Abstract
Phosphatidylinositol-3-kinase (PI3K)/Akt is an important cellular pathway that has been shown to participate in various replication steps of multiple viruses. In the present study, we compared the phosphorylation status of Akt during infection of MARC-145 cells and porcine alveolar macrophages (PAMs) with highly pathogenic PRRSV (HP-PRRSV) strain HuN4. We observed that biphasic activation of Akt was induced in at both the early stage (5, 15 and 30 min postinfection) and the late stage (12 and 24 h postinfection) of HP-PRRSV infection of MARC-145 cells, while an early-phase activation of Akt was found exclusively in virus-infected PAMs in vitro. Analysis with the PI3K-specific inhibitor LY294002 confirmed that PI3K acted as the upstream activator for the virus-induced activation of Akt. UV-irradiation-inactivated virus still induced the early event in PAMs but not in MARC-145 cells, suggesting that different mechanisms are employed for the early-stage induction of phosphorylated Akt within different cell cultures. We further demonstrated that FoxO1 and Bad, which serve as downstream targets of Akt, were phosphorylated in virus-infected MARC-145 cells. Moreover, the suppression of phosphorylated Akt with LY294002 significantly inhibited the virus-induced cytopathic effect (CPE) on MARC-145 cells, but it had a negligible effect on virus propagation. Collectively, our data provide new evidence of a novel role for the PI3K/Akt pathway in PRRSV infection of MARC-145 cells.
Keywords: Infectious Bursal Disease Virus, Severe Acute Respiratory Syndrome, Severe Acute Respiratory Syndrome Coronavirus, Infectious Progeny, Virus Entry Process
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) is a small, enveloped, positive-strand RNA virus belonging to the family Arteriviridae [9, 22]. Since it emerged in late 1980s in Europe and North America, it has caused significant economic losses to the pork industry worldwide [1, 26]. In 2006, an outbreak of HP-PRRSV described as “pig high fever disease” occurred in China and caused disastrous loses to the farmers [31, 39]. This disease is currently a major concern for the swine industry worldwide.
Phosphatidylinositol-3-kinase (PI3K)/Akt is a key signaling transduction pathway in the regulation of cell survival, cell proliferation and differentiation, and cell apoptosis [6–8, 10]. The activation of PI3K-Akt pathway promotes cell survival by the phosphorylation of numerous substrates such as glycogen synthase kinase-3 (GSK-3), FoxO1, Bad and mTOR [5, 11, 24, 36, 38]. Many viruses are known to manipulate this pathway in favor of their replication, such as influenza A virus [29], hepatitis B virus [16], hepatitis C virus [17], severe acute respiratory syndrome coronavirus (SARS-CoV) [23], Junín virus [19], human immunodeficiency virus type 1 [20], bovine herpesvirus type 1 [40] and infectious bursal disease virus [34]. Previous studies have shown that during PRRSV infection of porcine monocyte-derived dendritic cells (Mo-DCs), the PI3K/Akt pathway is activated at 90 min and 4 h postinfection (h p.i.), and it is inhibited at 12 h p.i. [37].
In vivo, the virus shows a very narrow cell tropism and infects a specific subpopulation of porcine macrophages [13, 33]. In vitro, efficient PRRSV replication is only observed in primary pig macrophages (e.g., alveolar macrophages), differentiated monocytes [13] or cells derived from African green monkey kidney, such as MARC-145 [18]. For easy manipulation, MARC-145 cells provide an important tool for the study of PRRSV replication. Previously, it was reported that the PI3K/Akt pathway is regulated by PRRSV in a complex manner in Mo-DCs [37], but whether this is a universal phenomenon in all permissive cells is unknown. A number of studies have shown that the PI3K/Akt signaling pathway is functionally dependent on downstream substrates of Akt such as FoxO1, Bad and mTOR for regulation of cell survival [3, 11, 15, 25]. The downstream targets of Akt controlled by PRRSV have not been identified. The objective of this study was to address how the PI3K/Akt pathway is modulated in infection of MARC-145 cells and PAMs with highly pathogenic PRRSV. We demonstrated that the PI3K/Akt pathway was activated by HP-PRRSV in a cell-culture-dependent manner and that the downstream targets FoxO1 and Bad were regulated through this pathway. In addition, we found that activated PI3K was required for the development of CPE in MARC-145 cells, but not for production of infectious progeny.
