Abstract
International travels and global human migration have had the unforeseen consequence of increasing the exposure of histopathologists in developed countries to the pathology of tropical infectious disease. The gastrointestinal tract (GIT) is often the primary site of infection due to the faecal-oral route of transmission and the high risk of exposure to contaminated water, food or soil when travelling to these regions. Whilst current microbiologic techniques are far more sensitive than histology in detecting infectious pathogens, the histopathologist nonetheless retains a pivotal role in diagnosing tropical GIT disease. This role entails evaluating endoscopic biopsies for any characteristic inflammatory pattern, identifying pathogens which may be present and excluding other look-alike pathologies. Recent advances in commercially available diagnostic modalities, including molecular techniques, have further broadened the scope of the histopathologist’s armamentarium. This review outlines a practical pattern-based approach to diagnosing tropical GIT infections in endoscopic material, so as to assist pathologists less familiar with this spectrum of pathology.
Keywords: Gastrointestinal tract, Tropical infections, Histopathology, Patterns
Introduction
The last 2 decades have seen a rising trend in international business and leisure travel with ecotourism, in particular, growing in popularity [1]. Many of these travel destinations are in the tropics, which provide the further allure of a warm climate. Together with the recent surge in global human migration and the fact that infectious organisms do not respect international borders, pathologists in developed countries are increasingly being exposed to a spectrum of often unfamiliar pathology [2].
The gastrointestinal tract (GIT) is a common primary site of tropical infections [3]. This is due to the direct faecal-oral route of pathogen transmission and the increased risk of ingestion of contaminated water, food, soil or organic material when visiting these regions [4]. Less frequently, skin exposure to a specific stage of a pathogen’s lifecycle in water or soil and secondary gut involvement by a systemic tropical disease can lead to GIT pathology.
This review focuses on GIT infections and provides a practical outline for a microscopic pattern-based approach to the evaluation of the endoscopic intestinal biopsy.
Epidemiological and clinical considerations
Travel to the hot and humid tropical and subtropical regions exposes the individual to a wide spectrum of infectious disease. As most of these countries are in the developing world, the traveller may further be exposed to limited infrastructure, minimal amenities and poor sanitation. These factors, combined with the unique regional infectious pathogens and, in some cases, characteristic intermediate host, place the international traveller at high risk for contracting a plethora of unusual GIT disease.
The most common tropical GIT infections are those causing diarrhoea. Visitors to developing countries are 9–151 times more likely to develop diarrhoeal illness when compared to the developed world, with up to 50% of individuals contracting traveller’s diarrhoea [4]. The highest risk is associated with visits to South America, Africa and South Asia [5, 6].
A causative microorganism is identified in less than 50% of patients with traveller’s diarrhoea [4, 7]. The commonest isolated pathogens are bacteria and parasites and, depending on the study, each represents up to two thirds of microorganisms identified [6, 8]. In that setting, viruses account for less than 15% of the pathogens (Table 1). Escherichia coli, Shigella and non-typhi Salmonella spp. are the most commonly detected bacterial pathogens, whilst giardiasis, amoebiasis and cryptosporidiosis are the most frequent parasitic infections [4, 6–8, 10, 11]. Infections caused by viruses and bacteria are mostly only of a few days duration [4]. When symptoms persist beyond 1 week, parasitic and some specific bacterial infections are usually implicated [12, 13].
Table 1.
Most frequently identified pathogens causing primary GIT infection associated with tropical travel [3, 7–11]
| Viral | Bacterial | Parasitic |
|---|---|---|
| Adenovirus | Campylobacter spp. | Ascaris lumbricoides |
| Enterovirus | Escherichia coli | Cryptosporidium parvum |
| Norwalk virus | Salmonella spp. | Entamoeba histolytica |
| Rotavirus | Shigella spp. | Giardia lamblia |
| Hookworms | ||
| Schistosoma spp. | ||
| Strongyloides stercoralis | ||
| Trichuris trichiura |
Tropical intestinal infections are usually acquired via a direct faecal-oral route, ingestion of contaminated water or foodstuffs. Schistosomiasis and most hookworm infections are exceptions, being contracted by skin exposure to parasitic larvae in contaminated water or soil. Most pathogens infect predominantly the intestines. Oesophageal involvement is extremely rare, and gastric involvement is usually only seen in disseminated or very severe parasitic infections. Interestingly, the traveller’s risk for contracting Helicobacter pylori-associated gastritis, which has an extremely high prevalence in developing countries and where co-infection with non-pylori Helicobacter spp. is frequent, is not increased [14–16].
Clinical features of GIT infections include acute or chronic watery, mucoid or bloody diarrhoea [4]. Other symptoms such as abdominal cramps and pain, nausea, vomiting, malabsorption, weight loss, intestinal haemorrhage and tenesmus are common. Finally, constipation, obstruction and even bowel perforation are reported. Associated fever usually implies an invasive infection or systemic pathogen dissemination. Children, the elderly and individuals on immunosuppressive medication have a higher risk of developing severe disease and associated complications [17]. Many individuals, however, may be entirely asymptomatic. Furthermore, detection of infections such as helminthiases may take months or even years as a result of a long incubation period (up to 12 weeks in schistosomiasis) and mostly asymptomatic clinical course.
The value of a pertinent history in diagnosing tropical GIT infections cannot be overemphasised. The patient’s presenting symptoms, recent travel history (including timing and destination), information about consumption of contaminated water, raw or undercooked foodstuffs, contact with soil and animals as well as participation in any outdoor activities (e.g. camping and swimming in natural bodies of water) ought to be ascertained, if not provided.
Pathology
Whilst current microbiologic and serologic methods are far more sensitive than microscopy in diagnosing gut infections, the histopathologist nonetheless retains a pivotal threefold role when assessing endoscopic biopsy material. This includes:
Recognition of a characteristic histologic pattern
Identification of etiologic pathogens
Exclusion of pathology demonstrating overlapping histologic features
The histology of many tropical GIT infections may be focal and/or subtle. As a result, examination of multiple histologic levels, use of appropriate special and immunohistochemical stains, and referral of biopsy material for in situ hybridisation (ISH) and polymerase chain reaction (PCR) testing may be required for a definitive diagnosis. Today, the histopathologist’s success in detecting etiologic microorganisms in biopsies has increased dramatically with advances in immunohistochemical and molecular techniques [18].
Primary tropical infections of the GIT
As infections affect predominantly the small and/or large intestine, evaluation of intestinal biopsy material has the highest diagnostic yield. The microscopic findings can be divided into several histologic patterns, namely (1) no or minimal non-specific change, (2) chronic non-destructive inflammation, often with intra-epithelial lymphocytosis, (3) acute ischaemic or pseudomembranous change, and active inflammation with a predominance of (4) neutrophils, (5) eosinophils, (6) lymphocytes and histiocytes, or (7) granulomas.
Despite overlap, these patterns serve as a useful guide for identifying the most likely offending pathogens and for excluding relevant differential diagnoses.
