Abstract
Background
We investigated the risk factors of radiation-induced thyroid dysfunction, then combined the clinical factors and optimum thyroid dosimetric parameters to predict the incidence rate of hypothyroidism (HT) and to guide individualized treatment.
Methods
A total of 206 patients with histologically proven nasopharyngeal carcinoma (NPC) treated at the Cancer Hospital of the University of Chinese Academy of Sciences between January 2015 and August 2018 were included. Dose–volume histogram (DVH) data, including mean dose, absolute volume, V20, V25, V30, V35, V40, V45, V50, V55, and V60 were extracted and used as dosimetric parameters. A logistic regression analysis model was built to identify predictors related to HT occurring within 2 years.
Results
Sex, N stage, thyroid volume, mean thyroid dose, and thyroid V20 and V50 were significantly different between patients with and without HT. Logistic regression analysis showed that N stage, thyroid volume, and thyroid V50 were independent predictors of HT. The radiosensitivity of the thyroid decreased as the thyroid volume increased. Patients with N stage > 1 had significantly higher HT incidence (37.38%) than patients with N stage ≤1 (13.11%). The incidence of HT was 54.55% in patients with thyroid V50 > 24% and was 34.15% in patients with thyroid V50 ≤ 24%.
Conclusions
The incidence of HT is significantly associated with N stage, thyroid volume, and thyroid V50. More attention should be paid to patients with NPC with thyroid volume ≤ 12.82 cm3 and advanced N stage disease.
Keywords: Nasopharyngeal carcinoma, Hypothyroidism, Radiation therapy
Background
Nasopharyngeal carcinoma (NPC) is common in southern China. Radiotherapy is the primary treatment, and the 5-year overall survival rate is up to about 80% [1, 2]. Radiation inevitably damages normal tissue while treating tumors. Radiation-induced thyroid abnormality is a common complication in patients with NPC who have undergone radiotherapy, and has been reported since 1929 [3]. In terms of the quality of life (QoL) of patients with NPC who have receive radiation, many clinical studies have shown that intensity-modulated radiotherapy (IMRT) can preserve many organs at risk (OARs) better than conventional radiotherapy and improve the patients’ QoL [4–6]. However, it is not easy to protect the pituitary and thyroid glands, as they are close to the primary tumor and neck nodal metastasis. However, it is worth noting that IMRT may result in a higher incidence of subclinical hypothyroidism (HT) than three-dimensional conformal radiotherapy (51.1% vs 27.3%) [7].
The incidence of HT in patients with NPC treated with IMRT ranges from 14.1 to 60% [8, 9], mostly occurring within 5 years after treatment. The incidence is high 1–2 years after radiotherapy [7, 10, 11]. The most common symptoms of HT are fatigue, lethargy, cold intolerance, weight gain, constipation, change in voice, and dry skin [12]. Serious HT may increase the risk of heart disease and influence the progression-free survival and overall survival of patients with cancer [11, 13]. Many studies have focused on the factors affecting thyroid function; nevertheless, the impact of radiation on HT related to the thyroid and pituitary glands remains poorly understood, and the incidence of HT has not been controlled effectively in patients with NPC post-IMRT [14]. The purpose of the present study was to investigate the risk factors related to radiation-induced thyroid dysfunction, then combine the clinical factors and optimum thyroid dosimetric parameters to predict the incidence of HT and guide individualized treatment.
Methods and materials
Patient selection
We collected the information of patients with histologically proven NPC from the Cancer Hospital of the University of Chinese Academy of Sciences between January 2015 and August 2018. We included patients with complete clinical information (including clinical characteristics, pre-radiotherapy biochemical results of normal thyroid function test), pathologically confirmed primary NPC, and follow-up for > 1 year or who developed HT within 1 year. Patients who had undergone chemotherapy or radiotherapy before treatment at our hospital or who had problems with the hypothalamic-pituitary-thyroid (HPT) axis were excluded. In total, 206 patients were included in this analysis.
Treatment
Chemotherapy
All patients received 2–3 cycles of TP (docetaxel/paclitaxel + cisplatin/nedaplatin), PF (cisplatin/nedaplatin + fluorouracil), or TPF (docetaxel/paclitaxel + cisplatin/nedaplatin + fluorouracil) induction chemotherapy, followed by IMRT with concurrent cisplatin or nedaplatin chemotherapy.
