Abstract
The aims of the present study were to determine the levels of bioaerosols including airborne culturable bacteria (total suspended bacteria, Gram-positive bacteria, Staphylococcus, Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), and Gram-negative bacteria), fungi, endotoxin, and viruses (influenza A, influenza B, respiratory syncytial virus types A/B, parainfluenza virus types 1/2/3, metapnemovirus, and adenovirus) and their seasonal variations in indoor air of residential apartments. Of the total suspended bacteria cultured in an indoor environment, Staphylococcus was dominant and occupied 49.0 to 61.3 % of indoor air. Among Staphylococcus, S. aureus were detected in 100 % of households' indoor air ranging from 4 to 140 CFU/m3, and 66 % of households were positive for MRSA ranging from 2 to 80 CFU/m3. Staphylococcus and S. aureus concentrations correlated with indoor temperature (adjusted β: 0.4440 and 0.403, p < 0.0001). Among respiratory viruses, adenovirus was detected in 14 (14 %) samples and influenza A virus was detected in 3 (3 %) samples regarding the indoor air of apartments. Adenovirus concentrations were generally higher in winter (mean concentration was 2,106 copies/m3) than in spring (mean concentration was 173 copies/m3), with concentrations ranging between 12 and 560 copies/m3. Also, a strong negative correlation between adenovirus concentrations and relative humidity in indoor air was observed (r = −0.808, p < 0.01). Furthermore, temperature also negatively correlated with adenovirus concentrations (r = −0.559, p < 0.05).
Keywords: Apartments' indoor air, Bioaerosols, Staphylococcus aureus, Methicillin-resistant S. aureus, Adenovirus, Seasonal influence
Introduction
Microorganisms are present everywhere in our environment: water, air, soil, plants, animals, and humans. When associated with air, they are defined as “airborne microorganisms” or “bioaerosols” (Di Giulio et al. 2010). Bioaerosols are generally complex mixtures that may include living organisms (bacteria, viruses, and fungi) as well as their fragments and toxins (Srikanth et al. 2008). They are generally recognized to cause various health effects such as infectious diseases, allergic reactions, and toxic manifestations (Kim and Kim 2007). Most airborne infectious diseases are caused by viruses (e.g., influenza, common colds, and measles). A few result from bacterial and fungal infection (e.g., tuberculosis and aspergillosis) (Burge 2001).
Hypersensitivity pneumonitis or extrinsic allergic alveolitis is an inflammatory airway disease caused by an unusual immune response to antigens like the cell wall components of some bacteria or fungi (Srikanth et al. 2008). Also, bacteria and fungi produce potent toxins such as endotoxin and mycotoxin that can have serious health effects including cancer, damage to the immune systems, and central nervous system malfunctions.
Exposure to bioaerosols continues to be of increasing interest to the community of those interested in indoor air quality and human health. Bioaerosols are spread easily through closed environments such as homes, schools, workplaces, and transport systems. However, most studies in the past on the monitoring of bioaerosols mainly focused on tracking of nosocomial infections (Elston and Barlow 2009; Kim et al. 2010; Sudharsanam et al. 2012).
On average, we are in our homes about 60 % of the time and are exposed to various bioaerosols. Bioaerosols in residential spaces can originate from outdoor air or from anthropogenic sources such as residents and their activities (Aydogdu et al. 2010). The most studied bioaerosols are airborne bacteria and fungi in residential spaces, and most are harmless and reside normally on the skin and mucous membranes of humans and other organisms.
Some reviewed studies have reported that the predominant bacteria species were Micrococcus spp., Staphylococcus spp., and Bacillus spp., and Penicillium spp., Aspergillus spp., and Cladosporium spp. were the most abundant mold genera in indoor air (Aydogdu et al. 2010; Gorny and Dutkiewicz 2002; Mentese et al. 2009). But recently, a study reported that Staphylococcus aureus (S. aureus) were present in indoor bioaerosol of general homes, which is considered to be one of the most common pathogens for outbreaks of food poisoning and an important cause of skin infections and invasive diseases (Lowy 1998). S. aureus is also the most prevalent cause of hospital- and community-acquired blood stream, skin and soft tissue, and lower respiratory infections (Diekema et al. 2001). Moreover, identified indoor S. aureus were particularly resistant to common antibiotics such as ampicillin, penicillin, and cefaclor (Gandara et al. 2006). Especially, methicillin-resistant S. aureus (MRSA) has become a significant and increasing cause of nosocomial infections and one of the most prevalent pathogens worldwide, with a significant economic impact on healthcare systems (Rhee and Woo 2010).
According to a report conducted by the National Health and Nutrition Examination Survey (NHANES) in 2001–2004, it is said that 28.6 to 32.4 % of healthy people were colonized in the nose with S. aureus, and 0.8 to 1.5 % were colonized with MRSA (Gorwitz et al. 2008). Direct human-to-human transmission via skin contact is one way for S. aureus and MRSA to spread, and transmission via environmental contamination of fomites or through air is another potential way that the organism can be acquired (Smith and Moritz 2010).
