells stably expressing either shLuc or shPK. ERK2 protein served as the loading control. (B) The pyruvate kinase activity was measured in HuH7 cells stably expressing either shLuc or shPK. (C and D) The pyruvate kinase activity in HuH7 cells was suppressed by transiently expressed SARS-CoV N protein. (C) Cell lysates were prepared from HuH7 cells transfected with empty vector (lane 1), or 2 μg (lane 2), 6 μg (lane 3), or 10 μg (lane 4) of vector expressing SARS-CoV N protein. Protein expression was detected using antibodies against PKL (upper panel), against myc tag to detect SARS-CoV N protein (middle panel), and against ERK2 protein to serve as a loading control (lower panel). (D) The pyruvate kinase activity was measured in HuH7 cells transfected with empty vector, or 2 μg, 6 μg, or 10 μg of vector expressing SARS-CoV N protein
Fig. 6.
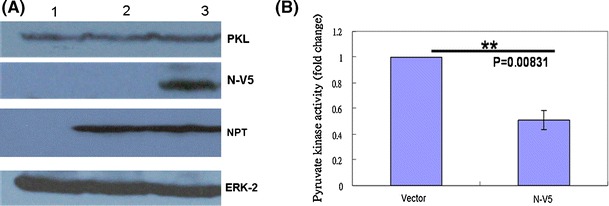
The pyruvate kinase activity in HuH7 cells was suppressed by stably expressed SARS-CoV N protein. (A) Cell lysates were prepared from HuH7 cells that were mock-transfected (lane 1), stably transfected with empty vector (lane 2), or transfected with the plasmid expressing SARS-CoV N protein (lane 3). Protein expression was detected using antibodies against PKL (upper panel), against V5 tag for SARS-CoV N protein, against NPT protein (selection marker), and against ERK2 as a loading control (lower panel). (B) The pyruvate kinase activity was measured in HuH7 cells stably transfected with empty vector or with the plasmid expressing SARS-CoV N protein
The PK activity in Vero E6 cells was not affected by SARS-CoV N protein in the transiently transfected system
Vero E6 cells are often used for the propagation of SARS-CoV [30]. In this study, we have demonstrated that SARS-CoV N protein can be detected as partially co-localized with the endogenous PK protein in Vero E6 cells (Fig. 4C). However, unlike HuH7 cells, the PK activity of Vero E6 cells was not affected by the presence of SARS-CoV N protein (data not shown). This is possibly due to the fact that domain C of the PK protein is highly variable across different PK protein sequences compared, for example, to the more highly conserved domain A [25].
In summary, pyruvate kinase (liver) was found to interact with SARS-CoV nucleocapsid protein in this study using the yeast two-hybrid system, GST pull-down assay, co-immunoprecipitation assay and confocal microscopy. Cellular pyruvate kinase activity in hepatoma cells was repressed by SARS-CoV nucleocapsid protein. Thus, our results suggest that SARS-CoV could reduce pyruvate kinase activity via its nucleocapsid protein, and this may in turn cause abnormal liver function.
Acknowledgments
We thank Dr. Li-Kuang Chen for providing SARS-CoV cDNA. RNAi reagents were obtained from the National RNAi Core Facility located at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, supported by grants from the NSC National Research Program for Genomic Medicine (NSC 94-3112-B-001-003 and NSC 94-3112-B-001-018-Y). This work was supported by grants from the National Science Council of Taiwan (NSC 98-2320-B-320-001-MY3) and from the Tzu Chi University (TCIRP96004-05) to Dr. Shih-Yen Lo.
Footnotes
W.-Y. Wei and H.-C. Li contribute equally to this work.