No or minimal non-specific change
Viral infections, cholera, enterotoxigenic (ETEC) and enteroaggregative (EAEC) Escherichia coli, giardiasis, most cases of food poisoning caused by enterotoxin-producing organisms such as Bacillus cereus and, less frequently, helminthic infections are associated with this pattern.
Predominant involvement of the small intestine causing acute watery, non-bloody diarrhoea with mild symptomatology is typical, and subsequently, the patients seldom undergo endoscopy due to the self-limiting nature of the disease. The striking exception is cholera, where the profuse watery diarrhoea may lead to severe and often life-threatening dehydration [19].
Tropical viral infections are caused by numerous viruses, including rotavirus, adenovirus, enterovirus, Norwalk virus (norovirus) and, less often, coronavirus, echovirus and astrovirus [4, 18, 20]. The role of rotavirus has diminished over the last decade due to the introduction of routine immunisation in many developed countries [21]. These GIT viral infections usually affect children and cause no significant histologic change, although villous blunting and broadening, surface epithelial vacuolisation and disarray, apoptosis, increase in lamina propria chronic inflammatory cells and lymphoid hyperplasia can be seen [22]. Infections such as those due to Norwalk virus may demonstrate an intra-epithelial lymphocytosis, but in contrast to gluten-sensitive enteropathy, it is typically mild and not associated with crypt hyperplasia. In adenovirus infection, focal epithelial apoptosis, nucleomegaly and nuclear inclusions—initially eosinophilic and later basophilic with “smudged” nuclei—may be identified and confirmed with immunohistochemistry or PCR (Fig. 1) [23].
Fig. 1.
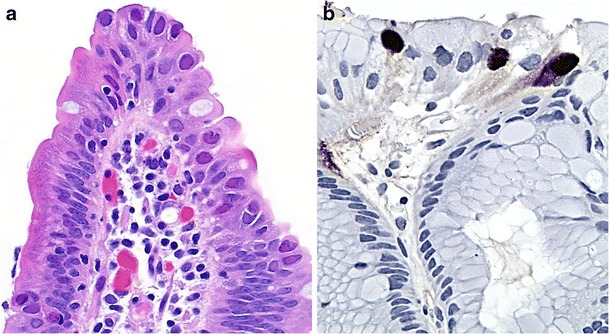
Gastrointestinal adenovirus infection. Epithelial disarray and scattered enteric epithelial intranuclear inclusions (a, ×1000). Confirmatory nuclear immunohistochemical positivity (clone 20/11 & 2/6, Cell Marque, Rocklin, California) on a stomach biopsy (b, ×1000)
Cholera, which is caused by Vibrio cholera serotype O1, can induce a profuse secretory-type diarrhoea due to the effect of the cholera toxin on enterocytes. Typically, small intestinal biopsies show no abnormality or mild non-specific changes such as mucosal edema and congestion, surface epithelial degeneration, mucin depletion and a mild lamina propria mononuclear cell increase [24].
ETEC infections, an important cause of traveller’s diarrhoea, seldom reveal any microscopic change [22]. In contrast, in EAEC infection, a layer of adherent, straight Gram-negative bacillary organisms may be identified on the surface of the large bowel epithelium [25]. These bacilli need to be differentiated from Brachyspira spp. (intestinal spirochaetosis), whose organisms have a corkscrew morphology and are positive on silver stains.
Infection with Giardia lamblia (a flagellate protozoan) often presents with acute severe foul smelling diarrhoea, whilst chronic infection may lead to persistent diarrhoea and malabsorption. Most intestinal biopsies show no histologic abnormality, but for the presence of parasites [26]. Organisms are readily identifiable on haematoxylin and eosin (H & E) staining, but are highlighted with a Giemsa stain. A periodic acid-Schiff-diastase (PAS-D) stain helps distinguish Giardia lamblia from mucin blobs. The organisms are pear- to sickle-shaped, binucleate and identified between villi, their random orientation likened to falling leaves (Fig. 2a). Trophozoite adherence to enterocytes is sometimes demonstrated (Fig. 2b). Gastric and colonic involvement occurs in severe infections, but should prompt exclusion of an underlying immune deficiency such as hypogammaglobulinaemia.
Fig. 2.
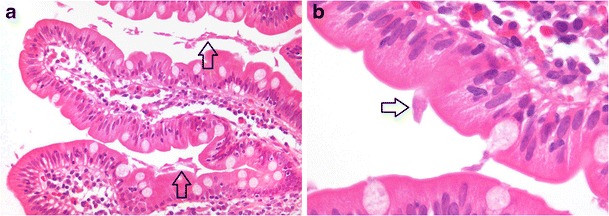
Enteric giardiasis with near normal mucosa, numerous intervillous trophozoites (a, arrows, ×200) and focal parasite attachment to the enterocyte luminal surface (b, arrow, ×1000)
Chronic non-destructive inflammation, often with intra-epithelial lymphocytosis
This pattern is characteristically associated with tropical sprue, although coccidial infections, giardiasis and some viral infections (discussed above) may be implicated. Microscopic pathology predominates in the small bowel.
Tropical sprue is an enigmatic small intestinal infectious disorder which may closely mimic coeliac disease [27, 28]. Patients usually present with malabsorption and biopsies reveal villous blunting, crypt hyperplasia, increased mucosal chronic inflammatory cells and eosinophils, as well as intra-epithelial lymphocytosis (IEL) (Fig. 3a, b) [29]. Diffuse and relatively uniform involvement of the small bowel, prominent deep (crypt) IEL and the rarity of total villous atrophy in tropical sprue allow microscopic distinction from coeliac disease and other protein hypersensitivities [30].
Fig. 3.
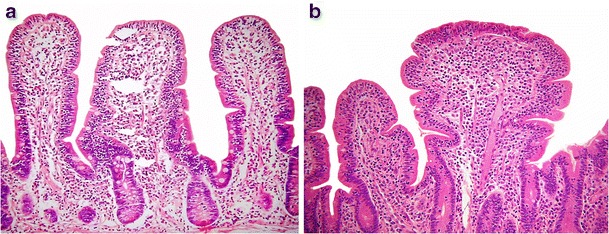
Tropical sprue showing a mucosal chronic inflammatory cell increase and villous blunting (a, ×100), as well as a striking intra-epithelial lymphocytosis (b, ×100)
Coccidiosis is caused, in order of decreasing frequency, by Cryptosporidium parvum, Cystisospora (formerly Isospora) belli, Cyclospora cayetanensis and, rarely, Sarcocystis hominis. The microsporidial organisms Enterocytozoon bieneusi and Encephalitozoon intestinalis are traditionally still included in this group, although recently reclassified as fungi [31]. The coccidians are common in tropical and subtropical countries, especially those with a high HIV burden, and are increasingly recognised as a cause of traveller’s diarrhoea [14, 32, 33]. Infection in immunocompetent patients is usually asymptomatic or mild and self-limiting. Small bowel histology may show variable degrees of villous blunting, epithelial disarray, crypt hyperplasia, patchy inflammatory cell infiltration and evidence of mild intra-epithelial lymphocytosis. Some cases may reveal active inflammation with neutrophil crypt abscesses and prominent eosinophils. A definitive diagnosis depends on demonstration of the parasite. Cryptosporidium parvum organisms are the most readily identifiable, as multiple 2- to 5-um basophilic spheres attached to the epithelial apical membrane (Fig. 4a). They stain with Warthin-Starry, Giemsa and Gram stains. Cystisospora belli organisms are identified as crescentic, elongated or round intra-epithelial forms measuring up to 20 μm and are positive on silver, Giemsa and periodic acid-Schiff (PAS) stains (Fig. 4b). Cyclospora cayetanensis are smaller, round or crescentic acid-fast organisms located in parasitophorous vacuoles in the upper part of epithelial cells (Fig. 4c) [34]. They are negative with PAS, silver and Gram stains. Microsporidial spores are the most difficult to identify, requiring careful search on Warthin-Starry, Gram and Giemsa stained sections for the small intracellular organisms, which may be confused with Paneth cell granules (Fig. 4d). Microscopic features of the predominantly zoonotic intestinal sarcosporidiasis are not well documented; small intestinal biopsy may demonstrate macrogametocyte and sporocyst parasitic forms similar in size to Cyclospora cayetanensis [35].
