Abstract
Porcine epidemic diarrhea virus (PEDV) is a highly contagious enteric pathogen of swine causing high mortality rates in piglets. PEDV outbreaks have occurred continuously in most swine-producing Asian countries and have recently emerged in the United States, leading to large economic losses for both the Asian and US pig industries. The spike (S) protein of PEDV consists of the S1 and S2 domains, responsible for virus binding and fusion, respectively. The involvement of the S1 domain in specific high-affinity interactions with the cellular receptor and induction of neutralizing antibodies in the natural host makes it a logical target for the development of effective vaccines and therapeutics against PEDV. Passive immunization by oral administration of egg yolk antibodies (IgY) obtained from immunized chickens provides an alternative source of specific antibodies for the prevention and treatment of PEDV in newborn piglets. In this study, we produced an IgY against the PEDV S1 protein and investigated its immunoprophylactic effect in neonatal piglets. A codon-optimized PEDV S1 gene consisting of amino acid residues 25–749 was synthesized and used to establish a stable porcine cell line constitutively expressing a recombinant PEDV S1 protein containing the chicken immunoglobulin Fc fragment at its C-terminus. The purified recombinant S1 protein was found to mediate potent immune responses in immunized hens. We next tested the ability of oral passive immunization with anti-PEDV S1 IgY to protect piglets against PEDV. Specific chicken IgY against the S1 protein was orally administered to neonatal piglets, and their responses subsequent to a virulent PEDV challenge were monitored. The results showed that oral administration of anti-PEDV S1 IgY efficiently protects neonatal piglets against PEDV, suggesting its potential as a prophylactic or therapeutic agent against acute PEDV infection.
Keywords: Porcine Epidemic Diarrhea Virus, Neutralize Antibody Titer, Acid Electrolytic Water, Neonatal Piglet, Porcine Epidemic Diarrhea Virus Infection
Introduction
Porcine epidemic diarrhea (PED) is characterized by acute enteritis and watery diarrhea followed by severe dehydration, leading to high mortality rates in neonatal piglets [5, 29, 33]. The disease was initially recognized in England in 1971 [27], and the causative agent, PED virus (PEDV), was not identified until 1978 [28]. PED epidemics were first reported in Asia in 1982, and PED has since continued to threaten swine health, causing substantial economic losses for the Asian swine industry [3, 11, 21, 30, 38]. In early 2013, sudden PED outbreaks occurred in the United States sweeping through the pork industry across the country [24, 35]. Subsequently, starting in late 2013, large-scale outbreaks of PED rapidly recurred in Korea, Taiwan, and Japan; a US-strain-like PEDV was found to be responsible for the recent outbreaks in those countries, raising global concerns regarding control measures for PED prevention [17–19, 22].
PEDV is a large enveloped virus possessing a single-stranded, positive-sense RNA genome of approximately 28 kb with a 5′ cap and a 3′ polyadenylated tail, belonging to the genus Alphacoronavirus within the family Coronaviridae of the order Nidovirales [28, 33]. The spike (S) protein of PEDV is a type I membrane glycoprotein consisting of 1,383 to 1,386 amino acids (aa), depending on the strain, that can be divided into the S1 (aa 1–735) and S2 (736–end) domains based on homology to the S proteins of other coronaviruses [6, 9, 15, 36]. Similar to other coronavirus S proteins, the PEDV S protein plays a critical role by interacting with the cellular receptor to mediate viral entry and inducing neutralizing antibodies in the natural host [1, 2]. More precisely, the S1 domain has been reported to contain the receptor-binding region and the main neutralizing epitopes [16, 37]. Furthermore, the S1 region has been established as a suitable target for determining genetic relatedness among PEDV isolates and for developing differential diagnostic assays and effective vaccines [4, 7, 15, 26].
In Korea, both modified live and killed vaccines against PEDV have been developed for disease control and use in the domestic market. However, the continued occurrence of PED nationwide has caused enormous financial losses for the Korean pork industry, raising questions regarding the efficacy of these commercial vaccines. This phenomenon appears to be due to genetic and antigenic differences between the vaccine strain and the strains prevalent in the field [15, 26]. Thus, the lack of effective vaccines increases the need for next-generation field-virus-based vaccines or control measures against PEDV infection. Passive immunization by oral administration of specific antibodies represents an attractive approach against gastrointestinal pathogens in both humans and animals [23]. Egg yolk immunoglobulin (IgY) from immunized chickens has been demonstrated to be a convenient large-scale source for specific antibodies; it has also been shown to be safe and effective against PEDV in newborn piglets [12].
The aim of the present study was to produce an IgY against the PEDV S1 protein and assess its immunoprophylactic properties in neonatal piglets. A codon-optimized PEDV S1 gene was used to establish a stable porcine cell line constitutively expressing a recombinant S1 protein. The recombinant S1 protein was capable of inducing efficient cytokine and antibody responses in immunized hens. Moreover, oral passive immunization using chicken IgY raised against the PEDV S1 protein was found to control and prevent PED post-challenge in suckling piglets.
Materials and methods
Cells, virus, and antibodies
HEK-293T cells (CRL-1573) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle medium (DMEM) with high glucose (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS; Invitrogen) and Antibiotic-Antimycotic Solution (100×; Invitrogen). PK-15 cells were grown in RPMI 1640 medium (Invitrogen) containing 10 % FBS and Antibiotic-Antimycotic Solution. Vero cells were cultured in alpha minimum essential medium (α-MEM; Invitrogen) with 10 % FBS and Antibiotic-Antimycotic Solution. The cells were maintained at 37 °C in a humidified 5 % CO2 atmosphere. The SM98-1 PEDV vaccine strain was obtained from the Korean Animal and Plant Quarantine Agency and propagated in Vero cells as described previously [8]. Challenge PEDV was prepared from intestinal contents obtained from a field case that was found to be free of other common etiologic agents of neonatal porcine diarrheal diseases, as described previously [26]. Briefly, a 4-day-old suckling piglet was inoculated orally with a small-intestine homogenate containing the field virus. Small-intestine tissues were collected and homogenized in a 10 % suspension with α-MEM using a MagNA Lyser (Roche Diagnostics, Mannheim, Germany) by three repetitions of 15 s at 7,000 rpm, and tissue suspensions were clarified by centrifugation for 10 min at 4,500×g (Hanil Centrifuge FLETA5, Incheon, South Korea). The clarified supernatant was filtered through a 0.22-μm-pore-size syringe filter (Millipore, Billerica, MA, USA), aliquoted, and stored at −80 °C until use as the crude challenge virus. Horseradish peroxidase (HRP) or fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA), and Alexa Fluor 488-conjugated secondary antibody was obtained from Molecular Probes (Carlsbad, CA, USA). The PEDV S-protein-specific monoclonal antibody (MAb) was a kind gift from Sang-Geon Yeo (Kyungpook National University, Daegu, South Korea), and the nucleocapsid (N)-protein-specific MAb was obtained from ChoongAng Vaccine Laboratory (CAVAC; Daejeon, South Korea).
