Abstract
Twenty-five serum samples of 22 free-living European wildcats (Felis silvestris) captured from 1991 to 1993 in central Spain were tested for evidence of exposure to seven feline pathogens. All the wildcats but one (95.4%) presented evidence of contact with at least one of the agents (mean = 2.2). Contact with feline leukemia virus (FeLV) was detected in 81% of the wildcats (antibodies, 77%; antigen p27, 15%). Antibodies to feline calicivirus (FCV, 80%), feline herpesvirus (FHV, 20%), feline parvovirus (FPV, 18%), and Chlamydophila sp. (27%) were also detected. Analyses were negative for feline immunodeficiency virus and feline coronavirus. The probability of having antibodies to FPV was inversely related with the concentration of serum cholesterol and with a morphometric index of body condition. Similarity in the composition of antibodies against disease agents (number and identity of detected and undetected antibodies) was significantly higher in pairs of female wildcats than in pairs of males or heterosexual pairs, suggesting that females had a more homogeneous exposure to pathogens. Seroprevalence for FHV was higher in males than in females. Antibodies to FHV and Chlamydophila sp. were more frequent in winter than in other seasons. In addition, the mean similarity of the pathogen community between pairs of serum samples was higher if both wildcats were caught during the same season than if they were not. Mean similarity was lowest when serum samples obtained in winter were compared with those from spring or summer. The results suggest that some agents probably had a reservoir in domestic cats and may cause some undetected morbidity/mortality in the studied wildcat population, whereas others, such as FeLV and FCV, may be enzootic.
Keywords: Felid, Feline leukemia, Feline respiratory disease, Seasonality, Serology
Introduction
The European wildcat (Felis silvestris Schreber 1777) is catalogued as vulnerable (IUCN 2007). The wildcat has a scattered distribution in Europe, where it has disappeared from much of its original distribution area, resulting in severe fragmentation of its populations. The Mediterranean area that includes the Iberian Peninsula represents one half of the distribution range of the species in Europe (Lozano et al. 2003). According to García-Perea (2002), the wildcat population in Spain could have declined and experienced considerable fragmentation. Acknowledged threats are persecution, habitat loss, and hybridization with domestic cats. However, the importance of other factors of decline has been so far neglected. In particular, an increasing concern exists about the role of infectious diseases as a cause of decline of populations of some wild species (Smith et al. 2006). Domestic cats are common in the dominant cultural landscapes of Mediterranean Europe, allowing for their interaction with wildlife and increasing the potential for disease transmission between domestic cats and wildcats. In fact, wildcats from a number of locations in northern Europe host viruses commonly found in domestic cats (McOrist et al. 1991; Artois and Remond 1994; Daniels et al. 1999; Leutenegger et al. 1999; Fromont et al. 2000). Contact with the pathogenic Feline leukemia virus (FeLV) was detected in all these studies. Fromont et al. (2000) also detected infections with the Feline immunodeficiency virus (FIV). In contrast, Račnik et al. (2008) failed to detect evidence of contact with FeLV and FIV in wildcats in Slovenia.
Excepting a few parasitological studies (Torres et al. 1989; Rodríguez and Carbonell 1998), no information is available about the disease agents infecting the European wildcat in the Iberian Peninsula or other areas of the Mediterranean region. FIV, FeLV, and other feline pathogens are known to infect domestic cats in Spain (Arjona et al. 2000; Solano-Gallego et al. 2006). These disease agents might not only decrease the persistence of local wildcat populations through increased mortality but also in more subtle ways, e.g., altering the behavior or reducing the body condition and fitness of infected individuals (Scott 1988). Furthermore, since the mechanisms of transmission between species of such disease agents are poorly known in the wild, wildcats could be involved in the epidemiology of infectious diseases that may pose a risk for sympatric rare endangered species, specially the Iberian lynx (Lynx pardinus), which have little contact with anthropic environments (Delibes et al. 2000).
The aims of the present study were (1) to assess the seroprevalence against feline disease agents in free-living wildcats and (2) to examine whether prevalence, number of detected agents, and similarity between wildcats in the composition of the pathogens they were exposed to were related with sex, season, and body condition.
Materials and methods
We studied a wildcat population in a 110-km2 area of eastern Toledo Mountains, southcentral Spain (39°18′ N, 3°45′ W). The study area consists of a mosaic of Mediterranean shrubland, cereal fields, and to a lesser extent, pastureland. Major land uses other than agriculture are game and sheep and goat husbandry. There are no towns or other forms of urban use, but the area contains scattered farms (0.1 farm/km2), most of them permanently inhabited, where free-roaming domestic cats and dogs are fairly common. Around 30 buildings aggregate in a 60-ha spot near the eastern end of the study area; a few are used as holiday houses; the other are virtually abandoned.