Fig. 4.
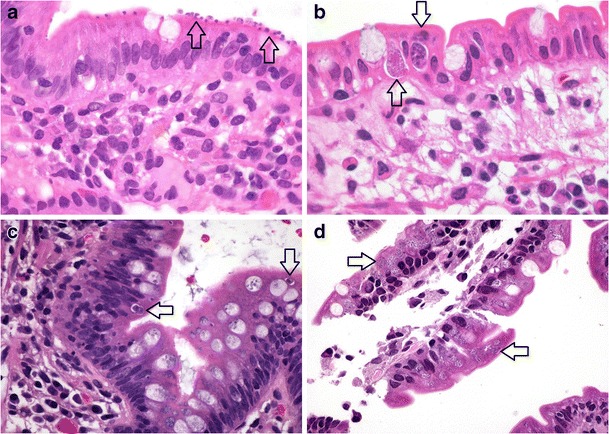
Enteric coccidial infections. Cryptosporidium parvum present as multiple small basophilic spherical organisms at the enterocyte apical surface (a, arrows, ×1000). Cystisospora belli showing epithelial disarray, with both sexual (macrogametocyte, lower arrow) and asexual (upper arrow) parasitic forms in epithelial parasitophorous vacuoles (b, ×1000). Round Cyclospora cayetanensis forms (arrows) present within enterocyte vacuoles (c, ×1000). Multiple small spherical microsporidial organisms (arrows) visible in enterocyte cytoplasm (d, ×1000)
Acute ischaemic or pseudomembranous change
Although typical of infections caused by enterohaemorrhagic Escherichia coli (EHEC), this pattern can also be seen with bacterial dysentery (especially Shigella spp.) and, rarely, amoebic dysentery (see below) [36]. The large intestine is the predominant site of involvement.
EHEC infection, particularly strain O157:H7, usually presents with a bloody diarrhoea and can lead to the haemolytic-uraemic syndrome and thrombotic thrombocytopaenic purpura [37]. Right-sided colonic involvement predominates. Endoscopic biopsies typically demonstrate acute ischaemic features, including mucosal erosion, edema and haemorrhage, crypt withering, lamina propria hyalinisation, capillary microthrombi and associated acute inflammatory exudates (Fig. 5a, b) [38]. EHEC infection must be distinguished from Clostridium difficile-associated colitis, which typically demonstrates a patchy distribution, mucin crypt distention and pronounced pseudomembrane formation with “volcano lesions”. More recently, Klebsiella oxytoca has also been documented as a cause of antibiotic-associated haemorrhagic colitis [39]. Correlation with the clinical history (including recent antimicrobial therapy), endoscopic findings as well as stool cultures and C. difficile toxin testing further help differentiate these three infections. Close clinical and endoscopic correlation helps to exclude the alternative possibilities of secondary gut involvement by systemic tropical infections associated with disseminated intravascular coagulation (DIC) and vasculopathy (see below), drugs and primary intestinal ischaemia.
Fig. 5.
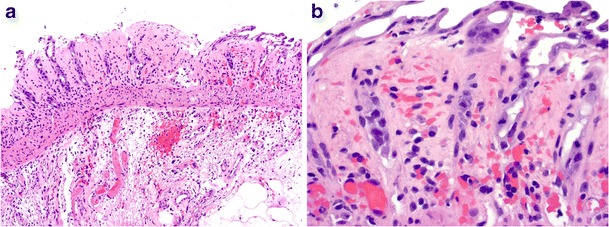
Enterohaemorrhagic Escherichia coli infection with mucosal erosion, prominent submucosal edema and fibrin thrombi (a, ×100). Note the withering crypts, lamina propria red cell extravasation and hyalinisation (b, ×400)
Active inflammation, neutrophils predominant
This pattern is characteristically associated with bacterial and amoebic dysentery.
Bacterial dysentery is caused by different organisms, including Shigella and non-typhi Salmonella spp. (50% of cases), Campylobacter spp., Yersinia spp. (especially Y.enterocolitica) and, less often, Aeromonas spp., Plesiomonas spp., enteroinvasive Escherichia coli (EIEC) and non-cholera Vibrio spp. [11, 13, 18, 40]. Patients typically present with acute bloody diarrhoea containing abundant leukocytes (pus). In the first 2 weeks, the pathology is dominated by neutrophil infiltration with acute self-limited/infectious-type colitis features. Emphasis is on superficial mucosal involvement, with patchy lamina propria neutrophil infiltration and clustering, cryptitis revealing epithelial degeneration, erosion and ulceration (Fig. 6 a–c) [41]. Later stages may have features of chronic active inflammation, however, and closely mimic idiopathic inflammatory bowel disease (IBD) (see below). The absence of pronounced crypt distortion and mucosal atrophy, surface villous change, granulomas, basal giant cells and lymphoid aggregates, a mixed lamina propria inflammatory infiltrate, as well as rarity of true crypt abscesses assist in excluding a diagnosis of IBD [42]. Histologic findings are of particular diagnostic importance in culture negative cases of bacterial dysentery [43].
Fig. 6.
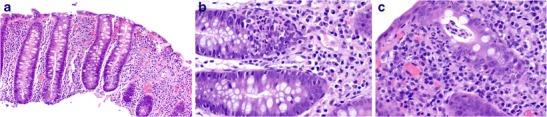
Bacterial dysentery. Colon biopsy demonstrating a preserved crypt architecture (a, ×100), prominent neutrophilic cryptitis (b, ×400) and early superficial crypt abscess formation (acute self-limited colitis) (c, ×400)
Amoebic dysentery is due to the protozoal parasite Entamoeba histolytica, although recent evidence suggests E. dispar may also be pathogenic [44]. Severe bloody diarrhoea is common, but patients may be asymptomatic or have mild and non-specific symptoms [45]. The caecum and right colon are typically affected, although involvement of the rest of the colorectum, appendix and small bowel, as well rupture with peritonitis, dissemination and metastatic abscesses (usually in the liver and lung) can occur. Amoebic dysentery is characterised by acute necrotizing inflammation, the degree of necrosis often disproportionate to the intensity of inflammation, and deep, frequently undermining (“flask-shaped”) ulcers [36]. Rarely, inflammatory mass (so-called amoeboma) formation may occur, particularly after inadequate therapy [46]. The parasite’s pale, small round nuclear morphology, intense PAS cytoplasmic positivity (due to glycogen) and negative CD68 immunoperoxidase staining distinguish it from histiocytes (Fig. 7a–d). Entamoeba spp. organisms are readily differentiated from the rarer, larger and ciliated protozoan, Balantidium coli.