Materials and methods
Viruses and cell cultures
The HP-PRRSV strain HuN4 used in the present study was isolated from a pig showing signs of “high fever syndrome” originating from a swine farm in the region of Hunan Province, China, in 2006 [31, 39]. A stock of HuN4 virus at passage 5 was prepared in MARC-145 cells, titrated, and stored at −70 °C until use.
MARC-145 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL) supplemented with 10 % fetal bovine serum (Gibco BRL) in an incubator at 37 °C containing 5 % CO2. The PAM cultures were obtained by broncho-alveolar lavage of 4-week-old domestic piglets from a PRRSV-negative herd affiliated with Shanghai Agricultural Science Institute. All animal experiments were conducted according to the guidelines and approved protocol of the Shanghai Veterinary Research Institutional Animal Care Committee. The PAMs were cultured with RPMI-1640 medium supplemented with 10 % fetal bovine serum. All of our experiments were conducted at Veterinary Laboratory Biosafety Level 2.
Antibodies and reagents
Rabbit monoclonal antibodies (mAb) recognizing phospho-Akt (Ser473), phospho-Bad (Ser136), phospho-FoxO1 (Ser256) and phospho-mTOR (Ser2448) were purchased from Cell Signaling Technology Inc. Mouse monoclonal β-actin antibody was obtained from Santa Cruz Biotechnology, Inc., Santa Cruz, Calif. Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG and anti-mouse IgG were purchased from Zhongshan Golden Bridge Biotechnology Co., Ltd. PI3K-specific inhibitor LY294002 was supplied by Cell Signaling Technology, and a 1000 × stock of this inhibitor was prepared with DMSO.
Cell treatment and virus infection
MARC-145 cells in 60-mm dishes were grown to 70 %-80 % confluence, whereupon they were subjected to serum starvation for 48 h in serum-free DMEM. Subsequently, growth-arrested cells were infected at a multiplicity of infection (MOI) of 5 with HuN4 virus or were mock infected with the same medium. One hour postinfection (p.i.), the cells were washed with phosphate-buffered saline (PBS, pH 7.4) and then cultured in fresh DMEM for various times as indicated.
For inhibitor experiments, MARC-145 cells were pretreated with the PI3K inhibitor ly294002 for 1 h. Cells were then infected with the virus at an MOI of 5 for 1 h, washed with PBS, placed in serum-free medium containing fresh inhibitor, and sustained for 23 h. DMEM containing 0.1 % DMSO (V/V) was used for the mock treatment unless otherwise specified.
Growth-arrested PAM cells cultured in 60-mm dishes were infected at an MOI of 5 with HuN4 or were mock infected with medium and then incubated for various times as indicated. The cells were then collected for Western blotting analysis.
UV irradiation of PRRSV HuN4 and the infection of both MARC-145 cells and PAMs with the inactivated virus
To inactivate HP-PRRSV by UV irradiation, the virus stocks were dispersed in 10-cm tissue culture dishes and placed directly under a UV lamp (20 W) for 30 min. Complete inactivation of the virus was confirmed by the titration on MARC-145 cells. Both MARC-145 cells and PAMs were exposed to UV-irradiation-inactivated virus to analyze the variation of phosphorylated Akt within 1 h p.i. as indicated.
Cell lysis and Western blot analysis
After HP-PRRSV infection for the lengths of time indicated, cells were washed with PBS and lysed with lysis buffer (1 % Triton X-100, 50 mM sodium chloride, 1 mM EDTA, 1 mM EGTA, 20 mM sodium fluoride, 20 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 0.5 μg/ml leupeptin, 1 mM benzamidine, and 1 mM sodium orthovanadate in 20 mM Tris-HCl, pH 8.0). The cell lysates were cleared by centrifugation for 15 min at 12,000g prior to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The separated proteins were transferred onto nitrocellulose membranes (Millipore). The membranes were blocked for 1 h at room temperature with skim milk solution (5 % in Tris-buffered saline containing 0.1 % Tween 20). Blots were incubated overnight at 4 °C with primary antibodies followed by incubation for 1 h with the secondary antibody (conjugated horseradish peroxidase). After extensive washing, the immunoreactive bands were detected by enhanced chemiluminescence (ECL) (Pierce) and subsequently reprobed for total protein loading using anti-β-actin antibody.