In the course of ecological studies, double-entrance box traps and padded no. 2 Victor coil-spring traps (Woodstream, Lititz, Pennsylvania) were placed during periods of 2–16 weeks across the study area. Trapping took place from winter (January–March) to summer (July–September), between 1991 and 1993. Twenty-two wildcats were caught and immobilized either with ketamine hydrochloride (Ketolar©, Parke-Davis, Spain) mixed with xylazine hydrochloride (Rompún©, Bayer, Spain), or with tiletamine-zolazepam (Zoletil©, Virbac, Spain). Wildcats were measured, weighed and classified as adults or juveniles (<1 year old), on the basis of tooth wear, as well as signs of pregnancy or current (milk) or past lactation (hairless areas around nipples). Coat patterns, size, weight, and spatial behavior indicated these animals were not domestic or feral cats. Our sample consisted of five adult males, 15 adult females, and two juvenile females. Whole blood was obtained from venipuncture of the cephalic veins. Samples were collected in serum separator tubes and allowed to clot. Then, they were centrifuged at 2,000 rpm for 15 min, and the serum was removed. A fraction was subjected to biochemical analysis, and the rest was kept frozen at −20°C until examined for the presence of antibodies to disease agents. Since three individuals were caught twice, 25 serum samples were obtained. For each wildcat, body condition was estimated in two ways: (1) the residual of a regression line of (log) body weight (g) against (log) head plus body length (mm), and (2) the concentration of total cholesterol (mg/dl) in the serum. In mammals, it is well known that the intake of animal proteins increases plasma total cholesterol (Forsythe et al. 1980; Sugano and Roba 1993). Thus, we hypothesized that a higher concentration of serum cholesterol indicates a higher protein intake and a better condition. Morphological and biochemical estimates of condition were statistically independent (r = 0.373, p = 0.106).
Serum samples were tested using enzyme-linked immunoassay (ELISA) using commercial kits provided by Ingenasa (Madrid, Spain) and EVL-Eurovet (Madrid, Spain) to the following disease agents: FIV (Ingezim FIV©), FeLV (Ingezim FeLV gp-70©), Feline coronavirus (FCoV; Ingezim FCoV©), Feline calicivirus (FCV; F1008-AB02-Feline calicivirus antibody ELISA©), Feline herpesvirus-1 (FHV; F107-AB02-Feline herpesvirus antibody ELISA©), and Chlamydophila sp. (F1009-AB01-Chlamydia antibody ELISA©). Presence of antibodies against feline parvovirus (FPV) were determined using a test for canine parvovirus (Ingezim CPV©) using anti-cat IgG as conjugate, following the recommendations of the manufacturer. Finally, the presence of FeLV p27 was determined with ELISA for antigen detection (Ingezim FeLV-Das©). Each procedure incorporated positive and negative control sera according to the manufacturer’s instructions.
We explored the univariate effects of sex, season, and body condition on the presence of antibodies against each considered pathogen with logistic regression. Then we fitted generalized linear models with Poisson errors to examine the effect of the same predictors on the number of disease agents per individual. Finally, we examined how similar the composition of pathogens was in each sample as a function of sex and season. For a given pair of serum samples A and B, we computed the simple matching coefficient (Krebs 1998), 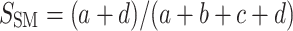 , where a is the number of pathogens that occur both in samples A and B, b is the number of antibodies in sample B but not in sample A, c is the number of antibodies in sample A but not in sample B, and d is the number of antibodies absent in both samples. We calculated this index of similarity in the identity of antibodies for all pairs of samples and compared the means of the simple matching coefficient between combinations of levels of sex and season with analysis of variance.
, where a is the number of pathogens that occur both in samples A and B, b is the number of antibodies in sample B but not in sample A, c is the number of antibodies in sample A but not in sample B, and d is the number of antibodies absent in both samples. We calculated this index of similarity in the identity of antibodies for all pairs of samples and compared the means of the simple matching coefficient between combinations of levels of sex and season with analysis of variance.
