Abstract
TiO2 photocatalysis with ultraviolet (UV-A) light has proven to be a highly effective process for complete inactivation of airborne microbes. However, the overall efficiency of the technology needs to be improved to make it more attractive as a defense against bio-terrorism. The present research investigates the enhancement in the rate of destruction of bacterial spores on metal (aluminum) and fabric (polyester) substrates with metal (silver)-doped titanium dioxide and compares it to conventional photocatalysis (TiO2 P25/+UV-A) and UV-A photolysis. Bacillus cereus bacterial spores were used as an index to demonstrate the enhanced disinfection efficiency. The results indicate complete inactivation of B. cereus spores with the enhanced photocatalyst. The enhanced spore destruction rate may be attributed to the highly oxidizing radicals generated by the doped TiO2.
Keywords: Photocatalysis, Metal-doped TiO2, Silver, Bacillus cereus spores, Fabric, UV-A
Introduction
The field of heterogeneous photocatalysis has been extensively studied in the last three decades, and this is illustrated by the vast numbers of scientific and technical publications on this topic. The semiconductor titanium dioxide (TiO2) is the most widely studied photocatalyst. The photosensitizing action of titanium dioxide was first observed in 1929, when as a pigment used in paint, it was found to be responsible for paint fading. This paint fading was because of the photodegradation of the polymer organic binder of the paint by the action of TiO2. However, work towards the development of photocatalytic theory, using titanium dioxide as a photocatalyst, gained momentum in the late 1960s [11].
The huge interest generated by photocatalysis has motivated several researchers to look into the basic mode of action of TiO2, which is now fairly well understood. TiO2 is a semiconductor with a band gap close to 3.2 eV, and UV light with wavelengths shorter than ∼ 380 nm photoactivates TiO2 by providing the band gap energy needed by an electron to jump from the valence band to the conduction band. This implies that when photons of UV-A light are absorbed on TiO2, they generate excited pairs of electrons and holes. The photogenerated holes react with the water to produce hydroxyl radicals (•OH), while the photogenerated electrons react with molecular oxygen to give superoxide radical anions (•O−2). These radicals so produced are highly reactive and they work together to completely oxidize the organic species. The attack by the •OH radical, in the presence of oxygen, thus initiates a complex cascade of oxidative reactions. The mechanism of the photocatalytic process has been extensively studied in the literature and several complex reaction pathways have been reported [7, 16, 29, 35]. Although the exact mechanism of the process and the reaction pathways are still not clear, the practical applications that these processes offer have fueled enormous commercial interest.
Photocatalytic oxidation as a technique for microbial disinfection was first demonstrated by Matsunaga et al. [27]. They investigated the effectiveness of photocatalytic oxidation of several microorganisms such as Lactobacillus acidophilus (gram positive bacteria), Saccharomyces cerevisiae (yeast), Escherichia coli (gram negative bacteria) and Chlorella vulgaris (green algae) in water. Their results show killing of the microbial cells in 60–120 min using a TiO2–Pt photocatalyst. They determined that the mode of action of the process was the photooxidation of Coenzyme A (CoA) leading to inhibition of cell respiration and thus cell death. The process was not entirely effective against Chlorella vulgaris though, because of its thicker cell wall [27]. This led to an extensive research in the field of photocatalytic oxidation for destruction of various microorganisms including bacteria, viruses, fungi, algae, and protozoa. Recent review articles [4, 10, 12, 28] provide a comprehensive coverage of the application of TiO2 photocatalysis to disinfection.
The pioneering work in the field of gas phase photocatalytic disinfection was done by Goswami et al. [13] when he along with his group developed a technology to completely inactivate biological contaminants in indoor air. A recirculating duct facility was used, wherein the bacteria (Serratia marcescens) was shown to be completely inactivated. Subsequent research conducted by Goswami et al. [14], with improved reactor designs, demonstrated 100 percent inactivation of Serratia marcescens bacteria in a much reduced time. Their group even reported inactivation of dust mite antigens by photocatalytic oxidation. Der p II was selected and its fast destruction demonstrated the ability of the photocatalytic technology to control allergies and diseases in the population [3].
