Abstract
We evaluated the efficiency of an air purifier using the single-chamber method for the effective removal of airborne Staphylococcus epidermidis, a nosocomial infection–causing bacterium. In this experiment, the bacterial strain S. epidermidis was injected using a nebulizer into the test chamber, which was similar to a consumer living space (60 m3). The microbial sampling was conducted via the air sampler method, and the reduction in S. epidermidis growth was monitored by performing three consecutive tests. Initially, a blank test was conducted to determine the natural decay rate and calibrate the experimental setup. After injecting the bacterial strain from 1240 to 11180 CFU per unit volume (m3), the natural decay rate showed a maximum deviation of 3.1% with a sampling error of 1.1% p at a confidence level of 95%. In addition, the particle size distribution in the test chamber was found to range from 0.3 to 5.0 μm, and a subsequent decrease in large-sized particles was observed with the operation of the air purifier, which is the size similar to that of suspended airborne bacteria. This can be used to assess the performance of the air purifier by calibrating the natural reduction value to the reduced operation value. Thus, the single-chamber technique is a promising approach for analyzing the removal efficacy of airborne bacteria from indoor air.
Keywords: Airborne bacteria, Bioaerosols, Air purifier, Single-chamber method, Staphylococcus epidermidis
Introduction
Recently, the infectious diseases caused by airborne bacteria and viruses are of primary global concern for both social and economic reasons. For example, severe acute respiratory syndrome (SARS) caused the deaths of nearly 700 people and affected almost 8000 during the first outbreak in 2002–2003; it eventually spread around 37 countries, causing a cost of $18 billion, primarily in Asia (Sørensen et al. 2006). Moreover, between 2009 and 2010, nearly 17,000 healthy adults died globally from influenza A (H1N1), and the seasonal influenza continues to kill many people annually (Tellier 2009; Chiu et al. 2017). Bioaerosols are airborne microorganisms that are either infectious or non-infectious in nature and that can easily spread to the environment via air in a short span of time (Ki Youn et al. 2010). With air as the transportation media, pathogenic bacteria, viruses, fungi, and biological fragments can instantly affect human health (Lee et al. 2008). Generally, these pathogenic bioaerosols enter and exit the respiratory system through breathing, coughing, laughing, sneezing, and personal contact (Shrivastava et al. 2013). Tuberculosis, whooping cough, meningitis, anthrax, and pneumonia are some of the most common examples of airborne bacterial diseases (Eickhoff 1996).
Recently, Staphylococcus, Bacillus, Corynebacterium, and Micrococcus have been recognized as the common genera which have contaminated the environments of a university (Di Giulio et al. 2010), hospitals (Okten and Asan 2012; Ki Youn et al. 2010), offices (Bonetta et al. 2010), and other human dwelling spaces (Frankel et al. 2012). Staphylococci are a common semi-pathogenic bacterial colonizer in the skin and mucous membranes of humans, and it can cause some minor skin diseases and a multitude of other diseases (Chessa et al. 2016; Lenart-Boron et al. 2017). Staphylococcus epidermidis belongs to the Gram-positive and coagulase-negative Staphylococci which are found in the human skin and mucous membrane (Otto 2009; Wieser and Busse 2000; Taylor et al. 2016); this is the major cause of nosocomial infections and is often the major infective agent in compromised patients, such as drug abusers, immuno-compromised patients, premature newborns, and the elderly (Cuong and Michael 2002; Okten and Asan 2012). The presence of infectious bioaerosols in indoor air is a major issue, and they should be controlled or removed to prevent contagious diseases (Aliabadi et al. 2011).
Generally, removal and disinfection are the two major techniques that have been adopted to control bioaerosols in the environment. In the removal method, an air purifier with filters (high-efficiency particulate air filter) is used to trap and eliminate infectious bioaerosols. In the disinfection method, disinfectants such as a light catalyzer, UV lamp, and chlorine are used to kill the airborne microorganisms (Pham and Lee 2015; Zacarías et al. 2012; Berry et al. 2007). The high-efficiency particulate air (HEPA) filtration technique is effectively used to reduce bioaerosols in laboratories and hospitals (Herrera-Cantú et al. 2017). The major mechanism of the high-efficiency air filter involves removing airborne microparticles from the indoor environment (Mittal et al. 2011). In addition, the indoor installation of an air purifier can significantly reduce airborne bacteria, molds, and mycobacteria. Wen et al. (2014) evaluated the filtration efficiency of a HEPA filter in a biosafety level 3 (BSL-3) laboratory using the aerosolized bacteria, Serratia marcescens, and reported the effective performance of the HEPA filter against these aerosolized bacteria. Moreover, Foarde (2010) used the single-pass method in a chamber filter to evaluate the collection efficiency of filters and the removal of non-biological particles.
The standard for air cleaners was developed in September 1984 by the American Home Appliances Association (AHAM) and reported the test method for particle removal performance of air cleaners. Since then, certification began in August 1985, and the American Heating, Refrigeration and Air-Conditioning Engineers (ASHRAE) standard marks the efficiency of the filter as 16 grades (Medical Advisory Secretariat 2005). The filter test and removal of particles range from 0.3 to 10.0 μm, and the higher-grade numbers show better performance in particle removal. Thus, considering this filtering efficiency, a reasonable test method is required for the removal of suspended bacteria in living spaces.
