Abstract
The objective of this study was to compare systemic and local cytokine profiles and neutrophil responses in patients with severe versus non-severe community-acquired pneumonia (CAP). Hospitalized patients with CAP were grouped according to the pneumonia severity index (PSI), as non-severe (PSI < 91 points) or severe (PSI ≥ 91 points). Blood and sputum samples were collected upon admission. Compared to non-severe CAP patients, the severe CAP group showed higher plasma levels of pro- and anti-inflammatory cytokines but in contrast, lower sputum concentrations of pro-inflammatory cytokines. Blood neutrophil functional responses were elevated in CAP patients compared to healthy controls. However, neutrophils from severe CAP patients showed reduced respiratory burst activity compared to the non-severe group. Results indicate that patients with severe CAP fail to mount a robust local pro-inflammatory response but exhibit instead a more substantial systemic inflammatory response, suggesting that a key driver of CAP severity may be the ability of the patient to generate an optimal local inflammatory response.
Electronic supplementary material
The online version of this article (doi:10.1007/s10753-014-9840-2) contains supplementary material, which is available to authorized users.
KEY WORDS: chemokines, community-acquired pneumonia, cytokines, inflammation, neutrophils, pneumonia severity
INTRODUCTION
Community-acquired pneumonia (CAP) is one of the major infectious disease-related causes of death in both developed and underdeveloped countries [1, 2]. CAP initiates with an inflammatory response elicited by resident alveolar macrophages in response to invading microorganisms [3]. These cells produce a cascade of pro-inflammatory cytokines and chemokines that vigorously recruit and activate blood leukocytes, particularly neutrophils, into the lungs, where they play a major role in curbing the infection [4].
In hospitalized CAP patients, severity of disease is influenced by the ability of the host′s immune response to control the pathogenic microorganism, as well as through regulation of the local pro-inflammatory response and neutrophil accumulation. During the inflammatory response, neutrophils undergo sequential release of various granule subtypes and enhance respiratory burst activity with production of reactive oxygen species, with the potential to cause tissue damage [5, 6]. Evidence suggests that failure to control excessive inflammation and/or neutrophil activation may result in exaggerated systemic inflammation and organ damage, leading to severe disease [7–10]. Data examining the association between local the inflammatory response and severity of disease are limited. Since severe CAP is associated with significantly higher mortality than non-severe CAP, a better understanding of the local and systemic inflammatory responses in hospitalized patients with severe CAP may be necessary to develop novel strategies for the management of these patients.
The objectives of this study were to characterize and contrast the lung and systemic cytokine profiles as well as blood neutrophil responses in patients with severe versus non-severe CAP at the time of hospital admission.
MATERIALS AND METHODS
Study Design
This was a prospective observational study of 40 hospitalized patients with CAP at the University of Louisville Hospital and the Louisville′s Veteran Administration Hospital from 01/04/2011 to 01/08/2012. The University of Louisville Human Subjects Program Protection Office and the Robley Rex Veterans Affairs Medical Center Institutional Review Boards approved this study prior to any data collection (approval nos.: 07.0182 and 0009, respectively).
Inclusion Criteria/Criteria for CAP
Patients with CAP, defined as evidence of a new pulmonary infiltrate at chest radiograph associated with at least one of the following: (1) new or increased cough; (2) fever or hypothermia; and (3) leukocytosis, left shift, or leucopenia, were included in this study following previous written consent. The inclusion and exclusion criteria and full case report forms for this study can be found at the Community Acquired Pneumonia Organization study site at www.caposite.com.
Exclusion Criteria
Unstable psychiatric or psychological condition rendering the subject unlikely to be cooperative or to complete the study requirements. Patients were excluded if they had a medical history that, in the investigator′s opinion, precluded subject compliance with the protocol. Immunosuppression was not part of the exclusion criteria. None of the patients included in this study was neutropenic (<1.7 × 103 neutrophils/mm3).
Healthy Control Group
In order to compare results of the plasma cytokines and neutrophil functional assays from CAP patients with those of healthy individuals, blood samples were also obtained from a control group (n = 12) of healthy adult donors (approved by the University of Louisville′s IRB #191.06).
Microbiologic Analysis
Testing of sputum samples, blood cultures, tracheal aspirates, pleural fluid; serology for respiratory viruses and atypical organisms; and urinary antigens for Legionella spp. and Streptococcus pneumoniae was performed according to standard clinical practice. The identification of microorganisms and susceptibility testing were performed according to standard methods [11].