Viruses are also probably the most common cause of infectious disease acquired within indoor environments. Viruses that cause colds, bronchiolitis, influenza, and other respiratory tract infections can be spread through aerolized droplets (Barker et al. 2001). Over 200 different viruses can be the cause of respiratory infections. Airborne viruses that are commonly associated with these infections include respiratory syncytial virus, influenza, parainfluenza, coronavirus, and adenoviruses (Ciencewicki and Jaspers 2007). However, there is only a limited amount of information currently available on the exposure to bioaerosols in apartments (Lee and Jo 2006). Especially, no studies have examined indoor levels of S. aureus, MRSA, and viruses in residential apartment buildings in Korea.
With regard to housing types in Korea, 45 % are single-family homes, 42 % are apartments, and 13 percent are categorized into other types. Most Koreans tend to prefer apartments because of the convenience associated with apartment life. Approximately 20 million people live in apartment buildings in Korea.
The aims of the present study were to determine the levels of bioaerosols including respirable S. aureus, MRSA, and viruses, and their seasonal variations regarding the indoor air of apartments. Moreover, the correlation between bioaerosols and the other monitoring items were evaluated using multiple linear regression analyses to verify the factors that affect the indoor air quality in apartments.
Materials and methods
Study site
This study was carried out from April 2010 to February 2011. In total, 25 households in high-rise apartment buildings were selected in the metropolitan area, Seoul and Kyonggi Province. All heads of households were requested to express agreement on the environmental sampling program and signed an informed consent to participate in the study. Prior to sampling, each home was inspected by a trained environmental health technician to document the type and condition of the house. Home characteristics are shown in Table 1.
Table 1.
Characteristics of the apartments involved in this study (n = 25)
| Characteristics | Division | Households, n (%) |
|---|---|---|
| Location | Seoul | 12 (48.0) |
| Gyeonggi Province | 13 (52.0) | |
| Number of family members | 2 | 4 (16.0) |
| 3–4 | 17 (68.0) | |
| 5–6 | 4 (16.0) | |
| Apartment area (m2) | 80–105 | 9 (36.0) |
| 110–125 | 8 (28.0) | |
| 125–215 | 9 (36.0) | |
| Period of residence | Less than 1 year | 6 (24.0) |
| 1–3 years | 8 (32.0) | |
| More than 3 years | 11 (44.0) | |
| Ventilation type | Natural | 13 (52.0) |
| Air purifier | 12 (48.0) | |
| Pets | Yes | 10 (40.0) |
| No | 14 (60.0) |
Air sampling and preparation
Bioaerosols investigated in this study were airborne culturable bacteria (total suspended bacteria, Gram-positive bacteria, and Gram-negative bacteria), fungi, endotoxin, and viruses. Among Gram-positive bacteria, Staphylococcus, S. aureus, and MRSA were measured in all indoor and outdoor air samples. The viruses investigated included those that cause respiratory diseases (influenza A/B, respiratory syncytial virus A/B, parainfluenza virus types 1/2/3, metapnemovirus, and adenovirus). On the other hand, volatile organic compounds, such as benzene, toluene, xylene, stylene, and formaldehyde, were measured in all apartments.
Air samplings were carried out every season: April to June for spring, July to September for summer, October to November for autumn, and January to February for winter. Sampling took place between noon and 6 p.m. and during normal human activities and habits. All indoor air sampling for bioaerosols were conducted simultaneously in a living room at a place well away from known or suspect sources. Simultaneously, outdoor air samples were taken 5–10 m away from the apartment complexes. All samples were collected in duplicate. During air sampling, temperature (°C) and the relative humidity (%) were measured with a portable weather station (SATO SK-L200T, Japan).
Airborne culturable bacteria and fungi
Airborne culturable bacteria and fungi were collected with an impactor sampler (MAS-100, Merck, Darmstadt, Germany) consisting of a plate containing 400 holes with a cut diameter of 0.74 μm. Air samples were nominally collected at a flow rate of 100 L/min for 2 min at a height of 1.5 m above ground level to coincide with the residents' breathing zone.
As nutrient media in petri dishes located on the air sampler, tryptic soy agar (Difco, USA), phenylethanol agar (5 % sheep blood), Mannitol salt agar (Difco, USA), MacConkey agar (Difco, USA), and Sabouraud Dextrose Agar (Difco, USA) were used for sampling total suspended bacteria, Gram-positive bacteria, Staphylococcus species, Gram-negative bacteria, and fungi, respectively. CHROMagar™Staph aureus, CHROMagar™MRSA, and CHROMagar™ECC (CHROMagar Paris, France) were applied for S. aureus, MRSA and coliform. Total suspended bacteria, S. aureus, MRSA, coliform, and Gram-negative bacteria were incubated at 37 °C for 48 h. Gram-positive bacteria were incubated at 37 °C for 48 h on the condition of 5 % CO2, and fungi were incubated at 25 °C for 72 h. Colony forming units (CFU) on each plate were counted by direct observation and recorded as total CFUs per plate per cubic meter (CFU/m3).
Respiratory viruses
Airborne viruses were collected with an SKC Biosampler (Eighty Four, PA, USA). The Biosampler impinger was filled with 20 ml of phosphate-buffered saline (PBS). The Biosampler was run at a sampling airflow of 12.5 L/min. The PBS in the SKC Biosampler was vortexed for 15 s, and 1.5 mL was transferred to a 1.7 mL Eppendorf tube.