In order to understand bioaerosols, they should be collected and identified through standardized sampling techniques with high collection rates. Bioaerosol sampling includes different methods such as impaction, impinger, and filtration (Maimaitireyimu et al. 2015). Among these, impaction is a widely used sampling technique with a high collection rate, through which the bioaerosols are directly collected in the culture medium. The Andersen sampler or slit sampler is used to collect the maximum concentrations of microbes or particle samples using the impaction method (Park et al. 2011; Normand et al. 2016).
In this study, we propose a new method to verify the removal efficiency of airborne bacteria. The ASHRAE standard is just a way to verify the filter, and the proposed method is to evaluate the product in a single chamber. The peculiarity of this study is that it has a built-in HEPA filter installed in a single chamber to evaluate an air cleaner and it prevents contaminations from anything other than the airborne bacteria we use in our experiments. So, we evaluated the efficiency of an air purifier on the removal of the injected bacterial strain, S. epidermidis, in a single-test chamber. In this method, the air purifier was installed and tested in a manner similar to a customer’s usage conditions. The microbial samples were collected from the chamber directly to the agar culture medium using the impaction sampling technique, and then the range of colony formation was analyzed. The natural decay rate, operational decay test, and particle distribution rate were analyzed, and the results were discussed in detail. Through these studies, the bacteria removal performance of the air cleaner was evaluated and it helps the consumers to check the performance in advance.
Materials and methods
Test chamber and air purifier (HEPA filter) device
The experiment was conducted in a single germless chamber with dimensions of 60.8 m3 (4.5 m (W)×4.5 m (L)×3 m (H)). The air purifier (HEPA filter) device capable of removing airborne particles was installed in the ceiling of the test chamber, and an air conditioning system was assembled to control the interior temperature and humidity (Fig. 1a). Moreover, a nebulizer (Devilbiss PulmoNeb, Compressor, Model 3655, USA) was set up to inject strains at a flow rate of 0.5 ml per minute. The mass median aerodynamic diameter (MMAD) is 5 μm. In addition, the system contains a device to control the moisture and spraying pressure (48.2 PSI), as well as filters to supply clean air. Figure 1b shows a schematic diagram of the air inbuilt purifier used in the experiment. The inbuilt air purifier consists of a primary filter, an activated carbon filter (it helps to remove ozone and odor inside the test chamber), and a HEPA filter for more effective air purification. In the typical experiment, the air purifier was installed in three different ways, which were all similar to a customer’s usage conditions. In the first one, a stand type was installed directly on the floor (Fig. 2a), while in the second one, a desktop type was installed at a height of 1.2 m from the floor (Fig. 2b) and a distance of 0.1 m from the wall. Finally, in the third one, wall-mounted or ceiling-embedded types were installed at 1.8 m from the floor of the experimental chamber (Fig. 2c).
Fig. 1.
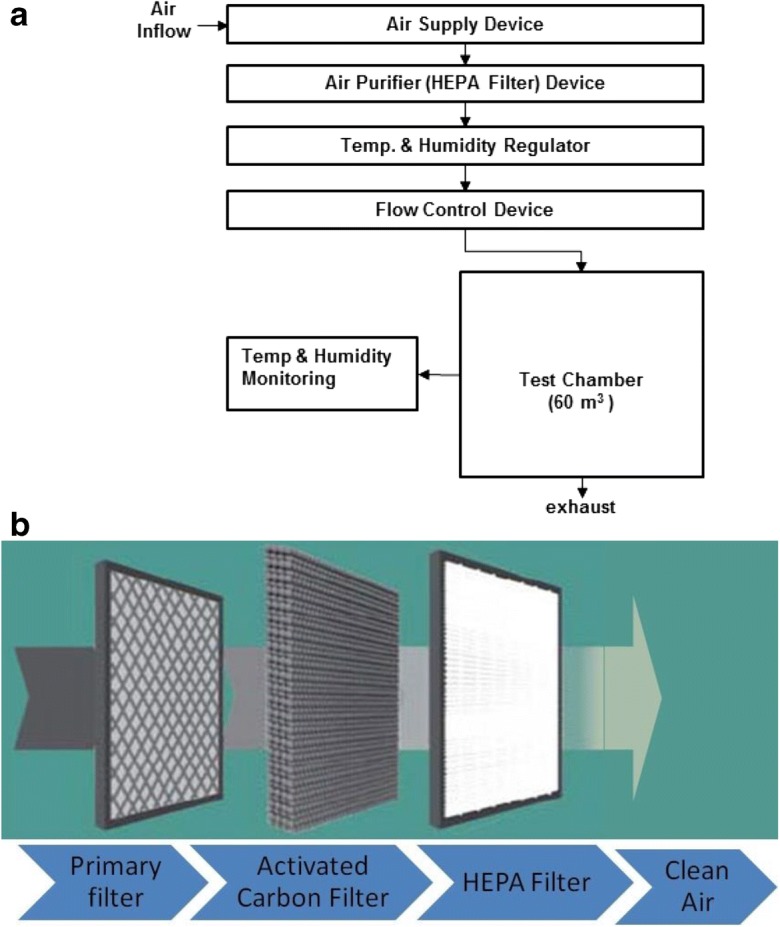
a Schematic flow chart of the test chamber 60.8 m3 (4.5 m (W) × 4.5 m (L) × 3 m (H)). The single chamber is connected with an air flow device, air filter (HEPA), temperature and humidity regulator, flow control device, and temperature and humidity monitoring device. b Typical types of filters used in air purifier device
Fig. 2.
