Abstract
Renal fibrosis leads to end-stage renal disease, but antifibrotic drugs are difficult to develop. Chronic kidney disease often results in muscle wasting, and thereby increases morbidity and mortality. In this work, adeno-associated virus (AAV)-mediated overexpressing miR-29a was hypothesized to counteract renal fibrosis and muscle wasting through muscle–kidney crosstalk in unilateral ureteral obstruction (UUO) mice. miR-29a level was downregulated in the kidney and skeletal muscle of UUO mice. The secretion of exosome-encapsulated miR-29a increased in cultured skeletal muscle satellite cells and HEK293 renal cells after stimulation with serum from UUO mice. This result was confirmed by qPCR and microRNA deep sequencing in the serum exosomes of mice with obstructed ureters. A recombinant AAV-miR-29a was generated to overexpress miR-29a and injected into the tibialis anterior muscle of the mice 2 weeks before UUO surgery. AAV-miR-29a abrogated the UUO-induced upregulation of YY1 and myostatin in skeletal muscles. Renal fibrosis was also partially improved in the UUO mice with intramuscular AAV-miR-29a transduction. AAV-miR-29a overexpression reversed the increase in transforming growth factor β, fibronectin, alpha-smooth muscle actin, and collagen 1A1 and 4A1 levels in the kidney of UUO mice. AAV-green fluorescent protein was applied to trace the AAV route in vivo, and fluorescence was significantly visible in the injected/uninjected muscles and in the kidneys. In conclusion, intramuscular AAV-miR-29a injection attenuates muscle wasting and ameliorates renal fibrosis by downregulating several fibrotic-related proteins in UUO mice.
Keywords: kidney fibrosis, YY1, MuRF1, αSMA, TGF-β3, TGF-β1
Introduction
Kidney fibrosis is a major common hallmark in the development and progression of organ damage in chronic kidney diseases (CKDs) and ultimately leads to end-stage renal failure.1 Muscle mass loss is a consequence of CKD that leads to muscle wasting, and results in increased morbidity and mortality.2,3 Recent studies suggest that attenuating skeletal muscle atrophy improves kidney recovery after injury by reducing renal fibrosis.4,5 The mechanism underlying the benefits of these muscle-derived microRNAs remains unknown, and the adeno-associated virus (AAV)-mediated microRNA transfer from skeletal muscles to other organs is poorly understood.
Muscle crosstalk with other organs such as bone, brain, heart, kidney, and adipose tissues has been widely reported.4,6–11 Crosstalk occurs between the skeletal muscle and the kidney in different mouse models.4,12 Muscle exercise has renoprotective effects, ameliorates cardiovascular parameters, improves clinical outcomes, and thereby can be a good model for muscle–organ crosstalk.13 Kokkinos et al. found that physical exercise attenuates the risk of developing CKD.14 Peng et al. found that muscle-specific peroxisome proliferator-activated receptor-γ coactivator-1α overexpression in mice reduces renal damage and fibrosis, thereby providing myokine-mediated muscle–kidney crosstalk.15 Hanatani et al. presented that Akt1-mediated skeletal muscle growth attenuates renal damage in experimental kidney disease.4 The treatment of cardiac injury also improves muscle damage.16 Muscle wasting therapy can enhance the function of other organs and serve as a major advantage in treating systemic diseases.17 However, the mechanism underlying the benefits of muscle wasting treatment for other organs is not well known.
Several potential mediators of crosstalk, including interleukin 6 (IL-6), IGF-1, and transforming growth factor β (TGF-β), have been identified.18–20 Injured renal epithelial cells could increase the production of TGF-β-containing exosomes, which initiate kidney fibrosis through activating fibroblasts.21 An increased level of TGF-β/myostatin cascade can suppress the insulin-IGF1/PI3K/Akt signaling pathway, leading to insulin resistance and muscle wasting. We previously found that an increase in TGF-β inhibits miR-29 promoter activity,22 leading to decreased miR-29 in the skeletal muscles and resulting in muscle atrophy.23 TGF-β is originally geared toward renal repair but could travel to the muscle and promote muscle atrophy. This proatrophic action might occur through Smad2/3 phosphorylation and/or by decreasing miR-29 and alter other fibrosis-related miRs. Our group is currently developing new therapeutic approaches to prevent muscle wasting through the inhibition of the transcription factor myostatin.20,24 This protein belongs to the TGF-β superfamily and inhibits muscle growth. An increased myostatin level is found in the muscles of diabetes mellitus mice and induces muscle atrophy.20,25
In this work, AAV-mediated microRNA, which is commonly used for muscular dystrophy treatment, is hypothesized to also attenuate kidney injury through exosome-mediated microRNA delivery. miR-29a (a well-known antifibrotic microRNA) and unilateral ureteral obstruction (UUO) mice (a well-established renal fibrosis model) were used to test our hypothesis. The role of miR-29a in muscle atrophy was examined by evaluating myostatin and muscle regeneration in UUO mice. The effects of intramuscular injection of AAV-miR-29a on renal fibrosis were determined. Finally, the possible mechanism that occurs when circulating exosomal miR-29a transfers from the skeletal muscle to the kidney was identified. The results could serve as a basis for the treatment of muscle atrophy to attenuate kidney damage.
