Abstract
Previous studies suggested that the otitis media (OM) complication rate of viral upper respiratory infection (vURI) is conditioned by genes affecting cytokine production. Two hundred and thirty children (114 male; 187 White, 25 Black; aged 1–9.3 years, average = 3.6 ± 1.6 years) were prospectively followed over the typical cold season for cold-like illness and OM. Nasopharyngeal secretion samples collected during cold-like illness and OM were assayed for upper respiratory viruses and buccal samples were assayed for TNFα (−308), IL-10(−1082, −819, −592), IL-6 (−174) and IFN-γ (+874) polymorphisms. Logistic regression was used to identify genotypes that predict OM coincident with RSV and rhinovirus (RV) infection. Of the 157 children with RV detection (79 male; 132 White, 13 Black, 12 Other; aged 3.6 ± 1.5 years), simple logistic regression identified age (B = −0.34, Z = −2.8, P < 0.01, OR = 0.71), IL-6 (B = −0.76, Z = −3.3, P < 0.01, OR = 0.47) and IL-10 (B = 0.49, Z = 2.0, P = 0.05, OR = 1.6) as significant predictors of OM coincidence. A more complex logistic regression model for RV detection that included selected OM risk factors identified these factors as well as the TNFα genotype, OM history, breastfeeding history and daily environment as significant predictors of OM coincidence. Of the 43 children with RSV detection (21 male; 35 White, 5 Black, 3 Other, aged 3.9 ± 1.7 years), logistic regression identified IL-10 (B = 1.05, Z = 2.0, P = 0.05, OR = 2.9) as a significant predictor of OM coincidence. New OM episodes coincident with evidence of RSV and RV infection were significantly more frequent in children with high production IL-10 phenotypes. The low production IL-6 and high production TNFα phenotypes also contributed to OM risk during RV detection. Cytokine polymorphisms may be one of an expectedly large number of genetic factors contributing to the known heritability of OM.
Keywords: RSV, Rhinovirus, Cytokine polymorphisms, Otitis media
Introduction
Otitis media (OM) is a very common disease in the pediatric population. New OM episodes can present with (acute OM, AOM) or without (OM with effusion, OME) signs and symptoms associated with bacterial infection of the middle ear [1]. OM onset as either AOM or OME is most often coincident with a cold-like illness (CLI) that is usually the symptomatic expression of viral upper respiratory infection (vURI) with rhinovirus (RV), RSV, influenza, parainfluenza, or adenovirus among others [2, 3]. Epidemiologic studies show that most OM episodes are short-lived and self-resolving with or without treatment, but that a subset persists as a long-standing asymptomatic condition, chronic OME [1, 4, 5]. Also, a subpopulation of children often labeled “otitis-prone” is characterized by numerous, repeated episodes of AOM (recurrent AOM, rAOM) [4]. A common treatment for both persistent OME and rAOM is tympanostomy tube insertion that was shown in clinical trials to be effective in resolving persistent OME and preventing recurrences of AOM [6, 7].
Twin/triplet studies document a high heritability (≈0.7) for rAOM [8–10] and for the percent of time with middle ear effusion at 3 and 5 years of age (a measure of chronic OME) [10]. Also, Daly et al. defined affected phenotype by the insertion of tympanostomy tubes (rAOM and chronic OME), and reported evidence of phenotype linkage to chromosomes 10q at marker D10S212 and 19q at marker D19S254, and conditional linkage to chromosome 3p between markers D3S4545 and D3S1259 [11].
Recognizing that pro- and anti-inflammatory cytokines modulate the host response to infection and other noxious stimuli, three reports compared the distributions of selected cytokine polymorphisms between control and otitis-prone populations. Joki-Erkkila and colleagues focused on the distributions of TNFα, IL-1α, IL-1β and IL-1ra polymorphisms and reported no between-group differences [12]. Patel et al. assayed for TNFα, IL-1β and IL-6 polymorphisms and reported significant between-group differences in the distributions of the TNFα and IL-6 genotypes [13]. More recently, Emonts et al. assayed for TNFα, IL-1 β, IL-4, IL-6, IL-8 and IL-10 polymorphisms and reported significant between-group differences in the distributions of the IL-6 and TNFα genotypes and an effect of the IL-10 genotype on AOM recurrence after pneumococcal vaccination [14].