Radiotherapy
All patients received radical IMRT. The patients were immobilized in the supine position with a head-neck-shoulder thermoplastic mask; computed tomography simulation (CT-sim) scan from the top of the head to the sternal angle was performed using a Philips Brilliance CT with 3-mm thickness; magnetic resonance imaging (MRI) scan was obtained in the same posture and immobilization mode with a Siemens Verio 3.0 T. The CT-sim and MRI scan images were transmitted into RayStation 4.0. PGTVnx+rn (planning gross primary tumor and retropharyngeal lymph node volume), PGTVnd (planning positive lymph nodes), PTV-1 (planning target volume 1), PTV-2, and OARs were contoured based on CT. According to RTOG (Radiation Therapy Oncology Group) 0615 [15], the PGTVnx+rn included the primary nasopharyngeal tumor and retropharyngeal lymph nodes with a prescription dose of 70.40 Gy at 2.20 Gy per fraction, the PGTVnd included positive lymph nodes with a prescription dose of 68.80 Gy at 2.15 Gy per fraction, the PTV-1 were high-risk areas included the primary nasopharyngeal tumor and nasopharyngeal mucosa with a prescription dose of 64.00 Gy at 2.00 Gy per fraction, the PTV-2 were low-risk areas with a prescription dose of 54.40 Gy at 1.70 Gy per fraction, included posterior maxillary sinus, pterygopalatine fossa, parapharyngeal space, skull base, part of posterior ethmoid sinus, bilateral lymphatic drainage area and so on. All patients were treated with daily fractions over 5 days per week using a linear accelerator (6–8 MV). The OAR dose constraints were as follows: the maximum temporal lobe dose was < 60 Gy or D1cc (the dose delivered volume of 1 cm3, Gy) was < 65 Gy, and the maximum pituitary dose was < 50 Gy; the dose constraints of other OARs were based on expert consensus, and there has been no thyroid dose constraint in clinical practice to date.
Dosimetric parameters
A senior doctor re-contoured the pituitary and thyroid. Dose–volume histogram (DVH) data, including the mean dose, absolute volume, V20, V25, V30, V35, V40, V45, V50, V55, and V60 (Vx: percentage volume of organ receiving more than x Gy,) were extracted and used in the statistical analysis.
Thyroid function test
Thyroid function was assessed by monitoring serum thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4). Hypothyroidism was defined as having TSH concentrations above the reference range (0.380~4.340 μIU/mL) and FT4 concentrations within or lower than the normal range (0.81–1.89 ng/dL). The above definitions cover both clinical and subclinical HT [12].
Statistical analysis
First, clinical characteristics stratified by HT were described via means and standard deviations (for normally distributed variables), interquartile range [median (Q25–Q75)] (for abnormally distributed variables), or frequency and percentages (for categorical variables), and their differences were compared by t-test, Mann-Whitney U test, or chi-square (or Fisher’s exact probability) test, respectively. Second, a logistic regression model was performed to examine which clinical and dosimetric parameters were related to the development of HT. Only characteristics significantly different in univariate analysis were included in the logistic regression model, and the independent predictors of HT were identified using backward elimination (P > 0.1 was excluded). Third, the dosimetric parameter cut-offs were calculated using the receiver operating characteristics (ROC) curve. Finally, we reclassified the significantly independent variables into binary variables according to the cut-offs, and evaluated their association with HT using the chi-square test (for categorical variables) or Cochran-Armitage trend test (for ordinal variables). P < 0.05 was considered statistically significant. All analyses were performed using R-3.6.0.
Results
Patients’ characteristics
Table 1 presents the descriptive statistics. Of the total 206 patients with NPC, 135 were male and 71 were female, and the patient age range was 25–82 years. The follow-up time was 6–48 months, with a median of 19 months. Overall, 50.49% of the patients (104/206) developed subclinical HT. The average thyroid and pituitary dose was 45.88 Gy (range, 42.86–47.98 Gy) and 51.91 Gy (range, 40.70–59.24 Gy), respectively. Sex, N stage, volume, average dose, and the thyroid V20 and V50 were significantly different between patients with and without HT. No differences were shown for other factors such as age, T stage, clinical stage, chemotherapy, and pituitary gland dosimetric parameters among the patients with and without HT.
Table 1.
