Abstract
The aim of this study is to estimate the incidence of the gastrointestinal stromal tumor after the previous diagnoses were confirmed and/or revised by both immunohistochemical and mutational analyses. We reviewed 17,858 surgically excised gastrointestinal lesions in our hospital from 1998 to 2004. All mesenchymal tumors were examined for CD117 expression by immunohistochemistry, and every CD117-negative mesenchymal tumors were further subjected to mutational analysis for KIT and PDGFRA exons. The results showed that approximately 35% of gastrointestinal stromal tumors were misdiagnosed if immunohistochemical analysis of CD117 expression was not performed; and approximately 15% misdiagnosed if mutation analysis was not available. Because approximately 4.72% of patients with gastrointestinal malignancies in Taiwan were treated in our hospital and the average of newly diagnosed gastrointestinal stromal tumors in our hospital was 14.33 cases per year, the estimated annual incidents of gastrointestinal stromal tumor in Taiwan were 303.60. Therefore, the annual incidence of gastrointestinal stromal tumor is 13.74 per million Taiwanese.
Keywords: Gastrointestinal stromal tumor, Incidence, CD117, KIT, PDGFRA
Introduction
Before the introduction of the term gastrointestinal (GI) stromal tumors (GISTs) two decades earlier, tumors of this kind were often diagnosed as smooth-muscle tumors. Afterwards, GIST was either used as an inclusive term to refer mesenchymal tumors regardless of their differentiation phenotype [1] or as an exclusive term for mesenchymal tumors that do not differentiate into Schwann cells or smooth-muscle cells [2]. The terminology was in flux and, for example, gastrointestinal autonomic nerve tumor, as known as plexosarcoma, was used when tumors of this kind show ultrastructural features of complex interdigitating cell processes, neurosecretory granules, and intermediate filaments. The estimated incidence of GIST, in the sense of such heterogeneous neoplasms, was approximately 6.8 per million [3].
In 1998, the CD117 expression in GISTs [4] was proposed as an important piece of evidence that links the histogenesis of GIST to interstitial cell of Cajal, which is the pacemaker cell for autonomic gut motility [5, 6]. Since then, the diagnosis of GIST has become specific, and requires the inclusion of immunopositivity for CD117 [7]. From this perspective, the incidence of CD117-positive GIST was approximately 11–14.5 per million [8, 9].
Recent studies have shown that gain-of-function mutation of KIT is the initial oncogenic step leading to the development of GIST [10]. Several investigators showed that 52–92% of GISTs harbored KIT mutation [11–13] and approximately 35% of GISTs lacking mutated KIT had PDGFRA mutation [14]. The discovery of mutations of these genes in GISTs is important from a therapeutic point of view because GISTs harboring such a mutation respond to imatinib mesylate (Glivec, Novartis Pharma, Basel, Switzerland) [15]. In addition to the therapeutic implication, mutation analysis can provide an additional diagnostic indicator for some GISTs that are immunonegative for CD117 [16, 17]. These CD117-negative GISTs are likely to be overlooked unless mutated KIT or PDGFRA genes are identified in the tumor. In this regard, the incidence of GIST, including the CD117-negative ones, remains to be estimated. These data are important to healthcare providers regarding the availability of imatinib mesylate, a remarkably effective [18] and expensive therapeutic agent for GISTs.
In this study, we aimed to determine the incidence of GIST, which, in the specific morphologic context, are positive for CD117 expression by immunohistochemistry (IHC) and/or the presence of KIT and PDGFRA mutations.
Materials and methods
Mackay Memorial Hospital (MMH) is a 2000-bed medical center in northern Taiwan. The pathology archives of all surgically excised specimens (excluding endoscopic biopsies) of the GI tract from 1998 to 2004 were reviewed. This study was conducted according to the guidelines of the Institutional Review Boards at MMH. The hematoxylin and eosin-stained along with immunostained sections of all mesenchymal lesions of the GI tract were retrieved.
