Abstract
Purpose
Results from recent clinical trials suggest that vitamin D efficacy against cancer may be influenced by body mass index. As suppression of parathyroid hormone (PTH) is one indicator of vitamin D efficacy, we examined to what extent doses of vitamin D3 supplementation suppress PTH levels in individuals with and without obesity.
Methods
A total of 328 healthy African Americans were randomized into the following four groups and treated for 3 months: placebo, 1000, 2000, or 4000 IU/day of vitamin D3 supplementation.
Results
Among the participants, 250 individuals with PTH measurements were included in the analysis. Obese individuals (n = 141) experienced a steep reduction of 3-month PTH from placebo to 1000 IU/day of vitamin D3 supplementation, but no further reduction at 2000 or 4000 IU/day. For non-obese individuals (n = 109), the reduction of 3-month PTH was approximately linear for increasing vitamin D3 doses. At supplementation of 2000 to 4000 IU/day, 3-month 25(OH)vitamin D levels were high in both non-obese and obese individuals, but the 3-month PTH levels remained about 10 pg/mL higher in individuals with obesity.
Conclusion
Our findings suggest that excess adiposity confers resistance to vitamin D efficacy in suppressing PTH levels, even when given at high doses.
Keywords: Vitamin D3 supplementation, circulating 25(OH)D, parathyroid hormone, obesity, cancer, effect modification
Introduction
Vitamin D has been hypothesized to reduce risks of cancer, diabetes, and cardiovascular disease, among other diseases, leading to the conduct of randomized trials. In the Vitamin D and Omega-3 Trial (VITAL), 2000 IU/day of vitamin D3 supplementation was associated with lower total cancer incidence in participants with normal body mass index (BMI) (HR = 0.76; 95% confidence interval, 0.63–0.90), but not among participants with overweight or obesity (P for interaction = 0.002)[1]. Moreover, in a trial of patients with metastatic colorectal cancer, the effect of high-dose vitamin D3 on progression-free survival was greater among patients with lower BMI (P for interaction = 0.04)[2]. Similar findings were reported in a recent large clinical trial of vitamin D supplementation and type 2 diabetes, which found that supplementation with 4000 IU/day of vitamin D3 did not significantly lower risk of type 2 diabetes in adults with prediabetes[3]. However, in subgroup analyses, risk reduction was observed in participants with BMI <30 kg/m2 (Hazard ratio = 0.71; 95% confidence interval, 0.53–0.95), but not in participants with BMI ≥30 kg/m2 (Hazard ratio = 0.97; 95% confidence interval, 0.80–1.17)[3]. These findings could be due to chance, but it is intriguing that a strong interaction in the same subgroup was observed in these three randomized trials of vitamin D. We considered other evidence for the plausibility that vitamin D efficacy would be influenced by body mass, with less effect in those with greater adiposity. It is known that obesity affects the vitamin D-parathyroid hormone (PTH) axis[4]. Suppression of PTH, an indicator of vitamin D efficacy, is less likely to occur at a given 25(OH)vitamin D (25(OH)D) level in obese than in lean individuals[5]. To date, there is limited research on dose-response effects of vitamin D on PTH levels according to BMI. Therefore, we examined whether relatively high doses of vitamin D3 supplementation can suppress PTH levels in individuals with obesity to similar levels as in individuals without obesity.
Methods
The parent study for this analysis was a prospective, randomized, double blind, placebo-controlled trial of oral vitamin D3 (cholecalciferol) supplementation in healthy African Americans (ClinicalTrials.gov: NCT00585637), described in detail elsewhere[6]. The project was approved by the Institutional Review Boards of Harvard T.H. Chan School of Public Health and Dana-Farber Cancer Institute. All procedures were followed in accordance with institutional guidelines.