Materials and Methods
Animals and UUO mouse model
Two-month-old male mice (C57BL/6J) were obtained from Jackson Laboratories (Bar Harbor, ME). For UUO surgery, the mice were anesthetized with intraperitoneal injection of a combination of 12 mg/kg xylazine and 60 mg/kg ketamine and placed in a prone position. An incision was made in the skin and the subcutaneous tissue along the length of the 11th rib using scissors. Muscles were divided, and pleura were carefully pushed upward, exposing the kidney. The left ureter was visualized and ligated with 4-0 silk at two points below the lower pole of the left kidney. Body weight and food intake were measured three times per week postsurgery. The mice were sacrificed at 7 and 14 days after UUO operation.
Virus and gene delivery
A type 9 recombinant AAV-expressing miR-29a gene (AAV-miR-29a) and a recombinant AAV encoding green fluorescent protein (GFP) (AAV-control) as the control vector were prepared by Emory Integrated Genomics Core. The stock virus titer for AAV-miR29a was 3.4 × 1014 and 6.5 × 1014 for AAV-control. Thereafter, 15 μL of viral preparation (1010) was injected into the tibialis anterior (TA) muscle of the left leg over 5 min at 2 weeks before UUO surgery.26
Culture of primary muscle satellite cells and HEK293 cells
Satellite cell isolation and culture were performed as previously reported.12 Fibroblast growth factor was removed for 2 days before the experiments to allow myotube formation.
miRNA sequencing
miRNA-Seq Library preparation and sequencing were performed as previously reported.11
Western blot analysis and antibodies
Total proteins were extracted using radioimmunoprecipitation assay buffer containing protease inhibitor cocktail. Equal amounts (20–50 μg/lane) were used for Western blot analysis.27,28 Primary antibodies: type I collagen (1:1,000), PTEN (1:1,000) were obtained from Santa Cruz; TGF-β (1:1,000) was purchased from R&D, and GAPDH (1:1,000) was from Millipore. TRIM63/MuRF1 (1:1,000) and Fibronectin (1:1,000) were from Sigma-Aldrich. Protein bands were detected using the Li--COR Odyssey infrared scanning system.
RNA extraction and quantitative real-time PCR
Total RNA from muscle and kidney were extracted using Tri-Reagent (Molecular Research, Inc., Cincinnati, OH). Real-time qPCR was used with the following PCR parameters: 94°C for 2 min, 45 cycles at 95°C for 15 s, and 60°C for 60 s. The mouse U6 gene and miR-103 were used as the standard for evaluating individual miRNA levels in the tissue and serum, respectively. miRNA expression was calculated as the difference between the threshold values of the two genes (ΔΔCT).
Muscle and kidney histology
Skeletal muscle immunohistology was performed in accordance with an established procedure.29 In brief, TA muscles were embedded in Tissue Freezing Media and cross-sections (10 μm thick) stained with an antilaminin antibody (1:50 dilution; Sigma-Aldrich) to evaluate cross-sectional area. At least 500 individual myofibers per muscle were measured. Immunohistochemical stains for kidney were performed on formalin-fixed and paraffin-embedded 3 μm sections. The ratio of primary antibodies against fibronectin was 1:200. Masson's trichrome staining was used to assess collagen levels in the kidney. All immunohistochemical analyses were repeated at least three times, and representative images were presented.
Statistical analysis
Data were presented as mean ± standard error. The statistical significance of the difference between the control and treatment groups was determined by simple ANOVA followed by Dunnett's multiple comparison tests. Student's t-test was used to identify significant differences between two groups. Statistical significance was defined as p-values <0.05.