Severity of Disease
As one of the most commonly used predictive scores [12], the PSI was used in this study to define CAP severity. Hospitalized patients were considered to have severe CAP if their PSI was Risk Class IV or V (91 points or higher). Patients with a PSI Risk Class of I–III (<91 points) were defined as having non-severe CAP.
Samples
Plasma Samples
Blood samples were obtained on the day of admission at the hospital. Venous blood was collected using sodium citrate vacutainer tubes. Following centrifugation at 300×g for 10 min, the plasma was separated by aspiration, aliquoted, and stored frozen at −80 °C until assayed.
Sputum Samples
Sputum samples were collected from a total of 15 CAP patients (7 with non-severe, 8 with severe CAP) on the day of admission to the hospital. Patients were instructed to rinse their mouth with water and collect any spontaneously-produced sputum into a 50-ml sterile disposable polypropylene centrifuge tube. The sample was kept at 4 °C and transported to the laboratory within 2 h of collection. Sputum samples were processed following the method described by Pizzichini et al. [13]. Upon receipt, sputum samples were diluted with an equal amount of a 0.1 % dithiothreitol (DTT) solution in PBS and incubated in a rocking platform for 15 min in order to digest the mucus. An equal volume of sterile saline was added followed by additional 5 min of incubation. The samples were then filtered through nylon gauze and centrifuged at 790×g for 10 min. The cell-free supernatants were then aliquoted and stored frozen at −80 °C until used for the measurement of cytokine levels.
Cytokine Measurements
The concentrations of interleukin (IL)-1β, IL-1 receptor antagonist (IL-1ra), IL-6, CXCL8 (IL-8), IL-10, IL-12p40, IL-17, interferon (IFN)γ, tumor necrosis factor (TNF)α, and CXCL10 (IP-10) in plasma and sputum samples were determined using Milliplex MAP Multiplex kits (EMD Millipore, Billerica, MA). Following thawing, plasma and processed sputum samples were centrifuged at 10,000×g for 5 min and the supernatants used in the assay according to the manufacturer′s instructions. In order to control for the potential interference of the DTT contained in the sputum samples on the measurement of the different cytokines in the Multiplex assay, we evaluated the effect of DTT by running samples of known cytokine concentrations in the presence and absence of DTT (at 0.0125 %, the same final concentration in the sputum samples). For eight out of the ten cytokines measured (IL-1β, IL-1ra, IL-6, IL-12p40, IL-17, IFNγ, TNFα, and CXCL8), there was little if any interference, with measurements in the presence of DTT being, on average, within ±25 % of controls. In the case of IL-10 and CXCL10, measurements in the presence of DTT showed average reductions of 50–60 % compared to controls. Comparisons of cytokine levels in the sputum samples between the different patient groups (severe vs. non-severe CAP) were performed only among similarly processed samples (e.g., in the presence of DTT).
Neutrophil Functional Studies
Neutrophil functional assays were performed using whole blood samples. Basal or formyl-methionyl-leucyl-phenylalanine (fMLF)-stimulated exocytosis were determined by measuring the plasma membrane expression of secretory vesicles (CD35, clone E11) and specific granules (CD66b, clone G10F5, both from BioLegend, San Diego, CA) on a FACSCalibur instrument (Becton Dickinson, Franklin Lakes, NJ). Following antibody treatment, red cells were lysed with BD red cell lysis buffer (Becton Dickinson), followed by two washes with 0.05 % sodium azide, and fixation in 1 % paraformaldehyde before analysis. Phagocytosis and phagocytosis-stimulated respiratory burst activity were measured using a flow cytometric assay based on the production of H2O2 stimulated by the phagocytosis of Staphylococcus aureus, as previously described [14]. Briefly, neutrophils (2 × 106 cells/ml) were incubated with 2′,7′-dichlorofluorescein diacetate (0.5 μM final concentration) for 10 min at 37 °C. Then, 50 μl aliquots of the cell suspension were sampled before and 10 min after the addition of opsonized, propidium iodide-labelled S. aureus (at a final concentration of 108 bacteria/ml). After fixation in 1 % paraformaldehyde, the cells were then analyzed for phagocytosis and H2O2 production by flow cytometry on a FACSCalibur instrument.
Statistics
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) and R version 2.15 (www.r-project.org). All cytokine and neutrophil data are presented as the median and intraquartile ranges. Data distribution was analyzed using the D′Agostino and Pearson omnibus normality test. Statistical comparisons between the groups were performed using the Mann–Whitney test. Correlation analyses were performed by the Spearman′s method. p values of ≤0.05 were considered statistically significant.
