Abstract
Incidents of contamination in biopharmaceutical production have highlighted the need to apply alternative or supplementary disinfection techniques. Ultraviolet (UV) irradiation is a well-established method for inactivating a broad range of microorganisms, and is therefore a good candidate as an orthogonal technique for disinfection. To apply UV as a safeguard against adventitious agents, the UV sensitivity of these target agents must be known so that the appropriate dose of UV may be applied to achieve the desired level of inactivation. This document compiles and reviews experimentally derived 254 nm sensitivities of organisms relevant to biopharmaceutical production. In general, different researchers have found similar sensitivity values despite a lack of uniformity in experimental design or standardized quantification techniques. Still, the lack of consistent methodologies has led to suspicious UV susceptibilities in certain instances, justifying the need to create a robust collection of sensitivity values that can be used in the design and sizing of UV systems for the inactivation of adventitious agents.
Keywords: Biopharmaceutical, Adventitious agent, Ultraviolet, Irradiation, Disinfection, Viral clearance
Introduction
There have been a number of publicized incidents in which biopharmaceutical production has experienced contamination with a virus or bacterial agent [6, 10, 24, 33, 72]. These contamination events are highly disruptive, requiring that production be halted, inventory be quarantined, and extensive testing be conducted to identify the nature and source of the contamination. The cost of a contamination event can be significant. For example, costs associated with a vesivirus 2117 contamination event at Genzyme in 2009 were estimated at more than $200 million including a fine of $175 million from the US FDA [3]. In addition, there is the direct or indirect cost to patients whose medications become unavailable.
Ultraviolet (UV) disinfection has been applied in drinking water for more than a century, with the first installation in Marseille in 1910, and the first US installation in Henderson KY in 1916 [66]. Many millions of people rely on drinking water disinfected with ultraviolet light, including the residents of New York City, USA, and Vancouver, Canada. Benefits of UV disinfection include the avoidance of chemical addition, as well as simple control and maintenance. The unique mode of action of UV, directly affecting the nucleic acids of an organism or virus, also makes it a desirable orthogonal methodology to complement technologies such as filtration or chemical inactivation. As a result, it is often considered a treatment method for small organisms or viruses that cannot be readily filtered or that are not susceptible to chemical disruption.
Biopharmaceutical manufacturers Genzyme and Sanofi Pasteur consider UV to be a promising prospective adventitious agent clearance technology to mitigate the risk of contamination from raw materials [44]. However, there is reluctance to implement it in processes because of the lack of available literature on the topic. Recently, an effort to correct this has been undertaken and our group has published research considering the effect of UV fluence on the composition and function of cell culture media [70, 117].
UV inactivation is generally achieved using lamps emitting at 254 nm. This wavelength, in the UVC range, is produced very efficiently using mercury vapor lamps, and it nearly corresponds to the nucleic acid absorption peak of about 265 nm. The absorbed energy can result in pyrimidine cyclobutane dimer formation; if the dimers are formed in a critical location and cannot be overcome by repair mechanisms, the organism cannot reproduce and is thereby inactivated. The amount of UV relevant to inactivation is properly denoted as fluence, which is the direction-independent irradiance-analog multiplied by the exposure time. The units of fluence are energy per unit area.
There have been attempts to predict the UV sensitivity of organisms by correlations with genome size, single-stranded vs. double-stranded, DNA vs. RNA, or detailed genomic analysis [57, 56, 64]. The model predictions have good general agreement with experiment, allowing the estimation of UV sensitivity for newly discovered organisms or in cases where experimental results are not available. However, even the most detailed mechanistic predictions by Kowalski [56] differ by up to a factor of 2 or 0.5 from specific experimental results. For that reason, this review considers only experimentally measured inactivation rates; model predictions are not included here.
Several review articles identify adventitious agents that are concerns for CHO cell processes based on known contamination events or known susceptibilities [12, 54, 74]. The objective of this study is to provide a critical review of the available UV inactivation literature for organisms that are crucial to the biopharmaceutical industry, and to provide a comprehensive list of the organisms studied along with their UV sensitivities. This work will hopefully be a valuable source of information in the design of UV disinfection equipment and experiments, as well as provide researchers a foundation to evaluate and analyze UV inactivation studies.
Methodology
Notes:
In the present work, definition and terminology are based on the IUPAC 2006 recommendations [18] (Table 1).
All plots were created in R, a programming language that facilitates statistical computing and graphic visualization (https://www.r-project.org/), using the ggplot2 package (http://docs.ggplot2.org/). Box-plot whiskers extend above the upper edge of the box to the highest value point (or below the lower edge of the box to the lowest value point) that is within 1.5 times the inter-quartile range.
Table 1.
Symbols and definitions
| Symbol | Name | Definition |
|---|---|---|
| Fo | Fluence | The integral of fluence rate and time. Units: energy/area, e.g. mJ/cm2. For UV disinfection, this is commonly referred to as “UV dose” |
| Eo | Fluence Rate | The radiant energy flux (power) from all directions passing through an infinitesimal spherical volume, divided by the cross-sectional area of the sphere. Units: power/area, e.g. mW/cm2 This is the spherical analog of irradiance, and is independent of direction |
| E (see note) | Irradiance | The radiant energy flux incident on a surface element, divided by the area of the element. Units: power/Area, e.g. mW/cm2. This quantity depends on the angle of incidence |
| D10 | For an organism with first-order UV inactivation kinetics, the Fluence required to decrease the number of viable organisms to 1/10 of the original number | |
| A | The optical absorption coefficient, in base 10. Used in the Beer–Lambert–Bouguere law. | |
| Ae | The optical absorption coefficient, in base e | |
| L | Length | The fluid path length through which UV radiation passes |
The same symbol is often used for both irradiance and fluence rate
Data selection and errors in UV inactivation studies
UV sensitivity is generally measured by applying a well-quantified, single-valued UV fluence to a fluid containing virus, and determining the resulting degree of inactivation of the virus. The process is repeated for a sequence of fluence values, and the resulting inactivation is plotted to determine the shape of the inactivation curve (the kinetics) and to minimize the impact of error in any one data point. For first-order kinetics, the D10 value (the fluence required for a single log reduction in infectivity) is readily determined from the slope of the resulting plot.
Quality assurance
The quality of data in a UV inactivation study should be checked by a simple plot of survival vs. UV fluence. For most organisms, the UV inactivation is well modeled by a first-order equation [66]. That is, the number of infective virus particles, N, is an exponential function of the applied fluence, F o multiplied by the original amount of infective virus, N 0:
| 1 |
When plotted as the log of the number of viable virus vs. the fluence, the data will form a straight line for many viruses. A simple check of data quality can be done by examining this log plot of the data. If the data deviates from the typical straight line, it may indicate that the experiment was flawed. In Fig. 1, we plotted data from two different investigators for the UV inactivation of feline calicivirus (FCV). It can be seen that the results from Park et al. [81] show first-order kinetics to about 5 log inactivation, while the results from Nuanualsuwan et al. [77] show tailing with a maximum inactivation of about 3 log. The work of Nuanualsuwan et al. [77] also shows tailing for ϕX174 phage at about 3 log, in contrast to the first-order kinetics reported by Battigelli et al. [9] even beyond 6 log inactivation (Fig. 1).
Fig. 1.
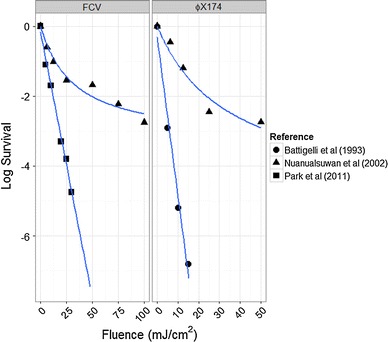
Inactivation data for feline calicivirus and ϕX174 phage from different research groups
Although some organisms may not exhibit first-order kinetics for UV inactivation, first-order kinetics are very common in response to UV irradiation. Any data set should be compared against other literature results to determine the expected inactivation kinetics for the virus, or for other viruses of the same family or genus. Deviation from expected kinetics may be the result of a number of factors, discussed below.
UV measurement errors
Examining the outliers in this literature review, it is apparent that some authors did not precisely apply single fluence values, but instead applied fluence distributions including higher and lower values than that quoted. Applying a well-quantified, mono-valued fluence requires careful design of the irradiation apparatus, careful fluid characterization, and usually careful mixing.
Fluence detectors are very difficult to design, construct, and calibrate. An ideal fluence sensor would have a spherical detector that was equally sensitive to radiation arriving from all directions (4π steradians). Some investigators have used small spherical containers of chemical actinometry solutions [83], or small coated plastic spheres that change fluorescence or color with UV fluence [13], but this approach does not permit real-time measurement. Creating a fluence detector with an electronic readout is nearly impossible. The solution to this dilemma is to use an irradiation geometry in which an irradiance sensor will suffice, since these are available from vendors, e.g. International Light Technologies and Solar Light, along with calibration traceable to U.S. National Institute of Standards (NIST) or other national laboratories.
