Abstract
The objective of this prospective study was to evaluate the clinical and laboratory parameters distinguishing viral from nonviral lower respiratory tract infection in elderly patients and to determine the yield of virological diagnostics in elderly patients with lower respiratory tract infection. The study was conducted in a 184-bed geriatric department in a university hospital during 4 winter months. All consecutive elderly persons admitted with a lower respiratory tract infection were included in the study. Clinical and laboratory parameters, a nasopharyngeal swab, and serological results for respiratory viruses were obtained for all participants. Available blood and sputum cultures were analysed. A total of 165 elderly persons (mean age, 82±6.8 years) were hospitalised with a lower respiratory tract infection. Familial flu-like illness (OR, 4.25; 95%CI, 1.4–13), better functionality (OR, 4; 95%CI, 1.3–14.15), and leucocyte count <1010/l (OR, 3; 95%CI, 1.3–7.1) were predictive for viral lower respiratory tract infection. Sixty (36.5%) definite diagnoses (positive blood culture, viral culture, or serological test) and seven (4.2%) probable diagnoses (positive sputum culture) were obtained. An early diagnosis (within 72 h) was possible in 38 (23%) and a late diagnosis in 29 (17.6%) participants. A nasopharyngeal swab contributed in 60.5% of the cases to an early diagnosis. Viral culture identified half (22/43) of the lower respiratory tract infections caused by influenza but only one of six lower respiratory tract infections caused by respiratory syncytial virus. In conclusion, a history of flu-like illness in family members and a total leucocyte count within normal limits makes a viral cause more likely in elderly people hospitalised with a lower respiratory tract infection during winter. Viral culture and rapid antigen detection are insensitive in elderly patients hospitalised with a lower respiratory tract infection.
Keywords: Influenza, Respiratory Syncytial Virus, Nursing Home Resident, Lower Respiratory Tract Infection, Respiratory Syncytial Virus Infection
Introduction
Lower respiratory tract infection (LRTI) is the primary cause of hospitalisation in elderly patients with infectious disease. Influenza virus and respiratory syncytial virus (RSV) are the most important viral causes of LRTI in the elderly. Both viruses cause excess hospitalisation, pneumonia, and mortality in the elderly during the winter [1]. These effects are even more pronounced in nursing home residents and persons with high-risk factors such as cardiopulmonary disease [2, 3, 4].
Laboratory confirmation of influenza and RSV is seldom done. The atypical presentation of LRTI in the elderly, the time span before results are available, the lack of etiological therapy for viral LRTI, and the need for early empirical antibiotic treatment when pneumonia is suspected hamper the use of virological diagnostics.
We conducted this study to investigate the possibility of recognizing viral pathogens in the elderly hospitalised with an LRTI and to evaluate the yield of standard virological diagnostics available in our centre.
Materials and Methods
Study Period
From 1 December 1997 to 31 March 1998, all consecutive patients over 69 years of age with an LRTI admitted to the geriatric ward (a total of 184 beds) of the University Hospital of Leuven were included in the study. Patients younger than 70 years of age or admitted to nongeriatric wards were excluded.
Data Collection
Demographic data, pre-illness data (contact history, comorbidities, antibiotic use, and mental, functional and vaccination status), illness data (date of onset, constitutional symptoms, upper respiratory tract infection, LRTI symptoms, clinical findings, and functional and mental status), baseline chemistry, and chest radiograph were obtained for each participant.
Definitions
LRTI was defined as the presence of at least two of the following symptoms, clinical signs, or radiographic findings: new or evolving cough, dyspnoea, sputum production, clinical signs of LRTI (rales, wheezing, bronchial breathing, crepitus, or silence), fever (≥38°C), or an infiltrate on chest radiograph. Pneumonia was defined as an LRTI with an infiltrate on chest radiograph. An acute exacerbation of chronic obstructive pulmonary disease (COPD) was defined as an LRTI in a patient with pre-existing COPD without an infiltrate on chest radiograph. Acute bronchitis was defined as an LRTI in a patient without COPD and without an infiltrate on chest radiograph.
