Abstract
Background
Viral encephalitis is characterised by diverse clinical and epidemiological features. Seizures are an important clinical manifestation and are associated with increased mortality and morbidity. Patients may have seizures during the acute illness or they may develop after recovery. There are no recommendations regarding the use of antiepileptic drugs for the primary or secondary prevention of seizures in patients with viral encephalitis.
This is an updated version of the original Cochrane review published in The Cochrane Library 2014, Issue 10.
Objectives
To assess the efficacy and tolerability of antiepileptic drugs for the primary and secondary prophylaxis of seizures in viral encephalitis. We had intended to answer the following questions.
1. Do antiepileptic drugs used routinely as primary prophylaxis for all patients with suspected or proven viral encephalitis reduce the risk of seizures during the acute illness and reduce neurological morbidity and mortality?
2. Do antiepileptic drugs used routinely as secondary prophylaxis for all patients who have had at least one seizure due to suspected or proven viral encephalitis reduce the risk of further seizures during the acute illness and reduce neurological morbidity and mortality?
Search methods
For the latest version of this review, we searched the Cochrane Epilepsy Group Specialized Register (11 April 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 11 April 2016), MEDLINE (Ovid 1946 to 11 April 2016), the WHO International Clinical Trials Registry Platform (ICTRP, 11 April 2016), and ClinicalTrials.gov (11 April 2016). We did not impose any language restrictions.
Selection criteria
Randomised and quasi‐randomised controlled trials in which patients were assigned to a treatment or control group (placebo or no drug).
Data collection and analysis
One review author (SP) searched the publications by title, abstract and keywords, and decided on their suitability for the review. For any studies where their suitability was unclear, the co‐authors (CR, BM) were consulted. The co‐authors (CR, BM) independently evaluated the selected studies. Since there were no included studies, we carried out no data analysis.
Main results
We did not find any randomised or quasi‐randomised controlled trials that compared the effects of antiepileptic drugs with placebo (or no drug) for the primary or secondary prevention of seizures in viral encephalitis. We identified two studies from the literature search where different antiepileptic drugs were used in patients with viral encephalitis, however both failed to meet the inclusion criteria.
Authors' conclusions
There is insufficient evidence to support or refute the routine use of antiepileptic drugs for the primary or secondary prevention of seizures in viral encephalitis. There is a need for adequately powered randomised controlled trials in patients with viral encephalitis to assess the efficacy and tolerability of antiepileptic drugs for the primary and secondary prophylaxis of seizures, which is an important clinical problem.
Keywords: Humans; Primary Prevention; Secondary Prevention; Anticonvulsants; Anticonvulsants/therapeutic use; Encephalitis, Viral; Encephalitis, Viral/complications; Seizures; Seizures/etiology; Seizures/prevention & control
Plain language summary
Antiepileptic drugs for the primary and secondary prevention of seizures in viral encephalitis
Background Viral encephalitis is characterised by inflammation and swelling of the brain and is caused by viral infection. Seizures can occur both during viral encephalitis and as a later consequence following resolution of the infection. Patients who have seizures during encephalitis are more likely to die or have a disability; some may also develop prolonged or repeated seizures, which can be very difficult to treat. As not all patients will develop seizures, it is unclear whether the use of antiepileptic drugs in patients with viral encephalitis before they have seizures can prevent further seizures and improve their outcome. It is also not clear whether the use of these drugs after the first seizure can prevent the occurrence of further seizures and long‐term epilepsy.
Results We did not find any high‐quality clinical trials that assessed whether the use of antiepileptic drugs in patients with no seizures or one seizure is more effective than placebo in preventing seizures and improving the outcome in viral encephalitis.
Conclusions Further research is needed to assess the efficacy and tolerability of antiepileptic drugs for the primary and secondary prophylaxis of seizures.
The evidence is current to April 2016.
Background
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews (Pandey 2014).
Description of the condition
Viral encephalitis is a broad group of rare and potentially fatal central nervous system infections, which can be caused by many different viruses. Depending upon the underlying viral aetiology, it can occur in either a sporadic or epidemic manner, with the most common global causes being herpes simplex virus (HSV) and Japanese encephalitis virus (JEV), respectively (Michael 2012). The annual incidence of viral encephalitis has been reported as being between 3.5 and 7.4/100,000 patient‐years (Johnson 1996; Koskiniemi 1997), with a relatively higher incidence in children aged between 1 and 15 years (10.5/100,000 child‐years) and infants (18.4/100,000 child‐years; Granerod 2007). However, due to the low sensitivity of clinical and laboratory tests and the difficulty of obtaining virological confirmation for all patients, a viral aetiology is proven in only 30% to 60% of cases (Misra 2008).
Seizures are a common clinical manifestation of viral encephalitis; their frequency depends, in part, upon the underlying viral aetiology (Michael 2012). Seizures may occur during or after the acute illness. As a result of brain damage, a minority of patients do develop symptomatic epilepsy after their recovery, which can lead to significant morbidity. The incidence of seizures in the acute stage is high in encephalitis due to HSV (perhaps up to 50%), while reports vary from 7% to 46% in encephalitis due to JEV (Kalita 2003; Misra 2008). Status epilepticus has also been reported, and control of seizures in this group may be particularly difficult (Misra 2008). In one study of 30 patients with status epilepticus due to encephalitis, the seizures continued in eight patients even after the administration of a third antiepileptic drug, and nine patients died (Kalita 2008). In a prospective study of 144 patients with encephalitis due to JEV, a history of convulsions was present in 59 patients (41%; Solomon 2002). A poor outcome, defined as death or severe neurological disability, was reported in 24 of 40 patients (62%) with witnessed seizures, compared to 26 of 104 patients (14%) with unwitnessed seizures (odds ratio (OR) 4.50; 95% confidence interval (CI) 1.94 to 10.52; P < 0.0001). Moreover, patients with status epilepticus had a higher risk of mortality compared to those with other seizures (P = 0.003). In the same study, patients with seizures were more likely to have features of elevated intracranial pressure and brain herniation. In a retrospective study of 103 patients with acute encephalitis, 28 of whom had a viral aetiology, those with status epilepticus were found to have a significantly increased risk of death (Thakur 2013).