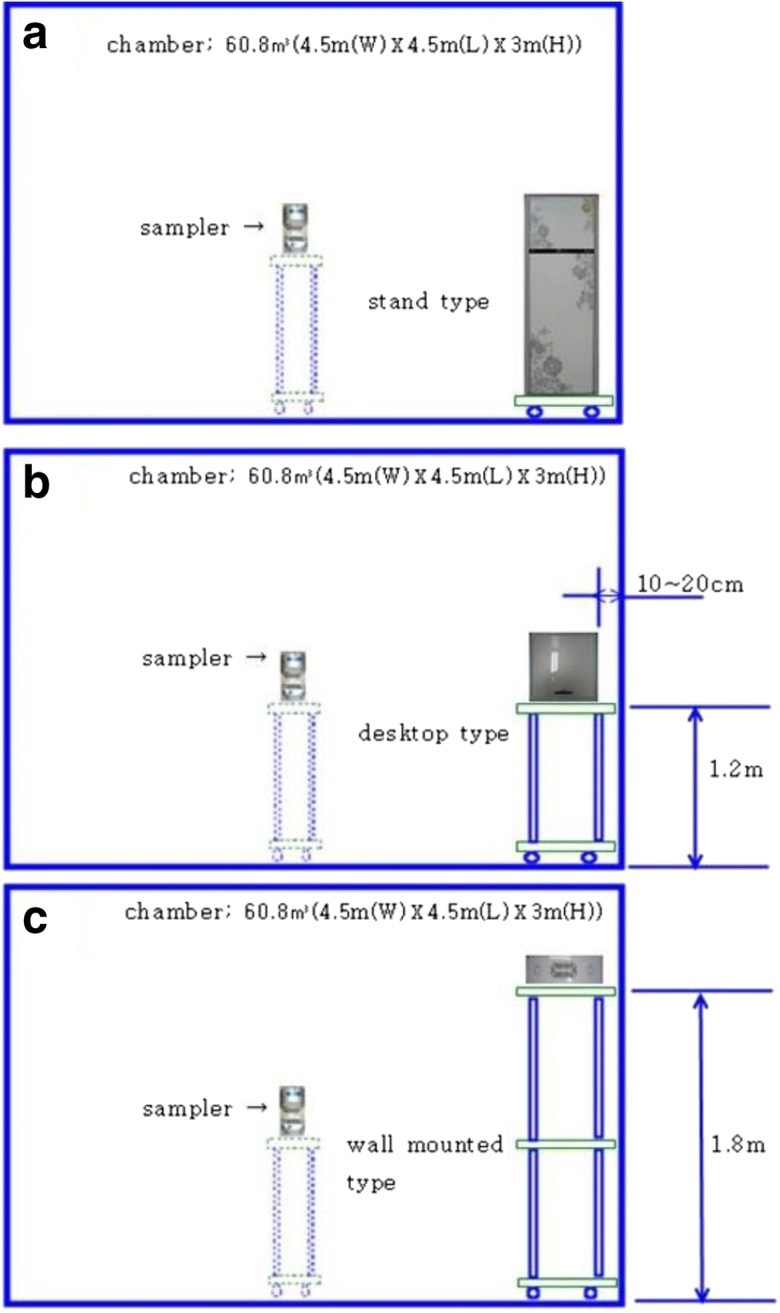
a Stand type unit setting. b Desktop type installed at a height of 1.2 m from the floor and a distance of 0.1 m from the wall. c Wall-mounted or ceiling-embedded types installed at 1.8 m from the floor
Test organism and air sampler
In consideration of the examiner’s safety, a non-pathogenic strand, Staphylococcus epidermidis (ATCC 12228), was selected as the representative strain. This selection was made because S. epidermidis is one of the signature microorganisms which is found at high rates indoors, and particularly in hospital air and catheters (Kelley and Gilbert 2013). Here, a nutrient solution is needed to incubate S. epidermidis and a nebulizer is utilized to spray into the chamber. The nominal flow of the microbial sampler (Merck, MAS-100 NT, USA) is 100 L/min. The head diameter is 10 cm, with a sampling volume of 100–1000 L/min. The sampling volume can be effectively controlled based on the required number of airborne microbes per unit volume.
Buffer solutions and culture media
The phosphate buffer solution (pH 7.2) was prepared by dissolving 34 g of potassium dihydrogen phosphate in 500 ml of distilled water. Then, 175 ml of sodium hydroxide solution was added so as to adjust the pH to ~ 7.1–7.3, and the solution was then made up to 1000 ml using distilled water. Next, the solution was stored in a refrigerator after undergoing a high-pressure steam sterilization. Prior to the experiment, the solution was diluted at 1:800 and sterilized at 121 °C for 15–20 min. The buffered peptone water (pH 7.0) was synthesized by mixing potassium dihydrogen phosphate 3.56 g, disodium hydrogen phosphate 18.23 g, sodium chloride 4.30 g, and peptone 1.0 g in 1000 ml of distilled water under heating. Then, the resultant buffered peptone water was sterilized in an autoclave at 121 °C for 15–20 min and the pH was adjusted to neutral.
