Abstract
High alpha-fetoprotein (AFP) > 1,000 ng/mL is associated with poor outcomes after liver transplantation (LT) for hepatocellular carcinoma (HCC). A new national policy has been implemented for AFP > 1,000 ng/mL requiring a decrease to < 500 ng/mL before LT, but there is a paucity of data on the optimal AFP threshold before LT. We aimed to evaluate the effects of a reduction in AFP from > 1,000 ng/mL to different AFP thresholds before LT on survival and HCC recurrence after LT using the United Network for Organ Sharing database. We identified 407 patients who underwent transplant between January 2005 and September 2015 and who had AFP > 1,000 ng/mL at least once before LT. The last AFP measurement before LT was > 1,000 ng/mL in 72.0%, decreased from > 1,000 to 101-499 ng/mL in 9.6%, and decreased to ≤ 100 ng/mL in 14.3%. Local-regional therapy was not performed in 45.4% of patients with AFP > 1,000 ng/mL at LT versus 12.8% of those with AFP of 101-499 ng/mL and 10.3% of those with AFP ≤ 100 ng/mL at LT (P < 0.001). Kaplan-Meier 5-year post-LT survival for those with AFP > 1,000 ng/mL at LT was 48.8% versus 67.0% for those with a decrease in AFP to 101-499 ng/mL (P < 0.001) and 88.4% for those with AFP ≤ 100 ng/mL at LT (P < 0.001). HCC recurrence probability at 5 years was 35.0% for patients with AFP > 1,000 ng/mL versus 13.3% for patients with AFP of 101-499 ng/mL and 7.2% for patients with AFP ≤ 100 ng/mL at LT (P < 0.001). In multivariable analysis, a decrease in the AFP to 101-499 ng/mL was associated with a > 2-fold reduction in posttransplant mortality (P = 0.01) and a nearly 3-fold reduction in HCC recurrence (P = 0.02) compared with AFP > 1,000 ng/mL at LT.
Conclusion:
Our results demonstrated significantly improved post-LT outcomes when restricting LT to patients with a reduction in AFP from > 1,000 to < 500 ng/mL, validating the recently implemented national policy.
Hepatocellular carcinoma (HCC) has emerged as the leading indication for liver transplantation (LT) and now accounts for more than 20% of all LT performed in the United States.(1,2) The Milan criteria (1 lesion ≤ 5 cm or 2-3 lesions ≤ 3 cm)(3) remain the gold standard for the selection of HCC candidates for LT despite mounting evidence that many factors predict post-LT outcomes besides tumor size and number.(4) In particular, elevated alpha-fetoprotein (AFP) is an important prognostic marker associated with the presence of microvascular invasion and worse tumor differentiation in the explant.(5–7) AFP levels as low as 16-20 ng/mL have been associated with worse post-LT outcomes compared with AFP levels below these cutoffs.(8,9) Although LT should be reserved for HCC patients who have a predicted 5-year survival comparable with non-HCC patients, using such a low AFP cutoff as a selection criterion for LT is not practical, as many HCC patients would need to be excluded from LT to prevent a single HCC recurrence.(7,10)
In contrast, several recent studies have demonstrated that AFP > 1,000 ng/mL among patients with HCC either within or beyond Milan criteria is associated with a very high risk of HCC recurrence and poor survival after LT.(6,7,11,12) In a large multi-center French study,(6) patients with AFP ≤ 100 ng/mL had 5-year post-LT survival of 68% compared with only 39% with AFP > 1,000 ng/mL. In a recent large European multicenter study, patients with AFP > 1,000 ng/mL only received a 7-month intention-to-treat survival benefit from transplant compared with 25 months for patients with AFP < 1,000 ng/mL.(12) Finally, in a study from the University of California, San Francisco (UCSF) of HCC patients meeting Milan criteria by imaging,(7) patients with AFP > 1,000 ng/mL had a 5-year recurrence-free survival of only 52%, versus 80% in those with an AFP ≤ 1,000. The authors determined that applying an AFP cutoff of > 1,000 ng/mL would result in exclusion of 5% of patients within Milan criteria from LT but allow for a 20% reduction in posttransplant HCC recurrence.(7)
Because post-LT survival among HCC patients with AFP > 1,000 ng/mL falls below a minimal acceptable threshold comparable with non-HCC patients, a new national policy has been implemented for those with an AFP > 1,000 ng/mL requiring a decrease to < 500 ng/mL with local-regional therapy (LRT) before LT can be undertaken.(13) Although this is clearly an important step forward, there is a paucity of data on the impact of reduction of AFP > 1,000 ng/mL to lower values on post-LT outcome. Additionally, the optimal AFP threshold before LT in this population is unknown. We therefore aimed to evaluate the effects of a reduction in AFP from > 1,000 ng/mL to different AFP thresholds before LT on survival and HCC recurrence after LT using the United Network for Organ Sharing (UNOS) database.
Patients and Methods
STUDY DESIGN AND PATIENT POPULATION
All patients in the UNOS database (Standard Transplant Analysis and Research files released in December 2016) aged 18 years and older with HCC who underwent LT between January 2005 and September 2015 who had at least one AFP value > 1,000 ng/mL while on the LT waiting list were included in this study. Of all HCC LT recipients during the study period, 3.8% met the study inclusion criteria based on AFP > ng/mL. Patients with radiographic tumor burden either within Milan criteria or UCSF down-staging criteria (1 lesion > 5 and ≤ 8 cm; 2-3 lesions, with at least one being > 3 cm and ≤ 5 cm and having total tumor diameter ≤ 8 cm; or 4-5 lesions each ≤ 3 cm with total tumor diameter ≤ 8 cm)(14) were included. Patients listed for a multiorgan transplant, those receiving a living donor, and those with either intrahepatic cholangiocarcinoma or mixed HCC/cholangiocarcinoma on explant were excluded from this study.
Study variables collected from the UNOS database included age, sex, race/ethnicity, cause of liver disease, Model for End Stage Liver Disease (MELD) and Child-Pugh score, radiographic size and number of HCC at initial listing with MELD exception and on the last imaging before LT, all available AFP measurements, the percentage of patients who underwent LRT, time from initial HCC MELD exception to LT, and the donor risk index (DRI). Per UNOS listing policy, patients underwent contrast-enhanced computed tomography or magnetic resonance imaging at a minimum of once every 3 months after LT listing. Explant pathology variables were collected for the subset of patients who had undergone transplant since April 2012, when UNOS began capturing HCC explant pathology data. These variables included histologic grade based on the modified Edmondson criteria (grade 1: well-differentiated; grade 2: moderately differentiated; and grade 3: poorly differentiated),(15) tumor stage, and presence of vascular invasion. Explant tumor staging was based on size and number of only viable tumors.