Results
Skeletal muscle and kidney release miR-29a into the serum through exosomes
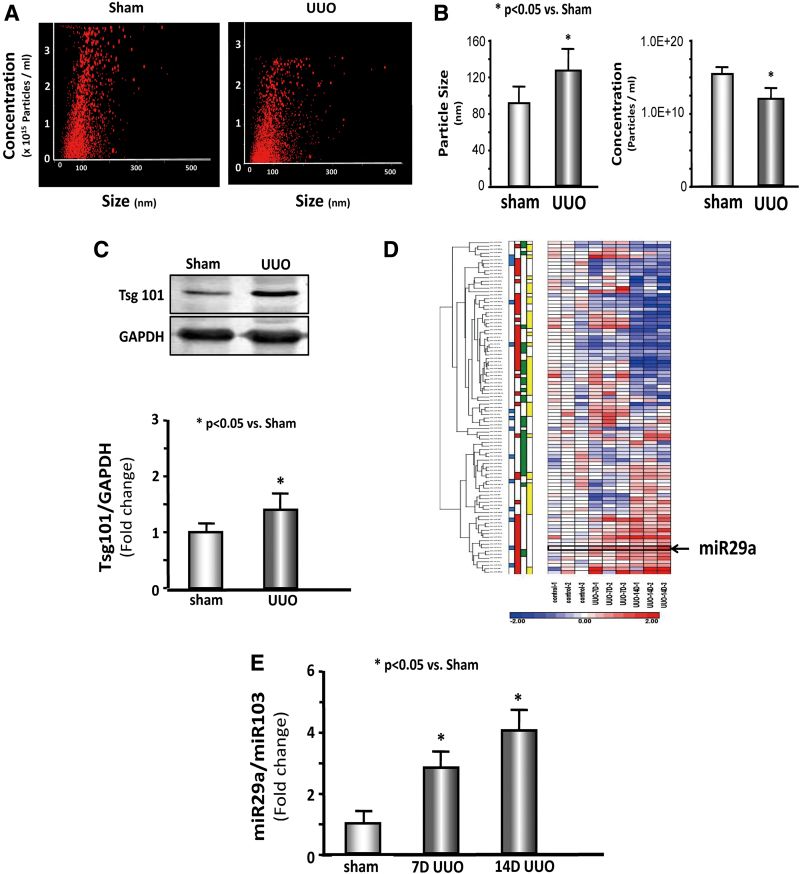
miRs were measured in the serum exosome to identify whether kidney disease altered microRNA in the serum. Serum exosome was isolated from sham and 7 days UUO mice and tested using a NanoSight instrument (Fig. 1A). The exosome size was significantly increased in the UUO serum; however, exosome concentration was decreased in UUO serum compared with shams (Fig. 1B). The exosome protein marker, Tsg101, was used to measure exosome abundance; Tsg101 was significantly increased in UUO serum exosome (Fig. 1C). miRNA deep sequencing was performed, and RNA was isolated from serum exosomes of 7-day and sham mice. A total of 53 miRs were altered in the serum exosomes from UUO mice compared with controls at 7 days (Fig. 1D and Supplementary Table S1). The expression of serum exosome miR-29a-3p was increased by 2.2-fold, miR-29c-3p was increased by 1.4-fold, and miR-29b was not statistically different. Real-time qPCR confirmed that the miR-29a-3p of serum exosome was increased 2.4-fold in 7 days and 4.1-fold in 14 days UUO mice versus sham mice (Fig. 1E).
Figure 1.
miR-29a-3p levels increased in the serum exosome of UUO mice. (A, B) Serum exosome size and concentration were measured by NanoSight Instruments in 7d UUO mice and sham mice (n = 5/group). (C) Expression and bar graph of Tsg101 in 7d UUO mice and sham mice (n = 9/group). (D) Heat map graph showing that miR-29a increased in serum exosomes from UUO mice compared with controls at 7 and 14 days. (E) Expression of miR-29a-3p/miR-103 (n = 9/group). 7d UUO, 7 days unilateral ureteral obstruction. Color images are available online.
miR-29a-3p was reduced in the kidney and skeletal muscle of UUO mice versus shams.12 Skeletal muscle satellite cells and human embryonic kidney cells (HEK293) were cultured, and treated with 5% pooled serum from UUO mice and normal mice serum for control to investigate this opposing change in miR-29a. The blood urea nitrogen level of the pooled serum was ∼60 mg/dL (vs. control levels of ∼18 mg/dL). UUO serum was replaced with exosome-free serum 48 h before cell harvest, and exosomes were isolated from the culture medium. The expression of miR-29a-3p was decreased by 20% in the lysates of satellite cells (Supplementary Fig. S1A) and decreased by 49% in HEK293 cells treated with UUO serum (Supplementary Fig. S1B). However, miR-29a-3p was 1.5-fold increased in the exosomes isolated from the culture medium of satellite cells (Supplementary Fig. S1C) and 1.9-fold increased in the culture medium of HEK293 cells (Supplementary Fig. S1D). These data suggest the possibility that skeletal muscle and kidney release exosome-carried miR-29a into the circulation in UUO mice.