Fig. 7.
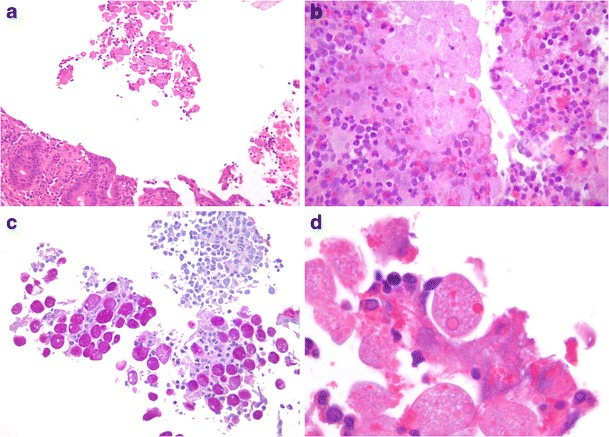
Caecal amoebic colitis. Entamoeba histolytica trophozoites may occur in small clusters (a, ×200), with associated necrotic debris and inflammatory cells, microscopically mimicking histiocytes (b, ×400). PAS staining highlights the parasites’ abundant intracytoplasmic glycogen (c, ×400). Trophozoites showing monotonous round pale nuclei and erythrophagocytosis (d, ×1000)
Active inflammation, eosinophils predominant
Parasitic infections caused by helminths (parasitic worms) and, less frequently, coccidians (discussed above) manifest with this microscopic pattern.
Helminth infections may be due to nematodes (roundworms), cestodes (flatworms) or trematodes (flukes). Ascariasis, trichuriasis, hookworm infection, strongyloidiasis and schistosomiasis are the commonest helminthiases [9]. Infections typically affect the small intestine, but spread to the large bowel, appendix and stomach can occur. Gastric involvement is most frequently seen with Strongyloides stercoralis hyperinfection, schistosomiasis and anisakiasis. The large bowel is the preferred site for enterobiasis and trichuriasis.
Biopsy findings in intestinal helminthiases include no/minimal microscopic change, mildly increased mucosal eosinophils and prominent active inflammation with abundant eosinophils, granulomas and ulceration. The features depend on the specific helminth and parasite burden. Cestode infections rarely cause any histologic abnormalities, whilst trematode infections are usually associated with pronounced inflammation. Invasive infections by nematodes cause prominent eosinophil infiltration, often present as small clusters deep in the mucosa and showing extensive degranulation. As helminthiases are rarely transient, associated inflammation may lead to chronic mucosal injury and even prominent fibrosis (see below).
The commonest nematode infections are ascariasis, trichuriasis, hookworm infection and enterobiasis (Fig. 8a). These parasites are often discovered incidentally on endoscopy or biopsy during workup for anaemia or malabsorption. In the absence of mucosal invasion, biopsy may reveal no abnormalities or minimal inflammatory changes and villous blunting. Intestinal capillariasis and Strongyloides stercoralis infection may merely reveal worms located in small bowel crypts. Invasive strongyloidiasis often leads to prominent mucosal edema, ulceration, inflammatory cell infiltration, granuloma formation and reactive mucosal hyperplasia. Infrequently, Strongyloides stercoralis autoinfection (hyperinfection) in immunocompetent patients results in a large organism burden and may affect the stomach and colon (Fig. 8b). Anisakiasis, in contrast, primarily affects the stomach and may cause pronounced inflammation with eosinophil microabscesses, necrosis, edema, haemorrhage, granuloma formation and mucosal hyperplasia.
Fig. 8.
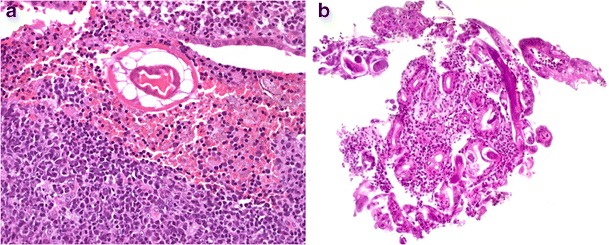
Nematode infections. Enterobius vermicularis, a roundworm also prevalent in developed countries, with characteristic cuticle, lateral alae and internal structure. The organism may be missed if associated with abundant faecal material and cellular debris (a, ×400). Gastric strongyloidiasis hyperinfection: adult nematode worms lying in gastric glands and showing associated active inflammation (b, ×200)
The commonest cestode infections are due to the adult worms of Taenia spp. and Hymenolepis nana (Fig. 9a, b). These parasites may evoke an increase in mucosal eosinophils (most often in relation to the worm and its attachment site). Sometimes, humans become an accidental intermediate host for Taenia solium. This occurs after ingestion of parasitic ova or gravid proglottids and leads to cysticercosis, the development of parasitic cysts (cysticerci, the larval stage of Taenia solium) at numerous extra-GIT sites, most commonly in skeletal muscle and the brain.
Fig. 9.
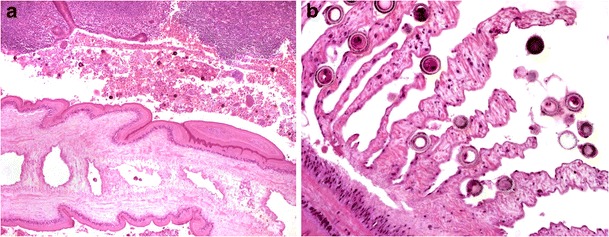
Cestode infection. Taenia spp. adult worm in the appendix lumen (a, ×100), showing internal structure of gravid proglottid with numerous characteristic thick-shelled ova (b, ×400)
Schistosomiasis is by far the commonest human trematode infection [47]. It differs from other gut helminthiases, as adult worms reside in intestinal veins and not the bowel lumen (Fig. 10a). The parasitic ova, however, are detected on biopsy and when viable, evoke an intense eosinophil-rich granulomatous response with fibrosis. The inflammation subsides when the ova degenerate and calcify. These changes may lead to mucosal granularity (sandpaper-like appearance) and even extensive polyp formation, particularly in the colorectum [48]. Table 2 summarises the worldwide distribution and ovum morphology of human schistosomes.
Fig. 10.