Viral RNA was isolated from collected aerosol samples using the MN (Macherey-Nagel, Germany) Viral RNA Isolation Kit. RNA was immediately transcribed into cDNA using RevertAid First Strand cDNA Synthesis Kit (Fermentas, Lithuania). cDNA was synthesized according to the kit manufacturer's instruction in a Thermal Cycler MJ Mini (Bio-Rad, USA). The PCR was performed in multiplex formats for all targets in a total reaction volume of 20 μL. To perform PCR reaction, Seeplex RV6 detection kit (Seegene, Korea) was used. Amplification was carried out under the following conditions using Thermal Cycler MJ Mini (Bio-Rad, USA): 40 cycles of 30 s at 94 °C, 120 s at 60 °C, and 120 s at 72 °C. The products were separated by electrophoresis in a 2 % agarose gel.
Among the virus-positive samples, we performed quantification by qPCR. The qPCR was performed with CFX 96 Real-Time System (Bio-Rad, USA) as follows: 20 s at 95 °C (initial denaturation), 5 s at 95 °C (amplification), and 20 s at 60 °C (extension). To determine the relative viral genome copy, a standard curve was generated from 10-fold serial dilutions of the Adeno standard template stock solution (5 × 108/ μL) and analyzed concurrently with all qPCR reactions. A negative control without template was also included in all real-time PCR reactions. All reactions were run in duplicate and averaged.
Endotoxin
Dust samples for endotoxin were collected at a flow rate of 20 L/min for 2 h by a cassette sampler mounted on a glass fiber filter (37 mm, SKC). Collected glass fiber filters were analyzed by the kinetic turbidimetric method.
Statistical method
The statistical analyses were performed using the SPSS program version 20 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov and Shapiro–Wilk statistical tests were employed to evaluate the normality of the data. Analysis of variance (ANOVA) was used for the comparison of seasonal concentrations of all culturable bioaerosols and endotoxin. A paired t test was employed for the comparison of the bioaerosol data sets of the indoor and outdoor air. Meanwhile, nonparametric test (Wilcoxon rank sum test) was performed to evaluate the differences of bioaerosols of two seasons (summer and winter).
Pearson's correlation coefficient was used to study the relationship among the microbial parameters analyzed. The correlation between bioaerosols and the other monitoring items were evaluated using multiple linear regression analyses to verify the factors that affect the indoor air quality in apartments. The criterion for significance in the procedures was p < 0.05.
Results
Airborne culturable bioaerosol and endotoxin
Table 2 presents the seasonal concentrations for culturable bioaerosol and endotoxin in indoor and outdoor air. The distribution of the airborne culturable bacteria (total suspended bacteria, Gram-positive bacteria, Staphylococcus, and S. aureus), fungi, and endotoxin were skewed to the right; therefore, the natural logarithm of the total concentration was used in all analyses.
Table 2.
Seasonal distributions of culturable bioaerosols and endotoxin levels in indoor and outdoor air of apartments (n = 25)
| Bioaerosols | Spring | Summer | Autumn | Winter | F value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | O | I | O | I | O | I | O | I | O | |
| Total suspended bacteria (CFU/m3)a | 383 | 174 | 673 | 139 | 547 | 195 | 280 | 76 | 10.134* | 5.964* |
| Gram-positive bacteria (CFU/m3)a | 251 | 50 | 378 | 36 | 335 | 46 | 106 | 26 | 12.528* | 3.660* |
| Staphylococcus (CFU/m3)a | 154 | 37 | 230 | 31 | 143 | 35 | 52 | 22 | 7.268* | 1.169 |
| S. aureus (CFU/m3)a | 45 | 11 | 45 | 7 | 27 | 9 | 27 | 9 | 7.406* | 1.739 |
| Methicillin-resistant S. aureus (CFU/m3)b | 2 | 1 | 11 | 3 | 9 | 2 | 6 | 2 | – | – |
| Gram-negative bacteria (CFU/m3)b | 8 | 3 | 3 | 5 | 5 | 4 | 0 | 0 | – | – |
| Coliform (CFU/m3)b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – |
| Fungi (CFU/m3)a | 180 | 210 | 219 | 203 | 177 | 231 | 96 | 76 | 11.981* | 17.194* |
| Endotoxin (EU/m3)a | 0.260 | 0.235 | 0.214 | 0.127 | 0.470 | 0.419 | 0.209 | 0.149 | 6.106* | 8.712* |
CFU colony forming unit, EU endotoxin unit, I indoor air, O outdoor air
*p < 0.05 by ANOVA test
aGeometric mean
bArithmetic mean
Seasonal concentrations of all culturable bioaerosols and endotoxin in indoor and outdoor air except Staphylococcus and S. aureus in outdoor air showed significant differences (p < 0.05). Geometric mean (GM) culturable bacterial concentrations (total suspended bacteria, Gram-positive bacteria, and Staphylococcus) observed in indoor air during summer were significantly higher than GMs for spring and winter (p < 0.05). The indoor/outdoor concentration ratios (I/O) for culturable bacteria, total suspended bacteria, Gram-positive bacteria, and Staphylococcus in summer were 4.84, 10.50, and 7.42, respectively. Whereas the I/O ratio for fungi was 1.08 in summer and ranged from 0.86 to 1.31 during all seasons. Of the total suspended bacteria cultured in the indoor environment, Gram-positive bacteria were occupied with 37.9 to 65.5 % through all seasons, but outdoor air showed the range from 23.6 to 34.2 %. Among the Gram-positive bacteria, Staphylococcus was dominant and occupied 49.0 to 61.3 % of indoor air and 74.0 to 86.1 % of outdoor air. Among Staphylococcus, S. aureus were detected in 100 % of households' indoor air ranging from 4 to 140 CFU/m3, and 66 % of households were positive for MRSA ranging from 2 to 80 CFU/m3. Gram-positive bacteria were present in less than 4 % of indoor and outdoor air. Among Gram-negative bacteria, coliform was cultured in neither indoor nor outdoor air during all seasons.