AAV-miR-29a attenuated the upregulation of muscle atrophy proteins in UUO mice
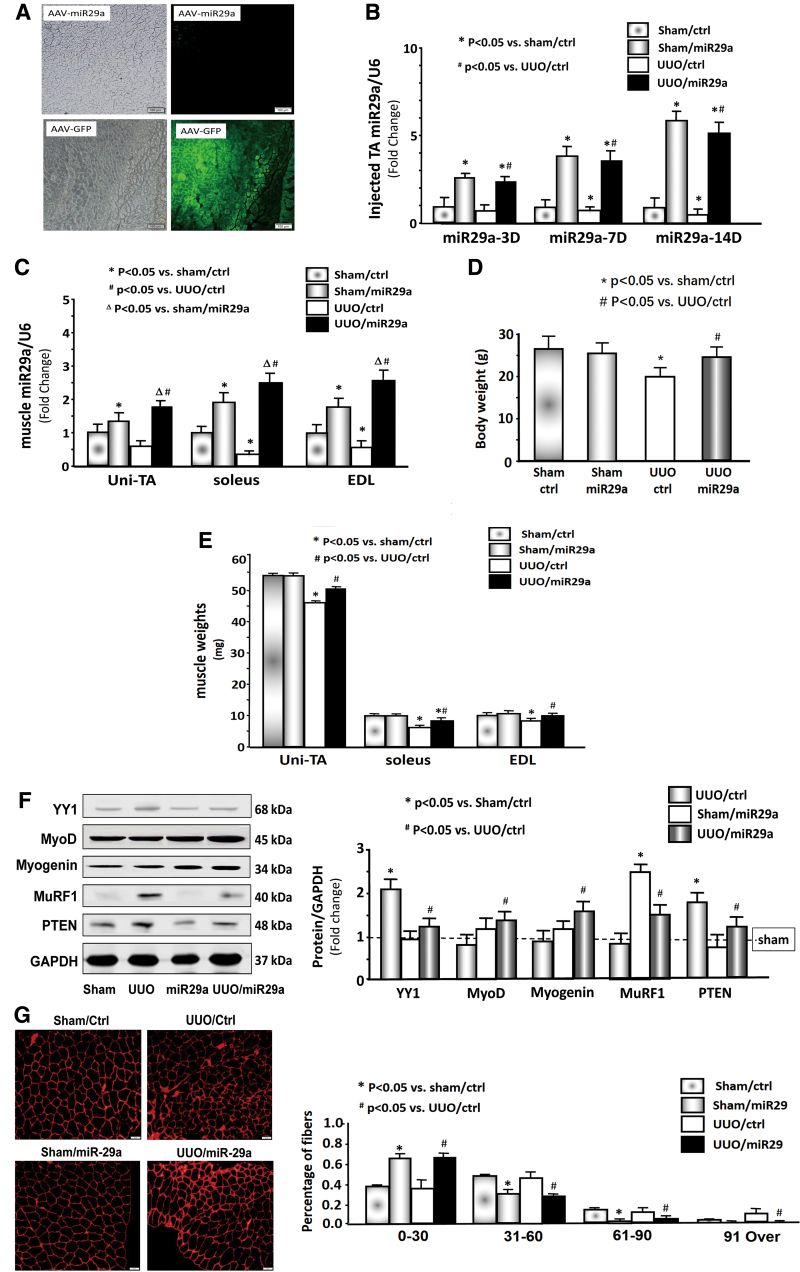
AAV-miR-29a and AAV-control (GFP) were injected into the TA muscle of mice. GFP was significantly visible in the TA muscle of AAV-control injected mice (Fig. 2A). In the muscle injected with AAV-miR29a, the expression of miR-29a-3p was increased 6-fold in the sham mice and 5.1-fold in the UUO muscle at 14 days (Fig. 2B). A time-dependent increase in miR-29a expression was observed. miR-29a expression also appeared to accumulate in the uninjected muscle of UUO mice because miR-29a-3p was increased in the contralateral TA muscle, soleus, and extensor digitorum longus (EDL) of the UUO mice compared with the levels of the muscle of sham mice (Fig. 2C).
Figure 2.
Provision of AAV-miR29a attenuated UUO-induced muscle loss. (A) Representative frozen cross-sections from TA muscles transduced with AAV in the normal control mice. The right panels show fluorescence microscopy images for detecting GFP expression; the left panels show bright-field microscopic images of muscle cross-section. The top panels show microscopy images in mice with AAV-miR-29 transduction. The bottom panels show microscopy images in mice with AAV-GFP transduction. (B) miR-29a/U6 expression in the TA muscle (n = 9/group). (C) miR-29a/U6 expression in the uninjected TA (Uni-TA), soleus, and EDL (n = 9/group). (D) Body weights and (E) muscle weights of sham-operated and UUO cohorts (n = 9/group). (F) Expression of YY1, myoD, myogenin, MuRF1, PTEN, and GAPDH. The bar graph shows the fold change of each protein band compared with the levels in sham mice (represented by a line at onefold) (n = 6/group). (G) The representative cross-sectional area of TA muscle of sham/ctr and UUO cohorts (n = 9/group). AAV, adeno-associated virus; EDL, extensor digitorum longus; GFP, green fluorescent protein; TA, tibialis anterior; UUO, unilateral ureteral obstruction. Color images are available online.