Past studies showed that the magnitude and duration of CLIs and the complication rates of vURIs are conditioned by host produced intracellular signaling chemicals including the cytokines, IL-6, IL-8, IL-10, TNFα and INFα [15]. To determine if cytokine polymorphisms affect OM risk during established virus infections, Gentile et al. assayed for IL-6, IL-8, IL-10, TNFα and INFγ polymorphisms in 77 infants hospitalized with RSV infection. They reported that INFγ genotype predicted OM coincidence, IL-10 genotype predicted pneumonia coincidence and IL-6 genotype predicted certain measures of illness severity [16]. In adults experimentally infected with RSV, the IL-6 genotype also predicted illness magnitude [17] and similar results were observed for adults experimentally infected with RV (Doyle, unpublished data).
Together, these observations suggest that polymorphisms in the promoter regions of certain pro- and anti-inflammatory cytokines predict a child’s risk for rAOM (otitis media proneness) and an infant’s risk for OM during RSV (and other?) virus infections. In this report, the effect of selected cytokine polymorphisms on the OM coincidence of RV and RSV infections in young children is explored.
Materials and methods
The data for this report were abstracted from those available for the first 4 years of an ongoing, 5-year study designed to characterize the causal relationships among vURIs, CLIs and OM in young children. The protocol was approved by the Institutional Review Boards at the University of Pittsburgh and the University of Virginia. Families at two study sites (Pittsburgh, Charlottesville) with two children between the ages of 1 and 5 years were recruited for participation by advertisement. Exclusion criteria included the presence in either child of a serious medical condition, a medical condition that predisposes to persistent OM, a non-intact or structurally abnormal tympanic membrane, a pre-existing sensorineural hearing loss, or an inability to cooperate sufficiently with the examination and test procedures. After affirmation of willingness to participate and acquisition of written informed consent, families were entered into the study in October with an anticipated follow-up through April of that year and were reimbursed for participation. The study subjects included the two index children and any older sibs less than 10 years of age who provided assent. The study data consist of demographic information, genotypes for selected cytokine polymorphisms, weekly assignments of the presence/absence of OM and detections of upper respiratory virus in the nasopharynx by PCR assay of nasal secretions. The hypothesis tested is that the selected cytokine polymorphisms affect the coincidence of new OM episodes during new detections of upper respiratory virus.
In all children, standard demographic information (age, race, sex) and, in a subset (those enrolled at Pittsburgh in years 1–4 and at Charlottesville in years 2–4), data for previously identified OM risk factors (breastfeeding history, daily environment, number of children in the family, past history of ear disease and exposure to tobacco smoke) were collected. A buccal swab was obtained, cells were isolated, and DNA was extracted and assayed for TNFα (−308 G/A), IL-10 (−1082 G/A, −819 T/C, −592 A/C), IL-6 (−174 G/C) and IFNγ (+874 A/T) polymorphisms as previously described [16]. For TNFα (−308), the G/A or A/A genotypes were assigned the high and G/G the low producer phenotype [18]; for IL-6 (−174) the G/G and G/C genotypes were assigned the high and C/C the low producer phenotype [19, 20]; for IFNγ (+874) the T/T genotype was assigned the high, T/A the intermediate and A/A the low production phenotype [16], and for IL-10 (−1082, −819, −592) the GCC/GCC haplotype was assigned the high, GCC/ACC or GCC/ATT the intermediate and ACC/ACC, ACC/ATA or ATA/ATA the low production phenotype [21, 22].
