Abstract
Human bocavirus (HBoV) has been detected primarily in children with acute lower respiratory tract disease (LRTD), but its occurrence, clinical profile, and role as a causative agent of RTD are not clear. The aim of this study was to investigate the prevalence and the potential clinical relevance of HBoV. Using molecular tests, we tested 1352 nasopharyngeal samples obtained between October 1, 2017 and April 30, 2018 from children up to the age of 16 with RTD for the presence of HBoV DNA and 20 other respiratory pathogens at three different hospitals in Belgium. HBoV was detected in 77 children with a median age of 10.6 months. Consecutive samples were available for 15 HBoV-positive children and showed persistent HBoV positivity in four of them. Monoinfection was observed in six infants. Four of them were born prematurely and were infected during hospitalization at the neonatal intensive care unit (NICU). Only one of these six monoinfected children was diagnosed with recurrent wheezing due to HBoV. This child was carried to term and had a high viral load. Coinfections, most frequently with rhinovirus (52.1%) and adenovirus (49.3%), were observed in 72 patients. In seventeen of them in which HBoV was present at high viral load or higher viral load than its copathogens, bronchi(oli)tis (n = 8), recurrent wheezing (n = 8) or episodic wheezing (n = 1) were diagnosed. Our results suggest that HBoV infection at high viral load in infants is associated with wheezing (P = 0.013, Cramer’s V = 0.613).
Electronic supplementary material
The online version of this article (10.1007/s00705-019-04396-6) contains supplementary material, which is available to authorized users.
Introduction
Respiratory tract diseases (RTDs) are a leading reason for morbidity in young children and are caused by a broad spectrum of microbial agents. Viruses account for the largest number of respiratory tract infections (RTIs). The so-called respiratory viruses include influenza A and B viruses (IAV, IBV), human parainfluenza viruses (HPIVs), human respiratory syncytial virus (HRSV), human adenoviruses (HAdVs), human rhinoviruses (HRVs), human coronaviruses (HCoVs), enteroviruses (EVs) and human parechoviruses (HPeVs). In the 21st century, using large-scale molecular virus screening, several novel viruses have been discovered in patients with respiratory infections. These viruses include human metapneumovirus (hMPV), polyomaviruses KI and WU, several coronaviruses (SARS-CoV, HCoV-NL63, HCoV-HKU1, MERS-CoV) and human bocavirus (HBoV) as described by Allander et al. in 2005 [1–3]. The DNA virus HBoV is a member of the family Parvoviridae, genus Bocaparvovirus. HBoV is classified into genotypes 1 through 4. HBoV1 is predominantly found in respiratory tract secretions from children with RTD, and HBoV2-4 are found mainly in stool samples from patients with gastroenteritis [3, 4]. HBoV is predominantly present in winter and spring [5]. The average prevalence of HBoV in respiratory tract samples ranges from 1.0% to 56.8%, depending on the country. The worldwide estimate for the total prevalence of HBoV in respiratory infections is 6.3% [6]. HBoV has been reported worldwide in all age groups; however, it has mainly been detected in children who presented at the hospital with RTD. A high HBoV viral load could be an etiologic agent for severe LRTI, and these patients may develop bronchitis and pneumonia with fever, cough and peribronchial infiltrates detected on a chest X-ray [7, 8]. Low viral loads in coinfection indicate more asymptomatic shedding [9]. Furthermore, HBoV has been suggested as a cause of pediatric gastrointestinal (GI) infection [10, 11] and might even have a causal role in encephalitis [12–15]. However, it has also been found in children with mild infections [16] and in asymptomatic ones, and therefore, the pathogenic role of HBoV is still under discussion [8, 17]. Classically, Koch’s postulates have been used to establish a causal relationship between viruses and disease [18]. As there is no animal model so far for HBoV, proving its clinical relevance is challenging. However, HBoV can replicate in human airway epithelium cultures [6, 19], and Deng et al. showed that HBoV1 induces damage to the airway epithelium (loss of cilia, disruption of the tight junction barrier, and a significant decrease in transepithelial electrical resistance) [20, 21]. Furthermore, serological diagnosis of HBoV has recently confirmed significant increases in IgG antibodies in children with pneumonia. These results support the idea that it is a true pathogen in RTI in children [22, 23]. Prolonged viral shedding has been described, about 2.5 months in outpatients and about 4.5 months to 1 year in hospitalized children, which probably explains why HBoV is detected in asymptomatic cases. This prolonged shedding may also explain why the rate of coinfection with other viruses is so high, ranging from 75% to 85% [24].
In this study, we retrospectively analyzed data for 1352 nasopharyngeal samples (NPSs, aspirates and swabs) that were molecularly tested for the presence of HBoV DNA. Our aim was to determine the prevalence of HBoV in children up to 16 years of age who presented at the hospital with RTD and to describe the clinical features of the infected children.
Materials and methods
Specimen collection
We retrospectively analyzed the data of the respiratory molecular screening of 1352 NPSs obtained between October 1, 2017 and April 30, 2018 from children up to the age of 16 who presented with RTD at three different hospitals in Belgium: Ghent University Hospital, AZ Sint-Jan, and AZ Sint-Lucas of Bruges. Since we wanted to describe the whole spectrum of disease attributed to HBoV, we choose to include all patients and use no exclusion criteria. Because of the retrospective nature of this study, there was no asymptomatic control group included. Three hundred two NPSs were collected and analyzed in the Ghent University Hospital, and 572 and 478 samples were collected at AZ Sint-Jan and AZ Sint-Lucas of Bruges, respectively, and analyzed at AZ Sint-Jan Hospital.