In 1999, Masaki and coworkers [26] reported destruction of bacteria and foul odor in air using stainless steel plates coated with thin films of TiO2. Greist et al. [15] demonstrated the capability of photocatalytic oxidation to destroy Bacillus anthracis (Anthrax) through the successful inactivation of Bacillus subtilis spore. Following the recent attack of SARS virus, Knight [20] studied a system, based on photocatalytic disinfection, to control the spread of infectious microorganisms such as SARS virus on flights. In 2003, Kuhn et al. [21] studied microbes relevant to hygiene including Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecium, and Candida albicans, and observed a 3–6-log reduction for these microorganisms (except for Candida albicans) in 60 min. Kim and his group [19] investigated the application of TiO2 photocatalysis to disinfect selected food-borne bacteria such as Salmonella choleraesuis subsp., Vibrio parahaemolyticus, and Listeria monocytogenes and demonstrated almost complete inactivation of the bacterial species in about 3 h. Recently, photocatalytic disinfection has also been shown to be effective against microcystin toxins produced by various cyanobacteria (blue–green algae) such as Microcystis, Anabaena, Oscillatoria, and Nostoc [22, 33].
Although photocatalytic disinfection has been demonstrated to be effective against a wide range of microbes, the kinetics of the process for complete inactivation and mineralization of microbes is comparatively slower as compared to conventional disinfection techniques. Jacoby et al. [17] studied photooxidation of E. coli in air, and found 54% mineralization in 75 h by measuring the carbon dioxide released. Also in their most recent studies, Wolfrum and Jacoby [37] demonstrated complete mineralization of E. coli, Micrococcus luteus, B. cereus (bacterial cells and spores), and Aspergillus niger spores by photocatalytic oxidation based on kinetic data and carbon mass balance. However, the inactivation and subsequent mineralization process was extremely slow.
The intent of this study is to enhance the overall rate of disinfection of the photocatalytic process, and to make it commercially more attractive as a defense against bio-terrorism. An efficient way to improve the kinetics of photocatalysis is the addition of transition metals to TiO2 [16]. Introduction of metal ions in the lattice of TiO2 has shown significant enhancement in the photcatalytic activity of TiO2 for the degradation of various organics. Iron (III)-doped TiO2 [8, 24], platinized TiO2 [6, 18], lanthanide metal ion doped TiO2 [36], chromium-, manganese- and cobalt-doped TiO2 [9], and silver (AG)-doped TiO2 [1, 23, 25] have all been successfully demonstrated as photocatalysts leading to an increased rate of destruction of organics.
Although there is extensive literature on the use of Ag-doped TiO2 for photocatalytic degradation of organics, its application for photocatalytic disinfection in gas phase has not been studied much. It is widely recognized that Ag+ ions possess antimicrobial properties and the work done by Sokmen et al. [34] demonstrated the enhancement in inactivation of E. coli in liquid phase using Ag-TiO2/UV system. The main aim of this study is to demonstrate the effectiveness of Ag-doped TiO2 photocatalyst for fast inactivation of highly resistant bacterial spores on surfaces in air.
Bacillus cereus bacterial spores were used and their percentage destruction determined as a function of time of exposure to the photocatalyst. Because of the high degree of resistance of bacterial spores to various disinfectants, and hence their slow kinetics of inactivation [2, 31], a logarithmic scale to plot inactivation was disregarded in favor of the less conventional method of plotting percentage destruction of spores. Destruction of B. cereus spores on both metal and fabric substrates with the Ag-doped TiO2 P25 (enhanced photocatalyst) was studied, and compared with conventional TiO2 photocatalysis and UV-A photolysis. The Ag+ ions from the dopant act as an electron trap in the photocatalysis process. This reduces the recombination between electrons and holes, and thus results in an increased availability of holes. The synergistic effect of the doped Ag+ ions, and highly oxidizing radicals generated by TiO2 photocatalysis process, may lead to a highly enhanced rate of microbial destruction.