Effect of PI3K inhibitor on virus replication
To analyze whether inhibition of PI3K by LY294002 would affect virus replication, serum-starved MARC-145 cells seeded in 24-well plates were pre-incubated with the inhibitors or mock pretreated with DMEM containing 0.1 % DMSO for 1 h at 37 °C and then infected with HuN4 virus for 1 h together with or without the inhibitor. After extensive washing with PBS, the cells were placed in DMEM with or without fresh inhibitor for 36 or 48 h. After two rounds of freezing and thawing, the infectious progeny was titrated with MARC-145 cells using a TCID50 assay.
Results
HP-PRRSV infection of MARC-145 cells leads to biphasic activation of the PI3K/Akt pathway
We first used the MARC-145 cell model to examine the kinetics of Akt phosphorylation at Ser473, which is required for Akt activation, to determine whether HP-PRRSV infection modulates the PI3K/Akt pathway. Cells that were mock infected with DMEM medium or infected with HP-PRRSV strain HuN4 were harvested at 5 min, 15 min, 30 min, 45 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h postinfection for Western blot analysis. It was found that Akt was activated in a biphasic manner after infection of MARC-145 cells with HP-PRRSV (Fig. 1A, top panel). The first phase of Akt activation occurred early (5 and 10 min p.i.) in virus infection (Fig. 1A). At 1 h p.i., the phosphorylation of Akt had returned to the basal level, and after a certain interval, Akt phosporylation increased again in the late stage of infection. The amount of phosphorylated Akt was evidently increased at 12 h p.i., and this level was sustained for the reminder of the investigation (24 h p.i.) (Fig. 1A, upper panel). The changes in Akt phosphorylation were not due to variation in the total amount of protein loaded (Fig. 1A, bottom panel).
Fig. 1.
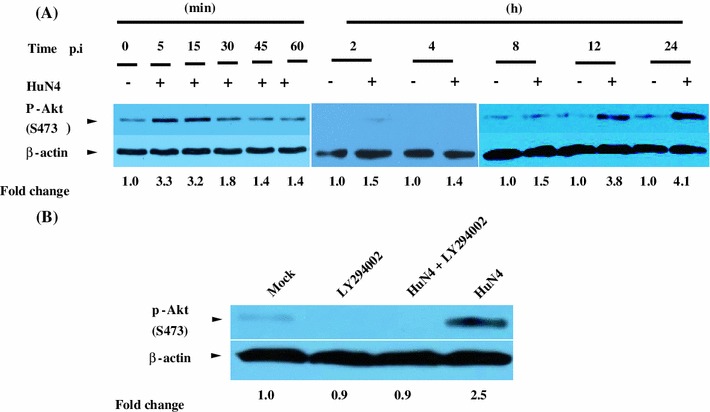
HP-PRRS strain HuN4 activates Akt via a PI3K-dependent pathway in MARC-145 cells. (A) Growth-arrested cells that were mock infected with DMEM or infected with HuN4 virus at a MOI of 5 were harvested at 5 min, 15 min, 30 min, 45 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h p.i. and processed for Western blotting. (B) Growth-arrested MARC-145 cells were first pretreated with LY294002 or mock pretreated with medium containing DMSO. They were then infected with the HuN4 virus at an MOI of 5 or mock-infected with DMEM. At 24 h p.i., the cell lysates were prepared and analyzed by Western blotting. The fold change of Akt phosphorylation is expressed as densitometric units (Image J 1.45 s, National Institute of Health, USA) of the band normalized to the β-actin level relative to the uninfected control. The results are representative of three independent experiments
Since Akt can be activated by both PI3K-dependent and PI3K-independent pathways [29], the role of activated PI3K in the virus-induced Akt activation was subsequently investigated. Here, the chemical LY294002, a potent and specific inhibitor of PI3K, was used. As demonstrated by immunoblot assay, 10 μM of LY294002 could efficiently inhibit the phosporylation of Akt induced by the virus at the late stage of infection (Fig. 1B). This indicated that the activated PI3K accounted for the virus-triggered Akt activation. Taken together, the data indicate that the PI3K/Akt pathway was activated in infection of MARC-145 cells by PRRSV.