Viral DNA extraction and real-time PCR amplification
The NPSs were tested in a routine setting for the presence of HBoV 1-4 DNA among 20 other infectious agents (IAV and IBV; human coronaviruses NL63 [HCoV-NL63], 229E [HCoV-229E], OC43 [HCoV-OC43] and HKU1 [HCoV-HKU1]; HPIV 1, 2, 3 and 4; hMPV A and B, HRV, HRSV A and B, HAdV, EV, HPeV, and Mycoplasma pneumoniae [MP]) using real-time PCR (RT-PCR) amplification by TaqMan® technology. The AZ Sint-Jan Hospital also tested for five viruses, 10 bacteria and two fungi. In the Ghent University Hospital, nucleic acid from NPSs was extracted using a NucliSENS EasyMAG (BioMérieux, France) automated extractor followed by a commercial multiplex RT-PCR assay (FTD Respiratory Pathogens 21, Fast Track Diagnostics, Luxembourg) according to manufacturer’s instructions. In the AZ Sint-Jan Hospital, nucleic acid extraction on a QIAsymphony (QIAGEN, Spain) automated extraction system according to the manufacturer’s instructions was followed by a singleplex RT-PCR using an in-house customized TaqMan® Array Card (TAC) (v13.0 premarket version Cambridge-Bruges) [25]. Because a universal primer for HBoV 1-4 was used, distinction between the different genotypes of HBoV was not possible, which is a limitation of our study. The detailed protocol for DNA extraction and RT-PCR amplification is available online as Online Resource 1.
Clinical interpretation of the HBoV PCR test results
HBoV results were categorized in three groups based on the quantitation cycle value (Cq value): Cq ≤ 22, strongly positive (high viral load); 22 < Cq ≤ 30, positive; 30 < Cq ≤ 40, weakly positive. We were especially interested in patients with HBoV in monoinfection or with HBoV present at a low Cq value (≤ 22) or a lower category of Cq value than its copathogens in cases of coinfection, since the fundamentals of RT-PCR allow us to understand that lower Cq values represent a higher viral load. The PCR results were analyzed together with the white blood cell count (WBC) and differentiation, the value of C-reactive protein (CRP), the clinical characteristics of the patient (URTD vs. LRTD, GI disease, fever), the duration of hospitalization, the need for oxygen therapy, and results of chest X-ray, if available. A history of underlying conditions such as cardiac disease, prematurity, chronic pulmonary disease or previous episodes of wheezing were studied if present. Bronchiolitis or wheezing in young children is a very common clinical problem, and definitions are often confusing. In this study, we define bronchiolitis as a constellation of clinical signs and symptoms occurring in children younger than 2 years, including a viral URT prodrome followed by increased respiratory effort and wheezing [26, 27]. We diagnosed children younger than two years of age with bronchiolitis if their history showed no previous episodes of wheezing and with “recurrent wheezing” if there had been previous episodes. Children older than two years of age with previous episodes of wheezing were diagnosed with episodic wheezing. Bronchopulmonary dysplasia (BPD) was defined as oxygen dependency beyond 28 days of life [28].
Statistical analysis
Statistical analysis was carried out using IBM SPSS Statistics 25. Categorical variables were compared using the chi-square test, and the continuous variables were compared using an independent t-test or one-way ANOVA. P-values less than 0.05 were considered significant.
Results
Detection of HBoV
Between October 1, 2017 and April 30, 2018, 1352 NPSs of 1153 infants and children with RTD were received for diagnostic evaluation. HBoV was detected in 87 (6.4%) samples from 77 children. The dataset for these positive patients is available as Online Resource 2. Out of the 87 samples, 13 (14.9%) were monoinfections and 74 (85.1%) were coinfections with one or more other viruses. The 13 single detections belonged to six infants, the 74 samples with coinfections belonged to 72 children. One child had multiple NPSs that were positive for HBoV in mono- and coinfection, so a total of 77 different children were included in this study (Fig. 1). Coinfections were most frequently seen with HRV (38 samples) and HAdV (36 samples). Coinfection of HBoV with bacteria or fungi was not seen.
Fig. 1.
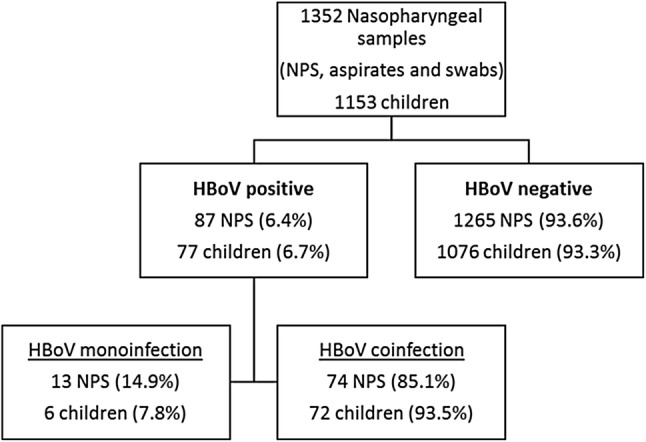
Flowchart of positivity of nasopharyngeal samples (NPS) for human bocavirus (HBoV) in monoinfection or coinfection in our pediatric study population
The HBoV viral load, expressed as a Cq-value, in children with a monoinfection (mean Cq, 26; range, 10–34) was not different from that in children with a coinfection (mean Cq, 28; range, 13-35) (P = 0.445). A high viral load (Cq ≤ 22) was observed in four of the 13 (30.8%) samples expressing HBoV alone and in 16 of the 74 (21.6%) samples with coinfection. Patients with a higher viral load were slightly younger, although this was not statistically significant (P = 0.051). The mean age of patients in the first (Cq ≤ 22), second (22 < Cq ≤ 30) and third category (30 < Cq ≤ 40) was 11.2, 11.2 and 16.8 months, respectively.
Persistent HBoV positivity
For 15 HBoV-positive children (4 monoinfections and 11 coinfections) various consecutive samples were taken because of a clinical need for them over a period of several weeks to several months (Table 1 and Fig. 2). Four children showed positivity for HBoV in consecutive samples. One child showed persistent HBoV positivity in seven separate determinations spread equally over a period of 97 days. The second child showed HBoV shedding in three determinations over a period of 35 days. The third child was HBoV positive two times within a period of 2 weeks; whereas in the last child, HBoV DNA could be detected in samples collected in October 2017 and in March 2018.