Results
All the wildcats but one (95.4%) presented evidences of contact with at least one of the pathogens (mean = 2.2 pathogens/individual). Four wildcats revealed antibodies for one agent (18.2%), 11 for two agents (50.0%), three for three agents (13.6%), two for four agents (9.1%), and one for five agents (4.5%). Seroprevalences are shown in Table 1. Exposure to FeLV and FCV was detected, respectively, in 81% and 80% of individuals. Antibodies against FPV, FHV, and Chlamydophila sp. were found in 20–30% of individuals. There was no evidence of contact with FIV or FCoV (Table 1).
Table 1.
Infectious agents for which evidences of contact were examined in a sample of European wildcat (F. silvestris) sera from southcentral Spain, ordered by decreasing prevalence
| Agent | Positive/tested | Prevalence | 95% CI |
|---|---|---|---|
| Feline leukemia virus | |||
| Antibodies | 17/22 | 0.77 | 0.59–0.88 |
| Antigen p27 | 3/20 | 0.15 | 0.04–0.37 |
| Total | 18/22 | 0.81 | 0.61–0.93 |
| Feline calicivirus | 16/20 | 0.80 | 0.57–0.93 |
| Chlamydophila sp. | 6/22 | 0.27 | 0.12–0.50 |
| Feline herpesvirus-1 | 4/20 | 0.20 | 0.07–0.42 |
| Feline parvovirus | 4/22 | 0.18 | 0.06–0.39 |
| Feline immunodeficiency virus | 0/22 | 0.00 | 0.00–0.15 |
| Feline coronavirus | 0/22 | 0.00 | 0.00–0.15 |
Antibodies against FeLV were detected in 17 wildcats. Sera of two other individuals yielded an absorbance value between the positive and negative cutoffs. In addition, three wildcats resulted positive for antigen detection. One of them presented both antigen and antibodies. A second individual was sampled twice and presented antigen and antibodies only in the second sample, 1 year later. The third wildcat was also sampled twice, and in the first sampling, both tests were negative, but 2 weeks later, it presented antigenemia but not antibodies (see Table 2 for these and other cases of seroconversion).
Table 2.
Serological results for wildcats sampled twice
| GM32 (♀, Ad) | GM47 (♀, Ad) | GM78 (♂, Ad) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st sampling | 2nd sampling | 1st sampling | 2nd sampling | 1st sampling | 2nd sampling | ||||
| May 3rd, 1991 | May 18th, 1991 | June 14th, 1992 | August 30th, 1993 | January 12th, 1994 | March 1st, 1994 | ||||
| FeLV Ab | Negative | → | Negative | Uncertain | → | Positive | Positive | → | Positive |
| FeLV p27 | Negative | → | Positive | Negative | → | Positive | Negative | → | Negative |
| FCV | Positive | → | Positive | Negative | → | Positive | Positive | → | Negative |
| FHV-1 | Negative | → | Negative | Negative | → | Negative | Positive | → | Positive |
| Chlamydophila sp. | Negative | → | Negative | Positive | → | Negative | Positive | → | Positive |
Code, sex, and age are given. All analyses were negative in these wildcats for FIV, FPV and FCoV
The prevalence of FHV in males was four times higher than in females, and these sexual differences were marginally significant (Table 3). Prevalence was similar in both sexes for other agents, except Chamydophila sp. whose proportion in males was more than twice as high as in females, though differences were not significant. The seroprevalence of FHV in wildcats caught in winter was significantly higher than in samples obtained in other seasons (Table 3). There was also a tendency for Chamydophila sp. to decrease its prevalence from winter to summer, whereas we found lower variation in seasonal prevalence for other agents. In general, the probability of having contacted a disease agent showed a weak association with indices of body condition. However, the probability of having antibodies against FPV had a significant inverse relationship with the concentration of serum cholesterol (Table 3). A similar trend was found between this probability and the morphometric index of body condition. No apparent clinical signs were observed in any of the studied wildcats at the time of handling.
Table 3.
Effects of sex and season on the prevalence of antibodies in wildcats sera
| Sex | G | p | Season | G | p | Condition | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Winter | Spring | Summer | W/L | p | Chol | p | |||||
| FeLV | 0.72 | 0.86 | 0.542 | 0.462 | 0.86 | 0.69 | 0.75 | 0.703 | 0.703 | + | 0.556 | + | 0.601 |
| FPV | 0.17 | 0.14 | 0.022 | 0.883 | 0.29 | 0.08 | 0.25 | 1.702 | 0.427 | − | 0.135 | − | 0.032 |
| FCV | 0.76 | 0.67 | 0.214 | 0.644 | 0.80 | 0.69 | 0.75 | 0.231 | 0.891 | − | 0.731 | − | 0.797 |
| FHV-1 | 0.12 | 0.50 | 3.452 | 0.063 | 0.80 | 0.08 | 0.00 | 11.527 | 0.003 | − | 0.957 | − | 0.139 |
| Chamydophila sp. | 0.17 | 0.43 | 1.773 | 0.183 | 0.43 | 0.23 | 0.00 | 3.386 | 0.184 | − | 0.589 | − | 0.318 |
The G statistic indicates the results of logistic regressions (sex, df = 1; season, df = 2). The sign of the logit coefficient and its significance are shown for the effects of two indices of body condition (df = 1)
W/L residuals of the regression log-weight vs. log-length, Chol serum cholesterol concentration
Poisson regression indicated that sex, season, and body condition did not explain a significant fraction of deviance in the number of types of antibodies found in serum samples.