HP-PRRSV activates Akt at the early stage of infection of PAMs
Since PAMs and monocytes are the major natural target cells during both acute and persistent PRRSV infection [13], it deserved to be investigated whether the virus controls the PI3K/Akt pathway during infection of PAMs. Here, the kinetics of phosphorylated Akt was determined in HP-PRRSV-infected PAMs in vitro. Serum-starved PAMs that were mock infected with RPMI-1640 medium or infected with HuN4 at 5 min, 15 min, 30 min, 45 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h postinfection were collected for Western blotting. As illustrated in Fig. 2, the amount of phosphorylated Akt increased dramatically from 5 to 30 min p.i. However, this activation was transient and quickly returned to the basal level at 45 min postinfection. In addition, the enhanced phosphorylation of Akt was not observed at the later stage. This suggested that Akt was only activated by HP-PRRSV at the early phase in the infection of PAMs, which is different from what was observed in MARC-145 cells.
Fig. 2.
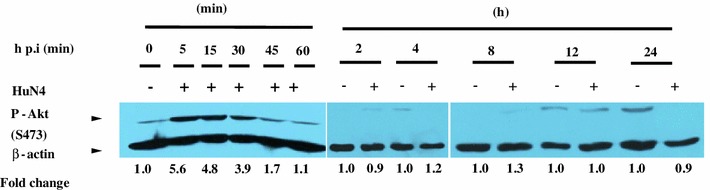
Activation of Akt in porcine alveolar macrophages (PAMs) that were infected with HP-PRRS strain HuN4. Growth-arrested PAMs were infected with the HuN4 virus at an MOI of 5 or mock-infected with RPMI medium. The infected PAMs were harvested at 5 min, 15 min, 30 min, 45 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h p.i. The cell lysates were prepared and analyzed by Western blotting. The fold change of Akt phosphorylation is expressed as densitometric units (Image J 1.45 s, National Institute of Health, USA) of the band normalized to the β-actin level relative to the uninfected control. The results are representative of three independent experiments
UV-irradiation-inactivated virus induces phosphorylation of Akt in PAMs
Since increased phosphorylation of Akt induced by HP-PRRSV was observed at 5 min and 15 min postinfection in virus-infected MARC-145 cells, and at 5, 15 and 30 min postinfection in PAMs, we hypothesized that the virus entry process accounted for this early-stage activation. Thus the UV-irradiated HP-PRRSV virus was employed to address this issue. Under the conditions described above, the virus could be completely inactivated (data not shown). Such inactivated virus fails to express viral proteins due to the formation of thymidine dimers, which prevent the transcription of viral genes but do not interfere with the capacity of the virus for receptor binding and entry into host cells by endocytosis [2]. As showed in Fig. 3, the exposure of cells to UV-irradiated virus led to phosphorylation of Akt at 5 and 15 min postinfection in PAMs (Fig. 3B), but not in MARC-145 cells (Fig. 3A). This indicates that virus-cell interactions during the binding or entry process are probably responsible for this event in PAMs, whereas in MARC-145 cells, a different mechanism may account for this activation.
Fig. 3.