A total of 328 African American men and women aged 30 to 80 years old living in Boston, MA were randomly assigned to one of four daily treatment arms: placebo, 1000 IU (25 mcg)/day, 2000 IU (50 mcg)/day, or 4000 IU (100 mcg)/day of vitamin D3 for 3 months. All tablets also contained 200 mg of calcium carbonate. To minimize the influence of sun exposure on vitamin D levels, the enrollment occurred during the late fall and winter months. The exclusion criteria included pre-existing disorders of calcium metabolism and parathyroid function (including prevalent hypercalcemia at baseline), type I diabetes, renal disease, sarcoidosis, concurrent active malignancies (other than non-melanoma skin cancer), cognitive impairment, active thyroid disease (e.g., Graves, Hashimoto’s, or thyroiditis), plans for vacation or extended travel to a sunny region during the supplementation phase of the study, and supplementation with vitamin D. Blood samples were collected at baseline and at 3 months. PTH was measured with stored blood samples using the DiaSorin intact PTH immunoradiometric assay. There were 250 individuals with PTH measurements and complete covariate information. Although the unavailability of blood samples in ~20% of participants could have influenced statistical power and precision, it was unlikely to cause bias.
For this analysis, the primary endpoint was 3-month change in serum PTH levels (from baseline to the end of the randomized vitamin D3 supplementation). Dose-response effect of vitamin D3 on PTH levels was evaluated to observe how PTH responds to different doses of vitamin D3.
To explore potential heterogeneity in the relationship, we performed a stratified analysis by obesity status (Obese, BMI ≥30 kg/m2 and Non-obese, BMI <30 kg/m2). Independent t-test was used when comparing the means of the two subgroups. If the variable of interest was not normally distributed, results from a non-parametric test (Wilcoxon rank-sum) and median values were reported instead. We used linear regression and tested for statistical interaction by adding a product term of the two variables in the model. Two-sided statistical significance was defined as P-value <0.05. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
In this study, 61, 65, 61, and 63 individuals were randomly assigned to placebo, 1000, 2000, and 4000 IU/day of vitamin D3 supplementation, respectively (Table). The compliance with supplementation in the population was 96.6% and did not differ significantly between treatment arms. In the stratified analysis, there were 141 participants with obesity (77.3% female) and 109 without obesity (57.8% female). The mean serum 25(OH)D level at baseline was 16.8 ng/mL among individuals with obesity and 17.4 ng/mL among individuals without obesity. While mean baseline 25(OH)D levels were not statistically different for the obese and non-obese groups (Independent t-test P-value = 0.66), baseline PTH level was statistically significantly higher in individuals with obesity compared to those without obesity (Median baseline PTH for obese: 39.3 pg/mL and for non-obese: 35.4 pg/mL; Wilcoxon rank-sum P-value = 0.0009).
Table.
Effect of vitamin D3 supplementation on PTH (pg/mL) and serum 25(OH)D level (ng/mL) during the treatment phase (Baseline to 3 months)a
| Vitamin D3 Dose (IU/day) |
|||||||
|---|---|---|---|---|---|---|---|
| Placebo | 1000 | 2000 | 4000 | 3 months change per 1000 IU/day of vitamin D3b | P-value | ||
| N at baseline | 61 | 65 | 61 | 63 | |||
| 25(OH)D, ng/mL, mean (SD) | |||||||
| Baseline | 16.1 (9.3) | 17.7 (9.1) | 16.3 (9.0) | 18.1 (9.1) | |||
| 3 months | 13.6 (7.7) | 27.9 (9.3) | 36.4 (11.2) | 47.5 (10.1) | |||