Functional status and mental status of the patient were assessed with scores derived from the literature [5, 6]. The scores range from 0 to 10. A functional score of less than four was considered indicative of functional independency. A mental score of less than eight was considered indicative of cognitive impairment.
Microbiological Assessment
Cultures for bacteriological investigations (blood and/or sputum) were taken on admission to the emergency department upon clinical judgment. A supplementary nasopharyngeal swab for viral culture and RSV antigen detection and an acute (on admission) and convalescent (after 4 weeks or earlier if discharged) blood sample for serological investigations for influenza A and B, parainfluenza 1, 2, and 3, RSV, adenovirus, Mycoplasma pneumoniae, and Chlamydia pneumoniae by complement fixation assay were taken. A definitive etiology for an LRTI was obtained by recovery of a pathogen in blood, by viral culture, by RSV antigen detection (early diagnostic tools yielding an etiologic diagnosis within 72 h after admission) or by a fourfold rise in serological titres (late diagnostic tool). A probable cause of LRTI was obtained by culture of a good-quality sputum sample (more polymorphonuclear cells than squamous epithelial cells and presence of 1 predominating pathogen).
Statistical Analysis
Student’s t test and the Mann-Whitney U test for continuous variables and the chi-square or Fisher’s exact test for categorical variables were used for univariate analysis. The statistically significant parameters were put into a stepwise logistic regression analysis to determine whether the clinical and laboratory features were associated with the presence or absence of an LRTI. A P value <0.05 was considered statistically significant. The results of the logistic regression are presented as odds ratios with the 95% confidence intervals. All analyses were performed with SAS 6.12 statistical software.
A written (in 79 of 165 patients) or oral (in 86 of 165 patients) witnessed informed consent was obtained from each participant prior to inclusion. The study protocol was approved by the ethics committee of the University Hospital of Leuven.
Results
A total of 165 patients out of 874 consecutive admissions to the geriatric ward during the study period exhibited LRTI symptoms (Fig. 1). These 165 patients were considered as the study population and had a mean age of 82 years (SD±6.8 years) and a male-to-female ratio of 0.85. The average length of hospital admission was 26 days (±19.4 days, range 5–107 days). The advanced age of the study population, the presence of comorbid disease, the functional and cognitive impairment with re-education within the same unit where initially admitted, and a significantly longer hospitalisation for patients (8/165) unable to return home and waiting for admission to a nursing home (50±13.4 days vs. 21±24.8 days, P<0.001) accounted for this long mean hospital stay. A total of 108 (65.5%) patients lived at home and 57 (34.5%) in nursing homes. Eighty-nine (54%) patients had pneumonia, 25 (15%) presented with exacerbations of pre-existing COPD, and 51 (31%) presented with acute bronchitis. Twenty-two (13%) patients died in hospital (Table 1).
Fig. 1.
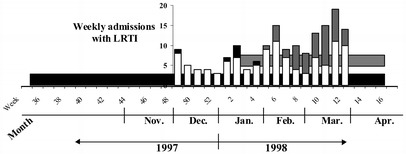
Influenza and RSV activity in the community, and hospitalisation for lower respiratory tract infection (LRTI) in the elderly. Horizontal bars show the RSV (black) and influenza (grey) activity in the community for the winter of 1997–1998 documented by the national surveillance system for acute respiratory tract infections in Belgium. Vertical bars show the weekly study inclusions with LRTI; a black top represents the LRTI caused by RSV and a grey top those caused by influenza
Table 1.