Plasmid construction
DNA manipulation and cloning were performed according to standard procedures [34]. Escherichia coli strain DH5α (RBC Bioscience, Taiwan) was used as the host for general cloning. The pFB-Neo-PEDV-rS1-Ig plasmid encoding a full-length, codon-optimized PEDV S1 gene (aa 24–735) was described previously [26]. The pcDNA3.1/Chicken Fc plasmid encoding the Fc domain of chicken IgG was a kind gift from Hyung-Kwan Jang (Chonbuk National University, Jeonju, South Korea). The chicken Fc (cFc) fragment from pcDNA3.1/Chicken Fc was subcloned into pFB-Neo-PEDV-rS1-Ig by replacing the Fc domain of human IgG1 to construct the gene expression plasmid, pFB-Neo-PEDV-rS1-cFc, which produces a chicken Fc-tagged fusion protein, rS1-cFc. The constructed plasmid was verified by nucleotide sequencing.
Generation of a stable PK-15 cell line expressing PEDV rS1-cFc
The retrovirus gene transfer system (Agilent Technologies, Santa Clara, CA, USA) was used to generate a cell line constitutively expressing the recombinant PEDV rS1-cFc gene or an empty retroviral vector as described elsewhere [15, 25]. Antibiotic-resistant continuous cell clones were examined by RT-PCR to verify the presence of the full-length rS1-cFc gene, and the positive clones (PK-rS1-cFc) were then amplified for subsequent analysis.
Immunofluorescence assay (IFA)
PK-rS1-cFc cells were grown on microscope coverslips placed in 6-well tissue culture plates. At 48 h post-seeding, the cells were fixed with 4 % paraformaldehyde for 10 min at room temperature (RT) and permeabilized with 0.2 % Triton X-100 in phosphate-buffered saline (PBS) at RT for 10 min. The cells were subsequently blocked with 1 % bovine serum albumin (Amresco, Solon, OH, USA) in PBS for 30 min at RT and then incubated with a goat anti-chicken IgG antibody conjugated to FITC. Finally, the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO, USA), and cell staining was visualized using a Leica DM IL LED fluorescence microscope (Leica, Wetzlar, Germany).
Protein purification
PK-rS1-cFc cells were grown in 100-mm-diameter tissue culture dishes to 90 % confluency in serum-free medium (OptiPRO SFM; Invitrogen). At 72 h post-seeding, the protein-containing cell culture supernatants were harvested, and soluble proteins were immunoprecipitated with Chicken IgY Precipitating Resin (GenScript, Piscataway, NJ, USA) according to the manufacturer’s protocol in the presence of protease inhibitors at 4 °C for 16 h. The beads were collected by centrifugation at 5,000×g (Eppendorf centrifuge 5415R, Hamburg, Germany) for 5 min at 4 °C and washed three times with 0.5 M NaCl in PBS. The samples were subsequently eluted with 50 mM sodium citrate/50 mM glycine (pH 2.0) and neutralized with 1 M Tris-HCl (pH 8.0). The purified proteins were concentrated with Amicon Ultracentrifugal filters 100K (Millipore). Protein concentration was measured using a Pierce BCA protein assay (Thermo Scientific, Rockford, IL, USA), and the final products were analyzed by western blotting to confirm target protein purification.
Protein gel staining and western blot analysis
The protein-containing cell culture supernatants or purified proteins as described above were mixed with NuPAGE 4× LDS sample buffer (Invitrogen) and boiled at 70 °C for 10 min. The proteins were separated on a NuPAGE 4–12 % gradient Bis-Tris gel (Invitrogen) under reducing conditions and stained using SimplyBlue SafeStain (Invitrogen) according to the manufacturer’s instructions or electrotransferred onto Immobilon-P polyvinylidene fluoride (PVDF) membranes (Millipore). The membranes were then blocked with 3% powdered skim milk (BD Biosciences, Belford, MA, USA) in TBS (10 mM Tris-HCl [pH 8.0], 150 mM NaCl) with 0.05 % Tween-20 (TBST) at 4 °C for 2 h and then reacted directly with the goat anti-chicken IgG HRP-conjugated secondary antibody or with anti-PEDV S MAb followed by the corresponding HRP-labeled secondary antibody at a 1:2,000 dilution for 2 h at 4 °C. Finally, the proteins were visualized using enhanced chemiluminescence reagents (GE Healthcare, Piscataway, NJ, USA) according to the manufacturer’s protocol.
Immunization of hens
Twenty 10-week-old White Leghorn hens were randomly allocated into three groups and immunized by intramuscular injection into the breast muscle with either the binary ethylenimine (BEI)-inactivated SM98-1 PEDV vaccine strain, 200 µg of the purified PEDV rS1-cFc protein resuspended in PBS, or PBS with a mineral oil-based adjuvant (Montanide ISA 70 VG; Seppic, Puteaux, France) (Table 1). The hens were then boosted three times with the same immunogens emulsified with adjuvant at 3-week intervals. Blood samples were collected prior to immunization, at each boost, and three weeks subsequent to the final boost. Eggs were collected for three weeks subsequent to the final boost.
Table 1.