References
- 1.Alvarez E, DeDiego ML, Nieto-Torres JL, Jimenez-Guardeno JM, Marcos-Villar L, Enjuanes L. The envelope protein of severe acute respiratory syndrome coronavirus interacts with the non-structural protein 3 and is ubiquitinated. Virology. 2010;402:281–291. doi: 10.1016/j.virol.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan-Yeung M, Yu WC. Outbreak of severe acute respiratory syndrome in Hong Kong special administrative region: case report. BMJ. 2003;326:850–852. doi: 10.1136/bmj.326.7394.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan HL, Kwan AC, To KF, Lai ST, Chan PK, Leung WK, Lee N, Wu A, Sung JJ. Clinical significance of hepatic derangement in severe acute respiratory syndrome. World J Gastroenterol. 2005;11:2148–2153. doi: 10.3748/wjg.v11.i14.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SC, Lo SY, Ma HC, Li HC. Expression and membrane integration of SARS-CoV E protein and its interaction with M protein. Virus Genes. 2009;38:365–371. doi: 10.1007/s11262-009-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui HJ, Tong XL, Li P, Hao YX, Chen XG, Li AG, Zhang ZY, Duan J, Zhen M, Zhang B, Hua CJ, Gong YW. Serum hepatic enzyme manifestations in patients with severe acute respiratory syndrome: retrospective analysis. World J Gastroenterol. 2004;10:1652–1655. doi: 10.3748/wjg.v10.i11.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, Cai J, Li X, Kang W, Weng D, Lu Y, Wu D, He L, Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enserink M. Infectious diseases. Clues to the animal origins of SARS. Science. 2003;300:1351. doi: 10.1126/science.300.5624.1351a. [DOI] [PubMed] [Google Scholar]
- 9.Farcas GA, Poutanen SM, Mazzulli T, Willey BM, Butany J, Asa SL, Faure P, Akhavan P, Low DE, Kain KC. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis. 2005;191:193–197. doi: 10.1086/426870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feksa LR, Cornelio A, Dutra-Filho CS, De Souza Wyse AT, Wajner M, Wannmacher CM. Inhibition of pyruvate kinase activity by cystine in brain cortex of rats. Brain Res. 2004;1012:93–100. doi: 10.1016/j.brainres.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Frieman MB, Chen J, Morrison TE, Whitmore A, Funkhouser W, Ward JM, Lamirande EW, Roberts A, Heise M, Subbarao K, Baric RS. SARS-CoV pathogenesis is regulated by a STAT1 dependent but a type I, II and III interferon receptor independent mechanism. PLoS Pathog. 2010;6:e1000849. doi: 10.1371/journal.ppat.1000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He R, Leeson A, Andonov A, Li Y, Bastien N, Cao J, Osiowy C, Dobie F, Cutts T, Ballantine M, Li X. Activation of AP-1 signal transduction pathway by SARS coronavirus nucleocapsid protein. Biochem Biophys Res Commun. 2003;311:870–876. doi: 10.1016/j.bbrc.2003.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann H, Hattermann K, Marzi A, Gramberg T, Geier M, Krumbiegel M, Kuate S, Uberla K, Niedrig M, Pohlmann S. S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J Virol. 2004;78:6134–6142. doi: 10.1128/JVI.78.12.6134-6142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh YC, Li HC, Chen SC, Lo SY. Interactions between M protein and other structural proteins of severe, acute respiratory syndrome-associated coronavirus. J Biomed Sci. 2008;15:707–717. doi: 10.1007/s11373-008-9278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1, 6-bisphosphate. Structure. 1998;6:195–210. doi: 10.1016/S0969-2126(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 18.Lai MM. SARS virus: the beginning of the unraveling of a new coronavirus. J Biomed Sci. 2003;10:664–675. doi: 10.1007/BF02256318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang ZW, Zhang LJ, Zhang SJ, Meng X, Li JQ, Song CZ, Sun L, Zhou YS, Dwyer DE. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS) Pathology. 2003;35:526–531. doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YN, Chen LK, Ma HC, Yang HH, Li HP, Lo SY. Thermal aggregation of SARS-CoV membrane protein. J Virol Methods. 2005;129:152–161. doi: 10.1016/j.jviromet.2005.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo H, Ye F, Chen K, Shen X, Jiang H. SR-rich motif plays a pivotal role in recombinant SARS coronavirus nucleocapsid protein multimerization. Biochemistry. 2005;44:15351–15358. doi: 10.1021/bi051122c. [DOI] [PubMed] [Google Scholar]