In a retrospective study of 45 children with encephalitis due to HSV, seizures occurred in 71% of 14 patients with a poor outcome, and in 56% of 26 patients with a good outcome (Hsieh 2007). Patients with acute encephalitis who develop status epilepticus and multifocal spikes on electroencephalography (EEG) may also have an increased risk of developing intractable epilepsy (Chen 2006). Following viral encephalitis, the risk of subsequent seizures is approximately 16 times that of the general population, and the risk may remain elevated for as long as 15 years following the acute episode. In one older study, patients who developed acute seizures during encephalitis had a 10% incidence of seizure by five years and a 22% incidence by 20 years, in comparison to 2% and 10%, respectively, in those without acute seizures (Annegers 1988). This is comparable to patients with severe head injury (Annegers 1980).
There are important predictors of early seizure in viral encephalitis, including younger age, lower level of consciousness, and cortical involvement on imaging (Misra 2008). High incidence of seizures in HSV encephalitis is thought to be mainly due to the involvement of the highly epileptogenic mesial temporal lobes, but it may also reflect other pathophysiological processes, such as haemorrhage, necrosis, and neuroimmunological processes. This may also partly explain the reportedly low incidence of seizures in encephalitis due to JEV. In Nipah encephalitis, early‐onset seizures have been reported in 24% of patients and late‐onset seizures in 50% of patients (Tan 2002). Late‐onset seizures in viral encephalitis may be due to cortical injury, which may possibly be greatest in the parietal and temporal lobes. In a retrospective study of seizure characteristics of patients with intractable epilepsy following encephalitis, it was found that the majority of these patients had neocortical foci (Marks 1992). In patients with La Crosse encephalitis, the incidence of later‐onset seizures is only 10% to 12% (Misra 2008). Therefore, there is a marked difference in the incidence of late‐onset seizures in different types of viral encephalitis.
Description of the intervention
Despite the high rate of seizures in some cases of viral encephalitis, and some evidence to suggest an association with poor outcome, there are no recommendations available regarding the use of antiepileptic drugs as primary or secondary prophylaxis, in patients with viral encephalitis (Michael 2012; Solomon 2012; Steiner 2010; Tunkel 2008). The majority of the current guidelines focus primarily on specific treatment for targeting the suspected or confirmed aetiology, with little emphasis on seizure management.
It is unclear why patients with viral encephalitis who develop seizures have a worse prognosis. It may be that the development of seizures is a proxy marker for those patients with the greatest brain injury, reflecting both viral cytopathy and neuroinflammatory processes. Alternatively, it may be that the seizures themselves cause additional brain damage, resulting in poorer outcomes, perhaps through excitotoxic injury, metabolic disturbances, cerebral oedema, elevated intracranial pressure, or metabolic disturbances, such as hypoxia or hypoglycaemia (Solomon 2002). If the latter is the case, then routine antiepileptic drug prophylaxis may potentially improve outcomes.
How the intervention might work
Theoretically, there is a case for primary and secondary prophylaxis with antiepileptic drugs in viral encephalitis. Antiepileptic drugs work by modifying different structures and processes involved in seizure development, such as ion channels, neurons, glia, and inhibitory and excitatory synapses. As seizures are associated with a worse outcome in viral encephalitis, reducing the frequency of seizures may improve the outcome. However, it remains unclear whether the prophylactic use of antiepileptic drugs can prevent the subsequent occurrence of seizures and influence immediate‐ and long‐term outcomes. Moreover, it is unclear which antiepileptic drugs should be used, and at what dosage. There is a relative lack of evidence‐based information on this subject, and it requires further study. Present treatment plans are based on clinical experience and on the data extrapolated from other acute neurological disorders.
Why it is important to do this review
This review intends to summarise the available information. For primary prevention, we aim to review whether the prophylactic administration of antiepileptic drugs in all patients with proven or suspected viral encephalitis is effective in preventing seizures, improving outcome, and reducing the risk of subsequent symptomatic epilepsy. For secondary prevention, we aim to review whether the use of antiepileptic drugs after a seizure in patients with proven or suspected viral encephalitis is effective in preventing further seizures, improving outcome and reducing the risk of subsequent symptomatic epilepsy. Using antiepileptic drugs for any indication carries a significant risk of side effects. Therefore, in order to inform treatment policy, we also need to know whether antiepileptic drugs do more harm than good. Moreover, blanket use of antiepileptic drugs may result in a worse overall outcome, as was identified when phenobarbital was used in children with cerebral malaria (Crawley 2000). Physicians are therefore not clear whether or not to treat a single seizure following viral encephalitis. Furthermore, intractable epilepsy following viral encephalitis often requires more than one antiepileptic drug. A favourable risk‐benefit ratio needs to be established before recommending the use of antiepileptic drugs for the primary prophylaxis of seizures in viral encephalitis. In addition, even if antiepileptic drug prophylaxis can improve outcomes, the best regimen and how long the antiepileptic drugs should be continued after the acute stage is unknown.
Objectives
To assess the efficacy and tolerability of antiepileptic drugs for the primary and secondary prophylaxis of seizures in viral encephalitis. We intended to answer the following questions.
