Abstract
The aim of this study was to collect and identify airborne bacteria in Norway, Sweden and Finland and to compare three different technologies for identifying collected airborne bacterial isolates: the “gold standard” method 16S rDNA sequencing, MALDI-TOF MS using the MALDI Biotyper 2.0 and the MIDI Sherlock® Microbial Identification System (MIDI MIS system). Airborne bacteria were collected during three different periods from May to October 2009 using air sampling directly on agar plates. A total of 140 isolates were collected during three sampling campaigns, and 74 % (103) of these isolates were analyzed by all three methods. The dominant genera in Norway and Finland were the gram-positive bacteria Bacillus and Staphylococcus, whereas the gram-negative bacterium Acinetobacter was the dominant genus in Sweden. Using 16S rDNA sequencing, MALDI-TOF MS and MIDI MIS analysis, 83, 79 and 75 %, respectively, of the isolates were identified and assigned to order or higher taxonomic levels. In this study, the MALDI-TOF MS combining with the MALDI Biotyper 2.0 classification tool was demonstrated to be a fast and reliable alternative for identifying the airborne bacterial isolates. These studies have increased knowledge about the airborne bacterial background in outdoor air, which can be useful for evaluating and improving the operational performance of biological detectors in various environments. To our knowledge, this is the first time that 16S rDNA sequencing, MALDI-TOF MS and MIDI MIS analysis technologies have been compared for their efficiency in identifying airborne bacteria.
Keywords: Airborne bacteria, Identification, 16S rDNA sequencing, MALDI-TOF MS, MIDI MIS analysis
Introduction
Bioaerosols have been studied in several indoor and outdoor environments addressing public and occupational health-related questions (e.g., indoor air quality control and health hazard assessments), as well as microbial ecology, atmospheric science and biodefense (Gilbert and Duchaine 2009; Mandal and Brandl 2011; Després et al. 2012). Microorganisms such as Mycobacterium tuberculosis, Legionella pneumophila, airborne viruses exemplified with SARS and influenza are of concern for the public health and associated with an airborne route of exposure (Stetzenbach et al. 2004). Disease causing bacteria or viruses can also be deliberately dispersed as bioaerosols (Atlas 2002; Levin and de Amorim 2003), and in the absence of reliable biological detector and surveillance systems, exposed individuals will not be aware of a biological incident until clinical symptoms appear and when they seek medical care. Knowledge about the microbial diversity in air is important for detector performance and for development of specific, selective and sensitive methods for detection and identification of specific airborne pathogenic bacteria.
In previous studies, airborne viable bacteria at Kjeller (Norway) and Sarpsborg, the latter at a biological treatment plant (Norway) were collected and classified to the genera level using 16S rDNA gene sequencing (Fykse et al. 2008; Blatny et al. 2011). Bacteria belonging to different genera were identified, and the dominant cultivable bacteria collected on agar in slit array was gram-positive Actinobacteria and Bacillus, whereas the dominating sequences identified in DNA samples extracted directly from collected aerosol samples was gram-negative Proteobacteria. Characterization of viable airborne bacteria at an underground subway in Oslo (Norway) showed that the major bacteria identified belonged to the genera Bacillus, Micrococcus and Staphylococcus (Dybwad et al. 2012).
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF MS) has successfully been used for microbial identification. Comparatively, the gold standard method 16S rDNA sequencing for identification of bacterial isolates is more time-consuming and less cost effective (Woo et al. 2003; Cherkaoui et al. 2010). For bacteria, the mass spectrum of unknown isolates is compared to a spectral library of reference strains and the species or genus is assigned based on the highest percent similarity to the reference spectral profiles (Sauer et al. 2008). Several publications have described the use of MALDI-TOF MS in routine clinical analysis (Eigner et al. 2009; Patel 2013). The MIDI MIS technique is based on fatty acid methyl ester analysis by gas chromatography (GC-FAME) and has been used to identify microbes in environmental and clinical samples (Slabbinck et al. 2009). The fatty acid profile (dependending on the growth medium used for culture) of a microbe is complex and unique to each bacterial strain, and hence, it can be used for identification purposes. In addition to identification, FAME analysis has been used for strain or subspecies level comparisons (Van den Velde et al. 2006). Previously, the MIDI MIS system has been successfully utilized for the differentiation of various strains of Bacillus thuringiensis (Adams et al. 2005). The cellular fatty acid profiles of 67 strains belonging to three different species of the genus Mycobacterium have been determined from clinical samples (Ozbek and Aktas 2003). However, to our knowledge, there are few articles describing the use of the MIDI MIS system and MALDI-TOF MS for identification of airborne bacteria (Chan et al. 2009; Dybwad et al. 2012, 2014).
The aim of the present study was to compare three different methods for their ability to identify the collected airborne bacterial isolates. These included the traditional 16S rDNA sequencing method and the more rapid screening methods MALDI-TOF MS combined with the MALDI Biotyper 2.0 and the MIDI MIS system. The viable airborne bacteria in Norway, Sweden and Finland collected at three different time point during the period May–October 2009 were identified and compared.