Clinical characteristics in patients with NPC treated with IMRT
| Variable | Patients | Statistic | P-value | |
|---|---|---|---|---|
| With HT (N = 104) | Without HT (N = 102) | |||
| Age (mean ± SD) | 50.62 ± 10.75 | 52.21 ± 10.67 | t = 1.070 | 0.289 |
| Sex | χ2 = 0.017 | 0.017* | ||
| Male | 60 (29.13) | 75 (36.41) | ||
| Female | 44 (21.36) | 27 (13.11) | ||
| T stage (2010UICC) | χ2 = 0.004 | 0.947 | ||
| T1–2 | 19 (9.22) | 19 (9.22) | ||
| T3–4 | 85 (41.26) | 83 (40.29) | ||
| N stage (2010UICC) | χ2 = 5.531 | 0.021* | ||
| N0–1 | 27 (13.11) | 42 (20.39) | ||
| N2–3 | 77 (37.38) | 60 (29.13) | ||
| M stage (2010UICC) | 0.498a | |||
| M0 | 98 (47.57) | 99 (48.06) | ||
| M1 | 6 (2.91) | 3 (1.46) | ||
| Clinical stage (2010UICC) | χ2 = 2.158 | 0.142 | ||
| I-II | 4 (1.94) | 9 (4.37) | ||
| III-IV | 100 (48.54) | 93 (45.15) | ||
| Nimotuzumab | χ2 = 0.004 | 0.947 | ||
| No | 85 (41.26) | 83 (40.29) | ||
| Yes | 19 (9.22) | 19 (9.22) | ||
| Neoadjuvant chemotherapy | χ2 = 1.500 | 0.221 | ||
| No | 4 (1.94) | 8 (3.88) | ||
| Yes | 100 (48.54) | 94 (45.63) | ||
| Thyroid volume (cm3) | 12.77 (10.79–16.13) | 15.88 (13.33–19.97) | Z = 4.091 | < 0.001*** |
| Mean dose of thyroid (Gy) | 46.08 (44.68–47.98) | 45.67 (42.86–47.05) | Z = -1.981 | 0.048* |
| V20 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | Z = -2.182 | 0.029* |
| V25 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | Z = -0.798 | 0.425 |
| V30 | 1.00 (0.97–1.00) | 0.99 (0.95–1.00) | Z = -1.697 | 0.090 |
| V35 | 0.93 (0.87–0.98) | 0.92 (0.78–0.96) | Z = -1.797 | 0.072 |
| V40 | 0.77 (0.69–0.85) | 0.76 (0.59–0.82) | Z = -1.808 | 0.071 |
| V45 | 0.56 (0.49–0.64) | 0.54 (0.40–0.60) | Z = -1.950 | 0.051 |
| V50 | 0.33 (0.28–0.41) | 0.32 (0.23–0.38) | Z = -2.090 | 0.037* |
| V55 | 0.11 (0.05–0.18) | 0.08 (0.05–0.15) | Z = -1.344 | 0.179 |
| V60 | 0.01 (0.00–0.04) | 0.00 (0.00–0.02) | Z = -1.322 | 0.186 |
| Pituitary volume (cm3) | 0.47 (0.36–0.55) | 0.43 (0.35–0.53) | Z = -1.259 | 0.208 |
| Mean dose of pituitary (Gy) | 51.04 (43.26–57.09) | 52.78 (40.70–59.24) | Z = 0.770 | 0.441 |
| V20 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | Z = 0.439 | 0.661 |
| V25 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | Z = -0.633 | 0.527 |
| V30 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | Z = -0.420 | 0.674 |
| V35 | 1.00 (0.88–1.00) | 1.00 (0.73–1.00) | Z = -0.892 | 0.372 |
| V40 | 1.00 (0.62–1.00) | 1.00 (0.5–1.00) | Z = -0.801 | 0.423 |
| V45 | 0.98 (0.41–1.00) | 1.00 (0.43–1.00) | Z = 0.501 | 0.616 |
| V50 | 0.52 (0.00–1.00) | 0.81 (0.00–1.00) | Z = 0.867 | 0.386 |
| V55 | 0.10 (0.00–0.56) | 0.39 (0.00–0.9) | Z = 1.247 | 0.213 |
| V60 | 0.00 (0.00–0.46) | 0.00 (0.00–0.53) | Z = 0.823 | 0.411 |
aP-value was calculated using Fisher’s exact probability; *P < 0.05, **P < 0.01, ***P < 0.001
Independent predictors of HT
The logistic regression analysis revealed that N stage, thyroid volume, and thyroid V50 were independent predictors of HT (Table 2). The risk of developing HT was significantly higher in the N2–3 cohort than in the N0–1 cohort (odds ratio [OR] = 1.91, 95% confidence interval [CI] = 1.02–3.57), and HT was highly prevalent in the thyroid V50 > 24% cohort (OR = 8.93, 95% CI = 0.89–89.76) and thyroid volume ≤ 12.82 cm3 cohort (OR = 0.89, 95% CI = 0.83–0.94).