| 25(OH)D differencec | −1.9 (5.3) | 10.2 (9.9) | 19.9 (11.2) | 29.7 (11.3) | 7.7 [6.8, 8.6] | <0.0001 | |
| Total PTH, pg/mL, mean (SD) | |||||||
| Baseline | 39.6 (14.8) | 41.4 (17.9) | 42.4 (21.0) | 43.5 (17.2) | |||
| 3 months | 44.2 (18.3) | 39.2 (14.5) | 35.6 (16.2) | 35.3 (15.1) | |||
| PTH differenced | 3.8 (16.3) | −4.0 (16.4) | −7.4 (14.0) | −8.2 (12.5) | −2.7 [−4.0, −1.4] | <0.0001 | |
| Stratified analysis | |||||||
| N at baseline | |||||||
| Obese | 31 | 36 | 33 | 41 | |||
| Non-obese | 30 | 29 | 28 | 22 | |||
| 25(OH)D, ng/mL, mean (SD) | |||||||
| Baseline | |||||||
| Obese | 15.5 (8.6) | 17.8 (8.3) | 15.0 (9.1) | 18.5 (9.2) | |||
| Non-obese | 16.7 (10.0) | 17.6 (10.1) | 17.8 (8.7) | 17.4 (9.3) | |||
| 3 months | |||||||
| Obese | 12.8 (7.0) | 28.2 (6.1) | 35.2 (8.1) | 45.0 (10.0) | |||
| Non-obese | 14.5 (8.5) | 27.6 (11.7) | 37.8 (14.1) | 52.4 (8.6) | |||
| 25(OH)D differencec | |||||||
| Obese | −1.8 (3.5) | 10.6 (9.5) | 20.2 (9.5) | 27.2 (10.4) | 6.8 [5.8, 7.9] | <0.0001 | |
| Non-obese | −2.1 (6.8) | 9.8 (10.4) | 19.5 (13.0) | 34.6 (11.5) | 9.1 [7.6, 10.6] | <0.0001 | |
| Total PTH, pg/mL, mean (SD) | |||||||
| Baseline | |||||||
| Obese | 43.1 (16.6) | 41.6 (19.6) | 49.0 (26.6) | 47.9 (18.0) | |||
| Non-obese | 36.1 (12.1) | 41.2 (15.9) | 34.6 (9.2) | 35.0 (11.7) | |||
| 3 months | |||||||
| Obese | 48.1 (19.9) | 38.9 (9.4) | 38.5 (19.8) | 38.9 (16.5) | |||
| Non-obese | 39.8 (15.5) | 39.4 (18.5) | 32.1 (9.7) | 28.1 (8.1) | |||
| PTH differenced | |||||||
| Obese | 4.3 (18.2) | −6.7 (18.2) | −11.3 (15.8) | −9.2 (13.7) | −2.8 [−4.7, −0.9] | 0.0045 | |
| Non-obese | 3.1 (14.2) | −1.1 (14.1) | −2.8 (9.9) | −6.0 (9.6) | −2.2 [−4.0, −0.4] | 0.016 | |
PTH, Parathyroid hormone; 25(OH)D, 25(OH)vitamin D; mean (SD), mean (Standard Deviation); IU, international unit; BMI, body mass index, kg/m2. Non-obese (BMI < 30) and obese (BMI ≥ 30). The numbers do not always sum to group totals due to missing information for some variables.
Month 3 – Month 0 change in PTH per 1000 IU/day of vitamin D3 supplementation, Mean [95% confidence interval]. Month 3 – Month 0 change in 25(OH)D per 1000 IU/day of vitamin D3 supplementation, Mean [95% confidence interval]
Month 3 – Month 0 difference in 25(OH)D
Month 3 – Month 0 difference in PTH
At 3 months after supplementation, the mean 25(OH)D level for individuals with obesity was 28.2, 35.2, and 45.0 ng/mL at 1000, 2000, and 4000 IU/day of vitamin D3, respectively (Table). Both obese and non-obese individuals attained similarly high 3-month 25(OH)D levels for increasing doses of vitamin D3 supplementation (Figure, left). In the Table, the 3-month change in PTH levels differed significantly by vitamin D3 dosage for both obese and non-obese individuals (Obese: −2.8 pg/mL for each additional 1000 IU/day of vitamin D3, P-value = 0.0045 and Non-obese: −2.2 pg/mL for each additional 1000 IU/day of vitamin D3, P-value = 0.016). While vitamin D3 supplementation at increasing doses progressively lowered PTH in both obese and non-obese participants, an approximate 10-unit difference in serum PTH levels at 3 months remained between the two groups. For individuals with obesity, there was a steep reduction of mean PTH at 3 months from placebo to 1000 IU/day (48.1 to 38.9 pg/mL) but no further reduction at 2000 or 4000 IU/day (Figure, right). For individuals without obesity, the reduction of PTH was approximately linear with a mean of 39.8, 39.4, 32.1, and 28.1 pg/mL for the placebo, 1000, 2000, and 4000 IU/day arms, respectively. However, the steepness of slope for obese and non-obese individuals was not statistically significantly different (P for interaction = 0.30).
Figure.