Materials and methods
The air sampling was performed at the Norwegian Defence Research Establishment (FFI), Kjeller, Norway (59°58′26.26N/11°02′48.38E), at the Defence Forces Technical Research Centre (PvTT), Lakiala, Finland (61°36′11.76N/23°29′26.28) and at the Swedish Defence Research Agency (FOI), Umeå, Sweden (63°51′0.83″N/20°19′53.21″E) during May to October 2009. The sampling periods (indicated as I, II and III) and the weather conditions during the sampling are specified in Table 1. Sweden performed only two sampling campaigns. The sampling site in Norway was a suburban agricultural area located 20 km north of Oslo city. The sampling site in Sweden was on the Umeå city border with suburban character, close to coniferous forest. In Finland, the sampling site was a woody terrain located in a suburban area 20 km southeast of Tampere. Briefly, airborne bacteria were sampled using slit samplers onto Trypticase soy agar (TSA) plates (Merck, Darmstadt, Germany). Three different methods were used to identify the colonies. The MIDI analyses were performed in Finland, and the 16S rDNA sequencing and MALDI-TOF MS analyses were performed in Norway.
Table 1.
Time period of sampling campaigns in 2009 and weather conditions
| Finland | Norway | Sweden | |
|---|---|---|---|
| Sampling I | May 12–14 | May 12–14 | May 12–14 |
| Sampling II | September 15–19 and 21–22 | September 10–12 | September 8–9 |
| Sampling III | October 27–30 | October 27–29 | No sampling |
| Weather | |||
| Sampling I | 8–14 °C, sunny and cloudy, no rain | 8–14 °C, 3–5 m/s wind, sunny and cloudy, one heavy rain shower | 4–15 °C, 1–7 m/s wind, 30–70 % RH sunny |
| Sampling II | 10–19 °C mainly sunny, no rain | 6–18 °C, <2 m/s wind, mainly sunny, no rain | 14–22 °C, 3–9 m/s wind, 35–95 % RH, cloudy |
| Sampling III | 8–14 °C | −4 to (+)4 °C, <2 m/s wind, sunny, no rain | No sampling |
Air sampling
Air sampling by impaction on agar plates was carried out during the same time period in Norway, Sweden and Finland in order to collect and identify viable airborne bacteria present in the three environments. Sampling of air was performed using the New Brunswick STA-203 (array) and STA 204 slit-to-agar samplers (at FFI and PvTT, respectively) or the Mattson–Garvin slit-to-agar sampler array (at FOI) (Ho et al. 2005; Blatny et al. 2011). Biological particles were impacted onto a 150 mm × 15 mm TSA agar plate in a slit sampler. The slit samplers were collecting air at a flow rate of 30 L/min. The STA-203 and the Mattson-Garvin sampler arrays consisted of ten samplers sequentially linked to each other. The STA-203 slit arrays were run for 3 × 8 h with eight samplers (two periods during daytime and one period during night), whereas the Mattson–Garvin slit array was run for 2 × 6 h with six samplers (daytime) and 1 × 10 h with ten samplers (night). A sampling time of 60 min on each agar plate was used (1,800 L of air). The STA 204 slit samplers were collecting air at a flow rate of 30 L/min for 60 min (1,800 L of air) before the agar plate was changed.
Growth conditions
After sampling, all plates were incubated at 30 °C for 24 h. The number of colonies was enumerated manually or using an automatic counter, ProtoCol (Kaiser; Germany). The number on each agar plate differed between no colonies and several hundred. A representative selection of morphologically distinct bacterial colonies were isolated and re-streaked two times on TSA agar to produce individual colonies and incubated as described before identification. A total of 140 colonies were isolated. The origin of the 140 isolates was the following: 71 isolates from Norway, 43 from Sweden and 26 from Finland. Of these isolates, 122 (87 %) were identified and confirmed by similar results for two of the three methods and 103 (74 %) were analyzed using all three methods for comparison of the three identification methods. When colonies were shipped to Finland and Norway for analysis, a single colony was transferred from the TSA agar onto an agar slant of TSA (1 mL) in cryo tubes (2 mL) and incubated at 30 °C for 24 h before transportation by mail. Upon arrival, the colonies were streaked out on TSA agar and incubated as described. However, some colonies were not able to grow after shipping or storage and therefore unable to be identified. Therefore, the number of analyzed isolates varies from the total number (140 as stated above) of colonies picked. The number of isolates analyzed by the three different methods is stated below. Isolates were kept for long-time storage at −80 °C in brain heart infusion broth (Oxoid) supplemented with 18 % glycerol (Merck). The number of colonies, CFU/m3 of air, reflects the ratio of airborne particles carrying viable organisms.
16S rDNA sequencing
The isolates were amplified using the 16S rRNA primers 27f/1492r and sequenced as described (Despres et al. 2007; Fykse et al. 2013). PCR amplicons were sequenced at the Eurofins MWG Operon (Ebersber, Germany). The sequence trace files were assembled, trimmed, aligned and manually checked using Bionumerics software 6.0 (Applied. Maths. Sint-Martens Latem, Belgium), and the sequences were classified using the Classifier and SeqMath tools at the Ribosomal Database Project (RDP) (Wang et al. 2007) and BLASTn databases via the online interface at National Center of Biotechnology and Information (NCBI). In general, taxonomic classification is based on sequence similarities of >98 %. From sampling campaign I, II and III, a total of 127 of the 140 isolates were sequenced and identified. Some sequences were not readable, and some isolates did not grow after storage and was therefore not sequenced.