RESULTS
Demographic, Clinical and Laboratory Findings
A total of 40 hospitalized patients with CAP were included in the study. Non-severe and severe CAP groups were made up of 19 and 21 patients, respectively. The main demographic, medical history, and clinical/laboratory characteristics of the two groups are summarized in Tables 1 and 2. There was a statistically significant difference (p = 0.035) in the ages of the two groups, with patients in the severe CAP group having a median age of 63.5 years vs. 51.5 years for the non-severe CAP group. No differences in gender or residence at a nursing home were observed. Cerebrovascular disease, congestive heart failure, and COPD were more frequent in the severe CAP group, but there were no significant differences with other underlying conditions. Blood urea nitrogen levels (p = 0.001) and peripheral blood neutrophil counts (p = 0.022) were higher in the severe CAP group. In-hospital or 30-day mortality and re-hospitalization were not significantly different between the groups. Patients in the non-severe CAP group were more likely to have a time to clinical stability (TCS) of less than 3 days compared to the severe CAP group (83 vs. 50 %, respectively; p = 0.046).
Table 1.
Patient Group Characteristics—Medical
| Variable | Severe CAP | Non-severe CAP | p value |
|---|---|---|---|
| n = 21 | n = 19 | ||
| n (%) | n (%) | ||
| Demographics | |||
| Age > 65 | 10 (48) | 2 (11) | 0.035 |
| Age, median (IQR) | 63.5 (20) | 51.5 (7.5) | <0.001 |
| Male gender | 19 (90) | 15 (79) | 1 |
| Nursing home resident | 1 (5) | 1 (5) | 1 |
| Past medical history | |||
| Smoking | 7 (33) | 9 (47) | 0.335 |
| Neurologic diseases | 0 (0) | 3 (16) | 0.089 |
| Cerebrovascular diseases | 6 (29) | 0 (0) | 0.022 |
| Chronic heart failure | 11 (52) | 2 (11) | 0.008 |
| COPD | 11 (52) | 3 (16) | 0.049 |
| Diabetes mellitus | 8 (38) | 4 (21) | 0.322 |
| HIV infection | 0 (0) | 3 (16) | 0.083 |
| Acute renal diseases | 3 (14) | 0 (0) | 0.238 |
| Chronic renal diseases | 3 (14) | 2 (11) | 1 |
| Hepatic diseases | 2 (10) | 0 (0) | 0.49 |
| Neoplastic diseases | 4 (19) | 0 (0) | 0.11 |
| Hyperlipidemia | 11 (52) | 4 (21) | 0.104 |
| Statin therapy | 8 (38) | 3 (16) | 0.286 |
| Clinical, laboratory and radiological findings | |||
| Altered mental status | 3 (14) | 0 (0) | 0.238 |
| Pleural effusion | 9 (43) | 3 (16) | 0.165 |
| ICU admission | 10 (48) | 3 (16) | 0.09 |
| HCAP | 5 (24) | 5 (27) | 0.731 |
| Pneumococcal Pna | 5 (24) | 4 (21) | 1 |
| Macrolide therapy | 5 (24) | 9 (47) | 0.101 |
| Early TCS (<3 days) | 11 (52) | 15 (79) | 0.046 |
| Clinical outcomes | |||
| In-hospital mortality | 2 (10) | 1 (5) | 1 |
| 30 day mortality | 1 (5) | 1 (5) | 1 |
| 30 day rehospitalization | 2 (10) | 1 (5) | 1 |
COPD chronic obstructive pulmonary disease, HCAP health care-associated pneumonia, Pna pneumonia
Table 2.
