Abstract
Background
The aim of the study was to evaluate the safety and feasibility of intra-arterial mitomycin C (MMC) infusion after selective internal radiation therapy (SIRT) using Yttrium-90 (90Y) resin microspheres in liver metastatic breast cancer (LMBC) patients.
Patients and methods
The prospective pilot study included LMBC patients from 2012–2018. Patients first received infusion of 90Y resin microspheres, after 6–8 weeks response to treatment was assessed by MRI, 18F-FDG PET/CT and laboratory tests. After exclusion of progressive disease, MMC infusion was administrated 8 weeks later in different dose cohorts; A: 6 mg in 1 cycle, B: 12 mg in 2 cycles, C: 24 mg in 2 cycles and D: maximum of 72 mg in 6 cycles. In cohort D the response was evaluated after every 2 cycles and continued after exclusion of progressive disease. Adverse events (AE) were reported according to CTCAE version 5.0.
Results
Sixteen patients received 90Y treatment. Four patients were excluded for MMC infusion, because of extra hepatic disease progression (n = 3) and clinical and biochemical instability (n = 1). That resulted in the following number of patient per cohort; A: 2, B: 1, C: 3 and D: 6. In 4 of the 12 patients (all cohort D) the maximum dose of MMC was adjusted due biochemical toxicities (n = 2) and progressive disease (n = 2). One grade 3 AE occurred after 90Y treatment consisting of a gastrointestinal ulcer whereby prolonged hospitalization was needed.
Conclusions
Sequential treatment of intra-arterial infusion of MMC after 90Y SIRT was feasible in 75% of the patients when MMC was administrated in different escalating dose cohorts. However, caution is needed to prevent reflux after 90Y SIRT in LMBC patients.
Key words: liver metastatic breast cancer, chemo resistant, intra-arterial therapy, radioembolization, selective internal radiation therapy, mitomycin C infusion
Introduction
Up to 50% of the metastatic breast cancer (mBC) patients eventually develop liver metastases which is associated with a poor overall survival ranging from 1–3 years.1,2 Liver metastatic breast cancer (LMBC) patients with limited (extra) hepatic disease can obtain a survival benefit from local treatment options, such as surgery and ablation.3, 4 Unfortunately, most LMBC patients have extended disease whereby systemic treatment by chemotherapy or anti-hormonal therapy, in hormone sensitive breast cancer, is indicated. Systemic treatment with chemotherapy has lengthened overall survival, but haematological, gastrointestinal and neurotoxicity are dose-limiting factors with systemic administration of these agents.5 Therapies with less systemic toxicity are therefore an attractive treatment option in LMBC patients refractory to systemic chemotherapy.6
Several intra-arterial therapies have been applied for LMBC patients, such as radioembolization (Yttrium-90 (90Y) resin microspheres), chemo embolization and chemo infusion, reported as effective and safe treatment options.7, 8, 9 Of these treatments, the best results are reported for intraarterial radioembolization, with a disease control rate of 72–91% and an overall survival of 6.6–13.6 months.7,10, 11, 12 For over 15 years, LMBC patients are treated by intra-arterial chemo infusion with mitomycin C (MMC) in our institute whereby disease control is obtained in 58% with low adverse events in a very heavily pre-treated cohort.9
Since primary breast cancer is sensitive for both systemic chemotherapy and external beam radiation therapy, LMBC patients might also benefit from the combined treatment of chemo infusion and selective internal radiation therapy (SIRT).
However, the sequential administration of these intra-arterial therapies e.g. SIRT and intra-arterial chemo infusion, has not yet been reported. In this pilot study we prospectively analysed the safety and feasibility of the sequential intra-arterial infusion of 90Y resin microspheres and MMC in patients refractory to conventional systemic chemotherapy.
Patients and methods
This prospective phase I pilot study of 16 patients was approved by the ethics committee of our hospital (S 53657). Patients were included from April 2012 until March 2018. All patients signed a written informed consent. Patient selection was performed by a multidisciplinary tumour board consisting of medical, surgical and radiation oncologists together with pathologists, nuclear medicine physicians and (interventional) radiologists.