Experimental design for chicken immunizationa
| Group | Antigen |
|---|---|
| 1 (n = 8) | Inactivated PEDV vaccineb |
| 2 (n = 8) | PEDV rS1-cFc immunogenc |
| 3 (n = 4) | Placebod |
aCorresponding antigens were given intramuscularly to 10-week-old hens, followed by three booster inoculations at 3-week intervals
bBEI-inactivated PEDV emulsified in ISA 70 adjuvant
cPurified PEDV rS1-cFc protein emulsified in ISA 70 adjuvant
dPBS emulsified in ISA 70 adjuvant
Isolation of IgY
Eggs collected from immunized hens were washed with diluted sodium hypochlorite solution (Yuhanclorox, Seoul, South Korea), disinfected with 70 % ethanol, and used for egg yolk fractionation. The separation of the yolk from the albumen and chalaza was performed as described previously [13]. The egg yolk material was mixed with distilled water (DW) at a 1:7 (v/v) ratio immediately followed by the addition of strong acid electrolytic water (pH < 2.0) at 0.02 % of the total volume. Samples were thoroughly whisked using a hand blender for approximately 10 min. The egg yolk mixture was kept at 4 °C for 24 h, and supernatants were then separated and clarified by centrifugation for 20 min at 3,000×g (Hanil Centrifuge FLETA5) to thoroughly remove lipids. The supernatants containing IgY was collected, lyophilized using a spray dryer and stored at 4 °C for further experiments.
Quantitative real-time RT-PCR
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using a standard gradient centrifugation purification protocol with Histopaque 1077 (Sigma) according to the manufacturer’s instructions. Total RNA was extracted from the PBMCs of hens using TRIzol Reagent (Invitrogen) and treated with recombinant DNase I (TaKaRa, Otsu, Japan) according to the manufacturer’s protocols. The concentrations of the extracted RNA were measured with a NanoVue spectrophotometer (GE Healthcare). Quantitative real-time RT-PCR was done using a Thermal Cycler Dice Real Time System (TaKaRa) with a One Step SYBR PrimeScript RT-PCR Kit (TaKaRa) and the following gene-specific primer sets as described previously [20, 31, 32]: chicken IFN-α forward, 5′-ATCCTGCTGCTCACGCTCCTTCT-3′; chicken IFN-α reverse, 5′-GGTGTTGCTGGTGTCCAGGATG-3′; chicken IFN-β forward, 5′-GCCTCCAGCTCCTTCAGAATACG-3′; chicken IFN-β reverse, 5′-CTGGATCTGGTTGAGGAGGCTGT-3′; chicken IFN-γ forward, 5′-AGCTGACGGTGGACCTATTATT-3′; chicken IFN-γ reverse, 5′-GGCTTTGCGCTGGATTC-3′; chicken IL-6 forward, 5′-CAAGGTGACGGAGGAGGAC-3′; chicken IL-6 reverse, 5′-TGGCGAGGAGGGATTTCT-3′; chicken IL-8 forward, 5′-CCAAGCACACCTCTCTTCCA-3′; chicken IL-8 reverse, 5′-GCAAGGTAGGACGCTGGTAA-3′; chicken TNF-α forward, 5′-GAAGCAGCGTTTGGGAGT-3′; chicken TNF-α reverse, 5′-GTTGTGGGACAGGGTAGG-3′; chicken glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-GGTGGTGCTAAGCGTGTTAT-3′; chicken GAPDH reverse, 5′-ACCTCTGTCATCTCTCCACA-3′. The steady-state mRNA levels of each cytokine gene were normalized against the level of chicken GAPDH mRNA, and the relative quantity (RQ) of mRNA accumulation was evaluated using the 2-ΔΔCt method. The relative fold change in the expression of each gene was then calculated by comparing pre-immune and immunized sera.
Serum neutralization
PEDV-specific neutralizing antibodies in the serum and IgY samples collected from hens in all groups were determined using a serum neutralization test in 96-well microtiter plates with the SM98-1 PEDV vaccine strain as previously described [14]. Briefly, individual virus stocks were diluted in serum-free α-MEM to a concentration of 200 plaque-forming units in a volume of 50 μl. The diluted virus was then mixed with 50 μl of a twofold serial dilution of individual inactivated sera or IgY solution dissolved in DW (100 mg/ml) in 96-well plates, and the mixture was incubated at 37 °C for 1 h. Next, approximately 1 × 104 Vero cells in 100 μl of α-MEM with 10 % FBS were added to each well, and the mixture was further maintained at 37 °C in a 5 % CO2 incubator for 3 to 4 days. The neutralization titer was calculated as the reciprocal of the highest serum dilution that inhibited virus-specific cytopathic effects in all duplicate wells.
Pig inoculation studies
The in vivo swine experiments described here were performed at the ImproAH Animal Facility under the guidelines established by the Institutional Animal Care and Use Committee. A total of 18 newborn piglets were obtained from seronegative pregnant sows at a commercial pig farm with no known prior PED outbreak or vaccination with PEDV. All animals were determined to be free of antibodies to PEDV, as well as to transmissible gastroenteritis virus and porcine reproductive and respiratory syndrome virus. No other animals aside from those included in the study were housed at the facility for the duration of the experiment. The design for the present pig experiment is outlined in Table 2. Piglets were randomly divided into four groups: group 1, administration of IgY against PEDV; group 2, administration of IgY against PEDV rS1-cFc; group 3, administration of IgY against placebo; group 4, control. The groups were housed in separate rooms, and no physical contact was allowed between the groups. All neonatal piglets except for animals in the control group were inoculated orally with 1 ml of the small-intestine homogenate containing 104 TCID50/ml PEDV field virus determined using real-time RT-PCR as described previously [10]. Following challenge exposure, piglets were administered orally with 2 ml of the corresponding IgY solution dissolved in DW (250 mg/ml) at 1 and 2 days post-inoculation (DPI).
Table 2.