Bilateral pneumatic otoscopy on enrolled children was scheduled at approximately weekly intervals at an “in home visit” (Pittsburgh) or at a study clinic visit (Charlottesville). At each observation time, both ears were examined by a validated otoscopist and classified as to the presence/absence of OM based on ratings of the tympanic membrane with respect to visibility, condition, position, appearance, color, vascularity, light reflex and mobility. A positive diagnosis for OM was made when middle ear effusion (with or without air/fluid level) was observed irrespective of the presence or absence of concurrent signs of ME infection. AOM was diagnosed by the presence of OM with concurrent signs of ME infection including parental report of ear pulling, otalgia, irritability and fussiness and otoscopic signs of erythema and/or white opacification (other than from scarring) of the tympanic membrane, bulging or fullness of the tympanic membrane, or otorrhea from a perforation of a previously intact tympanic membrane. OME was assigned to OM episodes without concurrent signs of infection [5]. Episodes of AOM but not OME were treated empirically with antibiotics. All examiners were blinded to parent-reported CLI, virus assay and cytokine genotype.
Nasal secretion samples were collected from the children during a parent-identified CLI episode in the child or in an enrolled sib, at the onset of a new OM episode in the child or in a sib and at random times during illness-free periods. Samples were assayed in batch for PCR detection of adenovirus, coronavirus, influenza virus (A,B), parainfluenza virus (types 1–3), RV and RSV using a protocol adapted from the commercially available Hexaplex procedure (Prodesse, Inc., Waukesha, WI) described previously [2]. Detections of the same virus within a 20-day period and without an intermittent observation of a different virus or “no detectable virus” were linked as a single virus detection.
A data map (temporal sequence for OM and overlaid virus detections) was constructed and read from entry to termination of each child’s participation. Because of the low detection frequencies for other assayed viruses and, to avoid biases associated with multiple same-virus detections for a long-standing infection and with disproportionate representation of those children with a greater number of independent same-virus detections, the reported analyses focus on the coincidence of OM for first detections of RV and RSV in each child. For each virus, the OM coincidence rate of a first virus detection was calculated as the sum over all subjects of associated new OM detections for that virus divided by the number of individuals with virus detection. Coincidence was defined as virus detection within 5 days before and 10 days after the day of onset of a new OM episode. This interval was chosen to correspond to the usual time for sampling nasal secretions during new CLI and/or OM episodes.
For each child with RV and RSV detections, the presence/absence of OM coincident with that detection was determined. Separately for RV and RSV detections, a simple logistic regression model was used to test for significant predictors of OM coincidence. There, age, sex, race and the phenotypes of the four assayed cytokines were entered as predictors. The larger number of RV detections allowed for a more complex logistic regression model that also included the OM risk factors. In the presentation of results, the format average ± standard deviation is used consistently. All statistical procedures were run using the NCSS 2000 Statistical Package (Kaysville, UT, USA).
Results
A total of 230 children (114 male; 187 White, 25 Black; aged 1.0–9.3 years, average = 3.6 ± 1.6 years), were enrolled and supplemental “risk factor” data were available for 205 of these children. RV was detected at least once in 157 children and RSV was detected in 43 children. A new OM episode was associated with 77 (49%) of the first RV detections and with 25 (58%) of the first RSV detections (P = 0.31, Fisher’s exact test). Twelve (16%) of the OM episodes associated with RV and 14 (56%) associated with RSV detection were classified as AOM (P < 0.01, Fisher’s exact test).
Table 1 reports the distributions of cytokine phenotypes for all subjects and for those with RV and RSV detections. Notably, all phenotypes were represented in these populations at reasonable frequencies for statistical analysis and, with the exception of IL-10 in the RSV subgroup, the frequencies of the phenotypes were remarkably similar among the populations. For IL-10, the low production phenotype appeared to be over-represented and the intermediate production phenotype under-represented in the RSV subgroup (P > 0.10, χ2 test). These results do not support an effect of cytokine phenotype on RSV or RV infection.
