Abstract
Despite being important conservation tools, tourism and research may cause transmission of pathogens to wild great apes. Investigating respiratory disease outbreaks in wild bonobos, we identified human respiratory syncytial virus and Streptococcus pneumoniae as causative agents. A One Health approach to disease control should become part of great ape programs.
Electronic supplementary material
The online version of this article (10.1007/s10393-018-1319-4) contains supplementary material, which is available to authorized users.
Keywords: Human respiratory syncytial virus, Streptococcus pneumoniae, Wild bonobo, DRC, Zoonoses
Research shows that chimpanzees and gorillas habituated to human presence can get infected with microorganisms of human origin. Most frequently, the transmission of respiratory pathogens has been described, resulting in some cases in high morbidity and considerable mortality (Köndgen et al. 2008, 2010, 2017; Kaur et al. 2008; Palacios et al. 2011; Grützmacher et al. 2016). Viruses identified so far are human respiratory syncytial virus (HRSV) and human metapneumovirus (HMPV), two common paramyxoviruses. These viruses are known to generally cause only mild disease in healthy human adults, but may lead to bronchiolitis and pneumonia, especially in young children (Falsey and Walsh 2000). In addition, in most great ape respiratory disease outbreaks described, Streptococcus (S.) pneumoniae was also involved in the disease development (Köndgen et al. 2017). S. pneumoniae is a common human pathogen, which can cause severe invasive pneumonia. Although primarily a human pathogen, it has also been shown to cause disease in other taxa, such as rodents, equids and nonhuman primates (Unwin et al. 2013).
Here, we report two outbreaks of respiratory disease in wild bonobos (Pan paniscus), endangered great apes that occurred in the Democratic Republic of the Congo (DRC) in 2014 and 2015.
The Study
The Malebo field site, where the two outbreaks occurred, is situated in the Bandundu Province, DRC (Fig. 1). In 2005, the WWF and the local NGO, Mbou-Mon-Tour started to habituate two bonobo groups (Nkala and Mpelu) to human presence, as part of a research and ecotourism program geared toward the conservation of the bonobos and the generation of supplemental income for local people. At the time of the first outbreak, neither of the groups had been fully habituated. Humans could not approach closer than approximately 20 meters. The region is not protected by law, but belongs to the local communities that traditionally do not hunt bonobos. However, community members do frequently enter the forest to hunt other animals, collect forest products or wash their clothes.
Fig. 1.
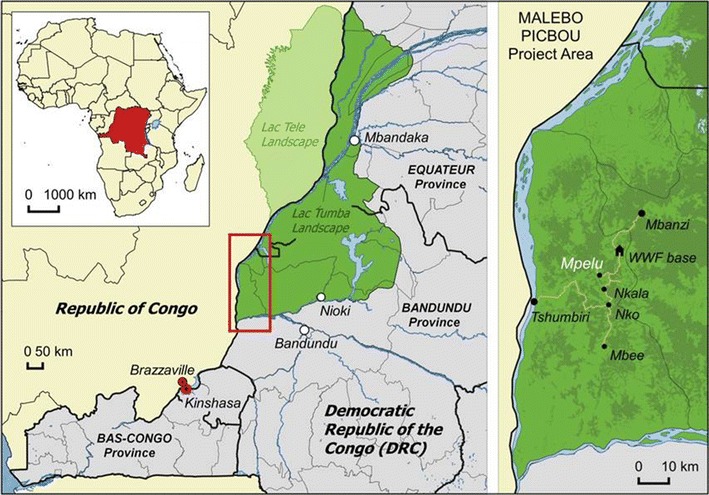
Location of the PICBOU project area.