MALDI-TOF MS
The isolates were classified/identified using a MicroFlex MALDI-TOF MS instrument in combination with the MALDI Biotyper 2.0 microbial identification system/platform (Bruker Daltronics, Bremen, Germany). The MALDI-TOF analysis is based on fingerprints of the mass spectra in the mass range 2,000–20,000 Da. In this range, the 16S rRNA binding proteins are represented. The spectra are compared to a reference database (Bruker Taxonomy) by the MALDI Biotyper 2.0 system, and putative genera or species is identified from the fingerprint of the bacteria. From sampling campaign I, II and III, a total of 127 (91 %) of the 140 isolates were analyzed. Some isolates failed to grow after storage and shipping and was therefore not identified. The analysis was performed as described (Dybwad et al. 2012). Briefly, a single colony from a TSA agar plate (kept at room temperature) was deposited on the 96 ground steel target plate as triplicate and air-dried. In the direct method, the target plate with the colonies was overlain with 1 µL matrix solution [4-hydroxy-a-cyanocinnamic acid (HCCA)]. The sample was air-dried and loaded into the MicroFlex instrument for data acquisition. The Biotyper 2.0 system was run in automatic classification mode, and the reference database used was the Bruker Taxonomy database (v3.1.1.0, containing the 3,740 library entries). Analysis of each sample takes 5–15 s. Some difficult-to-process isolates were extracted with ethanol/formic acid (Drevinek et al. 2012) to obtain better quality mass spectra. Score values proposed by the manufacturer were used to classify the isolates: >2.3 high probable species identification; > 2.0 < 2.3 probable species identification; > 1.7 < 2.0 probable genus identification; <1.7, not reliable identification.
MIDI MIS identification system
Isolated colonies from TSA agar were identified using the MIDI Sherlock® Microbial Identification System (MIDI MIS) (MIDI, Inc., Newark, Delaware, USA), which identifies bacteria by comparison of the whole cell fatty acid profiles between the samples and the system’s database. The samples for MIDI analysis were prepared precisely according to manufacturer’s instruction (http://www.midi-inc.com/index.html). Shortly, collected bacterial colonies were treated with saponin and sodium hydroxide/methanol mixture and methylation of free cellular fatty acid with a methanol/hydrochloride acid mixture. Then, methyl esters of the fatty acid were extracted with organic solvent and analyzed by gas chromatography. The fatty acid profile of an unknown sample is compared to the profiles in the library of reference strains. Different libraries are available for different growth conditions. An Agilent (Agilent Technologies, Santa Clara CA 95051 United States) 7890A gas chromatograph (GC) equipped with an Agilent 7683 autosampler, split–splitless inlet and flame ionization detector were used. The GC system was controlled with MIS Sherlock® (MIDI, Inc., Newark, DE, USA) and Agilent Chem Station software. The prepared samples were analyzed using the RTSBA6 library, which is aimed for environmental bacteria and contains fatty acid profiles for aerobes grown on TSA agar. From sampling campaign I, II and III, a total of 128 (91 %) of the 140 isolates were analyzed. Some isolates failed to grow after storage and shipping and was therefore not identified. Score values used for evaluation of the results: >0.500 means good match with library and reliable identification (with a separation of 0.100 between first and second choice), > 0.300 < 0.500 (>0.100 separation) may be a good match, but an atypical strain; <0.300 species not contained in the database.
Statistical analysis
The Chi-square test was used to investigate whether the biological diversity was similar between the different countries and the different dates. The test was also used to investigate the different methods’ ability to identify the isolates. The Chi-square test is used to determine whether there is a significant difference between the observed results and the expected (the hypothesis). The hypothesis in this study is that the diversity is similar on the different sites, the different periods and that the different methods have the same ability to identify the isolates. The significance level was set to p < 0.05.
Results
Weather conditions and bacterial concentrations
The weather conditions during the sampling periods at the three different sites were similar with a temperature range of −4 and 22 °C, and it was in general sunny and partly cloudy (see Table 1 for details). In Norway, it was one heavy rain shower during the first period, and during the last period, the temperature was below 0 °C.
The bacterial concentration (CFU/m3 air) at each sampling location during the different sampling periods varied from 0 to >1,000 CFU/m3, but the average numbers were low. In Norway, during sampling period I, II, III, the average airborne bacterial concentration was 17, 51 and 20 CFU/m3 of air, respectively. During sampling period I in Sweden, the airborne bacterial concentration was >90 CFU/m3. During sampling period II, the number was lower, in general <35 CFU/m3. In Finland, the bacterial concentration was low, below 20 CFU/m3 and varied between 0 and 40 CFU/m3. Typically, a “peak” of airborne bacteria was observed in the afternoon.
Identification of the bacterial isolates
The putative identity of the bacterial isolates included in this study was based on similar results for two of the three technologies applied in this study. Sixty-six percent of the 122 isolates were assigned to genus level, 6 % to family level, and 9 % to order level, whereas 19 % of the isolates could not be assigned to order level or higher (Table 2). In general, results from the air sampling performed in Norway, Sweden and Finland during the three time periods showed that the gram-positive Firmicutes were the predominant bacterial phylum identified, and identified genera were Bacillus and Staphylococcus. Species identified were S. saprophyticus, S. xylosus, S. succinius, S. cohnii, B. megaterium, B. subtilis and B. pumilus. The gram-positive bacterium Micrococcus was also identified in addition to the gram-negative bacteria Proteobacteria exemplified by Acinetobacter and Pseudomonas in this study (Fig. 1).