Materials and methods
Experimental facility
The disinfection study on metal and fabric substrates was done in a still air experimental facility as shown in Fig. 1. The experimental facility is a rectangular enclosure with dimensions of 1.12×0.74×0.51 m (44×29×20 in.). It consists of a bank of 43 EPR 3500 UV-A lamps (Southern New England Ultraviolet Company). Each lamp has a nominal power rating of 14 W, and emits approximately 1.5 W of UV-A radiation, predominately at 350 nm. 1. The enclosure consists of a vertically adjustable platform on which the substrate samples were placed. The stand was adjusted to a height so that the UV-A light intensity was 50 W/m2 on the surface of the samples. The UV-A light intensity was measured with an Eppley radiometer (model TUVR).
Fig. 1.
Experimental photocatalysis test facility
Photocatalyst
Titanium dioxide used in this study was Degussa P25. It had an approximate composition of 75% anatase and 25% rutile forms of TiO2, a BET surface area of 50 m2 /g, and a primary particle size of 20 nm (Degussa). The enhanced photocatalyst was prepared by doping TiO2 P25 with Ag by a proprietary process. A slurry of enhanced photocatalyst was coated onto the metal and fabric substrate. The metal substrate used in the study was sandblasted aluminum while the fabric substrate used was polyester. Each of the substrate samples used in the study was 50×50 mm (2×2 in.).
Culture media
BBLTM Columbia Broth was used as the culture media for B. cereus spores. For preparation of Columbia Broth, 35 g of the powder was dissolved in 1 l of sterilized water and mixed thoroughly. The suspension was warmed gently so that the solution was complete. The solution was then autoclaved at 121°C for 15 min without overheating.
Microorganism culture
Bacillus cereus (ATCC 2) bacterial spores were used as an index for disinfection study. For preparation of spores, B. cereus strains were cultivated in Columbia broth at 30°C for 12–18 h aerobically with vigorous agitation. Spores were then harvested and purified by lysozyme treatment and salt and detergent washes to remove the bacterial cells. Bacterial cells were collected by centrifugation at 10,000 g for 10 min at 4°C. The bacterial pellet was washed with 1/4 volume of 1 M KCl/0.5 M NaCl and incubated at 37°C for 60 min at 1/4 volume of Tris·Cl (50 mM, pH 7.2) containing lysozyme at 50 μg/mL. Finally, the spores were cleaned by alternate centrifugation (10,000 g, 10 min) and washing with: (1) NaCl (1 M); (2) deionized water; (3) SDS (0.05%); (4) TEP buffer (50 mM Tris·Cl buffer, pH 7.2. containing 10-mM EDTA and 2-mM phenylmethylsulfonyl fluoride); and (5) three washes with deionized water. After the lysozyme treatment, the spore suspension was heat shocked in a constant temperature bath at 80°C for 1 h to remove any bacterial cells left after the lysozyme treatment.
Experimental procedure
Destruction of B. cereus spores was studied for the following configurations:
Enhanced photocatalysis (Ag/-TiO2/-UV-A) on metal and fabric substrates
Conventional photocatalysis (TiO2/-UV-A) on metal and fabric substrates
Photolysis (UV-A) on metal and fabric substrates
Dark control on metal and fabric substrates
The experiments involved determining the increased destruction of B. cereus spores, on aluminum and polyester fabric substrates, with enhanced photocatalyst as compared to conventional photocatalysis, and photolysis under 350-nm UV-A light in still air conditions. Since this technology is being developed to be commercialized, no effort was made to observe the effect of germicidal UV-C (254 nm) radiation on the destruction of B. cereus spores, as it was considered a potential danger to the end user.
The UV-A light intensity was kept constant at 50 W/m2. The temperature in the still air facility was determined to be 24°C at the start of the experiment which increased by 2°C over the entire duration of the experiment. Previous experiments done by Goswami et al. [14] had shown that temperature is not a major factor in photocatalytic disinfection. Hence, no effort was made to determine the temperature directly on the surface of the substrate. Heat-shocked B. cereus spores with an initial concentration of 104 –105 cfu/mL were used. A study was performed to determine the appropriate concentration of B. cereus spores to be suspended and dried on the substrate. Several dilutions of the stock culture in sterilized water were deposited on the substrate to determine the initial concentration, which would give a detectable colony count on agar plates, after incubation. 0.1 mL of spore suspension with a concentration close to 5,000 cfu/mL proved to give reasonable colony counts. The spore concentration was diluted with sterilized water and vortexed for about 1 min to ensure uniform distribution of spores in the suspension, and no spore agglomeration.