Date: January 2005; Source: United Nations, 2008; Projection: WGS 84; Datum: Geographic Coordinate System
In May of 2014, a respiratory disease outbreak was observed in the Mpelu group, which consisted of approximately 40 bonobos prior to the outbreak. At least seven individuals were observed with severe symptoms, including coughing, nasal discharge, inappetence, emaciation and lethargy. Four animals were found dead during the course of this outbreak: three adults and one juvenile. Additional deaths cannot be ruled out because they can easily go unnoticed as the animals were not individually identified yet. In addition, tropical conditions can lead to rapid carcass breakdown, while dense vegetation hinders carcass location. Also the neighboring Nkala group, consisting of approximately 30 individuals, experienced a respiratory disease outbreak in April of 2015. At least 12 group members were observed with respiratory symptoms. Again, four bonobos were found dead: three adults and one infant. Samples were collected from three carcasses in each outbreak, including lung tissue samples, oral and rectal swabs (for details on sample collection and storage please see Technical Appendix). All carcasses are listed in Table 1. All but two carcasses from the second outbreak (bonobos #5 and #7) were found several days after the suspected time of death and, thus, were in advanced stages of decay.
Table 1.
Overview of Great Ape Samples and Results
| Species | Individual | Country | Group | Age | Sex | Material | Finding |
|---|---|---|---|---|---|---|---|
| Bonobo | #1 | DRC | Mpelu | Adult | F | Nasal/rectal swab | HRSV/S.pneu |
| Bonobo | #2 | DRC | Mpelu | Adult | M | Lung tissue | HRSV/S.pneu |
| Bonobo | #3 | DRC | Mpelu | Adult | M | Nasal swab | S.pneu |
| Bonobo | #4 | DRC | Mpelu | Juvenile | F | Lung tissue | HRSV/S.pneu |
| Bonobo | #5 | DRC | Nkala | Adult | M | Lung tissue | HRSV/S.pneu |
| Bonobo | #6 | DRC | Nkala | Adult | F | Lung tissue | HRSV/S.pneu |
| Bonobo | #7 | DRC | Nkala | Infant | M | Lung tissue | HRSV/S.pneu |
| Bonobo | #8 | DRC | Nkala | Adult | M | Nasal swab | HRSV/S.pneu |
DRC, The Democratic Republic of the Congo; S.pneu, Streptococcus pneumoniae.
Histology results from the lung tissue of bonobos #5 and #7 (second outbreak) revealed massive aggregates of neutrophilic granulocytes and cell detritus in alveoli and bronchial areas along with necrosis of alveolar and bronchial epithelial cells. Focally, aggregates of gram-positive cocci were seen within the alveoli (methods in Technical Appendix). These findings point to a high-grade, diffuse purulent bronchopneumonia of bacterial origin, while a primary viral infection seems likely.
All bonobo samples were screened for adenovirus, coronavirus, enterovirus, influenza virus A and B, HMPV, HRSV, measles virus, parainfluenza virus and rhinovirus using PCR-based methods (Köndgen et al. 2008; Technical Appendix). Additionally, a PCR specific for human coronavirus OC43 was performed (Vijgen et al. 2005). The latter was conducted because of the recent identification of this virus as the causative agent of a respiratory disease outbreak in wild habituated chimpanzees (data not shown).
HRSV was detected in both outbreaks: HRSV A in the Mpelu outbreak and HRSV B in the Nkala outbreak. Phylogenetic analyses performed in maximum likelihood, and Bayesian frameworks revealed that both HRSV sequences belonged to recent pandemic waves (Technical Appendix). Informing a relaxed molecular clock model with tip dates (Tan et al. 2012), we estimated that the bonobo-infecting HRSV A and B sequences both diverged from their closest human-infecting relatives in 2009 (95% highest posterior density: 2008–2010).
To investigate the spectrum of bacteria present at the time of death, lung tissue samples and nasal swabs were tested using a PCR targeting the bacterial 16S rRNA gene (Mugisha et al. 2014). Sequences pointing toward S. pneumoniae were generated from 1/17 cloned sequences. This was subsequently confirmed by a qPCR targeting the S. pneumoniae-specific autolysin gene (McAvin et al. 2001). No other relevant bacteria were detected. Samples did not allow the culturing of S. pneumoniae. Instead, further characterization was performed by multi locus sequence typing, generating ‘virtual clones’ (Chi et al. 2007; Technical Appendix). Sequence type (ST) 458 was determined—a known human ST. Consecutively, the serotypes associated with the determined ST were tested (Pai et al. 2006) and revealed the presence of serotype 3. Direct comparison to S. pneumoniae isolates circulating at the given location was not possible since no data exist from this region. In general, few data are available in the literature and in the S. pneumoniae MLST databases from the Democratic Republic of Congo. A wider study of the diversity of S. pneumoniae types in sub-Saharan Africa would be great importance.