1. Do antiepileptic drugs, used as routine primary prophylaxis for all patients with suspected or proven viral encephalitis, reduce the risk of seizures during the acute illness, and reduce neurological morbidity and mortality?
2. Do antiepileptic drugs, used as routine secondary prophylaxis for all patients who have had at least one seizure due to suspected or proven viral encephalitis, reduce the risk of further seizures during the acute illness, and reduce neurological morbidity and mortality?
Methods
Criteria for considering studies for this review
Types of studies
We considered all double‐blind, randomised and quasi‐randomised controlled trials in which patients were assigned to a 'treatment' or 'control' group (that is, placebo or no drug).
Types of participants
We used the World Health Organization (WHO) definition for viral encephalitis: "a person of any age, at any time of year, with an acute onset of fever and a change in mental status (including symptoms such as confusion, disorientation, coma, or inability to talk), a new onset of seizures (excluding simple febrile seizures), or both" (WHO 2006). Wehad planned to include studies in which the diagnosis of viral encephalitis was made using the Health Protection Agency criteria of "cerebrospinal fluid (CSF) examination documenting slightly raised protein levels, with a raised lymphocyte count (more than 5 but less than 500 X 10⁶ cells/L), and normal glucose levels, with the exception of mumps infection, advanced HSV, and lymphocytic choriomeningitis virus infection, where CSF glucose may be low (HPA 2011). We had planned to only include studies in which the diagnosis of viral aetiology was confirmed by methods such as polymerase chain reaction assays for HSV types 1 and 2, enteroviruses, varicella‐zoster, Epstein‐Barr virus, human herpes virus 6, cytomegalovirus, lymphocytic choriomeningitis and arboviruses (HPA 2011). We excluded studies in which patients had undergone a neurosurgical intervention for any indication. We considered all types of seizures, including simple and complex partial, with or without secondary generalisation.
Types of interventions
We had intended to include all trials where antiepileptic drugs were used in viral encephalitis and were compared with placebo or no treatment. We only considered drugs that appear in the list of antiepileptic drugs in the glossary section of the Cochrane Epilepsy Group module in The Cochrane Library (http://onlinelibrary.wiley.com/o/cochrane/clabout/articles/EPILEPSY/frame.html). We defined primary prophylaxis as the use of antiepileptic drugs to reduce the likelihood of seizures in patients who had viral encephalitis but had not had a seizure. We defined secondary prophylaxis as the use of antiepileptic drugs to reduce future seizures in patients with viral encephalitis who had had at least one seizure.
Types of outcome measures
We had intended to assess all primary and secondary outcomes in studies of both primary and secondary prophylaxis. We had also intended to perform an intention‐to‐treat (ITT) analysis.
Primary outcomes
Proportion of patients having a documented seizure during admission.
Average number of seizures per patient during admission.
Proportion of patients needing intensive care support for seizures during admission.
Change in outcome score from admission to discharge (Glasgow Outcome Scale score, Modified Rankin Scale score, Liverpool Outcome Score).
Proportion of patients remaining seizure‐free throughout the course of the follow‐up period.
Secondary outcomes
Proportion of patients who achieved seizure freedom at a defined follow‐up period after discharge.
Proportion of patients who achieved 50% seizure reduction in comparison to controls with acute encephalitis syndrome, who did not receive antiepileptic drugs during the acute period.
Proportion of patients who required one further antiepileptic drug at a defined follow‐up period after discharge.
Proportion of patients who required two further antiepileptic drugs over two years after discharge.
Average disability score at one year and two years after discharge.
Proportion of deaths after two years of discharge.
Quality of life as measured by a validated scale (e.g. SF‐36) at discharge and at one‐ and two‐year follow‐up.
Proportion of patients who experienced at least one side effect (skin rash, ataxia, cognitive or behavioural response, sedation, weight gain, sleep disturbance).
Any other adverse events or sequelae and tolerability.
Search methods for identification of studies
Electronic searches
Searches were run for the original review on 13 May 2014. For the latest update, we searched the following databases:
Cochrane Epilepsy Group Specialized Register (11 April 2016), using the search strategy outlined in Appendix 1;
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 11 April 2016), using the search strategy outlined in Appendix 2;
MEDLINE (Ovid, 1946 to 11 April 2016), using the search strategy outlined in Appendix 3;
World Health Organization International Clinical Trials Registry Platform search portal (ICTRP; searched on 11 April 2016), using the search string 'encephalitis AND seizure AND drug';
ClinicalTrials.gov (searched on 11 April 2016), using the search string 'encephalitis AND seizure AND drug'.
We did not impose any language restrictions.
Searching other resources
We checked the reference lists of the reports identified in our searches for additional reports of relevant studies. We contacted the authors and experts in the related field. We also searched conference proceedings (International Epilepsy Congress, European Congress on Epileptology and the American Epilepsy Society's Annual Meeting).
Data collection and analysis
Selection of studies
One review author (SP) searched the identified publications by title, abstract, and keywords, and decided on the suitability for inclusion in the review. For any studies where it was unclear whether they would be suitable for inclusion, the co‐authors (CR, BM) were consulted.
Two review authors (CR, BM) independently evaluated the full text of the selected studies. Three review authors (SP, CR, BM) independently assessed and discussed whether to include or exclude the studies and resolved any differences during mutual discussion.
Data extraction and management
We had planned to extract data on patient factors such as age, sex, seizure type(s), number of seizures prior to randomisation, presence of neurological deficits and signs at baseline, co‐morbidities, number and generic names of antiepileptic drugs, EEG and neuroimaging (computerised tomography (CT) or magnetic resonance imaging (MRI)) at baseline. We had planned to consider the following trial design aspects: sampling method, inclusion and exclusion criteria, method of diagnosis of encephalitis and epilepsy, method of randomisation, concealment of randomisation, blinding, matching drug aesthetics, stratification factors, treatment period, and description of withdrawals, drop‐outs and adverse events. See Appendix 4 for a full list of planned data extraction.