Enhanced photocatalysis
The aluminum substrates were coated with three coatings of the enhanced photocatalyst slurry in deionized water with intermittent drying of each coat at room temperature. For fabric substrates, TiO2 in pure or doped form was incorporated into the fibers of the polyester fabric. Then 0.1 mL of spore suspension was uniformly spread on each of the coated substrates using a sterilized (alcohol and a flame) glass rod, being careful not to push the spore suspension off the side of the substrate. The spore suspension was allowed to dry on the substrates in the dark for 12 h.
Conventional photocatalysis, photolysis and dark control
Experiments were performed on the uncoated substrates (dark control and photolysis), and TiO2-coated substrates (conventional photocatalysis), in the dark and exposure to UV-A light.
Each experiment included a batch of three samples of given substrate. For every photolysis and photocatalysis experimental run, a parallel dark run with same exposure time was carried out.
Each of the aluminum substrates was placed in the bottom of separate sterile petri dishes and 20 mL of liquid plate count agar poured over each sample. The spores were transferred from the substrates into the agar by shaking the petri dishes for 20 s, and subsequently removing the substrates from the agar by lifting them with a sterile needle. The spores were collected from the fabric substrates by vortexing these substrates in about 3 mL of sterilized water and finally plating this water in plate count agar. The petri dishes were inverted on solidification of the agar and incubated at 37°C for 16 h. Finally, the colonies on each petri dish were counted to determine the percentage destruction of spores relative to initial concentration.
Each experiment was repeated at least three times and three samples were taken at each time interval. Colonies were counted for each of these nine samples, and these colony counts were used to calculate the percentage destruction relative to initial colony count at each time interval.
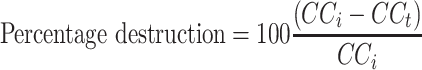 |
where CC i is the initial colony count and CC t is the colony count after a time interval ‘ t’. Average percentage destruction was then calculated and plotted as a function of time of exposure for each of the configurations.
Results and discussion
Metal substrate
Table 1 shows the average colony counts at each time interval for various experimental configurations on the metal substrate. As is evident, 0.1 mL of a 5,000 cfu/mL suspension of B. cereus spores gave an average initial colony count of 284 on plate count agar after recovery from the substrate. This is taken as the colony count relative to which destruction is calculated at various time intervals for all experiments. Average percentage destruction and standard deviation in percentage destruction are also included in the table.
Table 1.
Average colony counts and average percentage destruction of Bacillus cereus spores on metal substrate for various configurations
| Time of exposure (h) | Dark control | UV-A photolysis | Photocatalysis | Enhanced photocatalysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave. colony count | Ave. percentage dest. | SD percentage dest. | Ave. colony count | Ave. percentage dest. | SD percentage dest. | Ave. colony count | Ave. percentage dest. | SD percentage dest. | Ave. colony count | Ave. percentage dest. | SD percentage dest. | |
| 0 | 284 | 0.00 | 0.00 | 284 | 0.00 | 0.00 | 284 | 0.00 | 0.00 | 284 | 0.00 | 0.00 |
| 4 | 277 | 2.46 | 8.60 | 221 | 22.24 | 4.48 | 67 | 76.41 | 3.42 | 6 | 97.89 | 1.05 |
| 16 | 272 | 4.17 | 6.64 | 191 | 32.75 | 5.76 | 38 | 86.62 | 8.43 | 1 | 99.65 | 0.57 |
| 24 | 280 | 1.29 | 9.30 | 128 | 54.81 | 5.05 | 3 | 98.94 | 3.13 | 0 | 100.00 | 0.00 |
The spore destruction on metal with enhanced photocatalysis as compared to conventional photocatalysis, UV-A photolysis and under dark conditions is also demonstrated in Fig. 2. The error bars in the figure depict standard deviations. Percentage destruction of less than 2% with a standard deviation of about ±9% was observed even after 24 h in the dark. This indicated that B. cereus spores are not inactivated on a metal substrate.