Table 2.
Logistic regression analysis of factors influencing HT in patients with NPC treated with IMRT
| Variable | B | SE | P-value | OR (95%CI) |
|---|---|---|---|---|
| N-stage N2–3 (vs N0–1) | 0.65 | 0.32 | 0.04* | 1.91 (1.02–3.57) |
| Thyroid volume (cm3) | −0.12 | 0.03 | < 0.001*** | 0.89 (0.83–0.94) |
| Thyroid V50 | 2.19 | 1.18 | 0.06 | 8.93 (0.89–89.76) |
*P < 0.05, ***P < 0.001
ROC analysis
The ROC analysis (Fig. 1) suggested that thyroid volume was a good predictor of HT, with an area under the curve (AUC) of 0.67 (P < 0.001). The thyroid volume and thyroid V50 cut-offs were 12.82 cm3 and 24%, respectively. Thyroid radiosensitivity decreased with increasing thyroid volume, and thyroid volume < 12.82 cm3 was a risk factor. The incidence rate of HT was 37.31 and 75% in patients with thyroid volume > 12.82 cm3 and thyroid volume ≤ 12.82 cm3, respectively. The incidence rate of HT was 54.55 and 34.15% in patients with thyroid V50 > 24% and thyroid V50 ≤ 24%, respectively (Table 3).
Fig. 1.
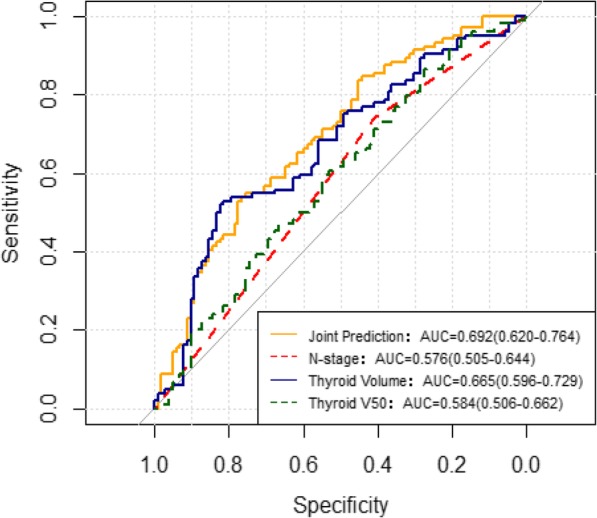
ROC analysis of 206 patients with NPC treated with IMRT
Table 3.
Associations of thyroid volume and thyroid V50 with HT
| Variable | HT | χ2 | P-value | ||
|---|---|---|---|---|---|
| No (%) | Yes (%) | ||||
| Thyroid volume | ≤12.82 cm3 | 18 | 54 (75.00) | 26.61 | < 0.001*** |
| > 12.82 cm3 | 84 | 50 (37.31) | |||
| Thyroid V50 | ≤0.24 | 27 | 14 (34.15) | 5.47 | 0.02* |
| > 0.24 | 75 | 90 (54.55) | |||
*P < 0.05, ***P < 0.001
Joint prediction for HT
The joint prediction model was based on two variables each, namely thyroid volume and thyroid V50, thyroid volume and N stage, and N stage and thyroid V50. An increased trend of HT incidence was observed with decreased thyroid volume while thyroid V50 increased (P < 0.001), and ranged from 29.03% (volume > 12.82 cm3 and V50 ≤ 24%) to 79.03% (volume ≤ 12.82 cm3 and V50 > 24%). Similar trends were also found in patients with decreased thyroid volume while N stage increased (P < 0.001), ranging from 31.25% (volume > 12.82 cm3 and N0–1) to 82.35% (volume > 12.82 cm3 and N2–3). Besides, the HT incidence rate increased as the thyroid V50 and N stage increased (p < 0.01), ranging from 14.29% (V50 ≤ 24% and N1–2) to 56.41% (V50 > 24% and N2–3) (Table 4).
Table 4.