Measures of treatment effect
We did not include any studies in this review, so we were unable to calculate measures of treatment effect. We had planned to assess treatment effect for the primary and secondary outcomes by calculating and reporting the odds ratio (with 95% confidence interval) for binary outcomes and the Mann‐Whitney U test or t‐test for continuous non‐parametric and parametric data, respectively.
Dealing with missing data
The Cochrane Epilepsy Group has approached the corresponding author of one excluded study for the raw data to undertake a subgroup analysis, however, we have not yet received a reply (Chen 2011).
Data synthesis
We were unable to perform a meta‐analysis, as no studies were included in the review.
Results
Description of studies
Results of the search
Figure 1 summarises the results of the searches and the process of screening and selecting studies for inclusion in the review. We screened 36 publications identified by the searches. We identified three publications that appeared to be relevant to our review and obtained the full papers (Chen 2011; Huang 2007; Zhang 2009). Huang 2007 and Zhang 2009 appeared to report on the same study. Neither Chen 2011 nor Huang 2007 fulfilled our inclusion criteria, therefore, we excluded them (see Excluded studies).
1.
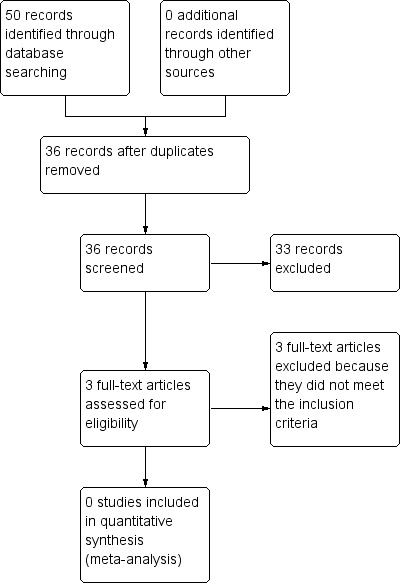
Study flow diagram.
Included studies
No studies are included in the review.
Excluded studies
We identified three publications of two studies, in which different antiepileptic drugs were used in patients with viral encephalitis. but none of them fulfilled our inclusion criteria and we excluded them (Chen 2011; Huang 2007a).
One study was an open‐label randomised controlled trial that reported on 67 adults with a clinical diagnosis of generalised convulsive status epilepticus, refractory to two doses of diazepam (Chen 2011). Patients were randomised to receive either more diazepam or valproate. Overall, there were no differences in the outcome between the two groups. Twenty‐two cases were due to viral encephalitis, although neither the process of diagnosing the encephalitis nor the aetiology were disclosed. Moreover, the outcome data for this group were not presented for subgroup analysis. The Cochrane Epilepsy Group has contacted the authors for these data, but to date have not received a response.
The second study reported on 96 children with viral encephalitis who were randomly allocated to the control (N = 40) or treatment groups (N = 56), however neither the method of randomisation nor blinding were described. It was also not clear from the publication whether all patients had viral encephalitis, what the aetiology was, how many received antiepileptic drugs as primary or secondary prophylaxis, or exactly which antiepileptic drugs were received by patients in each arm. There was also reference to undisclosed adjunctive therapies (Huang 2007).
Risk of bias in included studies
We had planned to use the following domains to assess the risk of bias:
• selection bias (sequence generation, allocation concealment)
• performance bias (blinding of study participants and personnel)
• detection bias
• reporting bias
• attrition bias
We had also planned to assess the potential impact of outcome reporting bias, by inputting an ORBIT table.
Since there were no included studies, we were unable to make any 'Risk of bias' assessments.
Effects of interventions
We did not include any studies in this review and therefore could not make an assessment of the effects of interventions.
Discussion
Summary of main results
The aim of this review was to assess the effects of antiepileptic drugs for the primary and secondary prophylaxis of seizures in viral encephalitis. No randomised or quasi‐randomised controlled trials with a placebo or no drug arm were identified.
We did identify two studies in which different antiepileptic drugs were used in patients with viral encephalitis; one in children, one in adults over 14 years. Although both studies reported they were randomised, there were no descriptions of the populations, interventions, randomisation processes, blinding, or co‐interventions. Neither study compared antiepileptic drugs against a placebo or no drug, which was the focus of our review.
Overall completeness and applicability of evidence
In the absence of any randomised or quasi‐randomised trials, no conclusions could be drawn regarding the overall completeness and applicability of the evidence.
Quality of the evidence
We identified no randomised or quasi‐randomised trials that either supported or refuted the use of antiepileptic drugs for the primary or secondary prevention of seizures in viral encephalitis.
Agreements and disagreements with other studies or reviews
Seizures are an important cause of mortality and morbidity in patients with viral encephalitis. Treatment of seizures in patients with viral encephalitis has been challenging and controversial (Michael 2012). There are currently no guidelines regarding the use of different antiepileptic drugs (Michael 2012; Solomon 2012). In a recent guideline published by the European Federation of Neurological Societies (EFNS), the only recommendation was to use phenytoin for the control of seizures in patients with viral encephalitis (Steiner 2010). It remains unclear whether antiepileptic drugs reduce the risk of seizures during the acute phase of the illness or decrease morbidity and mortality when used as primary prophylaxis. It is also unclear whether antiepileptic drugs reduce the risk of further seizures when used as secondary prophylaxis. Use of antiepileptic drugs carries an inherent risk of adverse events.