Similarity in the composition of antibodies against disease agents (number and identity of detected and undetected antibodies) was significantly higher in pairs of female wildcats than in pairs of males or heterosexual pairs (one-way analysis of variance (ANOVA), F 2,250 = 4.27, p = 0.015; Fig. 1). The mean (±SE) simple matching coefficient in pairs of the same sex (0.67 ± 0.02) was significantly higher than in wildcat pairs of different sex (0.60 ± 0.02; t = 2.48, df = 251, p = 0.014). There was a seasonal pattern of similarity too. The composition of antibodies in winter samples differed from the composition in samples collected in other seasons more than the composition of spring–summer pairs of serum samples and more than the composition of pairs of samples collected during the same season (one-way ANOVA, F 5,247 = 6.71, p < 0.001; Fig. 1). The mean (±SE) simple matching coefficient for pairs of wildcats caught during the same season (0.68 ± 0.02) was significantly higher than for pairs of wildcats caught in different seasons (0.61 ± 0.02; t = 2.55, df = 251, p = 0.011).
Fig. 1.

Mean (+SE) similarity in the composition of the pathogen community in pairs of wildcat serum samples according to sex (upper panel) and season (lower panel)
Discussion
Observed seroprevalences
Seroprevalence to the different studied disease agents in the wildcat population of Toledo Mountains was either in the range of, or higher than, values previously reported in Europe (Artois and Remond 1994; McOrist et al. 1991; Daniels et al. 1999; Leutenegger et al. 1999; Fromont et al. 2000). Active infections by FeLV were demonstrated in the studies quoted above. The FeLV antigenemia detected in the present study was in the range of the data by Fromont et al. (2000), McOrist et al. (1991), and Daniels et al. (1999) but was lower than that observed by Leutenegger et al. (1999). Our results may indicate that, as observed by McOrist et al. (1991) and in contrast with the observations in other wild felids (e.g. Citino 1986), FeLV may be self-sustained in the studied wildcat population. However, transmission from domestic cats may also occur. Feline leukemia virus is fatal in domestic cats, and mortality related to FeLV infection have been reported in a free-living cougar (Puma concolor; Jessup et al. 1993) and in a captive bobcat (Lynx rufus; Sleeman et al. 2001). Similar cases have not been recorded in the European wildcat. For example, Leutenegger et al. (1999) did not find clinical signs in FeLV-infected wildcats. As a rule, no evident signs were observed in the wildcats during this survey, either seropositive or infected. This may indicate that most of the wildcats may survive the infection. Nevertheless, some undetected mortality due to feline leukemia may occur.
Eighteen percent of the wildcats presented antibodies against FPV. This value was higher than those reported by Leutenegger et al. (1999) and Ostrowski et al. (2003) by means of the immunofluorescence assay. Feline parvovirus causes feline panleukopenia (FPL), and mortality due to this syndrome has been observed in other wild felids (e.g. in bobcat, Wassmer et al. 1988; or cougar, Roelke et al. 1993). This disease is markedly harmful in kittens (Barker and Parrish 2001). Thus, if wildcat kittens succumb often to FPL as observed in feral cats (van Rensburg et al. 1987), this disease may have a substantial impact on productivity and thus on population dynamics. We found a negative relationship between indices of wildcat condition and exposure to FPV. The analysis performed detects IgG, thus indicating past infection. Then, it is difficult to explain an effect of a past infection with wildcat condition at the time of sampling. Since, as said above, FPL is especially harmful in young cats, some incorrect development due to the disease could promote a subsequent impaired condition. Anyway, relatively poor condition in FPV-seropositive wildcats, together with the observed low seroprevalence, could be due to FPV-associated morbidity or mortality, although our results are inconclusive in this regard. It is not known whether FPV is self-sustained in the wildcat population or domestic cats act as a reservoir. This virus is extremely resistant and can survive for months in the environment (Greene 1998). Therefore, in addition to direct contact with a virus-shedding carnivore, habitat contamination by domestic cat feces may be a source of infection (Roelke et al. 1993). Additional difficulties to interpret the moderate FPV prevalence we found arise from potential specificity problems (Ostrowski et al. 2003), since the ELISA test utilized here does not allow determining if wildcats were infected by the feline or the canine parvovirus strain. Thus, not only domestic cats but also dogs and red foxes (Vulpes vulpes) could serve as sources of infection for wildcats, as suggested for the Iberian lynx (Roelke et al. 2008).