Additionally, 24 throat swabs from project staff and visitors who had been in potential contact with the bonobos were tested for respiratory viruses as described above. Notably, samples were taken at the onset of the 2015 outbreak and no tested person had shown respiratory symptoms. One sample tested positive for HMPV.
Conclusions
In this outbreak investigation, we detected a co-infection of HRSV and S. pneumoniae in bonobos that died of respiratory disease. This finding is similar to what has been described for wild chimpanzees and gorillas (Köndgen et al. 2008; Palacios et al. 2011). Beyond providing additional evidence for viral infections of human origin in wild great apes, the finding of a S. pneumoniae sequence type identical to a human type from Africa (see Technical Appendix for additional information) provides evidence that also the S. pneumoniae infection may have been acquired from humans. This finding is of central importance because in contrast to viral infections which are cleared from the hosts, S. pneumoniae infections may be maintained in the individual, community and, ultimately, in the population. A recent study, where S. pneumoniae of human origin was isolated from wild chimpanzees that died in a respiratory disease outbreak, supports this finding (Köndgen et al. 2017).
Previous reports on respiratory disease outbreaks have highlighted the risks of habituation for wild great apes. The transmission of human pathogens from those involved in following great apes (e.g., trackers, tourists, researchers) has long been the most parsimonious explanation. Although transmission from project staff cannot be excluded also in the outbreaks described here, transmission from villagers frequently entering the forest seems a more likely scenario. In this setting, hygiene measures for people involved in great ape programs—as suggested by the new IUCN guidelines (Gilardi et al. 2015) and as implemented by the local WWF project—will only reduce the risk of disease transmission by members of the project. Especially in these settings but in general in regions with high disease burden and insufficient health care, it is increasingly important to foster a One Health approach aimed simultaneously at reducing the disease burden in the local community (and domestic animals) and thereby the risk of disease to great ape populations. Ideally, this kind of approach involves stakeholders from all disciplines from health professionals, to veterinarians and biologists. Importantly, the local communities should be involved to design a realistic approach based on the local needs, local situation and respecting traditions. Such a One Health approach should comprise, e.g., general hygiene measures at the community level, but it may also include specific vaccination campaigns (e.g., anti-pneumococci) for people living in, and around great ape habitat. For example, the pneumococcal conjugate vaccine (PCV13) would have covered the serotype involved in both outbreaks.
Our results underline the necessity of health monitoring of wild great ape communities. They also demonstrate the importance of investigating the origin of diseases and the role habituation can play in detecting disease outbreaks that would otherwise go unnoticed. It also becomes evident that also the health status of the local communities as well as pathogens circulating locally in humans should be investigated in order to design solid and well targeted one health approaches. Based on these findings, more concrete conservation actions can be taken to protect human and animal health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank our collaborator, the local NGO Mbou-Mon-Tour for their support and the chiefs and communities of Nkala, Nko and Mpelu for giving access to their forests, and the trackers and the health monitor, Mame Ngono Tonton, for their hard work. We would like to thank Charles-Albert Petre, the Scientific Coordinator for the project for help with sample collection and the student, Maeva Vinot and the volunteers Kelly Mannion and Emmanuel Mahe who were present during the 2015 outbreak and helped to monitor the sick bonobos. Our thanks also go to the funders of the bonobo project, WWF Belgium and WWF Netherlands and to WWF Germany for providing the project with health monitoring equipment. We are also grateful for the support of the Institut Congolais pour la Conservation de la Nature (ICCN) and the Ministry of Environment, Conservation, Nature and Sustainable Development (MECNEDD). We finally thank Philipp Goeltenboth and Bruno Perodeau for organizational and logistical support and the Hans Böckler Stiftung, the ARCUS great ape fund, the EAZA Ape Conservation Fund and Zoo Leipzig for support and funding.