The seasonal variations in the temperature and relative humidity for indoor and outdoor air during the sampling period are shown in Table 3. The temperature range for the year was 20.6–29.1 °C in indoor air. The relative humidity for the year in both indoor and outdoor air was 24.7–69.2 %.
Table 3.
Seasonal temperature and relative humidity for 25 apartments according to seasons
| Seasons | Temperature (°C) | Relative humidity (%) | ||
|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | |
| Spring | 26.2 ± 2.3a | 25.9 ± 3.0 | 46.9 ± 8.6 | 42.1 ± 14.1 |
| Summer | 29.1 ± 1.8 | 29.0 ± 3.3 | 68.5 ± 7.6 | 69.2 ± 12.0 |
| Autumn | 22.7 ± 3.6 | 15.1 ± 1.2 | 39.8 ± 9.2 | 44.2 ± 8.5 |
| Winter | 20.6 ± 2.8 | 7.7 ± 3.08 | 24.7 ± 9.5 | 28.7 ± 8.9 |
aArithmetic mean ± standard deviation
Table 4 presents the factors affecting the indoor bioaerosol concentrations by multiple regression analysis which was performed to ascertain the effect of temperature and humidity. Increases in humidity as well as temperature were both associated with significantly higher culturable bacteria and fungi concentrations in indoor air. Total suspended bacteria and Gram-positive bacteria concentrations correlated with indoor humidity (adjusted β: 0.416, p < 0.0001), and Staphylococcus and S. aureus concentrations correlated with indoor temperature (adjusted β: 0.4440 and 0.403, p < 0.0001). Whereas fungi concentrations were associated with outdoor temperatures (adjusted β: 0.425, p < 0.0001).
Table 4.
Variables affecting bioaerosol concentrations in indoor air by multiple linear regression analysis
| Bioaerosol | Variables | Unstandardized coefficient (β) | Standardized coefficient (β) | Adjusted R 2 | p value |
|---|---|---|---|---|---|
| Total suspended bacteria | Indoor humidity | 0.018 | 0.416 | 0.164 | <0.0001 |
| Gram-positive bacteria | Indoor humidity | 0.021 | 0.416 | 0.165 | <0.0001 |
| Staphylococci | Indoor temperature | 0.126 | 0.440 | 0.185 | <0.0001 |
| S. aureus | Indoor temperature | 0.086 | 0.403 | 0.154 | <0.0001 |
| Fungi | Outdoor temperature | 0.028 | 0.425 | 0.172 | <0.0001 |
VOCs and formaldehyde concentrations showed independent associations with the bioaerosols (data not shown). All home characteristics shown in Table 1 demonstrated no significant association with the bioaerosols.
Virus
Table 5 presents the seasonal virus detection results in indoor air. Among investigated respiratory viruses, both adenovirus and influenza A virus were detected in a total of 100 aerosol samples in indoor air by multiplex polymerase chain reaction. Adenovirus was detected in 9 (36 %) of 25 aerosol samples in spring and in 4 (15 %) during the winter. Influenza A virus was only detected in three samples in winter. Other viruses, influenza B virus, parainfluenza virus, metapneumovirus, and respiratory syncytial virus, were not detected in all collected aerosol samples. Among the virus-positive samples, we performed adenovirus quantification by real-time polymerase chain reaction.
Table 5.
Number of positive aerosol samples for each type of respiratory viruses in indoor air (n = 25) by multiplex PCR
| Positive viruses | No. (%) of samples | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| Positive for human adenovirus | 9 | ND | 1 | 4 |
| Positive for influenza A | ND | ND | ND | 3 |
| Positive for influenza B | ND | ND | ND | ND |
| Positive for respiratory syncytial virus A/B | ND | ND | ND | ND |
| Positive for parainfluenza virus 1/2/3 | ND | ND | ND | ND |
| Positive for metapnemovirus | ND | ND | ND | ND |
| Detection rate (%) | 36.0 | 0 | 4.0 | 28.0 |
ND not detected
Figure 1 shows quantification result for adenovirus during spring, autumn, and winter in indoor air. Adenovirus concentrations were generally higher in winter (mean concentration was 2,106 copies/m3) than in spring (mean concentration was 173 copies/m3), with concentrations ranging between 12 to 560 copies/m3.
Fig. 1.
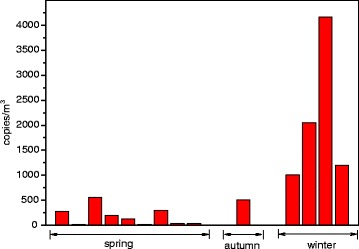
Aerosol concentrations of adenovirus detected in indoor air of apartments
Discussion
This study was carried out to determine the bioaerosols and endotoxin concentrations of the indoor and outdoor environment of residential apartments in Korea. Figure 2 presents the distribution of seasonal total suspended bacteria compared with categories of contamination indicated in the guidelines of Commission of European Communities for non-industrial indoor environments (CEC 1993). Nineteen (76 %) samples in summer and 14 (56 %) samples in autumn demonstrated high-level bacterial contamination, respectively. At 20 (80 %) sampling points in winter and 17 (68 %) in spring, however, the loads were intermediate level corresponding to 100–500 CFU/m3. The present data on what we obtained regarding the concentrations of culturable bacteria are similar to those revealed by a number previous indoor air studies in Korea and European countries (Gorny and Dutkiewicz 2002; Lee and Jo 2006).
