Abstract
Background
Various beneficial effects derived from neuraxial blocks have been reported. However, it is unclear whether these effects have an influence on perioperative mortality and major pulmonary/cardiovascular complications.
Objectives
Our primary objective was to summarize Cochrane systematic reviews that assess the effects of neuraxial blockade on perioperative rates of death, chest infection and myocardial infarction by integrating the evidence from all such reviews that have compared neuraxial blockade with or without general anaesthesia versus general anaesthesia alone for different types of surgery in various populations. Our secondary objective was to summarize the evidence on adverse effects (an adverse event for which a causal relation between the intervention and the event is at least a reasonable possibility) of neuraxial blockade. Within the reviews, studies were selected using the same criteria.
Methods
A search was performed in the Cochrane Database of Systematic Reviews on July 13, 2012. We have (1) included all Cochrane systematic reviews that examined participants of any age undergoing any type of surgical (open or endoscopic) procedure, (2) compared neuraxial blockade versus general anaesthesia alone for surgical anaesthesia or neuraxial blockade plus general anaesthesia versus general anaesthesia alone for surgical anaesthesia and (3) included death, chest infection, myocardial infarction and/or serious adverse events as outcomes. Neuraxial blockade could consist of epidural, caudal, spinal or combined spinal‐epidural techniques administered as a bolus or by continuous infusion. Studies included in these reviews were selected on the basis of the same criteria. Reviews and studies were selected independently by two review authors, who independently performed data extraction when data differed from one of the selected reviews. Data were analysed by using Review Manager Version 5.1 and Comprehensive Meta Analysis Version 2.2.044.
Main results
Nine Cochrane reviews were selected for this overview. Their scores on the Overview Quality Assessment Questionnaire varied from four to six of a maximal possible score of seven. Compared with general anaesthesia, neuraxial blockade reduced the zero to 30‐day mortality (risk ratio [RR] 0.71, 95% confidence interval [CI] 0.53 to 0.94; I2 = 0%) based on 20 studies that included 3006 participants. Neuraxial blockade also decreased the risk of pneumonia (RR 0.45, 95% CI 0.26 to 0.79; I2 = 0%) based on five studies that included 400 participants. No difference was detected in the risk of myocardial infarction between the two techniques (RR 1.17, 95% CI 0.57 to 2.37; I2 = 0%) based on six studies with 849 participants. Compared with general anaesthesia alone, the addition of a neuraxial block to general anaesthesia did not affect the zero to 30‐day mortality (RR 1.07, 95% CI 0.76 to 1.51; I2 = 0%) based on 18 studies with 3228 participants. No difference was detected in the risk of myocardial infarction between combined neuraxial blockade‐general anaesthesia and general anaesthesia alone (RR 0.69, 95% CI 0.44 to 1.09; I2 = 0%) based on eight studies that included 1580 participants. The addition of a neuraxial block to general anaesthesia reduced the risk of pneumonia (RR 0.69, 95% CI 0.49 to 0.98; I2 = 9%) after adjustment for publication bias and based on nine studies that included 2433 participants. The quality of the evidence was judged as moderate for all six comparisons.
No serious adverse events (seizure or cardiac arrest related to local anaesthetic toxicity, prolonged central or peripheral neurological injury lasting longer than one month or infection secondary to neuraxial blockade) were reported. The quality of the reporting score of complications related to neuraxial blocks was nine (four to 12 (median range)) of a possible maximum score of 14.
Authors' conclusions
Compared with general anaesthesia, a central neuraxial block may reduce the zero to 30‐day mortality for patients undergoing surgery with intermediate to high cardiac risk (level of evidence, moderate). Further research is required.
Keywords: Humans; Review Literature as Topic; Anesthesia, Epidural; Anesthesia, Epidural/adverse effects; Anesthesia, Epidural/methods; Anesthesia, General; Anesthesia, General/adverse effects; Anesthesia, Spinal; Anesthesia, Spinal/adverse effects; Anesthesia, Spinal/methods; Heart Arrest; Heart Arrest/prevention & control; Myocardial Infarction; Myocardial Infarction/mortality; Myocardial Infarction/prevention & control; Pneumonia; Pneumonia/mortality; Pneumonia/prevention & control; Postoperative Complications; Postoperative Complications/mortality; Postoperative Complications/prevention & control
Plain language summary
Effects of spinals and epidurals on perioperative death, myocardial infarction and pneumonia: an overview of Cochrane systematic reviews
Epidurals and spinals are anaesthetic techniques that block the transmission of painful stimuli from a surgical site to the brain at the level of the spinal cord. They allow the surgeon to perform surgery on the lower part of the abdomen (below the umbilicus) or on the lower limbs with no painful sensation while the person remains conscious. In this Cochrane overview, we summarized relevant randomized controlled trials from nine Cochrane systematic reviews, in which epidurals or spinals were compared as a method of replacing general anaesthesia or were added to general anaesthesia to reduce the quantity of narcotics or muscle relaxants required during general anaesthesia. The types of surgery included were caesarean section, abdominal surgery, repair of hip fracture, replacement of hip and knee joints and surgery to improve circulation in the legs.
When epidurals or spinals were used to replace general anaesthesia, the risk of dying during the surgery or within the following 30 days was reduced by approximately 29% (from 20 studies with 3006 participants). Also, the risk of developing pneumonia (chest infection) was reduced by 55% (from five studies with 400 participants). However, the risk of developing a myocardial infarction (heart attack) was the same for both anaesthetic techniques (from six studies with 849 participants).
When epidurals (and less frequently spinals) were used to reduce the quantity of other drugs required while general anaesthesia was used, the risk of dying during the surgery or within 30 days was the same for both anaesthetic techniques (from 18 studies with 3228 participants). Also, a difference was not detected for the risk of developing myocardial infarction (from eight studies with 1580 participants). The risk of developing pneumonia was reduced by approximately 30% when a correction was made for possible missing studies (from nine studies with 2433 participants).
No serious side effects (seizures, cardiac arrest, nerve damage lasting longer than one month or infection) were reported from the use of epidurals or spinals in these studies.
The quality of the evidence for all six comparisons was rated as moderate because of some imperfections in how the studies were carried out. Therefore further research is likely to have an important impact on our confidence in these results and may even change the results.
.
Background
Description of the condition
Despite major improvements in overall patient care (including anaesthetics and surgical techniques), postoperative death remains a reality. Death may occur following major infectious (superficial, deep, urinary tract or organ infection or sepsis), haematological (postoperative bleeding requiring transfusion, deep vein thrombosis, pulmonary embolus), cardiovascular (myocardial infarction, stroke), respiratory (pneumonia, unplanned intubation, prolonged mechanical ventilation), renal (acute kidney injury) and surgical (wound dehiscence, vascular graft loss) complications.
Description of the interventions
Regional blockade is commonly used to provide intraoperative anaesthesia and/or postoperative analgesia, with or without general anaesthesia, to patients undergoing surgery. Regional blockade refers to techniques in which conduction of painful stimuli is blocked at the level of the sensory nerve, the plexus or the spinal cord. Regional blockade at the level of the spinal cord is also described as neuraxial blockade. Unconsciousness and amnesia do not occur during regional blockade in the absence of complications or without sedation or general anaesthesia. For surgery, regional blockade may be used as an alternative to general anaesthesia or in combination with general anaesthesia as a replacement for opioids or neuromuscular blocking agents or to decrease the required doses of these two types of drugs. This overview examines neuraxial blockade used as a replacement for general anaesthesia during surgery or as a supplement to general anaesthesia during surgery.
How the intervention might work
Neuraxial blockade with or without general anaesthesia may reduce the incidence of some major complications that can lead to death such as pulmonary complications (Nishimori 2012), time to tracheal extubation (Guay 2006a; Nishimori 2012), cardiac dysrhythmias (Guay 2006a), venous thromboembolism (Parker 2004), blood transfusion (Guay 2006b), surgical site infection (Chang 2010) and acute kidney injury (Guay 2006a; Nishimori 2012). Maximal blood concentrations of stress response markers, such as epinephrine, norepinephrine, cortisol and glucose, are lower in patients for whom epidural analgesia is added to general anaesthesia (Guay 2006a).
Why it is important to do this overview
In 2000, Rodgers and colleagues published an extensive meta‐analysis that included data from 141 randomized controlled trials (RCTs) with 9559 participants and reported that intraoperative neuraxial blockade, with or without general anaesthesia, reduced the all‐cause mortality rate within 30 days of randomization (odds ratio (OR) 0.70; 95% confidence interval (CI) 0.54 to 0.90) compared with general anaesthesia alone (Rodgers 2000). The reported effect of neuraxial blockade on mortality from the meta‐analysis by Rodgers et al. may not reflect current practice. Furthermore, although Rodgers et al. concluded that a one‐third reduction in mortality rate would occur when a neuraxial block was added to general anaesthesia or when a neuraxial block was used to replace general anaesthesia, a wide CI (95% CI 0.53 to 1.41) around the former estimate of treatment effect precludes the conclusion that adding a neuraxial block to general anaesthesia reduces the mortality rate. Several Cochrane reviews have evaluated the effects of neuraxial blockade for various types of surgical populations. No synthesis of these reviews has been reported in an overview.
Objectives
Our primary objective was to summarize Cochrane systematic reviews that assess the effects of neuraxial blockade on perioperative rates of death, chest infection and myocardial infarction by integrating the evidence from all such reviews that have compared neuraxial blockade with or without general anaesthesia versus general anaesthesia alone for different types of surgery in various populations. Our secondary objective was to summarize the evidence on adverse effects (an adverse event for which a causal relation between the intervention and the event is at least a reasonable possibility) of neuraxial blockade. Within the reviews, studies were selected using the same criteria.
Methods
Criteria for considering reviews for inclusion
We considered all Cochrane systematic reviews that:
included RCTs;
examined participants of any age undergoing any type of surgical (open or endoscopic) procedure;
compared neuraxial blockade versus general anaesthesia alone for the surgical anaesthesia, or compared neuraxial blockade plus general anaesthesia versus general anaesthesia alone for the surgical anaesthesia; and
included death, chest infection, myocardial infarction or serious adverse events as outcomes.
Neuraxial blockade consisted of epidural, caudal, spinal or combined spinal‐epidural techniques administered as a bolus or by continuous infusion.
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews on July 13, 2012, using the following terms:
#1 MeSH descriptor Anesthesia, Epidural explode all trees
#2 MeSH descriptor Nerve Block explode all trees
#3 MeSH descriptor Anesthetics, Local explode all trees
#4 MeSH descriptor Anesthesia, Intravenous explode all trees
#5 MeSH descriptor Analgesia, Epidural explode all trees
#6 MeSH descriptor Anesthesia, Caudal explode all trees
#7 ((epidural or caudal or spinal or spinal?epidural) near (techniq* or administ* or bolus* or infusion*)) or an?esthesia
#8 (an?esthesia or block* or analgesia) near (regional or local or neuraxial or nerve or caudal or spinal or epidural or lumbar or general)
#9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
Data collection and analysis
We analysed the data using RevMan 5.1 (Review Manager Version 5.1) and Comprehensive Meta Analysis Version 2.2.044 (www.Meta‐Analysis.com).
Selection of reviews
One review author (JG) screened all abstracts of reviews identified by the search. The full reports of the potential reviews were obtained. Two review authors (JG, SK, NA, PC or SS) independently reviewed each report for inclusion.
Data extraction and management
From the included studies of selected reviews, studies were selected independently by two review authors (JG, SK, NA, PC or SS) using the same criteria as were used for the selection of reviews, with no language restriction. Data of selected studies were reextracted by one review author (JG) and were compared with the data included in the corresponding review. Any discrepancy was checked by a second review author (SK, NA, PC or SS).
Assessment of methodological quality of included reviews
Two of the review authors (JG and SK, NA, SS or PC) independently assessed the methodological quality of included reviews using a 10‐item index, the Overview Quality Assessment Questionnaire (OQAQ) (Oxman 1991). As the latest version of the risk of bias tool was unavailable when some of the Cochrane reviews were carried out, the methodological quality of included RCTs was reassessed using the current Cochrane tool for risk of bias.
Data synthesis
Studies were classified into two groups.
Neuraxial blockade versus general anaesthesia for the surgery.
Neuraxial blockade added to general anaesthesia versus general anaesthesia alone for the surgery.
Random‐effects models were used, and the effects were expressed as risk ratios (RRs). Heterogeneity was quantified by the I2 statistic, with the data entered in the direction (benefit or harm) yielding the lowest value. A value > 25% was used as the cutoff point for exploration. A priori factors chosen included the following.
American Society of Anesthesiologists (ASA) physical status (1 or 2 vs 3 or higher).
Age (< 18 years vs 18 to < 70 years vs 70 years or older).
Type of surgery (high vs intermediate cardiac risk vs low cardiac risk) (ACC/AHA 2007 Guidelines).
Type of neuraxial blockade (spinal vs epidural; lumbar vs thoracic epidural vs caudal).
Type of neuraxial drug (long‐acting opioid alone vs local anaesthetic alone vs local anaesthetic plus long‐acting opioid vs other adjuvants (e.g. clonidine, neostigmine, ketamine)).
Duration of neuraxial blockade (intraoperative only vs infusion continued for at least 48 hours after surgery).
Use of thromboprophylaxis (appropriate or not according to current standards).
Type of thromboprophylaxis (low‐molecular‐weight heparin, ximelagatran, fondaparinux or rivaroxaban vs regional blockade, pneumatic compression and aspirin vs warfarin).
Pregnancy.
Mode of analgesia in the control group (intravenous analgesia vs other routes).
For results in which the intervention produced an effect, a number needed to treat for an additional beneficial outcome (NNTB) or a number needed to treat for an additional harmful outcome (NNTH) was calculated that was based on the odds ratio (http://www.nntonline.net/visualrx/).
Publication bias was assessed by using a funnel plot followed by Duval and Tweedie's trim and fill technique, a classical fail‐safe number (alpha 0.05, two‐tails) or the regtest for each outcome.
The quality of the body of evidence for each outcome was judged as high, moderate or low according to the system developed by the GRADE Working Group (Atkins 2004; Guyatt 2011). The first consideration is the study design, with RCTs considered of higher quality than observational studies. The quality of the body of evidence is lower if the risk of bias of included studies is serious/very serious, some inconsistency is noted (I2 value), the demonstration of effect is indirect, imprecision in the results is evident (95% CI around the effect size) or a risk of publication bias is identified (classical fail‐safe number or funnel plot). The quality of the evidence is higher if the amplitude of the effect size is large/very large (< 0.5 or > 2.0 being large), evidence suggests a dose response or the possible effect of confounding factors would reduce a demonstrated effect or suggest a spurious effect when results show no effect. With high quality of evidence, further research is unlikely to change our confidence in the estimated effect. When the quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Three review authors (JG, SK and NLP) independently applied these criteria. Discrepancies were resolved by discussion.
For adverse effects of neuraxial blockade, all selected studies were assessed according to the seven criteria proposed by Stojadinovic A et al.: method of accrual, duration of data collection, definition of complication, morbidity and mortality rates, grade of complication severity, exclusion criteria and study follow‐up (Stojadinovic 2009). The following complications related to neuraxial blockade were sought specifically.
Mortality (anytime up to five years).
Seizure or cardiac arrest related to local anaesthetic toxicity (any significant prolonged neurological sequelae related to these events were to be described).
Prolonged central or peripheral neurological injury lasting longer than one month.
Infection secondary to neuraxial blockade.