The gastrointestinal cancers treated in MMH were identified by using International Classification of Diseases system (ICD-9 codes) as follows: code 150 for esophageal carcinoma, code 151 for gastric adenocarcinoma, code 152 for adenocarcinoma of the small intestine, and codes 153 and 154 for colorectal carcinoma.
IHC for CD117 expression was performed for all mesenchymal tumors of the GI tract. In brief, 5-μm representative sections of the specimens were deparaffinized with xylene, rehydrated through a series of graded alcohols, and reacted with antibody against CD117 (1:50 dilution; Dako, Carpinteria, CA). Immunoreaction was detected according to the manufacturer's instructions (Ventana Medical Systens, Tucson, AZ). Diaminobendizine containing hydrogen peroxide was employed as the chromogen. The positive immunostaining was defined if more than 15% of the tumor cells were strongly immunoreactive to CD117.
Tumor DNA, isolated from formalin-fixed paraffin-embedded tumors, were subjected to PCR amplification using eight pairs of oligonucleotide primers for exons 9, 11, 13, and 17 of KIT and exons 10, 12, 14, and 18 of PDGFRA according to previously described procedures [16]. The resultant amplicons were sequenced using the ABI PRISM BigDye terminator cycle sequencing ready reaction kit and ABI Prism 377 Genetic Analyzer (PE Applied Biosystems, Foster City, CA).
Results
Analysis of the original diagnoses of surgically excised specimens of the GI tract
From 1998 to 2004, there were 17,858 surgically excised specimens of the GI tract at our hospital (Table 1). Among them, 71.47% (n=12,764) were non-neoplastic lesions, 27.14% (n=4,847) epithelial tumors, and 0.94% (n=167) were mesenchymal tumors. The latter included 81 (48.5%) GISTs, 26 (15.6%) CD117-negative GISTs, and 60 (35.9%) non-GIST mesenchymal tumors.
Table 1.
Analysis of the surgically excised gastrointestinal lesions from 1998 to 2004
| Category | Year | Esophagus | Stomach | Small intestine | Appendix | Colorectum | Other | Total |
|---|---|---|---|---|---|---|---|---|
| Non-neoplastic | 1998 | 9 | 85 | 91 | 796 | 618 | 0 | 1,599 |
| 1999 | 10 | 94 | 126 | 811 | 563 | 0 | 1,604 | |
| 2000 | 4 | 39 | 113 | 816 | 806 | 0 | 1,778 | |
| 2001 | 14 | 69 | 134 | 900 | 1,136 | 0 | 2,253 | |
| 2002 | 6 | 94 | 46 | 835 | 1,262 | 0 | 2,243 | |
| 2003 | 8 | 73 | 127 | 695 | 654 | 0 | 1,557 | |
| 2004 | 11 | 78 | 91 | 796 | 754 | 0 | 1,730 | |
| Neoplastic | 1998 | 18 | 82 | 25 | 4 | 528 | 0 | 657 |
| 1999 | 19 | 76 | 28 | 7 | 592 | 1 | 723 | |
| 2000 | 25 | 50 | 23 | 0 | 419 | 1 | 518 | |
| 2001 | 28 | 100 | 22 | 8 | 436 | 2 | 596 | |
| 2002 | 30 | 109 | 15 | 5 | 449 | 1 | 609 | |