Joint prediction for HT
| Combined factors | HT | Z | P-value | |
|---|---|---|---|---|
| No (%) | Yes (%) | |||
| Combined thyroid volume and thyroid V50 | ||||
| Volume > 12.82 cm3 and V50 ≤ 24% | 22 (70.97) | 9 (29.03) | −5.45 | < 0.001*** |
| Volume > 12.82 cm3 and V50 > 24% | 62 (60.19) | 41 (39.81) | ||
| Volume ≤ 12.82 cm3 and V50 ≤ 24% | 5 (50.00) | 5 (50.00) | ||
| Volume ≤ 12.82 cm3 and V50 > 24% | 13 (20.97) | 49 (79.03) | ||
| Combined N stage and thyroid V50 | ||||
| N0–1 and V50 ≤ 24% | 18 (85.71) | 3 (14.29) | −2.86 | < 0.01** |
| N0–1 and V50 > 24% | 24 (50.00) | 24 (50.00) | ||
| N2–3 and V50 ≤ 24% | 9 (45.00) | 11 (55.00) | ||
| N2–3 and V50 > 24% | 51 (43.59) | 66 (56.41) | ||
| Combined thyroid volume and N stage | ||||
| Volume > 12.82 cm3 and N0–1 | 33 (68.75) | 15 (31.25) | −5.50 | < 0.001*** |
| Volume > 12.82 cm3 and N2–3 | 51 (59.30) | 35 (40.70) | ||
| Volume ≤ 12.82 cm3 and N0–1 | 9 (42.86) | 12 (57.14) | ||
| Volume ≤ 12.82 cm3 and N2–3 | 9 (17.65) | 42 (82.35) | ||
**P < 0.01, ***P < 0.001
Discussion
Hypothyroidism after radiotherapy for nasopharyngeal cancer is a common late complication. The incidence of HT was 78.4, 56.4, and 43.4% in patients with nasopharyngeal cancer after receiving radiotherapy for 1, 2, and 3 years, respectively [10], which is similar to our results. In the present study, there was 50.49% HT incidence after radiotherapy in patients with NPC, and it encompassed clinical and subclinical HT. It was obvious that the incidence of HT in patients with NPC was not controlled effectively despite all studies suggesting the correlation between thyroid dose–volume and HT. What we know so far is that the risk factors for radiation-induced HT are sex, age, T stage, N stage, chemotherapy, thyroid volume, and radiation dose [16–18]. However, the dosimetric parameter cut-offs related to HT remain unclear. In the present study, we not only analyzed the effect of dosimetric parameters on thyroid function, but also combined the clinical factors and thyroid dosimetric parameters to predict the incidence of HT.
Hypothyroidism and N stage
Wu et al. [17] found that N stage did not affect the incidence of HT, which is not consistent with our results. Our results show that patients with advanced N stage (N2 and N3) were at greater risk of developing HT. Here, the incidence rate of HT in the N0–1 and N2–3 groups was 13.11 and 37.38%, respectively. Logistic regression analysis showed that patients with N2–3 disease had 0.91 times increased risk of HT compared to patients with N0–1 disease. It might be because the thyroid gland in patients with cervical lymph node metastasis inevitably receives more radiation.
Hypothyroidism and thyroid volume
Thyroid volume is closely related to the incidence of HT [19, 20], and we found a clear relationship between thyroid volume and radiation-induced HT (Table 3). The incidence of HT was 37.31% in patients with thyroid volume ≥ 12.82 cm3, while it was 75.% in patients with thyroid volume < 12.82 cm3. Therefore, we postulated that when the thyroid volume is small, there may be insufficient functional thyroid submits to produce thyroid hormone. Therefore, TSH increases as a normal feedback mechanism from the pituitary gland, and these patients are prone to developing radiation-induced HT [10].