The UV irradiance must be accurately known so that fluence can be calculated. In their guideline for applying UV fluence, Bolton and Linden [15] recommend using a collimated beam apparatus. While this device does not actually collimate the UV radiation field, it effectively uses an aperture to ensure that UV only reaches the fluid from a single direction, directly above the fluid. This is important for two reasons: it ensures that the incident irradiance can be measured accurately, and it ensures that the attenuated radiation field in the fluid sample can be calculated accurately.
If the UV flux reaches the fluid sample from a variety of directions, most UV sensors cannot quantify the fluence rate. Most sensors will, by design, have a response that drops off as the angle of incidence deviates from the normal. A well-designed irradiance sensor will have a response that varies with the cosine of the angle of incidence. However, viruses do not respond differently to off-axis radiation or on-axis, so the fluence rate, not irradiance, is the appropriate measurement quantity. The solution is to have the UV reaching the sample only from a single angle, and to orient the UV sensor normal to this radiation. Under these conditions, irradiance and fluence rate are identical, so the irradiance reading will be correct. The attenuated radiation field in the fluid sample can then be readily calculated, since the geometry is simple.
It is also important to correct for the attenuation of UV fluence in the fluid sample in which the organism or virus is suspended. The radiation field in the fluid sample must be calculated from the fluid depth and absorbance. This is done using the measured irradiation at the fluid surface and the measured optical absorbance of the fluid at the relevant wavelength, usually 254 nm. The intensity in a non-scattering fluid may be calculated from the Beer–Lambert–Bouguer law [71]. Assuming that the fluid is well mixed and the irradiation time is long compared with the mixing time, the expression for the volume-average intensity can readily be found by integration. The equations governing average fluence in a well-mixed, semi-transparent media were published by Morowitz [73] expressing the average fluence rate, F avg, as:
| 2 |
where F 0 is the incident fluence rate, A e is the absorption coefficient (base e) at the wavelength of interest, and L is the fluid depth in the direction of the radiation field. Expressed as a function of the more common base-10 absorption coefficient, A, this equation becomes:
| 3 |
There are a number of other correction factors highlighted in the Bolton and Linden guideline, but this absorption factor is the most significant in many configurations used for inactivation studies, since suspensions of organisms and viruses often have strong absorbance in the UV range.
Other errors
Clumping can result in apparent deviations from the true inactivation kinetics. If organisms or viral particles are not fully dispersed, some will be present in clumps. This may result in an inactivation plot with a plateau as a result of shielding of the central virus or bacterium by those surrounding it, which will allow the central virus or bacterium to survive [106]. For example, if 1 in 1000 virus particles is fully shielded, the effect will be tailing above 3 log reductions, while fully dispersed virus will demonstrate intrinsic kinetics through higher inactivation levels. These effects were recognized and addressed by Furness [37] and by Das [30] in studying the UV inactivation kinetics of mycoplasma, in which they used sonication and filtration to ensure single-cell suspensions. As Furness [37] noted, “That the suspension contained only single cells was confirmed by demonstrating that the colony-forming units (CFU) were inactivated exponentially by ultraviolet irradiation”.
Fluid optics and poor mixing can also lead to tailing in dose–response curves. If the fluid sample is not adequately mixed, so that not all components of the sample receive the same integrated UV exposure (fluence), the less-irradiated fluid will dominate the results and can lead to a plateau in inactivation. As an extreme example, if one part in 1000 of the fluid receives zero fluence, then the inactivation cannot exceed 3 logs, even as the exposure time is increased. Similar, though less extreme, behavior can result from poor dose distribution: survival is dominated by the low-dose fluid regions. For this reason, it is preferable to conduct inactivation studies in relatively clear solutions to minimize intensity gradients and lessen the importance of mixing. Likewise, the irradiation device (collimated beam) should also be set up to minimize the UV intensity gradients across the surface of the fluid sample. This deviation from uniformity is denoted by the Petri factor in the method published by Bolton and Linden [15], and methods are provided to quantify the magnitude of the effect as well as recommendations for an acceptable range.
It is important to utilize a relevant assay to determine whether infectious virus remains after irradiation. Assays, such as tissue culture 50% infectious dose (TCID50), or plaque assays, will correctly assess infectivity; morphological changes to detector cells due to viral infection are the basis of the TCID50 assay and are responsible for plaque formation.
PCR methods have significant drawbacks in measuring the degree of disinfection, as they may be unable to distinguish between viable and inactivated organisms. The detection of specific sequences does not indicate the presence of an intact, infectious virion. Even the presence of a viral sequence in a host cell does not indicate infectious virus, since the virus might enter the host cell but still not be able to replicate. Care must be taken to ensure that the assay is quantifying infectious virus.
Randazzo et al. [82] and Ju et al. [51] have investigated the ability of PCR-based techniques to distinguish between viable and inactivated norovirus and E. coli, respectively. In both papers, the authors examined the ability of RT-qPCR (reverse-transcriptase, quantitative PCR), augmented by photoactivatable dye methods (propidium monoazide and ethidium monoazide), to discriminate between viable and inactivated organisms. RT-PCR amplifies RNA, and is thought therefore to detect only viable organisms that are expressing RNA (or an RNA virus). The dyes are furthermore intended to prevent amplification of nucleic acids from organisms with compromised capsids, since the dyes can penetrate to the nucleus and prevent amplification. Randazzo found that even the best combination of these dyes was only able to suppress about 2 log of thermally inactivated norovirus. Ju et al. [51] found similar issues, with up to a 7-log discrepancy between culture and qPCR results for E. coli inactivated by desiccation. In disinfection applications where 3 log or greater inactivation is expected, these methods will not correct the inherent over-estimation of viable virus by qPCR methods. Furthermore, UV disinfection will leave any capsid virtually intact, which will further limit the performance of these dye techniques. The authors of the present review do not recommend PCR methods for assessing UV disinfection.
UV sensitivities of bacteria and viruses
UV inactivation kinetics are often first order, and are commonly described by the D10 (the dose required to reduce the counts by one log10), defined above. A larger D10 value indicates that an organism is more resistant to UV inactivation. Considering pitfalls in methodology and overall consistency within multiple research labs, average D10 values based on experimental results are proposed (Table 2) based on a thorough review of the literature D10 values (Fig. 2, Fig. 3; Appendix Table 3). Unless addressed in the text below, the reported values in Table 2 are simply an arithmetic average of the available literature listed in the appendix.
Table 2.
Average dose per log10 inactivation of adventitious agents
| Family | Genome | Adventitious agent | D10 (mJ/cm2) |
|---|---|---|---|
| Enterobacteriaceae | dsDNA | E. coli | 1.9 |
| Mycoplasmataceae | dsDNA | Mycoplasma | 1.9 |
| Acholeplasmataceae | dsDNA | Acholeplasma | 2.5 |
| Leptospiraceae | dsDNA | Leptospira | 1.9 |
| Adenoviridae | dsDNA | Adenovirus | 42.3 |
| Arenaviridae | ssRNA | Junin virus | 3.0 |
| Arenaviridae | ssRNA | Lassa virus | 3.0 |
| Bunyaviridae | ssRNA | Cache Valley virus (CVV) | 20.8 |
| Bunyaviridae | ssRNA | Hanta virus | 2.8 |
| Bunyaviridae | ssRNA | Rift Valley fever virus | 2.8 |
| Caliciviridae | ssRNA | Canine calicivirus (CaCV) | 6.6 |
| Caliciviridae | ssRNA | Feline calicivirus (FCV) | 5.3 |
| Caliciviridae | ssRNA | Murine norovirus (MNV) | 8.3 |
| Circoviridae | Circular ssDNA | Porcine circovirus (PCV) | 17.8 |
| Filoviridae | ssRNA | Ebola virus | 1.7 |
| Filoviridae | ssRNA | Marburg virus | 1.7 |
| Flaviviridae | ssRNA | Bovine viral diarrhea virus | 12.2 |
| Flaviviridae | ssRNA | West Nile virus | 5.5 |
| Herpesviridae | dsDNA | Bovine herpesvirus 1 | 16.7 |
| Herpesviridae | dsDNA | Cytomegalovirus (CMV) | 4.8 |
| Herpesviridae | dsDNA | Pseudorabies virus | 8 |
| Leviviridae | ssRNA | Bacteriophage MS2 | 19.3 |
| Microviridae | Circular ssDNA | ϕX174 phage | 2.3 |
| Orthomyxoviridae | ssRNA | Influenza A virus | 4.5 |
| Paramyxoviridae | ssRNA | Parainfluenza Virus | 14.3 |
| Parvoviridae | ssDNA | Bovine parvovirus | 12.5 |
| Parvoviridae | ssDNA | Mouse minute virus (MMV) | 1.8 |
| Parvoviridae | ssDNA | Parvovirus H1 | 23.0 |
| Parvoviridae | ssDNA | Porcine parvovirus (PPV) | 2.0 |
| Picornaviridae | ssRNA | Cardiovirus A—encephalomyocarditis virus | 5.2 |
| Picornaviridae | ssRNA | Coxsackievirus | 10.4 |
| Picornaviridae | ssRNA | Echovirus | 9.1 |
| Picornaviridae | ssRNA | Foot and mouth disease virus | 22.2 |
| Picornaviridae | ssRNA | Hepatitis A virus (HAV) | 6.3 |
| Picornaviridae | ssRNA | Poliovirus (PV) | 8.0 |
| Polyomaviridae | Circular ssDNA | Mouse polyoma virus | 57.0 |
| Polyomaviridae | Circular ssDNA | Simian vacuolating virus (SV40) | 92.8 |
| Poxviridae | dsDNA | Variola virus | 2.5 |
| Reoviridae | dsRNA | Reovirus | 22.4 |
| Retroviridae | ssRNA | Murine leukemia and sarcoma viruses | 24.9 |
| Rhabdoviridae | ssRNA | Vesicular stomatitis virus | 2.3 |
| Togaviridae | ssRNA | Semliki forest virus | 11.1 |
| Togaviridae | ssRNA | Sindbis virus | 7.1 |
| Togaviridae | ssRNA | Equine encephalitis virus | 4.9 |
Fig. 2.