Table 2.
Taxonomic identification of bacterial isolates collected from TSA agar using 16S rDNA sequencing, MALDI-TOF MS and MIDI analysis
| No. of isolates classified to: | No. of isolates (%) |
|---|---|
| Genus | 81 (66) |
| Family | 7 (6) |
| Order | 11 (9) |
| Non-classified | 23 (19) |
| Total number of isolates | 122 (100) |
The putative identity of the isolates is confirmed by similar results for two of the three identification methods. A total of 122 isolates have been identified by two of the three analytical methods and are therefore included in the comparison in the table
Fig. 1.
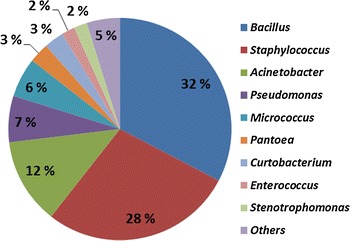
Characterization of the genera of the bacterial isolates collected in, Norway, Sweden and Finland at three different days are analyzed by 16S rDNA sequencing, MALDI-TOF MS and MIDI MIS analyses. The putative identity of the isolates (N = 122) is confirmed by similar results for two of the three methods
In Norway and Finland, the dominant genera identified were the gram-positive Bacillus, Staphylococcus and Micrococcus. In Sweden, the gram-negative bacteria Acinetobacter were identified as the dominant genus in addition to in chronological order Bacillus and Staphylococcus (Fig. 2). The airborne bacterial groups identified in Norway, Sweden and Finland was significantly different (p < 0.05). Comparison of the diversity of the bacterial genera collected at the three different sampling periods in Norway was also significantly different (Fig. 3) (p < 0.05). In the first sampling period in Norway, the Bacillus genus was dominating, whereas in the second and third sampling period, the Staphylococcus was the dominant genus.
Fig. 2.
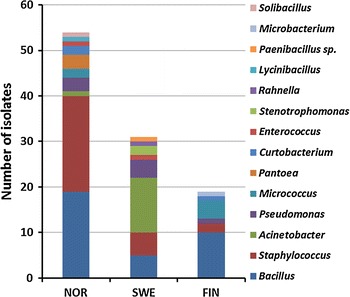
Comparison of the airborne bacteria in Norway, Sweden and Finland. p < 0.05 indicates a significant different diversity at the three different sites. The putative identity of the isolates (N = 122) is confirmed by similar results for two of the three methods
Fig. 3.
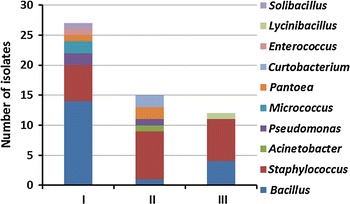
Comparison of airborne bacteria collected during the three different (I, II, III) sampling periods in Norway. p < 0.05 indicates a significant different diversity. The putative identity of the isolates (N = 71) is confirmed by similar results for two of the three methods
Comparison of 16S rDNA sequencing, MALDI-TOF MS and MIDI Sherlock Microbial Identification System
In order to assess the identity/disparity of the results obtained with 16S rDNA sequencing, MALD-TOF MS combined with the MALDI Biotyper 2.0 and the MIDI MIS System, the ability of the three methods to identify the 103 isolates was compared. A contingency table was used to investigate the different methods ability to identify the isolates. The number of isolates which the 16S sequencing, MALDI-TOF MS and MIDI MIS analysis method identified and failed to identify were counted and compared, and 83, 79 and 75 %, respectively, of the isolates were identified and assigned to order or higher taxonomic levels (Table 3). A Chi-square test was used to compare the ability of the three methods to identify the isolates. When 16S rDNA sequencing and MALDI-TOF MS was compared, 87 % of the isolates were assigned to genus level by both methods, whereas 7 % could not be assigned to the order level or higher (Fig. 4). A Chi-square test indicated no statistically difference (p = 0.078) between the two methods regarding their ability to identify the isolates. Comparison between 16S rDNA sequencing and MIDI MIS analysis showed that 70 % of the isolates was assigned to genus by both methods, whereas 19 % could not be assigned to the order level or higher. Statistical analysis revealed a significant difference of the two methods ability to identify the bacterial isolates (p = 0.00027). When MALDI-TOF MS and MIDI analysis was compared, 69 % of the isolates were assigned to similar genus by both methods, whereas 18 % of the isolates could not be assigned to the order level or higher. A significant difference between the two methods was identified (p = 0.031).
Table 3.
Comparison of 16S rDNA sequencing, MALD-TOF MS and MIDI MIS identification analysis of 103 bacterial isolates collected during the three periods in Norway, Sweden and Finland
| Methods | No. of isolates classified (%) | No. of isolates non-classified (%) |
|---|---|---|
| 16S r DNA sequencing | 85 (83) | 18 (17) |
| MALDI-TOF | 81 (79) | 22 (21) |
| MIDI | 77 (75) | 26 (25) |
The isolates were classified to order or higher. The 16S rDNA sequences were taxonomically identified by comparison with reference databases, the RDP database and the BLASTn database in NCBI Genbank. Only isolates identified using all three methods (N = 103) are included in this comparison
Fig. 4.