- 22.Ma HC, Fang CP, Hsieh YC, Chen SC, Li HC, Lo SY. Expression and membrane integration of SARS-CoV M protein. J Biomed Sci. 2008;15:301–310. doi: 10.1007/s11373-008-9235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma HC, Lin TW, Li H, Iguchi-Ariga SM, Ariga H, Chuang YL, Ou JH, Lo SY. Hepatitis C virus ARFP/F protein interacts with cellular MM-1 protein and enhances the gene trans-activation activity of c-Myc. J Biomed Sci. 2008;15:417–425. doi: 10.1007/s11373-008-9248-9. [DOI] [PubMed] [Google Scholar]
- 24.Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, Khattra J, Asano JK, Barber SA, Chan SY, Cloutier A, Coughlin SM, Freeman D, Girn N, Griffith OL, Leach SR, Mayo M, McDonald H, Montgomery SB, Pandoh PK, Petrescu AS, Robertson AG, Schein JE, Siddiqui A, Smailus DE, Stott JM, Yang GS, Plummer F, Andonov A, Artsob H, Bastien N, Bernard K, Booth TF, Bowness D, Czub M, Drebot M, Fernando L, Flick R, Garbutt M, Gray M, Grolla A, Jones S, Feldmann H, Meyers A, Kabani A, Li Y, Normand S, Stroher U, Tipples GA, Tyler S, Vogrig R, Ward D, Watson B, Brunham RC, Krajden M, Petric M, Skowronski DM, Upton C, Roper RL. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 25.Munoz ME, Ponce E. Pyruvate kinase: current status of regulatory and functional properties. Comp Biochem Physiol B Biochem Mol Biol. 2003;135:197–218. doi: 10.1016/S1096-4959(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 26.Ning Q, Liu M, Kongkham P, Lai MM, Marsden PA, Tseng J, Pereira B, Belyavskyi M, Leibowitz J, Phillips MJ, Levy G. The nucleocapsid protein of murine hepatitis virus type 3 induces transcription of the novel fgl2 prothrombinase gene. J Biol Chem. 1999;274:9930–9936. doi: 10.1074/jbc.274.15.9930. [DOI] [PubMed] [Google Scholar]
- 27.Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon LL, Guan Y, Nicholls JM, Yuen KY, Peiris JS. The aetiology, origins, and diagnosis of severe acute respiratory syndrome. Lancet Infect Dis. 2004;4:663–671. doi: 10.1016/S1473-3099(04)01172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Presek P, Reinacher M, Eigenbrodt E. Pyruvate kinase type M2 is phosphorylated at tyrosine residues in cells transformed by Rous sarcoma virus. FEBS Lett. 1988;242:194–198. doi: 10.1016/0014-5793(88)81014-7. [DOI] [PubMed] [Google Scholar]
- 30.Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Penaranda S, Bankamp B, Maher K, Chen MH, Tong S, Tamin A, Lowe L, Frace M, DeRisi JL, Chen Q, Wang D, Erdman DD, Peret TC, Burns C, Ksiazek TG, Rollin PE, Sanchez A, Liffick S, Holloway B, Limor J, McCaustland K, Olsen-Rasmussen M, Fouchier R, Gunther S, Osterhaus AD, Drosten C, Pallansch MA, Anderson LJ, Bellini WJ. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 31.Satija N, Lal SK. The molecular biology of SARS coronavirus. Ann NY Acad Sci. 2007;1102:26–38. doi: 10.1196/annals.1408.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi X, Gong E, Gao D, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Wu B, Fang W, Liao S, Wang S, Xie Z, Lu M, Hou L, Zhong H, Shao H, Li N, Liu C, Pei F, Yang J, Wang Y, Han Z, Zhang Q, You J, Zhu X, Gu J. Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am J Gastroenterol. 2005;100:169–176. doi: 10.1111/j.1572-0241.2005.40377.x. [DOI] [PubMed] [Google Scholar]
- 33.Surjit M, Liu B, Jameel S, Chow VT, Lal SK. The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochem J. 2004;383:13–18. doi: 10.1042/BJ20040984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surjit M, Liu B, Kumar P, Chow VT, Lal SK. The nucleocapsid protein of the SARS coronavirus is capable of self-association through a C-terminal 209 amino acid interaction domain. Biochem Biophys Res Commun. 2004;317:1030–1036. doi: 10.1016/j.bbrc.2004.03.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surjit M, Lal SK. The SARS-CoV nucleocapsid protein: a protein with multifarious activities. Infect Genet Evol. 2008;8:397–405. doi: 10.1016/j.meegid.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan YJ, Lim SG, Hong W. Characterization of viral proteins encoded by the SARS-coronavirus genome. Antiviral Res. 2005;65:69–78. doi: 10.1016/j.antiviral.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teoh KT, Siu YL, Chan WL, Schluter MA, Liu CJ, Peiris JS, Bruzzone R, Margolis B, Nal B. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol Biol Cell. 2010;21:3838–3852. doi: 10.1091/mbc.E10-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valentini G, Chiarelli LR, Fortin R, Dolzan M, Galizzi A, Abraham DJ, Wang C, Bianchi P, Zanella A, Mattevi A. Structure and function of human erythrocyte pyruvate kinase. Molecular basis of nonspherocytic hemolytic anemia. J Biol Chem. 2002;277:23807–23814. doi: 10.1074/jbc.M202107200. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Chiarelli LR, Bianchi P, Abraham DJ, Galizzi A, Mattevi A, Zanella A, Valentini G. Human erythrocyte pyruvate kinase: characterization of the recombinant enzyme and a mutant form (R510Q) causing nonspherocytic hemolytic anemia. Blood. 2001;98:3113–3120. doi: 10.1182/blood.V98.10.3113. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Rao S, Jiang C. Molecular pathogenesis of severe acute respiratory syndrome. Microbes Infect. 2007;9:119–126. doi: 10.1016/j.micinf.2006.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Li C, Zhang Q, Wang T, Li J, Guan W, Yu J, Liang M, Li D. Interactions of SARS coronavirus nucleocapsid protein with the host cell proteasome subunit p42. Virol J. 2010;7:99. doi: 10.1186/1743-422X-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SM, Wang CT. APOBEC3G cytidine deaminase association with coronavirus nucleocapsid protein. Virology. 2009;388:112–120. doi: 10.1016/j.virol.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu KL, Lu SN, Changchien CS, Chiu KW, Kuo CH, Chuah SK, Liu JW, Lin MC, Eng HL, Chen SS, Lee CM, Chen CL. Sequential changes of serum aminotransferase levels in patients with severe acute respiratory syndrome. Am J Trop Med Hyg. 2004;71:125–128. [PubMed] [Google Scholar]
- 44.Wu X, Zhou Y, Zhang K, Liu Q, Guo D. Isoform-specific interaction of pyruvate kinase with hepatitis C virus NS5B. FEBS Lett. 2008;582:2155–2160. doi: 10.1016/j.febslet.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 45.Xiao Y, Meng Q, Yin X, Guan Y, Liu Y, Li C, Wang M, Liu G, Tong T, Wang LF, Kong X, Wu D. Pathological changes in masked palm civets experimentally infected by severe acute respiratory syndrome (SARS) coronavirus. J Comp Pathol. 2008;138:171–179. doi: 10.1016/j.jcpa.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang CH, Li HC, Jiang JG, Hsu CF, Wang YJ, Lai MJ, Juang YL, Lo SY. Enterovirus type 71 2A protease functions as a transcriptional activator in yeast. J Biomed Sci. 2010;17:65. doi: 10.1186/1423-0127-17-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, Xu M, Yi JQ, Jia WD. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4:60–63. [PubMed] [Google Scholar]
- 48.Yeung KS, Meanwell NA. Recent developments in the virology and antiviral research of severe acute respiratory syndrome coronavirus. Infect Disord Drug Targets. 2007;7:29–41. doi: 10.2174/187152607780090739. [DOI] [PubMed] [Google Scholar]
- 49.You J, Dove BK, Enjuanes L, DeDiego ML, Alvarez E, Howell G, Heinen P, Zambon M, Hiscox JA. Subcellular localization of the severe acute respiratory syndrome coronavirus nucleocapsid protein. J Gen Virol. 2005;86:3303–3310. doi: 10.1099/vir.0.81076-0. [DOI] [PubMed] [Google Scholar]
- 50.Yu IM, Gustafson CL, Diao J, Burgner JW, 2nd, Li Z, Zhang J, Chen J. Recombinant severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein forms a dimer through its C-terminal domain. J Biol Chem. 2005;280:23280–23286. doi: 10.1074/jbc.M501015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Wu K, Wang D, Yue X, Song D, Zhu Y, Wu J. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology. 2007;365:324–335. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang YP, Zhang RW, Chang WS, Wang YY. Cxcl16 interact with SARS-CoV N protein in and out cell. Virol Sin. 2010;25:369–374. doi: 10.1007/s12250-010-3129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao X, Nicholls JM, Chen YG. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J Biol Chem. 2008;283:3272–3280. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou B, Liu J, Wang Q, Liu X, Li X, Li P, Ma Q, Cao C. The nucleocapsid protein of severe acute respiratory syndrome coronavirus inhibits cell cytokinesis and proliferation by interacting with translation elongation factor 1alpha. J Virol. 2008;82:6962–6971. doi: 10.1128/JVI.00133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwerschke W, Mazurek S, Massimi P, Banks L, Eigenbrodt E, Jansen-Durr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci USA. 1999;96:1291–1296. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]