HCC RECURRENCE
To identify patients with post-LT HCC recurrence, liver malignancy follow-up data and cause of death variables underwent physician review (author N.M.). Records indicating posttransplant recurrence of pretransplant malignancy or a cause of death indicating HCC or metastatic malignancy were classified as having HCC recurrence.
OUTCOMES AND STATISTICAL ANALYSIS
The primary outcome studied was post-LT survival, and the secondary outcome was post-LT HCC recurrence. Clinical and tumor characteristics were summarized using medians and interquartile ranges (IQRs) for continuous variables and proportions for categorical variables. Characteristics were stratified by AFP at LT category (≤ 100, 101-499, 500-1,000, and > 1,000 ng/mL) and compared with Kruskal-Wallis and Pearson chi-squared tests, as appropriate. Observed post-LT survival probabilities and HCC recurrence and 95% confidence intervals (CIs) were estimated at 5 years using the Kaplan-Meier method and compared by AFP at LT category using the log-rank test. For post-LT survival, patient follow-up was measured from the date of LT to death (event), with patients remaining alive censored at the date of retransplant or last follow-up. For HCC recurrence, patient follow-up was measured from the date of LT to HCC recurrence (event), with patients dying without HCC recurrence or remaining alive censored at the date of non-HCC death, retransplant, or last follow-up. The association of explanatory variables was explored using univariate and multivariable hazard ratios (HRs) and 95% CIs estimated by Cox proportional hazards regression for post-LT survival and recurrence. Explanatory variables with a univariate P value < 0.1 were included in the multivariable analysis, with the final model selected by backward elimination (P for removal > 0.05). Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and Stata/IC 14.2 (StataCorp, College Station, TX).
Results
FORMATION OF STUDY COHORT BASED ON AFP AT LT CATEGORIES
Among the 440 patients who met the inclusion and exclusion criteria, 33 (7.5%) were subsequently excluded because of not having any available AFP measurements within 90 days of LT. The remaining 407 patients formed the final cohort (Fig. 1) and were grouped into categories based on the last AFP before LT. AFP at LT was > 1,000 ng/mL in 293 patients (72.0%), decreased from > 1,000 to 500-1,000 ng/mL in 17 patients (4.2%), decreased to 101-499 ng/mL in 39 patients (9.6%), and decreased to ≤ 100 ng/mL at LT in 58 patients (14.3%). Of the 293 patients who had an AFP at LT > 1,000 ng/mL, for 192 (65.5%) this represented their only available AFP measurement while awaiting LT, whereas 101 (34.5%) had two or more AFP measurements on the waitlist. Of this latter group with multiple AFP measurements, the first AFP value > 1,000 ng/mL for 41 patients was their AFP at LT (i.e., rising AFP).
FIG. 1.
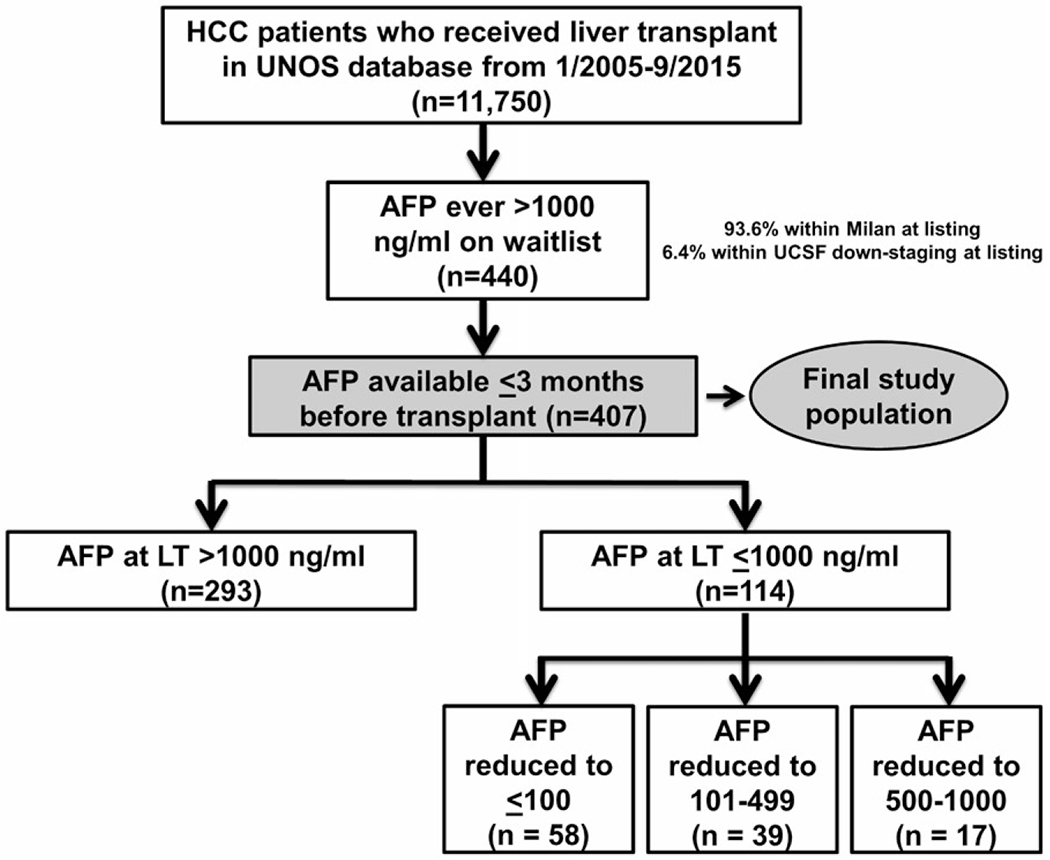
Flow diagram describing the formation of the study cohort stratified by AFP at transplant categories.
PATIENT CHARACTERISTICS
The baseline listing characteristics of the final study cohort stratified by AFP at LT category are summarized in Table 1. The median age at listing was 56 years (IQR: 52-61), and 70.5% were male. The most common races/ethnicities were Caucasian (58.5%), Asian (14.3%), Hispanic (12.8%), and Black (12.0%). Hepatitis C was the most common cause of liver disease (61.2%) followed by hepatitis B (10.3%). At the time of LT listing, the overall median MELD-Na score was 10 (IQR: 8-15), and 16.7% had Child C cirrhosis. The AFP at LT > 1,000 ng/mL group had the highest median MELD-Na (P = 0.004). Initial tumor stage was within Milan criteria in 93.6% and beyond Milan criteria but within the UCSF down-staging criteria in 6.4% and was similar between AFP at LT groups (P = 0.49).
TABLE 1.
