Abstract
Background and aims
The present investigation was carried out to determine the levels of blood serum components and inflammatory cytokines in diabetic rat models [Goto-Kakizaki (GK), Zucker, and streptozotocin (STZ)-induced Sprague Dawley (SD) rats] which underwent abdominal Low-Power Laser Irradiation (LPLI) and compare them with non-irradiated controls.
Methods
The animals were subdivided into the following groups: diabetic control rats (GK, Zucker, STZ) and diabetic rats treated with LPLI (GK + LPLI, Zucker + LPLI, and STZ + LPLI) (n = 7). The animals were irradiated three times weekly for 12 weeks in LPLI (830 nm) at a dose of 5 J/cm2 for 500 s.
Results
Body weight was significantly lowered in the Zucker- LPLI group compared to control at 10 weeks and this pattern was maintained until 12 weeks of age. TNF-α, IL-1I and IL-6 levels were significantly decreased (5.1 ± 1.1 vs 3.3 ± 0.5, p < 0.01; 43.6 ± 8.8 vs 27.1 ± 3.8, p < 0.01; 98.3 ± 15.8 vs 62.2 ± 12.1, p < 0.01) in the Zucker- LPLI group compared with the control rats. The small intestinal transit rates of charcoal meals were significantly decreased (58.1 ± 10.1 vs 73.4 ± 13.3, p < 0.05) in the Zucker-LPLI group compared with the control rats. Similarly, the serum levels of glucose, cholesterol and triglycerides of LPLI groups were decreased in comparison with that of diabetic control rats.
Conclusions
We suggest that abdominal LPLI can reduce body weight and LPLI could be applicable for use against diabetic-induced inflammatory factors.
Keywords: Diabetic rat models, Zucker rat, Low-Power Laser Irradiation (LPLI), Body weight gain, Inflammatory cytokines
Introduction
Diabetes has been classified into type 1 and type 2 diabetes, caused by failure of insulin secretion secondary to pancreatic cell destruction in the former and reduced insulin secretion or insulin resistance in latter. Among the diabetes patients in Japan, 90% have type 2 diabetes, 40% of which is developed due to insulin resistance caused by obesity. 1) Its pathogenesis is related to infiltration of inflammatory cells in the adipose tissue of obese patients. This is followed by release of inflammatory cytokines such as TNF-α and IL-6 which act on adipocytes to produce glucose and further reduce uptake ability and induce insulin resistance, leading to an increase in risk of type 2 diabetes. 2–6)
Anti-inflammatory effect of Low-Power Laser Irradiation (LPLI) has been established by several authors. We conducted LPLI experiments on knee synovial cells from patients with rheumatoid arthritis and observed a decrease in release of TNF-α, IL1-β. It was also reported that production of chemokine IL-8, which is known to be involved in proliferation, angiogenesis, and bone/cartilage destruction, was suppressed by laser irradiation. In addition, LPLI was found to suppress the bone resorption and destruction in the femur and rib cancellous bones of the collagen-induced arthritis model rat (an animal model of human rheumatoid arthritis). This was caused by suppressing the expression of inflammatory cytokine genes. 7–10)
In recent years, Kawano et al have shown that chronic inflammation in adipose tissue, especially abdominal visceral fat, has a major influence on the development of type 2 diabetes. In their experiments, they gave 60% high-fat diet to mice and observed increased production of Ccl2 which promotes macrophage accumulation in abdominal fat cells, thereby inducing chronic inflammation in the large intestine. In contrast, the Ccl2-deficient mice model, showed inhibition of chronic inflammation in the large intestine along-with improved insulin resistance and 30% reduction in blood glucose level. 11)
Hence this study was planned to evaluate the effectiveness of LPLI against type 2 diabetes by using a spontaneous type 2 diabetes model that is clinically and pathologically similar to human diabetes.
Materials and methods
Experimental rat model of diabetes
This experiment was approved by the Experimental Animal Ethics Committee of the Nihon University School of Dentistry at Matsudo (approval number 04-0034) and was conducted based on the laboratory animal guidelines of the institute. The experimental animals used were 7-week-old GK/Crlj male rats (non-obese, spontaneous type 2 diabetes model rats), 8-week-old Zucker (fa/fa) male rats (obese, spontaneous type 2 diabetes model rats) and 7-week-old Sprague Dawley (SD) male rats (SPF-specific pathogen-free animals) were purchased from Shizuoka Experimental Animals (Shizuoka, Japan). The animals were kept at room temperature (23 ± 1°C), humidity 60 ± 10%, light/dark cycle every 12 hours and fed on solid feed (MF®, Oriental Yeast Co., Ltd., Japan). The animals were freely allowed to use filtered tap water for drinking. After initial period of 1 week, the general condition was observed, and body weight was measured.
Streptozotocin-induction for diabetes in rats
Streptozotocin was dissolved in 0.1 M citrate buffer and administered into the jugular vein of SD male rats at a dose of 30 mg/kg. STZ rats were confirmed for diabetes onset after 2 days to 2 weeks of STZ administration with serum glucose concentration of 300 mg/dl or more and positive results for sugar in urine. STZ rats are experimental type 1 diabetes model rats 12, 13) in which STZ selectively destroys pancreatic β-cells, reducing the insulin secretion. They were divided into Control (Non-LPLI) group and LPLI group for the present experiment.