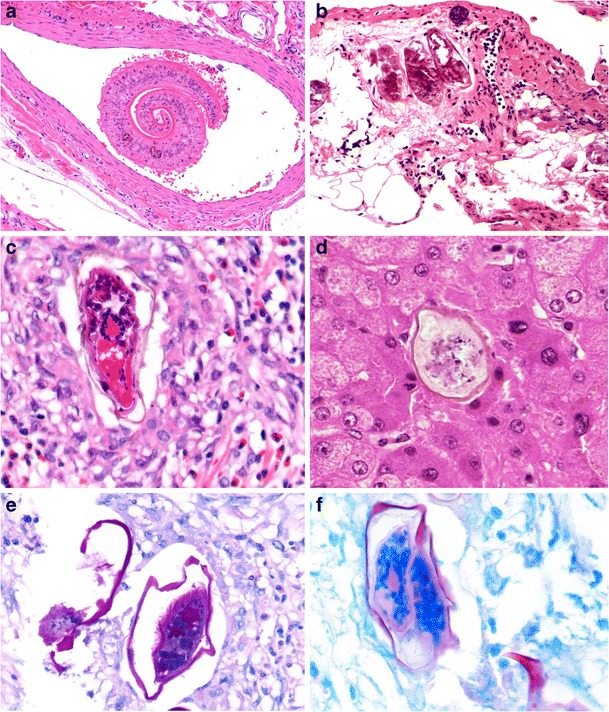
Intestinal schistosomiasis. Schistosoma mansoni worm residing in a mesenteric vein (a, ×100). Inert calcified elongated S. mansoni ova in rectal submucosa (b, ×200). Comparison of viable elongated S. mansoni ovum showing surrounding eosinophil-rich granulomatous inflammation (c, ×400) and smaller, ovoid S. mekongi ovum (in the liver) with small knob-like spine (d, ×400). S. mansoni ova showing PAS-positivity (e, ×400) and acid-fast staining of shell and spine (f, ×400)
Table 2.
Global distribution and ovum morphology of human Schistosoma spp.
| Geographic distribution | Size | Shape | Spine | |
|---|---|---|---|---|
| S. guineensis and related S. intercalatum | West and Central Africa | 140–240 × 50–85 μm | Elongated | Terminal |
| S. haematobium | Africa, Madagascar and Middle East | 110–170 × 40–70 μm | Elongated | Terminal |
| S. japonicum | China, East Asia and Philippines | 55–85 × 40–60 μm | Oval | Small lateral knob |
| S. mansoni | Africa, Middle East, parts of South America and Caribbean | 115–175 × 45–70 μm | Elongated | Lateral |
| S. mekongi | Southeast Asia, especially Mekong delta | 50–65 × 30–55 μm | Oval | Small lateral knob |
The morphology of schistosome ova can frequently be appreciated in histologic sections, but degenerative artefact and the plane of sectioning may compromise accurate evaluation (Fig. 10b–d). Whilst a Ziehl-Neelsen stain has traditionally been utilised to differentiate the ova based on the acid-fast nature of their shell and spine (the shell of S. haematobium and S. intercalatum is negative, that of S. mansoni and other species positive), this staining reaction is far from reliable (Fig. 10e, f) [49]. Although most human schistosome infections primarily affect the intestines, ova of Schistosoma haematobium (responsible for urogenital schistosomiasis) may occasionally be identified in the rectum and appendix. Human infection with predominantly zoonotic species such as S. matthei, S.malayensis and S. haematobium-S. bovis hybrid is infrequent [50, 51].
As helminths are very often not identified on biopsies, the non-infectious differential diagnoses to consider include allergic enterocolitis, primary eosinophilic gut disease, drug-induced inflammation and, in some cases, Crohn’s disease. A definitive diagnosis may require close correlation with the patient history, as well as relevant clinical, laboratory, endoscopic and microbiologic findings.
Active inflammation, lymphocytes and histiocytes predominant
This microscopic pattern is characteristic of typhoid (enteric) fever, which is caused by infection with Salmonella Typhi (previously enteritidis serovar typhi). Milder forms of the disease are caused by non-typhoid Salmonella spp. such as S. Enteritidis, Typhimurium and Paratyphi (paratyphoid).
The complex route of the Salmonella organisms through the body leads to the protean clinical manifestations of typhoid, including fever, headache, abdominal pain, skin rash, neutropaenia and a watery to bloody diarrhoea. The disease is often associated with significant systemic manifestations and may thus mimic extra-GIT/other systemic infections. The ileum, appendix and right colon are preferentially involved due to their high concentration of lymphoid tissue. Areas of ulceration, typically longitudinal along ileal Peyer’s patches, reveal prominent necrosis with a mixed infiltrate of lymphocytes, plasma cells and abundant histiocytes (Fig. 11a, b). The latter demonstrate phagocytosis of lymphocytes, erythrocytes and typhoid bacilli and are known as Mallory cells (Fig. 11c) [52]. Infiltrating neutrophils are scant, largely due to the associated neutropaenia [53, 54]. Complications are common and include bowel perforation.
Fig. 11.
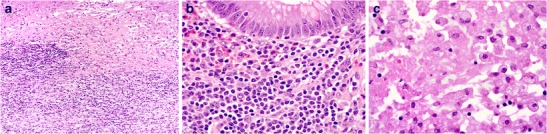
Ileocaecal involvement in typhoid fever. Ulceration with prominent necrosis (a, ×100) and associated infiltration by lymphocytes, histiocytes and plasma cells (b, ×400). Numerous histiocytes showing granular eosinophilic cytoplasm with ingestion of Salmonella spp. (c, ×400)
The histologic differential diagnoses include Whipple’s disease and secondary gut involvement by systemic infections (especially histoplasmosis and visceral leishmaniasis). The latter infections, however, demonstrate characteristic intracellular organisms and are not typically associated with prominent necrosis or ulceration. Crohn’s disease may be considered, although the necrosis, abundance of histiocytes, scant neutrophils and absence of granulomas rule out this diagnosis.
Active inflammation, granulomas predominant
Infection by Yersinia enterocolitica and Y. pseudotuberculosis (intestinal yersiniosis) is associated with this pattern, as are some helminthiases. The latter, however, are usually distinguished by their prominence of eosinophils.
Intestinal yersiniosis is an uncommon cause of traveller’s diarrhoea, although an increased risk for infection exists in patients with iron overload [55]. The organisms have a predilection for the ileum, right colon and appendix. Granulomatous inflammation is often accompanied by suppurative changes [56]. Although both species may be associated with prominent granulomas, this finding is more frequent and striking with Y. pseudotuberculosis infection. The granulomas demonstrate a prominent lymphoid cuff and often reveal central neutrophil microabscesses, associated neutrophil infiltration with mucosal ulceration (often aphthoid and in relation to lymphoid aggregates), histiocyte infiltration and prominent lymphoid hyperplasia (Fig. 12a, b) [57]. PAS, acid-fast and silver stains as well as careful clinical, endoscopic and microbiologic correlation help differentiate intestinal yersiniosis from secondary GIT involvement by mycobacterial and fungal infections (including rare cases of granulomatous histoplasmosis), as well as brucellosis. Exclusion of Crohn’s disease may be more difficult. Endoscopic evidence of mucosal cobblestoning and microscopic features of chronic mucosal injury (particularly if distant to the site of active inflammation) favour Crohn’s disease. Prominent appendiceal involvement strongly favours yersiniosis [58]. Close correlation with serology and microbiologic studies (culture and PCR) allows definitive distinction in most cases.