We detected evidence of exposure to the three main agents involved in the feline respiratory syndrome (FRS), i.e. FCV, FHV, and Chlamydophila sp. Contact with FCV and FHV was also detected by Artois and Remond (1994), Daniels et al. (1999), Leutenegger et al. (1999), and Ostrowski et al. (2003), but the prevalences we found are markedly higher than those reported in these studies. As far as we know, we report here for the first time antibodies to Chlamydophila sp. (or antigenically related agents) in a wild feline species. Only Paul-Murphy et al. (1994) tested but failed to detect antibodies to chlamydial agents in cougars. Again, it is unknown whether these agents are endemic in the studied wildcat population or wildcats became infected after contact with domestic cats. Riley et al. (2004) found higher titers to FCV in bobcats from areas where domestic cats occurred, suggesting that FCV might have been endemic in domestic cats but not in bobcats. In contrast, Roelke et al. (1993) believed that the virus may be present in Florida panthers, with transmission similar to that in the domestic cat (a persistently infected mother-to-offspring pathway). We believe that the moderate to high prevalence of disease agents related with FRS suggests that (1) contacts between domestic and wildcats, the most feasible way of transmission of FHV and Chlamydophila, are frequent, and (2) FCV may be endemic in this wildcat population, the most likely way of transmission being from mothers to kittens. The pathological significance of these agents to wildcats is unknown. In domestic cats, morbidity of FRS is considerable, although mortality is low (Greene 1998). Moderate or high seroprevalences suggest that this may be the case for wildcats, although some neonatal mortality may occur too.
The present study failed to demonstrate wildcat contact with FIV and FCoV. Both agents seem to be infrequent in other European wildcat populations (McOrist et al. 1991; Daniels et al. 1999; Leutenegger et al. 1999; Fromont et al. 2000). FIV might not be present in the studied population, although FIV antibodies are more likely to be found in adult, male cats (Sellon 1998), and only five individuals belonging to this cohort were included in the present survey. The prevalence of FIV in domestic cat populations in Spain is around 8% (Solano-Gallego et al. 2006; Arjona et al. 2000). Thus, the occurrence of unrecorded fatal cases of feline immunodeficiency in European wildcats from Spain cannot be discarded. The observed low seroprevalence to FCoV may be due to a probable absence of the agent in the domestic cats of the study area. According to Addie and Jarret (1998), FCoV is more common in domestic cats in catteries than in pet cats kept singly or in feral or stray cats. The domestic cats inhabiting our study area can be better described as feral cats than household cats.
Season and sex-related differences
We found a relatively high seroprevalence to FHV in winter. Assuming that interbreeding may not be uncommon, we believe that transmission of FHV (and perhaps of other agents) may be enhanced during the annual population peak of wildcats and domestic cats in summer-autumn, which may explain a higher seroprevalence in the following winter. No season-related differences in prevalence of any of the studied agents was recorded in previous studies regarding wildcat (McOrist et al. 1991; Daniels et al. 1999; Leutenegger et al. 1999; Fromont et al. 2000; Ostrowski et al. 2003) or other wild felines (e.g., Paul-Murphy et al. 1994). In red foxes from Australia, Robinson et al. (2005) observed that the seroprevalence to Canine adenovirus presented the highest seroprevalence in winter–spring, suggesting that foxes became infected in the mating season more probably than prior to dispersal, which is in agreement with our hypothesis for FHV. Related with this, the observed higher seroprevalence of FHV and Chlamydophila sp. in wildcat males may be also related with higher rates of intra- and inter-species encounters because males tend to move more during the mating season (Liberg, 1988).