Patient Group Characteristics—Laboratory Values
| Variable | Severe CAP | Non-severe CAP | p value |
|---|---|---|---|
| n = 21 | n = 19 | ||
| Median (IQR) | Median (IQR) | ||
| PaO2 (mmHg) | 67 (20.7) | 65 (9) | 0.258 |
| pH | 7.4 (0.2) | 7.4 (0) | 0.324 |
| Albumin (g/dl) | 3.5 (0.7) | 3.7 (0.7) | 0.976 |
| BNP (pg/ml) | 228.4 (454) | 42 (422) | 0.235 |
| BUN (mg/dl) | 24.5 (17.8) | 13.5 (6) | 0.001 |
| CRP (mg/l) | 6.9 (111.8) | 5.2 (90.8) | 0.397 |
| Procalcitonin (ng/ml) | 0.2 (4.9) | 0.1 (0.4) | 0.475 |
| Neutrophils (%) | 83.1 (8.9) | 76.8 (11.9) | 0.022 |
| Platelets (×103/μl) | 212 (66.5) | 242.5 (59.8) | 0.170 |
| WBC (×103/μl) | 11.9 (6.2) | 12.6 (6.8) | 0.807 |
| Temperature (oral—°F) | 99.2 (2.9) | 99.3 (1.9) | 0.902 |
| RR (breaths/min) | 22 (4.2) | 24 (7) | 0.794 |
| HR (beats/min) | 104.5 (21.2) | 101.5 (23.5) | 0.946 |
| Systolic BP (mmHg) | 122.5 (31.2) | 139.5 (50.5) | 0.37 |
| Na (mEq/l) | 137.5 (6) | 137 (4.8) | 0.702 |
| Hematocrit (%) | 37.5 (8.1) | 39.1 (5.5) | 0.849 |
| TCS (days) | 3.5 (3.8) | 2 (1) | 0.152 |
| LOS (days) | 6 (5) | 4 (2.8) | 0.442 |
| PSI (points) | 114.5 (32.5) | 59.5 (30) | <0.001 |
IQR interquartile range, PaO2 partial oxygen pressure, BNP brain natriuretic peptide, BUN blood urea nitrogen, CRP C-reactive protein, WBC white cell blood count, RR respiratory rate, HR heart rate, BP blood pressure, TCS time to clinical stability, LOS length of stay, PSI pneumonia severity index
The etiologic agent could not be identified by standard laboratory procedures in approximately half of the patients in each group (10 patients each) and in approximately a quarter of the cases in each group (5 patients), the etiologic agent was identified as S. pneumoniae (Table 3).
Table 3.
Etiologic Data
| ORGANISM | Severe CAP | Non-severe CAP |
|---|---|---|
| n (%) | n (%) | |
| No etiologic agent identified | 10 (48) | 10 (53) |
| Bacterial: | ||
| Citrobacter sp. | 1 (5) | |
| Haemophilus influenzae | 1 (5) | |
| Moraxella catarrhalis | 1 (5) | |
| S. pneumoniae | 5 (24) | 5 (26) |
| Pseudomonas aeruginosa | 1 (5) | |
| S. aureus (MSSA) | 2 (10) | |
| S. aureus (MRSA) | 1 (5) | |
| Viral: | ||
| Influenza virus A H3 | 1 (5) | |
| Coronavirus 229E | 1 (5) | |
| Protozoal: | ||
| Pneumocystis jirovecii | 1 (5) | |
MSSA methicillin-susceptible S. aureus, MRSA methicillin-resistant S. aureus
Plasma Cytokine Profiles
The plasma cytokine profiles for both groups are shown in Fig. 1 (data in table form in Supplementary material—Table 1). Generally, patients in the severe CAP group showed a pattern with median plasma concentrations of both pro- and anti-inflammatory cytokines that were higher in comparison with the non-severe CAP group and the healthy control group. The severe CAP group of patients had median plasma concentrations of IL-6, TNFα, and CXCL8 that were approximately twice as high as those of the non-severe CAP group. Moreover, while most patients in either group had undetectable concentrations of IL-1β in their plasma (≤0.1 pg/ml), when measurable, IL-1β tended to be higher in the severe CAP group. The differences in the median concentrations in the two groups were statistically significant (p < 0.05) for all of the above cytokines. No statistically significant differences were found for CXCL10 or IL-12p40. In the case of the anti-inflammatory cytokines, IL-1ra and IL-10, a similar pattern was observed, with the median concentrations for these cytokines being three to four times higher in the severe CAP group (p < 0.05). Finally, the median concentrations of adaptive immunity-related cytokines, IFNγ and IL-17, were also four to five times higher in the severe CAP group (p < 0.05).
Fig. 1.
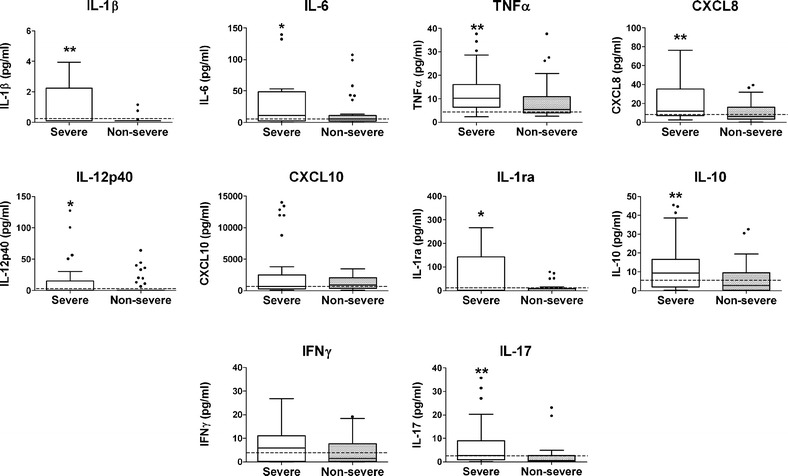
Plasma cytokine profiles in non-severe and severe CAP patient groups. Blood samples were obtained shortly after admission to the hospital and assayed for the indicated cytokines and chemokines. Box plots depict the 25–75 % interquartile range and the horizontal bar depicts the median. *p < 0.05; **p < 0.01. For comparison, the broken horizontal line represents the mean plus one SD of the values for the healthy control group. Median and IQR data are summarized in Supplementary Table 1.