Results
A total of 1158 titles/abstracts were screened. Of these, 304 were protocols, 844 were not relevant to neuraxial blockade used during surgery and one did not contain a control group with general anaesthesia. Therefore we retrieved and kept nine systematic reviews. These nine reviews included 117 trials. For Afolabi 2006, only three of 16 trials were retained (Dyer 2003; Hodgkinson 1980; Wallace 1995). Thirteen were excluded because they did not include any outcome of interest (Sener 2003; Datta 1983; Dick 1992; Hong 2003; Kavak 2001; Kolatat 1999; Korkmaz 2004; Lertakyamanee 1999; Mahajan 1992; Pence 2002; Petropoulos 2003; Yegin 2003) or had inadequate randomization (epidural and general anaesthesia used in alternate participants; Hollmen 1978). For Barbosa 2010, all four trials were retained (Bode 1996; Christopherson 1993; Cook 1986; Dodds 2007). For Choi 2003, of the 13 trials available, 12 were excluded because they evaluated different interventions (Bertini 1995; Capdevila 1999; Gustafsson 1986; Hendolin 1996; Hommeril 1994; Klasen 1999; Sharrock 1994; Weller 1991) or lacked any outcome of interest (D'Ambrosio 1999; Jorgensen 1991; Moiniche 1994; Singelyn 1998). Only one trial was retained (Wulf 1999). For Craven 2003, all four trials were excluded because they evaluated different interventions (William 2001) or lacked any outcome of interest (Krane 1995; Somri 1998; Welborn 1990). All 10 trials from Cyna 2008 were excluded because they did not include any outcome of interest (Bramwell 1982; Concha 1994; Gauntlett 2003; Lunn 1979; Mak 2001; Martin 1982; May 1982; Vater 1985; Weksler 2005; White 1983). For Jorgensen 2000, four of the 22 trials were retained (Cuschieri 1985; Liu 1995; Riwar 1992; Scheinin 1987). The remainder were excluded because they did not contain any outcome of interest (Ahn 1988; Cullen 1985; Rutberg 1984; Scott 1989; Wallin 1986; Wattwil 1989), studied different interventions (Asantila 1991; Beeby 1984; Brodner 2000; Cooper 1996; Delilkan 1993; Geddes 1991; George 1992; Lee 1988; Thoren 1989; Thorn 1992; Thorn 1996) or had inadequate randomization (Bredtmann 1990; anaesthetic technique attributed according to the date of surgery (even vs odd days)). For Nishimori 2012, one trial was excluded because it lacked any outcome of interest (Barre 1989). The remaining 12 trials were retained (Bois 1997; Boylan 1998; Broekema 1998; Davies 1993; Garnett 1996; Kataja 1991; Norman 1997; Norris 2001; Park 2001; Peyton 2003; Reinhart 1989; Yeager 1987). For Parker 2004, 13 of the 26 trials were retained (Berggren 1987; Bigler 1985; Couderc 1977; Davis 1981; Davis 1987; Juelsgaard 1998; McKenzie 1984; McLaren 1978; Racle 1986; Tasker 1983; Ungemach 1993; Valentin 1986; White 1980), and 13 were excluded for inappropriate randomization (Adams 1990; by the date of operation), lack of any outcome of interest (Biffoli 1998; Bredahl 1991; Brichant 1995; Brown 1994; Casati 2003; Kamitani 2003; Maurette 1988; Svartling 1986; Wajima 1995) or lack of any intervention of interest (de Visme 2000; Eyrolle 1998; Spreadbury 1980). For Werawatganon 2005, of the nine trials available, four were excluded because they did not include any outcome of interest (Allaire 1992; George 1994; Kowalski 1992; Tsui 1997), and two (Bois 1997; Boylan 1998) were already included from another review. Three new trials were added (Carli 2001; Paulsen 2001; Seeling 1991). Altogether, we retained 40 studies for the new analysis (Afolabi 2006: n = 3; Barbosa 2010: n= 4; Choi 2003: n = 1; Craven 2003: n = 0; Cyna 2008: n = 0; Jorgensen 2000: n = 4; Nishimori 2012: n = 12; Parker 2004: n = 13; Werawatganon 2005: n = 3). Half of these studies compared a neuraxial block versus general anaesthesia (Berggren 1987; Bigler 1985; Bode 1996; Christopherson 1993; Cook 1986; Couderc 1977; Davis 1981; Davis 1987; Dodds 2007; Dyer 2003; Hodgkinson 1980; Juelsgaard 1998; McKenzie 1984; McLaren 1978; Racle 1986; Tasker 1983; Ungemach 1993; Valentin 1986; Wallace 1995; Wulf 1999), and half compared neuraxial block plus general anaesthesia versus general anaesthesia alone (Bois 1997; Boylan 1998; Broekema 1998; Carli 2001; Cuschieri 1985; Davies 1993; Garnett 1996; Kataja 1991; Liu 1995; Norman 1997; Norris 2001; Park 2001; Paulsen 2001; Peyton 2003; Reinhart 1989; Riwar 1992; Scheinin 1987; Seeling 1991; White 1980; Yeager 1987). All retained trials studied adult participants undergoing surgery with an intermediate (Berggren 1987; Bigler 1985; Carli 2001; Couderc 1977; Cuschieri 1985; Davis 1981; Davis 1987; Dyer 2003; Hodgkinson 1980; Juelsgaard 1998; Liu 1995; McKenzie 1984; McLaren 1978; Paulsen 2001; Racle 1986; Riwar 1992; Scheinin 1987; Tasker 1983; Ungemach 1993; Valentin 1986; Wallace 1995; White 1980; Wulf 1999) or high (Bode 1996; Bois 1997; Boylan 1998; Christopherson 1993; Cook 1986; Davies 1993; Dodds 2007; Garnett 1996; Kataja 1991; Norman 1997; Norris 2001; Reinhart 1989) cardiac risk or with a mixture of both (Broekema 1998; Park 2001; Peyton 2003; Seeling 1991; Yeager 1987). These surgeries were performed on the lower limb (Berggren 1987; Bigler 1985; Bode 1996; Christopherson 1993; Cook 1986; Couderc 1977; Davis 1981; Davis 1987; Dodds 2007; Juelsgaard 1998; McKenzie 1984; McLaren 1978; Racle 1986; Tasker 1983; Ungemach 1993; Valentin 1986; White 1980; Wulf 1999), in the intra‐abdominal cavity (Bois 1997; Boylan 1998; Broekema 1998; Carli 2001; Cuschieri 1985; Davies 1993; Dyer 2003; Garnett 1996; Hodgkinson 1980; Kataja 1991; Liu 1995; Norman 1997; Norris 2001; Park 2001; Paulsen 2001; Peyton 2003; Reinhart 1989; Riwar 1992; Scheinin 1987; Seeling 1991; Wallace 1995) or at various parts of the body (Yeager 1987). Three trials studied pregnant women undergoing caesarean section (Dyer 2003; Hodgkinson 1980; Wallace 1995).
Description of included reviews
We included nine Cochrane reviews in this overview (Appendix 1). The first Cochrane review (Afolabi 2006) reported on 16 studies that included 1586 pregnant women undergoing caesarean section under epidural‐spinal anaesthesia versus general anaesthesia. Neuraxial blockade reduced maternal blood loss, but general anaesthesia was chosen preferentially for a further surgery by a higher number of participants. No maternal deaths were reported. The second review (Barbosa 2010) included four studies and 696 participants undergoing lower limb revascularization under epidural/spinal anaesthesia versus general anaesthesia. Neuraxial blockade reduced the incidence of pneumonia. The third review (Choi 2003) included 13 studies with 606 participants undergoing total hip or knee replacement with epidural or systemic analgesia. Epidural analgesia reduced early postoperative pain scores and the incidence of sedation but increased the rates of urinary retention, itching and low blood pressure. The fourth review (Craven 2003) included three studies with 108 preterm infants undergoing inguinal hernia repair under spinal versus general anaesthesia. When sedated infants were excluded, spinal anaesthesia reduced the incidence of postoperative apnoea. The fifth review (Cyna 2008) included 10 studies with 721 male children undergoing circumcision with the addition of a caudal block, a penile block or systemic analgesia. Compared with a penile block, a caudal block increased the incidence of leg weakness (motor block). The sixth review (Jorgensen 2000) included 22 studies with 1023 participants undergoing abdominal surgery with the addition of an epidural containing a local anaesthetic versus systemic or epidural opioids. An epidural with a local anaesthetic may have hastened the return of bowel function. The seventh review (Nishimori 2012) included 13 studies with 1224 participants undergoing elective open aortic abdominal surgery with the addition of epidural analgesia or systemic opioids. Epidural analgesia reduced the duration of tracheal intubation and mechanical ventilation by approximately 20%, and the overall risk of cardiovascular complications, myocardial infarction, acute respiratory failure (defined as extended need for mechanical ventilation), gastrointestinal complications and acute kidney injury, as well as pain scores, for up to three days. The eighth review (Parker 2004) included 22 studies with 2567 participants undergoing repair of a hip fracture. In this subpopulation, neuraxial blockade reduced the 30‐day mortality rate. The last review (Werawatganon 2005) included nine studies with 711 participants undergoing intra‐abdominal surgery with the addition of epidural analgesia or patient‐controlled intravenous opioids. Epidural analgesia reduced the six‐hour pain scores but increased the incidence of pruritus.
Methodological quality of included reviews
The overall quality of included reviews was average (Table 1). The quality of the 40 studies retained for reanalysis can be found in Figure 1.
1. Overview Quality Assessment Questionnaire.
| Item | Afolabi 2006 | Barbosa 2010 | Choi 2003 | Craven 2003 | Cyna 2008 | Jorgensen 2000 | Nishimori 2012 | Parker 2004 | Werawatganon 2005 |
| 1. Were the search methods reported? |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the search comprehensive? |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partially | Partially |
| 3. Were the inclusion criteria reported? |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Was selection bias avoided? |
Yes | Yes | Yes | Partially | Yes | Partially |
Yes | Partially | Yes |
| 5. Were the validity criteria reported? |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Was validity of the included studies assessed appropriately? |
Partially | Yes | Partially | Partially | Partially | Partially | Yes | Partially | Yes |
| 7. Were the methods used to combine studies reported? |
Yes | Yes | Partially | Partially |
Partially |
Partially |
Yes | Yes | Partially |
| 8. Were the findings combined appropriately? |
Partially | Yes | Partially | Partially | Partially | Partially |
Partially | Partially | Yes |
| 9. Were the conclusions supported by the reported data? |
Yes | Yes | Yes | Partially | Yes | Yes | Yes | Yes | Yes |
| 10. What was the overall scientific quality of the overview? (Likert scale from one to seven) |
Five | Six | Five | Four | Four | Five | Six | Five | Five |
Afolabi 2006: Recent studies are not included. “We updated the search of the Cochrane Pregnancy and Childbirth Group’s Trials Register on 1 October 2009 and added the results to the awaiting classification section". Did not use the new Cochrane scale, but the validity of the included trials was assessed appropriately. Nausea (I2 = 84%) and vomiting (I2 = 91%) should have been analysed with random‐effects models, and used/lack of prophylaxis and type of induction agent (propofol vs thiopental) could have been explored. For APGAR scores at one minute (I2 = 87%) and five minutes (I2 = 91%), the type of anaesthetic agents used (e.g. use of pancuronium in Kotalat’s study for one minute APGAR score of 6.7 ± 2.8) might have deserved to be mentioned. Publication bias assessment not performed.
Barbosa 2010: Publication bias assessment not performed.
Choi 2003: Last search in 2001. The quality of the studies was assessed with the JADAD score. High amount of heterogeneity for VAS scores at rest at four to six hours (I2 > 90%) analysed with fixed models. Publication bias assessment not performed.
Craven 2003: Last search in 2002. The definition for apnoea used in all studies differs from the protocol of the review. Moderate amount of heterogeneity (I2 = 60% and I2 = 65%) for apnoea/bradycardia analysed with fixed models. No exploration for heterogeneity because P value for heterogeneity not statistically significant (P value 0.20 and 0.06). Publication bias assessment not performed.
Cyna 2008: Last search 2008. Heterogeneity > 50% not explored. Publication bias assessment not performed.
Jorgensen 2000: Last search 1999, according to method section; inclusion of one study published in 2000. Inclusion of quasi‐randomized studies (as opposed to randomized studies only) in method section. The method section says: “Where heterogeneity in methodology, dosage of used drugs and type of surgery, across the reviewed studies prohibited a quantitative review, we restricted to perform a qualitative review.” Forest plots include quantitative analysis with I2 > 90%. Publication bias assessment not performed.
Nishimori 2012: Last search in 2010. For this review, the cutoff to use a random‐effects model was set at 30%, while the criterion prespecified for this overview was 25%. Publication bias assessment not performed.
Parker 2004: Last search 2004. The search for CENTRAL was limited to a part of it. Use of random‐effects models versus fixed‐effect models based on P value < 0.1 instead of on I2 values. Exclusion of studies on the basis of “neuroleptic technique” that could be considered total intravenous anaesthesia could be considered controversial. Publication bias assessment not performed.
Werawatganon 2005: Last search 2002. As the review title is “...for pain after intra‐abdominal surgery”, the reasons for inclusion of the term “labor” in the search (as opposed to caesarean section) are not obvious. It also is not obvious why studies with patient‐controlled epidural analgesia (which most of the time include a portion of basal continuous rate) were excluded instead of being studied as a subgroup, especially given the fact that IVPCA with and without a background infusion was included. Criteria to use a fixed‐effect or a random‐effects model or to decide when it was appropriate to explore heterogeneity were not predefined. Publication bias assessment not performed.
1.
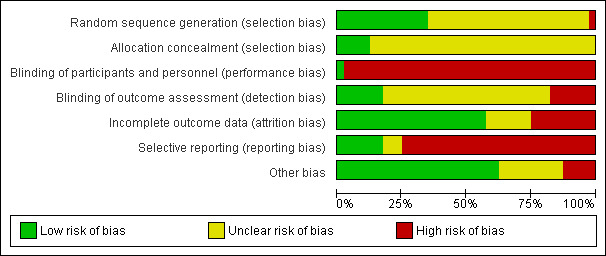
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Appendix 2 contains details supporting the judgement of the quality of included studies. Appendix 3 gives the reasons for exclusion of studies included in the previous reviews but excluded from our analysis.
Effect of interventions
Neuraxial blockade compared with general anaesthesia
Compared with general anaesthesia, neuraxial blockade reduced the zero to 30‐day mortality (RR 0.71, 95% CI 0.53 to 0.94; I2 = 0%; classical fail‐safe number = seven). The NNTB calculated on the OR was 44 (95% CI 27 to 228) for an incidence of 7.9% for general anaesthesia versus 5.2% for neuraxial blockade, based on 3006 participants (1570 for neuraxial blockade and 1436 for general anaesthesia). Cardiac risk was classified as intermediate for 76.5% (2300/3006) (intraperitoneal or orthopaedic surgery) and high for 23.5% (706/3006) (aortic or peripheral vascular surgery) of participants (Figure 2). With Duval and Tweedie's trim and fill analysis, the adjusted RR was 0.72 (95% CI 0.54 to 0.95) while looking for missing studies to the right and was unchanged while looking for missing studies to the left. Egger's regression intercept did not indicate a small‐study effect. Mortality data were available for 896 participants for the one to six‐month follow‐up (RR 1.52, 95% CI 0.89 to 2.62) and for 726 participants at six to 12‐month follow‐up (RR 1.27, 95% CI 0.74 to 2.17).
2.
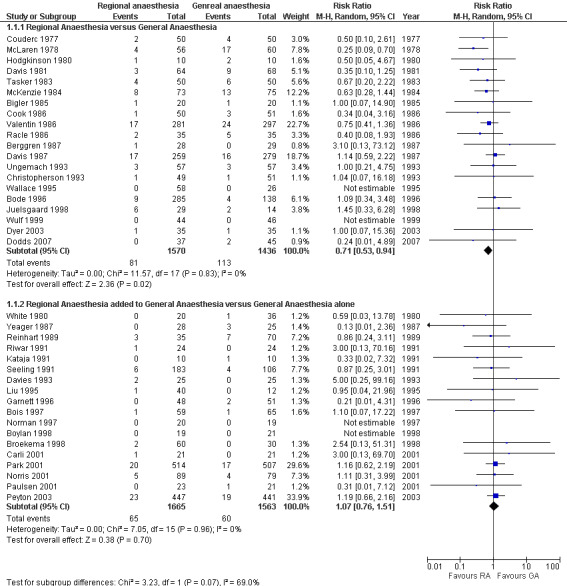
Forest plots for mortality zero to 30 days.
Neuraxial blockade also decreased the risk of pneumonia (RR 0.45, 95% CI 0.26 to 0.79; I2 = 0%; classical fail‐safe number = three) (Figure 3) based on 400 participants in studies published between 1981 and 1987. The NNTB was 11 (95% CI 8 to 27) for incidences of 7.6% and 16.8% for neuraxial blockade and general anaesthesia, respectively. Egger’s regression intercept did not indicate a small‐study effect. The RR adjusted for a possible publication bias was 0.44 (95% CI 0.26 to 0.73). No difference in the risk of myocardial infarction was noted between neuraxial blockade and general anaesthesia (RR 1.17, 95% CI 0.57 to 2.37; I2 = 0%) (Figure 4) based on 849 participants. No evidence of publication bias was found for this comparison.
3.
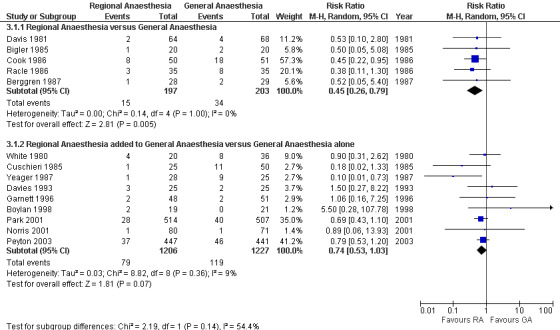
Forest plots for pneumonia zero to 30 days.
4.
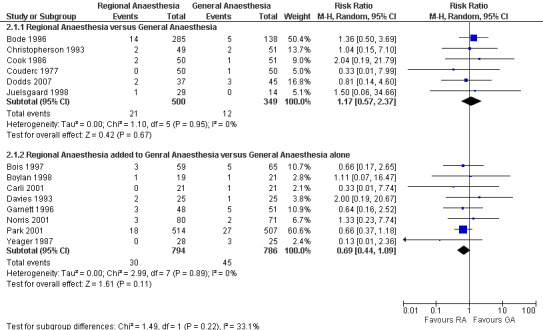
Forest plots for myocardial infarction zero to 30 days.
Neuraxial blockade plus general anaesthesia compared with general anaesthesia alone
Adding a neuraxial blockade to general anaesthesia did not affect the mortality risk (RR 1.07, 95% CI 0.76 to 1.51; I2 = 0%) (Figure 2) based on 3228 participants (1665 with a neuraxial block and 1563 without). With Duval and Tweedie's trim and fill analysis, the effect was almost unchanged (RR 1.13, 95% CI 0.80 to 1.59). The risk of myocardial infarction was not different between the two anaesthetic techniques (RR 0.69, 95% CI 0.44 to 1.09; I2 = 0%) (Figure 4) based on 1580 participants (794 with a neuraxial block and 786 without). The power to detect a 25% reduction in incidence from 5.7% was only 0.25 (α = 0.05, two‐sided test). With an adjustment for a possible publication bias, the RR would be 0.72 (95% CI 0.46 to 1.13).
Likewise, the addition of a neuraxial block did not change the risk of pneumonia when a random‐effects model was used (RR 0.74, 95% CI 0.53 to 1.03; I2 = 9%) (Figure 3) and was marginally suggestive of an effect when a fixed‐effect model was used (RR 0.74, 95% CI 0.56 to 0.98) based on 2433 participants. For the random‐effects model, the power to detect a 25% reduction is 0.58 (α = 0.05, two‐sided test) from an incidence of 9.5%. For the fixed‐effect model, the NNTB was 40 (95% CI 24 to 387). Egger’s regression intercept did not indicate a small‐study effect. The funnel plot revealed that two studies might be missing on the left side. With Duval and Tweedie's trim and fill analysis, the adjusted RR was 0.69 (95% CI 0.49 to 0.98) with a random‐effects model. If only studies with an a priori definition for the diagnosis of pneumonia were included (Cuschieri 1985; Davies 1993; Garnett 1996; Norris 2001; Park 2001; Peyton 2003; Yeager 1987), then adding a neuraxial block to general anaesthesia reduced the risk of pneumonia (RR 0.70, 95% CI 0.49 to 1.00). For the effect of neuraxial blockade on the risk of pneumonia by type of neuraxial block, the RR was 0.90 (95% CI 0.31 to 2.62) for spinal anaesthesia (White 1980), 5.5 (95% CI 0.28 to 107.78) for lumbar epidural analgesia (Boylan 1998), 0.64 (95% CI 0.17 to 2.47) for thoracic epidural analgesia (Cuschieri 1985; Davies 1993; Norris 2001) and 0.69 (95% CI 0.45 to 1.06) when lumbar or thoracic epidural analgesia could be used (Garnett 1996; Park 2001; Peyton 2003; Yeager 1987). All studies for this comparison included a local anaesthetic in the neuraxial block. No correlation was noted between the effect size (RR) and the mean age of participants included in the studies. A summary of the new findings is provided in Table 2.