Experimental design for PEDV challenge in pigletsa
| Group | Challenge + IgY |
|---|---|
| 1 (n = 6) | PEDV + anti-PEDV IgYb |
| 2 (n = 6) | PEDV + anti-rS1-cFc IgYc |
| 3 (n = 3) | PEDV + normal IgYd |
| 4 (n = 3) | No challenge + normal IgY (control) |
aAll neonatal piglets except the control group were inoculated orally with the small-intestine homogenate containing PEDV field virus and administered orally with IgY at 1 and 2 DPI
bIgY from the egg yolk of hens immunized against BEI-inactivated PEDV
cIgY from the egg yolk of hens immunized against purified PEDV rS1-cFc protein
dIgY from the egg yolk of hens immunized against PBS
Clinical signs of diarrhea and the mortality in challenged piglets were monitored daily throughout the study. Stool samples from all groups were collected daily with 16-inch, cotton-tipped swabs and subjected to RT-PCR using an i-TGEV/PEDV Detection Kit (iNtRON Biotechnology, Seongnam, South Korea) to detect the presence of PEDV. Fecal shedding of PEDV was measured by quantitative real-time RT-PCR as described above, and the results were analyzed using the system software as described previously [32].
All piglets from the challenged and control groups were euthanized at 5 DPI for post-mortem examination. Small-intestinal tissue specimens collected from each piglet (<3 mm thick) were fixed with 10 % formalin for 24 h at RT and then embedded in paraffin according to standard laboratory procedures. The formalin-fixed paraffin-embedded tissues were cut into 5- to 8-µm-thick sections on a microtome, floated in a 40 °C water bath containing DW, and transferred onto glass slides. The tissues were then deparaffinized in xylene for 20 min and washed in decreasing concentrations of ethanol (100 %, 95 %, 85 %, 70 %, and 50 %) for 3 min each. The deparaffinized intestinal tissue sections were stained with hematoxylin and eosin (H&E; Sigma) to observe histopathological lesions of PEDV infection and subjected to IFA using the PEDV N-specific MAb and goat anti-mouse IgG secondary antibody conjugated to Alexa Fluor 488 as described above.
Statistical analysis
The Student’s t-test was used for all statistical analyses and p-values of less than 0.05 were considered statistically significant.
Results
Generation of stable porcine-origin cell lines expressing the full-length, codon-optimized S1 protein
Previously, we synthesized a full-length, codon-optimized S1 gene and confirmed that codon optimization greatly enhanced the expression level of S1 upon transient transfection [26]. In this study, the codon-optimized S1 gene was used for stable transfection to produce preparative amounts of the S1 protein. To accomplish this, sublines of PK-15 cells were established that stably expressed the recombinant codon-optimized S1 under the control of a retroviral LTR promoter. Ten generated cell clones were initially collected and subjected to RT-PCR and western blot analysis to examine S1 expression at the mRNA and protein level, respectively (data not shown). Based on the results of the western blot analysis, one PK-rS1-cFc cell clone that consistently expressed the highest level of S1 was chosen for subsequent studies.
To characterize the PK-rS1-cFc cells, intracellular and extracellular expression levels of S1 were examined by immunofluorescence and western blotting. As shown in Fig. 1A, specific cell staining was clearly evident when PK-rS1-cFc cells were reacted with the anti-chicken IgG antibody, confirming a consistent high expression level of the S1 protein. Western blot analysis of cell culture supernatants revealed that the PK-rS1-cFc cells stably expressed and cumulatively secreted high levels of the approximately 180-kDa S1. The recombinant S1 protein expressed in the supernatants of stable PK-rS1-cFc cells was purified using chicken IgY precipitation resin beads. The purified S1 protein was detectable at a high level by SimplyBlue staining and was confirmed by immunoblotting with anti-chicken IgG antibody (Fig. 1B). Using our purification and concentration procedures, we were able to purify an average yield of 30 μg of S1 protein per 6 ml of PK-rSI-cFc cell culture supernatant cultivated in a 100-mm tissue culture dish for 72 h. In addition, the overall growth kinetics of S1 gene-expressing PK-15 cells were found to be similar to those of the parental PK-15 cells, indicating that S1 expression has no effect on cell growth (data not shown).
Fig. 1.
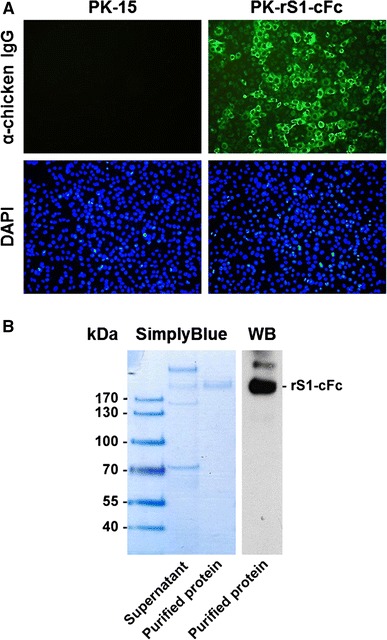
Constitutive expression of the recombinant S1 protein in PK-rS1-cFc cells. (A) Immunofluorescence assay for the rS1 protein. PK-15 or PK-rS1-cFc cells grown in a 6-well tissue culture plate were fixed with 4 % formaldehyde at 48 h post-seeding and incubated with anti-chicken IgG antibody (top panels). The cells were then counterstained with DAPI (bottom panels) and examined using a fluorescent microscope at 400× magnification. (B) Purification of the rS1 protein. The recombinant S1 protein was purified from serum-free medium of PK-rS1-cFc cells grown in a 100-mm tissue culture dish. The cell culture supernatant and the purified rS1 protein were resolved on a 4–12 % gradient Bis-Tris gel and stained with SimplyBlue solution (left panel) or electrotransferred onto a PVDF membrane (right panel). The membrane was blotted with a chicken IgG-specific antibody
Immunization of chickens with the recombinant S1-cFc immunogen
To produce IgY, hens assigned to the three groups were immunized intramuscularly, as outlined in Table 1. Blood samples were collected before immunization (pre-immune), at each boost, and 3 weeks after the final boost. PBMCs were prepared from blood samples obtained during the first and the last collections to evaluate the immunogenicity of each immunogen. This was conducted by examining the expression levels of immune-response genes upon immunization. Our results demonstrate that the expression of numerous immune-related genes, including IFN-α, IFN-β, IFN-γ, IL-6, and IL-8, was significantly altered by immunization of inactivated PEDV or S1-cFc compared to levels in the placebo-inoculated group (Fig. 2). These data indicate that the immunogens used in this study efficiently stimulated immune responses in chickens. Serum samples collected at each collection time were subjected to a serum neutralization test against the PEDV vaccine strain. Non-immunized hens showed only minimal neutralizing antibody titers, whereas immunized hens exhibited gradually increasing neutralizing antibody titers (Fig. 3A). Furthermore, hens immunized with inactivated PEDV (group 1) and S1-cFc protein antigen (group 2) at 3-week intervals produced similar neutralizing antibody titers ranging from 1:32 to 1:64 subsequent to the final immunization. In addition, egg yolk samples from each group were found to contain neutralizing antibody titers comparable to those in the corresponding serum samples, indicating the presence of neutralizing IgY antibodies (Fig. 3B).