Characteristics of the patients
| Characteristic | Place of residency | P-value | |
|---|---|---|---|
| Home (n=108) | Nursing home (n=57) | ||
| Mean age in years (±SD) | 81.4 (±6.6) | 83.4 (±7.1) | 0.069 |
| Sex (M/F) | 53/55 | 23/34 | 0.366 |
| LRTI (no. [%]) | |||
| Pneumonia | 56 (62.9) | 33 (37.1) | 0.564 |
| Bronchitis | 37 (72.5) | 14 (27.5) | 0.269 |
| AECOPD | 15 (60) | 10 (40) | 0.693 |
| Comorbidities, mean (±SD) | 1.8 (±1.2) | 2.3 (±1.3) | 0.005 |
| Mortality, no. (%) | 10 (9.3) | 12 (21.1) | 0.061 |
| Antibiotic therapya, no. (%) | |||
| Prior to admission | 30 (27.8) | 24 (42.1) | 0.070 |
| Functional statusb, mean (±SD) | |||
| Prior to admission | 1.3 (±2.3) | 4.7 (±3.3) | <0.001 |
| Mental status | |||
| Dementia, no. (%) | 7 (6.5) | 23 (40.4) | <0.001 |
| Hodkinson scorec, mean (±SD) | |||
| On admission | 8.7 (±2.7) | 4.7 (±4.5) | <0.001 |
| Leucocyte count (109/l), mean (±SD) | 9.95 (±5.1) | 11.5 (±6.6) | 0.108 |
| CRP (mg/l), mean (±SD) | 101.3 (±97.2) | 112.4 (±108.6) | 0.404 |
| Albumin (g/l), mean (±SD) | 33.7 (±5.8) | 30.6 (±5.1) | 0.023 |
| Ureum (mg/dl), mean (±SD) | 52.1 (±22.6) | 63.8 (±35) | 0.012 |
LRTI, lower respiratory tract infection; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CRP, C-reactive protein
aAntibiotic therapy: patients on antibiotics before hospitalisation
bFunctional score (0–10): normal score <4
cHodkinson score (0–10): normal score ≥8
Anamnestic and Clinical Data
The variables distinguishing proven viral LRTI from other LRTI (bacterial and of unknown aetiology) in a univariate analysis are listed in Table 2. In a stepwise logistic regression, familial flu-like illness (i.e. constitutional or RTI symptoms in relatives, not nursing home staff) (OR, 4.25; 95%CI, 1.4–13), better functionality (OR, 4; 95%CI, 1.3–14.15), and leucocyte count <1010/l (OR, 0.34; 95%CI, 0.14–0.81) remained independent predictors of viral LRTI in this population. Antibiotic use prior to hospitalisation (OR, 5.3; 95%CI, 2.05–13.73) also was associated with viral etiology, but this may be due to underestimation of bacterial causes in antibiotic-treated patients.
Table 2.
Proven viral LRTI versus LRTI caused by bacteria and of unknown origin
| Variable | Other (n=114) | Viral (n=51) | P value |
|---|---|---|---|
| Mean age in years (±SD) | 82.6 (±6.95) | 80.9 (±6.4) | 0.133 |
| Sex (M/F) | 56/58 | 20/31 | 0.312 |
| Residency, home/nursing home | 72/41 | 35/16 | 0.664 |
| Mortality, no. (%) | 19 (16.7) | 3 (5.9) | 0.091 |
| Pneumonia, no. (%) | 62 (54.4) | 27 (52.9) | 0.997 |
| Constitutional symptomsa, % | 85.6 | 39.4 | 0.952 |
| URT symptomsb, % | 30.3 | 13.7 | 0.933 |
| Familial flu-like illness, % | 8.9 | 28.6 | 0.007 |
| Antibiotic therapy on admission, % | 28.69 | 45.65 | 0.044 |
| Functional statusc prior to hospitalisation, mean (±SD) | 2.9 (±3.3) | 1.7 (±2.6) | 0.042 |
| Hodkinson scored on admission, mean (±SD) | 6.7 (±4.1) | 8.7 (±2.9) | 0.005 |
| Platelet count (109/l), mean (±SD) | 296.4 (±252.2) | 211.2 (±90.6) | 0.02 |
| Leucocyte count (109/l), mean (±SD) | 11.5 (±6.2) | 8.2 (±3.3) | <0.001 |
| LDH (U/l), mean (±SD) | 451.9 (±275.2) | 623.7 (±770.5) | 0.05 |
| Ureum (mg/dl), mean (±SD) | 9.4 (±20.9) | 59.2 (±30.1) | 0.04 |
LRTI, lower respiratory tract infection; URT, upper respiratory tract; LDH, lactate dehydrogenase
aAny of the following symptoms: headache, myalgia, arthralgia, asthenia, abdominal pain, anorexia, chills, emesis, or diarrhoea
bAny of the following symptoms: nasal congestion, periorbital pain, rhinorrhoea, earache, or sore throat
cFunctional score (0–10): normal score <4
dHodkinson score (0–10): normal score ≥8
The variables distinguishing influenza (n=43) and RSV (n=6) as a cause of LRTI in a univariate analysis were as follows: nursing home residency (30% in influenza LRTI vs. 83% in RSV LRTI; P=0.005), pneumonia (46.5% vs. 100%; P=0.02), functional status (mean functional score of 1.2±2 vs. 5.7±SD; P=0.008), and leucocyte count (mean of 7.8 [±3.1]×109/l vs. 10.9 [±3.1]×109/l; P=0.03). Due to the small sample size of the RSV group, no independent variable could distinguish between influenza and RSV in a logistic regression.