2. Summary of new findings.
| Neuraxial blockade compared with general anaesthesia for perioperative mortality, myocardial infarction or chest infection | ||||||
| Patient or population: patients of any age requiring surgery Settings: in‐hospital or ambulatory surgery Intervention: neuraxial blockade (RA) Comparison: general anaesthesia (GA) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| General anaesthesia (GA) | Neuraxial blockade (RA) | |||||
| RA versus GA: mortality Follow‐up: 30 days | Study population | RR 0.71 (0.53 to 0.94) | 3006 (20 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 79 per 1000 | 56 per 1000 (42 to 74) | |||||
| Low‐risk population | ||||||
| 20 per 1000 | 14 per 1000 (11 to 19) | |||||
| High‐risk population | ||||||
| 100 per 1000 | 71 per 1000 (53 to 94) | |||||
| RA versus GA: myocardial infarction Follow‐up: 30 days | Study population | RR 1.17 (0.57 to 2.37) | 849 (six studies) | ⊕⊕⊕⊝ moderate1 | ||
| 34 per 1000 | 40 per 1000 (19 to 81) | |||||
| Low‐risk population | ||||||
| 20 per 1000 | 23 per 1000 (11 to 47) | |||||
| High‐risk population | ||||||
| 60 per 1000 | 70 per 1000 (34 to 142) | |||||
| RA versus GA: pneumonia Follow‐up: 30 days | Study population | RR 0.45 (0.26 to 0.79) | 400 (five studies2) | ⊕⊕⊕⊝ moderate1,3,4 | ||
| 167 per 1000 | 75 per 1000 (43 to 132) | |||||
| Low‐risk population | ||||||
| 40 per 1000 | 18 per 1000 (10 to 32) | |||||
| High‐risk population | ||||||
| 200 per 1000 | 90 per 1000 (52 to 158) | |||||
| RA added to GA versus GA: mortality Follow‐up: 30 days | Study population | RR 1.07 (0.76 to 1.51) | 3228 (18 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 38 per 1000 | 41 per 1000 (29 to 57) | |||||
| Low‐risk population | ||||||
| 20 per 1000 | 21 per 1000 (15 to 30) | |||||
| High‐risk population | ||||||
| 60 per 1000 | 64 per 1000 (46 to 91) | |||||
| RA added to GA versus GA: myocardial infarction Follow‐up: 30 days | Study population | RR 0.69 (0.44 to 1.09) | 1580 (eight studies) | ⊕⊕⊕⊝ moderate1 | ||
| 57 per 1000 | 39 per 1000 (25 to 62) | |||||
| Low‐risk population | ||||||
| 20 per 1000 | 14 per 1000 (nine to 22) | |||||
| High‐risk population | ||||||
| 80 per 1000 | 55 per 1000 (35 to 87) | |||||
| RA added to GA versus GA: pneumonia Follow‐up: 30 days | Study population | RR 0.74 (0.53 to 1.03) | 2433 (10 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 95 per 1000 | 70 per 1000 (50 to 98) | |||||
| Low‐risk population | ||||||
| 40 per 1000 | 30 per 1000 (21 to 41) | |||||
| High‐risk population | ||||||
| 120 per 1000 | 89 per 1000 (64 to 124) | |||||
| *The assumed risk is based on the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RA: Regional anaesthesia; GA: General anaesthesia. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1Blinding. 2For the comparison RA versus GA, outcome pneumonia, studies were published between 1981 and 1987. 3Classical fail‐safe number = three. 4RR < 0.5. | ||||||
Adverse events
No serious adverse events were reported. The quality score for the reporting of complications related to neuraxial blockade was nine (median) (four to 12) (range) of a possible maximal score of 14.
Grade of evidence
The quality of the evidence was rated as moderate for all six comparisons (Table 2). Risk of bias introduced by study design was the reason for downgrading the quality from high to moderate, with the absence of blinding of outcome assessors being the most serious potentially avoidable concern. For the effect on pneumonia of the comparison of neuraxial blockade versus general anaesthesia, the small fail‐safe number (possibility of publication bias) was compensated for by the large (< 0.5) effect size.
Discussion
Summary of main results
Compared with general anaesthesia, neuraxial blockade reduced the mortality rate by approximately 2.5% (RR 0.72, 95% CI 0.54 to 0.95; I2 = 0%) (Figure 2) and the risk of perioperative pneumonia (RR 0.44, 95% CI 0.26 to 0.73; I2 = 0%) (Figure 3). Adding a neuraxial block to general anaesthesia may reduce the incidence of pneumonia (adjusted RR 0.69, 95% CI 0.49 to 0.98); however, this is less conclusive, as the results varied depending on whether the effect size was adjusted for a possible publication bias. We decided to use only random‐effects models regardless of the amount of heterogeneity, as we wanted to reduce the possibility of finding an effect where there was none. When heterogeneity is present, a random‐effects model will usually widen the confidence interval. The only comparison in which we saw statistical heterogeneity was the effect on the risk of pneumonia when a neuraxial block was added to general anaesthesia compared with the use of general anaesthesia alone (I2 = 9%). If data were pooled with a fixed‐effect model, then adding a neuraxial block to general anaesthesia reduced the incidence of pneumonia (RR 0.74, 95% CI 0.56 to 0.98), whereas no effect was detected if data were pooled with a random‐effects model (RR 0.74, 95% CI 0.53 to 1.03). However, when we included only the studies for which an a priori definition for the diagnosis of pneumonia was reported, the addition of neuraxial blockade to general anaesthesia reduced the risk of pneumonia regardless of the model used. None of the interventions (neuraxial blockade compared with general anaesthesia or neuraxial blockade added to general anaesthesia vs general anaesthesia alone) reduced the risk of myocardial infarction (Figure 4), but the power to detect a 25% risk reduction from the addition of an epidural to general anaesthesia was only 0.25 (α = 0.05, two‐sided test).
Overall completeness and applicability of evidence
When deciding which intervention to choose for a patient, one has to balance the benefits versus the risks. Although many studies provided an appropriate description of the techniques used, a clear mention of the presence or absence of complications related to the techniques with an adequate duration of follow‐up was lacking in many of the reports (median Stojadinovic's score nine/14). There is no doubt for the authors of this overview that complications will need to be evaluated in future trials. Currently, we have to rely on the data provided by the most recent large prospective studies to estimate the incidence of complications related to neuraxial blockade.
Quality of the evidence
The results of this overview are based on nine Cochrane reviews. The 40 studies retained for analysis are of good quality except for two criteria. First, blinding usually was not used in these studies. Given the potentially serious (although rare) side effects that can be associated with the insertion of an epidural catheter, many clinicians would consider insertion of an epidural catheter to be unethical if it is not used to provide neuraxial blockade. Some authors have tried to insert a "sham" catheter subcutaneously to circumvent this problem. Also, the need to administer opioids both epidurally and systemically would require extra vigilance for side effects. For the comparison of neuraxial blockade versus general anaesthesia, blinding of participants is not feasible and blinding of study personnel is unrealistic, at least for the intraoperative and immediate postanaesthetic periods.
Second, many of our studies suffered from the absence of reporting of side effects of neuraxial blocks, which resulted in degrading of the quality of the studies. Our goal was to invite the trial authors to make a clear statement on the complications of the techniques that they were studying.
Potential biases in the overview process
Using systematic reviews to find relevant studies to answer a question could be considered an unusual technique, but we do not think that this led us to "biased" results. First, all of the included systematic reviews used very comprehensive search strategies. Second, by using Duval and Tweedie's trim and fill analysis, we were able to quantify the effect sizes while taking any potential publication bias into account. Publication bias occurs when medical journals publish more studies favouring one intervention than studies favouring another one or a placebo. Publication bias would be particularly frequent for studies with a small sample size. When no publication bias is noted, if a graph is constructed with the standard error or the precision (one/standard error) on the y‐axis and the logarithm of the odds ratio on the x‐axis, then studies should be equally distributed on both sides of a vertical line passing through the effect size found (log odds ratio), and the entire graph should have the shape of a reversed funnel. Duval and Tweedie’s trim and fill analysis corrects the asymmetry by removing extremely small studies from the positive side (recomputing the effect size at each iteration until the funnel plot is symmetrical around the new effect size). The algorithm then adds the original studies back into the analysis and imputes a mirror image for each. The latter step does not modify the “new effect size” but corrects the variance that was falsely reduced by the first step. Duval and Tweedie’s trim and fill analysis yields an estimate of what would be the effect size (odds ratio, risk ratio, etc.) if no publication bias was present (Borenstein 2009).
Agreements and disagreements with other studies or reviews
In their meta‐analysis published in 2000, Rodgers et al. (Rodgers 2000) concluded that neuraxial blockade reduced the overall 30‐day mortality by approximately one‐third and that this would apply to trials in which neuraxial blockade was combined with general anaesthesia, as well as to trials in which neuraxial blockade was used alone. We, on the other hand, demonstrated that these two interventions are not equivalent (I2 for heterogeneity between the two interventions = 69%) (Figure 2). Using a neuraxial block as the sole anaesthetic technique reduced the 30‐day mortality rate, but adding a central neuraxial block to general anaesthesia did not have this effect.
Authors' conclusions
Implications for practice.
The findings of the present overview suggest that, compared with general anaesthesia, neuraxial block may reduce the 30‐day mortality rate (level of evidence: moderate) for adults undergoing a procedure with intermediate to high cardiac risk (peripheral vascular, intraperitoneal, orthopaedic and prostate surgery). The magnitude of this effect requires further exploration, as the overall quality of the included trials was moderate.
Implications for research.
Large high‐quality trials will be required to confirm or refute our results on effects on the mortality rate of using a neuraxial block as opposed to general anaesthesia. A larger sample size is required before any conclusions can be drawn regarding effects on the risk of myocardial infarction of adding an epidural to general anaesthesia. These trials should include appropriate follow‐up and descriptions of side effects to allow the reader to balance the risks and benefits of each technique.
What's new
| Date | Event | Description |
|---|---|---|
| 19 May 2016 | Amended | Co‐publication cited in 'Other published versions of this review' |
History
Protocol first published: Issue 9, 2012 Review first published: Issue 1, 2014
| Date | Event | Description |
|---|---|---|
| 27 January 2014 | Amended | Formatting problem sorted out in summary of new findings table |
Acknowledgements
The authors thank Mr Karen Hovhannisyan, Trials Search Co‐ordinator for the Cochrane Anaesthesia Review Group (CARG), for designing the search, and the University of Montreal for providing access to electronic databases and major medical journals. We also thank Dr Stephan Schwarz for providing translations of the two German articles in our systematic overview, Dr Helen Handoll for providing translations of some Japanese and Italian articles and Dr Mina Nishimori for granting us access to her data extraction sheets. Finally, we are indebted to Drs Mark Neuman (content editor), Marialena Trivella (statistical editor) and Lorne Becker, Denise Thompson, Jørn Wetterselv and Mina Nishimori (peer reviewers) for their help and editorial advice provided during the preparation of this overview.
Appendices
Appendix 1. Summary of major findings of the reviews included in this overview
| Authors | Date assessed as up‐to‐date | Number of studies/Number of participants | Population | Interventions and comparison interventions |
Major findings |
| Neuraxial blockade versus general anaesthesia | |||||
| Afolabi 2006 | 14 August 2006 | 16 1586 |
Pregnant women Caesarean section for any indication |
Epidural anaesthesia or Spinal anaesthesia versus General anaesthesia |
Neuraxial blockade reduces:
General anaesthesia:
|
| Barbosa 2010 | 9 June 2008 | Four 696 |
Adults Lower limb revascularization |
Epidural anaesthesia or Spinal anaesthesia versus General anaesthesia |
No difference:
Neuraxial blockade reduces:
|
| Craven 2003 | 31 March 2003 | Three 108 |
Preterm infants Inguinal herniorrhaphy |
Spinal anaesthesia versus General anaesthesia |
No difference in:
Excluding infants who received preoperative sedatives, neuraxial blockade:
|
| Parker 2004 | 10 June 2004 | 22 2567 |
Adults Hip fracture |
Epidural anaesthesia or Spinal anaesthesia versus General anaesthesia |
Neuraxial blockade:
|
| Neuraxial blockade added to general anaesthesia | |||||
| Choi 2003 | 13 May 2003 | 13 606 |
Adults Hip or knee replacement |
Epidural analgesia versus Systemic analgesia |
Neuraxial blockade:
|
| Cyna 2008 | 13 April 2008 | 10 721 |
Male children Circumcision |
Caudal epidural block versus Systemic analgesia or Dorsal nerve penile block |
Neuraxial blockade versus parenteral analgesia.
Neuraxial blockade versus dorsal nerve penile block.
Neuraxial blockade versus rectal or intravenous analgesia.
|
| Jorgensen 2000 | 31 August 2000 | 22 1023 |
Adults Abdominal surgery |
Epidural local anaesthetic versus Systemic opioids or Epidural opioids |
Substantial heterogeneity Neuraxial blockade with local anaesthetic versus systemic opioids:
Neuraxial blockade with local anaesthetic versus epidural opioid:
|
| Nishimori 2012 | 16 January 2011 | 13 1224 |
Adults Elective open abdominal aortic surgery |
Epidural analgesia versus Systemic opioid–based pain relief |
Neuraxial blockade (especially thoracic epidural):
|
| Werawatganon 2005 | 13 October 2004 | Nine 711 |
Adults Intra‐abdominal surgery |
Epidural analgesia versus Patient‐controlled analgesia with intravenous opioids |
Neuraxial blockade:
|
MD: mean difference; SMD: standardized mean difference.