Low-power laser irradiation (LPLI)
Low-power laser (LPL, ZH-M153DJP, wavelength of 830 nm, 5 J/cm2), distance between LPLI and rat abdominal digestive tract was 25 cm. Irradiation area was uniform with a diameter of 12 cm centering on the rat abdomen. The field and irradiation time were 500 seconds. The laser irradiation period was 12 weeks, and irradiation was carried out 36 times without anesthesia (Figure 1A, B; Figure 2A).
Figure 1:
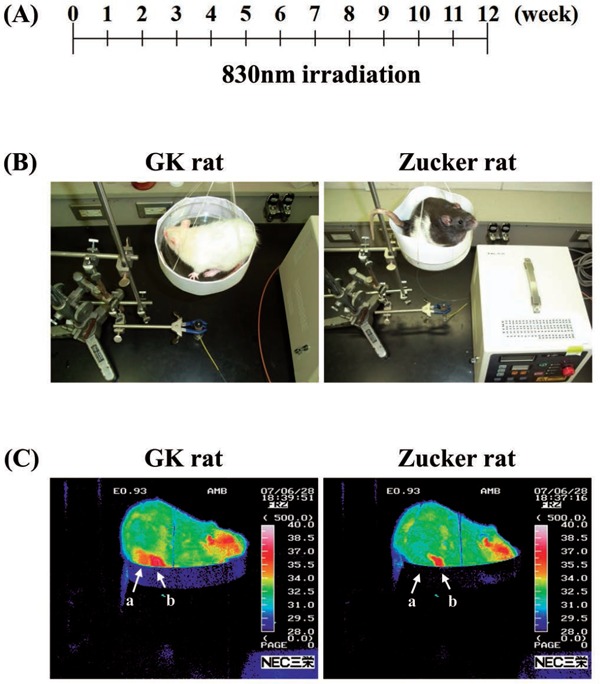
(A): Schema of experimental method. (B): Low-Power Laser Irradiation (LPLI) system. (C): LPLI system image. Photograph of LPLI in GK rat.
Figure 2:
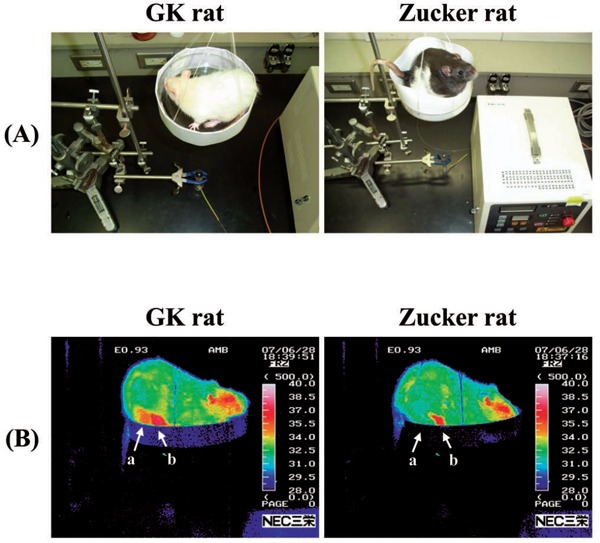
(A): Photograph of LPLI in GK and Zucker rats. (B): Thermographic images of abdominal digestive tract in rats at 2 weeks using infrared thermography.
Thermographic images of abdominal digestive tract using infrared thermography
The Infrared Thermograph (ITh) (TH9100MR, NEC Sanei Co., Ltd. Japan) was used to image the skin temperature of digestive tract in pseudo color. The distance between rat and ITh was always kept constant (30 cm) and its temperature was set to an upper threshold of 40°C and lower threshold of 28°C. The measurement was performed 30 minutes after LPLI at 25°C to 1°C in a room without air (Figure 2B). To maintain the uniformity of measurements, two points were indicated on abdomen as a and b were used as the average skin temperature in the abdominal digestive tract, once every two weeks.
Blood serum components and inflammatory cytokines
1 ml of blood sample was collected from the external jugular vein once a week. The sample was stored in centrifuge tube at room temperature for 30 minutes, immediately after collection. It was then centrifuged at 1,500 rpm for 15 minutes. Serum Glucose, total cholesterol and triglyceride concentrations were measured by enzymatic method (Test-Wako, Wako Pure Chemical Industries, Ltd. Japan). Blood insulin levels were determined using the Morinaga rat insulin measurement kit (Morinaga Institute of Science, Japan) and TNF-α, IL-1β nd IL-6 levels of inflammatory cytokines were measured using ELISA Kit (R & D System, Japan) before and after 12 weeks of LPLI.