Table 1.
Overview of results for 15 human bocavirus (HBoV)-positive children who were tested multiple times by PCR for respiratory pathogens during the 2017–2018 season
| Patient | Pathogen (identification + Cq value + date of PCR run) | Duration HBoV positivity (days) | Number of PCR assays performed | Number of samples positive for HBoV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Monoinfection | ||||||||||
| A | HBoV Cq 10 | HBoV Cq 23 | HBoV Cq 32 | HBoV Cq 32 | HBoV Cq 33 | HBoV Cq 34, HRV | HBoV Cq 30 | |||
| 23/12/17 | 8/01/18 | 15/01/18 | 5/02/18 | 14/02/18 | 1/03/18 | 30/03/18 | 97 | 7 | 7 | |
| B | HBoV Cq 30 | HBoV Cq 18 | HBoV Cq 34 | |||||||
| 1/01/18 | 15/01/18 | 5/02/18 | 35 | 3 | 3 | |||||
| C | HBoV Cq 34 | HRV | HRV | HRV | ||||||
| 25/12/17 | 15/02/18 | 13/04/18 | 20/04/18 | 4 | 1 | |||||
| D | HBoV Cq 34 | ND | ||||||||
| 21/02/18 | 31/03/18 | 2 | 1 | |||||||
| Coinfection | ||||||||||
| P | HBoV Cq 18, HAdV | HBoV Cq 32, HAdV, IBV, HPeV, HRV | 14 | 2 | 2 | |||||
| 15/12/17 | 29/12/17 | |||||||||
| X | HBoV Cq 35, HAdV, EV, HRV | HBoV Cq 33, HAdV | 148 | 2 | 2 | |||||
| 5/10/17 | 2/03/18 | |||||||||
| Y |
HBoV Cq 33, HAdV 9/10/17 |
HRV | HAdV, HRV | HAdV | 4 | 1 | ||||
| 3/11/17 | 16/11/17 | 27/12/17 | ||||||||
| Z |
HBoV Cq 34, HAdV, EV, HPeV 14/11/17 |
HRSV | 2 | 1 | ||||||
| 26/03/18 | ||||||||||
| AA | HRSV A, HRV | HBoV Cq 20, HAdV, HRV | 2 | 1 | ||||||
| 12/12/17 | 12/02/18 | |||||||||
| T | HCoV-OC43, HRSV A | HBoV Cq 17, HAdV, HRV | 2 | 1 | ||||||
| 2/01/18 | 30/04/18 | |||||||||
| AB |
HAdV, HRSV B, HRV 1/12/17 |
HBoV Cq 30, HAdV, EV, HRV 5/03/18 |
2 | 1 | ||||||
| AC |
HAdV, HRV 25/02/18 |
HBoV Cq 33, HAdV, HRV 28/02/18 |
2 | 1 | ||||||
| Q | HRSV A | HCoV-OC43 | HBoV Cq 22, HAdV, hMPV, HRV | 3 | 1 | |||||
| 21/11/17 | 17/12/18 | 1/02/18 | ||||||||
| AD | ND | ND |
HBoV Cq 30, HAdV, HRV 26/03/18 |
3 | 1 | |||||
| 28/02/18 | 7/03/18 | |||||||||
| AE | HRV | hMPV, HRV |
HBoV Cq 30, HRV 13/04/18 |
3 | 1 | |||||
| 26/01/18 | 12/03/18 | |||||||||
Positivity for HBoV was reflected in bold
HBoV, human bocavirus; HRV, human rhinovirus; ND, not detected; HADV, human adenovirus; IBV, influenza B virus; HPeV, human parechovirus; EV, enterovirus; HRSV, human respiratory syncytial virus; HCoV-OC43, human coronavirus OC43; hMPV, human metapneumovirus
Fig. 2.
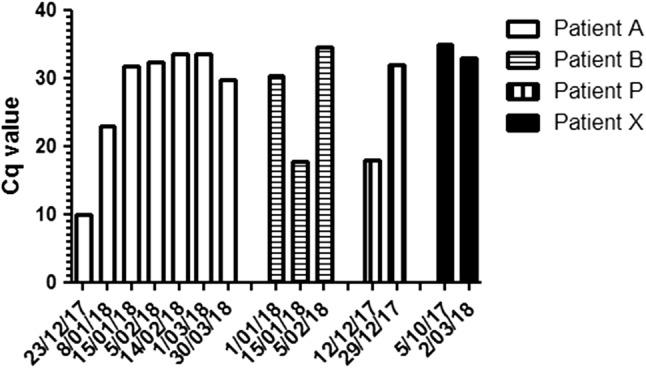
Persistent positivity for human bocavirus (HBoV) in four patients who were tested multiple times by PCR for respiratory pathogens during the 2017–2018 season
Clinical features associated with HBoV detection
The median age of the HBoV-positive children was 10.6 months (mean age, 13.2 months; range, 19 days–5.4 years). All but one of the children were younger than 3 years, and 62.8% of the children were younger than 12 months. The majority of the children (98.6%) were hospitalized in one of the three hospitals during the course of infection.
Monoinfection
The clinical characteristics of the six patients with respiratory episodes associated with single HBoV detection were analyzed in detail, and the results are shown in Table 2. The children had a median age of 2.6 months (mean age, 3.9 months; range, 19 days–11.5 months).
Table 2.