In the absence of any evidence from randomised or quasi‐randomised controlled trials, no recommendations can be made regarding the use of antiepileptic drugs as primary or secondary prophylaxis for seizures in patients with viral encephalitis.
Authors' conclusions
Implications for practice.
There is insufficient evidence to support or refute the use of antiepileptic drugs for the primary or secondary prophylaxis of seizures in patients with viral encephalitis.
Implications for research.
There is a need for well‐designed, randomised, double‐blind, placebo‐controlled trials of antiepileptic drugs as primary and secondary prophylaxis of seizures in viral encephalitis. Such studies should clearly establish the diagnosis and aetiology of viral encephalitis. Drug regimens should be clearly described and there should be adequate follow‐up, using established outcome measures. This research is desperately needed if we are to ascertain the efficacy and tolerability of antiepileptic drugs for the primary and secondary prophylaxis of seizures in viral encephalitis, to guide clinical practice in the treatment of this often devastating condition.
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2016 | New search has been performed | Searches updated 11 April 2016; no new trials identified. |
| 11 April 2016 | New citation required but conclusions have not changed | Conclusions remain the same. |
Acknowledgements
We thank the Cochrane Epilepsy Group for their continuous support. We also thank Mrs Tingting Liu, PhD student, Department of Clinical Infection, Microbiology and Immunology, Institute of Infection and Global Health, University of Liverpool, UK for translation of one of the publications from Chinese to English (Zhang 2009).
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 MeSH DESCRIPTOR Encephalitis, Viral Explode All
#2 (viral OR virus* OR virolog*) NEAR3 (encephaliti* OR meningoencephaliti*)
#3 (#1 OR #2) AND INREGISTER
Appendix 2. CENTRAL via CRSO search strategy
#1 MESH DESCRIPTOR Encephalitis, Viral EXPLODE ALL TREES WITH QUALIFIERS DT
#2 ((viral OR virus* OR virolog*) ADJ3 (encephaliti* OR meningoencephaliti*)):TI,AB,KY
#3 #1 OR #2
#4 MESH DESCRIPTOR Epilepsy EXPLODE ALL TREES WITH QUALIFIERS DT
#5 MESH DESCRIPTOR Anticonvulsants EXPLODE ALL TREES
#6 (antiepilep* or anti‐epilep* or anticonvuls* or anti‐convuls* or AED or AEDs):TI,AB,KY
#7 MESH DESCRIPTOR Midazolam EXPLODE ALL TREES
#8 MESH DESCRIPTOR Methazolamide EXPLODE ALL TREES
#9 MESH DESCRIPTOR Temazepam EXPLODE ALL TREES
#10 acetamide OR acetazolamid* OR azm OR diacarb OR diamox OR diazomid OR diluran OR glaupax
#11 barbexaclone
#12 beclamid*
#13 brivaracetam
#14 bromid*
#15 biston OR carbamazepin* OR carbatrol OR cbz OR epitol OR equetro OR neurotop OR tegretol OR teril OR timonil
#16 clobazam* OR frisium OR urbanyl
#17 antelepsin OR clonazepam* OR rivotril
#18 clorazepat* OR justum OR mendon OR trancap OR tranxene OR tranxilium
#19 diastat OR diazemuls OR diazepam* OR nervium OR relanium OR valium*
#20 divalproex*
#21 eslicarbazepin*
#22 ethosuximid* OR zarontin
#23 ethadion*
#24 felbamat* OR felbatol OR taloxa
#25 dilantin OR epanutin OR eptoin OR fenitoina OR phenytek OR phenytoin*
#26 gabapentin* OR neurontin
#27 ganaxolon*
#28 harkoseride OR lacosamid* OR vimpat
#29 epilepax OR lamictal OR lamotrigin*
#30 keppra OR levetiracetam* OR levitiracetam
#31 ativan OR lorazepam* OR lormetazepam OR temesta
#32 losigamon*
#33 Magnesium sulfat* or Magnesium sulphat*
#34 mephenytoin*
#35 mesuximid*
#36 luminal OR phenobarbit*
#37 dormicum OR dormire OR epistatus OR fulsed OR garen OR hypnovel OR ipnovel OR midazolam* OR nocturna OR setam OR terap OR versed
#38 methazolamid* OR neptazane
#39 nimetazepam
#40 arem OR benzalin OR gerson OR imeson OR mogadon OR nitrazepam OR radedorm
#41 ocbz OR oxcarbazepin* OR trileptal
#42 paraldehyde*
#43 paramethadion*
#44 e2007 OR fycompa OR perampanel*
#45 phenacemid*
#46 pheneturid*
#47 phensuximid*
#48 dilantin OR epanutin OR eptoin OR fenitoin* OR phenytek OR phenytoin*
#49 lyrica OR pregabalin*
#50 mysoline OR primidon* OR sertan
#51 progabid*
#52 remacemid*
#53 ezg OR ezogabin* OR retigabin* OR rtg
#54 rufinamid*
#55 seletracetam
#56 stiripentol
#57 sultiame OR sulthiame OR ospolot
#58 talampanel
#59 euhypnos OR norkotral OR restoril OR temazepam
#60 penthiobarbital OR pentothal OR thiopental* OR thiopentone OR tiopental OR tiopentale OR trapanal
#61 gabitril OR tiagabin*
#62 qudexy OR topamax OR topiramat* OR tpm
#63 trimethadion*
#64 valnoctamid*
#65 convulex OR depacon OR depakene OR depakine OR depakote OR dpa OR epilim OR epival OR stavzor OR valproate OR valproic OR vpa
#66 valpromid*
#67 gvg OR sabril OR vigabatrin
#68 zonegran OR zonisamid*
#69 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68
#70 #3 AND #69
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials published in Lefebvre 2011.