Baseline Listing Characteristics of the Study Population by AFP at LT Category (n = 407)
| Overall (n = 407) | AFP > 1,000 → ≤ 100 ng/mL (n = 58) | AFP > 1,000 → 101-499 ng/mL (n = 39) | AFP > 1,000 → 500-1,000 ng/mL (n = 17) | AFP > 1,000 ng/mL (n = 293) | P Value | |
|---|---|---|---|---|---|---|
| Age (IQR) | 56 (52-61) | 60 (54-63) | 56 (47-61) | 57 (53-60) | 56 (52-60) | 0.01 |
| Male (%) | 290 (70.5) | 38 (65.5) | 22 (56.4) | 15 (88.2) | 215 (73.4) | 0.04 |
| Ethnicity (%) | 0.44 | |||||
| Caucasian | 238 (58.5) | 29 (50.0) | 24 (61.5) | 10 (58.8) | 175 (59.7) | |
| Asian | 58 (14.3) | 16 (27.6) | 5 (12.8) | 3 (17.6) | 34 (11.6) | |
| Hispanic | 52 (12.8) | 6 (10.3) | 4 (10.3) | 2 (11.8) | 40 (13.7) | |
| Black | 49 (12.0) | 6 (10.3) | 5 (12.8) | 1 (5.9) | 37 (12.6) | |
| Other | 10 (2.5) | 1 (1.7) | 1(2.6) | 1 (5.9) | 7 (2.4) | |
| Cause (%) | 0.009 | |||||
| HCV | 249 (61.2) | 31 (53.4) | 22 (56.4) | 14 (82.3) | 182 (62.1) | |
| HBV | 42 (10.3) | 12 (20.7) | 1 (2.6) | 1 (5.9) | 28 (9.6) | |
| Alcohol | 16 (3.9) | 0 | 1 (2.6) | 0 | 15 (5.1) | |
| Autoimmune* | 10 (2.5) | 0 | 4 (10.3) | 0 | 6 (2.0) | |
| NASH | 8 (2.0) | 1 (1.7) | 1 (2.6) | 1 (5.9) | 5 (1.7) | |
| Other | 82 (20.1) | 14 (24.1) | 10 (25.6) | 1 (5.9) | 57 (19.5) | |
| MELD-Na (IQR) | 10 (8-15) | 9 (7-11) | 9 (7-13) | 10 (8-15) | 11 (8-16) | 0.004 |
| Child class (%)† | 0.07 | |||||
| A | 149 (36.7) | 27 (46.6) | 18 (46.2) | 8 (47.1) | 96 (32.9) | |
| B | 189 (46.6) | 25 (43.1) | 19 (48.7) | 5 (29.4) | 140 (47.9) | |
| C | 68 (16.7) | 6 (10.3) | 2 (5.1) | 4 (23.5) | 56 (19.2) | |
| Initial tumor stage (%) | 0.49 | |||||
| Milan | 381 (93.6) | 56 (96.6) | 38 (97.4) | 16 (94.1) | 271 (92.5) | |
| UCSF down-staging‡ | 26 (6.4) | 2 (3.4) | 1 (2.6) | 1 (5.9) | 22 (7.5) | |
| Total tumor diameter (cm) (IQR) | 3.0 (2.3-4.2) | 2.7 (2.2-3.5) | 3.0 (2.2-4.4) | 2.3 (2.0-2.8) | 3.2 (2.3-4.3) | 0.03 |
Includes autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis.
n= 406.
One lesion > 5 and ≤ 8 cm; 2-3 lesions, with at least one being > 3 cm and ≤ 5 cm and having total tumor diameter ≤ 8 cm; or 4-5 lesions, each ≤ 3 cm with total tumor diameter ≤ 8 cm.
LRT, WAIT TIME, AND TRANSPLANT CHARACTERISTICS
Waitlist-related and transplant-related characteristics are shown in Table 2. LRT was not performed in 45.4% of patients with AFP > 1,000 ng/mL at LT versus 12.8% of those with AFP of 101-499 ng/mL and 10.3% for those with AFP decreased to ≤ 100 ng/mL at LT (P < 0.001). Median overall wait time from initial HCC MELD exception to LT was 2.7 months (IQR: 0.9-6.0). Wait time from initial exception to LT was 1.5 months in the AFP at LT > 1,000 ng/mL group compared with 6.3 months in the AFP 101-499 ng/mL group and 7.9 months in the AFP at LT ≤ 100 ng/mL group (P < 0.001). When further stratifying the AFP > 1,000 ng/mL at LT group, median time from initial exception to LT for those with only one available AFP measurement was 1 month versus 3.9 months in those with at least two AFP measurements > 1,000 ng/mL and 5.2 months in those with multiple AFP measurements, with the first AFP value > 1,000 ng/mL being their AFP at LT (P < 0.001).
TABLE 2.
Waitlist and Transplant Characteristics of the Study Population by AFP at LT Category
| Overall (n = 407) | AFP > 1,000 → ≤ 100 ng/mL (n = 58) | AFP > 1,000 → 101-499 ng/mL (n = 39) | AFP > 1,000 → 500-1,000 ng/mL (n = 17) | AFP > 1,000 ng/mL (n = 293) | P Value | |
|---|---|---|---|---|---|---|
| Received LRT (%) | 260 (63.9) | 52 (89.7) | 34 (87.2) | 14 (82.3) | 160 (54.6) | < 0.001 |
| MELD-Na at LT (IQR) | 13 (9-18) | 11 (9-20) | 11 (8-18) | 13 (10-16) | 13 (9-19) | 0.27 |
| AFP at LT (ng/mL) (IQR) | 1,478 (552-2,781) | 14 (4-55) | 236 (149-437) | 634 (552-746) | 2,051 (1,385-3,776) | < 0.001 |
| Radiographic tumor stage at LT (%) | 0.050 | |||||
| Milan | 386 (95.3) | 58 (100) | 39 (100) | 17 (100) | 272 (93.5) | |
| UCSF Down-staging* | 19 (4.9) | 0 | 0 | 0 | 19 (6.5) | |
| Radiographic tumor number at LT (%) | < 0.001 | |||||
| 0 lesions | 60 (14.8) | 27 (46.6) | 6 (15.4) | 3 (17.7) | 24 (8.3) | |
| 1 lesion | 266 (65.7) | 29 (50.0) | 29 (74.4) | 11 (64.7) | 197 (67.7) | |
| 2 lesions | 53 (13.1) | 1 (1.7) | 4 (10.3) | 2 (11.8) | 46 (15.8) | |
| 3 lesions | 25 (6.2) | 1 (1.7) | 0 | 1 (5.9) | 23 (7.9) | |
| 4 lesions | 1 (0.2) | 0 | 0 | 0 | 1 (0.3) | |
| Total tumor diameter (cm) (IQR) | 3.0 (2.0-4.0) | 1.2 (0.0-2.2) | 2.6 (1.1-3.5) | 1.8 (1.0-2.9) | 3.3 (2.4-4.3) | < 0.001 |
| Time from initial HCC exception to LT (months) (IQR) | 2.9 (1.0-6.5) | 7.8 (5.1-12.6) | 6.3 (4.0-8.6) | 6.6 (5.6-10.6) | 1.5 (0.6-3.4) | < 0.001 |
| DRI (IQR) | 1.44 (1.15-1.72) | 1.41 (1.14-1.70) | 1.38 (1.10-1.77) | 1.64 (1.42-1.80) | 1.44 (1.16-1.72) | 0.27 |
One lesion > 5 and ≤ 8 cm; 2-3 lesions, with at least one being > 3 cm and ≤ 5 cm and having total tumor diameter ≤ 8 cm; or 4-5 lesions, each ≤ 3 cm with total tumor diameter ≤ 8 cm.