| 2003 | 22 | 78 | 28 | 0 | 692 | 0 | 820 | |
| 2004 | 33 | 95 | 34 | 0 | 1,007 | 2 | 1,171 | |
| 1. Epithelial | 1998 | 17 | 71 | 17 | 3 | 518 | 0 | 626 |
| 1999 | 19 | 66 | 12 | 7 | 579 | 0 | 683 | |
| 2000 | 24 | 38 | 11 | 0 | 409 | 0 | 482 | |
| 2001 | 28 | 86 | 4 | 8 | 430 | 0 | 556 | |
| 2002 | 27 | 96 | 8 | 5 | 440 | 0 | 576 | |
| 2003 | 20 | 69 | 23 | 0 | 683 | 0 | 795 | |
| 2004 | 31 | 84 | 20 | 0 | 994 | 0 | 1,129 | |
| Carcinoma | 1998 | 14 | 60 | 14 | 2 | 168 | 0 | 258 |
| 1999 | 18 | 54 | 7 | 1 | 177 | 0 | 257 | |
| 2000 | 19 | 33 | 11 | 0 | 84 | 0 | 147 | |
| 2001 | 21 | 70 | 0 | 0 | 96 | 0 | 187 | |
| 2002 | 22 | 70 | 6 | 0 | 120 | 0 | 218 | |
| 2003 | 17 | 54 | 12 | 0 | 264 | 0 | 347 | |
| 2004 | 25 | 69 | 14 | 0 | 305 | 0 | 413 | |
| 2. Mesenchymal | 1998 | 1 | 10 | 8 | 0 | 3 | 0 | 22a |
| 1999 | 0 | 7 | 13 | 0 | 3 | 1 | 24 | |
| 2000 | 1 | 9 | 10 | 0 | 5 | 1 | 26 | |
| 2001 | 0 | 13 | 11 | 0 | 0 | 2 | 26 | |
| 2002 | 3 | 11 | 6 | 0 | 4 | 1 | 25 | |
| 2003 | 2 | 9 | 2 | 0 | 3 | 0 | 16 | |
| 2004 | 2 | 10 | 13 | 0 | 1 | 2 | 28 | |
| GIST | 1998 | 0 | 7 | 7 | 0 | 0 | 0 | 14 (8)b |
| 1999 | 0 | 7 | 5 | 0 | 1 | 1 | 14 (10) | |
| 2000 | 0 | 5 | 7 | 0 | 2 | 1 | 15 (13) | |
| 2001 | 0 | 10 | 6 | 0 | 0 | 2 | 18 (15) | |
| 2002 | 0 | 9 | 3 | 0 | 1 | 1 | 15 (14) | |
| 2003 | 0 | 8 | 1 | 0 | 2 | 0 | 11 | |
| 2004 | 0 | 7 | 11 | 0 | 0 | 2 | 20 | |
| 3. Other tumors | 1998 | 0 | 1 | 0 | 1 | 7 | 0 | 9 |
| 1999 | 0 | 3 | 3 | 0 | 10 | 0 | 16 | |
| 2000 | 0 | 3 | 2 | 0 | 5 | 0 | 10 | |
| 2001 | 0 | 1 | 9 | 0 | 6 | 0 | 16 | |
| 2002 | 0 | 2 | 1 | 0 | 5 | 0 | 8 | |
| 2003 | 0 | 0 | 3 | 0 | 6 | 0 | 9 | |
| 2004 | 0 | 1 | 1 | 0 | 12 | 0 | 14 | |
| Total | 1998 | 27 | 167 | 116 | 800 | 1,146 | 0 | 2,256 |
| 1999 | 29 | 170 | 154 | 818 | 1,155 | 1 | 2,327 | |
| 2000 | 29 | 89 | 136 | 816 | 1,225 | 1 | 2,296 | |
| 2001 | 42 | 169 | 156 | 908 | 1,572 | 2 | 2,849 | |
| 2002 | 36 | 203 | 61 | 840 | 1,711 | 1 | 2,852 | |
| 2003 | 30 | 151 | 155 | 695 | 1,346 | 0 | 2,377 | |
| 2004 | 44 | 173 | 125 | 796 | 1,761 | 2 | 2,901 |
aGastrointestinal mesenchymal tumors including both GISTs and non-GISTs.
bNumbers in parentheses represent the cases originally diagnosed as GIST.
Reviewing the past diagnoses that were made before immunohistochemical analysis of CD117 expression was routinely used in our hospital for all mesenchymal tumors of the GI tract, we found that five cases of CD117-positive GISTs were misdiagnosed in 1998 and two cases were in 1999. Therefore, the misdiagnosis rate by morphologic assessment without the ancillary IHC for CD117 expression was approximately 36% (10/18) (Table 2).
Table 2.