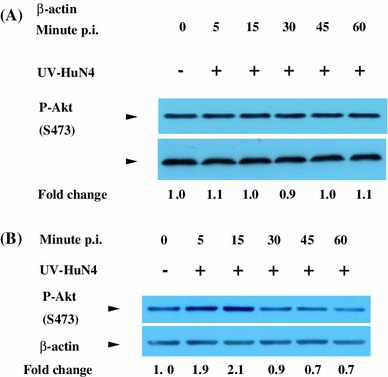
Regulation of Akt by UV-irradiation-inactivated virus at an early time. Serum-starved MARC-145 cells (A) and PAMs (B) were mock infected or infected with UV-irradiated HP-PRRSV and then processed for Western blotting at the time point indicated with phospho-Akt antibody. The fold change of Akt phosphorylation is expressed as densitometric units (Image J 1.45 s, National Institute of Health, USA) of the band normalized to the β-actin level relative to the uninfected control. Each experiment was repeated three times, and representative results are shown
FoxO1 and Bad are targeted by HP-PRRSV in a PI3K/Akt-dependent manner in MARC-145 cells
To investigate the mechanism by which HP-PRRSV promotes cell survival, some downstream targets of Akt in the late stage of infection of MARC-145 cells were analyzed by Western blotting using rabbit monoclonal antibodies against phospho-Bad (Ser136), phospho-FoxO1 (Ser256) and phospho-mTOR (Ser2448). Compared to the mock-infected control, virus replication resulted in significantly increased phosphorylation of both FoxO1 and Bad, while no change in mTOR was observed (Fig. 4A). Phosphorylation with both FoxO1 and Bad were most evident at 24 h p.i., which corresponds in time to the change in phosphorylated Akt. To address whether the change in phosphorylated FoxO1 and Bad was induced by the virus through the PI3K/Akt pathway, the PI3K-specific inhibitor LY294002 was employed for further investigation. As shown in Fig. 4B), treatment of MARC-145 cells with LY294002 significantly decreased the amount of phosphorylated FoxO1 and Bad induced by virus replication. This suggests that the virus manipulates the activity of both FoxO1 and Bad through the PI3K/Akt pathway.
Fig. 4.
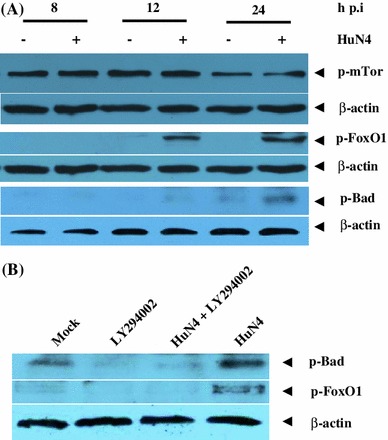
Downstream targets of Akt, FoxO1 and Bad, are regulated by HP-PRRSV strain HuN4. (A) Growth-arrested MARC-145 cells were infected with the HuN4 virus at an MOI of 5 or mock-infected with DMEM. The infected cells were harvested at different time points as indicated. Cell lysates were prepared and subjected to Western blotting. (B) Growth-arrested MARC-145 were pretreated with LY294002 or mock pretreated with medium containing DMSO. The pretreated cells were infected with the HuN4 virus at an MOI of 5 with or without inhibitor. At 24 h postinfection, cell lysates were prepared and analyzed by Western blotting. The results are representative of three independent experiments
Activated PI3K is essential for CPE formation, but not for PRRSV propagation
Since the PI3K/Akt pathway is universally controlled by PRRSV in all of the cell cultures investigated, the role it plays in virus replication was investigated in MARC-145 cells using LY294002, a PI3K/Akt pathway inhibitor. The cells were first treated and mock treated with the inhibitor and solvent, respectively, and then infected with the virus. The treatment of MARC-145 cells with LY294002 did not significantly inhibit virus replication (p > 0.05), as the titers were reduced 0.31 and 0.75 log10 at 36 and 48 h p.i., respectively (Fig. 5A). Unexpectedly, the virus-induced activation of the PI3K/Akt pathway was not essential for production of infectious PRRSV progeny virus.
Fig. 5.

Activated PI3K is required for CPE formation in infected MARC-145 cells but not for infectious progeny production. (A) Growth-arrested MARC-145 cells were pretreated with 10 μM LY294002 or diluent DMSO prior to infection with the HuN4 virus with or without the chemical. At 36 and 48 h p.i., infectious progeny were titrated (B) CPE formation was observed at 48 h p.i. Pictures were taken with a camera mounted on an inverted microscope (Leica DM IL LED). (a) Untreated normal MARC-145 cells, (b) HuN4-infected MARC-145 cells, (c) LY294002-treated MARC-145 cells, (d) LY294002-treated and HuN4 infected MARC-145 cells. These results are representative of three independent experiments
The cytopathic effect (CPE) in HuN4-infected MARC-145 cells is characterized by cell congregation and contraction [31]. Interestingly, in infected MARC-145 cells treated with LY294002, the CPE was significantly alleviated. Under normal conditions, PRRSV-infected MARC-145 cells showed considerable CPE at 48 h p.i. (Fig. 5B, b), but no CPE was observed in HuN4-infected MARC-145 cells when 10 μM of LY294002 was used to treat the cells (Fig. 5B, d). In addition, the inhibitor at a concentration of 10 μM could not induce CPE, and it was apparently not cytotoxic to MARC-145 cells, (Fig. 5B, c), which was confirmed by MTT assay (data not shown). Thus, these findings suggest that the pathway may be linked to HP-PRRSV-induced CPE formation in MARC-145 cells.