Fig. 2.
Comparison of enhanced photocatalytic (Ag-TiO2 P25/+UV-A), conventional photocatalytic (TiO2 P25/+UV-A), photolytic (UV-A) and dark destruction of Bacillus cereus spores on metal substrate (Error bars in the figure are standard deviations in average percentage destruction)
Complete spore destruction was not achieved even after 24 h of UV-A photolysis. The results showed 22% spore destruction in about 4 h and close to 55% destruction after 24 h of UV-A exposure. The percentage destruction data for UV-A photolysis had a standard deviation close to ±5%. Generally Bacillus endospores are highly resistant to UV radiation [30]. Riesenman and Nicholson [31] have shown that the Bacillus endospores possess a thick spore protein coating that provides resistance to sporicidal substances. They reported that UV-A wavelength becomes effective as a sporocide in case of mutation of spores that may lead to damage of this spore coat. Partially damaged spore protein coating may be a possible explanation of the destruction as observed with UV-A photolysis. The UV-A photolysis control test, however, was ineffective in complete destruction of B. cereus spores.
Conventional photocatalytic destruction of spores on metal was also studied wherein TiO2 P25 was used in conjunction with 350 nm UV-A (intensity kept constant at 50 W/m2) to inactivate B. cereus. Faster destruction was observed with almost 76% of the spores getting destroyed in the first 4 h and 99% in 24 h (standard deviation close to ±3%). A possible explanation for this reduced rate of destruction at longer time intervals could be that the spores being living entities may have varying resistance to attack by •OH radicals. Thus, the less resistant spores were destroyed in the first few hours and the more resistant ones survived for longer durations. It could also be due to the increasing amounts of dead spores and by-products generated by the photocatalytic oxidation of spores that may lead to quenching of •OH radicals and shielding of light [32]. Another explanation could be the possibility of spore agglomeration on metal substrate leading to reduced exposure to •OH radicals. The spore agglomeration could be avoided by adding a small quantity of surfactant to the spore suspension before dispersal on the samples. However, the use of a surfactant was avoided in this study as it may lead to germination of bacterial spores to vegetative bacterial cells, which are much easier to inactivate.
The experimental study with the enhanced photocatalyst (Ag-TiO2 P25/+UV-A) indicated extremely fast destruction of spores with close to 100% destruction evident at the first sampling time of 4-h irradiation. No spore colony count was observed at subsequent time intervals. The enhanced photocatalytic process thus leads to complete destruction of even hardy B. cereus spores in smaller exposure times. This marked improvement in destruction of B. cereus spores with enhanced photocatalysis could be attributed to the electron trapping by the doped Ag+ ions leading to reduced recombination and hence enhanced photocatalysis.
Fabric substrate
The average colony counts at different time intervals for various experimental configurations on fabric substrates are depicted in Table 2. The average initial colony count was determined to be 148 after recovery of the spores from the fabric substrate onto plate count agar. This recovery was from 0.1 mL of a 5,000 cfu/mL spore suspension dried on the fabric substrate. The average initial colony count as determined above was used to calculate percentage destruction at each subsequent time of exposure. Average percentage destruction and standard deviation in percentage destruction are also shown in Table 2.
Table 2.