Fig. 2.
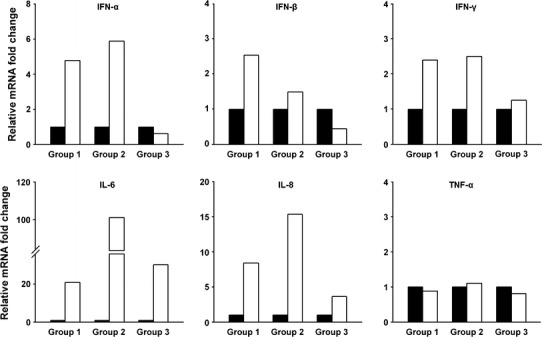
Expression patterns of cytokine gene mRNAs in immunized hens. PBMCs were separated from chicken blood samples collected prior to immunization (black bars) and 3 weeks after the final boost (white bars). The mRNA expression level of each gene, including chicken IFN-α, IFN-β, IFN-γ, IL-6, IL-8, and TNF-α was assessed by real-time RT-PCR and normalized to the expression of chicken GAPDH. Relative quantities (RQ) of mRNA accumulation were evaluated by the 2-ΔΔCt method, and the relative fold change in the expression of each cytokine gene was then calculated between pre-immune and immunized sera
Fig. 3.
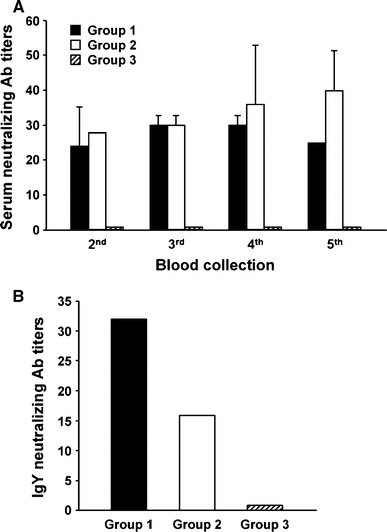
Virus-neutralizing antibody kinetics in sera and IgY from immunized hens. Chicken blood samples (A) collected at the indicated time points and IgY (B) extracted from egg yolk were subjected to a virus neutralization assay against SM98-1. The neutralizing antibody titers of the groups were plotted as a log2 scale. Values are representative of the mean from three independent experiments carried out in duplicate, and the error bars denote the standard deviations
Experimental infection of pigs with anti-PEDV S1 IgY
To evaluate the immunoprophylactic efficacy of anti-PEDV S1 IgY, neonatal 4- to 5-day-old PEDV-seronegative piglets were divided into four groups and challenged orally with wild-type PEDV followed by the respective antibody treatment at days 1 and 2 post-challenge. Animals in group 4 were not challenged with PEDV and given IgY orally from non-immunized chickens at the same time points as the other groups. Clinical observations of mortality and diarrhea in challenged piglets are summarized in Table 3. None of the piglets from group 4 died or developed any clinical signs of diarrhea. Additionally, fecal shedding of PEDV was also detected in the rectal swabs of these animals for the duration of the study (Fig. 4). In contrast, all piglets from the challenged groups (1–3), regardless of IgY administration, exhibited diarrhea that began at 1 or 2 days post-challenge and lasted throughout the challenge experiment. In group 3, one piglet died; the remaining piglets experienced severe watery diarrhea and shed PEDV in their feces, with a mean cycle threshold (Ct) value of 16.9 (range 14.8–18.6) during the study (Fig. 4). Although none of the piglets from groups 1 and 2 treated with IgY died during the challenge experiment, the number of piglets exhibiting diarrhea post-challenge varied depending on the group. All piglets from these groups showed mild-to-severe diarrhea lasting for the entire experiment but recovered from the diarrhea by the end of the study. Furthermore, fecal shedding of PEDV from these piglets was significantly lower than in group 3 piglets; PEDV RNA was detected in the fecal swab samples of all pigs in groups 1 and 2, with mean Ct values of 24.1 (range 23.1–24.8) and 21.9 (range 20.0–26.5), respectively (Fig. 4). However, despite the similar levels of diarrhea, the group 2 piglets shed slightly higher amounts of PEDV in their feces compared to those in group 1. Gross intestinal lesions consistent with viral enteritis, including thin-walled and fluid-content-dilated small intestines, were typically observed in all group 3 piglets; less-severe lesions were observed in piglets given either IgY (data not shown). Likewise, the majority of enterocytes over the entire villi in the control piglets with normal IgY treatment (group 3) were affected by PEDV, showing moderate-to-severe villous atrophy and destruction, while piglets from groups 1 and 2 exhibited mild intestinal lesions comparable to those in the non-challenged group, and viral antigens were only detected in their small intestines (Figs. 5 and 6). However, anti-PEDV S1 IgY treatment (group 2) was more efficacious than treatment with IgY raised against the whole virus (group 1) in reducing the overall severity of macroscopic and microscopic intestinal lesions in the piglets. Collectively, all immunoprophylactic methods used in this study were capable of protecting neonatal piglets against mortality and disease severity after challenge with a virulent PEDV.
Table 3.