Fig. 12.
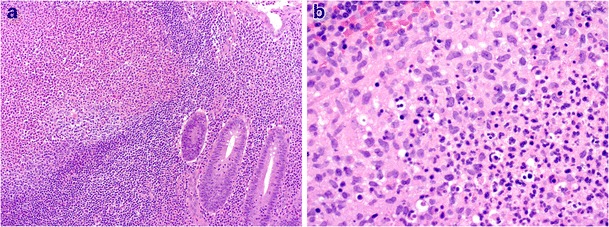
Enterocolic yersiniosis. Mucosal ulceration with lymphoid hyperplasia and acute suppurative inflammation, sometimes present in germinal centres (a, ×100). Mixed acute suppurative and granulomatous inflammation is common (b, ×400)
Histologic features of chronicity in primary tropical GIT infections
Some gut infections may lead to mucosal inflammation lasting longer than 2–4 weeks and result in microscopic evidence of chronicity (i.e. basal plasmacytosis, mucosal architectural distortion, atrophy, fibrosis and Paneth cell metaplasia). Such infections include helminthiases (e.g. schistosomiasis and strongyloidiasis), the late stage of bacterial dysentery (especially due to Shigella and non-typhi Salmonella spp), intestinal yersiniosis and, less often, other parasitic infections such as amoebiasis and coccidiosis, as well as some cases of typhoid fever [59–61]. Although the usually mild features of chronicity and characteristic associated inflammatory changes are helpful in confirming an infectious aetiology (discussed above), in some cases, distinction from idiopathic IBD may be impossible on histologic grounds alone. Careful correlation with all clinical, endoscopic, serologic and microbiologic findings, together with close follow-up, may be required for a definitive diagnosis.
Secondary GIT involvement by systemic infections
Occasionally, the gut is secondarily involved by systemic tropical infections. These patients usually present with prominent extra-GIT symptoms and the diagnosis of the systemic infection is commonly established by the time gut involvement occurs.
DIC with intestinal microvascular ischaemia and haemorrhage may be seen with viral haemorrhagic fevers (including Ebola) as well as malaria, particularly cases caused by Plasmodium falciparum [62, 63]. Zika virus infection has been associated with diarrhoea and abdominal pain, but the precise mechanism and associated gut pathology are as yet unknown [64].
Any part of the gut can be involved by the vasculitis/vasculopathy of rickettsiosis and leptospirosis, leading to diarrhoea and intestinal haemorrhage [65, 66]. Rare secondary involvement by brucellosis may mimic other granulomatous GIT disorders [67].
Gut infection by Trypanosoma cruzi (American trypanosomiasis) may cause inflammatory destruction of the myenteric plexus, but endoscopic biopsy pathology is non-specific [68]. Rare cases of secondary gut involvement by extra-intestinal parasitic infections such as sparganosis and fascioliasis have been reported [69, 70].
Conclusion
With international travel and human migration on the rise, pathologists in developed countries are increasingly being exposed to tropical infectious gastrointestinal pathology. By using a pattern-based approach to evaluate biopsy material (Fig. 13), possessing a knowledge of the most likely aetiologic pathogens and excluding look-alike, non-infectious pathology, the histopathologist retains a crucial diagnostic role despite the rapid technological advances in the field of microbiology.
Fig. 13.
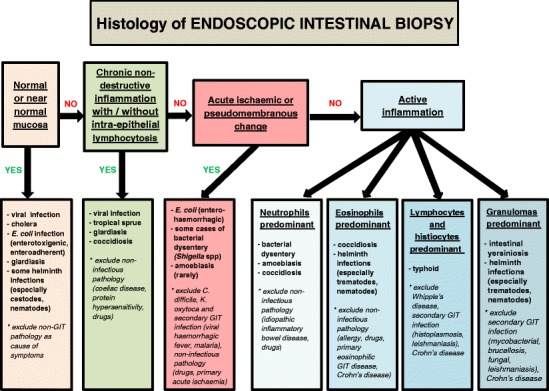
Microscopic pattern-based approach to a case of suspected tropical GIT infection
In some cases, a final diagnosis may only be possible after close collaboration between clinician, histopathologist and microbiologist. In this regard, careful correlation with the patient’s travel history and presentation, pertinent endoscopic findings, appropriate serology and stool microbiologic studies (including microscopy, culture, immunoassays and PCR investigations) is indicated. If uncertainty still exists about the diagnosis, case referral to a centre with expertise in infectious GIT pathology should always be considered. Most importantly, histopathologists in the developed world need to maintain a high level of microscopic vigilance when interpreting endoscopic biopsies in these changing times. Be warned: the GIT infection you have feared may well soon come across your table…!
Acknowledgements
The authors wish to thank Professor Joel Greenson (Department of Pathology, University of Michigan, Ann Arbor, USA) for contributing Figs. 1 and 3, Professor Rhonda Yantiss (Department of Pathology, Weill Cornell Medical College, New York, USA) for contributing Fig. 4c and d and Professor Willie van Heerden (Department of Oral Pathology, University of Pretoria, Pretoria, South Africa) for assistance with photography.
Compliance with ethical standards
The information contained in, and preparation of, this manuscript complies with the journal’s ethical standards.