Intramuscular injection of AAV-miR29a into the TA muscle prevented body weight loss and reversed the decrease of the soleus and EDL muscle mass in UUO mice; however, this treatment had no effect on body weight and muscle mass in sham mice (Fig. 2D, E). The effects of Exo/miR-29a on muscle regeneration-related protein markers were examined through Western blot analysis.23 The protein abundance of YY1, a transcription factor that inhibits muscle regeneration, was increased in UUO muscle. The muscle atrophy markers MuRF1 and PTEN were also increased. The muscle proliferation and differentiation markers myoD and myogenin were decreased in the muscle of UUO mice. miR-29a intervention retarded all these changes (Fig. 2F). Furthermore, the muscle cross-section area was decreased in UUO muscle; however, overexpressing miR-29a caused a shift to a large cross-sectional area compared with the results in UUO muscles (Fig. 2G). As such, injecting AAV-miR29a in TA muscle could attenuate muscle atrophy and body weight loss in UUO mice.
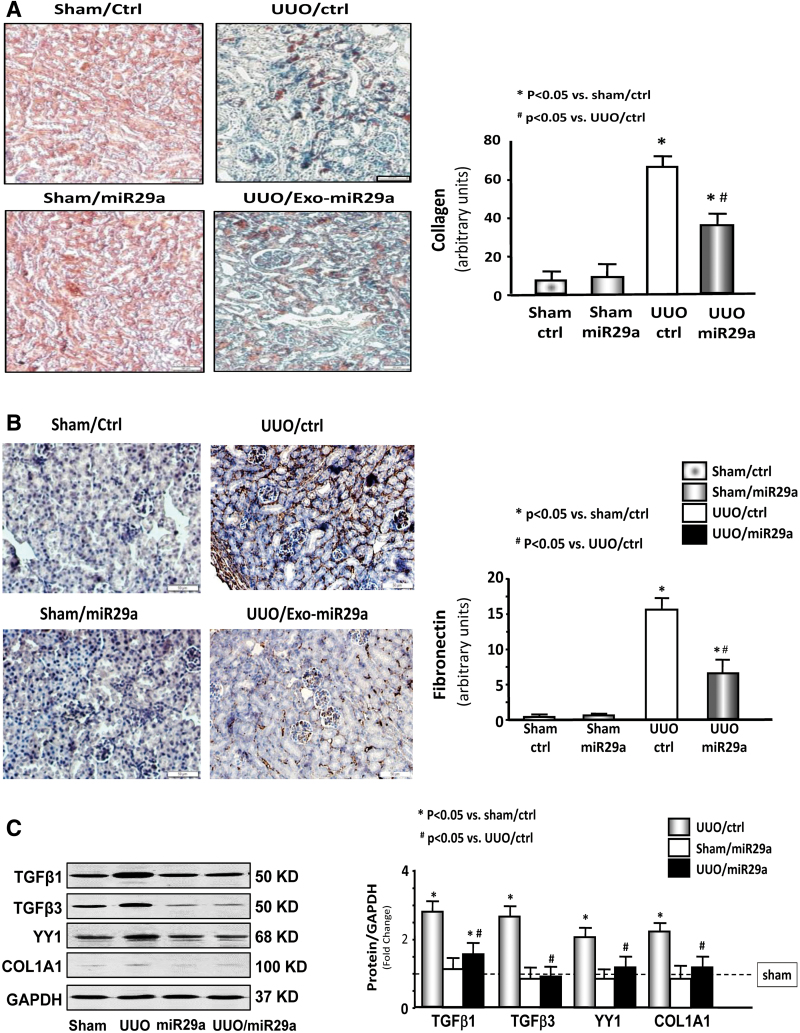
Intramuscular injection of AAV-miR-29a attenuated renal fibrosis in UUO mice
Collagen staining (indicated by blue color) as indicated by Masson's trichrome staining was sharply increased in the 14-day UUO kidney in the presence or absence of AAV-ctrl treatment. However, AAV-miR29a treatment significantly attenuated this change (Fig. 3A). Fibronectin is a glycoprotein of the extracellular matrix and closely associated with newly deposited collagen fibrils.30 The amount of fibronectin is significantly increased in the kidney of UUO mice and attenuated through AAV-miR29a treatment (Fig. 3B). In addition, miR-29a limited the increase in fibrosis-related proteins,31 including TGF-β1, TGF-β3, and collagen 1A1. Transcription factor YY1 (a profibrotic protein)32 is increased in the kidney of UUO mice and reduced after the intramuscular injection of AAV-miR29a (Fig. 3C). These results suggest that miR-29a overexpression in the skeletal muscle can improve renal fibrosis in UUO mice.