We also observed a similar pattern of exposure to agents in wildcats sampled in spring and summer, whereas animals sampled in winter presented a different pattern. These results indicate that there are clear seasonal-related variations in the exposure to the agents. We hypothesize that, during winter, some mortality of wildcats may be related with the exposure to one or more of the studied agents. This fact could be linked with the higher seroprevalence to FHV and Chlamydophila sp. in winter samples: selective mortality associated to these pathogens would explain why these agents showed lower seroprevalence in the following spring–summer. Although both pathogens course with low mortality in adult domestic cats (Gaskell and Dawson 1998), nothing is known about the clinical course of these infections in free-living wildcats. In some cases, a chronification of the disease in cats occurs, with clinical signs ranging from sneezing and nasal discharge to corneal edema and blindness (Gaskell and Dawson 1998). The latter may be critical for survival in the wild, and some mortality related to these agents cannot be discarded.
Interestingly, the identity of agents to which females presented antibodies, as well as the identity of agents to which females were not exposed, were more consistent than the composition of infectious agents in males. In addition, the similarity in the results of tests for multiple agents in pairs of the same sex was significantly higher than in wildcat pairs of different sex. As far as we know, no similar findings have been previously reported for any wild species. This result, far from giving simple differences in seroprevalence among sexes, presents evidence of a different pattern of exposure among females and males to the studied agents. We suggest that, whereas the behavior of the females exposes them to a particular set of agents and prevents exposure to others, the behavior of males (with a higher movement pattern, especially among juveniles during dispersal and adults during mating) makes more unpredictable the type of disease agents they face.
Conclusions
Our study confirmed that wildcats in central Spain were in contact with five of the studied common feline pathogens, some of which exhibited high seroprevalences. It has not been determined to what extent these agents pose a risk for wildcat populations, although a negative relationship between seroprevalence and condition was found for FPV. Therefore, our results indirectly support the hypothesis that diseases may contribute to reduce individual fitness. In addition, during the period when wildcats were captured, the extremely endangered Iberian lynx was still present in Toledo Mountains (Rodríguez et al. 1996) and thus potentially exposed to the studied pathogens. Since the causes of the marked regression of lynx populations in the last 30 years are multiple and have not been completely elucidated (Rodríguez and Delibes 2002) and very little is known about natural causes of mortality (Ferreras et al. 1992; Rodríguez and Delibes 2004), we believe that diseases might be considered as a potential contributing factor of decline in the Toledo Mountains lynx population during the 1980s.
Acknowledgments
J. Nicolás Guzmán and Giulia Crema assisted with wildcat trapping and sample collection. We thank Amparo Gomis at INAC (Ciudad Real, Spain) for performing biochemical analyses, J. Manuel Pérez de la Lastra at IREC (Ciudad Real, Spain) for laboratory assistance during sera analyses, and Jacques Delbecque (Ingenasa) and Silvia Simón (Eurovet Veterinaria S.L.) for information and advice about immunoassay performance. Field work was supported by the Spanish Ministry of Transportation, and lab work as well as manuscript preparation was funded by grants from Junta de Andalucía to A. Rodríguez. The trapping and sampling protocol followed the Spanish laws of animal welfare in scientific research (Real Decreto 1201/2005).
References
- Addie DD, Jarret O. Feline coronavirus infections. In: Greene CE, editor. Infectious diseases of dog and cat. Pennsylvania: W.B. Saunders; 1998. pp. 63–75. [Google Scholar]