Average colony counts and average percentage destruction of B. cereus spores on fabric substrate for various configurations
| Time of exposure (h) | Dark control | UV-A photolysis | Photocatalysis | Enhanced photocatalysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave. colony count | Ave. percentage dest. | SD percentage dest. | Ave. colony count | Ave. percentage dest. | SD percentage dest. | Ave. colony count | Ave. percentage dest. | SD percentage dest. | Ave. colony count | Ave. percentage dest. | SD percentage dest. | |
| 0 | 148 | 0.00 | 0.00 | 148 | 0.00 | 0.00 | 148 | 0.00 | 0.00 | 148 | 0.00 | 0.00 |
| 4 | 142 | 4.05 | 9.85 | 115 | 22.30 | 7.68 | 30 | 79.73 | 4.05 | 0 | 100.00 | 0.00 |
| 16 | 141 | 4.73 | 12.26 | 92 | 37.84 | 4.52 | 21 | 85.81 | 11.72 | 0 | 100.00 | 0.00 |
| 24 | 162 | 0.00 | 6.51 | 65 | 56.08 | 5.03 | 10 | 93.24 | 3.86 | 0 | 100.00 | 0.00 |
The spore destruction on fabric with enhanced photocatalysis as compared to conventional photocatalysis, photolysis and under dark conditions is illustrated in Fig. 3. The spore destruction in dark on the fabric substrate was insignificant. No appreciable destruction was observed as expected. The standard deviation of dark control destruction data was ±12% at the most. The photolytic destruction trend on fabric follows that on metal substrate close to 56% destruction with a ±5% standard deviation achieved in 24 h of UV-A exposure.
Fig. 3.
Comparison of enhanced photocatalytic (Ag-TiO2 P25/+UV-A), conventional photocatalytic (TiO2 P25/+UV-A), photolytic (UV-A), and dark destruction of B. cereus spores on fabric substrate (Error bars in the figure are standard deviations in average percentage destruction)
Conventional photocatalytic (TiO2 P25/+UV-A) destruction of spores on fabric substrate is also depicted. As experienced with metal substrate, the destruction rate tends to level off after the first 4 h. Close to 80% destruction was observed in the first 4 h, which increased to 96% destruction in 24 h. The rate of destruction leveled off after the first 4 h. The possibility of spores hiding inside the fibers of the fabric and surviving may be a possible explanation of this destruction trend. Also as mentioned before, the quenching of •OH radicals and shielding of light due to debris of dead spores may be a reason for this leveling off trend in TiO2 photocatalysis.
The increased photocatalytic destruction of B. cereus spores on a fabric substrate with the enhanced photocatalyst (Ag-TiO2 P25/+UV-A) as compared to the conventional TiO2 P25 photocatalysis and UV-A photolysis is clearly evident. The enhanced photocatalysis process destruction trend was similar to the destruction trend on metal substrate. 100% spore inactivation was achieved in 4 h of irradiation.
Conclusions
The enhanced photocatalysis process was presented and its effectiveness as an efficient technique for disinfection of surfaces was successfully demonstrated. Complete inactivation of B. cereus bacterial spores on metal and fabric substrates was reported. Control experiments involving conventional TiO2 photocatalytic, UV-A photolytic and dark destruction of spores showed that Ag-doped TiO2 photocatalysis results in much faster destruction kinetics as compared to these three control processes.
Footnotes
The spectrum of the black light used is a bell-shaped curve centered at 350 nm and extends from 300 to 400 nm
References
- 1.Arabatzis IM, Stergiopoulos T, Bernard MC, Labou D, Neophytides SG, Falaras P. Silver-modified titanium dioxide thin films for efficient photodegradation of methyl orange. Appl Catal B Environ. 2003;42:187–201. doi: 10.1016/S0926-3373(02)00233-3. [DOI] [Google Scholar]
- 2.Atrih A, Foster SJ. Bacterial endospores the ultimate survivors. Int Dairy J. 2002;12:217–223. doi: 10.1016/S0958-6946(01)00157-1. [DOI] [Google Scholar]