Figure 3.
Exogenous miR-29a in skeletal muscle can attenuate fibrosis progression in the UUO kidney. (A) Masson's trichrome staining of paraffin sections from kidneys of various groups as indicated. The bar graph shows the collagen amount (n = 6). (B) Immunostaining for fibronectin and bar graph showing the amounts of fibronectin in various groups as indicated (n = 6/group). (C) TGF-β1, TGF-β3, YY1, and collagen 1A1 expression in kidney lysates from different groups of mice. The bar graph shows the fold change of each protein band compared with levels in sham mice (represented by a line at onefold). (n = 9/group). TGF-β, transforming growth factor β; UUO, unilateral ureteral obstruction. Color images are available online.
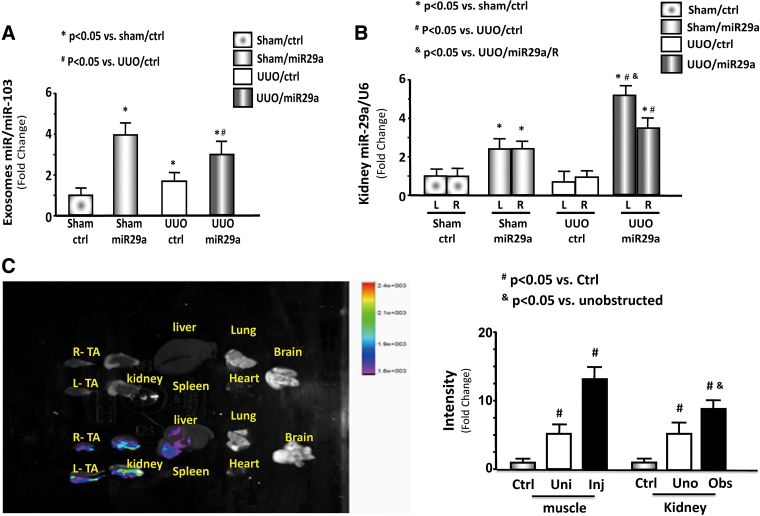
Evidence of muscle–kidney crosstalk
In serum exosome, miR-29a was significantly increased in the sham and UUO mice after AAV-miR29a transduction compared with AAV-ctrl (Fig. 4A). In the kidney, miR-29a expression was higher in the intramuscular injection of AAV-miR-29a than that of AAV-ctrl. The level of miR-29a was significantly higher in the obstructed kidney (left) than that in the unobstructed kidney (right) of the UUO mice (Fig. 4B). In addition, AAV-GFP was tracked with In-vivo Xtreme camera system. After TA injection with AAV-GFP, the fluorescence levels were increased in the kidney and noninjected muscle. Compared with other organs, UUO kidneys, including non-UUO kidney, showed the strongest fluorescence (Fig. 4C). These results suggest that miR-29a travels from the muscle to kidney possibly through exosome circulation.
Figure 4.
Evidence of miR-29a travel from the muscle to the kidney through exosome circulation of exosomes. (A) miR-29a/miR103 expression and bar graph showing miR expression in the serum exosomes of each group of mice. (n = 6/group). (B) Expression and bar graph of miR-29a/U6 in the right and left kidneys of each group of mice (n = 6/group). (C) Representative fluorescent organ images. Mice were injected in the left TA muscle with AAV/miR-ctrl (GFP positive). The fluorescence was assessed 14 days after injection. In each pair, the left kidney received UUO ligation (Obs = obstructed kidney), and the right was not obstructed (Uno = unobstructed kidney). The left TA muscle was transduced by AAV (Inj = injected muscle) and the right muscle was not (Uni = uninjected). Bar graph showing GFP intensity from AAV-GFP transduced mice compared with non-AAV injection mice (n = 3/group). AAV, adeno-associated virus; GFP, green fluorescent protein; TA, tibialis anterior; UUO, unilateral ureteral obstruction. Color images are available online.
Discussion
This study aimed to determine whether AAV-mediated miR-29a transfer can suppress muscle wasting and renal fibrosis. AAV-miR-29a attenuated muscle wasting and limited renal fibrosis by inhibiting YY1 and PTEN in skeletal muscle and YY1, TGF-β1, and TGF-β3 in the kidney in an UUO-induced kidney damage. Our observations provided a rationale that exogenous miR-29a has multiple functions in a kidney disease mouse model.