Microbiological Results
The yield of diagnostic techniques and different pathogens are listed in Table 3. No etiologic pathogen could be identified in 59.9% of LRTI cases. Blood culture was performed in 134 (81%) patients and yielded a definite diagnosis in 8 (6%) cases. There were six cases of bacteraemia caused by Streptococcus pneumoniae, one case caused by Staphylococcus aureus, and one case of gram-negative bacteraemia. Sputum was obtained in 79 (47.9%) patients. Only eight (10%) of the sputum-samples met the quality criteria.
Table 3.
Etiologic diagnoses
| Pathogen | Total diagnoses | Diagnostic accuracy | Timing of diagnosis after admission | Delay LRTI onset – admissionf | ||||
|---|---|---|---|---|---|---|---|---|
| Definitea | Probableb | Earlyc diagnosis | Lated diagnosis | ≤3 days (n=70) | 4–7 days (n=24) | >7 days (n=30) | ||
| diagnosis | diagnosis | |||||||
| Influenza | 43 | 43 (100%) | 0 | 22 (51.2%) | 21 (48.8%) | 20 (46.5%) | 7 (16.3%) | 9 (20.9%) |
| RSV | 6 | 6 (100%) | 0 | 1 (16.6%) | 5 (83.4%) | 1 (16.6%) | 0 | 1 (16.6%) |
| Parainfluenza | 2 | 2 (100%) | 0 | 0 | 2 (100%) | 2 (100%) | 0 | 0 |
| S. pneumoniae | 6 | 6 (100%) | 0 | 6 (100%) | 0 | 2 (33.3%) | 2 (33.3%) | 2 (33.4%) |
| S. aureus | 2 | 1 (50%) | 1 (50%) | 2 (100%) | 0 | 0 | 1 (50%) | 0 |
| Gram negativee | 7 | 1 (14%) | 6 (86%) | 7 (100%) | 0 | 3 (42.9%) | 1 (14.3%) | 2 (28.5%) |
| M. pneumoniae | 1 | 1 (100%) | 0 | 0 | 1 (100%) | 0 | 1 (100%) | 0 |
| Total (n=165) | 67 (40.1%) | 60 (36.4%) | 7 (4.2%) | 38 (23%) | 29 (17.6%) | 28 (17%) | 12 (7.3%) | 14 (8.5%) |
RSV, respiratory syncytial virus
aDefinite diagnosis: positive hemoculture, positive viral culture, fourfold rise in serological titres
bProbable diagnosis: good-quality sputum (i.e. more polymorphonuclear than squamous epithelial cells) and a sputum culture with 1 pathogen
cEarly diagnosis: established ≤72 h after admission
dLate diagnosis: established >72 h after admission (i.e. fourfold rise in serological titres)
eGram-negative: A. lwoffi 1 positive blood culture; H. influenzae 2, E. coli 3, E. cloacae 1 positive sputum cultures
fFor 41 patients, exact onset was indefinable
When admitted 1 week or later after the onset of LRTI, more patients were taking antibiotics on admission than those hospitalised within 1 week of LRTI onset (53% vs. 40%, P=0.014). However, there was no significant difference in diagnostic yield between patients hospitalised within 3 days, between 4 and 7 days, and after 7 days of LRTI onset.