Increase of GIST patients after using more sophisticate diagnostic modalities
| Tumors originally diagnosed as GIST | Tumors finally diagnosed as GIST | ||
|---|---|---|---|
| 1998 | 8 | 14 | 13 (CD117 positive) 1 (CD117 negative) |
| 1999 | 10 | 14 | 12 (CD117 positive) 2 (CD117 negative) |
| 2000a | 13 | 15 | 13 (CD117 positive) 2 (CD117 negative) |
| 2001 | 15 | 18 | 15 (CD117 positive) 3 (CD117 negative) |
| 2002b | 14 | 15 | 6 (CD117 positive) 9 (CD117 negative) |
| 2003 | 11 | 11 | 6 (CD117 positive) 5(CD117 negative) |
| 2004 | 20 | 20 | 16 (CD117 positive) 4 (CD117 negative) |
aImmunohistochemical analysis was available for suspected cases in our hospital since 2000.
bMutational analysis for KIT and PDGFRA has been employed since 2002 for suspected cases that were immunnonegative for CD117.
For every CD117-negative mesenchymal lesion of the GI tract that was morphologically indistinguishable from GIST, genomic KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 10, 12, 14, and 18) were sequenced. In the specific morphologic context, the KIT or PDGFRA mutant tumors were classified as GISTs regardless of the IHC results. Based on this approach, two cases of CD117-negative GISTs in 2000 and three cases in 2001 were misdiagnosed, indicating that there was approximately 15% (5/33) misdiagnosis rate if ancillary mutation analysis was not available (Table 2).
Among these 107 GISTs, the stomach was the most common site of tumor involvement, followed by the small intestine (Table 3). Approximately 8% (n=9) of GISTs were incidentally identified at surgery for adenocarcinoma (eight tumors) and lymphoma (one tumor).
Table 3.
Anatomical distribution of GISTs
| Esophagus | Stomach | Small bowel | Colorectum | Others | Subtotal | |
|---|---|---|---|---|---|---|
| 1998 | 0 | 7 | 7 | 0 | 0 | 14 |
| 1999 | 0 | 7 | 5 | 1 | 1 | 14 |
| 2000 | 0 | 6 | 7 | 2 | 0 | 15 |
| 2001 | 0 | 10 | 6 | 0 | 2 | 18 |
| 2002 | 0 | 9 | 4 | 1 | 1 | 15 |
| 2003 | 0 | 8 | 1 | 2 | 0 | 11 |
| 2004 | 0 | 7 | 11 | 0 | 2 | 20 |
| Subtotal | 0 | 54 | 41 | 6 | 6 | 107 |
| (percentage) | (0%) | (50.5%) | (38.1%) | (5.6%) | (5.6%) |
As shown in Fig. 1, there were 14.33 GIST patients per year from 1998 to 2000. Since 2001, our hospital has become a referral center of GIST patients and, thus, the annual case number dramatically increased thereafter except for 2003 when the emergence of an epidemic corona virus infection (severe acute respiratory syndrome, SARS) caused a nearly complete shutdown of our hospital for approximately 3 months. Therefore, the subsequent analysis of the incidence of GIST was based on data obtained between 1998 and 2000.
Fig. 1.
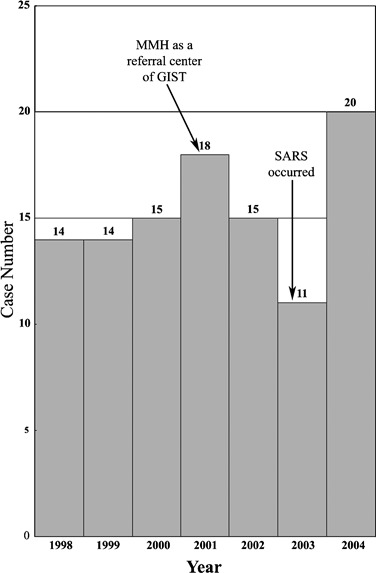
Incidents of gastrointestinal stromal tumor (GIST) in Mackay Memorial Hospital (MMH) from 1998 to 2004 appear to be constant before 2000. However, the case number was markedly influenced by exogenous factors such as reputation of managing GIST patients and unrelated epidemic corona viral infection (severe acute respiratory syndrome, SARS)
Incidence of GIST in Taiwan
To estimate the percentage of GI cancer patients in Taiwan treated at our hospital during the period between 1998 and 2000, we analyzed patients coded 150–154 in our hospital and those registered in a nationwide database (Department of Health, Executive Yuan, Taiwan). According to the public database, there were 10,725 cases of gastrointestinal cancer in 1998, 11,231 cases in 1999, and 11,844 cases in 2000 (Table 4).