Table 1.
Distribution of cytokine production phenotypes for the total population and for those children with RV and RSV detections
| All | RV | RSV | |||||
|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | ||
| TNFα | Low | 155 | 67 | 108 | 69 | 30 | 70 |
| High | 75 | 33 | 49 | 31 | 13 | 30 | |
| IL-6 | Low | 48 | 21 | 31 | 20 | 9 | 21 |
| High | 181 | 79 | 125 | 80 | 34 | 79 | |
| IL-10 | Low | 69 | 30 | 47 | 30 | 20 | 47 |
| Intermediate | 110 | 48 | 76 | 48 | 15 | 35 | |
| High | 49 | 22 | 34 | 22 | 8 | 19 | |
| IFNγ | Low | 76 | 33 | 52 | 33 | 15 | 35 |
| Intermediate | 111 | 48 | 76 | 48 | 20 | 47 | |
| High | 43 | 19 | 29 | 18 | 8 | 19 | |
Figure 1 shows the OM coincidence for first RV and RSV detections as a function of the TNFα, IL-10, IL-6 and IFNγ phenotypes. For both viruses, the high production IL-10 phenotype and the low production IL-6 phenotype were associated with greater OM coincidences. There were no apparent differences among the TNFα and the IFNγ phenotypes with respect to that measure. For the 157 children with RV detection (79 male; 132 White, 13 Black, 12 Other; aged 3.6 ± 1.5 years), the simple logistic regression model identified age (B = −0.34, Z = −2.8, P < 0.01, OR = 0.71), IL-6 (B = −0.76, Z = −3.3, P < 0.01, OR = 0.47) and IL-10 (B = 0.49, Z = 2.0, P = 0.05, OR = 1.6) as significant predictors of OM coincidence. Similarly, for the 43 children with RSV detection (21 male; 35 White, 5 Black, 3 Other, aged 3.9 ± 1.7 years), the simple logistic regression model identified IL-10 (B = 1.05, Z = 2.0, P = 0.05, OR = 2.9) as a significant predictor of OM coincidence.
Fig. 1.
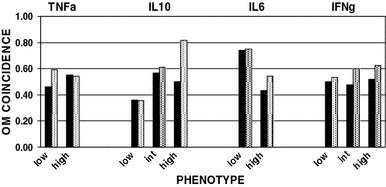
OM coincidence as a function of the TNFα, IL-10, IL-6 and IFNγ phenotypes for RV (black bars) and RSV (dotted bars) detections
For the RV subgroup, 138 children (71 male; 113 White, 13 Black, 12 Other; aged 3.5 ± 1.5 years) had supplemental data on the OM risk factors. By parent report, 41 (30%) children had a positive history for OM; the daily environment was listed as “home with the mother” for 60 (43%) children; the number of children in the family was 2 in 61, 3 in 51 and 4 or more for 19 of the children; only 20 (15%) children were exposed to tobacco smoke, and the majority of children (n = 109, 79%) were breastfed, with most (n = 74, 54%) being breastfed for 3 months or longer. Table 2 reports the significant predictors identified by the more complete logistic regression model for the coincidence of new OM episodes during first RV detection. Younger age, a past history of OM, daily environment that was “not home with the mother”, a longer period of breastfeeding, high production IL-10 and TNFα phenotypes and low production IL-6 phenotype were significant predictors of new OM episodes during RV detections.
Table 2.