The nutrient broth was prepared by dissolving 5.0 g peptone and 3.0 g meat extracts in 1000 ml of distilled water, the pH was adjusted to 7.0–7.4, and then the mixture was sterilized at 121 °C for 15 min. The nutrient agar was obtained by dissolving 15 g of purified agar in 1000 ml nutrient broth. After adjusting the pH to 6.8 ± 0.2, the mixture was sterilized at 121 °C for 15 min.
Distribution of airborne particles
The distribution characteristics of the airborne particles inside the chamber were subsequently investigated using a particle counter (Met One, Model 3313). In this analysis, the external air from the outdoor environment was directly introduced into a chamber without passing through a HEPA filter. Then, the chamber was sealed, and the movement characteristics of the airborne particles were measured over time from outside of the chamber via the tubing technique.
Natural decay test (blank tests)
The natural decay test was conducted before operating the air purifier. The following procedure was performed for the blank test: initially, air purifier (HEPA filter) device (Fig. 1b) in the chamber ceiling was operated for 1 h so as to remove the airborne bacteria and ozone (O(3)) inside the test chamber, where the temperature and humidity were regulated within 23 ± 1 °C and RH 50 ± 5% respectively. At this time, the UV lamps were turned on to sterilize the microbes which survived the purification of the HEPA filter. In this case, it is preferable to use activated carbon (AC) filters because a small amount of ozone may be generated. AC filters are used widely in air cleaning to remove ozone (Metts and Batterman 2006). Following this sterilization and removal of ozone, the HEPA filter device and UV lamp were turned off and the auxiliary fan inside the chamber was turned on. Subsequently, the test strand was injected at a rate of 1×105 CFU/ml (2 ml) using a nebulizer setup. Then, the auxiliary fan was turned off, and the chamber was left to stand for 1 h without any external disturbance. A microbial culture medium (petri dish) was attached on the air sampler inside the chamber and sampled for 1 min at a flow speed of 100 L/min, which was used as an initial value. Similarly, the chamber was left to stand for another 1 h and sampled for 5 min at a flow speed of 100 L/min, which was used as a final value. The initial and final values measured by this procedure were used as the blank test. After measuring the final value, the chamber was sterilized to remove the microbes by powering the UV lamp and operating the stirring plate.
Air purifier test (operational decay test)
In the air purifier evaluation test, the operation of the air purifier (on and off) was completely controlled from outside of the chamber. Similar to the natural decay test, the test chamber was calibrated to be free from airborne bacteria, and the initial value was measured after injecting the microbial strain. Then, the air purifier was operated for 1 h and the final value was measured. As the number of airborne microbes relatively decreases due to the high removal performance of air purifier, the sampling volume of the air sampler was increased to 500 L in order to acquire a more accurate final value.
Sampling of bacteria
Initially, the air sampler lid and cover were sterilized using an autoclave and 70% alcohol, and the moisture content was completely removed. An impact measurement method was used, in which the bioaerosols can directly contact the medium. The sample collection position was set at the center of the chamber at a height of 1.2 m from the floor. Each time of the test, the position of air sampler has to be maintained in the same position because the result depends upon the distance between the product and sampler. The air sampler can be moved or adjusted from the outside of the chamber by a conveyor without the test chamber needing to be opened. The amount of sample collected during the initial value measurement (the value measured 1 h after S. epidermidis injection) was 100 L, while the final value (the value measured 2 h after S. epidermidis injection) was 500 L.
Incubation
The medium with the airborne S. epidermidis was collected and cultivated for 24 h under an aerobic condition in 32–35 °C. The number of colonies in the petri dish was counted after 24 h of incubation. The medium was then carefully verified for the allowed range of number of colonies (≤ 400 CFU/petri dish) as well as contamination of the medium by other sources. The Feller table was applied to the value and corrected with the sample volume in order to obtain CFU value per unit volume (m3).
Colony count
Each colony grown in the petri dish was counted, and the number of colonies was corrected by a Feller table. In order to ensure the reliability of measurements and identical trends, the results were carefully reviewed. For accuracy management (QA/QC), the error range of the natural decay rate was recorded. In this unique study, cultivation was done for 24 h, and the minimum allowed colony was set to 8 CFU per petri dish while the maximum allowed range was fixed below 400 CFU per petri dish.
Calculation
The natural decay rate was calculated using Eq. (1).
| 1 |
where Bi is the natural decay rate, Ci is the measured value 1 h after injecting bacteria, and Ct is the measured value 2 h after injecting bacteria.
The initial value’s corrected value is calculated using Eq. (2)
| 2 |
where Sc is the initial value’s corrected value (CFU/m3), Bi is the natural decay rate, and Pt is the initial value (CFU/m3).
The airborne bacteria removal rate was defined as
| 3 |
where Ni is the airborne bacteria removal rate, Sc is the initial value’s corrected value, and Cn is the bacteria measured n hours after injecting bacteria under the operation of an air purifier.