Hypothyroidism and thyroid dose
Currently, scholars here and abroad hold different opinions on the relationship between thyroid dose–volume and HT. One study suggested that the thyroid minimum dose, mean dose, and V25–V60 are significantly associated with HT [21]. Sommat et al. [22] have suggested that the thyroid V40 is closely related to HT, where the incidence of radiation-induced HT in the thyroid V40 ≤ 85% and thyroid V40 > 85% groups was 21.4% (3/14) and 61.4% (54/88), respectively. However, with our large sample, we show that HT incidence in the thyroid V40 ≤ 85% group was 47.93% (81/169), which is not consistent with the earlier study. This difference may be related to the follow-up time, sample size, and treatment planning systems. Thyroid V50 is an important prognostic variable for radiation-induced HT in patients with NPC [23]. For example, Ling et al. [24] retrospectively analyzed 102 patients and recommended thyroid V50 < 50% as the optimal dose–volume limiting threshold for HT after radiotherapy. Sachdev et al. [25] believed that thyroid V50 > 60% was a risk factor associated with the incidence of HT. Zhai et al. [26] found that patients with thyroid mean dose ≥45 Gy had 4.9 times increased risk of HT than those receiving a lower mean dose, and the recommended plan optimization objectives were reducing the thyroid V45 and V50 to 50 and 35%, respectively. Their results are similar to ours in that the thyroid mean dose, V20, and V50 were significantly correlated with HT, and thyroid V50 ≤ 24% was an independent predictor of HT. The incidence of HT was 34.15% in patients with thyroid V50 ≤ 24% and was 54.55% in patients with thyroid V50 > 24%.
Hypothyroidism and combined factors
Based on the above results, more attention should paid to patients with small thyroid volume and advanced N stage. Therefore, we built combined factor models to guide individualized treatment. For each component model, thyroid volume and thyroid V50 (P < 0.001), thyroid volume and N stage (P < 0.001), and N stage and thyroid V50 (P < 0.01) were significantly different between patients with and without HT. Most previous studies focused only on thyroid dose, and neglected other clinical factors. Ours is the first retrospective study to consider both dosimetric variables and clinical factors such as N stage to predict the incidence of radiation-induced HT, which could guide physicians in forming individualized radiotherapy plans according to clinical factors and help reduce the HT occurrence rate in high-risk patients with NPC receiving IMRT.
Hypothyroidism and pituitary dose
The pituitary gland is the most important and complex endocrine gland. It secretes a variety of hormones, such as growth hormone, thyroid hormone, adrenocorticotrophic hormone, gonadotropin, and oxytocin. The pituitary gland is located in the sella turcica, which is just superior to the nasopharynx. It might receive a high dose during radiotherapy for NPC. Many researchers believe that patients with NPC have a higher risk of developing HT after radiotherapy than patients with other head and neck cancers, as the morbidity of HT is closely related to the pituitary function through the HPT axis. Lin [27] demonstrated that patients with NPC with high thyroid and pituitary doses (> 50 Gy) had the highest risk of thyroid abnormalities (83.3%), followed by patients with high thyroid dose and low pituitary dose (50%). Huang [18] reported that TSH and FT4 were correlated with the pituitary volume receiving doses of > 55 Gy. However, a recent study showed that the Dmean, V30, V40, V50 and V55 of the pituitary gland indicated no significant differences between the euthyroid and hypothyroid groups (P > 0.05) [28]. Similarly, we did not find a correlation for pituitary dose and radiation-induced damage to thyroid function. This may be because patients with secondary HT (those with low TSH and low FT4) related to pituitary-hypothalamus damage were not included in the study.
Hypothyroidism and other factors
In addition to thyroid volume, N stage, and thyroid V20 and V50, other factors such as sex, chemotherapy, age, and T stage were also considered HT-related. It remains controversial whether sex worsens the adverse effect of radiotherapy-induced HT; many researchers believe that female patients have a higher risk of HT .[19, 24, 29] Fan et al. [16] observed 2.03 times increased HT incidence in women relative to men among 14,893 patients with NPC and 16,105 patients with other head and neck cancers. Yet, others disagree: Bhandare et al. [30] reported that HT incidence in female patients was no higher than that in male patients. Our data support the former conclusion.
Zhai [26] reported that younger age (< 49 years) was a significant predictor for clinical HT after radiotherapy. Wu et al. [17] suggested that subclinical HT is strongly correlated with female patients or patients with lower T stage (T1–2 vs. T3–4). However, Luo et al. [29] identified chemotherapy as one of the most predictive factors for radiation-induced HT. In our research, we found that chemotherapy and T stage had no obvious statistically significant relation to the incidence of HT. This could have been caused by the small number of patients who did not receive chemotherapy and who had early T stage (T1–2) disease.