On the last reported imaging study before LT, 95.1% were within Milan criteria and 4.9% were within UCSF down-staging criteria. Patients in the AFP at LT > 1,000 ng/mL group were more likely to have radiographic tumor stage beyond Milan criteria (P = 0.04), had more lesions (P < 0.001), and had increased total tumor diameter (P < 0.001) on last imaging compared with the AFP at LT of 101-499 ng/mL and ≤ 100 ng/mL groups. In terms of complete radiographic response to LRT, 46.6% of patients in the AFP at LT ≤ 100 group had no remaining enhancing lesions on last imaging compared with 15.4% in the AFP 101-499 ng/mL group and 8.3% in the AFP > 1,000 ng/mL group. Median DRI was 1.44 (IQR: 1.15-1.72) and was similar among groups (P = 0.27).
Explant pathology data were available in the UNOS database in patients who underwent LT beginning in April 2012 (n = 65). Compared with patients with AFP at LT ≤ 100 ng/mL, patients in the AFP at LT > ng/mL group were less likely to have complete tumor necrosis on explant (10.0% vs. 38.9%, P = 0.03). AFP at LT > 1,000 ng/mL patients also had a numerically higher proportion with explant tumor burden beyond Milan criteria (27.5% vs. 11.1% for AFP ≤ 100 ng/mL) and microvascular invasion (17.5% vs. 5.6% for AFP ≤ 100 ng/mL), although these differences were not statistically significant (P Values > 0.2).
POSTTRANSPLANT SURVIVAL
Median post-LT follow-up time was 3.5 years (IQR: 1.2-7.0), and 170 patients (43.6%) died during the follow-up period. Overall post-LT survival was 84.8% (95% CI: 80.8-88.0) at 1 year and 56.9% (51.5-62.0) at 5 years. The Kaplan-Meier 5-year post-LT survival for those with AFP > 1,000 ng/mL at LT was 48.8% versus 67.0% for those with decrease in AFP to 101-499 ng/mL (P < 0.001) and 88.4% for those with decrease in AFP to ≤ 100 ng/mL before LT (P < 0.001) (Fig. 2A). The difference in survival between the groups with AFP of 101-499 ng/mL and AFP ≤ 100 ng/mL at LT was not statistically significant (P = 0.09). Median time to death in the AFP at LT > 1,000 ng/mL group was 1.4 years (IQR: 0.6-2.7) compared with 3.0 years (1.6-4.3) in the AFP 101-499 ng/mL group and 4.9 years (2.0-6.1) in the AFP ≤ 100 ng/mL group (P < 0.001). When further stratifying the AFP > 1,000 ng/mL at LT group, 5-year post-LT survival for those with only one available AFP measurement was 49.8% versus 43.8% in those with at least two AFP measurements > 1,000 ng/mL and 51.8% in those with multiple AFP measurements, with the first AFP value > 1,000 ng/mL being their AFP at LT (all P Values > 0.3) (Fig. 2B).
FIG. 2.
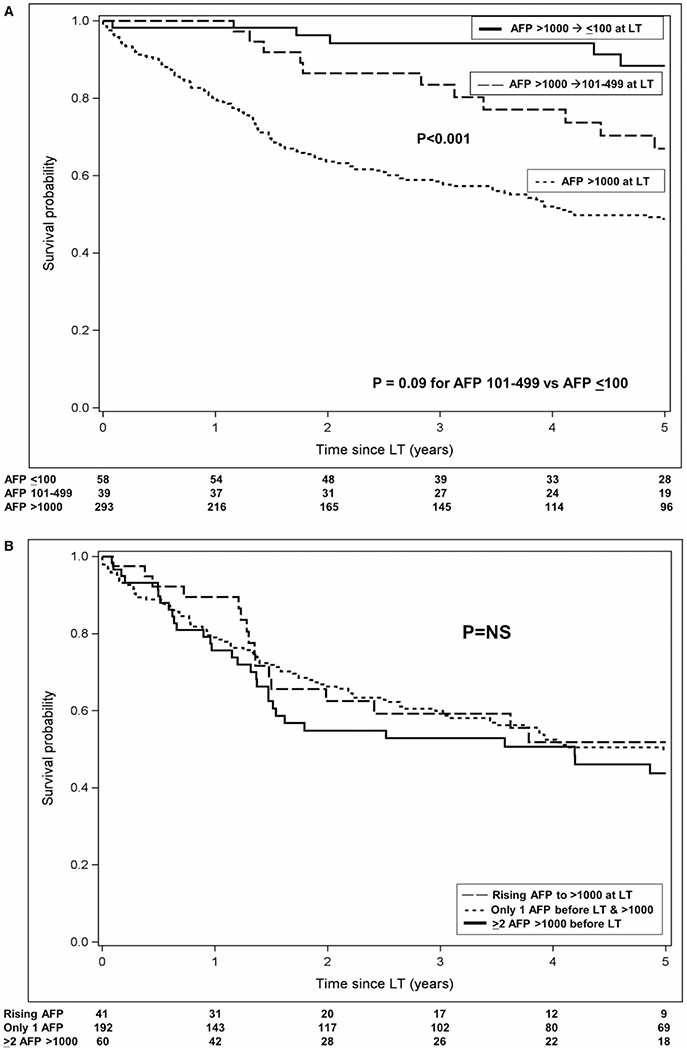
Observed post-LT survival probability (A) stratified by AFP at transplant category and (B) restricted to those with AFP at transplant > 1,000 ng/mL.