Discussion
Many viruses interfere with signaling pathways in their infected host cells to favor productive infection, which can also impact on the physiology of the host cell as well as pathogenesis. Therefore, the identification of cellular factors involved in virus replication is important for the design of novel antiviral strategies. In this regard, the manipulation of cellular pathways regulating cell apoptosis or survival affords great advantages to virus replication [35], and many viruses have been demonstrated to regulate cell survival via the regulation of the PI3K/Akt pathway [4, 14]. A previous report has indicated that the PI3K/Akt pathway is activated in the early phase and subsequently inhibited at 12 h p.i. during PRRSV infection of Mo-DCs [37]. Here, HP-PRRSV strain HuN4 was used to further investigate how and why this pathway is controlled by the virus. Here, we report that the activation of the PI3K/Akt pathway was significantly enhanced by the HuN4 virus at both the early and late stage of infection of MARC-145 cells, while a distinct pattern was adopted during infection of PAMs in which the phosphorylation of Akt occurred exclusively in the early stage, as early as 5 min p.i., and then returned to the basal level at 45 min p.i. (Figs. 1A and 2). When the cells were exposed to the UV-irradiation-inactivated virus, significantly increased phosphorylation of Akt was observed in PAMs, but not in MARC-145 cells. Entry of macrophages by PRRSV occurs via a few similar but also different mechanisms compared to entry into MARC-145 cells [12]. It could be inferred that the virus entry process in PAMs was enough to activate the PI3K/Akt pathway, while a different mechanism of activation was used in MARC-145 cells. It is possible that a different entry mechanism results in this difference in the regulation of Akt. Together with previous data reported by Zhang et al. [37], we generalize that PRRSV regulates the PI3K/Akt pathway by different mechanisms in Mo-DCs, PAMs and MARC-145 cells.
Various pro-apoptotic proteins have been identified as downstream targets of Akt, including the Bcl-2 family members Bad, GSK-3α/β, and mTOR and the FoxO1 transcription factor family members (FKHR) [5, 11, 24, 36, 38]. They are normally regulated in a PI3K/Akt-dependent manner. For example, the phosphorylation of Bad (Ser-136) was decreased but the phosphorylation of mTOR (Ser2448) and FoxO1 (Ser256) was increased by infection with varicella-zoster virus [27]. Similarly, it has been shown that the PI3K/Akt/mTOR cascade contributes to the establishment of persistent HCV infection [21]. In the present study, we demonstrated that both FoxO1 and Bad were regulated by PRRSV through the control of the PI3K/Akt pathway in the late stage of infection in MARC-145 cells (Fig. 4), and therefore the, PI3K/Akt/FoxO1 and PI3K/Akt/Bad cascades may be manipulated by PRRSV to control host-cell survival.
The PI3K/Akt pathway has been shown to play important roles in different steps of the life cycles of a variety of viruses [14]. For example, this pathway has been shown to be involved in the regulation of Ebola virus entry of host cells [28], to contribute to arenavirus budding [32], and to be involved in the control of synthesis of viral RNA of nonsegmented negative-stranded RNA viruses [30]. Unexpectedly, our investigation with the PI3K-specific inhibitor LY294002 showed that the PI3K/Akt pathway has a negligible effect on the titer of infectious PRRSV progeny in MARC-145 cells (p > 0.05), as determined at both 36 and 48 h p.i. (Fig. 5A), but inhibition of this pathway significantly blocked virus-induced CPE formation. To our knowledge, similar results have not been documented for other viruses. Therefore, it will be worthwhile in the future to investigate how the PI3K/Akt pathway regulates CPE formation during PRRSV infection.