In Table 1, the inclusion and exclusion criteria for this pilot study are shown. Figure 1 shows the treatment schedule of 90Y SIRT followed by MMC infusion in the different cohorts. The first 3 patients were allocated to cohort A, the second 3 patients to cohort B, the 3 patients thereafter to cohort C and the remaining of the patients in cohort D. Patients first underwent the 90Y treatment and in the absence of progression, the sequential intra-arterial hepatic MMC infusions were administrated 8 weeks later.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Histologically confirmed diagnosis of breast cancer | Bilirubin level > 1.5x upper limit normal |
| Radiological evidence of liver metastases | Transaminase (AST/ALT) > 2.5x upper limit normal |
| Liver only or liver predominant with stable extra-hepatic disease | Creatinine > 1.2x upper limit normal |
| Progressive under (multi-line) systemic chemo or hormonal therapy | Glomerular filtration rate < 60 mL/min/1.73 m2 |
| Eligible for intra-arterial therapy | Neutrophils < 1000/μL |
| Age > 18 years | Thrombocytes < 100x109/L |
| Karnofsky performance > 70 | Lung shunt fraction > 20% |
| Allergy to contrast media | |
| Active use of oral anticoagulation |
ALT = alanine aminotransferase; AST = aspartate aminotransferase
Figure 1.
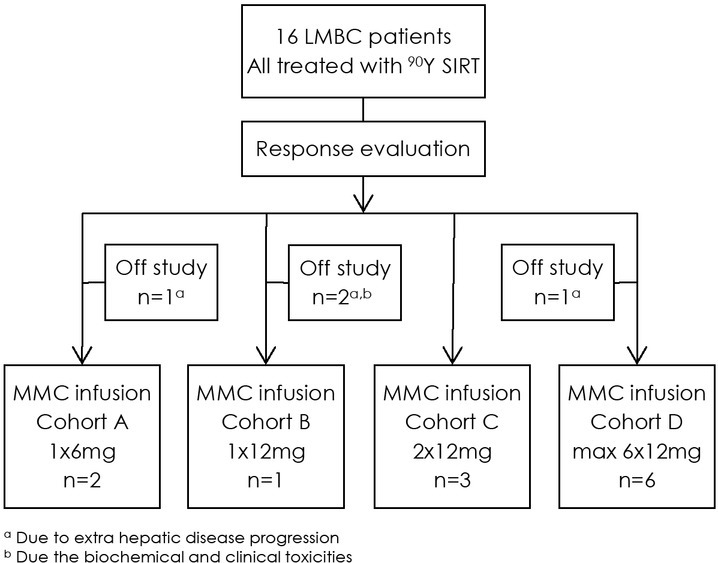
Treatment overview of the 16 patients treated with selective internal radiation therapy with Yttrium-90 containing microspheres (90Y SIRT) and mitomycin C (MMC) infusion in 4 escalating cohorts.
Radioembolization with Yttrium-90 (90Y) resin microspheres
All 90Y procedures were performed under local anaesthesia by or under direct supervision of an expert interventional radiologist (GM) and nuclear medicine physician (CD). Previously published work describes the details of the 90Y treatment.13 Briefly, patients first underwent a detailed baseline angiogram and embolization of enterohepatic arteries with micro-coils according to the expertise of the interventional radiologist. Technetium-99m-macroaggregated albumin (99mTC-MAA) was injected in the target vessels and a planar scintigraphy was performed to determine the lung shunt fraction. Two weeks later patients underwent bi-lobar or uni-lobar injection of 90Y resin microspheres (SIR spheres, Sirtex Inc, Cosgrove, Australia) under local anaesthesia. The activity of 90Y injected in the patient was calculated based on the body surface area method. In patients where it was possible to use the partition model, the partition model was used instead of the body surface area method. Dependent on the site of lesions, the 90Y resin microspheres were injected intra-arterially via a micro catheter to the right and left liver lobe (superselective). A Progreat® micro catheter (Terumo, Europe) or an anti-reflux catheter (Surefire Inc., Westminster, CO, USA) was used for injection of the microspheres. Patients received antiemetics and morphine derivates before 90Y injection when required.