Funding
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Leder K, Torresi J, Brownstein JS, et al. Travel-associated illness trends and clusters, 2000–2010. Emerg Infect Dis. 2015;19:1049–1073. doi: 10.3201/eid1907.121573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan MS, Osei-Kofi A, Omar A, et al. Pathogens, prejudice, and politics: the role of the global health community in the European refugee crisis. Lancet Infect Dis. 2016;16:e173–e177. doi: 10.1016/S1473-3099(16)30134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field V, Gautret P, Schlagenhauf P, et al. Travel and migration associated infectious diseases morbidity in Europe, 2008. BMC Infect Dis. 2010;10:330. doi: 10.1186/1471-2334-10-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher SM, McLaws ML, Ellis JT. Prevalence of gastrointestinal pathogens in developed and developing countries: systematic review and meta-analysis. J Public Health Res. 2013;2:42–53. doi: 10.4081/jphr.2013.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenwood Z, Black J, Weld L, et al. Gastrointestinal infection among international travellers globally. J Travel Med. 2008;15:221–228. doi: 10.1111/j.1708-8305.2008.00203.x. [DOI] [PubMed] [Google Scholar]
- 6.Swaminathan A, Torresi J, Schlagenhauf P, et al. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J Inf Secur. 2009;59:19–27. doi: 10.1016/j.jinf.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Zboromyrska Y, Hutardo JC, Salvador P, et al. Aetiology of traveller’s diarrhoea: evaluation of a multiplex PCR tool to detect different enteropathogens. Clin Microbiol Infect. 2014;20:O753–O759. doi: 10.1111/1469-0691.12621. [DOI] [PubMed] [Google Scholar]
- 8.Gascon J, Vila J, Valls ME, et al. Etiology of traveller’s diarrhea in Spanish travellers to developing countries. Eur J Epidemiol. 1993;9:217–223. doi: 10.1007/BF00158796. [DOI] [PubMed] [Google Scholar]
- 9.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlowski SW, Warren CA, Guerrant R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology. 2009;136:1874–1886. doi: 10.1053/j.gastro.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shakoor S, Zaidi AK, Hasan R. Tropical bacterial gastrointestinal infections. Infect Dis Clin N Am. 2012;26:437–453. doi: 10.1016/j.idc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Leder K. Advising travellers about management of travellers’ diarrhoea. Aust Fam Physician. 2015;44:34–37. [PubMed] [Google Scholar]
- 13.Vila J, Ruiz J, Gallardo F, Vargas M, Soler L, Figueras MJ, Gascon J. Aeromonas spp. and traveler’s diarrhea: clinical features and antimicrobial resistance. Emerg Infect Dis. 2003;9:552–555. doi: 10.3201/eid0905.020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slavik T. Human immunodeficiency virus–related gastrointestinal pathology: a Southern Africa perspective with review of the literature (part 1: infections) Arch Pathol Lab Med. 2012;136:305–315. doi: 10.5858/arpa.2011-0332-RA. [DOI] [PubMed] [Google Scholar]
- 15.Fritz EL, Slavik T, Delport W, Olivier B, van der Merwe SW. Incidence of Helicobacter felis and the effect of coinfection with Helicobacter pylori on the gastric mucosa in the African population. J Clin Microbiol. 2006;44:1692–1696. doi: 10.1128/JCM.44.5.1692-1696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindkvist P, Wadstrom T, Giesecke J. Helicobacter pylori infection and foreign travel. J Infect Dis. 1995;172:1135–1136. doi: 10.1093/infdis/172.4.1135. [DOI] [PubMed] [Google Scholar]
- 17.Heymann DL, editor. Control of communicable diseases manual. 19. American Public Health Association: Washington DC; 2008. [Google Scholar]
- 18.Lamps LW. Infective disorders of the gastrointestinal tract. Histopathology. 2007;50:55–63. doi: 10.1111/j.1365-2559.2006.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blacklock A, Sesay A, Kamara A, Kamara M, Blacklock C. Characteristics and clinical management of patients admitted to cholera wards in a regional referral hospital during the 2012 epidemic in Sierre Leone. Glob Health Action. 2015;8:25266. doi: 10.3402/gha.v8.25266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo NJ, Ajami ZD, Jiang FH, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010;48:1673–1676. doi: 10.1128/JCM.02072-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tate JE, Panozzo CA, Payne DC, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009;124:465–471. doi: 10.1542/peds.2008-3528. [DOI] [PubMed] [Google Scholar]
- 22.Geboes K. Inflammatory disorders of the small intestine. In: Shepherd NA, Warren BF, Williams GT, Greenson JK, Lauwers GY, Novelli MR, editors. Morson and Dawson’s gastrointestinal pathology. 5th. Chichester: Wiley-Blackwell; 2013. pp. 315–372. [Google Scholar]
- 23.Legrand F, Berrebi D, Houhou N, et al. Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplant. 2001;27:621–626. doi: 10.1038/sj.bmt.1702820. [DOI] [PubMed] [Google Scholar]
- 24.Pastore G, Schiraldi G, Fera G, Sforza E, Schiraldi O. A biopsy study of gastrointestinal mucosa in cholera patients during an epidemic in southern Italy. Am J Dig Dis. 1976;21:613–617. doi: 10.1007/BF01071953. [DOI] [PubMed] [Google Scholar]
- 25.Lamps LW. Surgical pathology of the gastrointestinal system: bacterial, fungal, viral, and parasitic infections. New York: Springer; 2009. pp. 37–42. [Google Scholar]
- 26.Oberhuber G, Kastner N, Stolte M. Giardiasis: a histologic analysis of 567 cases. Scand J Gastroenterol. 1997;32:48–51. doi: 10.3109/00365529709025062. [DOI] [PubMed] [Google Scholar]
- 27.Haghighi P, Wolf PL. Tropical sprue and subclinical enteropathy: a vision for the nineties. Crit Rev Clin Lab Sci. 1997;34:313–341. doi: 10.3109/10408369708998096. [DOI] [PubMed] [Google Scholar]
- 28.Ghoshal UC, Ghoshal U, Ayyagari A, et al. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J Gastroenterol Hepatol. 2003;18:540–547. doi: 10.1046/j.1440-1746.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathan MM, Ponniah J, Mathan VI. Epithelial cell renewal, turnover and relationship to morphologic abnormalities in jejunal mucosa in tropical sprue. Dig Dis Sci. 1986;31:586–592. doi: 10.1007/BF01318689. [DOI] [PubMed] [Google Scholar]
- 30.Cook GC. Aetiology and pathogenesis of postinfective tropical malabsorption (tropical sprue) Lancet. 1984;1:721–723. doi: 10.1016/S0140-6736(84)92231-1. [DOI] [PubMed] [Google Scholar]
- 31.Hibbett DS, Binder M, Bischof JF, et al. A higher-level phylogenetic classification of the fungi. Mycol Res. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Ackers JP. Gut Coccidia—Isospora, Cryptosporidium, Cyclospora and Sarcocystis. Semin Gastrointest Dis. 1997;8:33–44. [PubMed] [Google Scholar]
- 33.Goodgame R. Emerging causes of traveler’s diarrhea: Cryptosporidium, Cyclospora, Isospora, and Microsporidia. Curr Infect Dis Rep. 2003;5:66–73. doi: 10.1007/s11908-003-0067-x. [DOI] [PubMed] [Google Scholar]
- 34.Curry A, Smith HV. Emerging pathogens: Isospora, Cyclospora, and microsporidia. Parasitology. 1998;117:S143–S159. doi: 10.1017/S0031182099004904. [DOI] [PubMed] [Google Scholar]