Treatment of muscle wasting can improve the function of other organs, thereby promising a major therapeutic advantage in the treatment of systemic diseases.17 The benefit of exercise in improving clinical outcomes in a wide range of diseases is a good example of muscle–organ crosstalk.14
The three members in the miR-29 family are a, b, and c. The effects of miR-29 on muscle atrophy remain controversial (subset dependent). The present group and other researchers found that miR-29a was decreased in muscle atrophy models; an increase in miR-29a could prevent muscle atrophy in CKD, cancer, and Duchenne muscular dystrophy animal models.23,33,34 However, Li et al. found that overexpressing miR-29b contributes to muscle atrophy in culture C2C12 cells and in denervation-induced muscle atrophy.35 The different results are attributed to subset difference. In this study, miR-29a overexpression can ameliorate muscle wasting induced by UUO and attenuate renal fibrosis.
Our group found that miR-29 directly reduces TGF-β induced by targeting TGF-β3, thereby upregulating fibrosis signaling.12 In this study, the major benefit of miR-29a treatment for both muscle and kidney is the transcription factor YY1 inhibition. In the muscle, miR-29a decreases phosphatase and PTEN expression and YY1 protein levels. Both are negative regulators of myogenesis and muscle protein metabolism.36 YY1 plays a negative role in myogenesis by directly repressing the synthesis of various genes, including skeletal alpha-actin (α-actin),37 muscle creatine kinase (MCK), and myosin heavy chain IIb (MyHCIIb).38,39 The transcription factor YY1 occupies regulatory regions on these muscle-specific genes preventing transcription. Upon transcriptional activation, YY1 is displaced by serum response factor (SRF). SRF recruits the MyoD transcription factors, which initiate transcription of the muscle proteins (activated state).39 Hence, YY1 inhibition of satellite cell proliferation is the main mechanism leading to muscle wasting. Restoration of miR-29a in the kidney, at least in part, is through YY1 because it directly upregulates alpha smooth muscle actin and collagen32 and limits renal fibrosis.
This study found that serum microRNA levels might not reflect the levels in the organs. Decreasing miR-29a levels in the skeletal muscle and kidney reflect miR-29a secretion from the tissue into circulation. In a cell culture model, UUO serum enhanced miR-29a release from satellite cells through exosomes. This phenomenon could explain miR-29a decrease in organs and its increase in serum exosomes (Supplementary Fig. S1). Chen et al. have revealed that TGF-β overexpression during the progression of kidney fibrosis downregulates miR-29 and is consistent with the findings of this study.40 Conversely, miR-29 overexpression regulates TGF-β expression and attenuates fibrosis.22
In conclusion, this study suggests that treatment of muscle wasting through AAV-mediated miR-29a transfer will inhibit kidney fibrosis, thereby offering a potential treatment strategy to decrease morbidity and improve the quality of life of patients with multiple organ injury.
Supplementary Material
Disclaimer
The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH, the Department of Veterans Affairs, or the US Government.
Compliance with Ethics Requirements
All Institutional and National Guidelines for the care and use of animals were followed.
Authors' Contributions
H.W. and X.H.W. designed the study; B.W. and J.W. carried out experiments; W.H. and Y.Z. analyzed the data; A.Z., F.H., F.M., and Y.L. made the figures; J.D.K., X.H.W., and H.W. drafted and revised the article; all authors approved the final version of the article.
Author Disclosure
No competing financial interests exist.
Funding Information
Research reported in this publication was supported by grants from the National Natural Science Foundation of China (Nos. 81700650, 81700618, 31772690), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health under Award Number R01 AR060268 and American Heart Association Discover and Innovation Grants (17IBDG33780000) to X.H.W. and the Natural Science Foundation of Jiangsu Province (BK20181487) to B.W.