Experimental methods
GK and Zucker rats were divided into 2 groups (n = 7/group): non-irradiated (control group), and LPLI group (LPLI 3 times a week for 12 weeks). STZ rats were divided into 3 groups with 7 rats per group. The first group (control) had SD rats not treated with STZ, the second and third groups comprised of STZ-induced diabetic groups. The second group was non-irradiated diabetic group and the third was LPLI diabetic rat group. In all animals, the abdomen was shaved completely, and LPLI was irradiated mainly around the abdominal fat and pancreas. During the experimental period, the general condition of the animals was observed and daily food intake and water consumption, weekly body weight, serum glucose, total cholesterol and triglycerides were measured.
Small intestinal transit rates of charcoal meals in rats
The small intestinal transit rate was determined in rats by measuring the disappearance of charcoal from the small intestinal according to the method described by Portha et al. 14) 2.0 ml charcoal meal (10%, w/w) in 1% carboxymethyl cellulose was administered intragastrically using an orogastric canula. 30 minutes after receiving the charcoal meal, rats were sacrificed, and the small intestine was carefully removed. Small intestinal transit was measured from the pyloric sphincter to the ileocecal junction (A) and the distance traveled by the charcoal through the intestine (B) were measured. Small intestinal transit rate was calculated as the percentage of the distance traveled by the charcoal relative to the total length of the small intestine using the following formula: Small intestinal transit rate (%) = (B/A) ×100.
Statistical analysis
Experimental results were presented as mean ± standard deviation (Mean ± SD). The comparison between each group was tested for equal variance by F-test, and then the significant difference from the control group was tested by Student t-test or Welch t-test. A risk rate of less than 5% (p < 0.05) was determined as a statistically significant change.
Results
Body weight gain, drinking water intake, food consumption and food efficiency
The changes in body weight during the experiment have been shown in Figure 3. Both the GK and Zucker rats showed a smooth weight gain from the start to the end of the experiment. In the GK rats, there was no significant difference between the non-irradiated group and LPLI group. However, the body weight gain in LPLI group was less than non-irradiation group after 12th week (final body weight) in the Zucker rats (non-irradiated group: 706 ± 83 g; LPLI group: 588 ± 71 g. p < 0.05). In the SD rats, STZ group showed weight loss immediately after STZ administration compared to the control group. At 12 weeks, the weights in SD rats showed no significant difference between the non-irradiated group in the STZ group and LPLI STZ group (SD group: 478 ± 58 g; non-irradiated group: 352 ± 47 g; LPLI group: 375 ± 48 g; SD vs. p < 0.001).
Figure 3:
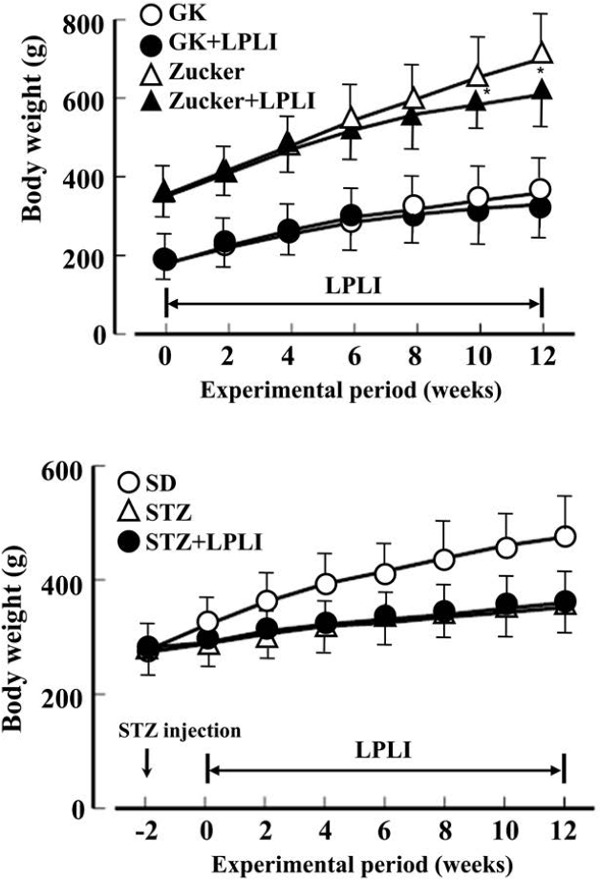
Effects of LPLI on body weight in GK, Zucker and streptozotocin-induced diabetic (STZ) rats. Data were shown as the mean ± SD of 7 animals in each group. *: p < 0.05 vs. no irradiation group.
Table 1 shows the average water consumption, feed intake, and feed efficiency (weight gain/feed intake) for each group during the experiment. There was no significant difference between the non-irradiated group and LPLI group in both Zucker and STZ groups. However, the results showed a significant decrease in LPLI group in feed efficiency compared with non-irradiation group in Zucker rats (non-irradiated group: 14.8 ± 3.1 g; LPLI: 10.1 ± 3.1 g; p < 0.05). In SD rats, the feed intake was approximately doubled, and the drinking water was approximately 7 times, showing a significant (p < 0.001) increase in the STZ group, and the feed efficiency decreased to 5:1.