1. exp Encephalitis, Viral/dt [Drug Therapy]
2. ((viral or virus$ or virolog$) adj3 (encephaliti$ or meningoencephaliti$)).tw.
3. 1 or 2
4. exp *Epilepsy/dt [Drug Therapy]
5. exp Anticonvulsants/
6. exp Midazolam/
7. exp Methazolamide/
8. exp Temazepam/
9. (antiepilep$ or anticonvulsant$ or AED$1).tw.
10. (Acetazolamid$ or Aedon or Aethosuximide or Alodorm or Amizepin$ or Ant?lepsin or Anxirloc or Arem or Ativan or Atretol or Avugane).tw.
11. (Baceca or Barbexaclon$ or Beclamid$ or Biston or Bomathal or Brivaracetam or Bromid$).tw.
12. (Calepsin or Carbagen or Carbamazepen* or Carbamazepin$ or Carbatrol or Carbazepin* or Carbelan or Castilium or CBZ or Celontin or Cerebyx or Chlonazepam or Chloracon or C?lorepin or Chlormethiazole or Clarmyl or Cloazepam or Clobam$ or Clobator or Clobazam or Clofritis or Clonazepam$ or Clonex or Clonopin or Clopax or Clorazepate or Convulex).tw.
13. (Dapaz or Dasuen or Delepsine or Depacon or Depak$ or Depamide or Deproic or Desitin or Diacomit or Diamox or Diastat or Diazepam or Difenilhidantoin$ or Dihydantoin or Dilantin or Dimethadione or Dimethyloxazolidinedione or Diphenin$ or Diphenylan or Diphenylhydantoin$ or Distraneurin or Divalpr$ or Dormicum).tw.
14. (Ecovia or Emeside or Epanutin or Epiject or Epilex or Epilim or Episenta or Epitol or Epival or Eptoin or Equanil or Equetro or Ergenyl or Erimin or Erlosamide or Eslicarbazepine or Estazolam or Ethadione or Ethosucci$ or Ethosuxi$ or Ethotoin or Ethylphenacemide or Etosuxi$ or Euhypnos or Exalief or Excegran or Ezogabine).tw.
15. (Fanatrex or Felbam$ or Felbatol or Fenitoin$ or Fenobarbit$ or Finlepsin or Fosphenytoin or Frisium or Fycompa).tw.
16. (Gabapentin$ or Gabapetin$ or Gabarone or Gabitril or Gabrene or Ganaxolone or Garene or Gralise or Grifoclobam).tw.
17. (Halogabide or Halogenide or Harkoseride or Hibicon or Hydroxydiazepam or Hypnovel).tw.
18. (Iktorivil or Inovelon or Insoma or Intensl or Karbamazepin or Karidium or Keppra or Klonopin or Kriadex).tw.
19. (Lacosamide or Lamict$ or Lamitor or Lamitrin or Lamogine or Lamotrigine or Lamotrine or Landsen or Levanxol or Levetiracetam or Lexin or Liskantin or Loraz or Lorazepam or Losigamone or Lucium or Luminal or Lyrica).tw.
20. (Magnesium sulfat$ or Magnesium sulphat$ or Mebaral or Medazepam or Mephenytoin or Mephobarbit$ or Mephyltaletten or Meprobamate or Meprospan or Mesantoin or Mesuximide or Methazolamide or Methsuximide or Methylacetazolamide or Methyloxazepam or Methylphenobarbit$ or Midazolam or Miltown or Mogadon or Mylepsinum or Mylproin or Mysoline or Mystan).tw.
21. (Neogab or Neptazane or Nesdonal or Neurontin or Neurotol or Nimetazepam or Nitrados or Nitrazadon or Nitrazepam or Nobrium or Nocturne or Noiafren or Norkotral or Normison or Normitab or Nortem or Novo‐Clopate or Nuctalon or Nupentin or Nydrane).tw.
22. (OCBZ or Onfi or Orfiril or Orlept or Ormodon or Ospolot or Oxcarbamazepin$ or Oxcarbazepin$ or Oxydiazepam).tw.
23. (Pacisyn or Paraldehyde or Paramethadione or Paxadorm or Paxam or Peganone or Penthiobarbital or Pentothal or Perampanel or Petinutin or Petril or Phemiton or Phenacemide or Pheneturide or Phenobarbit$ or Phensuximide or Phenylethylbarbit$ or Phenylethylmalonylurea or Phenytek or Phenytoin$ or Planum or Posedrine or Potiga or Pregabalin or Primidone or Prodilantin or Progabide or Prominal or Pronervon or Prosom or Prysoline).tw.
24. (Ravotril or Remacemide or Remestan or Remnos or Resimatil or Restoril or Retigabine or Riluzole or Rilutek or Riv?tril or Rudotel or Rufinamide or Rusedal).tw.
25. (Sabril or Seclar or Sederlona or Selenica or Seletracetam or Sentil or Sertan or Sibelium or Signopam or Sirtal or Sodipental or Somnite or Stavzor or Stazepin$ or Stedesa or Stiripentol or Sulthiam$ or Sultiam$).tw.
26. (Talampanel or Taloxa or Tasedan or Tegret?l or Telesmin or Temaze or Temazep$ or Temesta or Temtabs or Tenox or Teril or Thiomebumal or Thionembutal or Thiopental or Thiopentobarbit$ or Thiopentone or Tiagabin$ or Tiletamine or Timonil or Tiobarbit$ or Tipiram$ or Topamax or Topiram$ or Tranmep or Tranxene or Trapanal or Tridione or Trileptal or Trimethadione or Trobalt).tw.
27. (Urbadan or Urban?l).tw.