Significant predictors of OM coincidence of RV detection identified by logistic regression
| Predictor | B value | SE | Z value | P value | OR |
|---|---|---|---|---|---|
| Age | −0.50 | 0.17 | −2.98 | 0.00 | 0.61 |
| Breastfeeding | 1.57 | 0.51 | 3.07 | 0.00 | 4.78 |
| Environment | 0.43 | 0.19 | 2.30 | 0.02 | 1.54 |
| OM history | 1.05 | 0.46 | 2.29 | 0.02 | 2.87 |
| IL-6 phenotype | −0.89 | 0.30 | −2.98 | 0.00 | 0.41 |
| IL-10 phenotype | 0.91 | 0.34 | 2.67 | 0.01 | 2.47 |
| TNFα phenotype | 0.49 | 0.25 | 1.93 | 0.05 | 1.63 |
B Slope, SE standard error of B, Z Wald statistic, P value probability, OR Odds ratio
Discussion
Past studies documented the influence of environmental, demographic and genetic factors on the incidence and burden of OM in infants and children [23]. Other studies convincingly demonstrated that most new OM episodes are a complication of a vURI [2, 3]. Because illness expression and complication rates of a vURI can be linked to the local production of selected chemokines and cytokines [15], one pathway by which genes could influence OM risk is via polymorphisms that control the expression of those chemical signals. This is supported by a study of hospitalized infants aged 10–191 days pp with confirmed RSV infection. The results showed a significant association between the low production IFNγ phenotype and OM at the time of hospitalization as well as associations between the high production IL-10 phenotype and pneumonia and between the low production IL-6 phenotype and measures of illness severity [16].
The current study was designed to test the hypothesis that cytokine phenotypes predict the coincidence of OM during new detections of nasopharyngeal viruses (interpretable as vURIs caused by the viruses) in young children. The assayed polymorphisms, TNFα (−308), IL-10 (−1082, −819, −592), IL-6 (−174) and IFN-γ (+874), were chosen to represent those included in the earlier report [16]. The analyses focused on the OM coincidence of RV and RSV detections because of the small number of detections for other assayed viruses which severely limited the power of statistical testing. For both RV and RSV detections, a logistic regression model that included age, race, sex and the cytokine phenotypes identified the high production IL-10 phenotype as a significant predictor of new OM episodes. The large number of RV detections allowed for a more complex logistic regression model that included selected OM risk factors. As expected from previous reports [23], younger age, a positive history of OM and a daily environment outside of the home were significant predictors of OM coincidence. Unexpectedly, a positive history of breastfeeding also predicted OM coincidence which is directionally opposed to most previous reports. However, it should be noted that the majority of children in this study were breastfed and, consequently, the effect of breastfeeding may have resulted from random distributional linkage of breastfeeding with other, unmeasured, risk factors. None-the-less, that analysis identified the high production IL-10 and TNFα phenotypes and the low production IL-6 phenotype as significant predictors of OM coincidence.
With available data, the discrepancy between the results of the current study where OM coincidence of RSV infection was driven by the IL-10 haplotype and the previous study where that coincidence was driven by the IFNγ genotype cannot be explained. One possibility is that this reflects the difference in the ages of the study populations (young infants vs. children). Specifically, past studies show a role for IFNγ in suppressing RSV infection [24] and document a shift during early infancy from a TH2 (low IFNγ) biased to a TH1 (high IFNγ) biased immune response [25, 26]. There, the higher production IFNγ phenotype in young infants with a TH2 bias may allow for sufficient IFNγ to decrease RSV complications, but be inconsequential in older children with a TH1 bias where sufficient IFNγ is constitutively produced.
Others explored the role of cytokine polymorphisms as discriminators of the otitis-prone condition. The general format of these cross-sectional studies was to identify a group of children characterized by rAOM and a control group and then to compare the distributions of the cytokine genotypes between the two. For example, Joki-Erkkila et al. compared the distribution of TNFα (−308), IL-1α (−899), IL-1β (−511) and IL-1ra polymorphisms between 63 persons in 20 families with a high occurrence of rAOM and 400 unselected blood donors of unknown OM history [12]. While they reported no significant difference in the distribution of any of the genotypes, the study design was suboptimal given the lack of an age matched control group with no OM history and the power to detect true differences was low given the small sample size of the affected population.