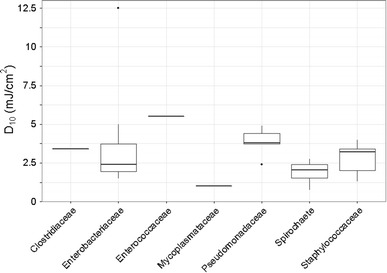
D10 values for bacterial families. Points plotted beyond the box-plot whiskers are outliers [104, 105]
Fig. 3.
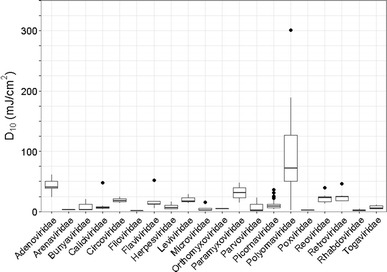
D10 values for viral families. Points plotted beyond the box-plot whiskers are outliers [104, 105]
Table 3.
Reported dose per log10 inactivation (D10) of adventitious agents
| Family | Adventitious agent | Serotype/strain | D10 (mJ/cm2) | References |
|---|---|---|---|---|
| Bacteria—Acholeplasmataceae | Acholeplasma | <14.3 | [57] | |
| Laidlawii, late logarithmic | 1 | [30] | ||
| Laidlawii, stationary | 1.5 | [30] | ||
| Laidlawii | 4.5 | [41] | ||
| Laidlawii JA1 | 3 | [76] | ||
| Laidlawii JA1 REP- | 2.25 | [76] | ||
| Bacteria—Clostridiaceae | Clostridium pasteurianum | 3.4 | [26] | |
| Clostridium perfringens | ≤42 | [92] | ||
| Bacteria—Enterobacteriaceae | Escherichia coli | 1.5 | [103] | |
| 2.1 | [23] | |||
| 1.25 | [45] | |||
| 1.9 | [121] | |||
| 2.8 | [50] | |||
| 2 | [95] | |||
| 1.4 | [96] | |||
| 2.5 | [115] | |||
| 1.4 | [80] | |||
| 3.7 | [100] | |||
| 1 | [116] | |||
| 1.4 | [114] | |||
| 1.5 | [102] | |||
| Salmonella anatum | 5 | [102] | ||
| Salmonella derby | 3.8 | [102] | ||
| Salmonella eteritidis | 2.5 | [102] | ||
| Salmonella infantis | 2 | [102] | ||
| Salmonella spp. | 5 strains | 1.8 | [116] | |
| Salmonella typhi | ATCC6539 | 1.8 | [23] | |
| Salmonella typhi | ATCC19430 | 2.1 | [114] | |
| Salmonella typhi | 2.3 | [102] | ||
| Salmonella typhi | 12.5 | [67] | ||
| Streptococcus faecalis | 5.5 | [45] | ||
| Bacteria—Mycoplasmataceae | Mycoplasma | 1 | [38] | |
| <14.3 | [57] | |||
| Arthritidis | 0.7 | [38] | ||
| Fermentans | 0.9 | [38] | ||
| Hominis type 1 | 0.7 | [38] | ||
| Orale type 1 | 1.1 | [38] | ||
| Orale type 2 | 0.6 | [38] | ||
| Pneumoniae | 0.8 | [38] | ||
| Salivarium | 1.1 | [38] | ||
| Orale | 1.1 | [37] | ||
| T210 | 0.3 | [39] | ||
| T-Pi | 0.4 | [39] | ||
| T960 | 0.6 | [39] | ||
| Orale | 2.2 | [5] | ||
| Buccale | 0.75 | [5] | ||
| Hominis | 1.2 | [5] | ||
| Fermentans | 2.1 | [5] | ||
| Salivarium | 1.8 | [5] | ||
| Gallisepticum A5969 | 3 | [42] | ||
| Gallisepticum A5969 | 4 | [41] | ||
| Bacteria—Pseudomonadaceae | Pseudomonas aeruginosa | PAO-1 | 2.4 | [21] |
| ATCC14207 | 3.7 | [2] | ||
| ATCC15442 | 3.8 | [2] | ||
| ATCC9027 | 3.8 | [2] | ||
| ATCC10145 | 4.6 | [2] | ||
| ATCC27853 | 4.9 | [2] | ||
| Bacteria—Spirochaete | Leptospira biflexa Patoc | 2.3 | [97] | |
| Leptospira illini 3055 | 2.75 | [97] | ||
| Leptospira interrogans | 0.75 | [97] | ||
| Leptospira biflexa Copenhagenii | 1.8 | [36] | ||
| Bacteria—Staphylococcaceae | Staphylococcus aureus | 112 | 1.3 | [43] |
| 112 | 3.4 | [43] | ||
| RN1349 | 2 | [8] | ||
| 3.2 | [100] | |||
| RN1349 | 4 | [8] | ||
| Prion | Bovine spongiform encephalopathy | Resistant | [55] | |
| Adenoviridae | Adenovirus | 41 | 23.6 | [69] |
| 40 | 30 | [69] | ||
| 1 | 34.5 | [79] | ||
| 6 | 38.5 | [79] | ||
| 2 | 39.7 | [40] | ||
| 2 | 40 | [79] | ||
| 5 and 2 | 40 | [34] | ||
| 2 | 42 | [62] | ||
| 5 and 7 | 42.5 | [22] | ||
| 40 | 50 | [101] | ||
| 54 | [110] | |||
| 40 | 54.3 | [79] | ||
| 5 | 61 | [52] | ||
| Junin virus | 3 | [64] | ||
| Lassa virus | 3 | [64] | ||
| Bunyaviridae | Cache Valley virus (CVV) | 20.8 | [90] | |
| Hanta virus | 2.8 | [64] | ||
| Rift Valley fever virus | 2.8 | [64] | ||
| Caliciviridae | Canine calicivirus (CaCV) | 48 | 6.6 | [31] |
| Feline calicivirus (FCV) | 4 | [31] | ||
| 4.8 | [103] | |||
| 6 | [101] | |||
| 6.3 | [81] | |||
| 47.9 | [77] | |||
| Murine norovirus (MNV) | 7.3 | [81] | ||
| 9.3 | [60] | |||
| Porcine circovirus (PCV) | 1 | 14 | [58] | |
| 2 | 18 to 25 | [85] | ||
| Filoviridae | Ebola virus | 1.7 | [64] | |
| Marburg virus | 1.7 | [64] | ||
| Flaviviridae | Bovine viral diarrhea virus | ≤17 | [7] | |
| 11.8 | [100] | |||
| 12.5 | [57] | |||
| 52 | [98] | |||
| 366 | [29] | |||
| West Nile virus | 5.5 | [64] | ||
| Herpesviridae | Bovine herpesvirus 1 | 16.7 | [57] | |
| Cytomegalovirus (CMV) | Murine | 4.6 | [93] | |
| C87 and AD169 | 5 | [4] | ||
| Pseudorabies virus | 8 | [100] | ||
| Leviviridae | Bacteriophage MS2 | 13.6 | [103] | |
| 14 | [69] | |||
| 15.9 | [60] | |||
| 16.4 | [84] | |||
| 17.5 | [81] | |||
| 21.7 | [31] | |||
| 23 | [78] | |||
| 23 | [101] | |||
| 28.9 | [79] | |||
| Microviridae | ϕX174 phage | 2.1 | [84] | |
| 2.3 | [9] | |||
| 2.5 | [95] | |||
| 15.5 | [77] | |||
| Orthomyxoviridae | Influenza A virus | H5N1 | 4.5 | [61] |
| Paramyxoviridae | Bovine parainfluenza virus | Type III | ≤48.2 | [7] |
| Mumps virus | Weak | [25] | ||
| Parainfluenza Virus | 3 | 14.3 | [57] | |
| Parvoviridae | Bovine parvovirus | 12.5 | [57] | |
| Mouse minute virus (MMV) | 0.8 | [46] | ||
| 1.7 | [11] | |||
| 1.7 | [87] | |||
| 1.7 | [86] | |||
| 2.2 | [108] | |||
| 2.3 | [58] | |||
| 2.5 | [111] | |||
| 20.8 | [90] | |||
| Parvovirus H1 | 23 | [27], [28] | ||
| Porcine parvovirus (PPV) | <18.2 | [57] | ||
| 1.8 | [58] | |||
| 2.3 | [110] | |||
| Picornaviridae | Cardiovirus A—Encephalomyocarditis virus (EMCV) | 4 | [20] | |
| 5.1 | [84] | |||
| 6.6 | [120] | |||
| Coxsackievirus | B5 | 7.3 | [9] | |
| B3 | 8.2 | [40] | ||
| B5 | 9 | [40] | ||
| A9 | 11.9 | [49] | ||
| B1 | 15.6 | [49] | ||
| Echovirus | 2 | 6.8 | [40] | |
| 12 | 7.4 | [81] | ||
| 1 | 8.3 | [40] | ||
| 1 | 10.8 | [49] | ||
| 11 | 12.2 | [49] | ||
| Foot and mouth disease virus | 12.5 | [57] | ||
| A132 | 19.7 | [78] | ||
| A-Sakol | 22.1 | [78] | ||
| O189 | 25.2 | [78] | ||
| AS1 | 31.3 | [78] | ||
| Hepatitis A virus (HAV) | HM-175 | 4 | [9] | |
| 4.5 | [110] | |||
| 7.5 | [112] | |||
| 9.2 | [109] | |||
| 36.5 | [77] | |||
| Poliovirus (PV) | 1 | 4.1 | [69] | |
| 4.7 | [103] | |||
| 6.5 | [45] | |||
| 1 | 7.7 | [40] | ||
| 1 | 8 | [79] | ||
| 3 | 10.3 | [49] | ||
| 1 | 11 | [49] | ||
| 2 | 12 | [49] | ||
| 1 | 24.1 | [77] | ||
| Polyomaviridae | Mouse polyoma virus | 47.6 | [107] | |
| 50 | [59] | |||
| 58.8 | [32] | |||
| 71.4 | [84] | |||
| 188.7 | [47] | |||
| Simian vacuolating virus (SV40) | 1.8 | [110] | ||
| 21.3 | [22] | |||
| 43.2 | [14] | |||
| 65 | [88] | |||
| 72 | [1] | |||
| 80 | [32] | |||
| 100 | [100] | |||
| 112 | [52] | |||
| 127 | [19] | |||
| 140 | [28] | |||
| 167 | [91] | |||
| 301 | [17] | |||
| 100 | [89] | |||
| Poxviridae | Variola Virus | 2.5 | [64] | |
| Reoviridae | Reovirus | 3 | 13.4 | [119] |
| 15.4 | [49] | |||
| 16.5 | [45] | |||
| 18.5 | [110] | |||
| 2 | 22.7 | [68] | ||
| 1 | 24 | [68] | ||
| 3 | 25 | [57] | ||
| 3 | 26.3 | [68] | ||
| 39.6 | [94] | |||
| Retroviridae | Murine leukemia virus | Moloney | 16.9 | [75] |
| Rauscher | 17.3 | [99] | ||
| Friend | 25 | [118] | ||
| Rauscher | 46 | [63] | ||
| Moloney | 18 | [53] | ||
| 24.9 | [71] | |||
| 26 | [110] | |||
| Rhabdoviridae | Vesicular stomatitis virus | 1 | [52] | |
| 1.2 | [84] | |||
| 4.8 | [100] | |||
| Togaviridae | Semliki forest virus | 11.1 | [113] | |
| Sindbis virus | 4.2 | [119] | ||