Overview of general information and laboratory and clinical parameters for the six children with human bocavirus (HBoV) in monoinfection
| General information | Laboratory parameters | Clinical parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gender | Age (months) | Prematurity (gestational age) | Medical department | Duration hospitalization (days) | WBC (*109/l) | CRP (mg/L) | First positivity HBoV (date + Cq value) | Fever (°C) | GI disorder | URTD | LRTD | Duration O2 therapy (days) | Chest RX | Medical history |
| A | M | 2.2 | 25 weeks | NICU UH Ghent | 206 | 14.0 | <0.6 | 23/12: 10 | No | No | No | Aggravation of CLD | Oxygen dependent at discharge | Peribronchial cuffing, atelectasis | BPD |
| B | F | 0.6 | 28 weeks | NICU UH Ghent | 69 | 11.7 | <0.6 | 1/01: 30 | No | No | No | Aggravation of CLD | 63 | / | BPD |
| C | F | 1.1 | 33 weeks | NICU UH Ghent | 3 | 6.9 | <0.6 | 25/12: 34 | No | No | No | Lower oxygen saturation | Oxygen dependent at discharge | Normal | Necrotizing enterocolitis |
| D | M | 3.0 | 26 weeks | NICU UH Ghent | 150 | 10.7 | <0.6 | 21/02: 34 | No | No | No | Aggravation of CLD | Oxygen dependent at discharge | / | BPD, pneumothorax |
| E | F | 4.9 | No | PICU UH Ghent | 4 | 12.3 | 6.0 ↑ | 15/02: 16 | No | No | No | Recurrent wheezing | 3 | Peribronchial cuffing, atelectasis | / |
| F | M | 11.5 | No | Pediatric ward AZ Sint-Jan | 3 | / | / | 3/02: 15 | Yes (38.4) | No | OMA | No | 0 | / | Recurrent OMA |
WBC, white blood cell count; CRP, C-reactive protein; GI disorder, gastrointestinal disorder; URTD, upper respiratory tract disease; LRTD, lower respiratory tract disease; M, male; F, female; NICU, neonatal intensive care unit; UH Ghent, Ghent University Hospital; CLD, chronic lung disease; BPD, bronchopulmonary dysplasia; PICU, pediatric intensive care unit; OMA, otitis media acuta
Nosocomial monoinfection
An important finding was that four out of the six children who were born prematurely at a gestational age of 25, 26, 28 and 33 weeks were still at the NICU of the Ghent University Hospital at the time of infection. Three of them got infected in the same week, so transmission probably occurred via the hospital staff. These premature patients were all treated in incubators and had had no previous contact with other children. The fact that they were in a protected area is probably the reason there was no coinfection. The patients born at 25, 26 and 28 weeks all developed BPD as a sequela of their premature birth. All three were intubated at birth, received surfactant, and needed long-term ventilator support. Two of them developed chronic oxygen dependency and were discharged with home oxygen therapy. The child born at a gestational age of 25 weeks needed conventional ventilation for 17 days, followed by 10 days of continuous positive airway pressure (CPAP) and long-term oxygen treatment. His respiratory condition was aggravated at an age of 70 days by infection with HBoV (Cq value, 10) with a need for repeated CPAP support and a higher oxygen supply. A chest X-ray at this time point showed peribronchial thickening and atelectasis. This child showed persistent HBoV PCR positivity in seven separate determinations (6 in monoinfection, 1 in coinfection). The other three premature infants expressed HBoV at higher Cq values (30–34) and had only mild deterioration of their respiratory condition. A child born at a gestational age of 26 weeks had severe BPD with a need for invasive ventilation (conventional and high-frequency oscillation) for 33 days followed by 18 days of CPAP. During the infection with HBoV, there was a higher oxygen need. A child born at a gestational age of 28 weeks needed conventional ventilation for 2 days followed by 3 days of oxygen supply by nasal canula. At day 19, infection with HBoV resulted in lower saturation and development of a need for extra oxygen until day 63. A child born at a gestational age of 33 weeks was transferred from another hospital to the NICU because of necrotizing enterocolitis. During her stay, there was an unexplained mild decline in saturation. A respiratory panel showed the presence of HBoV.
Community-acquired monoinfection
The fifth child was born at term and had no important pre-existing medical condition. This infant had been exclusively breastfed since birth. At the age of 4 months, she had a first episode of wheezing, and bronchiolitis was diagnosed. Further she had repetitive mild RTD, a finding that is very common in infants attending daycare. At an age of 4.9, months she presented at the emergency department with severe respiratory distress and a need for non-invasive respiratory support (high-flow O2 nasal cannula). She was admitted to the pediatric intensive care unit (PICU) for three days. Diagnosis of recurrent wheezing, caused by HBoV (Cq value, 16) was made. The sixth child had no LRTD and suffered from an otitis media. No pneumonia or GI disorders were observed in these six monoinfected children. There was no significant elevation of the white blood cell count or CRP value.
Community-acquired coinfection
The clinical characteristics of patients with HBoV in coinfection were analyzed in detail for a selected subpopulation. Seventeen cases in which HBoV was present at a Cq value ≤ 22 (n = 15) or at a lower Cq value than its copathogens (n = 2) were analyzed in more detail to access whether HBoV is a possible respiratory pathogen. An overview of the results can be found in Table 3. The median age of these 17 children was 11.8 months (range, 3.3–32.9 months). Five of these children had been born prematurely, with a gestational age ranging from 31 to 34 weeks. All children were hospitalized briefly in a pediatric ward at one of the three hospitals. Symptoms typically started with rhinitis and cough, which developed to tachypnea, wheezing, crackles, and sometimes the use of accessory muscles and nasal flaring. Bronchi(oli)tis (n = 8), recurrent wheezing (n = 8) and episodic wheezing (n = 1) were the leading diagnoses. Pneumonia was not observed. Chest X-rays were taken of 10 children, nine of which showed abnormalities (peribronchial thickening, atelectasis). Current evidence, however, does not support the routine use of chest radiography in children with bronchiolitis because abnormalities do not correlate well with disease severity. Fever was present in 76.5% of the cases, and hypoxia was present in 41.2% of them (duration of oxygen support varying from 1 to 5 days). Upper respiratory pathology such as conjunctivitis, rhinitis, pharyngitis, stomatitis and otitis media was present in 94.1% of the children. Gastrointestinal disorders, ranging in severity from decreased oral intake to vomiting and diarrhea, was observed in 11 children. There were only slight to moderate elevations in WBC count and CRP, compatible with viral infection.