Our results demonstrate that sex, N stage, thyroid volume, thyroid mean dose, and thyroid V20 and V50 were significantly different between patients with and without HT. Logistic regression analysis showed that N stage, thyroid volume, and thyroid V50 were independent predictors of HT. The strengths of our study are the large sample size as compared to other studies, the comparison with the baseline TSH level before and after treatment, the thyroid and pituitary gland counting by one physician, and being the first retrospective study to combine dosimetric variables and clinical factors. There are also some limitations to our findings. First, our patients lacked long-term follow-up (2 years). Second, all patients did not undergo thyroid function testing at the same time. Prospective studies should be conducted in the future to analyze the optimal limiting dose of the pituitary gland to further reduce the incidence of radiation-induced HT.
Conclusion
The incidence of HT in patients with NPC after IMRT was significantly associated with N stage, thyroid volume, and thyroid V50. Patients who received radiotherapy for the cervical lymph nodes were at greater risk of developing HT. However, given the limitation of the retrospective nature of our study, further prospective studies are needed to verify our conclusion. More attention should be paid to patients with thyroid volumes of ≤12.82 cm3 and with advanced N stage disease. Additionally, the pituitary dosimetric parameters did not show a statistically significant correlation with thyroid function.
Acknowledgements
Not applicable.
Abbreviations
- AUC
Area under the curve
- CI
Confidence interval
- CT
Computed tomography
- CT-sim
CT simulation
- D1cc
The dose delivered volume of 1 cm3
- DVH
Dose-volume histogram
- FT3
Free triiodothyronine
- FT4
Free thyroxine
- GTV
Gross tumor volume
- HPT
Hypothalamic-pituitary-thyroid
- HT
Hypothyroidism
- IMRT
Intensity-modulated radiotherapy
- MRI
Magnetic resonance imaging
- NPC
Nasopharyngeal carcinoma
- OARs
Organs at risk
- OR
Odds ratio
- PTV
Planning target volume
- QoL
Quality of life
- ROC
Receiver operating characteristic
- RTOG
Radiation Therapy Oncology Group
- TSH
Thyroid stimulating hormone
Authors’ contributions
LZ, YYC and ZHY analyzed and explained the related factors of radiation-induced HT, and were major contributors to writing the manuscript. JC and WS analyzed the data. SH, CJT, MC and ZLC provided clinical direction during project development and manuscript writing. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (81672971) and Key Technologies Research and Development Program (2017YFC0113201).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
The name of the ethics committee: Medical Ethics Committee of Zhejiang Cancer Hospital.
The committee’s reference number: IRB-2019-87.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhong-Hua Yu, Email: zhonghua_yu@126.com.
Yuan-Yuan Chen, Email: chenyy@zjcc.org.cn.
References
- 1.Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;15:631–637. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 2.Huang WB, Chan JYW, Liu DL, et al. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in southern China. Cancer. 2018;124:530–536. doi: 10.1002/cncr.31031. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal MB, Goldfine ID. Primary and secondary hypothyroidism in nasopharyngeal carcinoma. JAMA. 1976;236:1591–1593. doi: 10.1001/jama.1976.03270150025024. [DOI] [PubMed] [Google Scholar]
- 4.Huang TL, Chien CY, Tsai WL, et al. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck. 2016;38:1026–1032. doi: 10.1002/hed.24150. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Hu W, Wang W, et al. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;85:47–54. doi: 10.1016/j.ijrobp.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Chen YY, Zhao C, Wang J, et al. Intensity-modulated radiation therapy reduces radiation-induced trismus in patients with nasopharyngeal carcinoma: a prospective study with >5 years of follow-up. Cancer. 2011;117:2910–2916. doi: 10.1002/cncr.25773. [DOI] [PubMed] [Google Scholar]
- 7.Murthy V, Narang K, Ghosh-Laskar S, et al. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36:1573–1580. doi: 10.1002/hed.23482. [DOI] [PubMed] [Google Scholar]
- 8.Lee V, Chan SY, Choi CW, et al. Dosimetric predictors of hypothyroidism after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. Clin Oncol. 2016;28:52–60. doi: 10.1016/j.clon.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 9.McDowell LJ, Rock K, Xu W, et al. Long-term late toxicity, quality of life, and emotional distress in patients with nasopharyngeal carcinoma treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102:340–352. doi: 10.1016/j.ijrobp.2018.05.060. [DOI] [PubMed] [Google Scholar]