| 10 | [110] | |||
| Venezuelan equine encephalitis virus (VEEV) | 5.3 | [64] | ||
| Western equine encephalitis virus (WEEV) | 4.4 | [64] |
In general, the experimentally determined UV sensitivity values are consistent across multiple laboratories (Figs. 2, 3 and Table S1), which gives confidence in the reported values. Inconsistencies in the experimental data reviewed are discussed below (Fig. 4).
Fig. 4.

D10 values for adventitious agents that show inconsistencies in the data from different research laboratories. Points plotted beyond the box-plot whiskers are outliers [104, 105]
Bacteria
This review focused only on bacteria that are of concern as adventitious agents in biopharmaceutical production. More extensive reviews are available for bacteria and other organisms, including that of Hijnen [48]. Two organisms of particular interest as adventitious agents are mycoplasma and leptospira.
Mycoplasma and acholeplasma contaminations are relatively common, and since these bacteria lack a cell wall they are unaffected by many common antibiotics. They are approximately 0.1 µm in diameter, making them difficult to remove via typical sterilizing filtration. Several strains of mycoplasma and acholeplasma have been evaluated for sensitivity to UV irradiation. The one outlier in this data set is the result of Kurth [57], who reported only full clearance of mycoplasma. Therefore, the precise UV sensitivity cannot be determined from the data set of Kurth. Most experimental results show D10 values between 1 and 5 mJ/cm2, indicating that mycoplasma and acholeplasma are highly susceptible to UV inactivation.
Leptospira are a concern in biopharmaceutical production since their minor dimension of about 0.1 micron can allow them to penetrate many filters, especially those designed for bacteria, where the pore size is often about 0.2 micron. The D10 values for leptospira reported by Stamm and Charon [97] and by Fonseca [36] range from 1 to 3 mJ/cm2, indicating high susceptibility to UV disinfection. Stamm and Charon [97] provide only minimal information on the methodology and no discussion of the fluid optics, so it is difficult to determine whether the leptospira data are reliable. The authors of this review could not find any other quantitative data on the UV sensitivity of leptospira. This represents an area for additional research.
Escherichia coli has been studied by many investigators, and is readily inactivated by UV. Some strains have been found to have a shoulder in the inactivation curve, in which a low UV dose has a small impact below 1- or 2-log inactivation, with increasing impact at higher doses. For this reason, the D10 value in the summary table is based on the 4-log inactivation data, simply dividing the dose for 4-log inactivation by a factor of 4. This will be conservative for higher levels of inactivation. Since UV as a broad-spectrum inactivation method is likely to be sized for doses that would achieve more than 4-log inactivation of E. coli (a fluence of only 8 mJ/cm2) the authors feel that this is inherently conservative. Where an individual author reported values for multiple strains, only a single average value was used. This was intended to avoid giving extra emphasis to any individual author and their own systematic approach. The average D10 value for E. coli is 1.9 mJ/cm2, which confirms that this organism is highly susceptible to UV inactivation.
Feline calicivirus
Feline calicivirus has been studied by a number of investigators, and most find that it is relatively sensitive to UV inactivation, with an average D10 of about 5.3 mJ/cm2 (Fig. 4a). The outlier in this case is the result from Nuanualsuwan et al. [77], who report a value that is 9 × higher. Their D10 values reported for ϕX174, hepatitis A virus, and poliovirus are all significantly higher than those of other researchers. There are several concerns with the methodology reported by Nuanualsuwan et al. [77] that would cause erroneous results. First, they use a long lamp (91 cm) very close (12.5 cm) to the petri dish to be irradiated. This geometry can make it very difficult to obtain accurate flux measurements, and can cause shadowing near the edges of the dish. Combining the geometry and the lack of agitation, poor fluence uniformity is likely. The data presented display significant tailing at high fluences (Fig. 1), consistent with a lack of uniformity. Additionally, to determine the D10 values, the researchers used a trend line over the entire range of fluences, but a single log reduction in the virus actually occurs at a much lower UV fluence than presented. Therefore, we do not consider the D10 values reported by Nuanualsuwan et al. [77] to be accurate.
Porcine circovirus
Another organism of concern is porcine circovirus (PCV), which was detected in a commercial rotavirus vaccine (Rotarix®, GSK) [10], and can enter the biopharmaceutical production process through trypsin used in cell line development. Recent guidance from the European Medicines Agency [35] recommends the use of two complementary virus reduction steps for trypsin, including a final inactivation step of gamma, e-beam, or UV irradiation. UV sensitivity of PCV has been measured by Lackner et al. [58] and Remington [85], with a D10 range of 14–25 mJ/cm2. Although this range is large as a percentage, the moderate sensitivity reported by all investigators indicates that PCV can be readily inactivated by UV.
Bovine viral diarrhea virus
There is very little consistency in the D10 values reported for bovine viral diarrhea virus (BVDV), with a range of D10 values from 11.8 to 366 mJ/cm2 (Fig. 4b). Three of the five studies report a D10 value less than 20 mJ/cm2. The value of 366 mJ/cm2 reported by Daryany et al. [29] is remarkably high. Their experimental apparatus involved a UV lamp placed very close (10 cm) to the sample, and their methodology does not indicate the absorbance of the fluid or the presence of agitation, suggesting that the fluid was not stirred and that optical absorbance was not considered. These factors would lead to a lack of uniformity in fluence delivery and cause portions of the fluid to evade irradiation, increasing the apparent D10. The true D10 would be lower, but cannot be determined from the published information. A D10 value of 52 mJ/cm2 was reported by Steinmann et al. [98]. The fluid in this case was a platelet concentrate, which would have higher absorbance compared to the typical phosphate buffer. Minimal experimental information was provided for this study, and there was no indication that the fluid absorbance was considered in the calculated fluence delivery. Again, this would falsely increase the apparent resistance to UV disinfection, and the method would not correctly measure the inherent UV sensitivity. Based on these uncertainties, a consensus D10 value of 12.2 mJ/cm2 is recommended, but additional studies are warranted.