Table 3.
Overview of general information and laboratory and clinical parameters for the 17 children with human bocavirus (HBoV) in coinfection where HBoV was present at a low Cq value (≤ 22) or a lower Cq value than its copathogens
| General information | Laboratory parameters | Clinical parameters | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Gender | Age (months) | Prematurity (gestational age) | Duration hospitali-zation (days) | WBC (*109/l) | CRP (mg/L) | First positivity HBoV (date + Cq value) | High viral load CT ≤ 22 | Recent virosis 22<CT≤30 | Moderate signal 30<CT<40 | Fever (°C) | GI disorder | URTD | LRTD | Duration O2 therapy (days) | Chest RX | Medical history |
| G | M | 18.3 | 31 weeks | 5 | 12.9 | 16.5 ↑ | 5/01: 17 | HBoV | - | HAdV | 38.6 | Gastritis | Rhinitis | Recurrent wheezing | 4 | Peribronchial cuffing | / |
| H | F | 8.2 | 34 weeks | 5 | 11.2 | 9.9 ↑ | 7/04: 16 | HBoV | - | HCoV-HKU1, HPeV | No | No | Rhinitis, otitis, pharyngitis | Recurrent wheezing | 3 | Peribronchial cuffing, atelectasis | / |
| I | M | 6.7 | No | 6 | 19.7 ↑ | 4.2 | 17/04: 13 | HBoV | HAdV, HRV | - | No | No | Rhinitis | Recurrent wheezing | 2 | Peribronchial cuffing | / |
| J | M | 16.6 | No | 3 | 12.3 | 27.0 ↑ | 13/04: 16 | HBoV | HCoV-NL63 | HRV | 40.0 | No | Conjunctivitis | Bronchitis | 0 | Peribronchial cuffing | / |
| K | F | 11.9 | No | 2 | 12.3 | 11.0 ↑ | 25/03: 17 | HBoV | HRSV B, HRV | - | 40.0 | Gastritis | Rhinitis | Bronchiolitis | 0 | / | / |
| L | F | 11.9 | 34 weeks | 6 | 10.2 | 7.5 ↑ | 23/03: 14 | HBoV | HRV | - | No | No | Rhinitis | Bronchiolitis | 1 | / | / |
| M | M | 16.8 | No | 4 | 6.9 | 15.0 ↑ | 19/04: 18 | HBoV, IBV | EV, HRV | - | 39.2 | No | Stomatitis, conjunctivitis | Recurrent wheezing | 0 | / | / |
| N | F | 11.8 | No | 4 | 21.3 ↑ | 16.0 ↑ | 7/03: 14 | HBoV | - | IBV | No | No | Otitis, rhinitis, pharyngitis | Recurrent wheezing | 0 | / | / |
| O | F | 6.0 | No | 4 | 16.2 | 40.0 ↑ | 10/10: 14 | HBoV, HAdV | HRV | - | 39.4 | Gastritis | Conjunctivitis | Bronchitis | 0 | / | / |
| P | M | 4.7 | No | 3 | 20.5 ↑ | 26.4 ↑ | 15/12: 18 | HBoV | HAdV | - | 39.1 | Anorexia | Rhinitis | Bronchiolitis | 0 | Peribronchial cuffing | Esophageal atresia |
| Q | M | 8.4 | No | 6 | 13.7 | 9.4 ↑ | 1/02: 22 | HBoV | hMPV | HAdV, HRV | 39.5 | Anorexia, gastritis | Rhinitis | Recurrent wheezing | 5 | Peribronchial cuffing | / |
| R | M | 6.0 | No | 8 | 17.0 | 1.7 | 26/04: 22 | HBoV | HRV | - | 38.9 | Anorexia, gastritis, enteritis | Otitis, rhinitis | Bronchiolitis | 0 | / | / |
| S | F | 16.2 | 34 weeks | 3 | 13.4 | 12.7 ↑ | 3/04: 15 | HBoV | HCoV-NL63, HRV | - | 39.3 | Anorexia | Rhinitis | Recurrent wheezing | 0 | Peribronchial cuffing | / |
| T | M | 7.4 | No | 5 | 18.1 ↑ | 7.8 ↑ | 30/04: 17 | HBoV | HRV | HAdV | 39.4 | Anorexia, gastritis | Conjunctivitis | Bronchiolitis | 5 | Peribronchial cuffing | Eczema |
| U | M | 32.9 | No | 5 | 12.7 | 8.2 ↑ | 6/04: 16 | HBoV | - | HRV | 38.6 | Enteritis | No | Episodic wheezing | 4 | Normal | / |
| V | F | 16.2 | 34 weeks | 4 | 10.8 | 4.7 | 3/04: 23 | - | HBoV | HCoV-NL63 | 38.8 | Anorexia | Rhinitis | Recurrent wheezing | 0 | Peribronchial cuffing | / |
| W | M | 3.3 | No | 1 | 12.2 | 12.5 ↑ | 25/04: 28 | - | HBoV | HRV | 39.2 | Anorexia | Rhinitis | Bronchiolitis | 0 | / | / |
Positivity for HBoV was reflected in bold
WBC, white blood cell count; CRP, C-reactive protein; GI disorder, gastrointestinal disorder; URTD, upper respiratory tract disease; LRTD, lower respiratory tract disease; M, male; F, female; HAdV, human adenovirus; HCoV-HKU1, human coronavirus HKU1; HPeV, human parechovirus; HRV; human rhinovirus; ASD, atrial septum defect; HCoV-NL63, human coronavirus NL63; HRSV, human respiratory syncytial virus; IBV, influenza B virus; EV, enterovirus; hMPV, human metapneumovirus
Discussion
During the respiratory season 2017-2018, 1352 NPSs were received for molecular respiratory screening. HBoV was detected in 6.4% of the samples, which is in accordance with prevalence data described previously [6]. As the two hospitals use slightly different molecular techniques for diagnosis, infection frequencies can be biased. In agreement with previous studies [29], coinfection was common (85.1%), which might reflect the high prevalence of viral infections in young children and the prolonged shedding of HBoV [24]. Higher Cq values dominated. Only 23.0% of the specimens had Cq values ≤ 22. Competition or interference between viruses in the respiratory tract could explain the low viral loads observed in the majority of coinfections. In our study, HBoV viral loads were comparable between the monoinfected and coinfected samples, as was also found in previous studies [7, 23, 30]. However, the small number of samples in the monoinfected group makes it difficult to prove any statistical difference between the two.