Table 1: Effects of LPLI drinking water intake, food consumption and food efficiency in GK, Zucker and streptozotocin-induced diabetic (STZ) rats. Data were shown as the mean ± SD of 7 animals in each group. *: p < 0.05, vs. no irradiation group (control).
| Group | Drinking water intake (g/day/rat) | Food consumption (g/day/rat) | Food efficiency ratio (g/day/rat) | |
|---|---|---|---|---|
| GK | control | 15.2 ± 3.2 | 12.2 ± 3.4 | 12.6 ± 2.3 |
| LPLI | 19.1 ± 5.3 | 12.3 ± 3.9 | 10.8 ± 2.2 | |
| Zucker | control | 33.8 ± 6.8 | 26.8 ± 5.6 | 14.8 ± 3.1 |
| LPLI | 30.2 ± 6.7 | 24.4 ± 6.6 | 10.1 ± 3.1* | |
| SD | control | 17.8 ± 3.9 | 14.3 ± 3.8 | 15.4 ± 3.3 |
| LPLI | 96.7 ± 23.8 | 26.8 ± 4.7 | 3.0 ± 2.1 | |
| STZ+LPLI | 93.3 ± 31.1 | 26.1 ± 4.9 | 3.8 ± 2.2 |
Thermographic images of abdominal digestive tract
Figure 2 shows a thermal image of ITh of rat abdomen after LPLI in GK and Zucker group after 2 weeks. From the temperature distribution display image, comparing the non-irradiated group and LPLI in the GK, Zucker and STZ groups (31.3 ± 3.2 vs 35.8 ± 3.9, p < 0.05; 31.0 ± 3.3 vs 35.8 ± 3.6, p < 0.05; 30.7 ± 3.8 vs 36.5 ± 3.3°C, p < 0.05), LPLI groups showed significant increase in abdominal skin temperature (Table 2).
Table 2: Effects of LPLI on abdominal digestive tract temperature level in GK, Zucker, and streptozotocin-induced diabetic (STZ) rats at 2 weeks using infrared thermography. Data were shown as the mean ± SD of 7 animals in each group. *: p < 0.05, vs. no irradiation group (control).
| Group | Abdominal digestive tract Temperature (°C) |
|
|---|---|---|
| GK | control | 31.3 ± 3.2 |
| LPLI | 35.8 ± 3.9* | |
| Zucker | control | 31.0 ± 3.3 |
| LPLI | 35.8 ± 3.6* | |
| SD | control | 32.4 ± 3.1 |
| LPLI | 30.7 ± 3.8 | |
| STZ+LPLI | 36.5 ± 3.3* |
Blood serum components and inflammatory cytokines
The changes in blood glucose concentration during the experiment have been shown in Figure 4. In the 12-week GK group, the non-irradiated group and LPLI showed 282.4 ± 38.1 and 230.6 ± 31.8 mg/dl (p < 0.05), respectively, and LPLI showed a significant decrease. In the Zucker rats, the non-irradiated group and LPLI group showed 168.3 ± 28.3 and 126.1 ± 22.1 mg/dl (p < 0.05), respectively, and a significant decrease was observed in LPLI. The blood glucose concentration of SD rats, STZ non-irradiated group and STZ-LPLI group was 78.3 ± 11.2 mg / dl at the start of the experiment. Two days after STZ administration, it was 415.6 ± 88.2 mg/dl. After 12 weeks, the non-irradiation group and LPLI showed 520.8 ± 138.3 and 483.3 ± 106.4 mg/dl, respectively.
Figure 4:
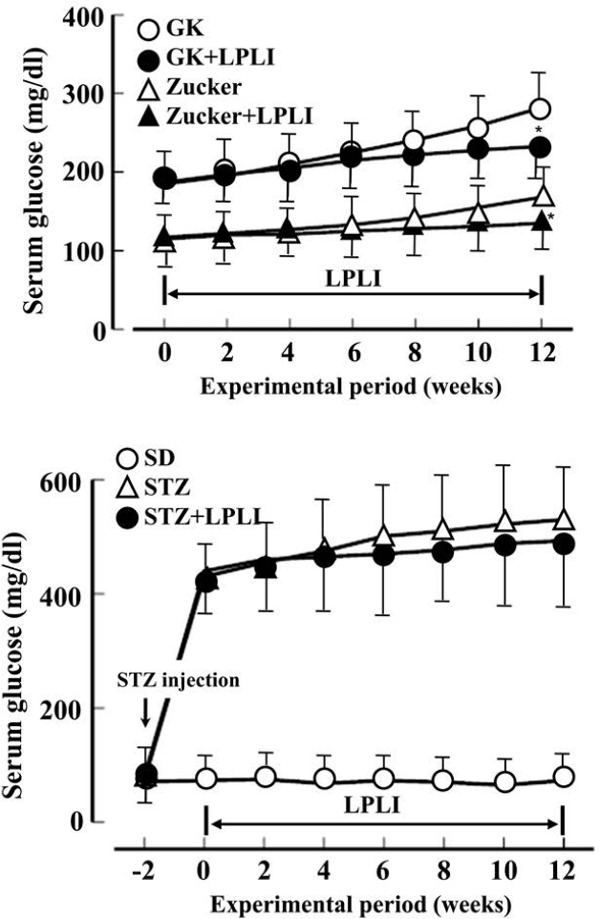
Effects of LPLI on serum glucose levels in GK, Zucker and streptozotocin-induced diabetic (STZ) rats. Data were shown as the mean ± SD of 7 animals in each group. *: p < 0.05 vs. no irradiation group.