In summary, our findings, together with a previous report of PRRSV regulating Akt in Mo-DCs, suggest that PI3K/Akt signaling is modulated by PRRSV in different ways in different cell types. FoxO1 and Bad were employed by the virus as downstream targets of the PI3K/Akt pathway. Unlike other viruses for which the control of this pathway contributes significantly to virus replication, for PRRSV, it is not important for generation of infectious progeny, but this modulation changes the physiology of the host cell, as revealed by the significant alleviation of CPE. This finding may offer a new and important insight into the role of PI3K/Akt in virus infection, or even in viral pathogenesis.
Acknowledgments
The study was supported by grant no. 2012ZL092 from Central Research Institutes of Basic Research Operations, grant no. U0931003 from NSFC-Guangdong Joint Foundation, grant no. 2009ZX08010-022B from the Ministry of Agriculture of China, and grant no. 09XD1405400 from the Excellent Scientist Program of Shanghai.
Footnotes
L. Zhu and S. Yang contributed equally to this work
References
- 1.Allende R, Laegreid WW, Kutish GF, Galeota JA, Wills RW, Osorio FA. Porcine reproductive and respiratory syndrome virus: description of persistence in individual pigs upon experimental infection. J Virol. 2000;74:10834–10837. doi: 10.1128/JVI.74.22.10834-10837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck MA, Chapman NM, McManus BM, Mullican JC, Tracy S. Secondary enterovirus infection in the murine model of myocarditis. Pathologic and immunologic aspects. Am J Pathol. 1990;136:669–681. [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 4.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/S0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 6.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 7.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 8.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia: Off J Leuk Soc Am, Leuk Res Fund, UK. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 9.Conzelmann KK, Visser N, Van Woensel P, Thiel HJ. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993;193:329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooray S. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- 11.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 12.Delrue I, Van Gorp H, Van Doorsselaere J, Delputte PL, Nauwynck HJ. Susceptible cell lines for the production of porcine reproductive and respiratory syndrome virus by stable transfection of sialoadhesin and CD163. BMC Biotechnol. 2010;10:48. doi: 10.1186/1472-6750-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan X, Nauwynck HJ, Pensaert MB. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV) Arch Virol. 1997;142:2483–2497. doi: 10.1007/s007050050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn EF, Connor JH. HijAkt: The PI3K/Akt pathway in virus replication and pathogenesis. Prog Mol Biol Transl Sci. 2012;106:223–250. doi: 10.1016/B978-0-12-396456-4.00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke TF, Cantley LC. Apoptosis. A bad kinase makes good. Nature. 1997;390:116–117. doi: 10.1038/36442. [DOI] [PubMed] [Google Scholar]
- 16.Guo H, Zhou T, Jiang D, Cuconati A, Xiao GH, Block TM, Guo JT. Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-akt signal transduction pathway. J Virol. 2007;81:10072–10080. doi: 10.1128/JVI.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida H, Tatsumi T, Hosui A, Nawa T, Kodama T, Shimizu S, Hikita H, Hiramatsu N, Kanto T, Hayashi N, Takehara T. Alterations in microRNA expression profile in HCV-infected hepatoma cells: involvement of miR-491 in regulation of HCV replication via the PI3 kinase/Akt pathway. Biochem Biophys Res Commun. 2011;412:92–97. doi: 10.1016/j.bbrc.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Kwang J, Yoon IJ, Joo HS, Frey ML. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- 19.Linero FN, Scolaro LA. Participation of the phosphatidylinositol 3-kinase/Akt pathway in Junin virus replication in vitro. Virus Res. 2009;145:166–170. doi: 10.1016/j.virusres.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas A, Kim Y, Rivera-Pabon O, Chae S, Kim DH, Kim B (2010) Targeting the PI3K/Akt cell survival pathway to induce cell death of HIV-1 infected macrophages with alkylphospholipid compounds. PloS one 5(9):e13121. doi:10.1371/journal.pone.0013121 [DOI] [PMC free article] [PubMed]