The median age of the HBoV-positive children was 10.6 months. All but one of the children were younger than 3 years of age. This is in agreement with previous studies in which it was observed that most HBoV infections occur between the ages of 6 months and 3 years [5, 23, 29]. This distribution is compatible with protection from infection by maternal antibodies. The mean age of children who express HBoV in monoinfection (3.9 months) is lower than the mean age of children with HBoV in coinfection (14.0 months) (P = 0.016). This makes perfect sense, as older children have had more time to be exposed to different respiratory viruses. Indeed, four 4 prematurely born patients with monoinfection were still hospitalized at the NICU at the time of infection. The HBoV viral load was higher among patients younger than 18 months. This is in concordance with findings of Zhou et al. [7].
Consecutive samples were available for 15 HBoV-positive children. Four children showed persistent HBoV PCR positivity. In one child with seven consecutive samples, a large decrease in viral load was observed after the initial detection, suggesting that a persistent low-level shedding of HBoV in respiratory secretions may follow the HBoV primary infection. This observation was also seen in the case of a second child in which the Cq value increased from 18 to 32 in a two-week period. The Cq values of a third child are indicative of reinfection in mid-January, as the Cq level begin January was much higher. In the last child, the observation of similar viral load levels at a 5-month interval could be attributed to protracted viral shedding or to reinfection. In four other children, the Cq value for HBoV was already high at the beginning, ranging from 33 to 34, and it is therefore not surprising that there was no persistence of HBoV in subsequent runs. Martin et al. found that HBoV sequences in consecutive samples taken from 12 children were not 100% identical and therefore were considered to be HBoV reinfections that contributed to long-term shedding [16]. Since this is a retrospective study, the NPSs are not available anymore in the laboratory, and we are therefore not able to perform sequencing to distinguish between reinfection and persistence. Long-term shedding, which is a feature shared by other human parvoviruses [31], might explain the high frequency of codetection of HBoV with other respiratory pathogens. Therefore, the positivity of HBoV does not necessary imply respiratory disease, and it is informative for clinicians to have an indication of the viral load.
Proving the clinical relevance and pathogenicity of HBoV is challenging, because fulfillment of Koch’s postulates is not possible and because of the high rate of coinfection. One might argue that HBoV is only an aggravating factor of respiratory disease, a persisting virus that is reactivated by the inflammatory process or an innocent bystander that is just detected by chance [32]. Many studies have confirmed the association between HBoV infections in hospitalized children and wheezing episodes [33–35], while other studies fail to find an obvious relationship between HBoV infection and distinct clinical manifestations [24]. In our monoinfected group, three infants were born at a gestational age of 25-28 weeks and already had weak lungs and an immature immune system as a result of their premature birth. All three met the consensus criteria for BPD. As it is known that children with BPD have an increased risk of developing severe disease when acquiring a respiratory viral infection, it is difficult to reliably attribute the observed clinical respiratory signs to the pathogenicity of HBoV. Nevertheless, we saw deterioration of the patients’ respiratory status with higher oxygen need and a need for more ventilator support at the time of infection in all three cases. In the monoinfected group, there was only one infant, who was 4.9 months old and without comorbidities, hospitalized at the PICU, for which there was a clear causal relationship between the presence of HBoV at a low Cq value and wheezing. Thus, in our study, only one child out of 77 HBoV-infected children needed hospitalization at the PICU because of severe acute respiratory distress due to HBoV infection. This is lower than the 3.93% observed in a study by Moesker et al. [17].
In order to assess whether HBoV showed characteristics of a respiratory pathogen in the coinfected group, we chose to focus in detail on cases in which HBoV was expressed at a high viral load or a higher viral load then it copathogens. In these patients, a viral URT prodrome followed by breathing difficulties and wheezing were the most frequently reported clinical signs, and bronchi(oli)tis and recurrent wheezing were the leading diagnoses. In this group, a relationship was observed between HBoV and LRTD. Some previous studies have found high viral loads to be associated with more-severe symptoms [9], while others have failed to find a clear relationship [5, 7, 23]. In our opinion, it is not possible to establish a clear relationship between viral load and severity of LRTD. In the past, many scoring systems have been developed in an attempt to quantify respiratory distress objectively, but few have demonstrated any predictive validity [36]. Since clinical parameters such as respiratory rates or use of accessory muscles were sometimes lacking in the medical files, it was not possible to make a 100% correct statement about severity. We may assume that all children who needed hospitalization, and certainly those who developed hypoxia with a need for extra oxygen or ventilator support, had rather severe clinical signs.