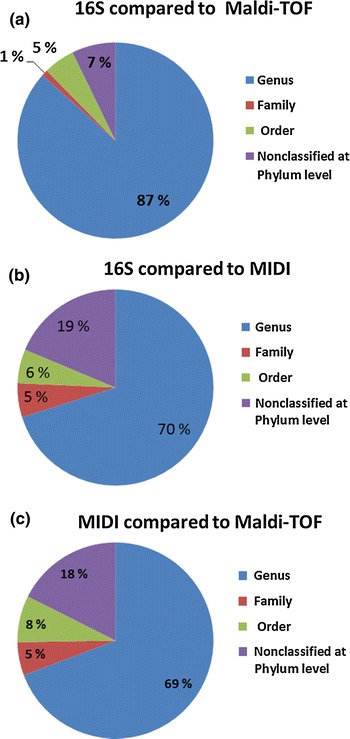
Comparison of the ability of the three methods, 16S rDNA sequencing, MALDI-TOF MS and MIDI MIS analysis for taxonomic assignment of the airborne bacterial isolates (N = 103) to order or higher. The comparison is based on the number of isolates classified to the taxonomic levels genus, family or order using the three methods. a Comparison of 16S rDNA sequencing and MALDI-TOF MS for assignment of 97 bacterial isolates to taxonomic level. b Comparison of 16S rDNA sequencing and MIDI MIS analysis for assignment of 107 bacterial isolates to taxonomic level. c Comparison of MIDI and MALDI-TOF for assignment of 91 bacterial isolates to taxonomic level
When comparing the three different methods for taxonomic identification, 23 % of the 103 isolates were affiliated to same species of Bacillus, Micrococcus, Acinetobacter, Enterococcus and Staphylococcus. The percentage of the 103 isolates affiliated to same genus was 45 %, and the percentage affiliated to same order were 17 %, whereas 15 % were not taxonomically assigned. There are several examples of mismatches between the three methods regarding affiliation of the isolates to genus. In one case, 16S rDNA sequencing suggested Pseudomonas, whereas MALDI-TOF suggested Bacillus. Other examples of mismatches are Bacillus suggested by 16S rDNA sequencing, Micrococcus by MIDI and Staphylococcus by MALDI-TOF; Curtobacterium suggested by 16S rDNA sequencing and MALDI-TOF whereas MIDI suggested Bacillus; Staphylococcus suggested by 16S rDNA sequencing, Salmonella by MIDI and no reliable identification by MALDI-TOF; and Staphylococcus suggested by 16S rDNA sequencing, Stenotrophomonas suggested by MIDI and Acinetobacter suggested by MALDI-TOF.
Discussion
The aim of this study was to investigate the naturally airborne bacteria at different sites on different time points and compare the ability of the three different identification technologies to identify the collected isolates. The selected locations were FFI at Kjeller, Norway, FOI in Umeå, Sweden, and PvTT in Lakiala, Finland. The selected technologies were 16S rDNA sequencing, MALDI-TOF MS and the MIDI MIS analysis. To our knowledge, this is the first time that these three methods have been compared regarding their ability to identify airborne bacterial isolates.
In Norway and Finland, the gram-positive Firmicutes represented by Bacillus and Staphylococcus were dominating. However, in Sweden, the gram-negative Gammaproteobacteria Acinetobacter was dominating. Cultivation-dependent techniques typically display a high proportion of gram-positive bacterial phyla (Atlas and Bartha 1997), and genera such as Micrococcus, Bacillus and Staphylococcus are commonly found in air samples from different sources (Mancinelli and Shulls 1978; Fang et al. 2007; Dybwad et al. 2012, 2014; Fykse et al. 2008). The results obtained in this work emphasize and supports these findings. However, it is challenging to predict how representative the microbial population identified in the present study are based on cultivation, when compared to the real airborne microbial community. It is difficult to find an optimal growth medium for a wide set of viable bacteria in air samples. In this study, TSA agar was chosen based on the requirements for the MIDI MIS analysis. Using different growth media, other bacterial species might have been recovered; however, gram-positive bacteria mainly identified in Norway and Finland are commonly identified in air samples. The results described in this study only represent a set of bacteria found during the three sampling periods in the three countries and are not representing the total bacterial diversity per se at the different sampling sites. Culture methods may also suffer from methodological constrains since bacterial groups can be overestimated, exemplified by Aeromonas and Acinetobacter showing high plating efficiency on nutrient-rich agar (Amann et al. 1995). Culture-independent methods have become important for analysis of bacterial diversity in the environment since <1 % of the bacteria in such communities is cultivable by standard laboratory methods (Amann et al. 1995). Since the year 2000, several culture-independent studies have been published and these studies show that the dominant-bacterial phyla differ in different locations and bacterial sequences affiliated to the gram-negative Proteobacteria are commonly found (Radosevich et al. 2002; Maron et al. 2005; Despres et al. 2007; Fierer et al. 2008), which support the findings of Acinetobacter in Sweden during the first sampling period. A recent study where culture-dependent and culture-independent methods were compared showed that the majority of the cloned sequences (60 %) from an aerosol sample were closely related to the cultured bacteria identified and sequences related to the Gammaproteobacteria, Actinobacteria, Bacteriodetes, and Firmicutes frequently appeared (Fahlgren et al. 2010). The Acinetobacter bacteria are widely found in nature, mostly in water and soil. However, they have also been isolated from the skin, throat, and various other sites in healthy people. Acinetobacter baumanni is often associated with hospital-acquired infections but does not typically colonize healthy people outside hospital settings (Peleg et al. 2008). It is difficult to explain the high level of Acinetobacter isolated in Sweden compared to Norway and Finland. However, during the first experiment in Sweden, the temporal increase in the level of total bacteria showed correlation with sudden wind direction changes (results not shown). This implies that materials may have been stirred up from the ground, e.g., soil or dust particles and that the origin possibly is from a rather localized source. The area around the sampling site in Sweden can be described as a suburban area close to coniferous forest. In general, the weather conditions during the sampling periods were also similar and can hardly explain the observed differences. However, it may also be due to cultivation biases that Acinetobacter would have been overestimated (Amann et al. 1995).