Hepatic intra-arterial chemo infusion with MMC
Six to eight weeks after the 90Y treatment, patients underwent response assessment by magnetic resonance imaging (MRI) of the liver and a whole body [18F]-FDG positron emission tomography/ computed tomography scan (18F-FDG PET/CT) together with laboratory tests. If patients showed no untreatable progression of disease on MRI and 18F-FDG PET-CT scan (bases on RECIST criteria 1.1) together with acceptable laboratory results (> 100x109 thrombocytes/L), the intra-arterial chemoinfusion of MMC could be administrated. Six to ten weeks after the 90Y procedure, patients received the first infusion of MMC, as previously described by our group.14 After local anaesthesia, vascular access was obtained by the right common femoral artery and, using a diagnostic 4-French catheter, a micro catheter was placed in the right and left hepatic arteries for injection of MMC in both liver lobes.
Ascending maximal doses of MMC were administrated in different cohorts. Patients were allocated to the MMC cohort before the 90Y procedure. The first cohort (A) received one cycle of 6mg MMC, the second cohort (B) received one cycle of 12mg MMC, the third cohort (C) received 2 cycles of 12mg of MMC and the fourth cohort (D) received the standard regime of MMC infusion consisting of a maximum of 6 cycles of each 12mg of MMC. Before and after the MMC infusion laboratory results were taken to determine whether the dose could be tolerated. The dose of MMC was adjusted when thrombocyte count was < 100x109 thrombocytes/L. In the last cohort, the effect of MMC was radiologically evaluated, by CT or MRI, after every two cycles. If these evaluations showed no further intra- or extra-hepatic disease progression, the patient was rescheduled for the next 2 cycles for a maximum of 6 cycles.
Outcomes
The primary outcomes of this study included the feasibility and safety of MMC infusion after 90Y SIRT. The feasibility was defined as the number of patients receiving the MMC infusion after 90Y treatment. The safety was determined by the number of grade 3 and higher adverse events after MMC and 90Y treatment and documented by the Common Terminology Criteria for Adverse Events version 5.0. For the response assessment the modified response evaluation criteria in solid tumours (mRE-CIST) was used to measure hepatic response by a maximum of two target lesions and categorized into four categories [Complete Response (CR), Partial Response (PR), Stable Disease (SD) and Progressive Disease (PD)].15
Statistical analysis
Patient and pre-treatment characteristics were presented as numbers, percentages and median time with range. Overall survival (OS) was calculated from work-up for 90Y treatment until death or loss to follow up by the Kaplan Meier estimates. All analyses were performed in SPSS (IBM Corp, version 25, Armonk, NY).
Results
Patient characteristics
Table 2 shows the patient characteristics of the 16 treated women. The median time from diagnosis of metastatic breast cancer to start of the study was 28 months (range 7.7–91.0 months). Patients were 26– 77 (median 59) years old at the start of the study.
Table 2.