- Arjona A, Escolar E, Soto I, Barquero N, Martín D, Gómez-Lucía E. Seroepidemiological survey of infection by feline leukemia virus and immunodeficiency virus in Madrid and correlation with some clinical aspects. J Clin Microbiol. 2000;38:3448–3449. doi: 10.1128/jcm.38.9.3448-3449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artois M, Remond M. Viral diseases as a threat to free-living wild cats (Felis silvestris) in continental Europe. Vet Rec. 1994;134:651–652. doi: 10.1136/vr.134.25.651. [DOI] [PubMed] [Google Scholar]
- Barker IK, Parrish CR. Parvovirus infections. In: Williams ES, Barker IA, editors. Infectious diseases of wild animals. 3. Iowa: Iowa State University Press; 2001. pp. 131–146. [Google Scholar]
- Citino SB. Transient FeLV viraemia in a clouded leopard. J Zoo Wildl Med. 1986;17:5–7. [Google Scholar]
- Daniels MJ, Golder MC, Jarrett O, Macdonald DW. Feline viruses in wildcats from Scotland. J Wildl Dis. 1999;35:121–124. doi: 10.7589/0090-3558-35.1.121. [DOI] [PubMed] [Google Scholar]
- Delibes M, Rodríguez A, Ferreras P. Action plan for the conservation of the Iberian lynx in Europe (Lynx pardinus). Nature and Environment Series 111. Strasbourg: Council of Europe; 2000. [Google Scholar]
- Ferreras P, Aldama JJ, Beltrán JF, Delibes M. Rates and causes of mortality in a fragmented population of Iberian lynx Felis pardina Temminck, 1824. Biol Conserv. 1992;61:197–202. doi: 10.1016/0006-3207(92)91116-A. [DOI] [Google Scholar]
- Forsythe WA, Miller ER, Hill GM, Romsos DR, Simpson RC. Effects of dietary protein and fat source on plasma cholesterol parameters, LCAT activity and amino acid levels and on tissue lipid content of growing pigs. J Nutr. 1980;110:2467–2479. doi: 10.1093/jn/110.12.2467. [DOI] [PubMed] [Google Scholar]
- Fromont E, Sager A, Leger F, Bourguemestre F, Jouquelet E, Stahl P, Pontier D, Artois M. Prevalence and pathogenicity of retroviruses in wildcats in France. Vet Rec. 2000;146:317–319. doi: 10.1136/vr.146.11.317. [DOI] [PubMed] [Google Scholar]
- García-Perea R (2002) Felis silvestris Schreber, 1775. In: Palomo LJ, Gisbert J (eds) Atlas de los mamíferos terrestres de España. Madrid, Dirección General de Conservación de la Naturaleza-SECEM-SECEMU: pp. 294–297
- Gaskell RM, Dawson S. Feline respiratory disease. In: Greene CE, editor. Infectious diseases of dog and cat. Pennsylvania: W.B. Saunders; 1998. pp. 106–115. [Google Scholar]
- Greene CE. Feline Panleukopenia. In: Greene CE, editor. Infectious diseases of dog and cat. Pennsylvania: W.B. Saunders; 1998. pp. 52–57. [Google Scholar]
- IUCN (2007) Felis silvestris. In: European Mammal Assessment. http://ec.europa.eu/ environment/nature/conservation/species/ema/species/felis_silvestris.htm. Downloaded on March 31st, 2008
- Jessup DA, Pettan KC, Lowenstine LJ, Pedersen NC. Feline leukemia virus infection and renal spirochetosis in free-ranging cougar (Felis concolor) J Zoo Wildl Med. 1993;24:73–79. [Google Scholar]
- Krebs CJ. Ecological methodology. 2. Menlo Park, CA: Addison Wesley Longman; 1998. [Google Scholar]
- Leutenegger CM, Hofmann-Lehmann R, Riols C, Liberek M, Worel G, Lups P, Fehr D, Hartmann M, Weilenmann P, Lutz H. Viral infections in free-living populations of the European wildcat. J Wildl Dis. 1999;35:678–686. doi: 10.7589/0090-3558-35.4.678. [DOI] [PubMed] [Google Scholar]
- Liberg O. Spatial organisation and reproductive tactics in the domestic cat and other felids. In: Turner DC, Bateson P, editors. The domestic cat: the biology of its behaviour. Cambridge: Cambridge University Press; 1988. pp. 83–98. [Google Scholar]
- Lozano J, Virgós E, Malo AF, Huertas DL, Casanovas JG. Importance of scrub-pastureland mosaics for wild-living cats occurrence in a Mediterranean area: implications for the conservation of the wildcat (Felis silvestris) Biodivers Conserv. 2003;12:921–935. doi: 10.1023/A:1022821708594. [DOI] [Google Scholar]
- McOrist S, Boid R, Jones TW, Easterbee N, Hubbard AL, Jarrett O. Some viral and protozool diseases in the European wildcat (Felis silvestris) J Wildl Dis. 1991;27:693–696. doi: 10.7589/0090-3558-27.4.693. [DOI] [PubMed] [Google Scholar]
- Ostrowski S, Van Vuuren M, Lenain DM, Durand A. A serologic survey of wild felids from Central West Saudi Arabia. J Wildl Dis. 2003;39:696–701. doi: 10.7589/0090-3558-39.3.696. [DOI] [PubMed] [Google Scholar]