The blood total cholesterol levels are shown in Table 3. The level in GK rats in the non-irradiated group and LPLI after 12 weeks was 373.8 ± 40.5 and 290.8 ± 32.9 mg/dl (p < 0.05). In the Zucker group, there was no significant difference between the non-irradiated group and LPLI at 622.7 ± 73.3 and 553.8 ± 72.7 mg/dl (p>0.05). Blood triglyceride concentration in LPLI decreased in all groups compared with the non-irradiated group during the experimental period, but this difference was not statistically significant (Table 3).
Table 3: Effects of LPLI on serum cholesterol and triglyceride levels in GK, Zucker, and streptozotocin-induced diabetic (STZ) rats. Data were shown as the mean ± SD of 7 animals in each group. *: p < 0.05 vs. no irradiation group.
| Group | Cholesterol (mg/dL) |
Tryglyceride (mg/dL) |
|
|---|---|---|---|
| GK | control | 373.8 ± 40.5 | 128.1 ± 16.6 |
| LPLI | 290.8 ± 32.9* | 98.7 ± 13.3 | |
| Zucker | control | 622.7 ± 73.3 | 520.6 ± 68.7 |
| LPLI | 553.8 ± 72.7 | 468.3 ± 65.8 | |
| SD | control | 483.5 ± 55.6 | 168.8 ± 23.3 |
| LPLI | 337.5 ± 40.2 | 131.6 ± 17.2 | |
| STZ+LPLI | 311.5 ± 39.9 | 110.8 ± 13.7 |
Table 4 shows the blood insulin concentrations after 12 weeks. In the GK rats, non-irradiated group and LPLI group showed 6.2 ± 1.7 and 5.1 ± 1.5 ng/ml (p < 0.05), highlighted a slight decrease in LPLI. In Zucker rats, the non-irradiated group and LPLI showed 11.8 ± 2.5 and 8.2 ± 1.8 ng/ml (p < 0.05), with significant decrease observed in LPLI. For the non-irradiated STZ group vs LPLI STZ group and non-irradiated STZ group vs non STZ treated SD rats, the insulin levels were 3.0 ± 0.5 vs 1.1 ± 0.2 ng/ml (p < 0.001); 3.0 ± 0.5 vs 1.3 ± 0.4 ng/ml (p < 0.001). Compared to the STZ untreated SD group, the STZ treated groups (both non-irradiated group and LPLI) showed a significant decrease. When the insulin levels in non-irradiated group and LPLI group were compared, the later showed an increase.
Table 4: Effects of LPLI on serum insulin levels in GK, Zucker and streptozotocin-induced diabetic (STZ) rats. Data were shown as the mean ± SD of 7 animals in each group. *: p < 0.05 vs. no irradiation group.
| Group | Serum insulin (ng/ml) |
|
|---|---|---|
| GK | control | 6.2 ± 1.7 |
| LPLI | 5.1 ± 1.5 | |
| Zucker | control | 11.8 ± 2.5 |
| LPLI | 8.2 ± 1.8* | |
| SD | control | 3.0 ± 0.5 |
| LPLI | 1.1 ± 0.2 | |
| STZ+LPLI | 1.3 ± 0.4 |
Blood TNF-α levels were 5.1 ± 1.1 and 3.3 ± 0.5 pg/ml (p < 0.01) in the non-irradiated group and LPLI in the Zucker rats, which showed a significant decrease in LPLI group. For the non-irradiated STZ group vs LPLI STZ group and non-irradiated STZ group vs non STZ treated SD rats, the levels were 1.9 ± 0.3 vs 5.8 ± 1.1 pg/ml (p < 0.001); 1.9 ± 0.3 vs 5.6 ± 1.2 pg/ml (p < 0.001). There was a significant increase in both the non-irradiated group and LPLI. However, there was no significant difference between the non-irradiated group in the STZ group and LPLI (Table 5).
Table 5: Anti-inflammatory effects of LPLI on serum TNF-á, IL-1I and IL-6 levels in GK, Zucker and streptozotocin-induced diabetic (STZ) rats at 12 weeks using infrared thermography. Data were shown as the mean ± SD of 7 animals in each group. *: p < 0.05, **: p < 0.01 vs. no irradiation group (control).