Fig. 2.
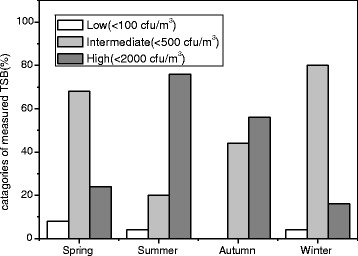
Distribution of seasonal total suspended bacteria compared with categories of contamination indicated in the guidelines of Commission of European Communities
The Korean government had established that the standard concentration of airborne bacteria in indoor air quality for public use facilities must not exceed 800 CFU/m3. Although all seasonal average concentrations of total suspended bacteria were below the standard concentration, 44 % samples in summer, 24 % in autumn, 16 % in spring, and 8 % in winter exceeded the standard approved by the Korean government. However, all seasonal samples did not exceed the residential limit value (RLV; 5 × 103 CFU/m3) in residential dwellings proposed by Dutkiewicz (Dutkiewicz and Górny 2002).
Among culturable bacteria we determined in our study, Gram-positive bacteria, mainly Staphylococcus, were found to be the most frequent bacteria. Staphylococcus is known to be the most common isolated genus in indoor air (Okten and Asan 2012). Among Staphylococcus, S. aureus has long been recognized as one of the most common human bacterial pathogens and causes of community-associated infections (Halablab et al. 2010; Philip et al. 2006). The household environment has been a generally unrecognized reservoir for S. aureus such as food items (shelves, sinks, and kitchen surfaces) and bedroom surfaces (pillows and bedding) (Davis et al. 2012). Recent Korean studies showed that about 50 % of the total airborne bacteria identified in general hospitals were S. aureus and 58.5 % isolated from clinical samples were MRSA (Kim et al. 2007, 2010).
In this study, the average concentrations of S. aureus in indoor air during each season ranged from 27 to 45 CFU/m3. These results were higher compared with the previously reported average concentration 15.4 CFU/m3 by Gandara et al., who investigated S. aureus in El Paso, Texas, from 24 one-story houses (Gandara et al. 2006). However, similar concentrations of S. aureus were observed in outdoor samples. The seasonal average concentrations of S. aureus in indoor air were obviously higher than those of outdoor air, which ranged from 3.0 to 4.1 times.
We found a significant difference between the total number of culturable bacteria in apartments with respect to season of the year (p < 0.001). As expected, the number of culturable bacteria was significantly higher in summer than in winter (p < 0.001). Relative humidity led to an increase in indoor airborne suspended bacteria and Gram-positive bacteria concentrations. Correlation coefficients were 0.395 (p < 0.01) and 0.316 (p < 0.01), respectively. Staphylococcus and S. aureus concentrations correlated with indoor temperature. Correlation coefficients were 0.335 (p < 0.01) and 0.294 (p < 0.01), respectively.
Several studies showed that the number of people and pets within households were associated with prevalences of S. aureus (Bramble et al. 2011; Faires et al. 2009; Hanselman et al. 2009; Weese and van Duijkeren 2010). Yet, no significant differences could be shown for the concentration of S. aureus in indoor air between the number of people and pets residing with family members in this study. However, the concentration of S. aureus were significantly higher within households having children (aged 0–17 years) than those not having children (p < 0.05).
Households are a potential space not only for S. aureus but also for MRSA. Scott et al. (2008) and Gandara et al. (2006) reported that 97–100 % of households were positive for S. aureus, and 26–39 % of homes were positive for MRSA. Lucet et al. (2009) reported that an estimated 20 % of people were persistent carriers who remain positive for MRSA for months or years. MRSA is one of the most prevalent dermatology pathogens in hospitals and is increasingly acquired in the community (Huang and Chen 2011). MRSA is usually considered a major nosocomial pathogen. In Korea, Kwon et al. investigated 13 hospitals with more than 400 beds and 160 community residences by collecting samples including the hands and nasal cavities of doctors, nurses, guardians, patients, and residents. Of the MRSA strain, 24.3 % was isolated from the medical and community environment (Kwon et al. 2007). In this study, S. aureus were detected in 100 % of households' indoor air, and 66 % of households were positive for MRSA. S. aureus and MRSA are usually transmitted by direct person to person contact; however, routes of indirect transmission might include environmental exposure through aerosols (Davis et al. 2012). Several case reports have implicated positive home environment for MRSA as persistent sources of infection (Uhlemann et al. 2011). There are many studies for people with MRSA in hospitals and communities (Davis et al. 2012). However, until now, insufficient studies have been conducted on MRSA in residential indoor air. In this study, although we did not isolate MRSA from family members and environmental surfaces, we suggest that household indoor air contamination from MRSA might contribute to colonization of healthy family members.