- 3.Beaudreau C, Hingorani SK, Goswami TK, Goswami DY (1998) Destruction of dust mite allergens using PhotechTM – photocatalytic technology for disinfection of indoor air. In: presented at Pan-American Workshop on Commercialization of Advanced Oxidation Technologies, Ontario, Canada
- 4.Blake DM, Maness P, Huang Z, Wolfrum EJ, Huang J, Jacoby WA. Application of the photocatalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells. Sep Purif Methods. 1999;28(1):1–50. doi: 10.1080/03602549909351643. [DOI] [Google Scholar]
- 5.Block SS. Disinfection, sterilization, and preservation. Philadelphia: Lea and Febiger; 1991. [Google Scholar]
- 6.Chen J, Ollis DF, Rulkens WH, Bruning H. Photocatalyzed oxidation of alcohols and organochlorides in the presence of native TiO2 and metallized TiO2 suspensions. Part (I): photocatalytic activity and pH influence. Water Res. 1999;33:661–668. doi: 10.1016/S0043-1354(98)00261-9. [DOI] [Google Scholar]
- 7.Davydov L, Smirtiotis PG. Quantification of the primary processes in aqueous heterogeneous photocatalysis using single-stage oxidation reactions. J Catal. 2000;191:105–116. doi: 10.1006/jcat.1999.2777. [DOI] [Google Scholar]
- 8.Dhananjeyan MR, Kandavelu V, Renganathan R. A study on the photocatalytic reactions of TiO2 with certain pyrimidine bases: effects of dopants (Fe3+) and calcination. J Mol Catal A Chem. 2000;151:217–223. doi: 10.1016/S1381-1169(99)00246-0. [DOI] [Google Scholar]
- 9.Dvoranova D, Brezova V, Mazur M, Malati MA. Investigation of metal-doped titanium dioxide photocatalysis. Appl Catal B Environ. 2002;37:91–105. doi: 10.1016/S0926-3373(01)00335-6. [DOI] [Google Scholar]
- 10.Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C Photochem Rev. 2000;1:1–21. doi: 10.1016/S1389-5567(00)00002-2. [DOI] [Google Scholar]
- 11.Gerischer H (1970) In: Eyring H, Henderson D, Jost W (eds) Physical chemistry – an advanced treatise. Academic, New York, pp 465–542
- 12.Goswami DY (1999) Recent developments in photocatalytic detoxification and disinfection of water and air. In: proceedings of the ISES 1999 Solar World Congress, Jerusalem, Israel
- 13.Goswami DY, Trivedi D, Block SS (1995) Photocatalytic disinfection of indoor air. Solar engineering, In: proceedings of the ASME International Solar Energy Conference, Hawaii, pp 421–430
- 14.Goswami DY, Trivedi D, Block SS. Photocatalytic disinfection of indoor air. J Solar Energy Eng. 1997;119:92–96. [Google Scholar]
- 15.Greist HT, Hingorani SK, Kelly K, Goswami DY (2002) Using scanning electron microscopy to visualize photocatalytic mineralization of airborne microorganisms. In: proceedings of the Indoor Air 2002, 9th International Conference on Indoor Air Quality and Climate, Monterey, California, pp 712–717
- 16.Hoffmann MR, Martin ST, Choi W, Bahnemann DW. Environmental applications of heterogeneous photocatalysis. Chem Rev. 1995;95:69. doi: 10.1021/cr00033a004. [DOI] [Google Scholar]
- 17.Jacoby WA, Maness PC, Wolfrum EJ, Blake DM, Fennell JA. Mineralization of bacterial cell mass on a photocatalytic surface in air. Environ Sci Technol. 1998;32:2650–2653. doi: 10.1021/es980036f. [DOI] [Google Scholar]
- 18.Kennedy JC, III, Datye AK. Photothermal heterogeneous oxidation of ethanol over Pt/TiO2. J Catal. 1998;179:375–389. doi: 10.1006/jcat.1998.2242. [DOI] [Google Scholar]
- 19.Kim B, Kim D, Cho D, Cho S. Bactericidal effect of TiO2 photocatalyst on food-borne pathogenic bacteria. Chemosphere. 2003;52:277–281. doi: 10.1016/S0045-6535(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 20.Knight H. Sars wars. Engineer. 2003;292:27–35. [Google Scholar]