Appendix 2. Characteristics of included studies
Characteristics of studies
Characteristics of included studies
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 57 patients undergoing emergent femoral fracture repair (< 72 hours) |
| Interventions |
Epidural anaesthesia (n = 28): test dose 5 mL followed by 9 to 21 mL of 2% prilocaine with epinephrine 5 mcg/mL through a catheter inserted at L3‐L4 or L4‐L5, then half the volume of 0.5% bupivacaine or prilocaine with epinephrine 75 minutes later. The catheter was removed at the end of the surgery General anaesthesia (n = 29): thiopental 3 to 4 mg/kg, succinylcholine, nitrous oxide, halothane and succinylcholine infusion |
| Outcomes | Mortality: One participant in the epidural group died on postoperative day one; three (group unspecified) died around five months after the surgery, for a total of four/57 deaths at one year Pneumonia: no a priori definition (x‐ray performed preoperatively and treated after the surgery) |
| Notes | Dextran and mobilization at postoperative day one used as thromboprophylaxis. No mention about presence/absence of complications related to regional or general anaesthesia |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors (for POCD) were blinded to the anaesthetic technique |
| Incomplete outcome data (attrition bias) | Low risk | Four participants lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Mortality given up to one year (although exact group not given). Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Unclear risk | Higher proportion of Ischaemic heart disease (18 vs 11) and of cerebrovascular disease (four vs two) in the epidural group |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 40 patients undergoing emergent femoral fracture repair (< 48 hours) |
| Interventions |
Spinal anaesthesia (n = 20): 3 mL of 0.75% bupivacaine at L3‐L4 General anaesthesia (n = 20): diazepam, fentanyl, nitrous oxide and pancuronium bromide |
| Outcomes | Mortality: The two deaths reported occurred early Pneumonia: no a priori criteria mentioned; diagnostic criteria unspecified |
| Notes | Early ambulation, no other thromboprophylaxis |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | High risk | Assessor (POCD) blinded to the anaesthetic technique used. Unspecified for the other outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropout or failed block reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether results apply to all enrolled participants |
| Other bias | Low risk | Groups well balanced for ASA physical status |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 423 patients undergoing elective peripheral vascular surgery (femoral or distal) |
| Interventions |
Spinal anaesthesia (n = 107): 16 to 20 mg of hyperbaric 1% tetracaine with 3 to 5 mg of phenylephrine at L3‐L4 or L4‐L5 Epidural anaesthesia (n = 96): at L2‐3 or L3‐4; 2% lidocaine followed by 0.5% bupivacaine to maintain a sensory level between T8 and T10. Epidural morphine was administered for the first 12 to 24 hours in 40% of participants General anaesthesia (n = 112): thiopental 2 to 4 mg/kg, fentanyl, succinylcholine, nitrous oxide, isoflurane or enflurane and vecuronium |
| Outcomes | Mortality: death occurring during the participant's hospitalization Myocardial infarction: ECG after surgery and daily for four days; CK every eight hours for 24 hours, then daily for three days; defined as new Q‐waves > 0.03 seconds with ↑ ST ≥ 1 mm in ≥ two leads or new ↓ ST ≥ 1 mm in ≥ two leads with ↑ CPK with > 5% MB fraction 68% (13/19) were silent, all occurred within four days. The study authors mention in the discussion that the rate of myocardial infarction might have been overestimated in light of the underlying pathology (CK‐MB elevation) Mortality was defined as cardiac death occurring during postoperative hospitalization |
| Notes | Unfractionated heparin 5000 units every 12 hours until ambulation and oral aspirin 81 mg daily until discharge thereafter. Presence/absence of complications from the anaesthetic technique are not mentioned |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done by computer program |
| Allocation concealment (selection bias) | Low risk | Placed in sealed envelopes. The envelopes were not opened until after eligible patients consented to participate in the study |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor (cardiologist) blinded to the anaesthetic technique used for myocardial infarction |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no missing outcome data reported in Bode 1996 (for 423 participants until hospital discharge or death). Missing data (surgical outcome) are not relevant to this overview |
| Selective reporting (reporting bias) | High risk | 423 participants selected from 705 consecutive eligible patients. Comment: no missing outcome data reported in Bode 1996 (for 423 participants until hospital discharge or death). Data analysed in intention‐to‐treat and per‐protocol. 32 failed blocks. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | From the review: "The Pierce 1997 was a post‐hoc analysis of the population recruited in Bode 1996 publication. Pierce presented graft function at 30 days in 264 of the 423 participants recruited into the original article." But for this overview, this does not apply. Slighlty less prior CHF (18.8% vs 27.3% and 28%) in the general anaesthesia group; otherwise, groups well matched |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 124 patients undergoing elective abdominal aortic surgery |
| Interventions |
General anaesthesia in both groups: midazolam, fentanyl, nitrous oxide, isoflurane and vecuronium Thoracic epidural anaesthesia (n = 59): T6‐T7 or T7‐T8 with 3 mL of 1.5% lidocaine with epinephrine 5 mcg/mL, followed (at completion of surgery) by 0.1 mL/kg of 0.125% bupivacaine with fentanyl 10 mcg/mL and an infusion adjusted for VAS scores ≦ three/10 (rest or movement) for 48 hours Morphine IVPCA (n = 65): adjusted for VAS scores ≦ three/10 (rest) for 48 hours |
| Outcomes | Mortality: during hospitalization Myocardial infarction: new Q‐waves ≧ 0.04 seconds' duration and ↓ ≧ 1 mm on the 12‐lead ECG, or CK‐MB ≧ 50 IU/L. Two‐lead Holter for 24 hours (validated by a cardiologist) |
| Notes | Heparin 0.5 mg/kg before infrarenal aortic cross‐clamping |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | Four with TEA and six with IV PCA excluded because of failure of Holter monitoring or epidural analgesia, or because of the use of analgesia not included in the protocol. One non‐Q myocardial infarction among the excluded TEA participants; no other complications in the excluded participants. This participant was included in the analysis of the overview |
| Selective reporting (reporting bias) | High risk | Data entered in intention‐to‐treat analysis for this overview. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Unclear risk | Slightly fewer smokers (33% vs 46%) in the epidural group; otherwise, groups well matched |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 40 patients undergoing elective abdominal aortic surgery |
| Interventions |
General anaesthesia in both groups: thiopental, fentanyl, nitrous oxide, isoflurane and neuromuscular blocking agents Lumbar epidural anaesthesia (n = 19): L2‐L3 or L3‐L4 with 2% lidocaine with epinephrine 5 mcg/mL, followed by 0.25% bupivacaine with morphine during the surgery and 0.125% bupivacaine with 0.1 mg/mL of morphine after the surgery adjusted for VAS scores four/10 (rest or movement) ≦ 48 hours Morphine IV PCA (n = 21) |
| Outcomes | Death: during hospitalization Myocardial infarction: no a priori definition. For the participant in the IVPCA group, MI with cardiogenic shock on the second postoperative day. For the participant in LEA, arm pain and elevated cardiac enzyme (value not provided) Pneumonia: no definition and no details (during hospitalization) |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Open randomized: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open study |
| Blinding of outcome assessment (detection bias) | Unclear risk | ST depression verified by a blinded assessor |
| Incomplete outcome data (attrition bias) | High risk | Failure to proceed to surgery as planned led to withdrawal. Two participants in LEA discontinued because of severe pruritus: One received bupivacaine‐fentanyl and the other IV PCA with meperidine at 30 hours. Naloxone for one participant in each group |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat (see above). Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Unclear risk | Higher blood loss (1610 mL vs 1017 mL) and longer surgical time (227 minutes vs 188 minutes) for IVPCA group |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 90 patients undergoing elective major abdominal surgery |
| Interventions |
General anaesthesia in both groups: thiopental or etomidate, sufentanil, nitrous oxide, isoflurane and vecuronium Thoracic epidural anaesthesia (n = 60): T7‐T8 or T8‐T9 with 2% lidocaine with epinephrine 5 mcg/mL, followed by 0.125% bupivacaine with sufentanil (n = 30) or morphine (n = 30) adjusted for VAS scores ≦ four at rest or six at movement for 48 hours Fentanyl infusion followed by IM injections (n = 30) |
| Outcomes | Mortality during hospitalization |
| Notes | Complications of epidural techniques recorded: no neurological sequelae |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned: no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded to epidural or IM |
| Blinding of outcome assessment (detection bias) | Low risk | Observer blinded to the route of administration. Sham epidural catheter on the skin, connected to an empty syringe in an infusion pump, which was covered to shield its content. The same cover was used for participants in the TEA group |
| Incomplete outcome data (attrition bias) | Low risk | No participant lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat for this overview |
| Other bias | Low risk | Groups well balanced |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 42 patients undergoing elective open colorectal surgery |
| Interventions |
General anaesthesia in both groups: thiopental, fentanyl, nitrous oxide, isoflurane and vecuronium Thoracic epidural anaesthesia (n = 21): T8 or T9 with 15 to 20 mL of 0.5% bupivacaine for a sensory block from T4 and S5, followed by 5 mL of 0.5 bupivacaine every hour during the surgery and epidural analgesia with 0.1% bupivacaine and fentanyl 2 mcg/mL adjusted for VAS scores < five/10 at rest for up to four days after the surgery Morphine IV PCA (n = 21): adjusted for VAS scores < five/10 at rest |
| Outcomes | Mortality Myocardial infarction: no definition provided |
| Notes | Antibiotic prophylaxis, early feeding and mobilization starting the day after surgery |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated at random: no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Data provided for all participants |
| Selective reporting (reporting bias) | Low risk | Data provided for measurements specified in the method section |
| Other bias | Low risk | Group well balanced |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 100 patients undergoing elective lower extremity revascularization |
| Interventions |
Lumbar epidural anaesthesia (n = 49): L2‐L3 or L3‐L4 with 3 mL of 1.5% lidocaine with epinephrine, followed by 7 mL of 0.75% bupivacaine through the needle and additional doses through a catheter during the surgery for sensory level at T8. Epidural fentanyl infusion for 24 hours after the surgery General anaesthesia (n = 51): with thiamylal, fentanyl, morphine, succinylcholine, tracheal lidocaine spray, nitrous oxide, enflurane and pancuronium. Morphine IV PCA after the surgery |
| Outcomes | Mortality: Data are available for zero to seven days and for zero to six months. For this overview, the zero to seven days was taken as zero to 30 days, and the difference between zero to seven and zero to six months was taken as one to six months Myocardial infarction (MI): based on preoperative 12‐lead ECG, day of surgery and on postoperative days one, two, three and seven; CK‐MB every six hours during ICU stay, then daily through postoperative day three; information on chest pain during the first seven days; autopsies/death certificates (blinded). MI diagnosed on Lipid Research Clinic, ECG according to Minnesota Code Pneumonia: defined as a new infiltrate on a chest x‐ray combined with the appearance of two of the following conditions within 24 hours of the radiological abnormality: a temperature greater than 38ºC, a leukocyte count above the normal range or the identification of a pathogen by sputum Gram stain or culture. Data not provided separately from sepsis |
| Notes | IV heparin according to the surgeon. When an infusion of IV heparin was given before the surgery, it was stopped four hours before the participant's arrival to the operating theatre. After surgery, heparin was administered to participants with diminished blood flow The trial was stopped prematurely by a monitoring committee (120 planned) on the basis of lower rate of regrafting/embolectomy in the epidural group (two vs nine) and no apparent benefit of general anaesthesia |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified (cardiac risk by the surgeon) within blocks of variable sizes arranged in random order |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned in the operating room immediately before surgery. Allocation was done immediately before the procedure, but unclear how sequence was concealed |
| Blinding of participants and personnel (performance bias) | High risk | Open study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Cardiologists assessing cardiac outcomes were blinded to the anaesthetic technique; all other outcomes were assessed openly |
| Incomplete outcome data (attrition bias) | High risk | Overall rate of missing data was 1.9% in participants assigned to the general anaesthesia regimen and 3.1% in participants assigned to epidural anaesthesia and analgesia |
| Selective reporting (reporting bias) | High risk | Intention‐to‐treat. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Unclear risk | Group well balanced, except slightly more diabetic participants in the GA group (41% vs 29%) |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 101 patients undergoing lower limb vascular surgery |
| Interventions |
Spinal anaesthesia (n = 50): 1.4 to 1.6 mL of hyperbaric 0.5% cinchocaine with 0.1 to 0.2 mL of epinephrine 1:1000 for a sensory level ≧ T10 General anaesthesia (n =51): thiopental, fentanyl, nitrous oxide, halothane and alcuronium or pancuronium bromide |
| Outcomes | Mortality: until discharge (all those deaths occurred within 30 days) Myocardial infarction: significant myocardial Ischaemic episode defined as ST segment depression > 1.0 mm on a correctly calibrated paper; followed for occurrence of chest pain consistent with Ischaemic heart disease. Real definition of myocardial infarction not provided Pneumonia: fever plus productive sputum or chest x‐ray changes |
| Notes | Heparin 5000 U IV before vascular clamping |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All data are available for the outcomes retained for this overview |
| Selective reporting (reporting bias) | Low risk | The outcome data were reported adequately. |
| Other bias | Low risk | Groups well balanced for ASA physical status scores and age (67.1 vs 66.4 years of age) |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 100 patients > 80 years of age undergoing emergent femoral fracture repair (< 24 hours of admission) |
| Interventions | Lumbar epidural anaesthesia (n = 50): single shot (n = 34) or continuous (n =16) with 0.5% bupivacaine with epinephrine 5 mcg/mL (with 2% lidocaine in some participants) General anaesthesia (n = 50): thiopental, nitrous oxide, dextromoramide or methoxyflurane, succinylcholine or pancuronium bromide |
| Outcomes | Mortality: Data are provided for zero to 11 days (kept as zero to 30 days) and at 11 days to three months (not kept) Myocardial infarction: ECG (ECG before and after [3 hours] the surgery and at 1, 3 and 10 days). New waves compatible with necrosis |
| Notes | Early mobilization and anti‐vitamin K from third postoperative day |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Drawing" (tirage au sort) |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Data are given for all participants |
| Selective reporting (reporting bias) | High risk | Data provided for measurements specified in the method section. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups well balanced for age, hypertension, abnormal ECG, vasculitis, chronic bronchitis with respiratory insufficiency, cerebrovascular accident, senility and Parkinson |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 75 consecutive patients < 75 years of age undergoing open cholecystectomy |
| Interventions | General anaesthesia in all participants with thiopental, intravenous narcotics and inhalational agents Thoracic epidural anaesthesia (n = 25): at the lower thoracic region, with an age‐related dose of 0.5% bupivacaine and kept for 12 hours Morphine IV infusion (n = 25) or IM (n = 25) |
| Outcomes | Pneumonia: Preoperative respiratory status was established by a questionnaire, a clinical examination and chest radiography. Chest infection was defined as pyrexia, production of purulent sputum, clinical signs of infection and radiological evidence of collapse persisting for longer than 72 hours. Antibiotics were given at the discretion of the clinician involved |
| Notes | 1.5 g cefuroxime was given intravenously before skin incision H Influenzae or S pneumoniae were isolated from eight/12 participants who developed chest infection No serious complications occurred in the epidural group |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized: no other details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Participants were assessed daily during the postoperative period by a single observer |
| Incomplete outcome data (attrition bias) | Unclear risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Attempts to insert the epidural catheter failed in four participants, who therefore received intermittent intramuscular morphine 10 mg as required. Data from these four participants were included in the epidural group for the purpose of analysis. The single chest infection of the TEA group developed in one of these technical failures |
| Other bias | Low risk | The groups were comparable in terms of physical characteristics, history of respiratory disease, smoking habits and duration of anaesthesia. Smokers 24/50 versus 11/25 and respiratory disease 20/50 and 11/25 (IV/IM and TEA, respectively) |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 50 consecutive patients scheduled for elective abdominal aortic surgery |
| Interventions | General anaesthesia in all participants with thiopental, fentanyl, nitrous oxide, enflurane and pancuronium bromide Thoracic epidural analgesia (n = 25): at T9‐T10 with 2 mL of 1.5% lidocaine with epinephrine 5 mcg/mL, followed by 5 mL hourly during surgery and 0.5% bupivacaine after surgery for 72 hours IV morphine analgesia (n = 25): 2 to 5 mg/h |
| Outcomes | Death Myocardial infarction: new Q‐waves, 0.04 seconds' duration, 1 mm amplitude; or increased CPK (MB) considered to be diagnostic of myocardial damage with/without ECG changes; or recent myocardial infarction at autopsy. CK‐MB die for three days Pneumonia: infiltatres on chest x‐ray, plus two of temperature > 38ºC, raised white blood cell count or positive sputum. Chest x‐ray for three days |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Independent anaesthetist |
| Incomplete outcome data (attrition bias) | Low risk | Data provided for all participants |
| Selective reporting (reporting bias) | High risk | One failed epidural kept in intention‐to‐treat. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | High risk | Groups well balanced for age, ASA physical status and preoperative coexisting diseases except chronic airways disease (five/25 vs 16/25 for control and TEA, respectively). |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 132 patients > 50 years of age undergoing emergent (< three days from injury) femoral fracture repair |