A definite aetiological diagnosis (by blood culture, nasopharyngeal swab, or serological investigation) was found in 60 of 165 (36.4%) of the patients presenting with an LRTI and a probable diagnosis (by sputum culture) in 7 of 165 (4.2%). Of these diagnoses, 56.7% (38/67) could be made within 72 h after admission by blood and/or sputum culture, viral culture, and antigen detection. The nasopharyngeal swab contributed 60.5% (23/38) of the early and 38.3% (23/60) of the definite etiological diagnoses. Of the LRTIs caused by influenza, 22 of 43 (51.2%) were detected by viral culture of a nasopharyngeal swab. Only one of the six RSV infections was documented by viral culture of a nasopharyngeal swab.
Direct antigen detection of RSV was negative in all cases.
Two serologically proven infections with parainfluenza and one with Mycoplasma pneumoniae were documented. No infection with Chlamydia pneumoniae or adenovirus was documented.
Superinfection (viral LRTI with 1 predominant bacterium in a good-quality sputum sample) was documented in three patients (2 with influenza and 1 with RSV). Mixed infection (good-quality sputum with more than 1 pathogen) was documented in three patients.
Discussion
Viral LRTI is an important cause of hospitalisation, pneumonia, and death in the elderly during winter. A definite etiological diagnosis of the LRTI may influence the management of these patients. In our analysis, no constitutional or respiratory symptom could adequately predict a viral cause of the LRTI. The combination of the three symptoms fever (≥38°C), acute onset (≤7 days), and cough have been shown to have a positive predictive value for the presence of influenza in 44% of elderly patients visiting their general practitioners with influenza-like illness and in 47% of elderly patients hospitalised with cardiopulmonary conditions during the influenza season [7, 8, 9].
The national surveillance system for acute respiratory tract infections in Belgium documents acute respiratory tract infections in sentinel practices and the aetiology in collaboration with a nationwide network of reference laboratories. During the study period, RSV infections were documented from the end of September until April, with a peak observed in late December. Influenza infections occurred from the end of January until April, with a peak occurring in late February (Fig. 1) [10].
In our study, the combination of these three symptoms had a predictive value of 26% for any viral LRTI during the whole study period. The predictive value of the symptom combination for influenza for the whole study period (influenza prevalence of 26%) was 30%, for the period that influenza was circulating in the community (prevalence 33%) 35%, and for the period since the first influenza hospitalisation (prevalence 40%) 40%. This demonstrates the need for active surveillance in the community and even in the hospital setting for influenza when influenza infection is sought based on clinical judgment. The lower predictive value in our analysis is partially due to the restriction of our population to LRTI in which fever (59%), cough (88%), and acute onset (76%) are prevalent and to the exclusion of influenza-like illness without LRTI symptoms and admissions for cardiac conditions that could have been caused by viral infections.
The prevalence of RSV LRTI in the study was 4%. This figure falls within the range documented (year, 2–5%; winter, 5–15%) in studies of RSV as an aetiology for hospitalisation in the elderly. Upper respiratory tract symptoms and wheezing seem to be more prevalent in RSV infections in these studies [11].
A comparison between influenza and RSV infections was not possible due to the low number of proven RSV infections. In studies comparing RSV and influenza in elderly patients, wheezing or therapy for bronchospasm was more prevalent in RSV infections while fever, constitutional symptoms, and gastrointestinal symptoms were more prevalent in influenza infections [12]. All patients with RSV in this study had pneumonia on admission, and five of the six patients were frail nursing home residents. The rate of pneumonia in elderly patients with RSV infection varies with the population studied. For community-dwelling elderly, the rate of pneumonia in RSV infection is estimated at 2–5% throughout the year and at 5–15% in winter, for nursing home residents at 10–20%, and for hospitalised elderly at 44–63% [11, 13].