In contrast, Patel et al. compared the distribution of TNFα (−308), IL-1β (+3953) and IL-6 (−174) polymorphisms between groups of children with and without a history of rAOM (otitis-prone) and reported that the high production TNFα and IL-6 phenotypes were significantly more frequent in the otitis-prone group [13]. These results compare favorably with those of the present study when recognizing that Patel et al. assigned the high production IL-6 phenotype to the less common C/C genotype, while we, like others assign the low production phenotype to that genotype [16, 17, 19, 20]. Thus, like OM coincidence during RV detection, membership in the otitis-prone group was predicted by the low production IL-6 and the high production TNFα phenotypes (using our phenotype assignments).
Emonts et al. studied 348 children aged 1–7 years all of whom had a history of rAOM in the previous year by parental report and were enrolled in a pneumococcal vaccine trial and 463 unselected adult controls with unknown OM history obtained from the blood bank. Of the children, 122 had 2–3 AOM episodes and 226 had ≥4 AOM episodes (defined for purposes of the study as otitis-prone). They assayed for TNFα (−238, −308, −376, −857, −863), IL-1β (−31), IL-4 (−524), IL-6 (−174), IL-8 (781) and IL-10 (−819, −1082) polymorphisms (as well as polymorphisms in other signaling molecules) and compared all genotype distributions between affected children and adult controls, and between the two groups of children. Contrary to Patel et al. (and our data), they reported a significant overrepresentation of the IL-6 (−174) G/G genotype in the rAOM children compared to control adults (but no difference between groups of affected children) and of the TNF (−238) G/G genotype and the (−376) G/G genotype but not the (−308) A/A genotype in otitis-prone children when compared with those who had 2–3 AOM episodes (but no differences between the rAOM group and the adult controls). The results of this study are difficult to interpret for a number of reasons including the suboptimal design that lacked matched-controls of known OM history, the fact that the associated P values were very close to 0.05 and were not corrected for the numerous comparisons done on the data set (adjustment for multiple comparisons would have eliminated the significance of all findings) and the internally inconsistent results where differences between groups of affected children were not generalizable to comparisons between all affected children and controls.
In conclusion, there is sufficient evidence to advance the hypotheses that cytokine genotypes affect the coincidence of OM during vURI and that cytokine genotypes discriminate between otitis-prone and control groups of children. A variety of study designs are applicable to testing these hypotheses including those that attempt to identify discriminators of the otitis-prone condition [12–14] and/or of children with chronic OME, and those that, like the present study, focus on possible mechanisms (relating cytokine polymorphisms to a pathway of disease pathogenesis) [14, 16, 27]. However, there are a large number of cytokine polymorphisms (as well as polymorphisms in other signaling molecules [14, 28–30]), and there are no clear rules for the judicious choice of which of these polymorphisms should be included in any given study. Moreover, the complexity of the signaling pathways involved in host inflammatory responses with feedback amplification and inhibition suggests that cytokine haplotypes be included as predictors, though this is hardly practical given the very large number of possible haplotypes. Guidance in these issues is expected when the data from more detailed genetic linkage studies of OM risk available, but it should be noted that none of the cytokine genes lie in the chromosomal regions identified as containing suspected genes affecting a child’s experience with tympanostomy tube insertion [11]. None-the-less, with these caveats and adequate justification for the choice of assayed polymorphisms, continued study along the lines advanced above is likely to yield important information regarding the genetic basis for OM susceptibility.
Acknowledgments
Supported in part by NIH grant DC005832. The authors thank Ellen Mandel, Margaretha L. Casselbrant, Harriette Wheatley and Ellen Reynolds for assisting with otoscopic examinations and sample procurement; Kathleen Ashe for assisting with the virology assays, and Adriana Zeevi and Cathy Lo for assisting with cytokine genotyping.