| Interventions | Spinal anaesthesia (n = 64) : 5 to 10 mg tetracaine in 6% dextrose with epinephrine 1:100,000 (n = 51) or 4.5 to 9 mg hyperbaric cinchocaine (n = 13) General anaesthesia (n = 68): diazepam, fentanyl, nitrous oxide and pancuronium bromide |
| Outcomes | Mortality: zero to 28 days Pneumonia: confirmed by chest x‐ray, include aspiration pneumonia |
| Notes | No thromboprophylaxis other than early mobilization and physiotherapy |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Data provided for all participants |
| Selective reporting (reporting bias) | High risk | Eight failed spinal converted to GA, not in intention‐to‐treat |
| Other bias | Low risk | Group well balanced for age, delayed injury‐surgery and incidence of major systemic disease. |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 538 patients > 55 years of age undergoing emergent femoral fracture repair |
| Interventions | Spinal anaesthesia (n = 259): with hypo‐/isobaric tetracaine, nupercaine or bupivacaine for spinal versus General anaesthesia (n = 279): with thiopental, fentanyl, nitrous oxide and non‐depolarizing neuromuscular blocking agents. Avoidance of halothane and droperidol as possible |
| Outcomes | Mortality (zero to 28 days) |
| Notes | Postoperative follow‐up from three to 30 months for a subgroup only (New Zealand participants representing 89% of the total; GA = 164; RA = 149). These figures were not retained (selective reporting). 279 participants randomly assigned to GA and 259 randomly assigned to RA for a total of 538 participants Total number of deaths for the first 28 days = 31 in Table V and 33 in the text. We retained 33 (17 for RA and 16 for GA) because those are the numbers closest to the percentage reported in the results in various sections of the text. |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated, the sexes being randomly assigned separately within each hospital. We considered it unlikely that a pseudo‐randomization technique could be different for women versus men |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | One anaesthesiologist/hospital |
| Incomplete outcome data (attrition bias) | Unclear risk | 11 excluded from the analysis from prespecified exclusion criteria |
| Selective reporting (reporting bias) | High risk | Intention‐to‐treat: 30 participants (11.5%) unsuccessful puncture and 14 (5.4%) incomplete block requiring additional analgesia. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Unclear risk | ASA physical status not reported separately for the two anaesthetic techniques |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 82 patients ≧ 50 years of age undergoing lower limb revascularization |
| Interventions |
Lumbar epidural anaesthesia (n = 37): 1.5% lidocaine with epinephrine for a sensory level at T6 for the surgery and 0.125% of bupivacaine with fentanyl 3 mcg/mL as an infusion in some participants General anaesthesia (n = 42): with thiopental, fentanyl, inhalational and neuromuscular blocking agents. IV opiates after the surgery |
| Outcomes | Mortality up to one year. Data provided for in‐hospital time (taken as zero to 30 days) and for zero to 12 months Myocardial infarction: defined as elevated creatine phosphokinase‐MB (CPK‐MB) in blood with new Q‐waves on the ECG or persistent electrocardiographic abnormalities and a diagnosis corroborated by an independent cardiologist Pneumonia: new infiltrate on chest x‐ray with temperature greater than 38 ºC, elevated white blood cell count and positive sputum culture. |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized non‐blinded clinical trial. Methods of randomization and blinding were not described. Number: 77 participants scheduled for femoral‐popliteal or femoral‐distal revascularization surgery, older than 49 years of age. Participants were randomly assigned to receive epidural or general anaesthesia. However, five participants had a second operation on the other leg for which they received the type of anaesthetic that they had not had for the first operation (a total of 82 operations). The trial authors report the number of outcomes for 82 operations, not for 77 participants. This had led to some ’unit of analysis’ problems |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded clinical trial |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded clinical trial |
| Incomplete outcome data (attrition bias) | Low risk | From the reviewer: "This study addressed two outcomes of interest for this review: mortality and myocardial infarction. No missing outcome data" |
| Selective reporting (reporting bias) | High risk | From the review: "Mortality was not reported during one year of follow up". From the protocol of the overview: "If one of the outcomes of interest to this overview was not an outcome in the study (not included in the method section), this will not be considered as selective reporting." Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Group well balanced |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 70 preeclamptic pregnant women undergoing emergent caesarean section for non‐reassuring fetal heart rate (< 100 or > 150 bpm; absent variability (< five bpm) of 60 minutes' duration; repetitive decelerations) Magnesium sulfate 4 G IV followed by 1 G/h IV |
| Interventions |
Spinal anaesthesia (n = 35): 1.8 mL of hyperbaric 0.5% bupivacaine with 10 mcg of fentanyl at L3‐L4 General anaesthesia (n = 35): thiopental 5 mg/kg, nitrous oxide, isoflurane and succinylcholine drip. intravenous magnesium sulfate for control of the pressor response to tracheal intubation |
| Outcomes | Mortality (fetal) |
| Notes | Fetal loss is included in this overview. There was an undiagnosed abruptio placentae, and haemodynamic reaction to laryngoscopy may aggravate this condition |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were said to have been randomly assigned by sealed envelopes. Unlikely to be quasi‐randomized if sealed envelopes were used |
| Allocation concealment (selection bias) | Low risk | Women were said to have been randomly assigned by sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment: The paediatrician was blinded to the type of anaesthesia used |
| Incomplete outcome data (attrition bias) | Low risk | No mothers were excluded but no data were provided for one neonate in the general anaesthesia group, as its mother suffered a stillbirth. Intention‐to‐treat: not stated (but all women remained in their allocated groups) |
| Selective reporting (reporting bias) | High risk | No data for one neonate in the general anaesthesia group, as its mother suffered a stillbirth from an abruptio placentae. This participant is included in this overview. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups seem well balanced for fetal heart rate abnormalities |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 99 patients undergoing elective aortic reconstructive surgery |
| Interventions | General anaesthesia for all participants with midazolam, fentanyl, isoflurane and pancuronium bromide Lumbar epidural anaesthesia (n = 48): at T12‐L1 loaded with 10 to 15 mL of 2% carbonated lidocaine and meperidine 2 mg/mL before incision, followed by an infusion of bupivacaine 0.1% with meperidine 2 mg/mL during and after the surgery until the morning of postoperative day two IV morphine infusion (n = 51): at 2 to 10 mg/h IV |
| Outcomes | Mortality (during hospitalization) Myocardial infarction: new Q‐waves on the ECG or an increase in creatinine phosphokinase (CK‐MB) > 5% of the rise in CPK and a minimum of 15 IU with or without ECG changes. ECG every 12 hours for 48 hours CPK‐MB every 12 hours for 72 hours Pneumonia: clinical findings suggestive of the diagnosis (temperature, positive sputum culture, pulmonary signs on physical examination) plus radiological findings to support the diagnosis |
| Notes | Study stopped by a committee for futility (Ischaemic events) |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 12 participants excluded for Holter failure |
| Selective reporting (reporting bias) | High risk | Data of excluded participants not reported. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Unclear risk | Higher number with history of CHF in the GA group (five vs zero) |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 20 pregnant women with preeclampsia/eclampsia treated with magnesium sulfate requiring emerging caesarean section |
| Interventions |
Lumbar epidural anaesthesia (n =10): with 12 mL of 0.75% bupivacaine at L1‐L2 or 20 mL at L3‐L4 General anaesthesia (n = 10) |
| Outcomes | Mortality (fetal) |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated: no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Data reported for all participants |
| Selective reporting (reporting bias) | High risk | Complications related to regional anaesthesia unspecified |
| Other bias | Low risk | Group well balanced for eclampsia/preeclampsia |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 43 patients with coronary heart disease undergoing emergent fracture hip repair |
| Interventions |
Spinal anaesthesia (n = 29): with incremental (n = 14; for a sensory level ≧ T10) or single shot (n = 15; 2.5 mL) 0.5% bupivacaine General anaesthesia (n = 14): with thiopental, fentanyl, nitrous oxide, enflurane and atracurium |
| Outcomes | Mortality (one month) Myocardial infarction: World Health Organization (WHO) criteria for 1998 The WHO European Myocardial Infarction registry criteria were based on clinical history, findings on the electrocardiogram (ECG), enzyme measurements in blood and postmortem findings. MI was diagnosed in the presence of one of the following:
or
or
or
In the revised WHO criteria used in the multi‐centre MONICA project conducted in the 1970s–80s, Minnesota coding was used to evaluate the ECG rather than the subjective methods of the above criteria. Explicit coding rules were defined for enzymes and symptoms. Most important, all possible situations with incomplete information on the ECG, enzymes or symptoms were covered |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor blinded to the anaesthetic technique for myocardial ischaemia |
| Incomplete outcome data (attrition bias) | High risk | 11 participants excluded for various reasons, data not provided |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups similar for ASA physical status |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 20 ASA 3 patients scheduled for aortic surgery |
| Interventions | General anaesthesia for all participants with diazepam, thiopental, fentanyl, nitrous oxide, isoflurane and pancuronium bromide Lumbar epidural analgesia (n = 10): at T12‐L1, 10 to 14 mL of 0.5% bupivacaine through the needle before catheter insertion, bupivacaine 0.5% at the end of surgery and up to 7 mL/h of bupivacaine 0.05% with fentanyl 10 mcg/mL after the surgery IV fentanyl during the surgery and oxycodone 3 to 5 mg IV (n = 10): on request and IV clonidine for hypertension after the surgery for the group without epidural |
| Outcomes | Death zero to 30 days |
| Notes | Heparinization for activated clotting time (ACT) 200 s |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Data reported for the 20 participants |
| Selective reporting (reporting bias) | High risk | Results provided for measurements mentioned in the method section. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups well balanced |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 54 patients scheduled to undergo elective open partial resection of the colon |
| Interventions | Genral anaesthesia for all participants (n = 54 [52 retained for analysis]): with Thoracic epidural anaesthesia (n = 40): at T8‐T10 with bupivacaine and morphine (n = 14), morphine alone (n = 12) or bupivacaine alone (n = 14) during and after the surgery adjusted for VAS scores < five/10 at rest Morphine IV PCA (n = 12) adjusted for VAS scores < five/10 at rest |
| Outcomes | Mortality during hospitalization (taken as zero to 30 days) |
| Notes | Early ambulation and feeding but specific thromboprophylaxis not reported |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization tables (one for each institution and stratified for the surgical site) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Double‐blinding of the type of solution in the epidural |
| Blinding of outcome assessment (detection bias) | High risk | Double‐blinding of the type of solution in the epidural |
| Incomplete outcome data (attrition bias) | High risk | Two participants withdrawn because the epidural could not be inserted |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat analysis |
| Other bias | Low risk | Groups well balanced for age and underlying pathology |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 150 patients undergoing femoral fracture repair |
| Interventions |
Spinal anaesthesia (n = 75): 1.3 to 1.5 mL of hyperbaric 0.5% cinchocaine at L3‐L4 or L4‐L5 General anaesthesia (n = 75): althesin, succinylcholine, nitrous oxide and halothane |
| Outcomes | Mortality: data taken from the legend of the graph |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated randomly: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | Fate at 12 months unknown for four/73 and two/75 participants for spinal and general anaesthesia, respectively |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat: Two participants were not included in the analysis because of failed block. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Group well balanced for age and interval between admission and surgery |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 116 patients undergoing femoral fracture repair |
| Interventions |
Spinal anaesthesia (n = 56): 0.5 mL of hyperbaric 0.5% cinchocaine at L3‐L4 General anaesthesia (n = 60): althesin, fentanyl, nitrous oxide and pancuronium bromide |
| Outcomes | Mortality |
| Notes | Methods come from a study published on a subset of the participants (n = 55); results were presented in a Table in a review article |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | High risk | Methods are reported for a subset of participants (55/116) only |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 42 males undergoing uncomplicated aortic replacement of an infrarenal abdominal aortic aneurysm |
| Interventions | General inhalational anaesthesia with fentanyl, nitrous oxide, enflurane and pancuronium bromide for all participants with or after the surgery Thoracic epidural analgesia (n = 22): at T9‐T10 or T10‐T11 loaded with bupivacaine for a sensory level of T4 and epidural morphine for at least 48 hours postoperatively Morphine IV PCA (n = 20) |
| Outcomes | Mortality in hospital taken as zero to 30 days |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned; no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | One participant in the IV PCA reoperated for gangrenous sigmoid colon, fate unknown |
| Selective reporting (reporting bias) | High risk | Two failed blocks and one reoperation in the PCA group excluded from the analysis |
| Other bias | Unclear risk | Groups well balanced for age, clamping time and hospital stay for the rest of the participants |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 168 patients undergoing elective abdominal aortic reconstructive surgery |
| Interventions | General anaesthesia with thiopental, fentanyl, nitrous oxide, enflurane and pancuronium bromide and thoracic epidural catheter for all participants Thoracic epidural anaesthesia (n = 85): intraoperative epidural anaesthesia (0.5% bupivacaine with fentanyl) followed by epidural analgesia (0.0625% bupivacaine and fentanyl 5 mcg/mL) (n = 46) or fentanyl IV PCA (n = 39) for 72 hours IV fentanyl (n = 75), followed by epidural analgesia (n = 38) or fentanyl IV PCA (n = 37) for 72 hours |
| Outcomes | Death (during hospitalization) Myocardial infarction: cardiac death and non‐fatal myocardial infarction determined by a blinded cardiologist ECG: preop, postop and at days one, two, three (interpreted according to Minnesota code) and seven; total CK and MB isoenzymes every six hours for 24 hours and then through postoperative day three; chest pain during first seven days. The diagnosis of MI required new Q‐waves of at least 0.04 seconds' duration and a minimum of 1 mm depth on 12‐lead electrocardiogram, or ischaemic electrocardiogram changes associated with an increase in creatinine phosphokinase with a greater than 5% MB fraction Pneumonia: Pneumonia was defined as a new infiltrate on chest radiograph combined with the appearance of two of the following conditions within 24 hours of the radiological abnormality: temperature greater than 38.5°C, leukocyte count greater than 10,000 or the identification of a pathogen by sputum Gram stain or culture. Treatment with an antibiotic was required for the diagnosis of pneumonia |
| Notes | The trial was stopped after 168 participants were randomly assigned by the monitoring committee for futility Heparin 100 U/kg before aortic cross‐clamping |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients consenting to enrolment were stratified by surgeon. Within strata, treatment regimens were assigned according to a randomization scheme containing variably sized, balanced blocks of treatment assignments |
| Allocation concealment (selection bias) | Low risk | The evening before surgery, the JHH Investigational Pharmacy determined the participant's treatment assignment and prepared the masked study medications |
| Blinding of participants and personnel (performance bias) | Low risk | Masked study medication delivered by the pharmacy |
| Blinding of outcome assessment (detection bias) | Low risk | Masked study medication delivered by the pharmacy |
| Incomplete outcome data (attrition bias) | High risk | Eight participants (two in each group) enrolled in the pilot study; their data have been added for mortality. For MI and pneumonia, the trial authors provided the data for participants who survived until hospital discharge |
| Selective reporting (reporting bias) | High risk | MI and pneumonia among participants who did not survive until hospital discharge not provided. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | High risk | Half the participants in the GA group (as remodelled for this overview) received postoperative epidural analgesia |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 1015 males undergoing abdominal aortic, open gastric, open biliary or open colon surgery |
| Interventions | General anaesthesia with thiopental, midazolam, fentanyl, nitrous oxide, isoflurane and vecuronium for all participants Thoracic or lumbar epidural anaesthesia (n = 514): 0.5% bupivacaine with epinephrine 5 mcg/mL for a sensory level ≧ T6, reinjected during the surgery and maintained as required for postoperative analgesia with morphine with/without local anaesthetics IV or IM opioids (n = 501) |
| Outcomes | Mortality: participant who died within 30 days after operation Myocardial infarction: an increase in serum concentration of the myocardial‐specific isoenzyme fractions of creatine kinase (CK‐MB) and lactic dehydrogenase, as evidenced by a ratio of CK‐MB/CPK of 5% or more AND/OR the following ECG changes: a typical new persistent elevation/depression of the ST segment and/or a new Q‐wave greater than 0.04 seconds in duration with its depth more than 25% of the amplitude of the succeeding R‐wave in limb leads, or any new Q‐wave in V1–V3. All study participants, in addition to the routine postoperative tests, had a 12‐lead electrocardiogram taken on the first and third postoperative days. Total creatine phosphokinase and MB isoenzymes were measured every 12 hours for three days after surgery Pneumonia: the presence of a new infiltrate on the chest x‐ray plus two of three clinical findings (a body temperature higher than 38°C, an abnormal elevation of white blood cell count and a pathogen identified in the sputum by Gram stain and culture), requiring intravenous antibiotic treatment |