Diarrhea in piglets in the challenge experiment
| Group | Piglet no. | Days after oral challenge | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| 1 | 1 | −a | − | ++a | ++ | ++ | +a |
| 2 | − | +++a | +++ | ++ | ++ | + | |
| 3 | − | − | +++ | +++ | +++ | + | |
| 4 | − | − | +++ | ++ | ++ | ++ | |
| 5 | − | + | +++ | +++ | + | + | |
| 6 | − | − | +++ | +++ | ++ | + | |
| 2 | 1 | − | +++ | ++ | +++ | +++ | + |
| 2 | − | − | ++ | ++ | + | + | |
| 3 | − | − | +++ | +++ | ++ | ++ | |
| 4 | − | − | ++ | ++ | ++ | + | |
| 5 | − | +++ | +++ | ++ | + | + | |
| 6 | − | +++ | +++ | ++ | ++ | ++ | |
| 3 | 1 | − | +++ | ++ | ++ | +++ | +++ |
| 2 | − | +++ | +++ | +++ | +++ | +++ | |
| 3 | − | +++ | Diedb | NDc | ND | ND | |
| 4 | 1 | − | − | − | − | − | − |
| 2 | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | |
a−, no diarrhea; +, mild diarrhea; ++, moderate diarrhea; +++, severe diarrhea
bMortality attributed to PEDV infection confirmed by RT-PCR and necropsy diagnosis
cNot determined due to the death of this piglet
Fig. 4.
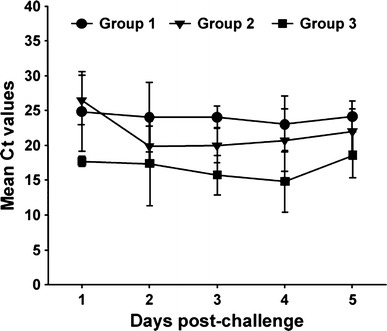
Viral shedding in feces of piglets. Viral RNA was extracted from fecal samples collected from piglets at 1 to 5 days post-challenge and subjected to real-time RT-PCR to determine the viral titers. Lower Ct value indicates higher viral titers. Values are representative of the mean from three independent experiments carried out in duplicate, and the error bars represent the standard deviations
Fig. 5.
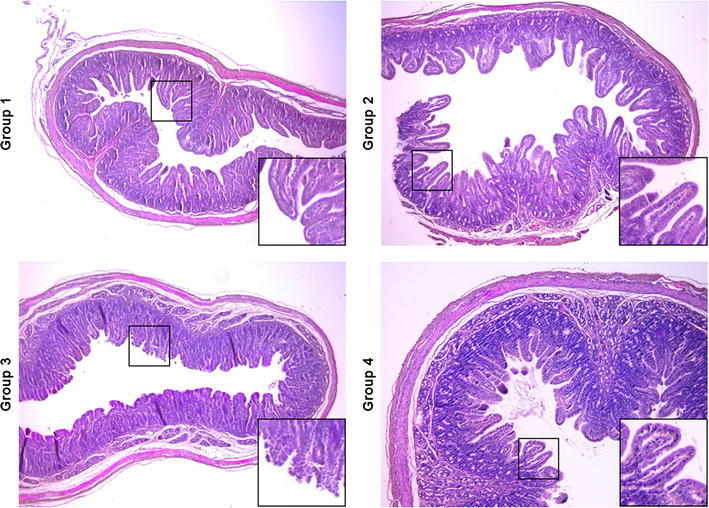
Microscopic lesions in small intestine tissues of piglets. Tissue specimens were collected from the small intestines of piglets from each group at the time of necropsy. The formalin-fixed and paraffin-embedded tissue sections were deparaffinized and stained with hematoxylin and eosin. The sections were examined using a light microscope at 40× magnification. The inset images are enlarged versions of parts of the picture
Fig. 6.
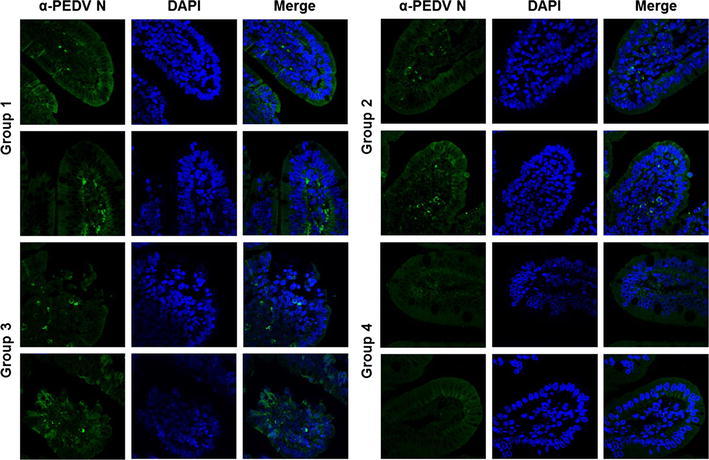
Detection of PEDV in small intestine tissues of piglets. Tissue specimens were prepared from small intestine of piglets from each group at the time of necropsy. The formalin-fixed and paraffin-embedded tissue sections were deparaffinized and subjected to immunofluorescence staining with an anti-PEDV N antibody. The sections were then counterstained with DAPI and examined using a fluorescence microscope at 200× magnification
Discussion
Vaccination against PEDV is one of the most important and effective prevention measures by passively transferring specific neutralizing antibodies present in vaccinated sows to their litters through colostrum and milk. Despite the availability of commercial attenuated and inactivated vaccines in Korea, PEDV continues to plague the domestic pork industry, raising issues regarding their protective efficacy. Recently, severe outbreaks of PEDV have re-emerged in Korea and have been estimated to affect over 40 % of pig farms, causing serious economic impact on producers and customers [17, 18]. Our previous studies suggested that antigenic and genetic variations between the vaccine virus and field PEDVs may be the cause of the incomplete efficacy or failure of vaccination, which appears to be responsible for the periodic outbreaks in domestic herds [15, 26]. Furthermore, a comparison of the amino acid sequence of the S1 domain of the PEDV S protein, which is associated with viral binding to host cell receptors and contains neutralizing epitopes [16, 37], has shown a difference of over 10 % between the vaccine strain and field isolates, suggesting the need for next-generation vaccine development [15, 17]. In addition to vaccination, artificial passive immunization using IgY has been used commercially as an alternative method for controlling PED by providing supportive immunity to neonatal piglets exposed to acute infection in Korea. However, opinions differ among swine practitioners and producers regarding the efficacy of this immunoprophylactic strategy. A similar debate regarding the commercial use of IgY may arise since the vaccine strain is also used to immunize chickens for the production of anti-PEDV IgY in Korea. We have previously demonstrated that the S1 protein of PEDV could be considered a potential candidate antigen for vaccination [26]. In the present study, the S1 protein of the field PEDV was used as an immunogen to inoculate hens, thereby producing anti-PEDV S1 IgY. We first aimed to stably express the full-length, codon-optimized S1 gene of PEDV in porcine-origin cells and to evaluate the immunogenicity and efficacy of IgY against the recombinant S1 protein. Subsequently, we were able to successfully generate a stable PK cell line continuously producing large amounts of the codon-optimized S1 protein tagged with cFc. Following the purification and concentration processes, approximately 30–40 μg of the recombinant S1-cFc protein could be consistently harvested from the culture supernatants of PK-rS1-cFc cells grown in a 100-mm culture dish.