Patient characteristics
| Patient Characteristics | N = 16 |
|---|---|
| Median months from diagnosis metastatic disease until start of study | 28 (8–91) |
| Median age at start study in years (range) | 59 (26–77) |
| Diagnosis of liver metastasis | |
| Synchronous | 2 (12.5%) |
| Metachronous | 14 (87.5%) |
| Hormone status of liver metastasis (n = 15) | |
| Estrogen receptor (positive) | 8 (53%) |
| Progesterone receptor (positive) | 8 (53%) |
| HER2Neu receptor (negative) | 15 (100%) |
| Triple negative receptor status | 6 (40%) |
| Tumor burden liver | |
| < 25% | 9 (56%) |
| 25%–50% | 6 (38%) |
| 50%–75% | 1 (6%) |
| Prior hepatic treatment | |
| Surgery/Ablation | 1 (6.3%) |
| Median number of chemotherapy regimens for stage 4 disease | 3 (0–8) |
| Extra-hepatic sites of metastases | |
| Yes | 9 (56%) |
| No | 7 (44%) |
| Number of additional metastatic sites | |
| 1 | 5 (31%) |
| 2 | 2 (13%) |
| ≥ 3 | 2 (13%) |
| Location of extrahepatic metastases | |
| Bone | 5 (31%) |
| Lung | 3 (19%) |
| Non-locoregional nodes | 4 (25%) |
| Brain | 2 (13%) |
A liver biopsy was taken before start of the study in 15 patients and showed a positive oestrogen receptor in 8 patients, positive progesterone receptor in 8 patients and a negative HER2-neu status in all patients (n = 15). Six patients had a triple negative receptor status. Tumour burden of the liver was < 25% in 9 patients and > 25% in 7 patients. Nine patients had extra-hepatic disease with a median of 1 site (range 0–4) and bone (n = 5) as most common site. Before inclusion, the median number of chemotherapy lines in the metastatic phase was 3 regimes consisting of anthracyclines in 75% and taxanes in 81%.
Treatment
Figure 1 shows a treatment overview of the 16 patients. All 16 patients underwent the 90Y procedure whereby pre-embolization of extra hepatic arteries was performed in 14/16 patients. A median dose of 1.68 GBq (range 1.043–2.140) was inserted in both liver lobes (n = 14) or one liver lobe (n = 2). At response evaluation five patients showed extra-hepatic disease progression, of whom, three patients were excluded for additional MMC infusion and two patients received the sequential MMC infusion, but with additional therapy of Exemestane (Aromasin®) for stabilization of their extra-hepatic disease. One patient was hospitalized after 90Y SIRT for 2.5 weeks because of the side effects of the 90Y treatment and excluded from further treatment.
Median time between 90Y SIRT and first MMC infusion was 2.1 months (range 1.7–12.8 months). In one patient an interval of 12.8 months occurred, because of PD in the liver after 90Y treatment. This patient first received systemic chemotherapy and after progression during this systemic chemotherapy the MMC infusion was administrated. A total of 12 patients were treated in four different cohorts of MMC doses after the 90Y treatment (Figure 1). In cohort D, the maximum of 6 cycles of 12 mg MMC was administrated in 2 patients. In the other four patients the dose was adjusted (n = 2) and discontinued (n = 2) due to hematologic toxicity (thrombocytopenia) and disease progression.
Adverse events
Table 3 shows the adverse events after the 90Y SIRT and MMC infusion. After the 90Y treatment one grade 3 adverse event occurred, consisted of a gastric ulcer treated with proton pump inhibitors and hospitalization. The most common grade 1/2 adverse events after 90Y SIRT were pain 7/16, nausea
Table 3.