| Group | TNF-α (pg/ml) | IL-1β (pg/ml) | IL-6 (pg/ml) | |
|---|---|---|---|---|
| GK | control | 3.9 ± 0.5 | 53.2 ± 10.6 | 106.5 ± 23.8 |
| LPLI | 3.3 ± 0.6 | 45.1 ± 6.7 | 78.1 ± 14.4* | |
| Zucker | control | 5.1 ± 1.1 | 43.6 ± 8.8 | 98.3 ± 15.8 |
| LPLI | 3.3 ± 0.5** | 27.1 ± 3.8** | 62.2 ± 12.1** | |
| SD | control | 1.9 ± 0.3 | 18.1 ± 2.3 | 41.8 ± 5.2 |
| LPLI | 5.8 ± 1.1 | 69.2 ± 11.1 | 120.0 ± 26.2 | |
| STZ+LPLI | 5.6 ± 1.2 | 66.8 ± 21.3 | 116.6 ± 15.3 |
Blood IL-1β levels among the Zucker rats, were 43.6 ± 8.8 and 27.1 ± 3.8 pg/ml (p < 0.01) in the non-irradiated group and LPLI respectively, with LPLI showing a significant decrease. Blood IL-6 concentrations in the GK rats at 12 weeks were 106.5 ± 23.8 and 78.1 ± 14.4 pg /ml (p < 0.05) in the non-irradiated group and LPLI. In the Zucker rats, the non-irradiated group and LPLI showed blood IL-6 levels of 98.3 ± 15.8 and 62.2 ± 12.1 pg/ml (p < 0.01), which decreased in LPLI group. Zucker's rat with LPLI showed significant suppression of blood inflammatory cytokines levels (TNF-α, IL-1β, IL-6) levels (p < 0.05, Table 5).
The small intestinal transit rates of charcoal meals were significantly decreased (58.1 ± 10.1 vs 73.4 ± 13.3, p < 0.05) in the Zucker-LPLI group compared with the control rats. The small intestinal transit rates showed no difference in GK and STZ rats (Table 6).
Table 6: Effects of LPLI on small intestinal transit rates of charcoal meals in GK, Zucker and streptozotocin-induced diabetic (STZ) rats. Data were shown as the mean ± SD of 7 animals in each group. *: p < 0.05 vs. no irradiation group.
| Group | Small intestinal transit (%) | |
|---|---|---|
| GK | control | 61.8 ± 15.3 |
| LPLI | 70.4 ± 12.6 | |
| Zucker | control | 58.1 ± 10.1 |
| LPLI | 73.4 ± 13.3* | |
| SD | control | 61.3 ± 12.7 |
| LPLI | 60.8 ± 13.3 | |
| STZ+LPLI | 66.7 ± 18.5 |
Discussion
The experimental designed involved GK (non-obese) and Zucker (obese) rats for Type 2 diabetes model and STZ-induced diabetic rats for type 1 diabetes model. The changes in body weight, blood biochemistry, inflammatory cytokines and temperature of abdominal wall were evaluated. The final weight in the Zucker rats was 706 ± 83 g and 588 ± 71 g in the non-irradiated group and LPLI, LPLI showed a significant weight loss. Extrapolation of this numerical data to the human body weight is decrease of 80 kg body weight to 66 kg. The LPK diet efficiency was low in both GK and Zucker rats, but no difference was observed between the non-irradiated group and LPLI. The rats did not show any loss of appetite or abnormal eating behavior after LPLI.
In STZ rats of type 1 diabetes model, the amount of insulin secretion from β cells was 1.1 ± 0.2 ng/ml in the non-irradiated group, whereas LPLI group showed an increase to 1.3 ± 0.4 ng/ml. This finding highlights that LPLI may have improved the function of islet β cells. The decrease in body weight and blood glucose level in LPLI group was also observed.
Abdominal skin temperature was measured using ITh. LPLI groups showed a significant (p < 0.05) difference from non-irradiated group in GK, Zucker and STZ-SD rats. ITh displays the thermal energy emitted from the living body as skin surface temperature and is widely used for testing various diseases that affect the body surface temperature, such as superficial acute inflammation, blood circulation disorders, chronic pain, inflammation, and tumor. 15) In the present experiment, LPLI improved blood flow in deep tissues and abdominal adipose tissues, this resulted in an increase in abdominal skin temperature.
Blood glucose levels were found to be lesser in LPLI groups as compared to non-irradiated groups. In the GK and Zucker rats, LPLI suppressed the increase in blood glucose levels compared to the non-irradiated groups from approximately 8 weeks of irradiation. GK rat is a naturally occurring non-obese diabetic animal model established by selective mating from Wistar rats. It is a type 2 diabetes model that develops diabetes at 8–10 weeks of age with abnormal pancreatic insulin secretion and insulin resistance. 16)
In the present experiment, blood glucose levels of Wistar rats and GK group at the same age were 73.5 ± 15.9 and 282.4 ± 38.1 mg/ml, respectively, and GK group developed hyperglycemic diabetes. The blood insulin levels in Wistar rats and GK groups were 2.5 ± 0.5 and 6.2 ± 1.7 pg/ml. Despite the fact that GK group secreted a sufficient amount of insulin, insulin resistance was observed. Zucker rats are type 2 diabetes with obesity, an animal model of type 2 diabetes that accounts for more than 90% of diabetes patients. Obesity is observed by weight gain and appearance from around 4 weeks of age which progresses rapidly by 10 weeks of age. Obesity leads to polyphagia, hyperlipidemia, hyperinsulinemia, hyperleptinemia and high fasting blood glucose level. Insulin resistance is a characteristic of type 2 diabetes and is responsible for hyperglycemia and hyperinsulinemia. 17, 18)
In the Zucker rats used in this experiment, the body weight at the end after 12 weeks was 706 ± 83 g, approximately twice that of normal rats of the same age, and the blood insulin level was 11.8 ± 2.5 pg/ml, approximately four times that of normal rats. The patient with type 2 diabetes show high insulin levels with obesity. In contrast, LPLI significantly decreased blood glucose and blood insulin levels in both GK and Zucker rats, suggesting that LPLI has the effect of improving insulin resistance.