- 21.Kuhn KP, Chaberny IF, Massholder K, Stickler M, Benz VW, Sonntag H, Erdinger L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UV-A light. Chemosphere. 2003;53:71–77. doi: 10.1016/S0045-6535(03)00362-X. [DOI] [PubMed] [Google Scholar]
- 22.Lawton LA, Robertson PKJ, Cornish BJPA, Marr IL, Jaspers M. Processes influencing surface interaction and photocatalytic destruction of microcystins on titanium dioxide photocatalysis. J Catal. 2003;213:109–113. doi: 10.1016/S0021-9517(02)00049-0. [DOI] [Google Scholar]
- 23.Lee W, Shen H-S, Dwight K, Wold A. Effect of Silver on the Photocatalytic activity of TiO2. J Solid State Chem. 1993;106:288–294. doi: 10.1006/jssc.1993.1288. [DOI] [Google Scholar]
- 24.Litter MI, Navio JA. Photocatalytic properties of iron-doped titania semiconductors. Journal of Photochemistry and Photobiology A Chemistry. 1996;98:171–181. doi: 10.1016/1010-6030(96)04343-2. [DOI] [Google Scholar]
- 25.Liu Y, Liu C-Y, Rong Q-H, Zhang Z. Chanracteristics of the silver-doped TiO2 nanoparticles. Appl Surf Sci. 2003;220:7–11. doi: 10.1016/S0169-4332(03)00836-5. [DOI] [Google Scholar]
- 26.Masaki Y, Masaude S, Ishida K. TiO2 photocatalyst for environmental purification. Sumitomo Met. 1999;50:26–32. [Google Scholar]
- 27.Matsunaga T, Tamoda R, Nakajima T, Wake H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett. 1985;29:211–214. doi: 10.1111/j.1574-6968.1985.tb00864.x. [DOI] [Google Scholar]
- 28.Mills A, Le Hunte S. An overview of semiconductor photocatalysis. J Photochem Photobiol A Chem. 1997;108:1–35. doi: 10.1016/S1010-6030(97)00118-4. [DOI] [Google Scholar]
- 29.Minero C. Kinetic analysis of photoinduced reactions at the water semiconductor interface. Catal Today. 1999;54:205–216. doi: 10.1016/S0920-5861(99)00183-2. [DOI] [Google Scholar]
- 30.Moeller R, Horneck G, Facius R, Stackebrandt E. Role of pigmentation in protecting Bacillus sp. endospores against environmental UV radiation. FEMS Microbiol Ecol. 2005;51:231–236. doi: 10.1016/j.femsec.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Riesenman PJ, Nicholson WL. Role of the spore coat layers in Bacillus subtillis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl Environ Microbiol. 2000;66:620–626. doi: 10.1128/AEM.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rincon A-G, Pulgarin C. Use of coaxial photocatalytic reactor (CAPHORE) in the TiO2 photo-assisted treatment of mixed E. coli and Bacillus sp. And bacterial community present in wastewater. Catal Today. 2005;101:331–344. doi: 10.1016/j.cattod.2005.03.022. [DOI] [Google Scholar]
- 33.Shephard GS, Stockenstrom S, de Villiers D, Engelbrecht WJ, Wessels GFS. Degradation of microcystin toxins in a falling film photocatalytic reactor with immobilized titanium dioxide catalyst. Water Res. 2002;36:140–146. doi: 10.1016/S0043-1354(01)00213-5. [DOI] [PubMed] [Google Scholar]
- 34.Sokmen M, Candan F, Sumer Z. Disinfection of E. coli by the Ag-TiO2/UV system: lipidperoxidation. J Photochem Photobiol A Chem. 2001;143:241–244. doi: 10.1016/S1010-6030(01)00497-X. [DOI] [Google Scholar]
- 35.Turchi CS, Ollis DF. Photocatalytic degradation of organic water contaminants; mechanisms involving hydroxyl radical attack. J Catal. 1990;122:178. doi: 10.1016/0021-9517(90)90269-P. [DOI] [Google Scholar]
- 36.Wang Y, Cheng H, Zhang L, Hao Y, Ma J, Xu B, Li W. The preparation, characterization, photoelectrochemical and photocatalytic properties of lanthanide metal-ion-doped TiO2 nanoparticles. J Mol Catal A Chem. 2000;151:205–216. doi: 10.1016/S1381-1169(99)00245-9. [DOI] [Google Scholar]
- 37.Wolfrum EJ, Huang J, Blake DM, Maness P, Huang Z, Fiest J. Photocatalytic oxidation of bacteria, bacterial and fungal spores, and model biofilm components to carbon dioxide on titanium dioxide-coated surfaces. Environ Sci Technol. 2002;36:3412–3419. doi: 10.1021/es011423j. [DOI] [PubMed] [Google Scholar]