Results and discussion
Generally, the filters in an air purifier can trap airborne particles by means of size exclusion, in which the air is forced through the filter and the particles are physically captured. In this present investigation, the size distribution of the particles inside the airtight chamber (analyzed using a particle counter) was found to be in the range of 0.3 to 5.0 μm (Fig. 3). The results indicated that the distribution of larger-sized particles decreased over time, due to the performance of the air purifier (Table 1). Here, particles near the size of a microbe (1 μm) were also observed to decrease by ~ 10% after 1 h of the experiment. Moreover, the particle size distribution of the bacterial strain also showed a similar decreasing pattern. However, the rates of decrease were higher for the test bacteria than for airborne particles; this may be attributed to the adhesion of microbes with liquid droplets. Therefore, the reduction rate is larger than that of the normal particles, even when the size of the microbes equals 1 μm. This is because the weights of the microbes increase when combined with liquid droplets, and the particle size has been estimated to be ≤ 5 μm. Consistent with our data, the microbial size has been shown to range between 1 and ≤ 7.5 μm and increase with increasing temperature (Tham and Zuraimi 2005). By contrast, a previous investigation on the size distribution of airborne heterotrophic bacteria has revealed aerodynamic diameters between 1.1 and 2.1 μm in one fraction respirable particulate matter and another at the coarse fraction with a diameter above 7 μm (Raisi et al. 2012). In addition, the distribution of bacteria has been controlled and influenced by the nebulizer used for the generation of bioaerosols. According to Kujundzic et al. (2006), the aerodynamic size distribution of bacteria generated with the nebulizer can vary due to the generation of particles with both larger and smaller aerodynamic diameters (0.3–0.6 μm). But, due to various complexities like coalescence in determining aerosol particle lifetime in the atmosphere, there are very few experimental studies that have been performed to research interparticle interactions (Bzdek and Reid 2017). Moreover, the behavior of the particles mainly depends upon the physicochemical properties like agglomeration state, shape, crystal structure, chemical composition, surface area, surface chemistry, surface charge, and porosity (Oberdörster et al. 2005).
Fig. 3.
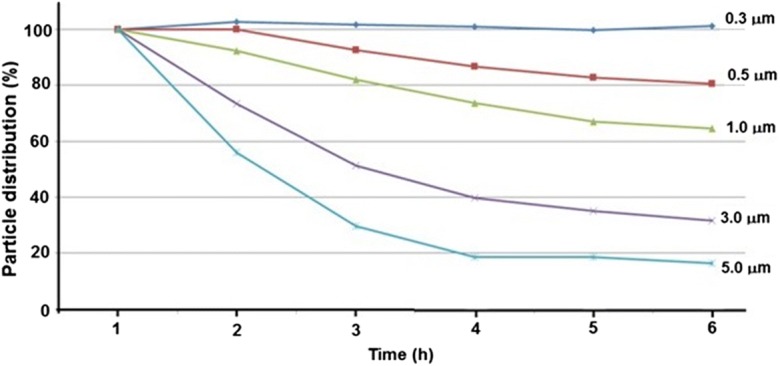
Distribution percentages of different size particles with respect to time (1 to 6 h)
Table 1.
Distributions of different size particles over time from 1 to 6 h
| Particle size (μm) | Time (h) | |||||
|---|---|---|---|---|---|---|
| 1:00 | 2:00 | 3:00 | 4:00 | 5:00 | 6:00 | |
| 0.3 | 1,247,053 | 1,279,256 | 1,269,741 | 1,259,171 | 1,243,333 | 1,261,025 |
| 0.5 | 196,298 | 196,215 | 181,629 | 170,027 | 162,282 | 158,351 |
| 1.0 | 17,981 | 16,597 | 14,734 | 13,271 | 12,047 | 11,602 |
| 3.0 | 548 | 402 | 281 | 218 | 192 | 173 |
| 5.0 | 91 | 51 | 27 | 17 | 17 | 15 |
The natural decay test was conducted seven times with bacterial concentrations varying from 1240 to 11,180 CFU/m3 (Table 2). The results indicated that the maximum deviation was 3.1% with the allowed sample error of ± 1.1% p at a 95% confidence level. It was observed that the bacteria decrease at a constant rate at constant intervals upon the injection of bacterial strain into the airtight chamber of constant space (Fig. 4a, b). Based on these observations, the results of the operational decay test showed the performance of the air purifier itself by correcting the natural decay rate. The efficiency of the air purifier on the removal of S. epidermidis was found to be similar to that reported by Wen et al. (2014), where S. marcescens was effectively filtered by a HEPA filter under the laboratory test unit. Before injecting the microbial samples into the chamber, the internal air in the chamber was cleaned and sterilized. The proliferation of bioaerosols was controlled when the air purifier (HEPA filter) device and UV filter were switched on before the natural decay test. The natural decay rate was observed to be in the range from 78.78 to 81.85% for 60 min. Here, UV is used to sterilize the air, and the particle flow through the HEPA filter comes into contact with the surfaces of the fibers by means of van der Waals forces and removed from the air stream (Taylor et al. 2016). In addition, the test unit (air purifier) was fixed in three different setups, specifically in stand, ceiling, and wall mount types, and they all showed similar removal efficiencies, which would help the product accommodate the lifestyle of the consumer.
Table 2.
Colony count per unit volume (m3) within one hour and the natural decay rate (%)
| S. No. | 0 min (CFU/m3) | 60 min (CFU/m3) | Natural decay (%) |
|---|---|---|---|
| 1 | 1240 | 260 | 79.03 |
| 2 | 2090 | 440 | 78.95 |
| 3 | 2460 | 490 | 80.08 |
| 4 | 2460 | 522 | 78.78 |
| 5 | 2810 | 510 | 81.85 |
| 6 | 5610 | 1068 | 80.96 |
| 7 | 11,180 | 2370 | 78.80 |
Fig. 4.