Pretransplant predictors of post-LT death in univariate analysis included higher MELD-Na, Child class C (vs. A), and higher DRI. Asian race/ethnicity, longer time from initial MELD exception to LT, and AFP at LT ≤ 100 ng/mL and 101-499 ng/mL were significant predictors of improved post-LT survival. AFP at LT of 500-1,000 ng/mL, age, sex, cause of liver disease, radiographic tumor stage or number at listing or at LT, and receipt of LRT were not significantly associated with post-LT survival on univariate analysis. In multivariable analysis, a decrease in the AFP at LT to 101-499 ng/mL was associated with a > 2-fold reduction in posttransplant mortality (HR: 0.47, 95% CI: 0.26-0.84, P = 0.01), whereas a decrease in AFP at LT to ≤ 100 ng/mL was associated with a nearly 4-fold reduction in post-LT mortality (HR: 0.27, 95% CI: 0.14-0.51, P < 0.001) compared with AFP at LT > 1,000 ng/mL. Higher DRI (HR: 1.47 per point, 95% CI: 1.02-2.13, P = 0.04) was associated with worse post-LT survival (Table 3).
TABLE 3.
Univariate and Multivariable Analyses of Predictors of Post-LT Death
| Predictor | HR (95% CI) | P Value |
|---|---|---|
| Univariate Analysis | ||
| AFP at LT group (ng/mL) | ||
| ≤ 100 (vs. > 1,000) | 0.27 (0.14-0.51) | <0.001 |
| 101-499 (vs. > 1,000) | 0.46 (0.26-0.84) | 0.01 |
| 500-1,000 (vs. > 1,000) | 0.54 (0.22-1.32) | 0.18 |
| Age (per year) | 1.02 (1.00-1.04) | 0.10 |
| Female (vs. male) | 0.99 (0.72-1.36) | 0.94 |
| Race/ethnicity | ||
| Asian (vs. Caucasian) | 0.55 (0.33-0.91) | 0.02 |
| Hispanic (vs. Caucasian) | 1.03 (0.66-1.62) | 0.89 |
| Black (vs. Caucasian) | 1.09 (0.70-1.70) | 0.70 |
| Cause of liver disease | ||
| Hepatitis B (vs. hepatitis C) | 0.56 (0.31-1.01) | 0.06 |
| Alcohol (vs. hepatitis C) | 1.49 (0.78-2.85) | 0.23 |
| NASH (vs. hepatitis C) | 0.24 (0.03-1.71) | 0.15 |
| Autoimmune (vs. hepatitis C) | 0.45 (0.14-1.42) | 0.17 |
| MELD-Na score (per point) | ||
| Listing | 1.03 (1.00-1.06) | 0.03 |
| LT | 1.03 (1.01-1.05) | 0.01 |
| Child class | ||
| Child C at listing (vs. Child A) | 1.87 (1.23-2.85) | 0.004 |
| Child B at listing (vs. Child A) | 1.27 (0.90-1.78) | 0.17 |
| Child C at LT (vs. Child A) | 1.65 (1.09-2.51) | 0.02 |
| Child B at LT (vs. Child A) | 1.49 (1.01-2.18) | 0.04 |
| Radiographic tumor stage | ||
| UCSF down-staging (vs. Milan) (initial) | 0.55 (0.26-1.17) | 0.12 |
| UCSF down-staging (vs. Milan) at LT | 1.22 (0.64-2.31) | 0.54 |
| Radiographic tumor number (per additional lesion) | ||
| At listing | 0.84 (0.67-1.06) | 0.14 |
| At LT | 1.02 (0.82-1.25) | 0.88 |
| Received LRT | 0.87 (0.64-1.18) | 0.38 |
| Time from initial HCC MELD exception to LT (per month) | 0.95 (0.92-0.99) | 0.01 |
| DRI (per point) | 1.43 (1.01-2.04) | 0.04 |
| Multivariable Analysis* | ||
| AFP at LT ≤ 100 ng/mL (vs. > 1,000 ng/mL) | 0.27 (0.14-0.51) | ≤0.001 |
| AFP at LT 101-499 ng/mL (vs. > 1,000 ng/mL) | 0.47 (0.26-0.84) | 0.01 |
| DRI (per point) | 1.47 (1.02-2.13) | 0.04 |
Variables removed by backward elimination: race/ethnicity (P = 0.71), cause of liver disease (P = 0.28), Child-Pugh class (P = 0.06), and time from initial exception to LT (P = 0.47).
POST-LT HCC RECURRENCE
HCC recurrence occurred in 23.6% at a median of 1.1 years (IQR: 0.5-1.8) from LT. Time to recurrence was similar among AFP at LT groups (P = 0.06) but was numerically longest in the AFP ≤ 100 ng/mL group at 1.7 years. Overall post-LT recurrence was 12.2% (95% CI: 9.2-16.0) at 1 year and 27.0% (22.5-32.3) at 5 years. The probability of HCC recurrence at 5 years was 35.0% for patients with AFP at LT > 1,000 ng/mL versus 13.3% for those with decrease in the AFP to 101-499 ng/mL (P < 0.001) and 7.2% for those with a decrease in AFP to ≤ 100 ng/mL (P < 0.001) (Fig. 3A). The difference in HCC recurrence rates between the latter two groups was not statistically significant (P = 0.38). When further stratifying the AFP > 1,000 ng/mL at LT group, 5-year post-LT recurrence for those with only one available AFP measurement was 33.8%, versus 40.3% in those with a rising AFP (P = 0.74) (Fig. 3B).
FIG. 3.