Supplementary Material
References
- 1. Zhou D, Liu Y. Renal fibrosis in 2015: understanding the mechanisms of kidney fibrosis. Nat Rev Nephrol 2016;12:68–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avram MM, Mittman N. Malnutrition in uremia. Semin Nephrol 1994;14:238–244 [PubMed] [Google Scholar]
- 3. Griffiths RD. Muscle mass, survival, and the elderly ICU patient. Nutrition 1996;12:456–458 [DOI] [PubMed] [Google Scholar]
- 4. Hanatani S, Izumiya Y, Araki S, et al. . Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J Am Soc Nephrol 2014;25:2800–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rondon-Berrios H, Wang Y, Mitch WE. Can muscle-kidney crosstalk slow progression of CKD? J Am Soc Nephrol 2014;25:2681–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo B, Zhang ZK, Liang C, et al. . Molecular communication from skeletal muscle to bone: a review for muscle-derived myokines regulating bone metabolism. Calcif Tissue Int 2017;100:184–192 [DOI] [PubMed] [Google Scholar]
- 7. Stanford KI, Goodyear LJ. Muscle-adipose tissue cross talk. Cold Spring Harbor Perspect Med 2017;8:a029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyabe M, Ohashi K, Shibata R, et al. . Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc Res 2014;103:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moon HY, Becke A, Berron D, et al. . Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab 2016;24:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paula FM, Leite NC, Vanzela EC, et al. . Exercise increases pancreatic beta-cell viability in a model of type 1 diabetes through IL-6 signaling. Faseb J 2015;29:1805–1816 [DOI] [PubMed] [Google Scholar]
- 11. Bin W, Aiqing Z, Haidong W, et al. . miR-26a limits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics 2019;9:1864–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang HD, Wang B, Zhang AQ, et al. . Exosome mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol Ther 2019;27:571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008;454:463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kokkinos P, Faselis C, Myers J, et al. . Exercise capacity and risk of chronic kidney disease in US veterans: a cohort study. Mayo Clin Proc 2015;90:461–468 [DOI] [PubMed] [Google Scholar]
- 15. Peng H, Wang Q, Lou T, et al. . Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidney. Nat Commun 2017;8:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aminzadeh MA, Rogers RG, Fournier M, et al. . Exosome-mediated benefits of cell therapy in mouse and human models of Duchenne Muscular Dystrophy. Stem Cell Rep 2018;10:942–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang XH, Mitch WE. Muscle wasting from kidney failure-a model for catabolic conditions. Int J Biochem Cell Biol 2013;45:2230–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang L, Du J, Hu Z, et al. . IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol 2009;20:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L, Wang XH, Wang H, et al. . Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol 2010;21:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L, Rajan V, Lin E, et al. . Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 2011;25:1653–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borges FT, Melo SA, Ozdemir BC, et al. . TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 2013;24:385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu Z, Klein JD, Mitch WE, et al. . Wang XH. MicroRNA-29 induces cellular senescence in aging muscle through multiple signaling pathways. Aging (Albany, NY) 2014;6:160–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang XH, Hu Z, Klein JD, et al. . Decreased miR-29 suppresses myogenesis in CKD. J Am Soc Nephrol 2011;22:2068–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu J, Li R, Workeneh B, et al. . Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int 2012;82:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang L, Pan J, Dong Y, et al. . Mitch WE. Stat3 activation links a C/EBPdelta to myostatin pathway to stimulate loss of muscle mass. Cell Metab 2013;18:368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang XH, Hu J, Du J, et al. . X-chromosome linked inhibitor of apoptosis protein inhibits muscle proteolysis in insulin-deficient mice. Gene Ther 2007;14:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Q, Du J, Hu Z, et al. . Evidence for adipose-muscle cross talk: opposing regulation of muscle proteolysis by adiponectin and Fatty acids. Endocrinology 2007;148:5696–5705 [DOI] [PubMed] [Google Scholar]
- 28. Su Z, Robinson A, Hu L, et al. . Acupuncture plus Low-Frequency Electrical Stimulation (Acu-LFES) attenuates diabetic myopathy by enhancing muscle regeneration. PLoS One 2015;10:e0134511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang B, Zhang C, Zhang A, et al. . MicroRNA-23a and microRNA-27a mimic exercise by ameliorating CKD-induced muscle atrophy. J Am Soc Nephrol 2017;28:2631–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giuffrida P, Pinzani M, Corazza GR, et al. . Biomarkers of intestinal fibrosis—one step towards clinical trials for stricturing inflammatory bowel disease. United Eur Gastroenterol J 2016;4:523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016;12:325–338 [DOI] [PubMed] [Google Scholar]
- 32. Guo J, Yao H, Lin X, et al. . IL-13 induces YY1 through the AKT pathway in lung fibroblasts. PLoS One 2015;10:e0119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zanotti S, Gibertini S, Curcio M, et al. . Opposing roles of miR-21 and miR-29 in the progression of fibrosis in Duchenne muscular dystrophy. Biochim Biophys Acta 2015;1852:1451–1464 [DOI] [PubMed] [Google Scholar]
- 34. Wang H, Garzon R, Sun H, et al. . NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 2008;14:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Chan MC, Yu Y, et al. . miR-29b contributes to multiple types of muscle atrophy. Nat Commun 2017;8:15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodman CA, McNally RM, Hoffmann FM, et al. . Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol Endocrinol 2013;27:1946–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee TC, Shi Y, Schwartz RJ. Displacement of BrdUrd-induced YY1 by serum response factor activates skeletal alpha-actin transcription in embryonic myoblasts. Proc Natl Acad Sci U S A 1992;89:9814–9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vincent CK, Gualberto A, Patel CV, et al. . Different regulatory sequences control creatine kinase-M gene expression in directly injected skeletal and cardiac muscle. Mol Cell Biol 1993;13:1264–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caretti G, Di Padova M, Micales B, et al. . The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev 2004;18:2627–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen HY, Zhong X, Huang XR, et al. . MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther 2014;22:842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.