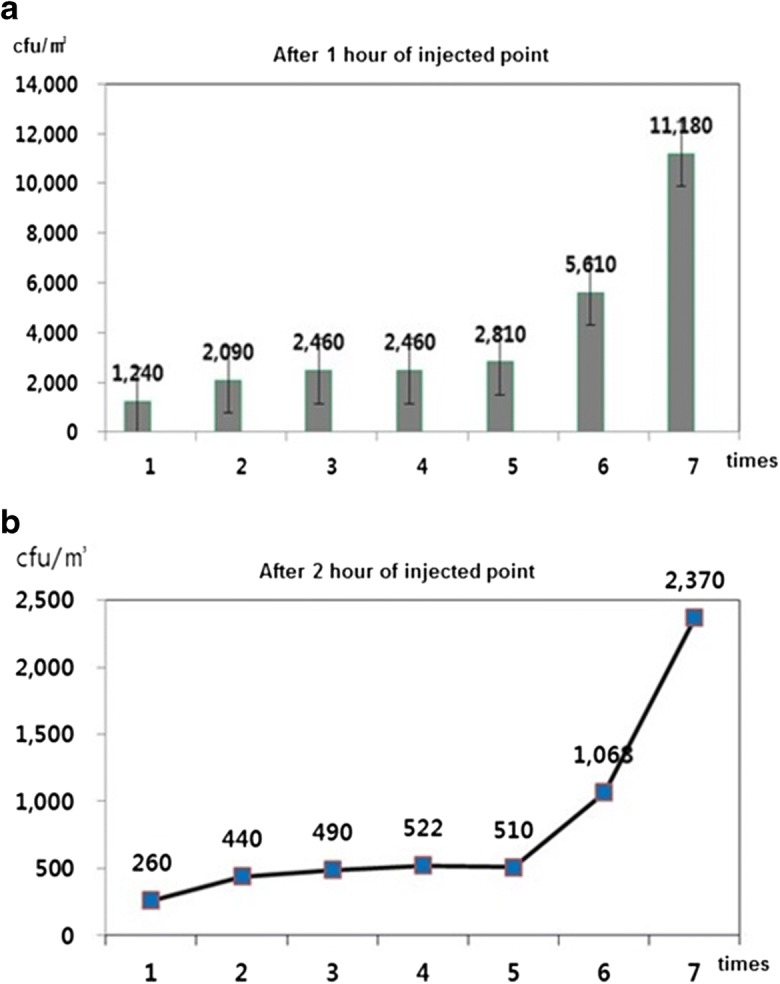
Survivability of S. epidermidis colony after 1 h (a) and 2 h (b) of nebulization
The S. epidermidis samples were collected and cultured in nutrient agar plates, and they all resulted in similar-shaped colonies in white color. The raised and cohesive colonies were approximately 1 to 2 mm in size after incubation at 37 °C for 24 h (Fig. 5a, b), and Fig. 5c and d show the negative and positive controls, respectively. Prior to the air purifier operation, the nebulized bacteria were found to be high and similar to the positive control. Once the airborne microbes were captured by the filter, the rate of suspended bacteria becomes very low or even zero. Thus, the HEPA filters built into air purifiers are made of intertwined fibers, where the smallest particles or microbes become trapped in three ways: interception, impaction, or diffusion. The smallest airborne microbes were most likely trapped in the fibers by means of diffusion. Once the microbes enter into the filter, they will colloid with the gas molecules and embed into the fibers of the filter (Herrera-Cantú et al. 2017). Here, the microbes suffer from lack of nutrients, and the high humidity in this environment leads to low proliferation. In addition, while selecting the test organism, the few criteria were observed, because the test device was a household air purifier; the most important challenge was that the organisms needed to be representative of the bacteria typically found in human dwelling spaces.
Fig. 5.
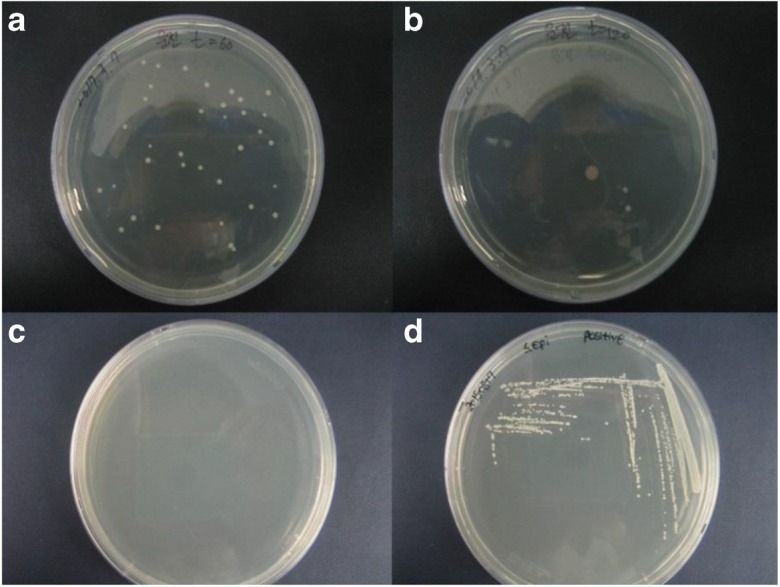
Colony of S. epidermidis after 24 h of incubation at 37 °C. a Before operation (t = 60 min). b After operation (t = 120 min). c Negative control. d Positive control
Conclusions
To summarize, the single-chamber technique was adopted to evaluate the efficiency of an air purifier on the removal of airborne bacteria. Programmed and periodic cleaning and sterilization activities of the chamber were performed as a pre-measure for the contaminations. The result suggests that the rate of decrease in microbes in a chamber with injected microbes after some fixed time was constant. The natural decrease rate had a correlation within the range of 1240–11,180 CFU of microbe density per 1 m3. By calibrating the operation value to the natural decay rate, the bacterial removal capability of the air purifier can be verified and the error of the resulting value can be minimized. Thus, this evaluation method for the air purifier device performance is considered to be promising for improving air filtration technology.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jung Hoon Lee and Jeong Yup Kim contributed equally to this work.