- 35.Fayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis species. Clin Microbiol Rev. 2015;28:295–311. doi: 10.1128/CMR.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandt H, Tamayo RP. Pathology of human amebiasis. Hum Pathol. 1970;1:351–385. doi: 10.1016/S0046-8177(70)80072-7. [DOI] [PubMed] [Google Scholar]
- 37.Page AV, Liles WC. Enterohemorrhagic Escherichia coli infections and the hemolytic-uremic syndrome. Med Clin North Am. 2013;97:681–695. doi: 10.1016/j.mcna.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Kelly J, Oryshak A, Wenetsek M, Grabiec J, Handy S (1990) The colonic pathology of E. coli O157 : H7 infection. Am J Surg Pathol 14:87–92. [DOI] [PubMed]
- 39.Högenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. New Engl J Med. 2006;355:2418–2426. doi: 10.1056/NEJMoa054765. [DOI] [PubMed] [Google Scholar]
- 40.Giddings SL, Stevens AM, Leung DT. Traveler’s diarrhea. Med Clin North Am. 2016;100:317–330. doi: 10.1016/j.mcna.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surawicz CM. The role of rectal biopsy in infectious colitis. Am J Surg Pathol. 1988;12(Suppl 1):82–88. [PubMed] [Google Scholar]
- 42.Surawicz CM, Belic L. Rectal biopsy helps to distinguish acute self-limited colitis from idiopathic inflammatory bowel disease. Gastroenterology. 1984;86:104–113. [PubMed] [Google Scholar]
- 43.Allison MC, Hamilton-Dutoit SJ, Dhillon AP, Pounder RE. The value of rectal biopsy in distinguishing self-limited colitis from early inflammatory bowel disease. Q J Med. 1987;65:985–995. [PubMed] [Google Scholar]
- 44.Oliveira FM, Neumann E, Gomes MA, Caliari MV. Entamoeba dispar: could it be pathogenic. Trop Parasitol. 2015;5:9–14. doi: 10.4103/2229-5070.149887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Variyam EP, Gogate P, Hassan M, Costerton WJ, Pillai S, Ward H, Jalan K. Nondysenteric intestinal amebiasis. Colonic morphology and search for Entamoeba histolytica adherence and invasion. Dig Dis Sci. 1989;34:732–740. doi: 10.1007/BF01540345. [DOI] [PubMed] [Google Scholar]
- 46.Hardin RE, Ferzli GS, Zenilman ME, Gadangi PK, Bowne WB. Invasive amebiasis and ameboma formation presenting as a rectal mass: an uncommon case of malignant masquerade at a western medical center. World J Gastroenterol. 2007;13:5659–5661. doi: 10.3748/wjg.v13.i42.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenwick A. The global burden of neglected tropical diseases. Public Health. 2012;126:233–236. doi: 10.1016/j.puhe.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 48.el-Masry NA, Farid Z, Bassily S, Kilpatrick ME, Watten RH (1986) Schistosomal colonic polyposis: clinical, radiological and parasitological study. J Trop Med Hyg 89:13–17. [PubMed]
- 49.Vieira S, Belo S, Hanschied T. Ziehl-Neelsen in schistosomiasis: much more than staining the shell and species identification. Am J Trop Med Hyg. 2016;94:699–700. doi: 10.4269/ajtmh.15-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soentjens P, Cnops L, Huyse T, et al. Diagnosis and clinical management of Schistosoma haematobium-schistosoma bovis hybrid infection in a cluster of travellers returning from Mali. Clin Infect Dis. 2016;63:1626–1629. doi: 10.1093/cid/ciw493. [DOI] [PubMed] [Google Scholar]
- 51.Greer GJ, Ow-Yang CK, Yong HS. Schistosoma malayensis n. sp.: a Schistosoma japonicum-complex schistosome from Peninsular Malaysia. J Parasitol. 1988;74:471–480. doi: 10.2307/3282058. [DOI] [PubMed] [Google Scholar]
- 52.Azad AK, Islam R, Salam MA, Alam AN, Islam M, Butler T. Comparison of clinical features and pathologic findings in fatal cases of typhoid fever during the initial and later stages of the disease. Am J Trop Med Hyg. 1997;56:490–493. doi: 10.4269/ajtmh.1997.56.490. [DOI] [PubMed] [Google Scholar]
- 53.Kraus MD, Amatya B, Kimula Y. Histopathology of typhoid enteritis: morphologic and immunophenotypic findings. Mod Pathol. 1999;12:949–955. [PubMed] [Google Scholar]
- 54.Mallouh AA, Sa’di AR. White blood cells and bone marrow in typhoid fever. Pediatric Infect Dis J. 1987;6:527–529. doi: 10.1097/00006454-198706000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Thwaites PA, Woods ML (2017) Sepsis and siderosis, Yersinia enterocolitica and hereditary haemochromatosis. BMJ Case Rep. doi:10.1136/bcr-2016-218185 [DOI] [PMC free article] [PubMed]
- 56.Gleason TH, Patterson SD. The pathology of Yersinia enterocolitica ileocolitis. Am J Surg Pathol. 1982;6:347–355. doi: 10.1097/00000478-198206000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Lamps LW, Madhusudhan KT, Greenson JK, et al. The role of Y. enterocolitica and Y. pseudotuberculosis in granulomatous appendicitis: a histologic and molecular study. Am J Surg Pathol. 2001;25:508–515. doi: 10.1097/00000478-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Dudley TH, Dean PJ. Idiopathic granulomatous appendicitis, or Crohn’s disease of the appendix revisited. Hum Pathol. 1993;24:595–601. doi: 10.1016/0046-8177(93)90238-C. [DOI] [PubMed] [Google Scholar]
- 59.Qu Z, Kundu UR, Abadeer RA, Wanger A. Strongyloides colitis is a lethal mimic of ulcerative colitis: the key morphologic differential diagnosis. Hum Pathol. 2009;40:572–577. doi: 10.1016/j.humpath.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Anand BS, Malhotra V, Bhattacharya SK, et al. Rectal histology in acute bacillary dysentery. Gastroenterology. 1986;90:654–660. doi: 10.1016/0016-5085(86)91120-0. [DOI] [PubMed] [Google Scholar]
- 61.Lamps LW. Update on infectious enterocolitides and the diseases that they mimic. Histopathology. 2015;66:3–14. doi: 10.1111/his.12582. [DOI] [PubMed] [Google Scholar]
- 62.Ansari AA. Clinical features and pathobiology of Ebola virus infection. J Autoimmun. 2014;55:1–9. doi: 10.1016/j.jaut.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Beeching NJ, Fenech M, Houlihan CF. Ebola virus disease. BMJ. 2014;349:g7348. doi: 10.1136/bmj.g7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.LaBeaud AD (2016) Zika virus infection: an overview. UpToDate.http://www.uptodate.com/contents/zika-virus-infection-an-overview. Accessed 11 December 2016.
- 65.Rovery C, Brouqui P, Raoult D. Questions on Mediterranean spotted fever a century after its discovery. Emerg Infect Dis. 2008;14:1360–1367. doi: 10.3201/eid1409.071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bal AM. Unusual clinical manifestations of leptospirosis. J Postgrad Med. 2005;51:179–183. [PubMed] [Google Scholar]
- 67.Jorens PG, Michielsen PP, Van den Enden EJ, et al. A rare cause of colitis—Brucella melitensis: report of a case. Dis Colon Rectum. 1991;34:194–196. doi: 10.1007/BF02049998. [DOI] [PubMed] [Google Scholar]
- 68.Todd IP, Porter NH, Morson BC, Smith B, Friedmann CA, Neal RA. Chagas’ disease of the colon and rectum. Gut. 1969;10:1009–1114. doi: 10.1136/gut.10.12.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JH, Yu JS, Park MS, Lee SI, Yang SW. Abdominal sparganosis presenting as an abscess with fistulous communication to the bowel. AJR Am J Roentgenol. 2005;185:1084–1085. doi: 10.2214/AJR.04.1680. [DOI] [PubMed] [Google Scholar]
- 70.Hassan HA, Majid RA, Rashid NG, et al. Eosinophilic granulomatous gastrointestinal and hepatic abscesses attributable to basidiobolomycosis and fasciolas: a simultaneous emergence in Iraqi Kurdistan. BMC Infect Dis. 2013;13:91. doi: 10.1186/1471-2334-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]