Sputum Cytokine Profiles
The sputum cytokine profiles are shown in Fig. 2 (data in table form in Supplementary material—Table 2). The concentrations of cytokines in the sputum were not only much higher, but their patterns did not mirror those observed in the plasma. When related to the severity groups, the patterns in sputum were opposite to those seen in plasma for several of the cytokines, with the median values being significantly higher in the non-severe CAP group, particularly for the pro-inflammatory cytokines. For example, median concentrations of IL-1β, IL-6, and TNFα were, respectively, 18-, 3-, and 9-times higher than those in the severe CAP group (p < 0.01 for IL-1β; p < 0.05 for IL-6; and TNFα). However, no significant differences were observed for the anti-inflammatory cytokines. In the case of adaptive-immunity-related cytokines, the median concentrations of IL-17 were found to be also higher in the non-severe CAP group (p < 0.01). No differences were observed in the concentrations of IFNγ.
Fig. 2.
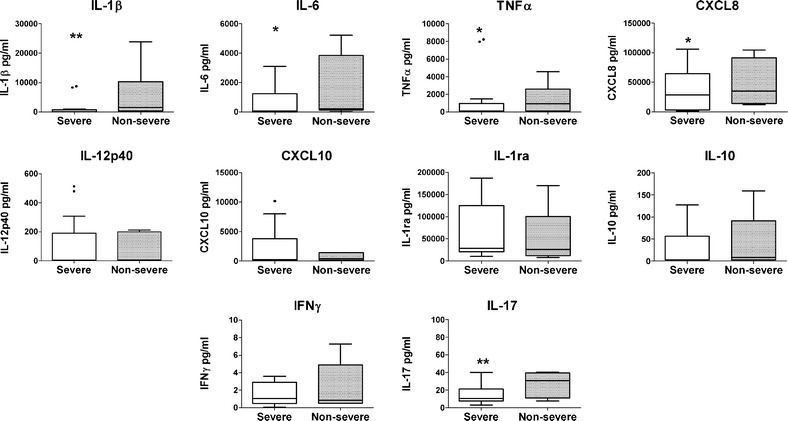
Sputum cytokine profiles in non-severe and severe CAP patient groups. Sputum samples were obtained shortly after admission to the hospital and processed and assayed for the indicated cytokines and chemokines. Box plots depict the 25–75 % interquartile range and the horizontal bar depicts the median. *p < 0.05; **p < 0.01. Median and IQR data are summarized in Supplementary Table 2.
Neutrophil Functional Studies
Table 4 shows that the basal and fMLF-stimulated expression of both granule markers CD35 and CD66b, as well as respiratory burst activity, were higher in the CAP groups (both non-severe and severe) compared to healthy donors, indicating that their neutrophils were primed/pre-activated in the CAP patients. Comparison between the non-severe and severe CAP groups showed a moderate but statistically-significant higher median basal level of CD66b, with no differences in fMLF-stimulated expression in severe CAP patients. In contrast, patients in the severe CAP group had significantly lower median levels of respiratory burst activity compared to the non-severe CAP group. Analysis of potential correlations between plasma cytokine levels and neutrophil functional studies indicated a moderate (Spearman′s r = 0.387) but statistically significant (p = 0.008) positive correlation between the neutrophil respiratory burst and plasma levels of CXCL10 when all patients′ samples were analyzed (Fig. 3).
Table 4.
Neutrophil Functional Studies
| Parameter | Control | Severe CAP | Non-severe CAP |
|---|---|---|---|
| CD35-basal | 82.0 (27.4) | 147.6 (147.6) | 122.4 (109.1) |
| CD35-stimulated | 180.6 (64.2) | 334.5 (186.3) | 399.5 (237.3) |
| CD66b-basal | 54.1 (20.7) | 71.3 (57.8)b | 55.9 (29.5) |
| CD66b-stimulated | 88.8 (38.3) | 156.2 (177.5) | 148.7 (95.7) |
| Respiratory bursta | 389.8 (192.9) | 492.6 (430.1)b | 730.8 (415.4) |
| Phagocytosis | 769.2 (593.2) | 1,064 (884.3) | 751.6 (940.5) |
Units: MFI values represent the median and interquartile range (IQR)
aPhagocytosis-stimulated
bStatistically significant differences between severe and non-severe CAP groups (p < 0.05)
Fig. 3.