| Notes | For participants undergoing abdominal aortic surgery, if frank blood was aspirated during the epidural procedure, the procedure was abandoned and rescheduled Heparin as required. Normal PT, PTT for epidural catheter removal. Antibiotics before surgery and for 24 hours after surgery |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using an adaptive randomization scheme, 13 within each of the 15 sites, we allocated participants to one of two treatment groups to balance between the groups the following prognostic variables: surgical type (aortic, gastric, biliary or colon), age (younger than 50 years, 50 to 70 years, older than 70 years) and Goldman index (≤12 versus ≥ 13). |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 37 participants withdrawn, leaving 489 for epidural and 495 for IV/IM groups. 11 lost to 30 days' follow‐up |
| Selective reporting (reporting bias) | High risk | Unclear; see above. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups well balanced, except more smokers among aortic participants with epidural, male only |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 44 patients undergoing elective open elective bowel resection |
| Interventions | General anaesthesia in both groups (no details) Thoracic epidural anaesthesia (n = 23): between T10 and T12; test dose with 3 mL 1.5% lidocaine with epinephrine 5 mcg/mL, followed by 100 mcg of fentanyl and 10 mL of 0.1% bupivacaine with fentanyl 5 mcg/mL at peritoneal closure and an infusion at 8 to 10 mL after the surgery IV morphine or meperidine PCA (n = 21): A background infusion could be added if required |
| Outcomes | Mortality: One participant died from an anastomotic leak after a prolonged hospital stay, exact day not reported, taken as zero to 30‐day mortality Pneumonia: "We had no patients that had any respiratory tract infections"; taken as 0 pneumonia |
| Notes | Antibiotic prophylaxis |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | The study was not blinded |
| Blinding of outcome assessment (detection bias) | High risk | The study was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Five participants removed from the analysis: One participant (TEA) required mechanical ventilation for 24 hours after surgery, three participants (PCA) were unable to provide pain scores and one participant (PCA) was found to have extensive bowel necrosis at laparotomy and did not undergo resection |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Unclear risk | Zero participants with diabetes in the TEA group versus six in the IVPCA group; not specified whether the death occurred in a diabetic participant |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 915 patients undergoing major abdominal surgery with one of nine defined comorbid states to identify high‐risk status |
| Interventions | General anaesthesia for all participants Epidural anaesthesia (n = 447): site of the epidural (to be selected by the attending anaesthetist to match the planned incision), epidural local anaesthetics and opioids during and after the operation (continuous infusions of bupivacaine or ropivacaine, supplemented with pethidine or fentanyl) for 72 hours IV (PCA or physician‐controlled) opioid infusions initially (n = 441) |
| Outcomes | Mortality: death from any cause within 30 days of surgery Pneumonia: new chest x‐ray infiltrate plus two or more of temperature 38°C, white cell count 12,000 and positive sputum culture |
| Notes | No major adverse consequences of epidural catheter insertion were reported. In no case was any catheter removed because of inflammation of the epidural site, and in no case were any serious complications of epidural catheter placement or adverse sequelae directly attributable to placement of the epidural catheter |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted random blocks with stratification by study centre |
| Allocation concealment (selection bias) | Low risk | Allocated by a central 24‐hour randomization service to control or epidural group |
| Blinding of participants and personnel (performance bias) | High risk | Masking participants and clinical staff for three days was impossible |
| Blinding of outcome assessment (detection bias) | Low risk | Data were encoded, entered into computer and analysed centrally in the Trial Secretariat at the University of Western Australia |
| Incomplete outcome data (attrition bias) | Low risk | Complete results |
| Selective reporting (reporting bias) | Low risk | 23 participants whose surgery was cancelled after randomization and four who were randomly assigned for an ineligible procedure were excluded from analysis. 19 participants who were listed for an eligible procedure at the time of randomization subsequently underwent a non‐eligible operation. By the intention‐to‐treat principle, these participants were included in the primary analysis |
| Other bias | High risk | Group well balanced. High failure rate of epidural at 72 hours: 183 inserted preoperatively and removed before 72 hours, thus 225 fully compliant with the protocol in the epidural group. Of 441 participants assigned to the control group, 19 (4.3%) had epidural analgesia established preoperatively or within 72 hours of surgery, and of those assigned to the epidural group, 29 (6.5%) did not receive an epidural |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 70 women > 75 years undergoing emergent femoral fracture repair (postponed 12 to 24 hours after admission) |
| Interventions |
Spinal anaesthesia (n = 35): 3 mL of hyperbaric 0.5% bupivacaine with epinephrine 5 mcg/mL at L3‐L4 General anaesthesia (n = 35): thiopental, fentanyl, lidocaine spray, nitrous oxide, enflurane and vecuronium |
| Outcomes | Mortality up to three months Pneumonia: clinical and radiological criteria |
| Notes | Thromboprophylaxis: out of bed at day one, physiotherapy unfractionated heparin (keeping normal PTT) |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly divided into two groups according to randomization table (permutations au hasard de Cochran et Cox) |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No failed block mentioned |
| Selective reporting (reporting bias) | High risk | Data provided for all enrolled participants. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups well balanced |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 105 patients undergoing elective abdominal aortic surgery |
| Interventions | General anaesthesia with thiopental, succinylcholine, nitrous oxide and pancuronium bromide for all participants Thoracic epidural anaesthesia (n = 35): at T7‐8 or T8‐9 with bupivacaine 0.5% for sensory blockade at levels between T3 and T5 (cephalad) and L2 and L3 (caudal) in combination with light general anaesthesia with diazepam. Bupivacaine 0.25% after the surgery Piritramide after the surgery (n = 70): taken as IV (not specifically written). Halothane (n = 30) or neuroleptanalgesia with fentanyl and droperidol (n = 40) |
| Outcomes | Mortality (during hospitalization) |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned: no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | High risk | Mortality data during hospitalization provided for all participants according to randomization group. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups well balanced for ASA physical status and duration of surgery |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 48 patients undergoing elective open colonic surgery |
| Interventions | General anaesthesia for all participants Lumbar epidural anaesthesia (n = 24): at L2‐L3 with bupivacaine 0.25% 6 to 12 mL/h (for T4‐T8 sensory level) for 48 hours IV pentazocine 10 mg/h for 48 hours (n = 24) |
| Outcomes | Mortality |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether random assignment was done before or after enrolment |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | High risk | Results seem complete but no mention about complications of epidurals |
| Other bias | Low risk | Groups seem well balanced |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 60 patients undergoing elective colonic surgery |
| Interventions | General anaesthesia for all participants with thiopental, fentanyl, nitrous oxide, enflurane and vecuronium The catheter was inserted with its tip at a level responding to the middle of the planned incision (n = 45). 4 mL of 0.5% bupivacaine, followed by 0.25% bupivacaine at 4 to 6 mL/h for 48 hours (n = 15) or epidural morphine 2 mg for 50 kg of body weight + 1 mg for each additional 10 kg of body weight daily for 48 hours (n = 15) or epidural morphine 2 to 6 mg/d as a continuous infusion for 48 hours (n = 15) IM oxycodone 0.15 mg/kg (n = 15) |
| Outcomes | Mortality in hospital, taken as zero to 30 days |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated, no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | High risk | Unlikely |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Results seem complete. No case of accidental dural puncture occurred during the study |
| Other bias | Low risk | Groups well balanced |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 292 patients undergoing infrarenal aortic bypass, gastric resection, gastrectomy, duodenum‐preserving pancreatic resection, Whipple's operation or cystectomy and neobladder formation |
| Interventions | General anaesthesia in all participants with midazolam, fentanyl, nitrous oxide, halothane, enflurane or isoflurane and pancuronium bromide Thoracic or lumbar epidural anaesthesia: 0.25% bupivacaine (n = 183) for the surgery and continued with a mixture of bupivacaine 0.25% and morphine (60 mcg/mL) at 0.1 mL/kg/h for 72 hours after the surgery (n = 93) or replaced by morphine alone 0.05 mg/kg in 10 mL of saline on request for 72 hours (n = 90) Morphine IV PCA (n =106) |
| Outcomes | Mortality in hospital, taken as zero to 30 days |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (from Geigy tables) and stratified for age, sex and type of operation |
| Allocation concealment (selection bias) | Unclear risk | Unclear; not mentioned |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | High risk | 47 dropouts not kept for the analysis: nine in the PCA group and 38 in the epidural groups (operation changed or not done, participant chose other anaesthetic method, epidural impossible or contraindicated) |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat. Complications of epidurals not mentioned |
| Other bias | Low risk | Groups well balanced |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 100 consecutive patients undergoing emergent femoral fracture repair |
| Interventions |
Spinal anaesthesia General anaesthesia |
| Outcomes | Mortality |
| Notes | Abstract, very little information, duration of follow‐up not mentioned, mortality numbers taken as zero to 30 days. The exact number of participants in each group not specified, taken as 50:50 |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random selection: no details |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not enough information to judge |
| Incomplete outcome data (attrition bias) | Unclear risk | Not enough information to judge |
| Selective reporting (reporting bias) | High risk | Not enough information to judge, presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Unclear risk | Not enough information to judge |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 114 patients undergoing hip surgery |
| Interventions |
Spinal anaesthesia (n = 57): 3 to 4 mL of hyperbaric 0.5% bupivacaine + prior three‐in‐one block General anaesthesia (n = 57): fentanyl, nitrous oxide and isoflurane |
| Outcomes | Mortality at two weeks, taken as zero to 30 days |
| Notes | Abstract |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned: no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No failed block reported |
| Selective reporting (reporting bias) | High risk | Data for mortality provided for both groups. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups said to be well balanced for age, ASA physical status and coexisting diseases |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 578 patients > 50 years undergoing femoral fracture repair |
| Interventions |
Spinal anaesthesia (n = 281): 3 to 4 mL of isobaric 0.5% bupivacaine General anaesthesia (n = 297): thiopental, succinylcholine (or gallamine), nitrous oxide, gallamine and either enflurane or droperidol and fentanyl |
| Outcomes | Mortality. Deaths taken from a National Registry up 10 months after inclusion of the last participant. Data retained up to nine months |
| Notes | Mobilization at day two or three, antiembolic stockings, no routine anticoagulant therapy and no antibiotics. Chest physiotherapy |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated: no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Two lost to follow‐up, excluded from the analysis. Data retained up to nine months only |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat; 84 excluded from the analysis after having been admitted into the study. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | High risk | More ASA 3 in the GA group and more ASA 4 in the spinal group. Mortality was related to ASA classification (P < 0.0005) |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | Pregnant women with severe preeclampsia undergoing emergent caesarean section for indications other than non‐reassuring fetal heart rate patterns |
| Interventions |
Neuraxial blockade (n = 54): lumbar epidural anaesthesia (n = 27): 18 to 23 mL of 2% lidocaine or 3% chloroprocaine in fractionated doses through a catheter for a sensory level at T4, or combined spinal‐epidural anaesthesia (n = 27) with 1.5 mL of hyperbaric 0.75% bupivacaine supplemented with additional boluses of 3 mL of 0.5% bupivacaine. Epidural fentanyl and morphine after delivery General anaesthesia (n = 26): thiopental, succinylcholine, lidocaine (1.5 mg/kg) and nitroglycerin (50‐mcg/kg boluses, maximum dose 200 mcg) before intubation, nitrous oxide, isoflurane 0.7%, atracurium or vecuronium. Fentanyl and morphine after delivery |
| Outcomes | Mortality (fetal) |
| Notes | Excluded if platelet count < 100,000/mm3. Specifically written: 84 live‐born infants and no neonatal deaths No serious maternal or fetal complications attributable to any of the three anaesthetic methods |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Six women withdrawn from participation. One failed epidural converted to GA. No serious maternal or fetal complications attributable to any of the three anaesthetic methods. 84 live‐born infants and no neonatal deaths |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether intention‐to‐treat |
| Other bias | Low risk | Groups well balanced for race, percentages of nulliparous women, blood pressures in labor. Higher birth weights for combined spinal‐epidural group |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | 60 patients undergoing emergent femoral fracture repair (< eight days from injury) |
| Interventions |
General anaesthesia (n = 40): thiopental, succinylcholine, lidocaine spray, fentanyl, nitrous oxide and halothane (n = 20) or a psoas compartment block with 30 mL of 2% plain mepivacaine (Chayen technique) and general anaesthesia with althesin, fentanyl and nitrous oxide (n = 20) Spinal anaesthesia with 0.6 to 0.8 mL of hyperbaric cinchocaine plus general anaesthesia with althesin, fentanyl and nitrous oxide (n = 20) |
| Outcomes | Mortality Pneumonia: no definition |
| Notes | Followed for four weeks (but the death occurred at 30 days and was recorded) |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Four participants withdrawn from results in the GA group |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat. Four failed psoas blocks withdrawn from the study |
| Other bias | Low risk | Groups well balanced for age and ASA physical status |
| Methods | Regional anaesthesia versus general anaesthesia |
| Participants | 90 patients undergoing unilateral total hip replacement |
| Interventions |
Lumbar epidural anaesthesia (n = 46): at L3‐L4, 3 mL 2% lidocaine and 0.1% ropivacaine for a sensory level ≧ T10 for the surgery and 0.2% ropivacaine for 48 hours after the surgery General anaesthesia (n = 45): thiopental or etomidate, fentanyl, nitrous oxide, isoflurane or enflurane and neuromuscular blocking agents. Morphine IV PCA after the surgery |
| Outcomes | Mortality |
| Notes | No participant died in the course of the study |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned: no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Mortality reported for all participants |
| Selective reporting (reporting bias) | Low risk | Not in intention‐to‐treat; one participant for whom the surgery was cancelled and one participant with a failed epidural who received general anaesthesia. Intention‐to‐treat for this overview |
| Other bias | Unclear risk | Groups said to be well balanced for ASA physical status, data not provided. Supported by Astra, Phase III approval study before marketing of ropivacaine |
| Methods | Regional anaesthesia added to general anaesthesia versus general anaesthesia |
| Participants | Patients scheduled for intrathoracic, intra‐abdominal or major (non‐cerebral) vascular surgery |
| Interventions | General anaesthesia for all participants according to the attending anaesthesiologist Thoracic or lumbar epidural anaesthesia with 0.75% bupivacaine or 1.5% lidocaine with epinephrine 5 mcg/mL intraoperatively for surgical anaesthesia and muscle relaxation. Analgesic concentrations of local anaesthetics and/or epidural administration of narcotics after the surgery Parenteral narcotics |
| Outcomes | Mortality: death that occurred in the hospital whIle a participant was recovering from the original surgical procedure or from a complication related to the original procedure Myocardial infarction: transmural myocardial infarction (defined as the appearance on the electrocardiogram of new Q‐waves at least 0.04 seconds in duration and 1 mm or more in depth); nontransmural myocardial infarction (diagnosed by a postoperative elevation of the serum lactate dehydrogenase and creatine phosphokinase and by a creatine phosphokinase isoenzyme pattern considered to be diagnostic of myocardial damage with or without ECG changes); recent myocardial infarction diagnosed at autopsy Pneumonia: new appearance of an infiltrate on chest x‐ray and new finding of two of three clinical criteria (temperature of 38°C or higher, an abnormal elevation of the white blood cell count or a sputum Gram stain and culture positive for a pathogen) |
| Notes |
Risk of bias table
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned. "The physicians and nurses caring for patients in this study were not informed of the outcome variables under consideration" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Three participants did not have a functioning epidural catheter: one because of technical failure, and two because a catheter was never inserted by independent decision of the anaesthesiologist in charge of the case |
| Selective reporting (reporting bias) | High risk | Not in intention‐to‐treat: Surgery originally scheduled for two participants in group II was cancelled after participants were randomly assigned. These two participants were eliminated from the study. Presence/absence of complications related to the anaesthetic techniques not reported |
| Other bias | Low risk | Groups well balanced for age, ASA physical status and type of surgery |
Footnotes
Appendix 3. Reasons for rejection of studies included in the selected reviews
Characteristics of excluded studies
| Reason for exclusion | "Quasi‐randomized trial" allocated by the date of operation |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | Wrong intervention (all participants had an epidural) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | Wrong intervention (no group with general anaesthesia) |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | Not randomly assigned: "Randomization was performed by allotting patients having surgery on odd numbered days to the group receiving TEA plus general anaesthesia (group I, n=57) while patients scheduled for surgery on even days received general anaesthesia without epidural block (group 11, n=59)" |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia alone (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | Local anaesthetics were not administered via the catheters in either group before the postoperative period (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | The study started postoperatively, the epidural was not used intraoperatively by the protocol (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | No group with general anaesthesia alone (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | Inadequate randomization, as women were allocated to groups alternately |