Table 4.
Patients with GI cancers surgically treated at MMH (a 2000-bed medical center) versus patients with GI cancers diagnosed in Taiwan
| 1998 | 1999 | 2000 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICD-9 CODE | Incidents in MMH | Incidents in Taiwan | Ratio (%) | Incidents in MMH | Incidents in Taiwan | Ratio (%) | Incidents in MMH | Incidents in Taiwan | Ratio (%) | |
| Esophagus | 150 | 50 | 933 | 5.36 | 41 | 969 | 4.23 | 55 | 1,052 | 5.23 |
| Gastric | 151 | 158 | 3,313 | 4.77 | 160 | 3,356 | 4.77 | 163 | 3,339 | 4.88 |
| Small intestine | 152 | 6 | 181 | 3.31 | 16 | 225 | 7.11 | 14 | 240 | 5.83 |
| Colorectal | 153 and 154 | 307 | 6,298 | 4.87 | 312 | 6,681 | 4.67 | 312 | 7,213 | 4.33 |
The incidents of esophageal cancers that were surgically treated at our hospital accounted for 5.36% (50/933) of the incidents with the same disease diagnosed in Taiwan. Along the same lines, the ratios of gastric, small intestinal, and colorectal cancers were 4.77, 3.31, and 4.87%, respectively. There was no statistical difference (P=0.68) among these ratios, suggesting that approximately 4.86% (521/10,725) of patients with GI malignancies in Taiwan were treated at our hospital in 1998 regardless of the anatomical locations of the cancers. Similarly, the ratios of esophageal, gastric, small intestinal, and colorectal cancers in 1999 were 4.23, 4.77, 7.11, and 4.67%, respectively, and were not significantly different among them (P=0.33). In 2000, these ratios were 5.23, 4.88, 5.83, and 4.33%, and were not statistically different (P=0.31).
Based on these calculations, approximately 4.72% (1,594/33,800) of GI cancer patients in Taiwan were treated at our hospital from 1998 to 2000. Because the clinical presentation of GISTs is indistinguishable from that of other GI cancers, it is not unreasonable to assume that GISTs that were newly diagnosed in our hospital also accounted for a similar percentage of the newly diagnosed GISTs in Taiwan. Therefore, the estimated number of GIST patients in Taiwan was 303.60 (14.33/4.72%) during the period 1998–2000. Because the average population in Taiwan was 22,099,217, the incidence of GIST would be approximately 13.74 per million Taiwanese (303.60/22,099,217).
Discussion
The incidence of GIST is known to be underestimated [19] because the definition has been evolving in the last decade and the diagnostic criteria have been modified accordingly. Including both immunohistochemical analysis of CD117 expression as well as mutational analysis of KIT and PDGFRA for the diagnosis of GISTs, we calculated that the annual incidence of GIST in Taiwan is 13.74 per million. Our estimated incidence is much higher than that of a recent study in the United States [3], which was based on the data of the National Cancer Institutes Surveillance, Epidemiology, and End-Results (SEER) registries. They reported an incidence rate of 6.8 per million and speculated that their incidence may be overestimated because of possible inclusion of non-GIST tumors in their calculation. Based on our finding that about 36% of GISTs would be misdiagnosed as other mesenchymal tumors if immunohistochemical and molecular analyses are not employed in the diagnosis, we believed the SEER's rate was in fact underestimated. However, it should be addressed that the SEER data did not include GISTs that were diagnosed as benign tumors; therefore, the SEER's rate probably reflected the incidence of GISTs with high or intermediate risk of malignancy.