Since humoral immunity is an important indicator for evaluating the effect of the S1-based immunogen used in this study, we immunized chickens with the S1-cFc antigen prepared from the cell culture supernatants of PK-rS1-cFc cells and investigated whether they developed cytokine and antibody responses. All of the proinflammatory cytokines tested, except for TNF-α, were stimulated in chickens immunized with either the whole virus or the S1-cFc antigen. Of these, the expression levels of IL-6 and IL-8 genes were distinctly enhanced in the S1-cFc-inoculated group compared with the inactivated PEDV-inoculated group. Furthermore, the chicken sera raised against S1-cFc contained higher levels of neutralizing antibody than those raised against the SM98-1 vaccine virus. However, the final levels of anti-PEDV IgY were lower in the eggs of the S1-cFc-immunized chickens compared to those of the SM98-1-inoculated group. These inconsistent results may be attributed to the use of the heterogeneous SM98-1 virus in the serum neutralization assay, which possesses a high degree of genetic variation in field isolates. Nevertheless, our data indicated that the recombinant S1 protein efficiently elicits immune responses in chickens. Subsequently, we tested the prophylactic efficacy of each IgY in suckling piglets post-challenge-exposure. Our data showed that, regardless of the IgY administered, all neonatal piglets treated with IgY survived after challenge with virulent PEDV, suggesting that the anti-PEDV S1 IgY provides effective passive immunity to prevent mortality comparable to whole-virus-based IgY. Despite the lower levels of virus shedding in the feces of animals treated with anti-PEDV IgY, the anti-S1 IgY-based strategy resulted in more-efficient protection than the anti-PEDV IgY procedure, as determined by the duration and severity of diarrhea and histopathological lesions. These results indicated that the administration of anti-S1 IgY could significantly reduce the mortality and clinical signs of piglets, suggesting that application of anti-S1 IgY is capable of partially blocking the virus from invading the small intestine. A more promising approach would be to use the entire field virus or its full-length S protein to produce IgY instead of using only the S1 domain, since the S protein contains multiple functional domains and neutralizing epitopes to efficiently stimulate non-susceptible hosts. For this purpose, the production and application of IgY against a Korean field isolate are currently under investigation.
In conclusion, to the best of our knowledge, this is the first study to evaluate the immunoprophylactic effect of IgY against the recombinant S1 protein of the field virus in a pig model. The results presented here indicate that the recombinant S1 protein can elicit cytokine and antibody responses and induce neutralizing antibodies in chickens. Furthermore, challenge experiments revealed that administration of anti-S1 IgY efficiently protected suckling piglets against field PEDV by providing passive immunity. PEDV infection causes high mortality rates (90–100 % in neonatal piglets under 7 days of age), and epidemiological observations indicate the rapid spread of the disease. In this circumstance, the preliminary results of the present study showed the potential of anti-PEDV S1 IgY application as a considerable measure against PEDV. Further experiments to optimize production procedures will be required to achieve higher titers of IgY; field studies on farms will be needed to better evaluate the efficacy of anti-S1 IgY. These studies will provide additional practical information for the future use of this alternative IgY method as a supplement to passive immunity against PED and other economically important viral diseases.
Acknowledgments
This research was supported by Technology Development Program for Bio-Industry, Ministry for Agriculture, Food and Rural Affairs, Republic of Korea (311007-05-1-HD120).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
References
- 1.Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang SH, Bae JL, Kang TJ, Kim J, Chung GH, Lim CW, Laude H, Yang MS, Jang YS. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol Cells. 2002;14:295–299. [PubMed] [Google Scholar]
- 3.Chen JF, Sun DB, Wang CB, Shi HY, Cui XC, Liu SW, Qiu HJ, Feng L. Molecular characterization and phylogenetic analysis of membrane protein genes of porcine epidemic diarrhea virus isolates in China. Virus Genes. 2008;36:355–364. doi: 10.1007/s11262-007-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Li G, Stasko J, Thomas JT, Stensland WR, Pillatzki AE, Gauger PC, Schwartz KJ, Madson D, Yoon KJ, Stevenson GW, Burrough ER, Harmon KM, Main RG, Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol. 2014;52:234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debouck P, Pensaert M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am J Vet Res. 1980;41:219–223. [PubMed] [Google Scholar]
- 6.Duarte M, Laude H. Sequence of the spike protein of the porcine epidemic diarrhoea virus. J Gen Virol. 1994;75:1195–1200. doi: 10.1099/0022-1317-75-5-1195. [DOI] [PubMed] [Google Scholar]
- 7.Gerber PF, Gong Q, Huang YW, Wang C, Holtkamp D, Opriessnig T. Detection of antibodies against porcine epidemic diarrhea virus in serum and colostrum by indirect ELISA. Vet J. 2014;202:33–36. doi: 10.1016/j.tvjl.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann M, Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J Clin Microbiol. 1988;26:2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackwood MW, Hilt DA, Callison SA, Lee CW, Plaza H, Wade E. Spike glycoprotein cleavage recognition site analysis of infectious bronchitis virus. Avian Dis. 2001;45:366–372. doi: 10.2307/1592976. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Kim IJ, Pyo HM, Tark DS, Song JY, Hyun BH. Multiplex real-time RT-PCR for the simultaneous detection and quantification of transmissible gastroenteritis virus and porcine epidemic diarrhea virus. J Virol Methods. 2007;146:172–177. doi: 10.1016/j.jviromet.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kweon CH, Kwon BJ, Jung TS, Kee YJ, Hur DH, Hwang EK, Rhee JC, An SH. Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean J Vet Res. 1993;33:249–254. [Google Scholar]