Also, the respiratory viruses, causes of common colds, sore throats, bronchitis, and influenza, have been known as major candidates for transmission by the airborne route. Airborne respiratory viruses are easily spread by droplets that can be inhaled directly or settle on surfaces. However, respiratory viruses are difficult to characterize in the airborne environment due to their small particle size and low concentration and the presence of a wide range of contaminants which can inhibit laboratory assays (Fabian et al. 2009). Some researchers have detected airborne respiratory viruses in the field, such as office environments, health centers, and hospital rooms (Blachere et al. 2009; Myatt et al. 2004; Tseng et al. 2010; Yang et al. 2011).
Wan et al. reported that approximately 18 % of the air samples from the pediatric emergency room of the Children's Hospital in Taiwan were found to contain adenovirus, and the detected concentrations of adenovirus in the air ranged from 48.4 to 461 copies/m3 (Wan et al. 2012). In another study, Yang et al. collected samples in the health center and the day care center, 33 % of the health center samples and 75 % of the day care center samples were confirmed to contain aerosolized influenza A virus (Yang et al. 2011). The average concentrations of influenza A virus was 1.6 × 104 copies/m3.
In this study, adenovirus was detected in 14 (14 %) samples and influenza A virus was detected in 3 (3 %) samples among 100 indoor aerosol samples which were seasonally collected from 25 households. Samples collected in spring had the highest detection rate (0.36) of adenovirus, and influenza virus A was detected only in winter. According to Korea Centers for Disease Control and Prevention (KCDC), outbreak of acute respiratory illness was significantly increased in South Korea in 2010, especially the detection rate of adenovirus from patients, which was more than eight times higher (20.0 %) than those of the 2.3 % annual average during the spring and fall of 2010 (KCDC 2011). In our study, the detection rate for adenovirus being significantly high compared with other viruses in spring was supported by the above observation of KCDC.
Mean adenovirus concentration was 767 copies/m3 and ranged from 12 to 4,164 copies/m3 during all seasons. This result was higher than the value by Wan et al. (2012) but somewhat lower than those measured in the day care center by Yang et al. (2011).
Until now, very limited data existed regarding the quantification of airborne viruses. However, it is known that viral concentrations higher than 100 copies/m3 could pose a health risk to people because the high copy number in the air does indeed suggest that transmission could occur (Tseng et al. 2010).
Seasonal adenovirus concentrations were significantly higher in winter than in spring (p < 0.01). Several publications describe the relation between survival of viruses and relative humidity and temperature (Casanova et al. 2010; Lowen et al. 2007). In general, enveloped viruses such as the influenza virus, which contains a lipid membrane, survive better at lower relative humidity and temperature, while nonenveloped ones such as polio virus tend to be more stable at higher relative humidity (Yang and Marr 2012). In this study, indoor mean relative humidity levels in winter and in spring were 24.9 % (ranging between 15.6 and 37.8 %) and 43.3 % (ranging between 24.2 and 59.4 %), respectively. Also, a strong negative correlation between adenovirus concentrations and relative humidity in indoor air was observed (r = −0.808, p < 0.01). Furthermore, temperature also negatively correlated with adenovirus concentrations (r = −0.559, p < 0.05).
According to the results of the study conducted by Myatt et al., aerosolized influenza virus survived best when the relative humidity was below 36 %, with a sudden decrease in survival of the virus when the relative humidity was raised above 49 % (Myatt et al. 2010).
In Korea, most of the apartments have central heating systems, which are regulated through central steam pipes by the building authorities. Also, natural ventilation is the most common mode for fresh air exchange in Korean houses, and people usually close the windows and doors of their homes for energy saving and security. Therefore, indoor relative humidity levels during heating seasons are very low in most of the Korean apartments. These conditions might be the possible cause for the greater detection of viruses in the indoor environment.
This study demonstrates baseline bioaerosol levels within apartments in the different seasons. These baselines will be useful in future studies as well as the assessment of indoor biological contamination issues.
The limitation of this study is the relatively small sample size. It is acknowledged that a small sample size resulted in a study with a lower statistical power and increased uncertainty. A larger sample size would likely provide more information. Additionally, the period of our sampling took place for only 1 year; a longer sampling period may allow for a more in-depth evaluation of trends within the bioaerosols.
Conclusion
The results obtained from this study showed that S. aureus were detected in 100 % of households' indoor air, and 66 % of households were positive for MRSA. Also, among respiratory viruses, adenovirus was detected in 14 (14 %) samples and influenza A virus was detected in 3 (3 %) samples.
S. aureus and MRSA have become a significant and increasing cause of community-associated infections and one of the most prevalent pathogens worldwide. Also, the respiratory viruses, causes of common colds, sore throats, bronchitis, and influenza, have been known as major candidates for transmission by the airborne route.
Although we did not investigate the people with illness caused by the viruses, S. aureus and MRSA, these results support the possibility that the risk of airborne disease to family members living in homes can exist, and airborne transmission may also occur through the indoor air of homes. Therefore, additional research is needed to understand the potential for the transmission of pathogens by aerosol.
Acknowledgments
This study was supported financially by a grant from GS Engineering & Construction Company and Woong Jin Coway Company.