Our estimated incidence (13.74 per million) is higher than that in Iceland, where the annual incidence of 11 per million was reported [8]. The difference between these two rates could be explained by our inclusion of CD117-negative GISTs if racial factors were ignored. The incidence of GIST in Taiwan is slightly lower than that in Sweden, where the annual incidence of 14.5 per million was reported [9]. The true annual incidence of GISTs in Sweden would probably be higher if CD117-negative GISTs were included. The slight difference in the annual incidences between Taiwan and Sweden could be explained, at least in part, by the autopsy cases. None of the GISTs analyzed in this study were identified from the autopsy cases, whereas 10% of GISTs in Sweden's study were incidentally identified at the time of autopsy [9]. In addition, the difference could also be explained by racial variation in that the SEER study pointed out a significant difference in incidence between Asian Pacific Islanders (10.3 per million) and Whites (6.0 per million) [3]. In this regard, the population in Taiwan comprises predominantly Han Chinese and a small portion of indigenous peoples that consist of nine aboriginal tribes of undetermined origin.
Some limitations of the study need to be mentioned. Because the specimens analyzed in this study were surgically excised tumors for symptomatic treatment, the incidence reported here should be interpreted in this context. Therefore, our rate should theoretically be lower than that in Japan, where GISTs are more likely to be diagnosed and removed through endoscopic biopsy than other countries.
In conclusion, based on immunohistochemical and molecular analyses, we report here that the incidence of GIST in Taiwanese is approximately 13.7 per million, similar to those of Scandinavia. Our study also indicates that caution should be exercised when a reported incidence of GIST is interpreted because the rate may decrease if ancillary diagnostic tests are not used and increase if incidentally identified cases, such as autopsies and small biopsies, are included. True incidence of GIST may finally be determined after accurate rates are reported on various ethnic groups and racial factors are clarified.
Acknowledgments
Supported in part by Novartis Oncology, Taiwan, to Dr. Chin-Yuan Tzen and by intramural grant from Mackay Memorial Hospital to Dr. Chi-Yuan Tzen.
References
- 1.Schaldenbrand JD, Appelman HD. Solitary solid stromal gastrointestinal tumors in von Recklinghausen's disease with minimal smooth muscle differentiation. Hum Pathol. 1984;15:229–232. doi: 10.1016/S0046-8177(84)80184-7. [DOI] [PubMed] [Google Scholar]
- 2.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 4.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 6.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CD,, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 8.Geir T, Hjörtur GG, Magnús KM, Jón GJ. Gastrointestinal stromal tumors in Iceland, 1990–2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005;117:289–293. doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–495. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 11.Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA, Demetri GD, Fletcher CD, Fletcher JA. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. [PubMed] [Google Scholar]
- 12.Hirota S, Nishida T, Isozaki K, Taniguchi M, Nakamura J, Okazaki T, Kitamura Y. Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J Pathol. 2001;193:505–510. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH818>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, Sobin LH, Miettinen M. Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. A study of 200 cases. Am J Pathol. 2000;157:1091–1095. doi: 10.1016/S0002-9440(10)64623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van Den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 16.Tzen CY, Mau BL. Analysis of CD117-negative gastrointestinal stromal tumors. World J Gastroenterol. 2005;11:1052–1055. doi: 10.3748/wjg.v11.i7.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai S, Hasegawa T, Sakuma Y, Takazawa Y, Motegi A, Nakajima T, Saito K, Fukayama M, Shimoda T. Myxoid epithelioid gastrointestinal stromal tumor (GIST) with mast cell infiltrations: a subtype of GIST with mutations of platelet-derived growth factor receptor alpha gene. Hum Pathol. 2004;35:1223–1230. doi: 10.1016/j.humpath.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20:1692–1703. doi: 10.1200/JCO.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 19.de Silva CM, Reid R. gastrointestinal stromal tumors (GIST): C-kit mutations, cd117 expression, differential diagnosis and targeted cancer therapy with imatinib. Pathol Oncol Res. 2003;9:13–19. doi: 10.1007/BF03033708. [DOI] [PubMed] [Google Scholar]