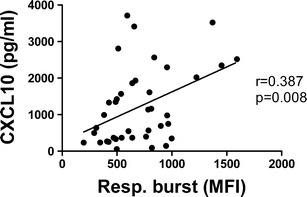
Correlation between the neutrophil respiratory burst and plasma levels of CXCL10.
DISCUSSION
This study indicates that, at the time of hospital admission, patients with severe CAP have a decreased local inflammatory response and an exaggerated systemic inflammatory response when compared to patients with non-severe CAP. This observation suggests that patients with severe CAP may have a lower and suboptimal lung inflammatory response resulting in an inability to control the microbial invasion at the local level.
In our analysis of the plasma cytokine response, we found that the patients with severe CAP presented with significantly higher levels of both pro- and anti-inflammatory cytokines, arguing against a potential skewing of the systemic response towards anti-inflammatory or immunosuppressive cytokines. Although it was expected that the higher systemic levels of pro- and anti-inflammatory cytokines would correlate with more severe pneumonia, we found that hospitalized patients with non-severe CAP had significantly higher levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNFα) and IL-17 in the sputum. Interestingly, no significant differences between the two groups were observed in the sputum concentrations of chemokines (CXCL8 and CXCL10) or anti-inflammatory cytokines (IL-1ra and IL-10). Taken together, these observations suggest that the local (sputum) pro-inflammatory response in hospitalized patients with non-severe CAP was more robust than in hospitalized patients with severe CAP.
Despite the activated status of neutrophils in hospitalized patients with CAP compared to healthy controls, neutrophils in patients with severe CAP showed moderately but significantly reduced levels of respiratory burst activity when compared to patients with non-severe CAP. This is consistent with reduced bactericidal capacity and an inability to control bacterial infection. Indeed, reduced neutrophil respiratory burst activity has been reported in cases of sepsis [15]. The causes for the reduced respiratory burst in hospitalized patients with severe CAP are not clear. Nevertheless, the positive and statistically significant correlation found between neutrophil respiratory burst activity and the systemic levels of CXCL10 is interesting. A role for ROS in the activation of signaling mechanisms, including STAT-1 phosphorylation, involved in the secretion of CXCL10 has been reported [16]. Moreover, a reduced production of CXCL10 may also correlate with decreased ability to attract T lymphocytes, particularly the Th1 subset, to the lungs, therefore further compromising the ability to adequately control infection [17].
Our study is in line with previous reports of positive associations between higher serum pro-inflammatory and anti-inflammatory cytokine levels with measures of pneumonia severity, such as the pneumonia severity index, CRB, or CRB-65 scores [7, 18–23]. However, our findings of higher pro-inflammatory cytokine levels in the sputum of non-severe vs. severe CAP patients have not been, to our knowledge, previously reported. A study by Paats et al. [23] found significantly higher levels of IL-6, CXCL8, and IFNγ in the bronchoalveolar lavage (BAL) fluids of pneumonia patients compared to healthy controls but no correlations with disease severity scores (PSI or CRB-65). However, it is not clear how closely cytokine levels in sputum correlate with those in BAL. Because of the heavy predominance of neutrophils in sputum compared to BAL, cytokine levels in the former may be more representative of a neutrophil response. A separate study by Lee et al. [21] did find significantly higher levels of TNFα in BAL from patients with severe CAP who died versus those that survived. Comparisons with our studies are not possible since all of the patients analyzed in Lee′s study had severe CAP.
Our study had relatively few numbers of patients and sputum samples, which limits the ability of our study to generalize, and prevented adjustment for confounding effects. Another potential limitation of our study was the inclusion of CAP patients with different microbial etiologies. It has been reported that different microorganisms may elicit different cytokine activation patterns in CAP with the lowest inflammatory patterns being associated with CAP of unknown etiology and the highest with S. pneumoniae and Enterobacteriaceae [24].