28. (Valance or Valcote or Valium or Valnoctamide or Valparin or Valpro$ or Versed or Vigabatrin$ or Vimpat or Visano or VPA).tw.
29. (Xilep or Zalkote or Zarontin or Zebinix or Zonegran or Zonisamid$).tw.
30. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
32. clinical trials as topic.sh.
33. trial.ti.
34. 31 or 32 or 33
35. exp animals/ not humans.sh.
36. 34 not 35
37. 3 and 30 and 36
38. remove duplicates from 37
Appendix 4. Checklist of items considered in data collection or data extraction
Source
Eligibility
Methods
Participants
Interventions
Outcomes
For each outcome of interest:
Results
For each outcome of interest:
Miscellaneous
|
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2011 | No other data are presented for subgroup analysis comparing drug effectiveness between the treatment groups; longitudinal follow‐up and outcome scores are not provided |
| Huang 2007 | Study has many methodological flaws: it is not clear that all the patients had encephalitis and the aetiology is unknown; it is not clear what proportion were treated with primary or secondary prophylaxis; there is significant heterogeneity in the management and details are not presented; outcome is only significant with an arbitrary outcome score |
| Zhang 2009 | additional publication for Huang 2007; excluded for the same reasons |
Details of the excluded studies are provided in Table 1.
1. Excluded studies.
| Reference | Type of study | Type of participants | Types of intervention | Types of outcome measures | Conclusion |
| Huang 2007a | Randomisation and blinding methodology not disclosed | Only 88/96 had a CSF pleocytosis and no neuroimaging data are provided to establish the WHO criteria for encephalitis. Aetiology is unknown for all patients. 74 'acute' and 22 'sub‐acute' but no definitions given. All convulsive SE; unclear who was treated before/after development. Control (n = 40) or treatment (n = 56) | Control: chlorpromazine (Wintermin) and promethazine (Phenergan) (0.5 mg/kg intramuscular < 25 mg); 100 g/L chloral hydrate (0.5 mg/kg enema < 15 mL); phenobarbital (Luminal) (5 mg/kg intramuscular < 150 mg); diazepam (0.3 mg/kg intramuscular < 10 mg), alternately delivered with convulsions. Exact drugs received not described Intervention: 'large' doses (not specified) of chlorpromazine and promethazine to keep patient in 'lethargy' (not specified) for a few days (not specified). Some also received chloral hydrate, phenobarbital and diazepam every 4 to 6 hours according to their half‐life. The exact details of the distribution of these drugs between groups are not given. A blanket and unsupported statement that the "usage was the same as the control group" is included. Anticonvulsants were given for 2 days, even when no convulsions occurred |
None of our primary or secondary outcome measures is assessed | Study has many methodological flaws: it is not clear that all the patients had encephalitis and the aetiology is unknown; it is not clear what proportion were treated with primary or secondary prophylaxis; there is significant heterogeneity in the management and details are not presented; outcome is only significant with an arbitrary outcome score |
| Chen 2011 | Open‐label randomised controlled trial of secondary prophylaxis. Randomisation methodology not disclosed | Adults (> 14 years) with clinically diagnosed convulsive SE, who failed intravenous diazepam (0.2 mg/kg) twice with a 10‐minute interval. 121 screened, 67 failed diazepam, 1 dropped out. 36 in diazepam group and 30 in valproate group; 10 (28%) and 12 (40%) due to 'viral encephalitis'. Data to establish WHO criteria and aetiology are not provided | Group 1: 3rd bolus of diazepam (0.2 mg/kg, 5 mg/minute) then infusion (4 mg/hour; increased every 3 minutes by 1 µg/kg until seizures controlled or max < 1 hour) Group 2: sodium valproate bolus (intravenous 30 mg/kg, 6 mg/minute) then infusion (1 to 2 mg/kg/hour until seizures controlled, and > 6 hours) |
None of our primary or secondary outcomes are reported. EEG (> 6 hours), control of seizures defined by 2 electroencephalographers; blinding not stated. No significant difference in resolution of seizures < 1 hour or recurrence < 24 hours. Control in 'viral encephalitis' was lower 4 (18%) than for the other causes (x 2 = 18.089, P < 0.01, OR 0.009; 95% CI 0.026 to 0.329) | No other data are presented for subgroup analysis comparing drug effectiveness between the treatment groups; longitudinal follow‐up and outcome scores are not provided |
CI: confidence interval CSF: cerebrospinal fluid EEG: electroencephalography OR: odds ratio SE: status epilepticus WHO: World Health Organization
Contributions of authors
The first review author (SP) searched the retrieved publications by title, abstract and keywords, and decided their suitability for the review; where this was unclear, the other review authors (CR, BM) reviewed this. Two review authors (CR, BM) independently evaluated the selected studies. All review authors (SP, CR and BM) discussed the studies that appeared to meet the inclusion criteria and made a decision about included and excluded studies. All the authors (SP, CR, BM) jointly wrote the first draft and resolved disagreements by mutual discussion.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Dr Sanjay Pandey: none
Dr Chaturbhuj Rathore: none
Dr Benedict Michael is an NIHR Doctoral Research Fellow
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Chen 2011 {published data only}
- Chen WB, Gao R, Su YY, Zhao JW, Zhang YZ, Wang L, et al. Valproate versus diazepam for generalized convulsive status epilepticus: a pilot study. European Journal of Neurology 2011;18(12):1391‐6. [DOI] [PubMed] [Google Scholar]
Huang 2007 {published data only}
- Huang Y, Yang L, Wang S, Wang G. Alterative application of five anticonvulsants according to the half life for the treatment of status epilepticus in children with severe viral encephalitis. Neural Regeneration Research 2007;2(9):561‐4. [Google Scholar]
Zhang 2009 {published data only}
- Zhang SY, Huang YZ, Yang LB, Zhang SS, Wang ZG, Jiang Y. Evaluation of therapeutic effect of reformed subhibernation therapy for status epilepticus in children with severe viral encephalitis. Journal of Jilin University Medicine 2009;35(5):928‐31. [Google Scholar]
Additional references
Annegers 1980
- Annegers JF, Grabow JD, Groover RV, Laws ER, Elveback LR, Kurland LT. Seizures after head trauma: a population study. Neurology 1980;30:683‐9. [DOI] [PubMed] [Google Scholar]
Annegers 1988
- Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology 1988;38(9):1407‐10. [DOI] [PubMed] [Google Scholar]
Chen 2006
- Chen YJ, Fang PC, Chow JC. Clinical characteristics and prognostic factors of postencephalitic epilepsy in children. Journal of Child Neurology 2006;21(12):1047‐51. [DOI] [PubMed] [Google Scholar]
Crawley 2000
- Crawley J, Waruiru C, Mithwani S, Mwangi I, Watkins W, Ouma D, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet 2000;355(9205):701‐6. [DOI] [PubMed] [Google Scholar]
Granerod 2007
- Granerod J, Crowcroft NS. The epidemiology of acute encephalitis. Neuropsychological Rehabilitation 2007;17(4‐5):406‐28. [DOI] [PubMed] [Google Scholar]
HPA 2011
- Health Protection Agency. Investigation of Viral Encephalitis and Meningitis. UK Standards for Microbiology Investigations. G 4 Issue 2.2. 2011. Available from http://www.hpa.org.uk/SMI/pdf.