- 10.Lertbutsayanukul C, Kitpanit S, Prayongrat A, et al. Validation of previously reported predictors for radiation-induced hypothyroidism in nasopharyngeal cancer patients treated with intensity-modulated radiation therapy, a post hoc analysis from a phase III randomized trial [J] J Radiat Res. 2018;59:446–455. doi: 10.1093/jrr/rry036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil VM, Noronha V, Joshi A, et al. Influence of hypothyroidism after Chemoradiation on outcomes in head and neck Cancer. Clin Oncol. 2018;30:675. doi: 10.1016/j.clon.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550–1562. doi: 10.1016/S0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1–5. doi: 10.1016/j.radonc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee NY, Zhang Q, Pfister DG, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan CY, Lin CS, Chao HL, et al. Risk of hypothyroidism among patients with nasopharyngeal carcinoma treated with radiation therapy: a population-based cohort study. Radiother Oncol. 2017;123:394–400. doi: 10.1016/j.radonc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Wu YH, Wang HM, Chen HH, et al. Hypothyroidism after radiotherapy for nasopharyngeal cancer patients. Int J Radiat Oncol Biol Phys. 2010;76:1133–1139. doi: 10.1016/j.ijrobp.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Wang X, Hu C, et al. Hypothalamic-pituitary-thyroid dysfunction induced by intensity-modulated radiotherapy (IMRT) for adult patients with nasopharyngeal carcinoma. Med Oncol. 2013;30:710. doi: 10.1007/s12032-013-0710-9. [DOI] [PubMed] [Google Scholar]
- 19.Alterio D, Jereczek-Fossa BA, Franchi B, et al. Thyroid disorders in patients treated with radiotherapy for head-and-neck cancer: a retrospective analysis of seventy-three patients. Int J Radiat Oncol Biol Phys. 2007;67:144–150. doi: 10.1016/j.ijrobp.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 20.Boomsma MJ, Bijl HP, Christianen ME, et al. A prospective cohort study on radiation-induced hypothyroidism: development of an NTCP model. Int J Radiat Oncol Biol Phys. 2012;84:351–356. doi: 10.1016/j.ijrobp.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Luo R, Li M, Yang Z, et al. Nomogram for radiation-induced hypothyroidism prediction in nasopharyngeal carcinoma after treatment. Br J Radiol. 2017;90:1070. doi: 10.1259/bjr.20160686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommat K, Ong WS, Hussain A, et al. Thyroid V40 predicts primary hypothyroidism after intensity modulated radiation therapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2017;98:574–580. doi: 10.1016/j.ijrobp.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Prpic M, Kruljac I, Kust D, et al. Dose-volume derived Nomogram as a reliable predictor of radiotherapy-induced hypothyroidism in head and neck Cancer patients. Radiol Oncol. 2019;53:488–496. doi: 10.2478/raon-2019-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling S, Bhatt AD, Brown NV, et al. Correlative study of dose to thyroid and incidence of subsequent dysfunction after head and neck radiation. Head Neck. 2017;39:548–554. doi: 10.1002/hed.24643. [DOI] [PubMed] [Google Scholar]
- 25.Sachdev S, Refaat T, Bacchus ID, et al. Thyroid V50 highly predictive of hypothyroidism in head-and-neck Cancer patients treated with intensity-modulated radiotherapy (IMRT) Am J Clin Oncol. 2017;40:413–417. doi: 10.1097/COC.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 26.Zhai RP, Kong FF, Du CR, et al. Radiation-induced hypothyroidism after IMRT for nasopharyngeal carcinoma: clinical and dosimetric predictors in a prospective cohort study. Oral Oncol. 2017;68:44–49. doi: 10.1016/j.oraloncology.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Lin Z, Wang X, Xie W, et al. Evaluation of clinical hypothyroidism risk due to irradiation of thyroid and pituitary glands in radiotherapy of nasopharyngeal cancer patients. J Med Imaging Radiat Oncol. 2013;57:713–718. doi: 10.1111/1754-9485.12074. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Shao Z, Tang T, et al. A dosimetric study on radiation-induced hypothyroidism following intensity-modulated radiotherapy in patients with nasopharyngeal carcinoma. Oncol Lett. 2018;16:6126–6132. doi: 10.3892/ol.2018.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo R, Wu VWC, He B, et al. Development of a normal tissue complication probability (NTCP) model for radiation-induced hypothyroidism in nasopharyngeal carcinoma patients. BMC Cancer. 2018;18:575. doi: 10.1186/s12885-018-4348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhandare N, Kennedy L, Malyapa RS, et al. Primary and central hypothyroidism after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2007;68:1131–1139. doi: 10.1016/j.ijrobp.2007.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