- 12.Kweon CH, Kwon BJ, Woo SR, Kim JM, Woo GH, Son DH, Hur W, Lee YS. Immunoprophylactic effect of chicken egg yolk immunoglobulin (IgY) against porcine epidemic diarrhea virus (PEDV) in piglets. J Vet Med Sci. 2000;62:961–964. doi: 10.1292/jvms.62.961. [DOI] [PubMed] [Google Scholar]
- 13.Laca A, Paredes B, Díaz M. A method of egg yolk fractionation. Characterization of fractions. Food Hydrocoll. 2010;24:434–443. doi: 10.1016/j.foodhyd.2009.11.010. [DOI] [Google Scholar]
- 14.Lee C, Hodgins D, Calvert JG, Welch SK, Jolie R, Yoo D. Mutations within the nuclear localization signal of the porcine reproductive and respiratory syndrome virus nucleocapsid protein attenuate virus replication. Virology. 2006;346:238–250. doi: 10.1016/j.virol.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DK, Park CK, Kim SH, Lee C. Heterogeneity in spike protein genes of porcine epidemic diarrhea viruses isolated in Korea. Virus Res. 2010;149:175–182. doi: 10.1016/j.virusres.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DK, Cha SY, Lee C. The N-terminal region of the porcine epidemic diarrhea virus spike protein is important for the receptor binding. Korean J Microbiol Biotechnol. 2011;39:140–145. [Google Scholar]
- 17.Lee S, Lee C. Outbreak-related porcine epidemic diarrhea virus strains similar to US strains, South Korea, 2013. Emerg Infect Dis. 2014;20:1223–1226. doi: 10.3201/eid2007.140294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Ko DH, Kwak SK, Lim CH, Moon SU, Lee DS, Lee C. Reemergence of porcine epidemic diarrhea virus on Jeju Island. Korean J Vet Res. 2014;54:185–188. doi: 10.14405/kjvr.2014.54.3.185. [DOI] [Google Scholar]
- 19.Lee S, Park GS, Shin JH, Lee C. Full-genome sequence analysis of a variant strain of porcine epidemic diarrhea virus in South Korea. Genome Announc. 2014;2:e01116-14. doi: 10.1128/genomeA.01116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YJ, Lee C. Cytokine production in immortalized porcine alveolar macrophages infected with porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2012;150:213–220. doi: 10.1016/j.vetimm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Li H, Liu Y, Pan Y, Deng F, Song Y, Tang X, He Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect Dis. 2012;18:1350–1353. doi: 10.3201/eid1803.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CN, Chung WB, Chang SW, Wen CC, Liu H, Chien CH, Chiou MT. US-like strain of porcine epidemic diarrhea virus outbreaks in Taiwan, 2013–2014. J Vet Med Sci. 2014;76:1297–1299. doi: 10.1292/jvms.14-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mine Y, Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. J Med Food. 2002;5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- 24.Mole B. Deadly pig virus slips through US borders. Nature. 2013;499:388. doi: 10.1038/499388a. [DOI] [PubMed] [Google Scholar]
- 25.Nam E, Lee C. Contribution of the porcine aminopeptidase N (CD13) receptor density to porcine epidemic diarrhea virus infection. Vet Microbiol. 2010;144:41–50. doi: 10.1016/j.vetmic.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh J, Lee KW, Choi HW, Lee C. Immunogenicity and protective efficacy of recombinant S1 domain of the porcine epidemic diarrhea virus spike protein. Arch Virol. 2014;159:2977–2987. doi: 10.1007/s00705-014-2163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oldham J. Letter to the editor. Pig Farming. 1972;10:72–73. [Google Scholar]
- 28.Pensaert MB, de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pijpers A, van Nieuwstadt AP, Terpstra C, Verheijden JH. Porcine epidemic diarrhoea virus as a cause of persistent diarrhoea in a herd of breeding and finishing pigs. Vet Rec. 1993;132:129–131. doi: 10.1136/vr.132.6.129. [DOI] [PubMed] [Google Scholar]
- 30.Puranaveja S, Poolperm P, Lertwatcharasarakul P, Kesdaengsakonwut S, Boonsoongnern A, Urairong K, Kitikoon P, Choojai P, Kedkovid R, Teankum K, Thanawongnuwech R. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg Infect Dis. 2009;15:1112–1115. doi: 10.3201/eid1507.081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagong M, Lee C. Porcine reproductive and respiratory syndrome virus nucleocapsid protein modulates interferon-β production by inhibiting IRF3 activation in immortalized porcine alveolar macrophages. Arch Virol. 2011;156:2187–2195. doi: 10.1007/s00705-011-1116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sagong M, Park CK, Kim SH, Lee KK, Lee OS, Lee DS, Cha SY, Lee C. Human telomerase reverse transcriptase-immortalized porcine monomyeloid cell lines for the production of porcine reproductive and respiratory syndrome virus. J Virol Methods. 2012;179:26–32. doi: 10.1016/j.jviromet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Saif LJ, Pensaert MB, Sestak K, Yeo SG, Jung K. Coronaviruses. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of swine. 10. Ames, IA: Wiley-Blackwell; 2012. pp. 501–524. [Google Scholar]
- 34.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 35.Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- 36.Sturman LS, Holmes KV. Proteolytic cleavage of peplomeric glycoprotein E2 of MHV yields two 90K subunits and activates cell fusion. Adv Exp Med Biol. 1984;173:25–35. doi: 10.1007/978-1-4615-9373-7_3. [DOI] [PubMed] [Google Scholar]
- 37.Sun DB, Feng L, Shi HY, Chen JF, Liu SW, Chen HY, Wang YF. Spike protein region (aa 636789) of porcine epidemic diarrhea virus is essential for induction of neutralizing antibodies. Acta Virol. 2007;51:149–156. [PubMed] [Google Scholar]
- 38.Takahashi K, Okada K, Ohshima K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nippon Juigaku Zasshi. 1983;45:829–832. doi: 10.1292/jvms1939.45.829. [DOI] [PubMed] [Google Scholar]