The use of the PSI score to define severe CAP is not without its limitations. A recent meta-analysis indicates that some of the most commonly-used scores such as PSI and CURB-65, previously derived and validated to predict 30-day mortality, have only moderate performance to predict ICU admission [12]. Moreover, it needs to be remarked that several factors influencing the PSI score may have direct effects on the inflammatory/immune response and, thus, be partially responsible for the observed results. Our two PSI-based severity groups differed significantly in terms of age and several co-morbidities. Aging is known to affect immune function at multiple levels, with both innate and adaptive immune responses being compromised with increased age [25–27]. Multiple studies, both in mice and humans, have analyzed the effect of senescence in macrophages and neutrophils, which are the main cells involved in the innate responses during CAP. Generally, macrophages and neutrophils from older individuals have been found to have deficits in the expression of Toll-like receptors (TLRs), opsonization and phagocytosis, bacterial killing, chemotactic responses, and production of cytokines and chemokines [25–27]. However, age by itself does not seem to be the only factor accounting for the differences observed in our study since comparison of elderly (>65 years old) vs. non-elderly patients showed no significant differences in neutrophil functional parameters (results not shown).
Another limitation to our study is the inclusion of patients with conditions potentially affecting their immunocompetence status. The presence of co-morbidities such as congestive heart failure, cerebrovascular diseases, and COPD, which are characterized by the presence of chronic inflammatory responses, neoplasia, and therapeutic corticosteroid use, may result not only in several defects in the function of immune cells, but also in the activation of several immunoregulatory mechanisms, including immunosuppressive cytokines and myeloid and lymphocytic regulatory cells [28]. Further studies are needed to better understand the contribution of the immune defects associated with these co-morbidities to the local lung response in CAP. In this regard, targeting of immunosuppressive mechanisms, particularly in the lung, may thus have a potential benefit for high risk CAP patients.
A common pathophysiological explanation of the inflammatory response in patients with severe CAP proposes that, in response to invading microorganisms, first there is an initial exaggerated local inflammatory response. When the excessive local inflammatory response fills the pulmonary compartment, there is a spill of cytokines and inflammation into the systemic circulation, generating the systemic inflammatory response that is associated with severe CAP. The data from our study seem to indicate that, an exaggerated local inflammatory response is not associated with severe CAP, and on the contrary, patients with severe CAP have a significantly lower pulmonary inflammatory response in relation to patients with non-severe CAP. In agreement with the literature [7, 18–23], we found that patients with severe CAP have a significantly higher level of systemic inflammation, but the initial step to reach this systemic inflammation may not be an excessive local pulmonary inflammation. It can be hypothesized that, patients who are able to produce an adequate local pulmonary response are able to contain the microorganisms, do not develop an exaggerated systemic response, and present with a non-severe CAP. If a first response at the local pulmonary level is not sufficient due to some local level of immune deficiency, then a second response at a systemic level is elicited as the last attempt to control the infection. If this paradigm were to be correct, and patients with severe CAP have dual inflammatory responses with a low pulmonary response and a high systemic response, then the therapeutic management of inflammation in patients with severe CAP should be different for the lung and for the systemic circulation.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(DOC 31 kb)
(DOC 74 kb)
Acknowledgments
Financial Disclosures
Dr. Uriarte was supported by NIH grant K99/R00 HL087924.
Footnotes
Rafael Fernandez-Botran and Silvia. M. Uriarte contributed equally to this work.
REFERENCES
- 1.Armstrong G, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. Journal of the American Medical Association. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 2.Almirall J, Bolibar I, Vidal J, Sauca G, Coll P, Niklasson B, et al. Epidemiology of community-acquired pneumonia in adults; a population-based study. European Respiratory Journal. 2000;15:757–763. doi: 10.1034/j.1399-3003.2000.15d21.x. [DOI] [PubMed] [Google Scholar]
- 3.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infection and Immunity. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolling UK, Hansen F, Braun J, Rink L, Katus HA, Dalhoff K. Leukocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax. 2001;56:121–125. doi: 10.1136/thorax.56.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends in Immunology. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. Journal of Leukocyte Biology. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 7.Antunes G, Evans SA, Lordan JL, Frew AJ. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. European Respiratory Journal. 2002;20:990–995. doi: 10.1183/09031936.02.00295102. [DOI] [PubMed] [Google Scholar]