- Paul-Murphy J, Work T, Hunter D, Mcfie E, Fjelline D. Serologic survey and serum biochemical reference ranges of the free-ranging mountain lion (Felis concolor) in California. J Wildl Dis. 1994;30:205–215. doi: 10.7589/0090-3558-30.2.205. [DOI] [PubMed] [Google Scholar]
- Racnik J, Skrbinsek T, Potocnik H, Kos I, Tozon N. Viral infections in wild-living European wildcats in Slovenia. Eur J Wildl Res. 2008;54:767–770. doi: 10.1007/s10344-008-0202-y. [DOI] [Google Scholar]
- Riley SP, Foley J, Chomel B. Exposure to feline and canine pathogens in bobcats and gray foxes in urban and rural zones of a national park in California. J Wildl Dis. 2004;40:11–22. doi: 10.7589/0090-3558-40.1.11. [DOI] [PubMed] [Google Scholar]
- Robinson AJ, Crerar SK, Waight-Sharma N, Müller WJ, Bradley MP. Prevalence of serum antibodies to canine adenovirus and canine herpesvirus in the European red fox (Vulpes vulpes) in Australia. Aust Vet J. 2005;83:356–361. doi: 10.1111/j.1751-0813.2005.tb15634.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez A, Carbonell E. Gastrointestinal parasites of the Iberian lynx and other wild carnivores from central Spain. Acta Parasitol. 1998;43:128–136. [Google Scholar]
- Rodríguez A, Delibes M. Internal structure and patterns of contraction in the geographic range of the Iberian lynx. Ecography. 2002;25:314–328. doi: 10.1034/j.1600-0587.2002.250308.x. [DOI] [Google Scholar]
- Rodríguez A, Delibes M. Patterns and causes of non-natural mortality in the Iberian lynx during a 40 year period of range contraction. Biol Conserv. 2004;118:151–161. doi: 10.1016/j.biocon.2003.07.018. [DOI] [Google Scholar]
- Rodríguez A, Crema G, Delibes M. Use of non-wildlife passages across a high speed railway by terrestrial vertebrates. J Appl Ecol. 1996;33:1527–1540. doi: 10.2307/2404791. [DOI] [Google Scholar]
- Roelke ME, Forrester DJ, Jacobson ER, Kollias GV, Barr MC, Evermann JF, Pirtle EG. Seroprevalence of infectious disease agents in free-ranging Florida panthers (Felis concolor coryi) J Wildl Dis. 1993;29:36–49. doi: 10.7589/0090-3558-29.1.36. [DOI] [PubMed] [Google Scholar]
- Roelke M, Johnson WE, Millán J, Palomares F, Revilla E, Rodríguez A, Calzada J, Ferreras P, León-Vizcaíno L, Delibes M, O’Brien SJ. Exposure to disease agents in the endangered Iberian lynx (Lynx pardinus) Eur J Wildl Res. 2008;54:171–178. doi: 10.1007/s10344-007-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott ME. The impact of infection and disease on animal populations: implications for conservation biology. Conserv Biol. 1988;2:40–65. doi: 10.1111/j.1523-1739.1988.tb00334.x. [DOI] [Google Scholar]
- Sellon RH. Feline immunodeficiency virus infection. In: Greene CE, editor. Infectious diseases of dog and cat. Pennsylvania: W.B. Saunders; 1998. pp. 84–96. [Google Scholar]
- Sleeman JM, Keane JM, Johnson JS, Brown RJ, Woude SV. Feline leukemia virus in a captive bobcat. J Wildl Dis. 2001;37:194–200. doi: 10.7589/0090-3558-37.1.194. [DOI] [PubMed] [Google Scholar]
- Smith KF, Sax DF, Lafferty KD. Evidence for the role of infectious disease in species extinction and endangerment. Conserv Biol. 2006;20:1349–1357. doi: 10.1111/j.1523-1739.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Hegarty B, Espada Y, Llull J, Breitschwerdt E. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol. 2006;118:274–277. doi: 10.1016/j.vetmic.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Sugano M, Roba K. Dietary protein and lipid metabolism: a multi functional effect. Ann N Y Acad Sci. 1993;676:215–222. doi: 10.1111/j.1749-6632.1993.tb38736.x. [DOI] [PubMed] [Google Scholar]
- Torres J, Casanova JC, Feliú C, Gisbert J, Manfredi MT. Contribución al conocimiento de la cestodofauna de Felis silvestris Schreber, 1776 (Carnivora: Felidae) en la Península Ibérica. Rev Iber Parasitol. 1989;49:307–312. [Google Scholar]
- Van Rensburg PJJ, Skinner JD, van Aarde HJ. Effects of feline panleucopaenia on the population characteristics of feral cats on Marion Island. J Appl Ecol. 1987;24:63–73. doi: 10.2307/2403787. [DOI] [Google Scholar]
- Wassmer DA, Guenther DD, Layne JN. Ecology of the bobcat in Florida. Bull Fla State Mus Biol Sci. 1988;3:159–228. [Google Scholar]