- 21.Mannova P, Beretta L. Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J Virol. 2005;79:8742–8749. doi: 10.1128/JVI.79.14.8742-8749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizutani T, Fukushi S, Saijo M, Kurane I, Morikawa S. JNK and PI3K/Akt signaling pathways are required for establishing persistent SARS-CoV infection in Vero E6 cells. Biochimica et biophysica acta. 2005;1741:4–10. doi: 10.1016/j.bbadis.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/S1534-5807(02)00401-X. [DOI] [PubMed] [Google Scholar]
- 25.Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–431. doi: 10.1042/0264-6021:3440427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005;227:385–392. doi: 10.2460/javma.2005.227.385. [DOI] [PubMed] [Google Scholar]
- 27.Rahaus M, Desloges N, Wolff MH. Varicella-zoster virus requires a functional PI3K/Akt/GSK-3alpha/beta signaling cascade for efficient replication. Cell Signal. 2007;19:312–320. doi: 10.1016/j.cellsig.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Saeed MF, Kolokoltsov AA, Freiberg AN, Holbrook MR, Davey RA. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 2008;4:e1000141. doi: 10.1371/journal.ppat.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin YK, Liu Q, Tikoo SK, Babiuk LA, Zhou Y. Effect of the phosphatidylinositol 3-kinase/Akt pathway on influenza A virus propagation. J Gen Virol. 2007;88:942–950. doi: 10.1099/vir.0.82483-0. [DOI] [PubMed] [Google Scholar]
- 30.Sun M, Fuentes SM, Timani K, Sun D, Murphy C, Lin Y, August A, Teng MN, He B. Akt plays a critical role in replication of nonsegmented negative-stranded RNA viruses. J Virol. 2008;82:105–114. doi: 10.1128/JVI.01520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong GZ, Zhou YJ, Hao XF, Tian ZJ, An TQ, Qiu HJ. Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg Infect Dis. 2007;13:1434–1436. doi: 10.3201/eid1309.070399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urata S, Ngo N, de la Torre JC. The PI3K/Akt pathway contributes to arenavirus budding. J Virol. 2012;86:4578–4585. doi: 10.1128/JVI.06604-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Breedam W, Delputte PL, Van Gorp H, Misinzo G, Vanderheijden N, Duan X, Nauwynck HJ. Porcine reproductive and respiratory syndrome virus entry into the porcine macrophage. J Gen Virol. 2010;91:1659–1667. doi: 10.1099/vir.0.020503-0. [DOI] [PubMed] [Google Scholar]
- 34.Wei L, Hou L, Zhu S, Wang J, Zhou J, Liu J. Infectious bursal disease virus activates the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway by interaction of VP5 protein with the p85alpha subunit of PI3K. Virology. 2011;417:211–220. doi: 10.1016/j.virol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Wei L, Zhu S, Wang J, Liu J. Activation of the PI3K/Akt signaling pathway during porcine circovirus type 2-infection facilitates cell survival and viral replication. J Virol. 2012;86:13589–13597. doi: 10.1128/JVI.01697-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3–3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/S0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Wang X. A dual effect of porcine reproductive and respiratory syndrome virus replication on the phosphatidylinositol-3-kinase-dependent Akt pathway. Arch Virol. 2010;155:571–575. doi: 10.1007/s00705-010-0611-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhou FQ, Snider WD. Cell biology. GSK-3beta and microtubule assembly in axons. Science. 2005;308:211–214. doi: 10.1126/science.1110301. [DOI] [PubMed] [Google Scholar]
- 39.Zhou YJ, Hao XF, Tian ZJ, Tong GZ, Yoo D, An TQ, Zhou T, Li GX, Qiu HJ, Wei TC, Yuan XF. Highly virulent porcine reproductive and respiratory syndrome virus emerged in China. Transbound Emerg Dis. 2008;55:152–164. doi: 10.1111/j.1865-1682.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhu L, Ding X, Zhu X, Meng S, Wang J, Zhou H, Duan Q, Tao J, Schifferli DM, Zhu G. Biphasic activation of PI3K/Akt and MAPK/Erk1/2 signaling pathways in bovine herpesvirus type 1 infection of MDBK cells. Vet Res. 2011;42:57. doi: 10.1186/1297-9716-42-57. [DOI] [PMC free article] [PubMed] [Google Scholar]