We identified additional variables that are associated with a viral origin of LRTI. The exposure to family members with flu-like illness distinguishes viral from nonviral infections. Other caregivers in close contact with elderly can also transmit viruses, but this information was not available. Contact (and travel) history is a valuable tool, as has been shown in the recent battle against the SARS epidemics in China and Canada [14].
A functional independency prior to admission also was a risk factor for hospitalisation with a viral LRTI. It is well known that hospitalisation rates for influenza and pneumonia are five times higher in elderly without high-risk conditions than in young adults (13–23 per105 persons/year vs. 125–228 per 105 persons/year) and are even higher in elderly with high-risk conditions (399–518/105 persons/year) [2, 15].
For RSV-associated pneumonia, the hospitalisation rate is 40–180/105 persons/year in all elderly and 50–230/105 persons/year in nursing home residents [3]. An association between high-risk conditions, poor functionality, and bacterial infection is possible.
A nonelevated leucocyte count (<1010/l) was associated with viral LRTI. This has been documented in other studies as well for both influenza and RSV [12, 16].
The aetiological diagnosis of LRTI in hospitalised elderly is hampered by many obstacles. In 60% of LRTIs in our study, no causative pathogen was identified. The impact of antibiotic treatment before diagnostic sampling and the low sensitivity of the diagnostic tools used will be discussed below. Other pathogens could have been present. Up to 9% of winter hospitalisations for cardiopulmonary conditions in the elderly are caused by rhinovirus and coronavirus [17]. Legionella pneumophila and Mycobacterium tuberculosis also cause LRTI. Diagnostic tools to identify these pathogens were not used in this study. Finally, misclassification of symptomatic heart failure and pulmonary disease (embolism, cancer, and interstitial lung disease) as an LRTI is possible.
The sputum samples were difficult to obtain (49% of the study population) and of poor quality, since only 10% of the sputum samples met the quality criteria. Many patients (33%) already received antibiotics prior to admission. Although a good-quality sputum can predict the bacterial aetiology of pneumonia, the yield of these samples is diminished in the elderly (≥75 years), in antibiotic pretreated patients, and in mild-to-moderate (rather than severe) pneumonia [18, 19]. Oropharyngeal colonisation with gram-negative bacilli can be misleading [19]. Therefore, the usefulness of routine sputum culture in this population must be questioned. At least there should be a selection for macroscopically purulent samples of patients not treated with antibiotics.
Six percent of blood cultures yielded a definite diagnosis. Pretreatment with antibiotics and less severe pneumonia are also associated with a reduced diagnostic yield of blood cultures [20, 21].
Serological diagnosis with a fourfold rise in titers is retrospective and, upon admission, high acute titers can already be present in many patients since the LRTI developed an average of 5 days before admission. Moreover, previous infections or vaccination can produce circulating antibodies as well.
The serologic assay used was a complement fixation. A higher sensitivity could be obtained with an enzyme immunoassay, as documented for influenza and RSV [22, 23].
Direct demonstration of the viral pathogen is also difficult. Half of the serologically proven influenza LRTIs and only one of six RSV LRTIs were culture positive. This corresponds with findings in other studies examining viral culture yields for the identification of influenza and RSV. This is attributable to a shorter duration and lower titre of viral shedding in the elderly compared with children and adults [24, 25, 26].
Rapid antigen detection with immunofluorescence or enzyme immunoassay is less sensitive than viral culture [13, 24, 27, 28]. In our study, the RSV antigen detection enzyme immunoassay revealed no RSV infection. The results of these rapid antigen detection assays depend on the age of the studied population (good sensitivity [75–95%] in children), the immune-status of the population, the type of specimen studied, the time of collection after disease onset, and the sample processing [29, 30].
Treatment of influenza with amantadine, rimantidine, or neuraminidase inhibitors must start within 48 h after disease onset to be effective. In our study, only 39% of the patients with influenza LRTI presented within 2 days after disease onset. When there is a high probability of influenza, antiviral therapy and precautionary measures to reduce nosocomial spread in closed settings like nursing homes and hospitals can be started in patients presenting with the symptoms described above [31].