Mouse minute virus
One particularly important adventitious agent is mouse minute virus (MMV), which is a potential contaminant in biopharmaceutical production, and is known to be resistant to physico-chemical treatment [16]. It is very sensitive to UV inactivation, with a consensus D10 of about 1.8 mJ/cm2 (Fig. 4a). The outlier in this data is the report of Schleh et al. [90]. In this publication, full viral clearance was achieved, so the actual D10 value is lower than the calculated value. Also, minimal information about the experiments was provided, making it difficult to critically assess the methodology (the same is true for the D10 value of Cache Valley virus determined from the same publication).
Mouse polyoma virus
For mouse polyoma virus (MPyV), there are four publications that report similar results, with a consensus D10 value of 57 mJ/cm2, but Heberman and Ting [47] report a value roughly three times higher (Fig. 4b). Heberman and Ting [47] report the surface energy as the fluence and do not consider the absorbance of the fluid, both of which could lead to erroneous D10 values that are likely too high.
Simian vacuolating virus
The results for simian vacuolating virus type 40 (SV40) represent an interesting case since there have been numerous UV irradiation studies with a wide range of D10 values reported (Fig. 4b). There is an outlier on both the high- and low-sensitivity side. The average D10 without these two values is 92.8 mJ/cm2, compared with the low of only 1.8 mJ/cm2 from Wang et al. [110] and a high value of 301 mJ/cm2 for Bourre et al. [17]. The authors of the present review have measured the D10 for SV40 to be approximately 100 mJ/cm2 [89], consistent with the consensus literature value. SV40 may represent a worst case design organism for UV inactivation, with the highest resistance to UV inactivation of the organisms considered.
Correlations with nucleic acid
In contrast to previous assumptions by other authors about UV irradiation, such as single-stranded genomes being more sensitive to UV than double-stranded genomes [68], it is evident (Fig. 5) that UV resistance does not simply correlate with the overall nature of the genome. The most resistant viruses have a range of genome types: SV40 (ssDNA, 92.8 mJ/cm2), mouse polyoma virus (ssDNA, 57.0 mJ/cm2), and adenovirus (dsDNA, 42.3 mJ/cm2). Therefore, the UV sensitivity cannot be simply correlated to nucleic acid conformation. More successful models, such as the proprietary model from Kowalski [56], take into account more details including locations and frequency of pyrimidines.
Fig. 5.
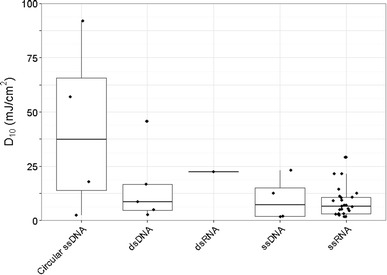
D10 values grouped by nucleic acid type. For a better appreciation of the number of studies contributing to the conclusions around the relationship between D10 values and nucleic acid type, all data points have been included in this figure using the geom_jitter (http://docs.ggplot2.org/current/geom_jitter.html) command in ggplot2
Discussion and conclusions
Although UV disinfection has been broadly applied industrially for both drinking water (high UV transparency) and municipal wastewater (lower UV transparency), it is not widely accepted for biopharmaceutical applications. The only commercially available UV system designed for biopharmaceuticals, Sartorius UVivatec, is too large for most cell line development and too small for most full-scale batch production.
Compared to drinking water and wastewater, UV treatment of cell culture media is complicated by the opacity of the fluid as well as the requisite to minimize the UV dose delivery to avoid damaging essential components of the media. Therefore, biopharmaceutical applications would necessitate custom-designed UV equipment. Similarly designed systems for fluids with high optical absorbance and stringent regulations are those for apple cider (the FDA-approved Cidersure by Headwater Foods, Rochester NY) and sugar solutions (Aquafine LS HX by Aquafine Corporation, Valencia CA).
As the biopharmaceutical industry progresses to disposable production equipment, UV technology could be well disposed to provide a sterile, disposable disinfection system. The authors of the present review have been using a family of prototype reactors in which the wetted part consists of disposable Teflon, and the design has been scaled from internal volumes of 27–500 mL [70] with corresponding increases in flow. The same design could be readily scaled further, achieving a range of disposable UV reactors suitable for perfusion, or for parallel batch production. Knowledge of the UV sensitivity of adventitious agents is crucial for efficient sizing and operation of UV reactors for biopharmaceutical applications.
High log reductions are readily achievable with UV disinfection. In water treatment, phages are commonly used for verifying UV fluence [106]. Phage stocks such as MS2 or T1UV are available at titres of up to 1011 PFU/ml and are inoculated into test fluids that are then treated with UV equipment. Typical titres of 108 PFU/ml may be fully inactivated, or more commonly the test is designed to have a countable titre after treatment, so that the fluence can be calculated from the log inactivation and known UV sensitivity of the phage [65]. Particle-association is one of the primary causes of deviation from first-order kinetics with phage and other virus. If the fluid to be treated, such as a cell culture medium, does not contain particles that could shelter viruses, then first-order kinetics can be expected up to 8 log10 inactivation or more.
The present review has found that experimentally determined 254 nm sensitivity values for many adventitious agents in the biopharmaceutical industry have been reported and are fairly consistent between different research groups. Based on the experimental data available in the literature, outliers and explanations for the inconsistent results have been highlighted. Reviewing the available data, average D10 sensitivity values have been established that can be used to design and size UV disinfection systems to inactivate specific agents. Additionally, some of the common pitfalls in experimental design of UV treatment have been identified, such as neglecting to consider the fluid absorbance, not having a proper light distribution on the sample surface, and considering only the fluence intensity at the surface of the sample rather than within the liquid.
Most of the adventitious agents that have been identified as concerns for CHO cell processes are included in this review, but reliable UV sensitivity data for the following agents were not readily available and merit further study: bluetongue virus, bovine coronavirus, bovine polyoma virus, bovine respiratory syncytial virus, epizootic hemorrhagic disease virus, monkey pox virus, mycobacteria, and vesivirus 2117.
Acknowledgements
This work was supported in part by NSERC ENGAGE Plus (EGP2 460753-13) and NSERC Collaborative Research and Development (CRDTJ 48483-2015) grant to MGA, and a MITACS Accelerate Post-Doctoral Fellowship to SMM. Thank you to Kathryn Remington, of BioReliance, for consultation regarding virology. Thank you to Mark Kustermans, Ismail Gobulukoglu, and Priyanka Saxena of Trojan Technologies for their contributions to the data collected for this review.
Appendix
See Table 3
References
- 1.Aaronson SA. Effect of ultraviolet irradiation on the survival of simian virus 40 functions in human and mouse cells. J Virol. 1970;6(4):393–399. doi: 10.1128/jvi.6.4.393-399.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abshire RL, Dunton H. Resistance of selected strains of Pseudomonas aeruginosa to low-intensity ultraviolet radiation. Appl Environ Microbiol. 1981;41:1419–1423. doi: 10.1128/aem.41.6.1419-1423.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ailworth E, Weisman R (2009) Virus shuts Genzyme plant, holds up drugs for 8000. Boston Globe. http://www.boston.com/business/healthcare/articles/2009/06/17/genzyme_temporarily_halts_production_on_2_key_drugs/. Accessed Nov 2015
- 4.Albrecht T, St. Jeor SC, Funk FD, Rapp F. Multiplicity reactivation of human cytomegalovirus inactivated by ultra-violet light. Int J Radiat Biol Relat Stud Phys Chem Med. 1974;26(5):445–454. doi: 10.1080/09553007414551471. [DOI] [PubMed] [Google Scholar]
- 5.Aoki S, Ito S, Watanabe T. UV survival of human mycoplasmas: evidence of dark reactivation in mycoplasma buccale. Microbiol Immunol. 1979;23(3):147–158. doi: 10.1111/j.1348-0421.1979.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 6.Aranha H. Virus safety of biopharmaceuticals: absence of evidence is not evidence of absence. Contract Pharma. 2011;13:82–87. [Google Scholar]