References
- Aliabadi AA, Rogak SN, Bartlett KH, Green SI. Preventing airborne disease transmission: review of methods for ventilation design in health care facilities. Advances in Preventive Medicine. 2011;2011:124064. doi: 10.4061/2011/124064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D, Mainelis G, Fennell D. Effect of an ionic air cleaner on indoor/outdoor particle ratios in a residential environment. Aerosol Science and Technology. 2007;41(3):315–328. doi: 10.1080/02786820701199702. [DOI] [Google Scholar]
- Bonetta S, Bonetta S, Mosso S, Sampo S, Carraro E. Assessment of microbiological indoor air quality in an Italian office building equipped with an HVAC system. Environmental Monitering Assessment. 2010;161(1-4):473–483. doi: 10.1007/s10661-009-0761-8. [DOI] [PubMed] [Google Scholar]
- Bzdek BR, Reid JP. Perspective: aerosol microphysics: from molecules to the chemical physics of aerosols. J. Chem. Phys. 2017;147:220901. doi: 10.1063/1.5002641. [DOI] [PubMed] [Google Scholar]
- Chessa D, Ganau G, Spiga L, Bulla A, Mazzarello V, Campus GV, Rubino S. Staphylococcus aureus and Staphylococcus epidermidis virulence strains as causative agents of persistent infections in breast implants. PLoS One. 2016;11(1):e0146668. doi: 10.1371/journal.pone.0146668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S, Black CL, Yue X, Greby SM, Laney AS, Campbell AP, de Perio MA. Working with influenza-like illness: presenteeism among US health care personnel during the 2014-2015 influenza season. American journal of infection control. 2017;45(11):1254–1258. doi: 10.1016/j.ajic.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuong V, Michael O. Staphylococcus epidermidis infections. Microbes and infection. 2002;4:481–489. doi: 10.1016/S1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- Di Giulio M, Grande R, Di Campli E, Di Bartolomeo S, Cellini L. Indoor air quality in university environments. Environmental Monitering Assessment. 2010;170(1-4):509–517. doi: 10.1007/s10661-009-1252-7. [DOI] [PubMed] [Google Scholar]
- Eickhoff TC. Airborne disease: including chemical and biological warfare. American Journal of Epidemiology. 1996;144(8):S39–S46. doi: 10.1093/aje/144.Supplement_8.S39. [DOI] [PubMed] [Google Scholar]
- Foarde KK. Development of a method for measuring single-pass bioaerosol removal efficiencies of a room air cleaner. Aerosol Science and Technology. 2010;30(2):223–234. doi: 10.1080/027868299304804. [DOI] [Google Scholar]
- Frankel M, Beko G, Timm M, Gustavsen S, Hansen EW, Madsen AM. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Applied and environmental microbiology. 2012;78(23):8289–8297. doi: 10.1128/AEM.02069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Cantú I, García-Aguilar K, Pedraza-Gress E, Vázquez-López E, García-Mar JJ, Flores-González LA, et al. Quantic analysis of the adherence of a gram-negative bacteria in a HEPA filter. International Journal of Advanced Engineering, Management and Science. 2017;3(12):1122–1125. doi: 10.24001/ijaems.3.12.5. [DOI] [Google Scholar]
- Kelley ST, Gilbert JA. Studying the microbiology of the indoor environment. Genome biology. 2013;14(2):202. doi: 10.1186/gb-2013-14-2-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki Youn K, Yoon Shin K, Daekeun K. Distribution characteristics of airborne bacteria nad fungi in the general hospital of Korea. Industrial Health. 2010;48:236–243. doi: 10.2486/indhealth.48.236. [DOI] [PubMed] [Google Scholar]
- Kujundzic E, Hernandez M, Miller SL. Particle size distributions and concentrations of airborne endotoxin using novel collection methods in homes during the winter and summer seasons. Indoor Air. 2006;16(3):216–226. doi: 10.1111/j.1600-0668.2005.00419.x. [DOI] [PubMed] [Google Scholar]
- Lee BU, Sun HY, Ji J-H, Bae G-N. Inactivation of S. epidermidis, B. subtilis, and E. coli bacteria bioaerosols deposited on a filter utilizing airborne silver nanoparticles. Journal of Microbiology and Biotechnology. 2008;18(1):176–182. [PubMed] [Google Scholar]
- Lenart-Boron A, Wolny-Koladka K, Juraszek K, Kasprowicz A. Phenotypic and molecular assessment of antimicrobial resistance profile of airborne Staphylococcus spp. isolated from flats in Krakow. Aerobiologia (Bologna) 2017;33(3):435–444. doi: 10.1007/s10453-017-9481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaitireyimu W, Lu X, Patrick KHL, Zhi N. Development and laboratory evaluation of a compact swirling aerosol sampler (SAS) for collection of atmospheric bioaerosols. Atmospheric Pollution Research. 2015;6(4):556–561. doi: 10.5094/APR.2015.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Advisory Secretariat. (2005). Air cleaning technologies: an evidence-based analysis. Ontario Health Technology Assessment Series, 5(17), 1–52. [PMC free article] [PubMed]