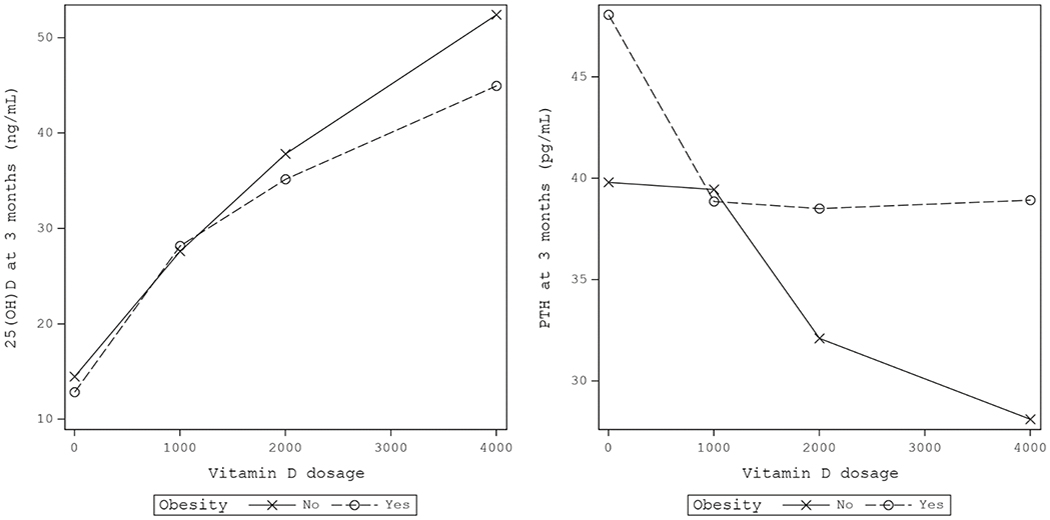
(Left) Mean 25(OH)vitamin D (25(OH)D) level at 3 months for different doses of vitamin D3 supplementation, stratified by obesity status. (Right) Mean parathyroid hormone (PTH) level at 3 months for different doses of vitamin D3 supplementation, stratified by obesity status
Conclusions
The threshold for vitamin D sufficiency remains controversial, but has been defined by some as the lowest serum concentration of 25(OH)D that maximally suppresses PTH secretion[7]. In this trial, even though increases in vitamin D3 dose lowered PTH in both non-obese and obese participants, higher PTH levels in individuals with obesity persisted even at 4000 IU/day of vitamin D3 and 45 ng/mL of attained 25(OH)D. Although the interaction was not statistically significant, these results suggest that excess adiposity confers some resistance to the efficacy of vitamin D in suppressing PTH levels even when given at high doses.
Similar to our findings and results from the randomized trials on vitamin D supplementation[1–3], a recently published randomized trial on calcium supplementation and colorectal adenoma showed apparent difference in calcium efficacy for normal versus overweight or obese individuals[8]. 1200 mg/day of calcium supplementation reduced risk of colorectal adenoma among individuals with normal BMI, but not among those with overweight or obesity. The results from our study and the trial on calcium supplementation suggest that BMI not only modifies the efficacy of vitamin D but also the efficacy of calcium.
In a meta-analysis that investigated PTH response to vitamin D3 supplementation in the general adult population, vitamin D3 supplementation of 3000 IU/day for one year effectively suppressed serum PTH levels[5]. However, for individuals with obesity, a recent meta-analysis of clinical trials reported that while 4000 IU/day of vitamin D3 supplementation maximally increased serum 25(OH)D levels, only 1000 IU/day was needed to significantly suppress serum PTH levels[9]. This pattern was observed in our study, whereby 1000 IU/day appeared to maximally reduce PTH in individuals with obesity, despite 25(OH)D levels increasing progressively with higher vitamin D3 doses. In individuals without obesity, the achieved PTH level with higher doses was approximately 10 pg/mL lower than in individuals with obesity.
The main strength of this study was the randomized design and high doses of vitamin D3 supplementation tested. A limitation was the small sample size, which possibly led to insufficient power to detect whether the slope of PTH levels at increasing vitamin D3 doses differed by obesity status, thereby requiring confirmatory studies. Nonetheless, our data suggest that PTH levels remain elevated in individuals with obesity compared to individuals without obesity even at relatively high doses of vitamin D3 (2000~4000 IU/day).