In conclusion, HBoV is frequently found in NPSs of children with RTD. In our study, we found that HBoV infection at high viral load in infants is associated with bronchi(oli)tis and recurrent or episodic wheezing (P = 0.013, Cramer’s V = 0.613).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1 Protocol for DNA extraction and real-time PCR amplification (pdf, 162 kB) (PDF 160 kb)
Online Resource 2 Dataset of the 87 nasopharyngeal samples positive for human bocavirus (xls. 447 kB) (XLSX 94 kb)
Abbreviations
- BPD
Bronchopulmonary dysplasia
- CLD
Chronic lung disease
- CPAP
Continuous positive airway pressure
- Cq
Quantitation cycle
- CRP
C-reactive protein
- EV
Enterovirus
- GI
Gastrointestinal
- HAdV
Human adenoviruses
- HBoV
Human bocavirus
- HCoV-229E
Human coronavirus 229E
- HCoV-HKU1
Human coronavirus HKU1
- HCoV-NL63
Human coronavirus NL63
- HCoV-OC43
Human coronavirus OC43
- hMPV
Human metapneumovirus
- HRV
Human rhinoviruses
- HPeV
Human parechovirus
- HPIV
Human parainfluenza viruses
- HRSV
Human respiratory syncytial virus
- IAV
Human influenza A virus
- IBV
Human influenza B virus
- LRTD
Lower respiratory tract disease
- MP
Mycoplasma pneumoniae
- ND
Not detected
- NICU
Neonatal intensive care unit
- NPS
Nasopharyngeal samples
- PICU
Pediatric intensive care unit
- RTD
Respiratory tract disease
- RTI
Respiratory tract infections
- RT-PCR
Real-time PCR
- SARI
Severe acute respiratory infection
- UH
University Hospital
- URTD
Upper respiratory tract disease
- WBC
White blood cell count
Author contributions
VV analyzed and interpreted the laboratory and clinical data for all patients of the three participating hospitals included in this study and wrote this manuscript. The microbiologists MR, KF and WV provided the laboratory data from AZ Sint-Jan and AZ Sint-Lucas Hospital of Bruges and helped with the interpretation of the data. The pediatricians SD, KS and FC provided the clinical data from the Ghent University Hospital, the AZ Sint-Jan and AZ Sint-Lucas Hospital of Bruges, respectively, and helped with the interpretation of the data. The study was supervised and coordinated by professor EP. All authors read and approved the final manuscript.
Funding
No funding was received for this study.
Data availability
The data generated and/or analyzed during the current study are available online: Online Resource 2 Dataset of the 87 nasopharyngeal samples positive for human bocavirus (xls. 447 kB).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the three participating hospitals under Belgian Registration number B670201836996.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vanessa Verbeke, Email: vanessa.verbeke@uzgent.be.
Marijke Reynders, Email: marijke.reynders@azsintjan.be.
Katelijne Floré, Email: katelijne.flore@azsintjan.be.
Wouter Vandewal, Email: wouter.vandewal@stlucas.be.
Sara Debulpaep, Email: sara.debulpaep@uzgent.be.
Kate Sauer, Email: kate.sauer@azsintjan.be.
Frederik Cardoen, Email: frederik.cardoen@stlucas.be.
Elizaveta Padalko, Phone: +32 (0)9/332 21 08, Email: Elizaveta.Padalko@uzgent.be.
References
- 1.Fouchier RA, Rimmelzwaan GF, Kuiken T, Osterhaus AD. Newer respiratory virus infections: human metapneumovirus, avian influenza virus, and human coronaviruses. Curr Opin Infect Dis. 2005;18(2):141–146. doi: 10.1097/01.qco.0000160903.56566.84. [DOI] [PubMed] [Google Scholar]
- 2.Woo PCY, Lau SKP, Chu CM, Chan KH, Tsoi HW, Huang Y, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allander T, Tammi MT, Eriksson M, et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor A, Simmonds P, Slikas E, Li L, Bodhidatta L, Sethabutr O, et al. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J Infect Dis. 2010;201(11):1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva PE, Figueiredo CA, Luchs A, de Paiva TM, Pinho MAB, Paulino RS, et al. Human bocavirus in hospitalized children under 5 years with acute respiratory infection, Sao Paulo, Brazil, 2010. Arch Virol. 2018;163(5):1325–1330. doi: 10.1007/s00705-017-3694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guido M, Tumolo MR, Verri T, Romano A, Serio F, De Giorgi M, et al. Human bocavirus: current knowledge and future challenges. World J Gastroenterol. 2016;22(39):8684–8697. doi: 10.3748/wjg.v22.i39.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou JY, Peng Y, Peng XY, Gao HC, Sun YP, Xie LY, et al. Human bocavirus and human metapneumovirus in hospitalized children with lower respiratory tract illness in Changsha, China. Influ Other Respir Viruses. 2018;12(2):279–286. doi: 10.1111/irv.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaberg R, Ampofo K, Tardif KD, Stockmann C, Simmon KE, Hymas W, et al. Human bocavirus capsid messenger RNA detection in children with pneumonia. J Infect Dis. 2017;216(6):688–696. doi: 10.1093/infdis/jix352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang W, Yin F, Zhou W, Yan Y, Ji W. Clinical significance of different virus load of human bocavirus in patients with lower respiratory tract infection. Sci Rep. 2016;6:20246. doi: 10.1038/srep20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peltola V, Soderlund-Venermo M, Jartti T. Human bocavirus infections. Pediatr Infect Dis J. 2013;32(2):178–179. doi: 10.1097/INF.0b013e31827fef67. [DOI] [PubMed] [Google Scholar]
- 11.Lekana-Douki SE, Behillil S, Enouf V, Leroy EM, Berthet N. Detection of human bocavirus-1 in both nasal and stool specimens from children under 5 years old with influenza-like illnesses or diarrhea in Gabon. BMC Res Notes. 2018;11(1):495. doi: 10.1186/s13104-018-3605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitui MT, Tabib SM, Matsumoto T, Khanam W, Ahmed S, Mori D, et al. Detection of human bocavirus in the cerebrospinal fluid of children with encephalitis. Clin Infect Dis. 2012;54(7):964–967. doi: 10.1093/cid/cir957. [DOI] [PubMed] [Google Scholar]