Number of grade 1 (G1), grade 2 (G2) and grade 3 (G3) adverse events after treatment of selective internal radiation therapy by 90Y labeled microspheres infusion (90Y SIRT) and mitomycin C (MMC) infusion within 30 days. No grade 3 adverse events occurred after MMC infusion
| Treatment | 90Y SIRT | MMC Cohort A 1x6mg |
MMC Cohort B 1x12mg |
MMC Cohort C 2x12 mg |
MMC Cohort D max 6x12mg |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse events | G1 | G2 | G3 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 |
| Clinical | |||||||||||
| Fatigue | 2/16 | - | - | - | - | - | - | 3/3 | - | 2/6 | - |
| Pain | 5/16 | 2/16 | - | - | - | - | - | - | - | 1/6 | - |
| Nausea | 3/16 | 2/16 | - | - | - | - | - | - | - | 1/6 | - |
| Emesis | 2/16 | 2/16 | - | - | - | - | - | - | - | 1/6 | - |
| Gastrointestinal ulcer | - | 4/16 | 1/16 | - | - | - | - | - | - | - | - |
| Biochemical | |||||||||||
| Leukopenia | 2/16 | - | - | - | - | - | - | 2/3 | - | 4/6 | 2/6 |
| Thrombocytopenia | 3/16 | 1/16 | - | 1/2 | - | 1/1 | - | 3/3 | - | 4/6 | 2/6 |
| Anaemia | - | - | - | - | - | - | - | - | - | 4/6 | - |
| Increased aspartate aminotransferase | 5/16 | 1/16 | - | - | - | - | - | - | - | 2/6 | - |
| Increased alanine aminotransferase | 3/16 | 1/16 | - | - | - | - | - | - | - | 1/6 | - |
| Increased bilirubin | - | - | - | - | - | - | - | 1/3 | 1/3 | - | - |
| Increased alkaline phosphatase | 2/16 | 1/16 | - | - | - | - | - | 1/3 | - | 2/6 | - |
| Decreased eGFR | - | 1/16 | - | - | - | - | - | - | - | - | - |
| Increased gamma-glutamyl transferase | 4/16 | 1/16 | - | - | - | - | - | 1/3 | - | 4/6 | - |
eGFR = estimated glomerular filtration rate
5/16 and ulcer 4/16. An elevation in liver function test (aspartate, alanine and gamma-glutamyl transferase) was seen in 8/16 patients.
No grade 3 or higher adverse events occurred after MMC infusion. The most common grade 1/2 adverse event was thrombocytopenia in 11/12 patients and leukopenia in 8/12 patients.
Response, follow up and survival
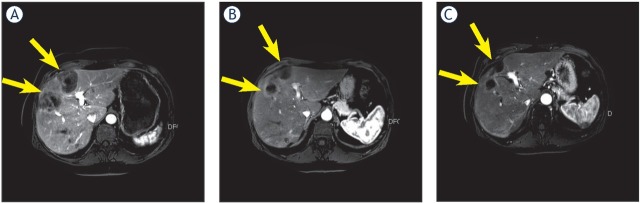
Response evaluation of the liver after 6 to 8 weeks after 90Y treatment (n = 16) revealed PR in 10 patients and SD in 6 patients (Figure 2). Extra hepatic response evaluation showed PD in 5 patients and SD in 11 patients. Additional hepatic response of MMC infusion after 90Y treatment was PR in 4/12 patient, SD in 5/12 patients and PD in 3/12 patients.
Figure 2.
Hepatic response on magnetic resonance imaging (MRI) before 90Y treatment (A), after 90Y treatment (B) and after 2 cycles of MMC infusion (C). C is a partial response relative to A. Arrows indicate the liver metastases.
After the study, nine patients were eligible to receive systemic chemotherapy and one patient was eligible for surgery and underwent a segmentectomy. The median OS was 12.6 months (95% CI 10.23–15.0).
Discussion
Intra-arterial therapy is a treatment option in patients with chemo-refractory liver dominant metastatic breast cancer.6 In our institute MMC infusions are performed for almost 20 years for chemo refractory LMBC whereby a disease control of 58% is obtained with low grade adverse events.9 In the present study we investigated whether MMC infusion is feasible and safe after 90Y SIRT.
The sequential treatment of 90Y followed by MMC was possible in 75% (12/16) of the enrolled patients. Only low-grade adverse events (grade 1 and 2) were observed after the MMC infusions. In addition, the gradual escalation of the MMC infusions, ranging from 6 mg in 1 cycle to a maximum of 72 mg in 6 cycles, caused no additional adverse events.
During the regular MMC protocol of our institute, patients can receive a maximum of 72 mg in 6 cycles whereby close monitoring is performed to adjust, postpone or stop the MMC infusion after each cycle.9 In current study, the same protocol was followed in the last cohort, subsequently 4 patients did not receive all 6 MMC cycles. Thus, this pilot shows that the regular protocol of MMC can be followed after prior treatment with 90Y. Patients maintained their performance status after the sequential treatments, and so 9/12 patients were eligible for systemic therapy afterwards and 1/12 even for hepatic surgery. Therefore, this combination treatment can be used as a drug holiday from systemic treatment.