| Reason for exclusion | Randomization started after the surgery (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia alone (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with regional anaesthesia (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group with general anaesthesia alone (wrong intervention) |
| Reason for exclusion | No group with general anaesthesia alone (wrong intervention) |
| Reason for exclusion | No group with general anaesthesia alone (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | No group randomly assigned to general anaesthesia (wrong intervention) |
| Reason for exclusion | No outcome of interest |
| Reason for exclusion | Wrong intervention (no group with general anaesthesia alone) |
| Reason for exclusion | No outcome of interest |
Footnotes
Contributions of authors
Conceiving of the review: Joanne Guay (JG), Peter Choi (PC), Santhanam Suresh (SS), Natalie Albert (NA), Sandra Kopp (SK), and Nathan Leon Pace (NLP).
Co‐ordinating the review, screening search results and organizing retrieval of papers: JG.
Screening retrieved papers against inclusion criteria: JG, SK, NA, PC and SS.
Abstracting data from papers: JG, SK, NA, PC and SS.Providing data management for the review: JG and NLP.
Grading the evidence: JG, SK and NLP.
Sources of support
Internal sources
-
The Cochrane Anaesthesia Review Group, Denmark.
The search strategy was designed by Mr Karen Hovhannisyan, Trials Search Co‐ordinator for the Cochrane Anaesthesia Review Group, Rigshospitalet, Dept. 3342, Blegdamsvej 9, 2100 Copenhagen, Denmark
External sources
-
University of Montreal, Canada.
Access to electronic databases and to major medical journals was provided by the University of Montreal, Montreal, Quebec, Canada
Declarations of interest
Joanne Guay: I had no direct relationship with any equipment manufacturer or pharmaceutical company for longer than five years. I hold no stocks or shares other than mutual funds. I did not act as an expert witness for longer than five years. During the last five years, I have received fees as speaker for two lectures given at the University of Dalhousie: one on regional anaesthesia for carotid endarterectomy and the other on local anaesthetic–related methaemoglobinaemia. I am the editor of a multi‐author textbook on anaesthesia (including notions on general and regional anaesthesia). Sandra Kopp: no conflict of interest. Natalie Albert: no conflict of interest. Peter Choi: I have published a prior systematic review included in this Cochrane overview. I have had no participation in judging the quality of my own review or in selecting studies or extracting data from studies pertaining to my review. Nathan Leon Pace: no competing interests. Santhanam Suresh: I have interests in the following: Foundation for Anesthesia Education and Research (FAER) (funded research); Orthopedic Knowledge Online (consultant fees); NIH Sub‐Contract/University of Colorado (funded research); NIH Sub‐Contract, Boston Children’s Hospital (funded research); Sonosite, Inc (equipment support); Philips Healthcare (equipment support); and GE Healthcare (equipment support).
Edited (no change to conclusions)
References
References to included reviews
Afolabi 2006
- Afolabi BB, Lesi FE, Merah NA. Regional versus general anaesthesia for caesarean section. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004350.pub2] [DOI] [PubMed] [Google Scholar]
Barbosa 2010
- Barbosa FT, Jucá MJ, Castro AA, Cavalcante JC. Neuraxial anaesthesia for lower‐limb revascularization. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007083.pub2] [DOI] [PubMed] [Google Scholar]
Choi 2003
- Choi PT, Bhandari M, Scott J, Douketis JD. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003071] [DOI] [PMC free article] [PubMed] [Google Scholar]
Craven 2003
- Craven PD, Badawi N, Henderson‐Smart DJ, O'Brien M. Regional (spinal, epidural, caudal) versus general anaesthesia in preterm infants undergoing inguinal herniorrhaphy in early infancy. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003669] [DOI] [PubMed] [Google Scholar]
Cyna 2008
- Cyna AM, Middleton P. Caudal epidural block versus other methods of postoperative pain relief for circumcision in boys. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003005.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorgensen 2000
- Jorgensen H, Wetterslev J, Moiniche S, Dahl JB. Epidural local anaesthetics versus opioid‐based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001893] [DOI] [PubMed] [Google Scholar]
Nishimori 2012
- Nishimori M, Low JH, Zheng H, Ballantyne JC. Epidural pain relief versus systemic opioid‐based pain relief for abdominal aortic surgery. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD005059.pub3] [DOI] [PubMed] [Google Scholar]
Parker 2004
- Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD000521.pub2] [DOI] [PubMed] [Google Scholar]
Werawatganon 2005
- Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra‐abdominal surgery. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004088.pub2] [DOI] [PubMed] [Google Scholar]
Additional references
ACC/AHA 2007 Guidelines
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery), American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society for Vascular Surgery, Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007;116(17):e418‐99. [DOI] [PubMed] [Google Scholar]
Adams 1990
- Adams HA, Wolf C, Michaelis G, Hempelmann G. [Postoperative course and endocrine stress reaction of geriatric patients with para‐articular hip fractures. Prospective randomized study comparing spinal anesthesia and halothane intubation narcosis] [Postoperativer Verlauf und endokrine Stress‐Reaktion geriatrischer Patienten mit huftnahen Frakturen. Prospektiv‐randomisierte Studie zum Vergleich von Spinalanasthesien und Halothan‐Intubationsnarkosen]. Anasthesie, Intensivtherapie, Notfallmedizin 1990;25(4):263‐70. [PUBMED: 2171375] [PubMed] [Google Scholar]
Ahn 1988
- Ahn H, Bronge A, Johansson K, Ygge H, Lindhagen J. Effect of continuous postoperative epidural analgesia on intestinal motility. The British Journal of Surgery 1988;75(12):1176‐8. [PUBMED: 3233467] [DOI] [PubMed] [Google Scholar]
Allaire 1992
- Allaire PH, Messick JM Jr, Oesterling JE, Byer DE, Myers RP, Lieber MM, et al. A prospective randomized comparison of epidural infusion of fentanyl and intravenous administration of morphine by patient‐controlled analgesia after radical retropubic prostatectomy. Mayo Clinic proceedings. Mayo Clinic 1992;67(11):1031‐41. [PUBMED: 1434863] [DOI] [PubMed] [Google Scholar]
Asantila 1991
- Asantila R, Eklund P, Rosenberg PH. Continuous epidural infusion of bupivacaine and morphine for postoperative analgesia after hysterectomy. Acta Anaesthesiologica Scandinavica 1991;35(6):513‐7. [PUBMED: 1897347] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Services Research 2004;4(1):38. [PUBMED: 15615589] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barre 1989
- Barre E, Raimbault E, Coriat P, Low Koune JP, Baron JF, Viars P. [Monitoring of mixed venous oxygen saturation in aortic surgery: value and limits] [Monitorage de la SvO2 en chirurgie aortique: interet et limites.]. Annales Francaises d'Anesthesie et de Reanimation 1989;8(6):688‐95. [PUBMED: 2699176] [DOI] [PubMed] [Google Scholar]
Beeby 1984
- Beeby D, MacIntosh KC, Bailey M, Welch DB. Postoperative analgesia for Caesarean section using epidural methadone. Anaesthesia 1984;39(1):61‐3. [PUBMED: 6696222] [DOI] [PubMed] [Google Scholar]
Berggren 1987
- Berggren D, Gustafson Y, Eriksson B, Bucht G, Hansson LI, Reiz S, et al. Postoperative confusion after anesthesia in elderly patients with femoral neck fractures. Anesthesia and Analgesia 1987;66(6):497‐504. [PUBMED: 3578861] [PubMed] [Google Scholar]
Bertini 1995
- Bertini L, Tagariello V, Molino FM, Posteraro CM, Mancini S, Rossignoli L. [Patient‐controlled postoperative analgesia in orthopedic surgery: epidural PCA versus intravenous PCA] [Analgesia postoperatoria controllata dal paziente in chirurgia ortopedica: PCA epidurale versus PCA endovenosa.]. Minerva Anestesiologica 1995;61(7‐8):319‐28. [PUBMED: 8948744] [PubMed] [Google Scholar]
Biffoli 1998
- Biffoli F, Piacentino V, Meconcelli G, Guidi F, Dal Poggetto L, Bacci I, et al. [The effect of anesthesiologic technique on the mental state of elderly patients submitted for orthopedic surgery of the lower limbs] [Influenza della condotta anestesiologica sullo stato mentale di soggetti anziani sottoposti a chirurgia ortopedica dell'arto inferiore.]. Minerva Anestesiologica 1998;64(1‐2):13‐9. [PUBMED: 9658786] [PubMed] [Google Scholar]
Bigler 1985
- Bigler D, Adelhoj B, Petring OU, Pederson NO, Busch P, Kalhke P. Mental function and morbidity after acute hip surgery during spinal and general anaesthesia. Anaesthesia 1985;40(7):672‐6. [PUBMED: 4025772] [DOI] [PubMed] [Google Scholar]
Bode 1996
- Bode RH Jr, Lewis KP, Zarich SW, Pierce ET, Roberts M, Kowalchuk GJ, et al. Cardiac outcome after peripheral vascular surgery. Comparison of general and regional anesthesia. Anesthesiology 1996;84(1):3‐13. [PUBMED: 8572352] [DOI] [PubMed] [Google Scholar]
Bois 1997
- Bois S, Couture P, Boudreault D, Lacombe P, Fugere F, Girard D, et al. Epidural analgesia and intravenous patient‐controlled analgesia result in similar rates of postoperative myocardial ischemia after aortic surgery. Anesthesia and Analgesia 1997;85(6):1233‐9. [PUBMED: 9390586] [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta‐analysis. Padstow, Cornwall: Wiley, 2009. [Google Scholar]
Boylan 1998
- Boylan JF, Katz J, Kavanagh BP, Klinck JR, Cheng DC, DeMajo WC, et al. Epidural bupivacaine‐morphine analgesia versus patient‐controlled analgesia following abdominal aortic surgery: analgesic, respiratory, and myocardial effects. Anesthesiology 1998;89(3):585‐93. [PUBMED: 9743393] [DOI] [PubMed] [Google Scholar]
Bramwell 1982
- Bramwell RG, Bullen C, Radford P. Caudal block for postoperative analgesia in children. Anaesthesia 1982;37(10):1024‐8. [PUBMED: 6127970] [DOI] [PubMed] [Google Scholar]
Bredahl 1991
- Bredahl C, Hindsholm KB, Frandsen PC. Changes in body heat during hip fracture surgery: a comparison of spinal analgesia and general anaesthesia. Acta Anaesthesiologica Scandinavica 1991;35(6):548‐52. [PUBMED: 1897353] [DOI] [PubMed] [Google Scholar]
Bredtmann 1990
- Bredtmann RD, Herden HN, Teichmann W, Moecke HP, Kniesel B, Baetgen R, et al. Epidural analgesia in colonic surgery: results of a randomized prospective study. The British Journal of Surgery 1990;77(6):638‐42. [PUBMED: 2099750] [DOI] [PubMed] [Google Scholar]
Brichant 1995
- Brichant JF, Blom‐Peters L, Buffels R, Lamy M. Central neural blockage failed to decrease deep venous thrombosis in patients undergoing hip surgery and receiving low molecular weight heparin. British Journal of Anaesthesia 1995;74(Suppl 1):75. [Google Scholar]
Brodner 2000
- Brodner G, Mertes N, Aken H, Mollhoff T, Zahl M, Wirtz S, et al. What concentration of sufentanil should be combined with ropivacaine 0.2% wt/vol for postoperative patient‐controlled epidural analgesia?. Anesthesia and Analgesia 2000;90(3):649‐57. [PUBMED: 10702452] [DOI] [PubMed] [Google Scholar]
Broekema 1998
- Broekema AA, Veen A, Fidler V, Gielen MJ, Hennis PJ. Postoperative analgesia with intramuscular morphine at fixed rate versus epidural morphine or sufentanil and bupivacaine in patients undergoing major abdominal surgery. Anesthesia and Analgesia 1998;87(6):1346‐53. [PUBMED: 9842825] [PubMed] [Google Scholar]
Brown 1994
- Brown AG, Visram AR, Jones RD, Irwin MG, Bacon‐Shone J. Preoperative and postoperative oxygen saturation in the elderly following spinal or general anaesthesia—an audit of current practice. Anaesthesia and Intensive Care 1994;22(2):150‐4. [PUBMED: 8210017] [DOI] [PubMed] [Google Scholar]
Capdevila 1999
- Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999;91(1):8‐15. [PUBMED: 10422923] [DOI] [PubMed] [Google Scholar]
Carli 2001
- Carli F, Trudel JL, Belliveau P. The effect of intraoperative thoracic epidural anesthesia and postoperative analgesia on bowel function after colorectal surgery: a prospective, randomized trial. Diseases of the Colon and Rectum 2001;44(8):1083‐9. [PUBMED: 11535845] [DOI] [PubMed] [Google Scholar]
Casati 2003
- Casati A, Aldegheri G, Vinciguerra E, Marsan A, Fraschini G, Torri G. Randomized comparison between sevoflurane anaesthesia and unilateral spinal anaesthesia in elderly patients undergoing orthopaedic surgery. European Journal of Anaesthesiology 2003;20(8):640‐6. [PUBMED: 12932066] [DOI] [PubMed] [Google Scholar]
Chang 2010
- Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population‐based study. Anesthesiology 2010;113(2):279‐84. [PUBMED: 20657202] [DOI] [PubMed] [Google Scholar]
Christopherson 1993
- Christopherson R, Beattie C, Frank SM, Norris EJ, Meinert CL, Gottlieb SO, et al. Perioperative morbidity in patients randomized to epidural or general anesthesia for lower extremity vascular surgery. Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology 1993;79(3):422‐34. [PUBMED: 8363066] [DOI] [PubMed] [Google Scholar]
Concha 1994
- Concha M, Gonzalez A, Gonzalez J, Vergara R. [Postoperative analgesia for ambulatory surgery in children: a comparison of 2 techniques] [Analgesie postoperatoire pour la chirurgie ambulatoire chez l'enfant: comparaison de deux techniques.]. Cahiers d'Anesthesiologie 1994;42(3):339‐42. [PUBMED: 7812859] [PubMed] [Google Scholar]
Cook 1986
- Cook PT, Davies MJ, Cronin KD, Moran P. A prospective randomised trial comparing spinal anaesthesia using hyperbaric cinchocaine with general anaesthesia for lower limb vascular surgery. Anaesthesia and Intensive Care 1986;14(4):373‐80. [PUBMED: 3551674] [DOI] [PubMed] [Google Scholar]
Cooper 1996
- Cooper DW, Ryall DM, McHardy FE, Lindsay SL, Eldabe SS. Patient‐controlled extradural analgesia with bupivacaine, fentanyl, or a mixture of both, after Caesarean section. British Journal of Anaesthesia 1996;76(5):611‐5. [PUBMED: 8688256] [DOI] [PubMed] [Google Scholar]
Couderc 1977
- Couderc E, Mauge F, Duvaldestin P, Desmonts JM. [Comparative results of general and peridural anesthesia for hip surgery in the very old patient] [Resultats comparatifs de l'anesthesie generale et peridurale chez le grand vieillard dans la chirurgie de la hanche.]. Anesthesie, Analgesie, Reanimation 1977;34(5):987‐97. [PUBMED: 613869] [PubMed] [Google Scholar]
Cullen 1985
- Cullen ML, Staren ED, el‐Ganzouri A, Logas WG, Ivankovich AD, Economou SG. Continuous epidural infusion for analgesia after major abdominal operations: a randomized, prospective, double‐blind study. Surgery 1985;98(4):718‐28. [PUBMED: 3901375] [PubMed] [Google Scholar]
Cuschieri 1985
- Cuschieri RJ, Morran CG, Howie JC, McArdle CS. Postoperative pain and pulmonary complications: comparison of three analgesic regimens. The British Journal of Surgery 1985;72(6):495‐8. [PUBMED: 4016522] [DOI] [PubMed] [Google Scholar]
D'Ambrosio 1999
- D'Ambrosio A, Borghi B, Damato A, D'Amato G, Antonacci D, Valeri F. Reducing perioperative blood loss in patients undergoing total hip arthroplasty. The International Journal of Artificial Organs 1999;22(1):47‐51. [PUBMED: 10098585] [PubMed] [Google Scholar]
Datta 1983
- Datta S, Carr DB, Lambert DH, Morrison J, Naulty JS, Fischer J, et al. Anesthesia for cesarean delivery: relationship of maternal and fetal plasma B‐endorphin concentrations to different types of anesthesia. Anesthesiology 1983;59:A418. [Google Scholar]
Davies 1993
- Davies MJ, Silbert BS, Mooney PJ, Dysart RH, Meads AC. Combined epidural and general anaesthesia versus general anaesthesia for abdominal aortic surgery: a prospective randomised trial. Anaesthesia and Intensive Care 1993;21(6):790‐4. [PUBMED: 8122735] [DOI] [PubMed] [Google Scholar]
Davis 1981
- Davis FM, Laurenson VG. Spinal anaesthesia or general anaesthesia for emergency hip surgery in elderly patients. Anaesthesia and Intensive Care 1981;9(4):352‐8. [PUBMED: 6797318] [DOI] [PubMed] [Google Scholar]
Davis 1987
- Davis FM, Woolner DF, Frampton C, Wilkinson A, Grant A, Harrison RT, et al. Prospective, multi‐centre trial of mortality following general or spinal anaesthesia for hip fracture surgery in the elderly. British Journal of Anaesthesia 1987;59(9):1080‐8. [PUBMED: 3311100] [DOI] [PubMed] [Google Scholar]
de Visme 2000
- Visme V, Picart F, Jouan R, Legrand A, Savry C, Morin V. Combined lumbar and sacral plexus block compared with plain bupivacaine spinal anesthesia for hip fractures in the elderly. Regional Anesthesia and Pain Medicine 2000;25(2):158‐62. [PUBMED: 10746528] [DOI] [PubMed] [Google Scholar]
Delilkan 1993
- Delilkan AE, Vijayan R. Epidural tramadol for postoperative pain relief. Anaesthesia 1993;48(4):328‐31. [PUBMED: 8494137] [DOI] [PubMed] [Google Scholar]
Dick 1992
- Dick W, Traub E, Kraus H, Tollner U, Burghard R, Muck J. General anaesthesia versus epidural anaesthesia for primary caesarean section—a comparative study. European Journal of Anaesthesiology 1992;9(1):15‐21. [PUBMED: 1735394] [PubMed] [Google Scholar]
Dodds 2007
- Dodds TM, Fillinger MP, Walsh DB, Surgenor SD, Mandel D, Yeager MP. Clinical outcomes after lower extremity revascularization: a comparison of epidural and general anaesthesia. The Journal of Applied Research 2007;7(3):238‐49. [NIM UID: 101132979] [Google Scholar]
Dyer 2003
- Dyer RA, Els I, Farbas J, Torr GJ, Schoeman LK, James MF. Prospective, randomized trial comparing general with spinal anesthesia for cesarean delivery in preeclamptic patients with a nonreassuring fetal heart trace. Anesthesiology 2003;99(3):561‐9; discussion 5A‐6A. [PUBMED: 12960539] [DOI] [PubMed] [Google Scholar]
Eyrolle 1998
- Eyrolle L, Zetlaoui P, Belbachir A, Rosencher N, Conseiller C. Regional anaesthesia for femoral neck fracture surgery: comparison of lumbar plexus block and spinal anaesthesia. British Journal of Anaesthesia 1998;80(Suppl 1):112. [Google Scholar]
Garnett 1996
- Garnett RL, MacIntyre A, Lindsay P, Barber GG, Cole CW, Hajjar G, et al. Perioperative ischaemia in aortic surgery: combined epidural/general anaesthesia and epidural analgesia vs general anaesthesia and i.v. analgesia. Canadian Journal of Anaesthesia = Journal Canadien d'Anesthesie 1996;43(8):769‐77. [PUBMED: 8840054] [DOI] [PubMed] [Google Scholar]
Gauntlett 2003
- Gauntlett I. A comparison between local anaesthetic dorsal nerve block and caudal bupivacaine with ketamine for paediatric circumcision. Paediatric Anaesthesia 2003;13(1):38‐42. [PUBMED: 12535037] [DOI] [PubMed] [Google Scholar]
Geddes 1991
- Geddes SM, Thorburn J, Logan RW. Gastric emptying following caesarean section and the effect of epidural fentanyl. Anaesthesia 1991;46(12):1016‐8. [PUBMED: 1781524] [DOI] [PubMed] [Google Scholar]
George 1992
- George KA, Chisakuta AM, Gamble JA, Browne GA. Thoracic epidural infusion for postoperative pain relief following abdominal aortic surgery: bupivacaine, fentanyl or a mixture of both?. Anaesthesia 1992;47(5):388‐94. [PUBMED: 1599061] [DOI] [PubMed] [Google Scholar]
George 1994
- George KA, Wright PM, Chisakuta AM, Rao NV. Thoracic epidural analgesia compared with patient controlled intravenous morphine after upper abdominal surgery. Acta Anaesthesiologica Scandinavica 1994;38(8):808‐12. [PUBMED: 7887102] [DOI] [PubMed] [Google Scholar]
Guay 2006a
- Guay J. The benefits of adding epidural analgesia to general anesthesia: a metaanalysis. Journal of Anesthesia 2006;20(4):335‐40. [PUBMED: 17072704] [DOI] [PubMed] [Google Scholar]
Guay 2006b
- Guay J. The effect of neuraxial blocks on surgical blood loss and blood transfusion requirements: a meta‐analysis. Journal of Clinical Anesthesia 2006;18(2):124‐8. [PUBMED: 16563330] [DOI] [PubMed] [Google Scholar]
Gustafsson 1986
- Gustafsson LL, Johannisson J, Garle M. Extradural and parenteral pethidine as analgesia after total hip replacement: effects and kinetics. A controlled clinical study. European Journal of Clinical Pharmacology 1986;29(5):529‐34. [PUBMED: 3956558] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Hendolin 1996