In conclusion, our results show that during the winter a viral infection is an important cause of LRTI requiring hospitalisation in elderly people. A history of familial flu-like illness and a nonelevated leucocyte count suggests a viral etiology. Etiological diagnosis can be obtained in 40% of the patients, mostly by serological tests or viral culture of a nasopharyngeal swab. Better diagnostic tools are required to identify bacterial and viral causes of LRTI in order to more adequately stratify initial therapeutic and preventive approaches. Molecular diagnostic tests (e.g., real-time polymerase chain reaction) may offer new opportunities in this respect.
References
- 1.Fleming Lancet. 1993;342:1507. doi: 10.1016/s0140-6736(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 2.Simonsen J Infect Dis. 2000;181:831. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 3.Han J Infect Dis. 1999;179:25. [Google Scholar]
- 4.Falsey J Am Geriatr Soc. 1995;43:30. doi: 10.1111/j.1532-5415.1995.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickinson Lancet. 1990;335:778. doi: 10.1016/0140-6736(90)90881-5. [DOI] [PubMed] [Google Scholar]
- 6.Hodkinson Age Ageing. 1972;1:233. doi: 10.1093/ageing/1.4.233. [DOI] [PubMed] [Google Scholar]
- 7.Govaert Fam Practice. 1998;15:16. doi: 10.1093/fampra/15.1.16. [DOI] [PubMed] [Google Scholar]
- 8.Walsh J Am Geriatr Soc. 2002;50:1498. doi: 10.1046/j.1532-5415.2002.50404.x. [DOI] [PubMed] [Google Scholar]
- 9.Zambon Arch Intern Med. 2001;161:2116. doi: 10.1001/archinte.161.17.2116. [DOI] [PubMed] [Google Scholar]
- 10.Snacken Vax Info. 1998;22:6. [Google Scholar]
- 11.Falsey J Infect Dis. 1995;172:389. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 12.Wald J Am Geriatr Soc. 1995;43:170. doi: 10.1111/j.1532-5415.1995.tb06384.x. [DOI] [PubMed] [Google Scholar]
- 13.Falsey Clin Microbiol Rev. 2000;13:371. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poutanen N Engl J Med. 2003;348:1995. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 15.Centers MMWR. 2002;51:1. [Google Scholar]
- 16.Hulson J Fam Pract. 2001;50:1051. [PubMed] [Google Scholar]
- 17.Falsey J Infect Dis. 2002;185:1338. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roson Clin Infect Dis. 2000;31:869. doi: 10.1086/318151. [DOI] [PubMed] [Google Scholar]
- 19.Ewig Chest. 2002;121:1486. doi: 10.1378/chest.121.5.1486. [DOI] [PubMed] [Google Scholar]
- 20.Waterer Respir Med. 2001;95:78. doi: 10.1053/rmed.2000.0977. [DOI] [PubMed] [Google Scholar]
- 21.Glerant Respir Med. 1999;93:208. doi: 10.1016/s0954-6111(99)90010-0. [DOI] [PubMed] [Google Scholar]
- 22.Steinhoff J Clin Microbiol. 1980;12:447. doi: 10.1128/jcm.12.3.447-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julkunen J Virol Methods. 1984;9:7. doi: 10.1016/0166-0934(84)90078-8. [DOI] [PubMed] [Google Scholar]
- 24.Rebelo-de-Andrade Epidemiol Infect. 2000;124:515. doi: 10.1017/s0950268899003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falsey J Clin Microbiol. 2002;40:817. doi: 10.1128/JCM.40.3.817-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins J Infection. 2002;45:10. [Google Scholar]
- 27.Falsey J Am Geriatr Soc. 1996;44:71. doi: 10.1111/j.1532-5415.1996.tb05641.x. [DOI] [PubMed] [Google Scholar]
- 28.Steininger J Clin Microbiol. 2002;40:2051. doi: 10.1128/JCM.40.6.2051-2056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrmann J Clin Microbiol. 2001;39:134. [Google Scholar]
- 30.Van Clin Infect Dis. 2002;34:177. [Google Scholar]
- 31.Sintchenko J Clin Virol. 2002;25:15. [Google Scholar]