We did observe a high number of gastrointestinal ulcers after 90Y SIRT (n = 5, 31%). All these patients underwent pre-embolization and received a bilobar treatment in the left and right hepatic artery. Despite these precautions, the ulcers occurred probably due to reflux and non-target deposition of the 90Y resin microspheres which is a known risk of 90Y treatment for LMBC.16 After the introduction of an anti-reflux catheter in the last 6 patients, no more ulcers occurred. According to the literature, several factors are associated with reflux of 90Y resulting in gastrointestinal ulcering, namely prior administration of monoclonal antibodies (i.e. bevacuzimab, trastuzumab), the type of 90Y micro-spheres and the dose of 90Y.10, 17 In the present study no correlations were found for prior monoclonal antibodies treatment and the dose of 90Y with the development of gastrointestinal ulcers. The development of the gastrointestinal ulcers in the current study was probably caused by several pretreatment and treatment related factors. The use of the anti-reflux catheter showed improvement with no further reported gastrointestinal ulceration. Therefore, caution is needed in future studies.
The median OS of radioembolization in LMBC patients ranges from 6.6–13.6 months, which is in line with the median OS of 12.6 in this study.10, 11,18, 19, 20 The median OS was higher compared to earlier studies where patients received only MMC infusion (12.6 versus 7.6 months).9,14 However, the inclusion criteria for this study were more strict than our routine indications for MMC infusion. Consequently, patients had less extensive local and extra hepatic disease with a better performance status compared to our previous reported cohort of patients treated with MMC infusion only.
The main limitation of this study consists of small patient number in the cohorts and the long inclusion period.
Conclusions
In conclusion, the sequential treatment of intra-arterial infusion of MMC after 90Y therapy is feasible with limited low-grade adverse events after MMC. The regular MMC protocol can be followed with adjustment of MMC dose based on clinical, radiological and biochemical parameters. However, caution is needed to prevent reflux after 90Y SIRT in LMBC patients. Further research is needed to show whether the combination of 90Y and MMC has a benefit over the treatment of MMC or 90Y alone in LMBC patients.
Disclosure
The institution of HW received consulting fees from Sirtex. GM is consult/has an advisory role for Sirtex. BA, EK, RD, CD, RBT, KP, PN declare no conflict of interest.
References
- 1.Eichbaum MHR, Kaltwasser M, Bruckner T, De Rossi TM, Schneeweiss A, Sohn C. Prognostic factors for patients with liver metastases from breast cancer. Breast Cancer Res Treat. 2006;96:53–62. doi: 10.1007/s10549-005-9039-1. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz A, Sebagh M, Wicherts DA, Castro-Benitez C, Van Hillegersberg R, Paule B. Long-term survival and cure model following liver resection for breast cancer metastases. Breast Cancer Res and Treat. 2018;170:89–100. doi: 10.1007/s10549-018-4714-1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golse N, Adam R. Liver metastases from breast cancer: what role for surgery? Indications and results. Clin Breast Cancer. 2017;17:256–65. doi: 10.1016/j.clbc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Ghersi D, Wilcken N, Simes RJ. A systematic review of taxane-containing regimens for metastatic breast cancer. Br J Cancer. 2005;93:293–301. doi: 10.1038/sj.bjc.6602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon AC, Uddin OM, Riaz A, Salem R, Lewandowski RJ. Making the case: intra-arterial therapy for less common metastases. Semin Intervent Radiol. 