- 8.Bordon J, Aliberti S, Fernandez-Botran R, Uriarte SM, Rane MJ, Duvvuri PD, et al. Understanding the roles of cytokines, neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. International Journal of Infectious Diseases. 2013;17:e76–e83. doi: 10.1016/j.ijid.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Chollet-Martin P, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. Subpopulation of hyperresponsive polymorphonuclear neutrophils in patients with adult respiratory distress syndrome. Role of cytokine production. American Review of Respiratory Diseases. 1992;146:990–996. doi: 10.1164/ajrccm/146.4.990. [DOI] [PubMed] [Google Scholar]
- 10.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Molecular Medicine. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balows A, Haussler WJ, Herrmann KL, et al. Manual of clinical microbiology. 5. Washington DC: American Society of Microbiology; 1991. pp. 147–150. [Google Scholar]
- 12.Marti C, Garin N, Grosgurin O, Poncet A, Combescure C, Carballo S, Perrier A. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Critical Care. 2012;16:R141. doi: 10.1186/cc11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, Gleich GJ, Dolovich J, Hargreave FE. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. American Journal of Respiratory and Critical Care Medicine. 1996;154:308–317. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 14.Ward RA, Nakamura M, McLeish KR. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. Journal of Biological Chemistry. 2000;275:36713–36719. doi: 10.1074/jbc.M003017200. [DOI] [PubMed] [Google Scholar]
- 15.Pascual C, Kerzai W, Meier-Hellmann A, Bredle D, Reinhart KA. A controlled study of leukocyte activation in septic patients. Intensive Care Medicine. 1997;23:743–748. doi: 10.1007/s001340050403. [DOI] [PubMed] [Google Scholar]
- 16.Yang C-S, Kim JJ, Lee SJ, Hwang JH, Lee C-H, Lee M-S, Jo E-K. TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal inducer and activator of transcription-1. Journal of Immunology. 2013;190:6368–6377. doi: 10.4049/jimmunol.1202574. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X, Moore TA, Newstead MW, Deng JC, Lukacs NW, Standiford TJ. IP-10 mediates selective mononuclear cell accumulation and activation in response to intrapulmonary transgenic expression and during adenovirus-induced pulmonary inflammation. Journal of Interferon and Cytokine Research. 2005;25:103–112. doi: 10.1089/jir.2005.25.103. [DOI] [PubMed] [Google Scholar]
- 18.Igonin AA, Armstrong VW, Shipkova M, Lazareva NB, Kukes VG, Oellerich M. Circulating cytokines as markers of systemic inflammatory response in severe community-acquired pneumonia. Clinical Biochemistry. 2004;37:204–209. doi: 10.1016/j.clinbiochem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y-L, Chen W, Chen L-Y, Chen C-H, Lin Y-C, Liang S-J, Shih C-M. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. Journal of Critical Care. 2010;25:176.e7–176.e13. doi: 10.1016/j.jcrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Endeman H, Meijvis SCA, Rijkers GT, van Velzen-Blad H, van Moorsel CH, Grutters JC, Biesma DH. Systemic cytokine response in patients with community-acquired pneumonia. European Respiratory Journal. 2011;37:1431–1438. doi: 10.1183/09031936.00074410. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez P, Ferrer M, Mart V, Reyes S, Martinez R, Menendez R, et al. Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Critical Care Medicine. 2011;39:2211–2217. doi: 10.1097/CCM.0b013e3182257445. [DOI] [PubMed] [Google Scholar]
- 22.Zobel K, Martus P, Pletz MW, Ewig S, Prediger M, Welte T, Bühling F. Interleukin 6, lipopolysaccharide-binding protein and interleukin 10 in the prediction of risk and etiologic patterns in patients with community-acquired pneumonia: results from the German competence network CAPNETZ. BMC Pulmonary Medicine. 2012;12:6. doi: 10.1186/1471-2466-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paats MS, Bergen IM, Hanselaar WEJJ, Groeninx van Zoelen EC, Hoogsteden HC, Hendriks RW, van der Eerden MM. Local and systemic cytokine profiles in nonsevere and severe community-acquired pneumonia. European Respiratory Journal. 2013;41:1378–1385. doi: 10.1183/09031936.00060112. [DOI] [PubMed] [Google Scholar]
- 24.Menendez R, Sahuquillo-Arce JM, Reyes S, Martinez R, Polverino E, Cilloniz C, et al. Cytokine activation patterns and biomarkers are influenced by microorganisms in community-acquired pneumonia. Chest. 2012;141:1537–1545. doi: 10.1378/chest.11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenisch C, Patrutta S, Daxböck F, Krause R, Hörl W. Effect of age on human neutrophil function. Journal of Leukocyte Biology. 2000;67:40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 26.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3(4):161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 27.Brubaker AL, RendonL JL, Ramirez L, Choudry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous would infection with advanced age. Journal of Immunology. 2013;120:1–12. doi: 10.4049/jimmunol.1201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Seminars in Cancer Biology. 2012;22:307–318. doi: 10.1016/j.semcancer.2012.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 31 kb)
(DOC 74 kb)