Hsieh 2007
- Hsieh WB, Chiu NC, Hu KC, Ho CS, Huang FY. Outcome of herpes simplex encephalitis in children. Journal of Microbiology, Immunology and Infection 2007;40:34‐8. [PubMed] [Google Scholar]
Johnson 1996
- Johnson RT. Acute encephalitis. Clinical Infectious Diseases 1996;23:219‐24. [DOI] [PubMed] [Google Scholar]
Kalita 2003
- Kalita J, Misra UK, Pandey S, Dhole TN. A comparison of clinical and radiological findings in adults and children with Japanese encephalitis. Archives of Neurology 2003;60:1760‐4. [DOI] [PubMed] [Google Scholar]
Kalita 2008
- Kalita J, Nair PP, Misra UK. Status epilepticus in encephalitis: a study of clinical findings, magnetic resonance imaging, and response to antiepileptic drugs. Journal of Neurovirology 2008;14(5):412‐7. [DOI] [PubMed] [Google Scholar]
Koskiniemi 1997
- Koskiniemi M, Korppi M, Mustonen K, Rantala H, Muttilainen M, Herrgård E, et al. Epidemiology of encephalitis in children. A prospective multicentre study. European Journal of Pediatrics 1997;156(7):541‐5. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org/.
Marks 1992
- Marks DA, Kim J, Spencer DD, Spencer SS. Characteristics of intractable seizures following meningitis and encephalitis. Neurology 1992;42:1513‐8. [DOI] [PubMed] [Google Scholar]
Michael 2012
- Michael BD, Solomon T. Seizures and encephalitis: clinical features, management, and potential pathophysiologic mechanisms. Epilepsia 2012;53 Suppl(4):63‐71. [DOI] [PubMed] [Google Scholar]
Misra 2008
- Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia 2008;49 Suppl 6:13‐8. [DOI] [PubMed] [Google Scholar]
Solomon 2002
- Solomon T, Dung NM, Kneen R, Thao le TT, Gainsborough M, Nisalak A, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain 2002;125(Pt 5):1084‐93. [DOI] [PubMed] [Google Scholar]
Solomon 2012
- Solomon T, Michael BD, Smith PE, Sanderson F, Davies NW, Hart IJ, et al. National Encephalitis Guidelines Development and Stakeholder Groups. Management of suspected viral encephalitis in adults: Association of British Neurologists and British Infection Association National Guideline. Journal of Infection 2012;64(4):347‐73. [DOI] [PubMed] [Google Scholar]
Steiner 2010
- Steiner I, Budka H, Chaudhuri A, Koskiniemi M, Sainio K, Salonen O, et al. Viral meningoencephalitis: a review of diagnostic methods and guidelines for management. European Journal of Neurology 2010;17:999‐e57. [DOI] [PubMed] [Google Scholar]
Tan 2002
- Tan CT, Goh KJ, Wong KT, Sarji SA, Chua KB, Chew NK, et al. Relapsed and late‐onset Nipah encephalitis. Annals of Neurology 2002;51:703‐8. [DOI] [PubMed] [Google Scholar]
Thakur 2013
- Thakur KT, Motta M, Asemota AO, Kirsch HL, Benavides DR, Schneider EB, et al. Predictors of outcome in acute encephalitis. Neurology 2013;81(9):793‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunkel 2008
- Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases 2008;47:303‐27. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. Recommended standards for surveillance of selected vaccine‐preventable diseases. Available from: http://www.who.int/vaccines‐documents/DocsPDF06/843.pdf. Geneva: World Health Organization, 2006. [Google Scholar]
References to other published versions of this review
Pandey 2012
- Pandey S, Rathore C, Michael B. Antiepileptic drugs for the primary and secondary prevention of seizures in viral encephalitis. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010247] [DOI] [Google Scholar]
Pandey 2014
- Pandey S, Rathore C, Michael BD. Antiepileptic drugs for the primary and secondary prevention of seizures in viral encephalitis. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD010247.pub2] [DOI] [PubMed] [Google Scholar]