- 7.Bae JE, Jeong EK, Lee JI, Kim IS, Kim JS. Evaluation of viral inactivation efficacy of a continuous flow ultraviolet-C reactor (UVivatec) Korean J Microbiol Biotechnol. 2009;37(4):377–382. [Google Scholar]
- 8.Barreto HM, Siqueira-Junior JP. Protective effect of furocoumarins against 254-nm ultraviolet in Staphylococcus aureus. Curr Microbiol. 2006;52(1):40–44. doi: 10.1007/s00284-005-0078-y. [DOI] [PubMed] [Google Scholar]
- 9.Battigelli D, Sobsey M, Lobe D. The inactivation of hepatitis A virus and other model viruses by UV irradiation. Wat Sci Technol. 1993;27(3–4):339–342. [Google Scholar]
- 10.Baylis SA, Finsterbusch T, Bannert N, Blümel J, Mankertz A. Analysis of porcine circovirus type 1 detected in Rotarix vaccine. Vaccine. 2011;29(4):690–697. doi: 10.1016/j.vaccine.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann K (2014) UV-C irradiation: a new viral inactivation method for biopharmaceuticals. Amer Pharm Rev 17(6)
- 12.Berting A, Farcet MR, Kreil TR. Virus susceptibility of Chinese hamster ovary (CHO) cells and detection of viral contaminations by adventitious agent testing. Biotechnol Bioeng. 2010;106(4):598–607. doi: 10.1002/bit.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blatchley ER, III, Chan P, Lee CC, Mofidi A, Scheible OK, Shen C. Validation of medium-pressure UV disinfection reactors by Lagrangian actinometry using dyed microspheres. Water Res. 2008;43(5):1370–1380. doi: 10.1016/j.watres.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Bockstahler LE, Lytle CD. Radiation enhanced reactivation of nuclear replicating mammalian viruses. Photochem Photobiol. 1977;25(5):477–482. doi: 10.1111/j.1751-1097.1977.tb09173.x. [DOI] [PubMed] [Google Scholar]
- 15.Bolton JR, Linden KG. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J Environ Eng. 2003;129(3):209–215. doi: 10.1061/(ASCE)0733-9372(2003)129:3(209). [DOI] [Google Scholar]
- 16.Boschetti N, Wyss K, Mischler A, Hostettler T, Kempf C. Stability of minute virus of mice against temperature and sodium hydroxide. Biologicals. 2003;31(3):181–185. doi: 10.1016/S1045-1056(03)00037-X. [DOI] [PubMed] [Google Scholar]
- 17.Bourre F, Benoit A, Sarasin A. Respective roles of pyrimidine dimer and pyrimidine (6-4) pyrimidone photoproducts in UV mutagenesis of simian virus 40 DNA in mammalian cells. J Virol. 1989;63(11):4520–4524. doi: 10.1128/jvi.63.11.4520-4524.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braslavsky SE. Glossary of terms used in photochemistry 3rd edition. J Pure Appl Chem. 2007;79(3):293–465. doi: 10.1351/pac200779030293. [DOI] [Google Scholar]
- 19.Brown TC, Cerutti PA. Ultraviolet radiation inactivates SV40 by disrupting at least four genetic functions. EMBO J. 1986;5(1):197–203. doi: 10.1002/j.1460-2075.1986.tb04196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caillet-Fauquet P, Di Giambattista M, Draps ML, Sandras F, Branckaert T, De Launoit Y, et al. Continuous-flow UVC irradiation: a new, effective, protein activity-preserving system for inactivating bacteria and viruses, including erythrovirus B19. J Virol Methods. 2004;118(2):131–139. doi: 10.1016/j.jviromet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Cairns G, Kerr KG, Beggs CB, Sleigh PA, Mooney L, Keig P, et al. Susceptibility of Burkholderia cepacia and other pathogens of importance in cystic fibrosis to UV light. Lett Appl Microbiol. 2001;32(3):135–138. doi: 10.1046/j.1472-765x.2001.00874.x. [DOI] [PubMed] [Google Scholar]
- 22.Cameron KR, Tomkins LM, Eglin RP, Ross LJN, Wildy P, Russell WC. The effects of ultraviolet and ionizing radiation on herpesviruses, SV40 and adenoviruses in relation to the small-plaque effect. Arch Virol. 1979;62(1):31–40. doi: 10.1007/BF01314901. [DOI] [PubMed] [Google Scholar]
- 23.Chang JCH, Osoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG, et al. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 1985;49(6):1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Bergevin J, Kiss R, Walker G, Battistoni T, Lufburrow P, et al. Case study: a novel bacterial contamination in cell culture production—Leptospira licerasiae. PDA J Pharm Sci Technol. 2012;66(6):580–591. doi: 10.5731/pdajpst.2012.00892. [DOI] [PubMed] [Google Scholar]
- 25.Chu LW, Morgan HR. Studies of the hemolysis of red blood cells by mumps virus I. The development of mumps virus hemolysin and its inactivation by certain physical and chemical agents. J Exp Med. 1950;91(4):393–402. doi: 10.1084/jem.91.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clauß M. Higher effectiveness of photoinactivation of bacterial spores, UV resistant vegetative bacteria and mold spores with 222 nm compared to 254 nm wavelength. Acta Hydroch Hydrob. 2006;34(6):525–532. doi: 10.1002/aheh.200600650. [DOI] [Google Scholar]
- 27.Cornelis JJ, Rommelaere J. Direct and indirect effects of ultraviolet light on the mutagenesis of parvovirus H-1 in human cells. EMBO J. 1982;1(6):693–699. doi: 10.1002/j.1460-2075.1982.tb01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornelis JJ, Su ZZ, Ward DC, Rommelaere J. Indirect induction of mutagenesis of intact parvovirus H-1 in mammalian cells treated with UV light or with UV-irradiated H-1 or simian virus 40. Proc Natl Acad Sci. 1981;78(7):4480–4484. doi: 10.1073/pnas.78.7.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daryany MKA, Hosseini SM, Raie M, Fakharie J, Zareh A. Study on continuous (254 nm) and pulsed UV (266 and 355 nm) lights on BVD virus inactivation and its effects on biological properties of fetal bovine serum. J Photoch Photobio B. 2009;94(2):120–124. doi: 10.1016/j.jphotobiol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Das J, Maniloff J, Bhattacharjee S. Dark and light repair in ultraviolet irradiated acholeplasma laidlawii. Biochim Biophys Acta. 1972;259(2):189–197. doi: 10.1016/0005-2787(72)90058-5. [DOI] [PubMed] [Google Scholar]
- 31.de Roda Husman AM, Bijkerk P, Lodder W, Van Den Berg H, Pribil W, Cabaj A, et al. Calicivirus inactivation by nonionizing (253.7-nanometer-wavelength [UV]) and ionizing (gamma) radiation. Appl Environ Microbiol. 2004;70(9):5089–5093. doi: 10.1128/AEM.70.9.5089-5093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Defendi V, Jensen F. Oncogenicity by DNA tumor viruses. Science. 1967;157:703–705. doi: 10.1126/science.157.3789.703. [DOI] [PubMed] [Google Scholar]
- 33.Drexler HG, Uphoff CC. Mycoplasma contamination of cell cultures: incidence, sources, effects, detection, elimination, prevention. Cytotechnology. 2002;39(2):75–90. doi: 10.1023/A:1022913015916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durance CS, Hoffman R, Andrews RC, Brown M. Applications of ultraviolet light for inactivation of adenovirus. P Water Environ Fed. 2005;1:1–12. doi: 10.2175/193864705783978294. [DOI] [Google Scholar]
- 35.European Medicines Agency (2015) Guideline on the use of porcine trypsin used in the manufacture of human biological medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500162147.pdf. Accessed Nov 2015
- 36.Fonseca LS, da Silva JB, Milanez JS, Monteiro-Vitorello CB, Momo L, et al. Leptospira interrogans serovar copenhageni harbors two lexA genes involved in SOS response. PLoS One. 2013;8(10):e76419. doi: 10.1371/journal.pone.0076419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furness G. Analysis of the growth cycle of mycoplasma orale by synchronized division and by ultraviolet irradiation. J Infec Diseases. 1968;118(4):436–442. doi: 10.1093/infdis/118.4.436. [DOI] [PubMed] [Google Scholar]
- 38.Furness G. Differential responses of single cells and aggregates of mycoplasmas to ultraviolet irradiation. Appl Microbiol. 1969;18(3):360–364. doi: 10.1128/am.18.3.360-364.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furness G. T-Mycoplasmas: growth patterns and physical characteristics of some human strains. J Infect Dis. 1975;132(5):592–596. doi: 10.1093/infdis/132.5.592. [DOI] [PubMed] [Google Scholar]
- 40.Gerba CP, Gramos DM, Nwachuku N. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl Enviro Microbiol. 2002;168(10):5167–5169. doi: 10.1128/AEM.68.10.5167-5169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh A, Das J, Manilof J. Lack of repair of ultraviolet light damage in mycoplasms gallisepticum. J Mol Biol. 1977;116(2):337–344. doi: 10.1016/0022-2836(77)90221-2. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh A, Das J, Manilof J. Effect of acriliflavine on ultraviolet inactivation of Acholeplasma laidlawii. Biochim Biophys Acta. 1978;543(4):570–575. doi: 10.1016/0304-4165(78)90311-2. [DOI] [PubMed] [Google Scholar]