Obesity is the most common cause of lifestyle-related diseases such as diabetes. In obese conditions, macrophages infiltrate between enlarged visceral fat cells, resulting in chronic inflammation. Hotamisligil et al. showed that TNF-α is a kind of adipocytokine secreted from adipocytes and is secreted excessively from fat cells of obese people or obese animal models and phosphorylates the serine residue of IRS-1. We reported that insulin resistance was induced as a result of decreasing tyrosine phosphorylation of the insulin receptor and inhibiting insulin action. 19–21) Clinical data have also reported that macrophages are activated under hyperglycemia in type 2 diabetic patients and produce excessive amounts of inflammatory cytokines such as TNF-IV and IL-6. 22, 23) In obese individuals, blood levels of adipose tissue-derived TNF-IV and IL-6 increase, and their blood levels decrease with decreasing body weight. 24, 25)
In obese visceral adipocytes, the expression of multiple inflammatory cytokines such as IL-1β 26) and IL-6 27) is also increased, synergistically suppressing insulin signal and enhancing insulin resistance. In this study, TNF-α levels in Zucker rats were about three times higher than normal rats, LPLI significantly decreased TNF-IV by 25%, IL1-IV and IL-6 by 30% as compared to the non-irradiated group.
The evaluation of various inflammatory model animals has revealed an anti-inflammatory action of LED 28, 29) and LPLI. 30, 31) In the oral region, LPLI (wavelength 660 nm) in the salivary gland of STZ diabetic rats suppressed the increase in HMGB1/AGE/RAGE gene expression by the NF-κ pathway, which is a transcription factor of inflammatory response along-with Bax and Caspase-3, inhibiting this activity. It also reduced diabetes-induced apoptosis and improved the salivary secretion in STZ diabetic rats. 32)
In this study, GK and Zucker rats, which are clinically and pathologically similar to human diabetes type 2, and STZ-induced diabetic rats, which are type 1 diabetes rat models, were used to focus on subcutaneous adipose tissue in the abdomen. In addition, it was observed that LPLI significantly suppressed the increase of blood inflammatory cytokine levels in Zucker rats and was effective in improving diabetes accompanied by obesity. LPLI is also presumed to be involved in the suppression of inflammatory cytokine production from abdominal adipocytes and associated weight loss in Zucker rats. Animal models are useful alternatives for diseases commonly observed in humans in studies of disease pathogenesis and therapeutic intervention. The obese Zucker rat and the nonobese GK rat are frequently used for studies on type 2 diabetes. The etiology and development of type 2 diabetes in humans are likely as disparate as the disease in numerous procedures with experimental animals. Therefore, it is indispensable for identifying the reason for the differences in both animal models which may provide a foundation for type 2 diabetes progression and may yield unique insights into the risk factors of type 2 diabetes in humans.
Conclusion
LPLI suppressed body weight gain in Zucker rats, a model of obese spontaneous type 2 diabetes, and increased the levels of blood components and inflammatory cytokines TNF-α, IL-1β, and IL-6. It is hence suggested that this is effective in improving the insulin resistance, increasing insulin secretion and reduction in body weight of type 2 diabetes rat model while no significant effect on type 1 diabetes rat model.
Acknowledgements
We would like to thank the staff of the animal facility for care of the rats. This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflicts of interest
The article has not been published elsewhere. The authors declare no conflict of interest.