- Hendolin H, Nuutinen L, Kokki H, Tuomisto L. Does morphine premedication influence the pain and consumption of postoperative analgesics after total knee arthroplasty?. Acta Anaesthesiologica Scandinavica 1996;40(1):81‐5. [PUBMED: 8904263] [DOI] [PubMed] [Google Scholar]
Hodgkinson 1980
- Hodgkinson R, Husain FJ, Hayashi RH. Systemic and pulmonary blood pressure during caesarean section in parturients with gestational hypertension. Canadian Anaesthetists' Society Journal 1980;27(4):389‐94. [PUBMED: 7407669] [DOI] [PubMed] [Google Scholar]
Hollmen 1978
- Hollmen AI, Jouppila R, Koivisto M, Maatta L, Pihlajaniemi R, Puukka M, et al. Neurologic activity of infants following anesthesia for cesarean section. Anesthesiology 1978;48(5):350‐6. [PUBMED: 646154] [DOI] [PubMed] [Google Scholar]
Hommeril 1994
- Hommeril JL, Bernard JM, Gouin F, Pinaud M. Ketoprofen for pain after hip and knee arthroplasty. British Journal of Anaesthesia 1994;72(4):383‐7. [PUBMED: 8155435] [DOI] [PubMed] [Google Scholar]
Hong 2003
- Hong JY, Jee YS, Yoon HJ, Kim SM. Comparison of general and epidural anesthesia in elective cesarean section for placenta previa totalis: maternal hemodynamics, blood loss and neonatal outcome. International Journal of Obstetric Anesthesia 2003;12(1):12‐6. [PUBMED: 15676315] [DOI] [PubMed] [Google Scholar]
Jorgensen 1991
- Jorgensen LN, Rasmussen LS, Nielsen PT, Leffers A, Albrecht‐Beste E. Antithrombotic efficacy of continuous extradural analgesia after knee replacement. British Journal of Anaesthesia 1991;66(1):8‐12. [PUBMED: 1997063] [DOI] [PubMed] [Google Scholar]
Juelsgaard 1998
- Juelsgaard P, Sand NP, Felsby S, Dalsgaard J, Jakobsen KB, Brink O, et al. Perioperative myocardial ischaemia in patients undergoing surgery for fractured hip randomized to incremental spinal, single‐dose spinal or general anaesthesia. European Journal of Anaesthesiology 1998;15(6):656‐63. [PUBMED: 9884850] [DOI] [PubMed] [Google Scholar]
Kamitani 2003
- Kamitani K, Higuchi A, Asahi T, Yoshida H. [Postoperative delirium after general anesthesia vs. spinal anesthesia in geriatric patients]. Masui. The Japanese Journal of Anesthesiology 2003;52(9):972‐5. [PUBMED: 14531256] [PubMed] [Google Scholar]
Kataja 1991
- Kataja J. Thoracolumbar epidural anaesthesia and isoflurane to prevent hypertension and tachycardia in patients undergoing abdominal aortic surgery. European Journal of Anaesthesiology 1991;8(6):427‐36. [PUBMED: 1765040] [PubMed] [Google Scholar]
Kavak 2001
- Kavak ZN, Basgul A, Ceyhan N. Short‐term outcome of newborn infants: spinal versus general anesthesia for elective cesarean section. A prospective randomized study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;100(1):50‐4. [PUBMED: 11728657] [DOI] [PubMed] [Google Scholar]
Klasen 1999
- Klasen JA, Opitz SA, Melzer C, Thiel A, Hempelmann G. Intraarticular, epidural, and intravenous analgesia after total knee arthroplasty. Acta Anaesthesiologica Scandinavica 1999;43(10):1021‐6. [PUBMED: 10593465] [DOI] [PubMed] [Google Scholar]
Kolatat 1999
- Kolatat T, Somboonnanonda A, Lertakyamanee J, Chinachot T, Tritrakarn T, Muangkasem J. Effects of general and regional anesthesia on the neonate (a prospective, randomized trial). Journal of the Medical Association of Thailand = Chotmaihet Thangphaet 1999;82(1):40‐5. [PUBMED: 10087737] [PubMed] [Google Scholar]
Korkmaz 2004
- Korkmaz F, Eksioglu B, Hanci A, Basgul A. Effects of general and regional anesthesia on the neonate (a prospective, randomized trial). Journal of the Medical Association of Thailand 2004;82(Suppl 2):77. [PubMed] [Google Scholar]
Kowalski 1992
- Kowalski S, Ong B, Bell D, Ostryzniuk T, Serrette C, Wasylak T, et al. Postoperative analgesia and pulmonary function following upper abdominal surgery: epidural fentanyl versus PCA morphine. Canadian Journal of Anaesthesia 1992;39(5 II Suppl):A58. [Google Scholar]
Krane 1995
- Krane EJ, Haberkern CM, Jacobson LE. Postoperative apnea, bradycardia, and oxygen desaturation in formerly premature infants: prospective comparison of spinal and general anesthesia. Anesthesia and Analgesia 1995;80(1):7‐13. [PUBMED: 7802303] [DOI] [PubMed] [Google Scholar]
Lee 1988
- Lee A, Simpson D, Whitfield A, Scott DB. Postoperative analgesia by continuous extradural infusion of bupivacaine and diamorphine. British Journal of Anaesthesia 1988;60(7):845‐50. [PUBMED: 3293643] [DOI] [PubMed] [Google Scholar]
Lertakyamanee 1999
- Lertakyamanee J, Chinachoti T, Tritrakarn T, Muangkasem J, Somboonnanonda A, Kolatat T. Comparison of general and regional anesthesia for cesarean section: success rate, blood loss and satisfaction from a randomized trial. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet 1999;82(7):672‐80. [PUBMED: 10511769] [PubMed] [Google Scholar]
Liu 1995
- Liu SS, Carpenter RL, Mackey DC, Thirlby RC, Rupp SM, Shine TS, et al. Effects of perioperative analgesic technique on rate of recovery after colon surgery. Anesthesiology 1995;83(4):757‐65. [PUBMED: 7574055] [DOI] [PubMed] [Google Scholar]
Lunn 1979
- Lunn JN. Postoperative analgesia after circumcision. A randomized comparison between caudal analgesia and intramuscular morphine in boys. Anaesthesia 1979;34(6):552‐4. [PUBMED: 484815] [DOI] [PubMed] [Google Scholar]
Mahajan 1992
- Mahajan J, Mahajan RP, Singh MM, Anand NK. Anaesthetic technique for elective caesarean section and neurobehavioural status of newborns. International Journal of Obstetric Anesthesia 1992;2(2):89‐93. [PUBMED: 15636857] [DOI] [PubMed] [Google Scholar]
Mak 2001
- Mak MY‐L, Philip AE, Cho SC, Chan JT‐M. Postoperative analgesia in children day surgery circumcision: comparison of three methods. Annals of the College of Surgeons of Hong Kong 2001;5:146‐50. [Google Scholar]
Martin 1982
- Martin LV. Postoperative analgesia after circumcision in children. British Journal of Anaesthesia 1982;54(12):1263‐6. [PUBMED: 7171413] [DOI] [PubMed] [Google Scholar]
Maurette 1988
- Maurette P, Castagnera L, Vivier C, Erny P. [Comparative repercussions of general and spinal anesthesia on the psychological functions of the aged subject] [Repercussions comparees de l'anesthesie generale et de la rachianesthesie sur les fonctions psychiques du sujet age.]. Annales Francaises d'Anesthesie et de Reanimation 1988;7(4):305‐8. [PUBMED: 3202339] [DOI] [PubMed] [Google Scholar]
May 1982
- May AE, Wandless J, James RH. Analgesia for circumcision in children. A comparison of caudal bupivacaine and intramuscular buprenorphine. Acta Anaesthesiologica Scandinavica 1982;26(4):331‐3. [PUBMED: 7124308] [DOI] [PubMed] [Google Scholar]
McKenzie 1984
- McKenzie PJ, Wishart HY, Smith G. Long‐term outcome after repair of fractured neck of femur. Comparison of subarachnoid and general anaesthesia. British Journal of Anaesthesia 1984;56(6):581‐5. [PUBMED: 6721969] [DOI] [PubMed] [Google Scholar]
McLaren 1978
- McLaren AD, Stockwell MC, Reid VT. Anaesthetic techniques for surgical correction of fractured neck of femur. A comparative study of spinal and general anaesthesia in the elderly. Anaesthesia 1978;33(1):10‐4. [PUBMED: 626331] [DOI] [PubMed] [Google Scholar]
Moiniche 1994
- Moiniche S, Hjortso NC, Hansen BL, Dahl JB, Rosenberg J, Gebuhr P, et al. The effect of balanced analgesia on early convalescence after major orthopaedic surgery. Acta Anaesthesiologica Scandinavica 1994;38(4):328‐35. [PUBMED: 8067218] [DOI] [PubMed] [Google Scholar]
Norman 1997
- Norman JG, Fink GW. The effects of epidural anesthesia on the neuroendocrine response to major surgical stress: a randomized prospective trial. The American Surgeon 1997;63(1):75‐80. [PUBMED: 8985076] [PubMed] [Google Scholar]
Norris 2001
- Norris EJ, Beattie C, Perler BA, Martinez EA, Meinert CL, Anderson GF, et al. Double‐masked randomized trial comparing alternate combinations of intraoperative anesthesia and postoperative analgesia in abdominal aortic surgery. Anesthesiology 2001;95(5):1054‐67. [PUBMED: 11684971] [DOI] [PubMed] [Google Scholar]
Oxman 1991
- Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. Journal of Clinical Epidemiology 1991;44(11):1271‐8. [PUBMED: 1834807] [DOI] [PubMed] [Google Scholar]
Park 2001
- Park WY, Thompson JS, Lee KK. Effect of epidural anesthesia and analgesia on perioperative outcome: a randomized, controlled Veterans Affairs cooperative study. Annals of Surgery 2001;234(4):560‐9; discussion 569‐71. [PUBMED: 11573049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paulsen 2001
- Paulsen EK, Porter MG, Helmer SD, Linhardt PW, Kliewer ML. Thoracic epidural versus patient‐controlled analgesia in elective bowel resections. American Journal of Surgery 2001;182(6):570‐7. [PUBMED: 11839319] [DOI] [PubMed] [Google Scholar]
Pence 2002
- Pence S, Kocoglu H, Balat O, Balat A. The effect of delivery on umbilical arterial cord blood gases and lipid peroxides: comparison of vaginal delivery and cesarean section. Clinical and Experimental Obstetrics and Gynecology 2002;29(3):212‐4. [PUBMED: 12519045] [PubMed] [Google Scholar]
Petropoulos 2003
- Petropoulos G, Siristatidis C, Salamalekis E, Creatsas G. Spinal and epidural versus general anesthesia for elective cesarean section at term: effect on the acid‐base status of the mother and newborn. The Journal of Maternal‐Fetal and Neonatal Medicine 2003;13(4):260‐6. [PUBMED: 12854928] [DOI] [PubMed] [Google Scholar]
Peyton 2003
- Peyton PJ, Myles PS, Silbert BS, Rigg JA, Jamrozik K, Parsons R. Perioperative epidural analgesia and outcome after major abdominal surgery in high‐risk patients. Anesthesia and Analgesia 2003;96(2):548. [PUBMED: 12538211] [DOI] [PubMed] [Google Scholar]
Racle 1986
- Racle JP, Benkhadra A, Poy JY, Gleizal B, Gaudray A. [Comparative study of general and spinal anesthesia in elderly women in hip surgery] [Etude comparative de l'anesthesie generale et de la rachianesthesie chez la femme agee dans la chirurgie de la hanche.]. Annales Francaises d'Anesthesie et de Reanimation 1986;5(1):24‐30. [PUBMED: 3706840] [DOI] [PubMed] [Google Scholar]
Reinhart 1989
- Reinhart K, Foehring U, Kersting T, Schaefer M, Bredle D, Hirner A, et al. Effects of thoracic epidural anesthesia on systemic hemodynamic function and systemic oxygen supply‐demand relationship. Anesthesia and Analgesia 1989;69(3):360‐9. [PUBMED: 2774232] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Riwar 1992
- Riwar A, Schar B, Grotzinger U. [Effect of continuous postoperative analgesia with peridural bupivacaine on intestinal motility following colorectal resection] [Effekt der kontinuierlichen postoperativen Analgesie mit Bupivacain peridural auf die Darmmotilitat nach kolorektalen Resektionen]. Helvetica Chirurgica Acta 1992;58(5):729‐33. [PUBMED: 1592646] [PubMed] [Google Scholar]
Rodgers 2000
- Rodgers A, Walker N, Schug S, McKee A, Kehlet H, Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ (Clinical research ed.) 2000;321(7275):1493. [PUBMED: 11118174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutberg 1984
- Rutberg H, Hakanson E, Anderberg B, Jorfeldt L, Martensson J, Schildt B. Effects of the extradural administration of morphine, or bupivacaine, on the endocrine response to upper abdominal surgery. British Journal of Anaesthesia 1984;56(3):233‐8. [PUBMED: 6704272] [DOI] [PubMed] [Google Scholar]
Scheinin 1987
- Scheinin B, Asantila R, Orko R. The effect of bupivacaine and morphine on pain and bowel function after colonic surgery. Acta Anaesthesiologica Scandinavica 1987;31(2):161‐4. [PUBMED: 3564873] [DOI] [PubMed] [Google Scholar]
Scott 1989
- Scott NB, Mogensen T, Bigler D, Lund C, Kehlet H. Continuous thoracic extradural 0.5% bupivacaine with or without morphine: effect on quality of blockade, lung function and the surgical stress response. British Journal of Anaesthesia 1989;62(3):253‐7. [PUBMED: 2649123] [DOI] [PubMed] [Google Scholar]
Seeling 1991
- Seeling W, Bothner U, Eifert B, Rockemann M, Schreiber M, Schurmann W, et al. [Patient‐controlled analgesia versus epidural analgesia using bupivacaine or morphine following major abdominal surgery. No difference in postoperative morbidity] [Patientenkontrollierte Analgesie versus Epiduralanalgesie mit Bupivacain oder Morphin nach grossen abdominellen Eingriffen. Kein Unterschied in der postoperativen Morbiditat.]. Der Anaesthesist 1991;40(11):614‐23. [PUBMED: 1755532] [PubMed] [Google Scholar]
Sener 2003
- Sener EB, Guldogus F, Karakaya D, Baris S, Kocamanoglu S, Tur A. Comparison of neonatal effects of epidural and general anesthesia for cesarean section. Gynecologic and Obstetric Investigation 2003;55(1):41‐5. [PUBMED: 12624551] [DOI] [PubMed] [Google Scholar]
Sharrock 1994
- Sharrock NE, Urquhart BL, Ganz S, Williams‐Russo PG. Epidural infusions of bupivacaine and fentanyl do not improve rehabilitation following one‐stage bilateral total knee arthroplasty. Annals of the Academy of Medicine, Singapore 1994;23(6 Suppl):3‐9. [PUBMED: 7710234] [PubMed] [Google Scholar]
Singelyn 1998
- Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient‐controlled analgesia with morphine, continuous epidural analgesia, and continuous three‐in‐one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesthesia and Analgesia 1998;87(1):88‐92. [PUBMED: 9661552] [DOI] [PubMed] [Google Scholar]
Somri 1998
- Somri M, Gaitini L, Vaida S, Collins G, Sabo E, Mogilner G. Postoperative outcome in high‐risk infants undergoing herniorrhaphy: comparison between spinal and general anaesthesia. Anaesthesia 1998;53(8):762‐6. [PUBMED: 9797520] [DOI] [PubMed] [Google Scholar]
Spreadbury 1980
- Spreadbury TH. Anaesthetic techniques for surgical correction of fractured neck of femur. A comparative study of ketamine and relaxant anaesthesia in elderly women. Anaesthesia 1980;35(2):208‐14. [PUBMED: 7386839] [DOI] [PubMed] [Google Scholar]
Stojadinovic 2009
- Stojadinovic A, Shockey SM, Croll SM, Buckenmaier CC 3rd. Quality of reporting of regional anesthesia outcomes in the literature. Pain Medicine (Malden, MA) 2009;10(6):1123‐31. [PUBMED: 19671083] [DOI] [PubMed] [Google Scholar]
Svartling 1986
- Svartling N, Lehtinen AM, Tarkkanen L. The effect of anaesthesia on changes in blood pressure and plasma cortisol levels induced by cementation with methylmethacrylate. Acta Anaesthesiologica Scandinavica 1986;30(3):247‐52. [PUBMED: 3739582] [DOI] [PubMed] [Google Scholar]
Tasker 1983
- Tasker TPB, Raitt DG, Kohn RLJ, Vater M, Crashaw C. Subarachnoid block or general anaesthesia? A study of the stress response during and after surgery for prosthetic replacement of fractured neck of femur. Journal of Bone and Joint Surgery (British Volume) 1993;65:660. [Google Scholar]
Thoren 1989
- Thoren T, Sundberg A, Wattwil M, Garvill JE, Jurgensen U. Effects of epidural bupivacaine and epidural morphine on bowel function and pain after hysterectomy. Acta Anaesthesiologica Scandinavica 1989;33(2):181‐5. [PUBMED: 2922985] [DOI] [PubMed] [Google Scholar]
Thorn 1992
- Thorn SE, Wattwil M, Naslund I. Postoperative epidural morphine, but not epidural bupivacaine, delays gastric emptying on the first day after cholecystectomy. Regional Anesthesia 1992;17(2):91‐4. [PUBMED: 1581266] [PubMed] [Google Scholar]
Thorn 1996
- Thorn SE, Wickbom G, Philipson L, Leissner P, Wattwil M. Myoelectric activity in the stomach and duodenum after epidural administration of morphine or bupivacaine. Acta Anaesthesiologica Scandinavica 1996;40(7):773‐8. [PUBMED: 8874561] [DOI] [PubMed] [Google Scholar]
Tsui 1997
- Tsui SL, Lee DK, Ng KF, Chan TY, Chan WS, Lo JW. Epidural infusion of bupivacaine 0.0625% plus fentanyl 3.3 micrograms/ml provides better postoperative analgesia than patient‐controlled analgesia with intravenous morphine after gynaecological laparotomy. Anaesthesia and Intensive Care 1997;25(5):476‐81. [PUBMED: 9352758] [DOI] [PubMed] [Google Scholar]
Ungemach 1993
- Ungemach JW, Andres FJ, Eggert E, Schoder K. The role of anaesthesia in geriatric patients with hip fractures: a prospective study. European Journal of Anaesthesiology 1993;10(5):380. [Google Scholar]
Valentin 1986
- Valentin N, Lomholt B, Jensen JS, Hejgaard N, Kreiner S. Spinal or general anaesthesia for surgery of the fractured hip? A prospective study of mortality in 578 patients. British Journal of Anaesthesia 1986;58(3):284‐91. [PUBMED: 3947489] [DOI] [PubMed] [Google Scholar]
Vater 1985
- Vater M, Wandless J. Caudal or dorsal nerve block? A comparison of two local anaesthetic techniques for postoperative analgesia following day case circumcision. Acta Anaesthesiologica Scandinavica 1985;29(2):175‐9. [PUBMED: 3976329] [DOI] [PubMed] [Google Scholar]
Wajima 1995
- Wajima Z, Kurosawa H, Inoue T, Yoshikawa T, Ishikawa G, Shitara T, et al. [Changes in dementia rating scale scores of elderly patients with femoral neck fracture during perioperative period]. Masui. The Japanese Journal of Anesthesiology 1995;44(11):1489‐97. [PUBMED: 8544286] [PubMed] [Google Scholar]
Wallace 1995
- Wallace DH, Leveno KJ, Cunningham FG, Giesecke AH, Shearer VE, Sidawi JE. Randomized comparison of general and regional anesthesia for cesarean delivery in pregnancies complicated by severe preeclampsia. Obstetrics and Gynecology 1995;86(2):193‐9. [PUBMED: 7617349] [DOI] [PubMed] [Google Scholar]
Wallin 1986
- Wallin G, Cassuto J, Hogstrom S, Rimback G, Faxen A, Tollesson PO. Failure of epidural anesthesia to prevent postoperative paralytic ileus. Anesthesiology 1986;65(3):292‐7. [PUBMED: 3755875] [PubMed] [Google Scholar]
Wattwil 1989
- Wattwil M, Thoren T, Hennerdal S, Garvill JE. Epidural analgesia with bupivacaine reduces postoperative paralytic ileus after hysterectomy. Anesthesia and Analgesia 1989;68(3):353‐8. [PUBMED: 2919775] [PubMed] [Google Scholar]
Weksler 2005
- Weksler N, Atias I, Klein M, Rosenztsveig V, Ovadia L, Gurman GM. Is penile block better than caudal epidural block for postcircumcision analgesia?. Journal of Anesthesia 2005;19(1):36‐9. [PUBMED: 15674514] [DOI] [PubMed] [Google Scholar]
Welborn 1990
- Welborn LG, Rice LJ, Hannallah RS, Broadman LM, Ruttimann UE, Fink R. Postoperative apnea in former preterm infants: prospective comparison of spinal and general anesthesia. Anesthesiology 1990;72(5):838‐42. [PUBMED: 2187377] [DOI] [PubMed] [Google Scholar]
Weller 1991
- Weller R, Rosenblum M, Conard P, Gross JB. Comparison of epidural and patient‐controlled intravenous morphine following joint replacement surgery. Canadian Journal of Anaesthesia = Journal Canadien d'Anesthesie 1991;38(5):582‐6. [PUBMED: 1934205] [DOI] [PubMed] [Google Scholar]
White 1980
- White IW, Chappell WA. Anaesthesia for surgical correction of fractured femoral neck. A comparison of three techniques. Anaesthesia 1980;35(11):1107‐10. [PUBMED: 7446914] [DOI] [PubMed] [Google Scholar]
White 1983
- White J, Harrison B, Richmond P, Procter A, Curran J. Postoperative analgesia for circumcision. BMJ (Clinical research ed.) 1983;286(6382):1934. [PUBMED: 6407641] [DOI] [PMC free article] [PubMed] [Google Scholar]
William 2001
- William JM, Stoddart PA, Williams SA, Wolf AR. Post‐operative recovery after inguinal herniotomy in ex‐premature infants: comparison between sevoflurane and spinal anaesthesia. British Journal of Anaesthesia 2001;86(3):366‐71. [PUBMED: 11573526] [DOI] [PubMed] [Google Scholar]
Wulf 1999
- Wulf H, Biscoping J, Beland B, Bachmann‐Mennenga B, Motsch J. Ropivacaine epidural anesthesia and analgesia versus general anesthesia and intravenous patient‐controlled analgesia with morphine in the perioperative management of hip replacement. Ropivacaine Hip Replacement Multicenter Study Group. Anesthesia and Analgesia 1999;89(1):111‐6. [PUBMED: 10389787] [DOI] [PubMed] [Google Scholar]
Yeager 1987
- Yeager MP, Glass DD, Neff RK, Brinck‐Johnsen T. Epidural anesthesia and analgesia in high‐risk surgical patients. Anesthesiology 1987;66(6):729‐36. [PUBMED: 3296854] [DOI] [PubMed] [Google Scholar]
Yegin 2003
- Yegin A, Ertug Z, Yilmaz M, Erman M. The effects of epidural anesthesia and general anesthesia on newborns at cesarean section. Turkish Journal of Medical Sciences 2003;33:311‐4. [Google Scholar]
References to other published versions of this review
Guay 2012
- Guay J, Choi P, Suresh S, Ganapathy S, Albert N, Kopp S, et al. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guay 2014
- Guay J, Choi PT, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial anesthesia for the prevention of postoperative mortality and major morbidity: an overview of cochrane systematic reviews. Anesthesia and Analgesia 2014;119(3):716‐25. [DOI: 10.1213/ANE.0000000000000339; PUBMED: 24977635] [DOI] [PubMed] [Google Scholar]