- 43.Goering RV, Pattee PA. Mutants of Staphylococcus aureus with increased sensitivity to ultraviolet radiation. J Bacteriol. 1971;106(1):157–161. doi: 10.1128/jb.106.1.157-161.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goetschalckx S, Fabre V, Wynants M, Bertaux L, Plavsic M, Boussif O, et al. A holistic biosafety risk mitigation approach. Amer Pharm Rev. 2014;17(4):48–56. [Google Scholar]
- 45.Harris GD, Adams VD, Sorenson DL, Curtis MS. Ultraviolet inactivation of selected bacteria and viruses with photoreactivation of the bacteria. Water Res. 1987;21(6):687–692. doi: 10.1016/0043-1354(87)90080-7. [DOI] [Google Scholar]
- 46.Harris RE, Coleman PH, Morahan PS. Stability of minute virus of mice to chemical and physical agents. Appl Microbiol. 1974;28(3):351–354. doi: 10.1128/am.28.3.351-354.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herberman RB, Ting RC. Dissociation of multiple polyoma virus functions by ultraviolet irradiation. Exp Biol Med. 1969;131(2):461–464. doi: 10.3181/00379727-131-33902. [DOI] [PubMed] [Google Scholar]
- 48.Hijnen WAM, Beerendonk EF, Medema GJ. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 2006;40:3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 49.Hill WF, Hamblet FE, Benton WH, Akin EW. Ultraviolet devitalization of eight selected enteric viruses in estuarine water. Appl Microbiol. 1970;19(5):805–812. doi: 10.1128/am.19.5.805-812.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoyer O. Testing performance and monitoring of UV systems for drinking water disinfection. Water Supply. 1998;16(1–2):424–429. [Google Scholar]
- 51.Ju W, Moyne AL, Marco ML. RNA-based detection does not accurately enumerate living Escherichia coli O157:H7 cells on plants. Front Microbiol. 2016;7:233. doi: 10.3389/fmicb.2016.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kallenbach NR, Cornelius PA, Negus D, Montgomerie D, Englander S. Inactivation of viruses by ultraviolet light. Curr St Hematol Blood Transfus. 1988;56:70–82. doi: 10.1159/000416558. [DOI] [PubMed] [Google Scholar]
- 53.Kelloff G, Aaronson SA, Gilden RV. Inactivation of murine sarcoma and leukemia viruses by ultra-violet irradiation. Virology. 1970;42:1133–1135. doi: 10.1016/0042-6822(70)90361-2. [DOI] [PubMed] [Google Scholar]
- 54.Kerr A, Nims R. Adventitious viruses detected in biopharmaceutical bulk harvest samples over a 10 Year Period. PDA J Pharm Sci Tech. 2010;64(5):481–485. [PubMed] [Google Scholar]
- 55.Kimberlin RH. Bovine spongiform encephalopathy. Rev Sci Tech OIE. 1992;11(2):347–390. doi: 10.20506/rst.11.2.608. [DOI] [PubMed] [Google Scholar]
- 56.Kowalski W, Bahnfleth W, Hernandez M. A genomic model for predicting the ultraviolet susceptibility of viruses. IUVA News. 2009;11(2):15–28. [Google Scholar]
- 57.Kurth J, Waldmann R, Heith J, Mausbach K, Burian R. Efficient inactivation of viruses and mycoplasma in animal sera using UVC irradiation. Devel Biol Stand. 1998;99:111–118. [PubMed] [Google Scholar]
- 58.Lackner C, Leydold SM, Modrof J, Farcet MR, Grillberger L, Schäfer B, et al. Reduction of spiked porcine circovirus during the manufacture of a vero cell-derived vaccine. Vaccine. 2014;32(18):2056–2061. doi: 10.1016/j.vaccine.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Latarjet R, Cramer R, Montagnier L. Inactivation, by UV-, X-, and γ-radiations, of the infecting and transforming capacities of polyoma virus. Virology. 1967;33(1):104–111. doi: 10.1016/0042-6822(67)90098-0. [DOI] [PubMed] [Google Scholar]
- 60.Lee J, Zoh K, Ko G. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl Environ Microbiol. 2008;74(7):2111–2117. doi: 10.1128/AEM.02442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lénès D, Deboosere N, Ménard-Szczebara F, Jossent J, Alexandre V, Machinal C, et al. Assessment of the removal and inactivation of influenza viruses H5N1 and H1N1 by drinking water treatment. Water Res. 2010;44(8):2473–2486. doi: 10.1016/j.watres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 62.Linden KG, Scheible K, Shen C, Shin GA, Lee JK, Posy P. Demonstrating 4-log adenovirus inactivation in a medium pressure UV reactor. P Water Environ Fed. 2009;1:287–288. doi: 10.2175/193864709793848040. [DOI] [Google Scholar]
- 63.Lovinger GG, Ling HP, Gilden RV, Hatanaka M. Effect of UV light on RNA directed DNA polymerase activity of murine oncornaviruses. J Virol. 1975;15(5):1273–1275. doi: 10.1128/jvi.15.5.1273-1275.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lytle CD, Sagripanti JL. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J Virol. 2005;79(22):14244–14252. doi: 10.1128/JVI.79.22.14244-14252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mackey ED (2004) Bridging pilot-scale testing to full-scale design of UV disinfection systems. American Water Works Association
- 66.Masschelein WJ, Rice RG. Ultraviolet light in water and wastewater sanitation. Boca Raton, FL, USA: CRC Press; 2002. [Google Scholar]
- 67.Maya C, Beltran N, Jimenez B, Bonilla P. Evaluation of the UV disinfection process in bacteria and amphizoic amoebae inactivation. Water Supply. 2003;3(4):285–291. [Google Scholar]
- 68.McClain ME, Spendlove RS. Multiplicity reactivation of reovirus particles after exposure to ultraviolet light. J Bacteriol. 1966;92(5):1422–1429. doi: 10.1128/jb.92.5.1422-1429.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng QS, Gerba CP. Comparative inactivation of enteric adenoviruses, poliovirus and coliphages by ultraviolet irradiation. Water Res. 1996;30(11):2665–2668. doi: 10.1016/S0043-1354(96)00179-0. [DOI] [Google Scholar]
- 70.Meunier SM, Todorovic B, Dare EV, Begum A, Guillemette S, Wenger A, Saxena P, Campbell JL, Sasges M, Aucoin MG. Impact of dissolved oxygen during UV-irradiation on the chemical composition and function of CHO cell culture media. PLoS One. 2016;11(3):e0150957. doi: 10.1371/journal.pone.0150957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller FP, Vandome AG, McBrewster J (2009) Beer-Lambert Law. VDM publishing, Saarbrucken, Germany. ISBN 6130200609, 9786130200602
- 72.Moody M, Alves W, Varghese J, Khan F. Mouse minute virus (MMV) contamination—a case study: detection, root cause determination, and corrective actions. PDA J Pharm Sci Technol. 2011;65(6):580–588. doi: 10.5731/pdajpst.2011.00824. [DOI] [PubMed] [Google Scholar]
- 73.Morowitz HJ. Absorption effects in volume irradiation of microorganisms. Science. 1950;111(2879):229–230. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- 74.Nims R, Presente E, Sofer G, Phillips C, Chang A. Adventitious agents: concerns and testing for biopharmaceuticals. Biotech Bioprocess Ser. 2005;29:143. [Google Scholar]
- 75.Nomura S, Bassin RH, Turner W, Haapala DK, Fischinger PJ. Ultraviolet inactivation of moloney leukaemia virus: relative target size required for virus replication and rescue of “defective” murine sarcoma virus. J Gen Virol. 1972;14:213–217. doi: 10.1099/0022-1317-14-2-213. [DOI] [PubMed] [Google Scholar]
- 76.Nowak J, Das J, Maniloff J. Characterization of an Acholeplasma laidlawii variant with a REP- phenotype. J Bacteriol. 1976;127:832–836. doi: 10.1128/jb.127.2.832-836.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nuanualsuwan S, Mariam T, Himathongkham S, Cliver DO. Ultraviolet inactivation of feline calicivirus, human enteric viruses, and coliphages. Photochem Photobiol. 2002;76(4):406–410. doi: 10.1562/0031-8655(2002)0760406UIOFCH2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 78.Nuanualsuwan S, Thongtha P, Kamolsiripichaiporn S, Subharat S. UV inactivation and model of UV inactivation of foot-and-mouth disease viruses in suspension. Int J Food Microbiol. 2008;127(1):84–90. doi: 10.1016/j.ijfoodmicro.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 79.Nwachuku N, Gerba CP, Oswald A, Mashadi FD. Comparative inactivation of adenovirus serotypes by UV light disinfection. Appl Environ Microbiol. 2005;71(9):5633–5636. doi: 10.1128/AEM.71.9.5633-5636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Otaki M, Okuda A, Tajima K, Iwasaki T, Kinoshita S, Ohgaki S. Inactivation differences of microorganisms by low pressure UV and pulsed xenon lamps. Wat Sci Technol. 2003;47(3):185–190. [PubMed] [Google Scholar]
- 81.Park GW, Linden KG, Sobsey MD. Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Lett Appl Microbiol. 2011;52(2):162–167. doi: 10.1111/j.1472-765X.2010.02982.x. [DOI] [PubMed] [Google Scholar]
- 82.Randazzo W, Lopez-Galvez F, Allende A, Aznar R, Sanchez G. Evaluation of viability PCR performance for assessing norovirus infectivity in fresh-cut vegetables and irrigation water. Int J Food Microb. 2016;229:1–6. doi: 10.1016/j.ijfoodmicro.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 83.Rahn RO, Bolton J, Stefan MI. The iodide/iodate actinometer in UV disinfection determination of the fluence rate distribution in UV reactors. Photochem Photobiol. 2006;82(2):611–615. doi: 10.1562/2005-06-10-RN-570. [DOI] [PubMed] [Google Scholar]