References
- Chi F, Leider M, Leendertz F, Bergmann C, Boesch C, Schenk S, et al. New Streptococcus pneumoniae clones in deceased wild chimpanzees. Journal of Bacteriology. 2007;189:6085–6088. doi: 10.1128/JB.00468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clinical Microbiology Reviews. 2000;13:371–384. doi: 10.1128/CMR.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KV, Gillespie TR, Leendertz FH, Macfie EJ, Travis DA, Whittier CA, et al. Best Practice Guidelines for Health Monitoring and Disease Control in Great Ape Populations. Gland: IUCN SSC Primate Specialist Group; 2015. p. 56. [Google Scholar]
- Grützmacher KS, Köndgen S, Keil V, Todd A, Feistner A, Herbinger I, et al. Codetection of respiratory syncytial virus in habituated wild western lowland gorillas and humans during a respiratory disease outbreak. EcoHealth. 2016;19:1–2. doi: 10.1007/s10393-016-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Singh J, Tong S, Humphrey C, Clevenger D, Tan W, et al. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. American Journal of Primatology. 2008;70:755–765. doi: 10.1002/ajp.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köndgen S, Kühl H, N’Goran PK, Walsh PD, Schenk S, Ernst N, et al. Pandemic human viruses cause decline of endangered great apes. Current Biology. 2008;18:260–264. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Köndgen S, Schenk S, Pauli G, Boesch C, Leendertz FH. Noninvasive monitoring of respiratory viruses in wild chimpanzees. EcoHealth. 2010;7:332–341. doi: 10.1007/s10393-010-0340-z. [DOI] [PubMed] [Google Scholar]
- Köndgen S, Calvignac-Spencer S, Grützmacher K, Keil V, Mätz-Rensing K, Nowak K, et al. Evidence for Human Streptococcus pneumoniae in wild and captive chimpanzees: A potential threat to wild populations. Scientific Reports. 2017;7:14581. doi: 10.1038/s41598-017-14769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvin JC, Reilly PA, Roudabush RM, Barnes WJ, Salmen A, Jackson GW, et al. Sensitive and specific method for rapid identification of Streptococcus pneumoniae using real-time fluorescence PCR. Journal of Clinical Microbiology. 2001;39:3446–3451. doi: 10.1128/JCM.39.10.3446-3451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugisha L, Köndgen S, Kaddu-Mulindwa D, Gaffikin L, Leendertz FH. Nasopharyngeal colonization by potentially pathogenic bacteria found in healthy semi-captive wild-born chimpanzees in Uganda. American Journal of Primatology. 2014;76(2):103–110. doi: 10.1002/ajp.22212. [DOI] [PubMed] [Google Scholar]
- Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. Journal of Clinical Microbiology. 2006;44:124–131. doi: 10.1128/JCM.44.1.124-131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G, Lowenstine LJ, Cranfield MR, Gilardi VK, Spelman L, Lukasik-Braum M, et al. Human metapneumovirus infection in wild mountain gorillas, Rwanda. Emerging Infectious Diseases. 2011;17:711–713. doi: 10.3201/eid1704.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Lemey P, Houspie L, Viveen MC, Jansen NJ, Van Loon AM, et al. Genetic variability among complete human respiratory syncytial virus subgroup A genomes: bridging molecular evolutionary dynamics and epidemiology. PLoS ONE. 2012;7:e51439. doi: 10.1371/journal.pone.0051439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin S, Chatterton J, Chantrey J. Management of severe respiratory tract disease caused by human respiratory syncytial virus and Streptococcus pneumoniae in captive chimpanzees (Pan troglodytes) Journal of Zoo and Wildlife Medicine. 2013;44:105–115. doi: 10.1638/1042-7260-44.1.105. [DOI] [PubMed] [Google Scholar]
- Vijgen L, Keyaerts E, Moe E, Maes P, Duson G, Van Ranst M. Development of one-step, real-time, quantitative reverse transcriptase PCR assays for absolute quantitation of human coronaviruses OC43 and 229E. Journal of Clinical Microbiology. 2005;43:5452–5456. doi: 10.1128/JCM.43.11.5452-5456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