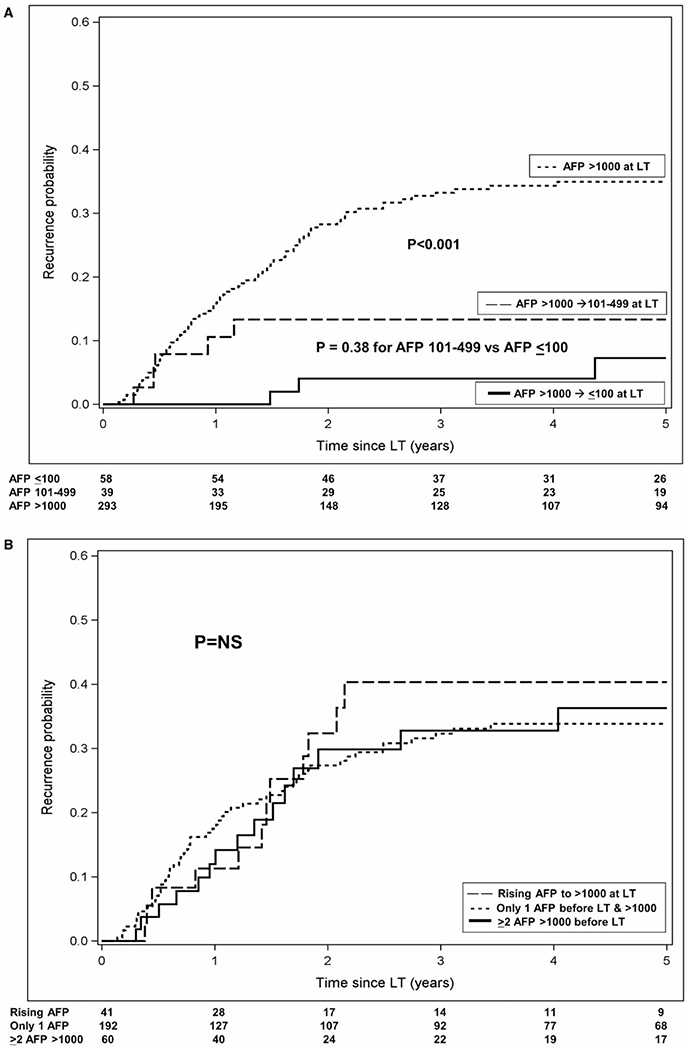
Observed post-LT HCC recurrence probability (A) stratified by AFP at transplant category and (B) restricted to those with AFP at transplant > 1,000 ng/mL.
The results of univariate and multivariable analysis of predictors of post-LT HCC recurrence are summarized in Table 4. Pretransplant predictors of post-LT recurrence in univariate analysis included radiographic tumor stage within UCSF down-staging criteria at LT (vs. Milan), alcoholic liver disease, and Child class B (vs. A). AFP at LT groups ≤ 100 ng/mL and 101-499 ng/mL and increasing time from initial MELD exception to LT were significant predictors of decreased post-LT recurrence. AFP at LT of 500-1,000 ng/mL, age, sex, race/ethnicity, MELD-Na score, radiographic tumor number at listing or LT, receipt of LRT, and DRI were not predictive of post-LT recurrence on univariate analysis. In multivariable analysis, compared with AFP > 1,000 ng/mL at LT, a decrease in the AFP to 101-499 ng/mL was associated with a nearly 3-fold reduction in HCC recurrence (HR: 0.35, 95% CI: 0.14-0.86, P = 0.02), with an even lower risk of recurrence predicted with a decrease in the AFP to ≤ 100 ng/mL (HR: 0.14, 95% CI: 0.04-0.43, P = 0.001).
TABLE 4.
Univariate and Multivariable Analyses of Predictors of Post-LT HCC Recurrence
| Predictor | HR (95% CI) | P Value |
|---|---|---|
| Univariate Analysis | ||
| AFP at LT group (ng/mL) | ||
| ≤ 100 (vs. > 1,000) | 0.14 (0.04-0.43) | 0.001 |
| 101-499 (vs. > 1,000) | 0.35 (0.14-0.86) | 0.02 |
| 500-1,000 (vs. > 1,000) | 0.18 (0.03-1.27) | 0.09 |
| Age (per year) | 1.02 (0.99-1.05) | 0.19 |
| Female (vs. male) | 0.75 (0.47-1.21) | 0.24 |
| Race/ethnicity | ||
| Asian (vs. Caucasian) | 0.61 (0.31-1.17) | 0.14 |
| Hispanic (vs. Caucasian) | 1.10 (0.61-1.96) | 0.76 |
| Black (vs. Caucasian) | 0.66 (0.32-1.39) | 0.27 |
| Cause of liver disease | ||
| Hepatitis B (vs. hepatitis C) | 0.51 (0.22-1.17) | 0.11 |
| Alcohol (vs. hepatitis C) | 2.50 (1.24-5.03) | 0.01 |
| NASH (vs. hepatitis C) | 0.49 (0.07-3.56) | 0.48 |
| Autoimmune (vs. hepatitis C) | 0.29 (0.04-2.07) | 0.22 |
| MELD-Na Score (per point) | ||
| At listing | 1.01 (0.97-1.05) | 0.56 |
| At LT | 1.01 (0.98-1.04) | 0.48 |
| Child class | ||
| Child C at listing (vs. Child A) | 1.39 (0.76-2.53) | 0.29 |
| Child B at listing (vs. Child A) | 1.16 (0.73-1.82) | 0.53 |
| Child C at LT (vs. Child A) | 1.67 (0.93-3.00) | 0.09 |
| Child B at LT (vs. Child A) | 1.73 (1.02-2.95) | 0.04 |
| Radiographic tumor stage | ||
| UCSF down-staging (vs. Milan) (initial) | 0.92 (0.40-2.11) | 0.85 |
| UCSF down-staging (vs. Milan) at LT | 2.11 (1.06-4.20) | 0.03 |
| Radiographic tumor number (per additional lesion) | ||
| At listing | 0.89 (0.66-1.21) | 0.47 |
| At LT | 0.98 (0.74-1.30) | 0.89 |
| Received LRT | 0.77 (0.51-1.17) | 0.22 |
| Time from initial HCC MELD exception to LT (per month) | 0.92 (0.87-0.98) | 0.005 |
| DRI (per point) | 1.32 (0.81-2.17) | 0.27 |
| Multivariable Analysis* | ||
| AFP at LT ≤ 100 ng/mL (vs. > 1,000 ng/mL) | 0.14 (0.04-0.43) | 0.001 |
| AFP at LT 101-499 ng/mL (vs. > 1,000 ng/mL) | 0.35 (0.14-0.86) | 0.02 |
Variables removed by backward elimination: cause of liver disease (P = 0.06), UCSF down-staging vs. Milan at LT (P = 0.27), Child-Pugh class (P = 0.08), and time from initial exception to LT (P = 0.71).
HIGHEST EVER AFP
While awaiting LT, nearly half (49.4%) of the study cohort had a peak AFP of 1,001-1,999 ng/mL, whereas 18.2% had a peak AFP of > 5,000 ng/mL. There were no significant differences observed in post-LT survival by “highest ever AFP” categories (1,001-1,999, 2,000-2,999, 3,000-3,999, 4,000-4,999, and ≥ 5,000 ng/mL). For example, probability of post-LT survival at 3 years was 71.0% (95% CI: 63.8-77.0) for those with a highest ever AFP of 1,001-1,999 ng/mL compared with 76.0% (57.6-87.2) for those with a peak AFP of 3,000-3,999 ng/mL and 59.7% (47.4-70.0) for peak AFP ≥ 5,000 ng/mL (Fig. 4A). Similarly, there were also no significant differences observed in the probability of post-LT HCC recurrence using the same highest ever AFP categories (Fig. 4B).
FIG. 4.