- 84.Rauth AM. The physical state of viral nucleic acid and the sensitivity of viruses to ultraviolet light. Biophys J. 1965;5(3):257–273. doi: 10.1016/S0006-3495(65)86715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Remington K (2014) Use of porcine circovirus as a challenge for filtration and other virus risk mitigation steps. 17th Planova Workshop, Washington, DC, 12–13 Jun 2014
- 86.Rommelaere J, Ward DC. Effect of UV-irradiation on DNA replication of the parvovirus minute-virus-of-mice in mouse fibroblasts. Nucleic Acids Res. 1982;10(8):2577–2596. doi: 10.1093/nar/10.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rommelaere J, Vos J-M, Cornelis JJ, Ward DC. UV-enhanced reactivation of minute-virus-of-mice: stimulation of a late step in the viral life cycle. Photochem Photobiol. 1981;33:845–854. doi: 10.1111/j.1751-1097.1981.tb05502.x. [DOI] [PubMed] [Google Scholar]
- 88.Sarasin AR, Hanawalt PC. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc Natl Acad Sci. 1978;75(1):346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sasges M, Remington K (2015) Clearance of adventitious agents from cell culture media in a flow-through UV-C reactor, presented at International Society for Bioprocess Technology Fall meeting Sept 2015
- 90.Schleh M, Lawrence B, Park T, Rosenthal S, Hart R, Dehghani H. Effectiveness of upstream barrier technologies for inactivation of adventitious contaminants of cell culture. Amer Pharm Rev. 2010;13(7):72. [Google Scholar]
- 91.Seemayer NH, Defendi V. Analysis of minimal functions of simian virus 40 II. Enhancement of oncogenic transformation in vitro by UV irradiation. J Virol. 1973;12(6):1265–1271. doi: 10.1128/jvi.12.6.1265-1271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seltsam A, Müller TH. UVC irradiation for pathogen reduction of platelet concentrates and plasma. Transfus Med Hemother. 2011;38(1):43–54. doi: 10.1159/000323845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shanley JD. Ultraviolet irradiation of murine cytomegalovirus. J Gen Virol. 1982;63:251–254. doi: 10.1099/0022-1317-63-1-251. [DOI] [PubMed] [Google Scholar]
- 94.Shaw JE, Cox DC. Early inhibition of cellular DNA synthesis by high multiplicities of infectious and UV-inactivated reovirus. J Virol. 1973;12(4):704–710. doi: 10.1128/jvi.12.4.704-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sommer R, Haider T, Cabaj A, Pribil W, Lhotsky M. Time dose reciprocity in UV disinfection of water. Water Sci Technol. 1998;38(12):145–150. doi: 10.1016/S0273-1223(98)00816-6. [DOI] [Google Scholar]
- 96.Sommer R, Lhotsky M, Haider T, Cabaj A. UV inactivation, liquid-holding recovery, and photoreactivation of E. coli O157 and other pathogenic E.coli strains in water. J Food Prot. 2000;63(8):1015–1020. doi: 10.4315/0362-028X-63.8.1015. [DOI] [PubMed] [Google Scholar]
- 97.Stamm L, Charon N. Sensitivity of pathogenic and free-living Leptospira to UV radiation and mitomycin C. Appl Environ Microbiol. 1988;54(3):728–733. doi: 10.1128/aem.54.3.728-733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinmann E, Gravemann U, Friesland M, Doerrbecker J, Müller TH, Pietschmann T, Seltsam A. Two pathogen reduction technologies—methylene blue plus light and shortwave ultraviolet light—effectively inactivate hepatitis C virus in blood products. Transfusion. 2013;53(5):1010–1018. doi: 10.1111/j.1537-2995.2012.03858.x. [DOI] [PubMed] [Google Scholar]
- 99.Stull H, Gazdar A. Stability of Rauscher leukemia virus under certain laboratory conditions. P Soc Exp Biol Med. 1976;152:554–556. doi: 10.3181/00379727-152-39438. [DOI] [PubMed] [Google Scholar]
- 100.Terpstra FG, Van’t Wout AB, Schuitemaker H, Van Engelenburg FA, Dekkers DW, Verhaar R, et al. Potential and limitation of UVC irradiation for the inactivation of pathogens in platelet concentrates. Transfusion. 2008;48(2):304–313. doi: 10.1111/j.1537-2995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 101.Thurston-Enriquez JA, Haas CN, Jacangelo J, Riley K, Gerba CP. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl Environ Microbiol. 2003;69(1):577–582. doi: 10.1128/AEM.69.1.577-582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tosa K, Hirata T (1998) Photoreactivation of Salmonella following UV disinfection. In: Proc IAWQ 19th Biennial Int Conf 10
- 103.Tree J, Adams M, Lees D. Disinfection of feline calicivirus (a surrogate for norovirus) in wastewaters. J Appl Microbiol. 2005;98:155–162. doi: 10.1111/j.1365-2672.2004.02442.x. [DOI] [PubMed] [Google Scholar]
- 104.Tukey JW (1970) Plots of Relationship. In: Exploratory data analysis (limited preliminary edition), vol. 1, Ch. 5. Addison-Wesley Publishing Co, Reading
- 105.Tukey JW. Exploratory Data Analysis. 1. Reading: Addison-Wesley Publishing Co; 1977. [Google Scholar]
- 106.United States Environmental Protection Agency (2006) Ultraviolet disinfection guidance manual for the final long term 2 enhanced surface water treatment rule. EPA 815-R-06-007 Nov 2006
- 107.van der Eb AJ, Cohen JA. The effect of UV-irradiation on the plaque-forming ability of single-and double-stranded polyoma virus DNA. Biochem Bioph Res Com. 1967;28(2):284–288. doi: 10.1016/0006-291X(67)90442-1. [DOI] [PubMed] [Google Scholar]
- 108.Vos JM, Cornelis JJ, Limbosch S, Zampetti-Bosseler F, Rommelaere J. UV-irradiation of related mouse hybrid cells: similar increase in capacity to replicate intact minute-virus-of-mice but differential enhancement of survival of UV-irradiated virus. Mutat Res. 1981;83(2):171–178. doi: 10.1016/0027-5107(81)90002-6. [DOI] [PubMed] [Google Scholar]
- 109.Wang C-H, Tschen S-Y, Flehmig B. Antigenicity of hepatitis A virus after ultra-violet irradiation. Vaccine. 1995;13(9):835–840. doi: 10.1016/0264-410X(94)00054-Q. [DOI] [PubMed] [Google Scholar]
- 110.Wang J, Mauser A, Chao SF, Remington K, Treckmann R, Kaiser K, Pifat D, Hotta J. Virus inactivation and protein recovery in a novel ultraviolet-C reactor. Vox Sang. 2004;86(4):230–238. doi: 10.1111/j.0042-9007.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 111.Weaver B, Rosenthal S. Viral risk mitigation for mammalian cell culture media. PDA J Pharm Sci Tech. 2010;64(5):436–439. [PubMed] [Google Scholar]
- 112.Weidenmann A, Fischer B, Straub U, Wang C-H, Flehmig B, Schoenen D. Disinfection of hepatitis A virus and MS-2 coliphage in water by ultraviolet irradiation: comparison of UV-susceptibility. Water Sci Technol. 1993;27(3–4):335–338. [Google Scholar]
- 113.White J, Matlin K, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilson BR, Roessler PF, Van Dellen E, Abbaszadegan M, Gerba CP (1992) Coliphage MS-2 as a UV water disinfection efficacy test surrogate for bacterial and viral pathogens. In: Proceedings of the water quality technol ogy conference. American Water Works Association, Toronto, Ontario, Canada 15–19 Nov 1992
- 115.Wu Y, Clevenger T, Deng B. Impacts of goethite particles on UV disinfection of drinking water. Appl Environ Microbiol. 2005;71(7):4140–4143. doi: 10.1128/AEM.71.7.4140-4143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yaun BR, Sumner SS, Eifert JD, Marcy JE. Response of Salmonella and E. coli O157: H7 to UV energy. J Food Protect. 2003;66(6):1071–1073. doi: 10.4315/0362-028X-66.6.1071. [DOI] [PubMed] [Google Scholar]
- 117.Yen S, Sokolenko S, Manocha B, Patras A, Daynouri-Pancino F, Blondeel EJM, Sasges M, Aucoin MG. Treating cell culture media with UV irradiation against adventitious agents: minimal impact on CHO performance. Biotechnol Prog. 2014;30(5):1190–1195. doi: 10.1002/btpr.1942. [DOI] [PubMed] [Google Scholar]
- 118.Yoshikura H. Ultraviolet inactivation of murine leukemia and sarcoma viruses. Int J Cancer. 1971;7:131–140. doi: 10.1002/ijc.2910070115. [DOI] [PubMed] [Google Scholar]
- 119.Zavadova Z, Libikova H. Comparison of the sensitivity to ultraviolet irradiation of reovirus 3 and some viruses of the Kemerovo group. Acta Virol. 1975;19:88–90. [PubMed] [Google Scholar]
- 120.Zavadova Z, Gresland L, Rosenbergova M. Inactivation of single- and double-stranded ribonucleic acid of encephalomyocarditis virus by ultraviolet light. Acta Virol. 1968;12(6):515–522. [PubMed] [Google Scholar]
- 121.Zimmer JL, Slawson RM. Potential repair of E. coli DNA following exposure to UV radiation from both medium- and low-pressure UV sources used in drinking water treatment. Appl Environ Microbiol. 2002;68(7):3293–3299. doi: 10.1128/AEM.68.7.3293-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