References
- 1.Marchant CD, Shurin PA, Turczyk VA, Wasikowski DE, Tutihasi MA, Kinney SE. Course and outcome of otitis media in early infancy: a prospective study. J Pediatr. 1984;104:826–831. doi: 10.1016/S0022-3476(84)80475-8. [DOI] [PubMed] [Google Scholar]
- 2.Winther B, Alper CM, Mandel EM, Doyle WJ, Hendley JO. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119:1069–1075. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- 3.Winther B, Doyle WJ, Alper CM. A high prevalence of new onset otitis media during parent diagnosed common colds. Int J Pediatr Otorhinolaryngol. 2006;70:1725–1730. doi: 10.1016/j.ijporl.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Mandel EM, Doyle WJ, Winther B, Alper CM. The incidence, prevalence and burden of OM in unselected children aged 1–8 years followed by weekly otoscopy through the “common cold” season. Int J Pediatr Otorhinolaryngol. 2008;72:491–499. doi: 10.1016/j.ijporl.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandel EM, Rockette HE, Bluestone CD, Paradise JL, Nozza RJ. Myringotomy with and without tympanostomy tubes for chronic otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1989;115:1217–1224. doi: 10.1001/archotol.1989.01860340071020. [DOI] [PubMed] [Google Scholar]
- 7.Mandel EM, Rockette HE, Bluestone CD, Paradise JL, Nozza RJ. Efficacy of myringotomy with and without tympanostomy tubes for chronic otitis media with effusion. Pediatr Infect Dis J. 1992;11:270–277. doi: 10.1097/00006454-199204000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kvaerner KJ, Tambs K, Harris JR, Magnus P. Distribution and heritability of recurrent ear infections. Ann Otol Rhinol Laryngol. 1997;106:624–632. doi: 10.1177/000348949710600802. [DOI] [PubMed] [Google Scholar]
- 9.Casselbrant ML, Mandel EM, Fall PA, et al. The heritability of otitis media: a twin and triplet study. JAMA. 1999;282:2125–2130. doi: 10.1001/jama.282.22.2125. [DOI] [PubMed] [Google Scholar]
- 10.Casselbrant ML, Mandel EM, Rockette HE, et al. The genetic component of middle ear disease in the first 5 years of life. Arch Otolaryngol Head Neck Surg. 2004;130:273–278. doi: 10.1001/archotol.130.3.273. [DOI] [PubMed] [Google Scholar]
- 11.Daly KA, Brown WM, Segade F, et al. Chronic and recurrent otitis media: a genome scan for susceptibility loci. Am J Hum Genet. 2004;75:988–997. doi: 10.1086/426061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joki-Erkkila VP, Puhakka H, Hurme M. Cytokine gene polymorphism in recurrent acute otitis media. Arch Otolaryngol Head Neck Surg. 2002;128:17–20. doi: 10.1001/archotol.128.1.17. [DOI] [PubMed] [Google Scholar]
- 13.Patel JA, Nair S, Revai K, et al. Association of proinflammatory cytokine gene polymorphisms with susceptibility to otitis media. Pediatrics. 2006;118:2273–2279. doi: 10.1542/peds.2006-0764. [DOI] [PubMed] [Google Scholar]
- 14.Emonts M, Veenhoven RH, Wiertsema SP, et al. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics. 2007;120:814–823. doi: 10.1542/peds.2007-0524. [DOI] [PubMed] [Google Scholar]
- 15.Doyle WJ, Skoner DP, Gentile D. Nasal cytokines as mediators of illness during the common cold. Curr Allergy Asthma Rep. 2005;5:173–181. doi: 10.1007/s11882-005-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentile DA, Doyle WJ, Zeevi A, et al. Cytokine gene polymorphisms moderate illness severity in infants with respiratory syncytial virus infection. Hum Immunol. 2003;64:338–344. doi: 10.1016/S0198-8859(02)00827-3. [DOI] [PubMed] [Google Scholar]