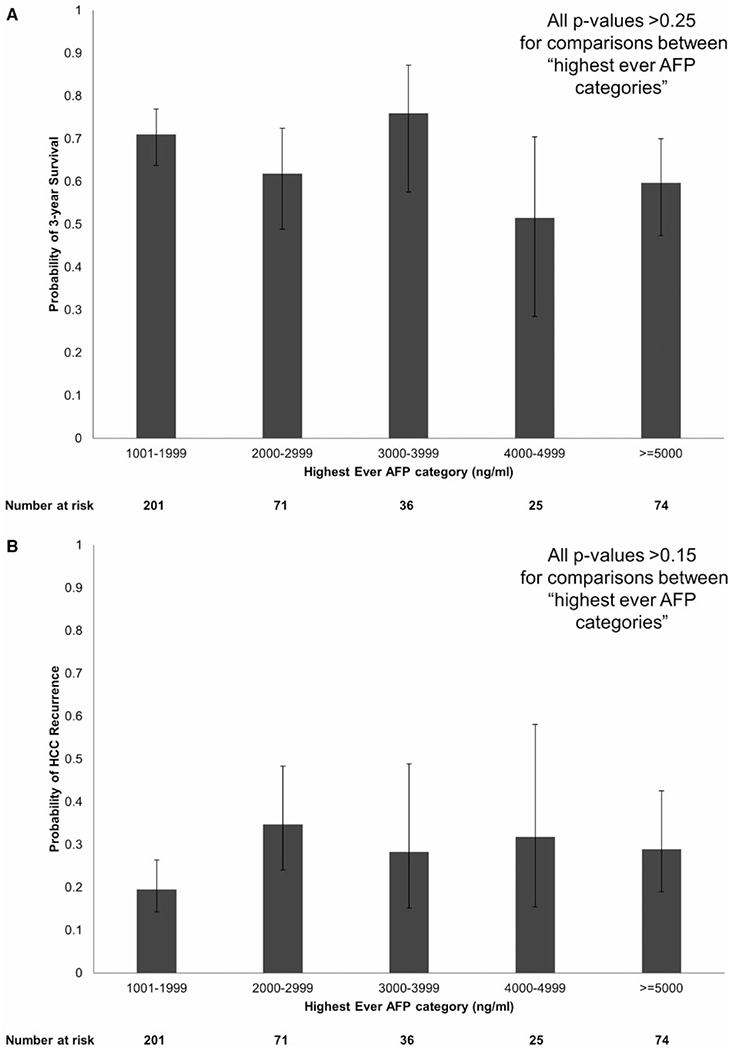
Probability of (A) 3-year post-LT survival and (B) HCC recurrence stratified by highest ever AFP categories.
Discussion
HCC is now a leading indication for LT in the United States,(1) and given the ongoing organ shortages, improving patient selection to maximize transplant survival benefit has become critically important. Rather than relying on tumor burden alone, many have advocated using markers of tumor biology to refine selection criteria.(4,16) The Extended Toronto Criteria mandate tumor biopsy to exclude those with poorly differentiated tumor grade from LT consideration.(17) The National Cancer Center Korea Criteria(18) and Kyoto extended criteria(19) use [18F] fluorodeoxyglucose positron emission tomography scan and des-gamma-carboxy prothrombin, respectively, as part of their selection criteria. Response to LRT has garnered significant interest for those both within and beyond Milan criteria. Although successful tumor down-staging can select patients with more favorable tumor biology who are likely to do well after LT,(14,20,21) HCC patients with progressive disease after LRT have poor survival(22–24) and a greater risk for post-LT HCC recurrence.(25) Nevertheless, radiographic response to LRT(26) may be subjective and difficult to implement as a standardized criterion on a broad scale. If LT centers are already inaccurately reporting tumor size for transplant listing purposes,(27) reporting biases in terms of response to LRT will undoubtedly emerge as well.
A plethora of studies have demonstrated AFP to be an important prognostic marker in LT for HCC. Several studies have suggested particularly poor post-LT outcome in patients with AFP > 1,000 ng/mL going into LT,(6,7) although these studies are limited by relatively small numbers of patients in this subset. A new national policy has recently been implemented in which patients with HCC and AFP > ng/mL are required to show a decrease in AFP to < 500 ng/mL with LRT before they can proceed with LT.(13) Despite this recent policy change, little is known about the impact of reduction of AFP > 1,000 ng/mL to lower thresholds on post-LT outcome.
In this largest study to date involving 407 patients with at least one AFP > 1,000 ng/mL while on the LT waiting list, we have confirmed poor post-LT outcome in nearly 300 HCC patients with the last AFP > 1,000 ng/mL within 3 months before LT, with observed 5-year post-LT survival of 49% and post-LT HCC recurrence probability of 35%. Interestingly, within this subgroup with AFP > 1,000 ng/mL at the time of LT, we did not observe significant differences in post-LT survival based on whether patients on the waitlist had only one AFP measurement versus multiple AFP measurements or if they had a rising AFP to > 1,000 ng/mL at LT. Importantly, a significant decrease in AFP from > 1,000 to 101-499 ng/mL before LT was associated with a greater than 2-fold reduction in post-LT mortality and an almost 3-fold reduction in HCC recurrence, thus validating the recently implemented national policy. The benefits of AFP reduction on post-LT outcomes appeared even greater in the patients with a decrease in AFP from > 1,000 to < 100 ng/mL before LT. We feel that the new policy requiring a reduction from > 1,000 to < 500 ng/mL is a good starting point. Whether the AFP threshold should be further reduced to < 100 ng/mL and whether those with AFP of 500-1,000 ng/mL require reduction in AFP to a lower threshold need further study.
The findings in the present study highlight the shifting focus in the selection of HCC patients for LT in recent years. There have been multiple studies suggesting that rapid LT for HCC can lead to decreased post-LT survival by allowing for LT in patients who otherwise may have had waitlist dropout due to aggressive tumor biology.(2,28–30) Our findings demonstrate that in HCC patients with AFP ever > 1,000 ng/mL, their peak AFP is much less important than the AFP at the time of LT. We have clearly demonstrated a significant impact of AFP reduction from > 1,000 to < 500 ng/mL at LT, leading to improved survival along with decreased HCC recurrence and time to death, which would be expected to improve transplant survival benefit. However, it takes time and LRT to achieve these goals. Not surprisingly, LRT was reported in just over half of the patients with AFP > 1,000 ng/mL at LT versus nearly 90% of those with an AFP reduction to < 500 ng/mL at LT. Additionally, median time from initial MELD exception to LT was shortest in the AFP > 1,000 ng/mL group and longest in the AFP ≤ 100 ng/mL group. A period of observation is required for evaluating tumor response to LRT and changes in AFP before LT. This concept has been filtered into the “ablate and wait” strategy for candidate selection(31) and provides further justification for the 6-month mandatory delay in awarding HCC exception points. An important message is that patients with an AFP > 1,000 ng/mL should be closely observed over time to demonstrate response to LRT with significant reduction in AFP before they are considered acceptable candidates for LT.