- 13.Yu JM, Chen QQ, Hao YX, Yu T, Zeng SZ, Wu XB, et al. Identification of human bocaviruses in the cerebrospinal fluid of children hospitalized with encephalitis in China. J Clin Virol. 2013;57(4):374–377. doi: 10.1016/j.jcv.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Mori D, Ranawaka U, Yamada K, Rajindrajith S, Miya K, Perera HK, et al. Human bocavirus in patients with encephalitis, Sri Lanka, 2009–2010. Emerg Infect Dis. 2013;19(11):1859–1862. doi: 10.3201/eid1911.121548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akturk H, Sik G, Salman N, Sutcu M, Tatli B, Ciblak MA, et al. Atypical presentation of human bocavirus: severe respiratory tract infection complicated with encephalopathy. J Med Virol. 2015;87(11):1831–1838. doi: 10.1002/jmv.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin ET, Kuypers J, McRoberts JP, Englund JA, Zerr DM. Human bocavirus 1 primary infection and shedding in infants. J Infect Dis. 2015;212(4):516–524. doi: 10.1093/infdis/jiv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moesker FM, van Kampen JJ, van der Eijk AA, van Rossum AM, de Hoog M, Schutten M, et al. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin Microbiol Infect. 2015;21(10):964. doi: 10.1016/j.cmi.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivers TM. Viruses and Koch’s postulates. J Bacteriol. 1937;33(1):1–12. doi: 10.1128/jb.33.1.1-12.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijkman R, Koekkoek SM, Molenkamp R, Schildgen O, van der Hoek L. Human bocavirus can be cultured in differentiated human airway epithelial cells. J Virol. 2009;83(15):7739–7748. doi: 10.1128/JVI.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng X, Li Y, Qiu J. Human bocavirus 1 infects commercially available primary human airway epithelium cultures productively. J Virol Methods. 2014;195:112–119. doi: 10.1016/j.jviromet.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng X, Yan Z, Cheng F, Engelhardt JF, Qiu J. Replication of an autonomous human parvovirus in non-dividing human airway epithelium is facilitated through the DNA damage and repair pathways. PLoS Pathog. 2016;12(1):e1005399. doi: 10.1371/journal.ppat.1005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korppi M, Jartti T, Hedman K, et al. Serologic diagnosis of human bocavirus infection in children. Pediatr Infect Dis J. 2010;29(4):387. doi: 10.1097/INF.0b013e3181ce8e81. [DOI] [PubMed] [Google Scholar]
- 23.Ding XF, Zhang B, Zhong LL, Xie LY, Xiao NG. Relationship between viral load of human bocavirus and clinical characteristics in children with acute lower respiratory tract infection. Zhongguo dang dai er ke za zhi Chin J Contemp Pediatr. 2017;19(3):327–330. doi: 10.7499/j.issn.1008-8830.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin ET, Fairchok MP, Kuypers J, Magaret A, Zerr DM, Wald A, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis. 2010;201(11):1625–1632. doi: 10.1086/652405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steensels D, Reynders M, Descheemaeker P, Curran MD, Jacobs F, Denis O, et al. Clinical evaluation of a multi-parameter customized respiratory TaqMan((R)) array card compared to conventional methods in immunocompromised patients. J Clin Virol. 2015;72:36–41. doi: 10.1016/j.jcv.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricci V, Nunes VD, Murphy MS, Cunningham S, Team GDGT. GUIDELINES bronchiolitis in children: summary of NICE guidance. BMJ Br Med J. 2015;350:1–3. doi: 10.1136/bmj.h1. [DOI] [PubMed] [Google Scholar]
- 27.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:E1474–E1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 28.Bancalari E, del Moral T. Bronchopulmonary dysplasia and surfactant. Biol Neonate. 2001;80:7–13. doi: 10.1159/000047170. [DOI] [PubMed] [Google Scholar]
- 29.Calvo C, Garcia-Garcia ML, Pozo F, Carballo D, Martinez-Monteserin E, Casas I. Infections and coinfections by respiratory human bocavirus during eight seasons in hospitalized children. J Med Virol. 2016;88(12):2052–2058. doi: 10.1002/jmv.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljubin-Sternak S, Mestrovic T, Ivkovic-Jurekovic I, Tesovic G, Mlinaric-Galinovic G, Luksic I, et al. High detection rates of human bocavirus in infants and small children with lower respiratory tract infection from croatia. Clin Lab. 2019;65(1):1–4. doi: 10.7754/Clin.Lab.2018.180702. [DOI] [PubMed] [Google Scholar]
- 31.Lindblom A, Isa A, Norbeck O, Wolf S, Johansson B, Broliden K, et al. Slow clearance of human parvovirus B19 viremia following acute infection. Clin Infect Dis. 2005;41(8):1201–1203. doi: 10.1086/444503. [DOI] [PubMed] [Google Scholar]
- 32.Weissbrich B, Neske F, Schubert J, Tollmann F, Blath K, Blessing K, et al. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis. 2006;6:109–116. doi: 10.1186/1471-2334-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.do Amaral de Leon C, Amantea SL, Pilger DA, Cantarelli V. Clinical and epidemiologic profile of lower respiratory tract infections associated with human bocavirus. Pediatr Pulmonol. 2013;48(11):1112–1118. doi: 10.1002/ppul.22732. [DOI] [PubMed] [Google Scholar]
- 34.Esposito S, Daleno C, Prunotto G, Scala A, Tagliabue C, Borzani I, et al. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influ Other Respir Viruses. 2013;7(1):18–26. doi: 10.1111/j.1750-2659.2012.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Cai F, Wu X, Wu T, Su X, Shi Y. Incidence of viral infection detected by PCR and real-time PCR in childhood community-acquired pneumonia: a meta-analysis. Respirology. 2015;20(3):405–412. doi: 10.1111/resp.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Destino L, Weisgerber MC, Soung P, Bakalarski D, Yan K, Rehborg R, et al. Validity of respiratory scores in bronchiolitis. Hosp Pediatr. 2012;2(4):202–209. doi: 10.1542/hpeds.2012-0013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1 Protocol for DNA extraction and real-time PCR amplification (pdf, 162 kB) (PDF 160 kb)
Online Resource 2 Dataset of the 87 nasopharyngeal samples positive for human bocavirus (xls. 447 kB) (XLSX 94 kb)
Data Availability Statement
The data generated and/or analyzed during the current study are available online: Online Resource 2 Dataset of the 87 nasopharyngeal samples positive for human bocavirus (xls. 447 kB).