The present study has increased the knowledge about the airborne bacterial background in outdoor air. The performance of a biological detector, including sensitivity and false positive/negative rate, is dependent on the sensor’s operating environment such as the microbial diversity in air. False negative and positive results may occur from detection of non-pathogenic closely related species (Kuske 2006; Brodie et al. 2007).
The gold standard identification method, 16S rDNA sequencing, was compared to the more rapid screening methods MALDI-TOF MS (Benagli et al. 2011) and MIDI MIS Identification System (Slabbinck et al. 2009). In general, all three technologies turned out to be useful methods for identification of bacterial isolates collected from air. The 16S rDNA sequencing method is time-consuming and more laborious compared to MALDI-TOF MS and MIDI analyses. MALDI-TOF MS and MIDI MIS reduce the time required for identification of bacterial isolates since sample preparation and analysis time are shorter (minutes–hours) compared to 16S rDNA sequencing, which includes PCR, sequencing of the PCR amplicons, sequence evaluation and trimming and taxonomic analysis (days). When comparing the ability of the 16S rDNA sequencing and MALDI-TOF MS to identify and assign the bacterial isolates to a taxonomic level, no significant difference between the two technologies appeared, and 87 % of the isolates were assigned to similar genus. This result is in agreement with the conclusion in the study of Dybwad et al. 2012. A significant difference between the 16S rDNA sequencing and MIDI analysis was identified, and a higher percentage of the isolates were not assigned to a taxonomic level (compared to 16S rDNA sequencing and MALDI-TOF) when using the MIDI identification system. One explanation for this difference might be differences in the databases used in the various identification methods. In general, the Biotyper 2.0 used for MALDI-TOF MS have been used extensively for analysis of clinical isolates (Eigner et al. 2009; Patel 2013), and MALDI-TOF MS is well established in clinical laboratories and is used as a high throughput rapid identification method of clinical isolates (Benagli et al. 2011). The database used for MALDI-TOF MS analysis needs to be expanded by several environmental bacteria, which will greatly benefit the use of this method for identification of environmental isolates (Dybwad et al. 2014). The database used for MIDI MIS analysis is for environmental aerobic bacteria. However, an advantage of MALDI-TOF MS compared to MIDI analysis is that the culture media have no effect on the identification. The MIDI analyzes is based on fatty acid methyl ester analysis by gas chromatography, and the database used is based on the use of TSA agar (Slabbinck et al. 2009). The present results are in agreement with other studies showing that the MALDI-TOF MS method is comparable to the 16S rDNA sequencing method for identification of bacterial isolates (Seng et al. 2013). In the case of identification and classification of pathogenic and spoilage bacteria in sea food, the MALDI-TOF MS analysis demonstrated to be a competent bacterial typing tool, and in some cases, MALDI-TOF MS had more discriminating potential than 16S rDNA sequencing (Böhme et al. 2013).
In this study, MALDI-TOF MS using the Biotyper 2.0 has proven to be a fast, accurate and reliable technique for taxonomic identification of airborne bacterial isolates. These results highlight the use of MALDI-TOF as an alternative to the more costly and time-consuming gold standard method 16S rDNA sequencing for identification of cultural environmental bacteria. The MIDI method was also useful; however, the percentages of the isolates identified and classified to genus level were lower, and this method is dependent on the growth media used. In conclusion, this study, where bacterial isolates collected from air was identified, showed that the 16S rDNA sequencing and the MALDI-TOF MS analysis had similar discriminating power, whereas the MIDI method turned out to have less discriminating power in this comparison using the databases specified under the present conditions. The present studies are also important for developing realistic testing and evaluation schemes for biological detection systems by providing microbial background information that can be used to mimic operational environment in aerosol test chambers (Berchebru et al. 2014).
Acknowledgments
This study was financed by the Norwegian Defence Research Establishment (FFI), Oslo University Hospital, Defence Forces Technical Research Centre, CBRN Defence (PvTT), Finland and by the Swedish Defence Research Agency (FOI), Sweden.