There are several limitations of the present study. Although the overall cohort included 407 patients with at least one AFP measurement > 1,000 ng/mL on the waitlist, the relatively small sample size of the 114 patients who had a reduction in AFP to < 1,000 ng/mL before LT might have limited our ability to detect meaningful differences between groups. This is especially true for the small group of only 17 patients with an AFP at LT of 500-1,000 ng/mL. There was a lack of detailed information in the UNOS database on the effects of LRT, a known risk factor for HCC recurrence.(22,23,25) However, monitoring the change in AFP in those with multiple AFP measurements and assessing tumor burden on subsequent exception applications when present may in part account for the effects of preoperative LRT. There is likely underreporting of HCC recurrence in the UNOS database,(32) as no mandate requires centers to report HCC recurrence. Although there do not appear to be any systematic center-specific differences,(33) the potential for underreporting HCC recurrence explains why we have chosen post-LT survival rather than HCC recurrence as our primary outcome. We were not able to capture prelisting AFP history such as, for example, those with an AFP > 1,000 ng/mL and subsequent reduction to < 1,000 ng/mL before listing. However, we only excluded 7.5% of the study cohort because of missing information on AFP at the time of LT. Finally, explant pathology results were only available for the 17% of patients who underwent LT after April 2012.
In conclusion, this study demonstrated significantly improved post-LT survival and decreased HCC recurrence when restricting LT to patients with a reduction in AFP from > 1,000 to < 500 ng/mL before LT, validating the recently approved national policy. Whether the AFP threshold should be further reduced to < 100 ng/mL and whether those with initial AFP of 500-1,000 ng/mL require reduction in AFP to a lower threshold before LT require further study.
Acknowledgments
Supported by the Clinical and Translational Core of the University of California, San Francisco Liver Center (P30 DK026473).
Potential conflict of interest: Drs. Mehta and Yao have received research grant support from FUJIFILM Wako.
Abbreviations:
- AFP
alpha-fetoprotein
- CI
confidence interval
- DRI
donor risk index
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- IQR
interquartile range
- LRT
local-regional therapy
- LT
liver transplantation
- MELD
Modelfor End Stage Liver Disease
- UCSF
University of California, San Francisco
- UNOS
United Network for Organ Sharing
Footnotes
This study was presented in part at the 2017 Annual Meeting of the American Association for the Study of Liver Disease in the Presidential Plenary Session.
REFERENCES
- 1).Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transpl 2017;17(Suppl 1):174–251. [DOI] [PubMed] [Google Scholar]
- 2).Halazun KJ, Patzer RE, Rana AA, Verna EC, Griesemer AD, Parsons RF, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology 2014;60:1957–1962. [DOI] [PubMed] [Google Scholar]
- 3).Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 4).Mehta N, Yao FY. Moving past “One size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl 2013;19:1055–1058. [DOI] [PubMed] [Google Scholar]
- 5).Fujiki M, Takada Y, Ogura Y, Oike F, Kaido T, Teramukai S, et al. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transpl 2009;9:2362–2371. [DOI] [PubMed] [Google Scholar]
- 6).Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986–994. [DOI] [PubMed] [Google Scholar]
- 7).Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1,000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl 2013;19:634–645. [DOI] [PubMed] [Google Scholar]
- 9).Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Thera 2012;35:987–999. [DOI] [PubMed] [Google Scholar]
- 12).Lai Q, Vitale A, Iesari S, Finkenstedt A, Mennini G, Spoletini G, et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017;66:1910–1919. [DOI] [PubMed] [Google Scholar]
- 13).U.S. Department of Health and Human Services. OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. https://optn.transplant.hrsa.gov/media/1922/liver_hcc_criteria_for_auto_approval_20160815.pdf. Accessed December 13, 2018.
- 14).Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 16).Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017;14:203–217. [DOI] [PubMed] [Google Scholar]
- 17).Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, et al. The Extended Toronto Criteria for liver transplantation in patients with hepatocellular carcinoma: a prospective validation study. Hepatology 2016;64:2077–2088. [DOI] [PubMed] [Google Scholar]
- 18).Lee SD, Lee B, Kim SH, Joo J, Kim SK, Kim YK, et al. Proposal of new expanded selection criteria using total tumor size and (18) F-fluorodeoxyglucose - positron emission tomography/computed tomography for living donor liver transplantation in patients with hepatocellular carcinoma: The National Cancer Center Korea criteria. World J Transplant 2016;6:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013;154:1053–1060. [DOI] [PubMed] [Google Scholar]
- 20).Mehta N, Guy J, Frenette CT, Dodge JL, Osorio RW, Minteer WB, et al. Excellent outcomes of liver transplantation following down staging of hepatocellular carcinoma to within Milan criteria-a multi-center study. Clin Gastroenterol Hepatol 2018;16:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Yao FY, Fidelman N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: Where do we stand with tumor down-staging? Hepatology 2016;63:1014–1025. [DOI] [PubMed] [Google Scholar]
- 22).Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 2006;12:1260–1267. [DOI] [PubMed] [Google Scholar]
- 23).Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl 2013;19:1108–1118. [DOI] [PubMed] [Google Scholar]
- 24).Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19:1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Kim DJ, Clark PJ, Heimbach J, Rosen C, Sanchez W, Watt K, et al. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transpl 2014;14:1383–1390. [DOI] [PubMed] [Google Scholar]
- 26).Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liv Dis 2010;30:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Samoylova ML, Nigrini MJ, Dodge JL, Roberts JP. Biases in the reporting of HCC tumor sizes on the liver transplant waiting list. Hepatology 2017;66:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Samoylova ML, Dodge JL, Yao FY, Roberts JP. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 2014;20:937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Schlansky B, Chen Y, Scott DL, Austin D, Naugler WE. Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry. Liver Transpl 2014;20:1045–1056. [DOI] [PubMed] [Google Scholar]
- 30).Mehta N, Heimbach J, Lee D, Dodge JL, Harnois D, Burns J, et al. Wait time of less than 6 and greater than 18 months predicts hepatocellular carcinoma recurrence after liver transplantation: proposing a wait time “sweet spot”. Transplantation 2017;101:2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl 2010;16:925–929. [DOI] [PubMed] [Google Scholar]
- 32).Mehta N, Dodge JL, Roberts JP, Yao FY. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transpl 2018;18:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing database. Liver Transpl 2013;19:1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]