Our findings suggest a need for further mechanistic research but add to the biologic plausibility that obese individuals may be less responsive to the effect of vitamin D3 supplementation. The suppression of PTH secretion has often been used as an indicator of vitamin D sufficiency[7], although whether this relates to effects of vitamin D on conditions such as cancer and diabetes remains unknown. Whether differences in vitamin D sensitivity, free or bioavailable 25(OH)D levels[10], vitamin D receptor activity, gene expression, or other factors account for the heterogeneity in results[1–3] by BMI level in recent trials warrants further study.
Acknowledgements
Financial Support: The parent trial of this study was funded by the National Cancer Institute (U01CA138962 [Dr. Chandler]; P50CA127003; R01CA205406 [Dr. Ng]), the American Cancer Society Mentored Research Scholar Award (127524-MRSG-15-012-01-CNE [Dr. Chandler]), and the American Society of Clinical Oncology Career Development Award (Dr. Ng). These funding sources had no role in the conception or conduct of the study, took no part in the data collection or analysis, and had no role in the drafting, review, or approval of the article. ClinicalTrials.gov: NCT00585637
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE (2019) Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. The New England journal of medicine 380 (1):33–44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM, Rubinson DA, Schrag D, Miksad R, Bullock AJ, Allen J, Zuckerman D, Chan E, Chan JA, Wolpin BM, Constantine M, Weckstein DJ, Faggen MA, Thomas CA, Kournioti C, Yuan C, Ganser C, Wilkinson B, Mackintosh C, Zheng H, Hollis BW, Meyerhardt JA, Fuchs CS (2019) Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. Jama 321 (14):1370–1379. doi: 10.1001/jama.2019.2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O’Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M (2019) Vitamin D Supplementation and Prevention of Type 2 Diabetes. The New England journal of medicine 381 (6):520–530. doi: 10.1056/NEJMoa1900906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung YS, Wu D, Smith D, Meydani SN, Han SN (2018) Dysregulated 1,25-dihydroxyvitamin D levels in high-fat diet-induced obesity can be restored by changing to a lower-fat diet in mice. Nutrition research (New York, NY) 53:51–60. doi: 10.1016/j.nutres.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 5.Moslehi N, Shab-Bidar S, Mirmiran P, Hosseinpanah F, Azizi F (2015) Determinants of parathyroid hormone response to vitamin D supplementation: a systematic review and meta-analysis of randomised controlled trials. The British journal of nutrition 114 (9):1360–1374. doi: 10.1017/s0007114515003189 [DOI] [PubMed] [Google Scholar]
- 6.Chandler PD, Agboola F, Ng K, Scott JB, Drake BF, Bennett GG, Chan AT, Hollis BW, Emmons KM, Fuchs CS, Giovannucci EL (2015) Reduction of Parathyroid Hormone with Vitamin D Supplementation in Blacks: A Randomized Controlled Trial. BMC nutrition 1. doi: 10.1186/s40795-015-0024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saliba W, Barnett O, Rennert HS, Lavi I, Rennert G (2011) The relationship between serum 25(OH)D and parathyroid hormone levels. The American journal of medicine 124 (12):1165–1170. doi: 10.1016/j.amjmed.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 8.Barry EL, Lund JL, Westreich D, Mott LA, Ahnen DJ, Beck GJ, Bostick RM, Bresalier RS, Burke CA, Church TR, Rees JR, Robertson DJ, Baron JA (2019) Body mass index, calcium supplementation and risk of colorectal adenomas. Int J Cancer 144 (3):448–458. doi: 10.1002/ijc.31803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotito A, Teramoto M, Cheung M, Becker K, Sukumar D (2017) Serum Parathyroid Hormone Responses to Vitamin D Supplementation in Overweight/Obese Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 9 (3). doi: 10.3390/nu9030241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbs DC, Fedirko V, Um C, Gross MD, Thyagarajan B, Bostick RM (2018) Associations of Circulating 25-Hydroxyvitamin D3 Concentrations With Incident, Sporadic Colorectal Adenoma Risk According to Common Vitamin D-Binding Protein Isoforms. Am J Epidemiol 187 (9):1923–1930. doi: 10.1093/aje/kwy102 [DOI] [PMC free article] [PubMed] [Google Scholar]