References
- Adams DJ, Gurr S, Hogge J. Cellular fatty-acid analysis of Bacillus thuringiensis var. kurstaki commercial preparations. Journal of Agricultural and Food Chemistry. 2005;53(3):512–517. doi: 10.1021/jf0485685. [DOI] [PubMed] [Google Scholar]
- Amann R, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiological Review. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas RM. Bioterriorism: From threat to reality. Annual Review of Microbiology. 2002;56:167–185. doi: 10.1146/annurev.micro.56.012302.160616. [DOI] [PubMed] [Google Scholar]
- Atlas RM, Bartha R. Micobial ecology: Fundamentals and applications. San Fransico, CA: Benjamin/Cummings Publishing Co., Inc.; 1997. [Google Scholar]
- Benagli C, Rossi V, Dolina M, Tonolla M, Petrini O. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for the identification of clinically relevant bacteria. PLoS ONE. 2011;6:e16424. doi: 10.1371/journal.pone.0016424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchebru L, Rameil P, Gaudin J-C, Gausson S, Larigauderie G, Pujol C, Morel Y, Ramisse V. Normalization of test and evaluation of biothreat detection systems: Overcoming microbial air content fluctuations by using a standardized reagent bacterial mixture. Journal of Microbiological Methods. 2014;105:141–145. doi: 10.1016/j.mimet.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Blatny JM, Ho J, Skogan G, Fykse EM, Aarskaug T, Waagen V. Airborne Legionella bacteria from pulp waste treatment plant: Aerosol particles characterized as aggregates and their potential hazard. Aerobiologia. 2011;27:147–162. doi: 10.1007/s10453-010-9184-9. [DOI] [Google Scholar]
- Böhme K, Fernández-No IC, Pazos M, Gallardo JM, Barros-Velázquez J, Cañas B, Calo-Mat P. Identification and classification of seafood-borne pathogenic and spoilage bacteria: 16S rRNA sequencing versus MALDI-TOF MS fingerprinting. Electrophoresis. 2013;34:877–887. doi: 10.1002/elps.201200532. [DOI] [PubMed] [Google Scholar]
- Brodie EL, DeSantis TZ, Parker JP, Zubietta IX, Piceno YM, Andersen GL. Urban aerosols harbor diverse and dynamic bacterial populations. Proceedings of National Academic Science. 2007;104:299–304. doi: 10.1073/pnas.0608255104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PL, Yu PH, Cheng YW, Chan CY, Wong PK. Comprehensive characterization of indoor airborne bacterial profile. Journal of Environmental Science. 2009;21:1148–1152. doi: 10.1016/S1001-0742(08)62395-5. [DOI] [PubMed] [Google Scholar]
- Cherkaoui A, Hibbs J, Emonet S, Tangomo M, Girard M, Francois P, Schrenzel J. Comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry methods with conventional phenotypic identification for routine identification of bacteria to the species level. Journal of Clinical Microbiology. 2010;48:1169–1175. doi: 10.1128/JCM.01881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després, V. R., Huffman, J. A., Burrows, S. M., Hoose, C., Safatov, A. S., Buryak, G., et al. (2012). Primary biological aerosol particles in the atmosphere: A review. Tellus B, 64, 1–58.
- Despres VR, Nowoisky JF, Klose M, Conrad R, Andreae MO, Poschl U. Characterization of primary biogenic aerosol particles in urban, rural, and high-alpine air by DNA sequence and restriction fragment analysis of ribosomal RNA genes. Biogeosciences. 2007;4:1127–1141. doi: 10.5194/bg-4-1127-2007. [DOI] [Google Scholar]
- Drevinek M, Dresler J, Klimentova J, Pisa L, Hubalek M. Evaluation of sample preparation methods for MALDI-TOF MS identification of highly dangerous bacteria. Letters in Applied Microbiology. 2012;55(1):40–46. doi: 10.1111/j.1472-765X.2012.03255.x. [DOI] [PubMed] [Google Scholar]
- Dybwad M, Granum PE, Bruheim P, Blatny JM. Characterization of airborne bacteria at an underground subway station. Applied and Environmental Microbiology. 2012;78:1917–1929. doi: 10.1128/AEM.07212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybwad M, Skogan G, Blatny JM. Temporal variability of the bioaerosol background at a subway station: Concentration level, size distribution and diversity of airborne bacteria. Applied and Environmental Microbiology. 2014;80:257–270. doi: 10.1128/AEM.02849-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigner U, Holfelder M, Oberdorfer K, Betz-Wild U, Bertsch D, Fahr AM. Performance of a matrix-assisted laser desorption ionization-time-of-flight mass spectrometry system for the identification of bacterial isolates in the clinical routine laboratory. Clinical Laboratory. 2009;55:289–296. [PubMed] [Google Scholar]
- Fahlgren C, Hagström A, Nilsson D, Zweifel UL. Annual variations in the diversity, viability, and origin of airborne bacteria. Applied and Environmental Microbiology. 2010;76:3015–3025. doi: 10.1128/AEM.02092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Ouyang Z, Zheng H, Wang X, Hu L. Culturable airborne bacteria in outdoor environments in Beijing, China. Microbial Ecology. 2007;54:487–496. doi: 10.1007/s00248-007-9216-3. [DOI] [PubMed] [Google Scholar]