- 17.Gentile DA, Doyle WJ, Zeevi A, Piltcher O, Skoner DP. Cytokine gene polymorphisms moderate responses to respiratory syncytial virus in adults. Hum Immunol. 2003;64:93–98. doi: 10.1016/S0198-8859(02)00705-X. [DOI] [PubMed] [Google Scholar]
- 18.Heesen M, Kunz D, Bachmann-Mennenga B, Merk HF, Bloemeke B. Linkage disequilibrium between tumor necrosis factor (TNF)-alpha-308 G/A promoter and TNF-beta NcoI polymorphisms: association with TNF-alpha response of granulocytes to endotoxin stimulation. Crit Care Med. 2003;31:211–214. doi: 10.1097/00003246-200301000-00032. [DOI] [PubMed] [Google Scholar]
- 19.Brull DJ, Montgomery HE, Sanders J, et al. Interleukin-6 gene −174 g>c and −572 g>c promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol. 2001;21:1458–1463. doi: 10.1161/hq0901.094280. [DOI] [PubMed] [Google Scholar]
- 20.Burzotta F, Iacoviello L, Di Castelnuovo A, et al. Relation of the −174 G/C polymorphism of interleukin-6 to interleukin-6 plasma levels and to length of hospitalization after surgical coronary revascularization. Am J Cardiol. 2001;88:1125–1128. doi: 10.1016/S0002-9149(01)02046-X. [DOI] [PubMed] [Google Scholar]
- 21.Kilpinen S, Huhtala H, Hurme M. The combination of the interleukin-1alpha (IL-1alpha-889) genotype and the interleukin-10 (IL-10 ATA) haplotype is associated with increased interleukin-10 (IL-10) plasma levels in healthy individuals. Eur Cytokine Netw. 2002;13:66–71. [PubMed] [Google Scholar]
- 22.Karjalainen J, Hulkkonen J, Nieminen MM, et al. Interleukin-10 gene promoter region polymorphism is associated with eosinophil count and circulating immunoglobulin E in adult asthma. Clin Exp Allergy. 2003;33:78–83. doi: 10.1046/j.1365-2222.2003.01577.x. [DOI] [PubMed] [Google Scholar]
- 23.Daly KA, Rovers MM, Hoffman HJ, et al. Recent advances in otitis media. 1. Epidemiology, natural history, and risk factors. Ann Otol Rhinol Laryngol Suppl. 2005;194:8–15. doi: 10.1177/00034894051140s104. [DOI] [PubMed] [Google Scholar]
- 24.Bont L, Heijnen CJ, Kavelaars A, et al. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J. 1999;14:144–149. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 25.Adkins B. Development of neonatal Th1/Th2 function. Int Rev Immunol. 2000;19:157–171. doi: 10.3109/08830180009088503. [DOI] [PubMed] [Google Scholar]
- 26.Protonotariou E, Malamitsi-Puchner A, Rizos D, et al. Age-related differentiations of Th1/Th2 cytokines in newborn infants. Mediators Inflamm. 2004;13:89–92. doi: 10.1080/09629350410001688468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiertsema SP, Baynam G, Khoo SK, et al. Impact of genetic variants in IL-4, IL-4 RA and IL-13 on the anti-pneumococcal antibody response. Vaccine. 2007;25:306–313. doi: 10.1016/j.vaccine.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Wiertsema SP, Herpers BL, Veenhoven RH, et al. Functional polymorphisms in the mannan-binding lectin 2 gene: effect on MBL levels and otitis media. J Allergy Clin Immunol. 2006;117:1344–1350. doi: 10.1016/j.jaci.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Wiertsema SP, Khoo SK, Baynam G, et al. Association of CD14 promoter polymorphism with otitis media and pneumococcal vaccine responses. Clin Vaccine Immunol. 2006;13:892–897. doi: 10.1128/CVI.00100-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emonts M, Wiertsema SP, Veenhoven RH, et al. The 4G/4G plasminogen activator inhibitor-1 genotype is associated with frequent recurrence of acute otitis media. Pediatrics. 2007;120:e317–e323. doi: 10.1542/peds.2006-1390. [DOI] [PubMed] [Google Scholar]