References
- Aydogdu H, Asan A, Otkun MT. Indoor and outdoor airborne bacteria in child day-care centers in Edirne City (Turkey), seasonal distribution and influence of meteorological factors. Environmental Monitoring and Assessment. 2010;164(1–4):53–66. doi: 10.1007/s10661-009-0874-0. [DOI] [PubMed] [Google Scholar]
- Barker J, Stevens D, Bloomfield SF. Spread and prevention of some common viral infections in community facilities and domestic homes. Journal of Applied Microbiology. 2001;91(1):7–21. doi: 10.1046/j.1365-2672.2001.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachere FM, Lindsley WG, Pearce TA, Anderson SE, Fisher M, Khakoo R, Meade BJ, Lander O, Davis S, Thewlis RE, Celik I, Chen BT, Beezhold DH. Measurement of airborne influenza virus in a hospital emergency department. Clinical Infectious Diseases. 2009;48(4):438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- Bramble M, Morris D, Tolomeo P. Potential role of pet animals in household transmission of methicillin-resistant Staphylococcus aureus: a narrative review. Vector Borne and Zoonotic Diseases. 2011;11:617–620. doi: 10.1089/vbz.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge H. Biological agents. In: Scott Baker JD, McCallum D, editors. Residential exposure assessment: A sourcebook. New York: Plenum; 2001. pp. 245–261. [Google Scholar]
- Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Applied and Environmental Microbiology. 2010;76(9):2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEC. (1993). Indoor air quality & its impact on man. Biological particles in indoor environments. EUR 14988 EN. Commission of the European communities, Luxembourg.
- Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhalation Toxicology. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- Davis MF, Iverson SA, Baron P, Vasse A, Silbergeld EK, Lautenbach E, Morris DO. Household transmission of meticillin-resistant Staphylococcus aureus and other staphylococci. Lancet Infectious Diseases. 2012;12(9):703–716. doi: 10.1016/S1473-3099(12)70156-1. [DOI] [PubMed] [Google Scholar]
- Di Giulio M, Grande R, Di Campli E, Di Bartolomeo S, Cellini L. Indoor air quality in university environments. Environmental Monitoring and Assessment. 2010;170(1–4):509–517. doi: 10.1007/s10661-009-1252-7. [DOI] [PubMed] [Google Scholar]
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M, Grp SP. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clinical Infectious Diseases. 2001;32:S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- Dutkiewicz J, Górny R. Biological factors hazardous to human health: classification and criteria of exposure assessment. Medycyna Pracy. 2002;53(1):29–39. [PubMed] [Google Scholar]
- Elston JWT, Barlow GD. Community-associated MRSA in the United Kingdom. Journal of Infection. 2009;59(3):149–155. doi: 10.1016/j.jinf.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Fabian P, McDevitt JJ, Lee WM, Houseman EA, Milton DK. An optimized method to detect influenza virus and human rhinovirus from exhaled breath and the airborne environment. Journal of Environmental Monitoring. 2009;11(2):314–317. doi: 10.1039/b813520g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faires MC, Tater KC, Weese JS. An investigation of methicillin-resistant Staphylococcus aureus colonization in people and pets in the same household with an infected person or infected pet. Journal of the American Veterinary Medical Association. 2009;235:540–543. doi: 10.2460/javma.235.5.540. [DOI] [PubMed] [Google Scholar]
- Gandara A, Mota LC, Flores C, Perez HR, Green CF, Gibbs SG. Isolation of Staphylococcus aureus and antibiotic-resistant Staphylococcus aureus from residential indoor bioaerosols. Environmental Health Perspectives. 2006;114(12):1859–1864. doi: 10.1289/ehp.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny RL, Dutkiewicz J. Bacterial and fungal aerosols in indoor environment in Central and Eastern European countries. Annals of Agricultural and Environmental Medicine. 2002;9(1):17–23. [PubMed] [Google Scholar]
- Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. Journal of Infectious Diseases. 2008;197(9):1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- Halablab MA, Hijazi SM, Fawzi MA, Araj GF. Staphylococcus aureus nasal carriage rate and associated risk factors in individuals in the community. Epidemiology and Infection. 2010;138(5):702–706. doi: 10.1017/S0950268809991233. [DOI] [PubMed] [Google Scholar]
- Hanselman BA, Kruth SA, Rousseau J, Weese JS. Coagulase positive staphylococcal colonization of humans and their household pets. Canadian Veterinary Journal. 2009;50:954–958. [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Chen CJ. Community-associated meticillin-resistant Staphylococcus aureus in children in Taiwan, 2000s. International Journal of Antimicrobial Agents. 2011;38(1):2–8. doi: 10.1016/j.ijantimicag.2011.01.011. [DOI] [PubMed] [Google Scholar]
- KCDC Public health weekly report. Korea Centers for Disease Control. 2011;4(15):269. [Google Scholar]
- Kim KY, Kim CN. Airborne microbiological characteristics in public buildings of Korea. Building and Environment. 2007;42(5):2188–2196. doi: 10.1016/j.buildenv.2006.04.013. [DOI] [Google Scholar]
- Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, Park WB, Kim SH, Bang JH, Kim DM, Park KU, Shin S, Lee MS, Choi HJ, Kim NJ, Kim EC, Oh MD, Kim HB, Choe KW. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. Journal of Antimicrobial Chemotherapy. 2007;60(5):1108–1114. doi: 10.1093/jac/dkm309. [DOI] [PubMed] [Google Scholar]
- Kim KY, Kim YS, Kim D. Distribution characteristics of airborne bacteria and fungi in the general hospitals of Korea. Industrial Health. 2010;48(2):236–243. doi: 10.2486/indhealth.48.236. [DOI] [PubMed] [Google Scholar]
- Kwon Y-I, Kim T-W, Kim H-Y, Chang Y-H, Kwak H-S, Woo G-J, Chung Y-H. Monitoring of methicillin resistant Staphylococcus aureus from medical environment in Korea. Korean Journal of Microbiology and Biotechnology. 2007;35(2):158–162. [Google Scholar]
- Lee JH, Jo WK. Characteristics of indoor and outdoor bioaerosols at Korean high-rise apartment buildings. Environmental Research. 2006;101(1):11–17. doi: 10.1016/j.envres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathogens. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. New England Journal of Medicine. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Lucet JC, Paoletti X, Demontpion C, Degrave M, Vanjak D, Vincent C, Andremont A, Jarlier V, Mentre F, Nicolas-Chanoine MH. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Archives of Internal Medicine. 2009;169(15):1372–1378. doi: 10.1001/archinternmed.2009.217. [DOI] [PubMed] [Google Scholar]
- Mentese S, Arisoy M, Rad AY, Gullu G. Bacteria and fungi levels in various indoor and outdoor environments in Ankara, Turkey. Clean-Soil Air Water. 2009;37(6):487–493. doi: 10.1002/clen.200800220. [DOI] [Google Scholar]
- Myatt TA, Johnston SL, Zuo Z, Wand M, Kebadze T, Rudnick S, Milton DK. Detection of airborne rhinovirus and its relation to outdoor air supply in office environments. American Journal of Respiratory and Critical Care Medicine. 2004;169(11):1187–1190. doi: 10.1164/rccm.200306-760OC. [DOI] [PubMed] [Google Scholar]
- Myatt TA, Kaufman MH, Allen JG, MacIntosh DL, Fabian MP, McDevitt JJ. Modeling the airborne survival of influenza virus in a residential setting: the impacts of home humidification. Environmental Health. 2010;9:55. doi: 10.1186/1476-069X-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okten S, Asan A. Airborne fungi and bacteria in indoor and outdoor environment of the Pediatric Unit of Edirne Government Hospital. Environmental Monitoring and Assessment. 2012;184(3):1739–1751. doi: 10.1007/s10661-011-2075-x. [DOI] [PubMed] [Google Scholar]
- Philip LG, Susan XL, Elaine LL. A U.S. population-based survey of Staphylococcus aureus colonization. Annals of International Medicine. 2006;144(5):318–325. doi: 10.7326/0003-4819-144-5-200603070-00006. [DOI] [PubMed] [Google Scholar]
- Rhee CH, Woo GJ. Emergence and characterization of foodborne methicillin-resistant Staphylococcus aureus in Korea. Journal of Food Protection. 2010;73(12):2285–2290. doi: 10.4315/0362-028x-73.12.2285. [DOI] [PubMed] [Google Scholar]
- Scott E, Duty S, Callahan M. A pilot study to isolate Staphylococcus aureus and methicillin-resistant S aureus from environmental surfaces in the home. American Journal of Infection Control. 2008;36(6):458–460. doi: 10.1016/j.ajic.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Smith TC, Moritz ED. The environment as a factor in methicillin-resistant Staphylococcus aureus transmission. Reviews on Environmental Health. 2010;25(2):121–134. doi: 10.1515/REVEH.2010.25.2.121. [DOI] [PubMed] [Google Scholar]
- Srikanth P, Sudharsanam S, Steinberg R. Bio-aerosols in indoor environment: composition, health effects and analysis. Indian Journal of Medical Microbiology. 2008;26(4):302–312. doi: 10.4103/0255-0857.43555. [DOI] [PubMed] [Google Scholar]
- Sudharsanam S, Swaminathan S, Ramalingam A, Thangavel G, Annamalai R, Steinberg R, Balakrishnan K, Srikanth P. Characterization of indoor bioaerosols from a hospital ward in a tropical setting. African Health Sciences. 2012;12(2):217–225. doi: 10.4314/ahs.v12i2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CC, Chang LY, Li CS. Detection of airborne viruses in a pediatrics department measured using real-time qPCR coupled to an air-sampling filter method. Journal of Environmental Health. 2010;73(4):22–28. [PubMed] [Google Scholar]
- Uhlemann, A., Knox, J., Miller, M., Hafer, C., Vasquez, G., Ryan, M., Vavagiakis, P., Shi, Q., & Lowy, F. D. (2011). The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureus USA300: a case–control study. PLoS One, 1–9. [DOI] [PMC free article] [PubMed]
- Wan G. H., Huang C. G., Huang Y. C., Huang J. P., Yang S. L., Lin T. Y., Tsao K. C. Surveillance of airborne adenovirus and Mycoplasma pneumoniae in a hospital pediatric department. Plos One. 2012;7(3):1–5. doi: 10.1371/journal.pone.0033974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese JS, van Duijkeren E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Veterinary Microbiology. 2010;140(3–4):418–429. doi: 10.1016/j.vetmic.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Yang W, Marr LC. Mechanisms by which ambient humidity may affect viruses in aerosols. Applied and Environmental Microbiology. 2012;78(19):6781–6788. doi: 10.1128/AEM.01658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Elankumaran S, Marr LC. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. Journal of the Royal Society Interface. 2011;8(61):1176–1184. doi: 10.1098/rsif.2010.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]