- Fierer N, Liu Z, Rodríguez-Hernández M, Knight R, Henn M, Hernandez MT. Short-term temporal variability in airborne bacterial and fungal populations. Applied and Environmental Microbiology. 2008;74:200–207. doi: 10.1128/AEM.01467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fykse EM, Aarskaug T, Trane I, Blatny JM. Legionella and non-Legionella bacteria in a biological treatment plant. Canadian Journal of Microbiology. 2013;59:102–109. doi: 10.1139/cjm-2012-0166. [DOI] [PubMed] [Google Scholar]
- Fykse EM, Langseth B, Skogan G, Olsen JS, Blatny JM. A study of the diversity of viable airborne bacteria—Use of real time PCR for detection of bioterror agents in air. Journal of Applied Microbiology. 2008;105:351–358. doi: 10.1111/j.1365-2672.2008.03750.x. [DOI] [PubMed] [Google Scholar]
- Gilbert Y, Duchaine C. Bioaerosols in industrial environments: A review. Canadian Journal of Civil Engineering. 2009;2009(36):1873–1886. doi: 10.1139/L09-117. [DOI] [Google Scholar]
- Ho J, Spence M, Duncan S. An approach towards characterizing a reference sampler for culturable biological particle measurement. Journal of Aerosol Science. 2005;36:557–573. doi: 10.1016/j.jaerosci.2004.11.020. [DOI] [Google Scholar]
- Kuske CR. Current and emerging technologies for the study of bacteria in outdoor air. Current Opinion of Biotechnology. 2006;17:291–296. doi: 10.1016/j.copbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Levin DB, De Amorim GV. Potential aerosol dissemination of biological weapons: Lessons from biological control of insects. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science. 2003;1:37–42. doi: 10.1089/15387130360514814. [DOI] [PubMed] [Google Scholar]
- Mancinelli RL, Shulls WA. Airborne bacteria in an urban environment. Applied and Environmental Microbiology. 1978;35:1095–1101. doi: 10.1128/aem.35.6.1095-1101.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal J, Brandl H. Bioaerosols in indoor environment—A review with special reference to residential and occupational locations. Open Environmental and Biological Monitoring Journal. 2011;2011(4):83–96. [Google Scholar]
- Maron PA, Lejon DPH, Carvalho E, Bizet K, Lemanceau P, Ranjard L, Mougel C. Assessing genetic structure and diversity of airborne bacterial communities by DNA fingerprinting and 16S rDNA clone library. Atmospheric Environment. 2005;39(20):3687–3695. doi: 10.1016/j.atmosenv.2005.03.002. [DOI] [Google Scholar]
- Ozbek A, Aktas O. Identification of three strains of Mycobacterium species isolated from clinical samples using fatty acid methyl ester profiling. Journal of International Medical Research. 2003;31(2):133–140. doi: 10.1177/147323000303100210. [DOI] [PubMed] [Google Scholar]
- Patel R. Matrix assisted laser desorption ionization-time of flight mass spectrometry in clinical microbiology. Clinical Infectious Diseases. 2013;57(4):564–572. doi: 10.1093/cid/cit247. [DOI] [PubMed] [Google Scholar]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a successful pathogen. Clinical Microbiological Review. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radosevich JL, Wilson WJ, Shinn JH, DeSantis TZ, Andersen GL. Development of a high-volume aerosol collection system for the identification of airborne microorganisms. Letters in Applied Microbiology. 2002;34:162–167. doi: 10.1046/j.1472-765x.2002.01048.x. [DOI] [PubMed] [Google Scholar]
- Sauer S, Freiwald A, Maier T, Kube M, Reinhardt R, Kostrzewa M, Geider K. Classification and identification of bacteria by mass spectrometry and computational analysis. PLoS ONE. 2008;3(7):e2843. doi: 10.1371/journal.pone.0002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng P, Abat C, Rolain JM, Colson P, Lagier JC, Gouriet F, Fournier PE, Drancourt M, La Scola B, Raoult D. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: Impact of MALDI-TOF mass spectrometry. Journal of Clinical Microbiology. 2013;51(7):2182–2194. doi: 10.1128/JCM.00492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbinck B, De Baets B, Dawyndt P, De Vos P. Towards large-scale FAME-based bacterial species identification using machine learning techniques. Systemic and Applied Microbiology. 2009;32(3):163–176. doi: 10.1016/j.syapm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Stetzenbach LD, Buttner MP, Cruz P. Detection and enumeration of airborne biocontaminants. Current Opinion in Biotechnology. 2004;15:170–174. doi: 10.1016/j.copbio.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Van den Velde S, Lagrou K, Desmet K, Wauters G, Verhaegen J. Species identification of corynebacteria by cellular fatty acid analysis. Diagnostic Microbiology and Infectious Disease Journal. 2006;54(2):99–104. doi: 10.1016/j.diagmicrobio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PCY, Ng KHL, Lau SKP, Yip K, Fung AMY, Leung K, Tam DMW, Que T, Yuen K. Usefulness of the MicroSeq 500 16S ribosomal DNA-based bacterial identification system for identification of clinically significant bacterial isolates with ambiguous biochemical profiles. Journal of Clinical Microbiology. 2003;41:1996–2001. doi: 10.1128/JCM.41.5.1996-2001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