References
- 1: Osborn O, Olefsky JM: The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med, 2012; 18: 363-374. [DOI] [PubMed] [Google Scholar]
- 2: Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibei RL, et al. : Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest, 2003; 112: 1796-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3: Lumeng CN, Bodzin JL, Saltiel AR: Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest, 2007; 117: 175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4: Tilg H, Moschen AR: Inflammatory mechanisms in the regulation of insulin resistance. Mol Med, 2008; 14: 222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5: Hotamisligil GS: Inflammation and metabolic disorders. Nature, 2006; 444: 860-867. [DOI] [PubMed] [Google Scholar]
- 6: Muoio DM, Newgard CB: Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta- cell failure in type 2 diabetes. Nat Rev Mol Cell Biol, 2008; 9: 193-205. [DOI] [PubMed] [Google Scholar]
- 7: Zhang L, Zhao J, Kuboyama N, Abiko Y: Low-level laser irradiation treatment reduces CCL2 expression in rat rheumatoid synovia via a chemokine signaling pathway. Lasers Med Sci, 2011; 26: 707-717. [DOI] [PubMed] [Google Scholar]
- 8: Kuboyama N, Ohta M, Abiko Y, et al. : Anti-rheumatic activities of free electron laser irradiation on arthritis in rats. JJSLM, 32: 108-115, 2011. [Google Scholar]
- 9: Kuboyama N, Ohta M, Sato Y, Abiko Y: Anti-inflammatory activities of light emitting diode irradiation on collagen-induced arthritis in mice. JJSLM, 33: 19-25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10: Kuboyama N, Abiko Y: Reduction of monocyte chemoattractant protein-1 expression in rheumatoid arthritis rat joints with light-emitting diode phototherapy. Laser Therapy, 2012; 21: 177-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11: Kawano Y, Nakae J, Watanabe N, Kikuchi T, Tateya S, et al. : Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-dependent Manner. Cell Me-tab, 2016; 24: 295-310. [DOI] [PubMed] [Google Scholar]
- 12: Rakieten N, Rakieten ML, Nadkarni MV: Studies on the diabetogenic action of streptozotocin. Cancer Chemother Rep, 1963; 29: 91-98. [PubMed] [Google Scholar]
- 13: Szkudelski T: The mechanism of alloxan and streptozotocin in B cell of the rat pancreas. Physiol Res, 2001; 50: 537-546. [PubMed] [Google Scholar]
- 14: Portha B, Serradas P, Bailbe D, Suzuki K, Goto Y, et al. : :-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type 2 diabetes. Diabetes, 1991; 40: 486-491. [DOI] [PubMed] [Google Scholar]
- 15: Nakamura K, Mochizuki K, Ishii Y: Thermography for osteoarthritis of the knee. J Physical Medicine, 1996; 7: 266-271. [Google Scholar]
- 16: Goto Y, Kakizaki M, Masaki N: Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med, 1976; 119: 85-90. [DOI] [PubMed] [Google Scholar]
- 17: DeFronzo RA: Retinal Hazard from Blue Light Emitting Diode. Diabetologia, 1992, 35: 389-397.1516769 [Google Scholar]
- 18: Doxey DL, Nares S, Park B, Trieu C, Cutler CW, et al. : Diabetes-induced impairment of macrophage cytokine release in a rat model: potential role of serum lipids. Life Sci, 1998; 63: 1127-1136. [DOI] [PubMed] [Google Scholar]
- 19: Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science, 1993; 259: 87-91. [DOI] [PubMed] [Google Scholar]
- 20: Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM: Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest, 1995; 95: 2409-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21: Hotamisligil GS, Perald P, Budavari A, Ellis R, White MF, et al. : IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science, 1996; 271: 665-668. [DOI] [PubMed] [Google Scholar]
- 22: Devaraj S, Venugopal SK, Singh U, Jialal I: Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta}. Diabetes, 2005; 54: 85-91. [DOI] [PubMed] [Google Scholar]
- 23: Guha M, Bai W, Nadler JL, Natarajan R: Molecular mechanisms of TNF alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and independent pathways. J Biol Chem, 2000; 273: 17728-17739. [DOI] [PubMed] [Google Scholar]
- 24: Kopp HP, Kopp CW, Festa A, Krzyzanowska K, Kriwanek S, et al. : Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol, 2003; 23: 1042-1047. [DOI] [PubMed] [Google Scholar]
- 25: Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, et al. : Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res, 2003; 11: 1048-1054. [DOI] [PubMed] [Google Scholar]
- 26: Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, et al. : Intereukin-1 receptor antagonist in type 2 diabetes mellitus. N Engl J Med, 2007; 356: 1517-1526. [DOI] [PubMed] [Google Scholar]
- 27: Rotter V, Nagaev I, Smith U: Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor--, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem, 2003; 278: 45777-45784. [DOI] [PubMed] [Google Scholar]
- 28: Xavier M, David DR, de Souza RA, Arrieiro AN, Miranda H, et al. : Anti-inflammatory effects of low-level light emitting diode therapy on Achilles tendinitis in rats. Lasers Surg Med, 2010; 42: 553-558. [DOI] [PubMed] [Google Scholar]
- 29: Lim W, Lee S, Kim I, Chung M, Kim M, et al. : The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med, 2007; 39: 614-621. [DOI] [PubMed] [Google Scholar]
- 30: Safavi SM, Kazemi B, Esmaeili M, Fallah A, Modarresi A, et al. : Effects of low-level He-Ne laser irradiation on the gene expression of IL-1β, TNF-α, IFN-γ, TGF-β, bFGF and PDGF in rat's gingival. Lasers Med, 2008; 23: 331-335. [DOI] [PubMed] [Google Scholar]
- 31: Rabelo SB, Villaverde AB, Nicolau R, Salgado MC, Melo Mda S, et al. : Comparison between wound healing in induced diabetic and nondiabetic rats after low-level laser therapy. Photomed Laser Surg, 2006; 24: 474-479. [DOI] [PubMed] [Google Scholar]
- 32: Fukuoka CY, Simoes A, Uchiyama T, Arana-Chavez VE, Abiko Y, et al. : The effects of low-power laser irradiation on inflammation and apoptosis in submandibular glands of diabetes- induced rats. PLoS One, 2017; 12: 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