2017;34:132–9. doi: 10.1055/s-0037-1601852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smits ML, Prince JF, Rosenbaum CE, van den Hoven AF, Nijsen JF, Zonnenberg BA. Intra-arterial radioembolization of breast cancer liver metastases: a structured review. Eur J Pharmacol. 2013;709:37–42. doi: 10.1016/j.ejphar.2012.11.067. et al. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Zhang J, Ji S, Shao G, Zhao K, Wang Z. Transarterial chemoembolisation for breast cancer with liver metastasis: a systematic review. Breast. 2017;36:25–30. doi: 10.1016/j.breast.2017.09.001. et al. [DOI] [PubMed] [Google Scholar]
- 9.Aarts BM, Klompenhouwer EG, Dresen RC, Laenen A, Beets-Tan RGH, Punie K. Intra-arterial mitomycin C infusion in a large cohort of advanced liver metastatic breast cancer patients: safety, efficacy and factors influencing survival. Breast Cancer Res Treat. 2019;176:597–605. doi: 10.1007/s10549-019-05254-4. et al. [DOI] [PubMed] [Google Scholar]
- 10.Fendler WP, Lechner H, Todica A, Paprottka KJ, Paprottka PM, Jakobs TF. Safety, efficacy and prognostic factors after radioembolization of hepatic metastases from breast cancer: a large single center experience in 81 patients. J Nucl Med. 2016;57:517–23. doi: 10.2967/jnumed.115.165050. et al. [DOI] [PubMed] [Google Scholar]
- 11.Pieper CC, Meyer C, Wilhelm KE, Block W, Nadal J, Ahmadzadehfar H. Yttrium-90 radioembolization of advanced, unresectable breast cancer liver metastases-a single-center experience. J Vasc Interv Radiol. 2016;27:1305–15. doi: 10.1016/j.jvir.2016.05.028. et al. [DOI] [PubMed] [Google Scholar]
- 12.Gordon AC, Salem R, Lewandowski RJ. Yttrium-90 radioembolization for breast cancer liver metastases. J Vasc Interv Radiol. 2016;27:1316–9. doi: 10.1016/j.jvir.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Maleux G, Deroose C, Laenen A, Verslype C, Heye S, Haustermans K. Yttrium-90 radioembolization for the treatment of chemorefractory colorectal liver metastases: technical results, clinical outcome and factors potentially influencing survival. Acta Oncologica. 2016;55:486–95. doi: 10.3109/0284186X.2015.1101151. et al. [DOI] [PubMed] [Google Scholar]
- 14.Maes T, Wildiers H, Heye S, Demey W, Maleux G, Neven P. Intrahepatic mitomycin C bolus infusion in the treatment of extensive liver metastases of breast cancer. Breast Cancer Res Treat. 2008;110:135–42. doi: 10.1007/s10549-007-9707-4. et al. [DOI] [PubMed] [Google Scholar]
- 15.Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K. Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics. 2013;33:1323–41. doi: 10.1148/rg.335125214. [DOI] [PubMed] [Google Scholar]
- 16.Pieper CC, Willinek WA, Thomas D, Ahmadzadehfar H, Essler M, Nadal J. Incidence and risk factors of early arterial blood flow stasis during first radioembolization of primary and secondary liver malignancy using resin microspheres: an initial single-center analysis. Eur Radiology. 2016;26:2779–89. doi: 10.1007/s00330-015-4076-6. et al. [DOI] [PubMed] [Google Scholar]
- 17.Collins J, Salem R. Hepatic radioembolization complicated by gastrointestinal ulceration. Semin Intervent Radiol. 2011;28:240–5. doi: 10.1055/s-0031-1280673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena A, Kapoor J, Meteling B, Morris DL, Bester L. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol. 2014;21:1296–303. doi: 10.1245/s10434-013-3436-1. [DOI] [PubMed] [Google Scholar]
- 19.Bangash AK, Atassi B, Kaklamani V, Rhee TK, Yu M, Lewandowski RJ. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol. 2007;18:621–8. doi: 10.1016/j.jvir.2007.02.019. et al. [DOI] [PubMed] [Google Scholar]
- 20.Jakobs TF, Hoffmann RT, Fischer T, Stemmler HJ, Tatsch K, La Fougere C. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008;19:683–90. doi: 10.1016/j.jvir.2008.01.009. et al. [DOI] [PubMed] [Google Scholar]