- Metts TA, Batterman SA. Effect of VOC loading on the ozone removal efficiency of activated carbon filters. Chemosphere. 2006;62(1):34–44. doi: 10.1016/j.chemosphere.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Mittal H, Parks SR, Pottage T, Walker JT, Bennett AM. Survival of microorganisms on HEPA filters. Applied Biosafety. 2011;16(3):163–166. doi: 10.1177/153567601101600305. [DOI] [Google Scholar]
- Normand AC, Ranque S, Cassagne C, Gaudart J, Sallah K, Charpin DA, et al. Comparison of air impaction and electrostatic dust collector sampling methods to assess airborne fungal contamination in public buildings. Annals of Work Exposures and Health. 2016;60(2):161–175. doi: 10.1093/annhyg/mev075. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle and Fibre Toxicology. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okten S, Asan A. Airborne fungi and bacteria in indoor and outdoor environment of the Pediatric Unit of Edirne Government Hospital. Environmental Monitering and Assessment. 2012;184(3):1739–1751. doi: 10.1007/s10661-011-2075-x. [DOI] [PubMed] [Google Scholar]
- Otto M. Staphylococcus epidermidis-the ‘accidental’ pathogen. Nature Reviews Microbiology. 2009;7(8):555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CW, Yoon KY, Do Kim Y, Park JH, Hwang J. Effects of condensational growth on culturability of airborne bacteria: implications for sampling and control of bioaerosols. Journal of Aerosol Science. 2011;42:213–223. doi: 10.1016/j.jaerosci.2011.01.006. [DOI] [Google Scholar]
- Pham T-D, Lee B-K. Disinfection of Staphylococcus aureus in indoor aerosols using Cu–TiO2 deposited on glass fiber under visible light irradiation. Journal of Photochemistry and Photobiology A: Chemistry. 2015;307-308:16–22. doi: 10.1016/j.jphotochem.2015.04.002. [DOI] [Google Scholar]
- Raisi L, Aleksandropoulou V, Lazaridis M, Katsivela E. Size distribution of viable, cultivable, airborne microbes and their relationship to particulate matter concentrations and meteorological conditions in a Mediterranean site. Aerobiologia. 2012;29(2):233–248. doi: 10.1007/s10453-012-9276-9. [DOI] [Google Scholar]
- Shrivastava SR, Shrivastava PS, Ramasamy J. Airborne infection control in healthcare settings. Infection Ecology and Epidemiology. 2013;3(1):21411. doi: 10.3402/iee.v3i0.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen MD, Sørensen B, Gonzalez-Dosal R, Melchjorsen CJ, Weibel J, Wang J, Jun CW, Huanming Y, Kristensen P. Severe acute respiratory syndrome (SARS) Annals of the New York Academy of Sciences. 2006;1067:500–505. doi: 10.1196/annals.1354.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, McCollister B, Park D. Highly bactericidal polyurethane effective against both normal and drug-resistant bacteria: potential use as an air filter coating. Applied Biochemistry and Biotechnology. 2016;178(5):1053–1067. doi: 10.1007/s12010-015-1928-0. [DOI] [PubMed] [Google Scholar]
- Tellier R. Aerosol transmission of influenza A virus: a review of new studies. Journal of the Royal Society Interface. 2009;6(Suppl 6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham KW, Zuraimi MS. Size relationship between airborne viable bacteria and particles in a controlled indoor environment study. Indoor Air. 2005;15(9):48–57. doi: 10.1111/j.1600-0668.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- Wen Z, Yang W, Li N, Wang J, Hu L, Li J, et al. Assessment of the risk of infectious aerosols leaking to the environment from BSL-3 laboratory HEPA air filtration systems using model bacterial aerosols. Particuology. 2014;13:82–87. doi: 10.1016/j.partic.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser M, Busse H-J. Rapid identification of Staphylococcus epidermidis. International Journal of Systematic and Evolutionary Microbiology. 2000;50:1087–1093. doi: 10.1099/00207713-50-3-1087. [DOI] [PubMed] [Google Scholar]
- Zacarías SM, Satuf ML, Vaccari MC, Alfano OM. Efficiency evaluation of different TiO2 coatings on the photocatalytic inactivation of airborne bacterial spores. Industrial and Engineering Chemistry Research. 2012;51(42):13599–13608. doi: 10.1021/ie3009956. [DOI] [Google Scholar]


