Abstract
Acute viral respiratory infections are the most common infections in humans. Co-infection with different respiratory viruses is well documented but not necessarily well understood. The aim of this study was to utilise laboratory data from the winter season following the 2009 influenza A(H1N1) outbreak to investigate rates of respiratory virus co-infections, virus prevalence in different age groups and temporal variations in virus detection. The Health Protection Agency Public Health Laboratory (HPA PHL) Birmingham, UK, routinely uses polymerase chain reaction (PCR) to detect common respiratory viruses. The results from specimens received for respiratory virus investigations from late September 2009 to April 2010 were analysed. A total of 4,821 specimen results were analysed. Of these, 323 (13.2 %) had co-detections of two viruses, 22 (0.9 %) had three viruses and four (0.2 %) had four viruses. Reciprocal patterns of positive or negative associations between different virus pairs were found. Statistical analysis confirmed the significance of negative associations between influenza A and human metapneumovirus (HMPV), and influenza A and rhinovirus. Positive associations between parainfluenza with rhinovirus, rhinovirus with respiratory syncytial virus (RSV) and adenovirus with rhinovirus, parainfluenza and RSV were also significant. Age and temporal distributions of the different viruses were typical. This study found that the co-detection of different respiratory viruses is not random and most associations are reciprocal, either positively or negatively. The pandemic strain of influenza A(H1N1) was notable in that it was the least likely to be co-detected with another respiratory virus.
Keywords: Influenza, Respiratory Syncytial Virus, Respiratory Virus, Respiratory Viral Infection, Laboratory Information Management System
Introduction
Respiratory viral infections are a large cause of morbidity and mortality worldwide. Acute viral respiratory infections are the most common infections in man [1, 2]. Young children have, on average, 6–8 infections per year and adults have 2–4 infections per year [3].
The most common viruses that infect the respiratory tract include influenza A and B, rhinoviruses, coronaviruses, respiratory syncytial viruses (RSVs), parainfluenza viruses, adenoviruses, enteroviruses, human metapneumovirus (HMPV) and human bocavirus (HboV) [3, 4]. Acute upper respiratory tract infections (AURTIs) often include symptoms which can overlap with those of mild influenza and include pharyngitis, rhinorrhoea, cough, sneezing, fever, malaise and myalgia [3]. Most of the AURTI viruses can also cause lower respiratory tract infections [1].
Diagnostic methods for respiratory viruses include viral culture, serology, antigen detection and nucleic acid amplification techniques (NAATs), such as polymerase chain reaction (PCR). Virus culture has long been the “gold standard” and can detect a wide range of viruses, including previously unknown viruses. It also confirms that what is detected is viable. Culture is, however, labour-intensive and slow to produce results. Likewise, serology to detect antibody response to infection may only produce positive results up to two weeks after infection. These two techniques, therefore, have little routine diagnostic value, as most infections will have resolved before the results are available [1, 5]. Immunofluorescence/antigen detection tests can allow rapid detection but are subjective, rely on the experience of investigators and lack sensitivity [1, 5].
NAAT tests including multiplex assays which allow testing for several viruses at once and the detection of multiple infections are now becoming the standard method of laboratory diagnosis for respiratory infections. NAATs have the advantages of speed, sensitivity and specificity, which allows more prompt treatment, infection control and prophylaxis, where appropriate [1, 5, 6]. During the study period, the standard routine test for respiratory viruses diagnosis used by the Health Protection Agency Public Health Laboratory (HPA PHL) Birmingham, UK, was a panel of multiplex real-time PCR assays detecting influenzas A and B, swine lineage influenza A(H1N1), RSV A and B, HMPV, parainfluenzas 1, 2, 3 and 4, rhinovirus and adenovirus.
During the winter of 2009/2010, the results of the routine respiratory virus PCR workload were analysed to investigate the prevalence and impact of the most common respiratory viruses in the West Midlands region, England. The results were compared with national figures for England and virus prevalence in different age groups, temporal variation and rates of co-infection were also investigated.
Materials and methods
Specimens were received from patients with acute respiratory illness from hospitals and general practitioners within the West Midlands region of central England, UK. Specimens included nasopharyngeal aspirates, nose and throat swabs, and bronchoalveolar lavages.
Respiratory virus detection was carried out using real-time PCR for influenza A, influenza B, RSV, HMPV, parainfluenza viruses, rhinovirus and adenovirus, as previously described [7].
Data on all specimens received at HPA PHL Birmingham for respiratory virus investigations from late September 2009 to April 2010 were downloaded from the local laboratory information management system. The downloaded data included patient identifiers, specimen receipt dates, location at which specimens were taken, laboratory reference numbers and respiratory virus PCR detection test results.
Duplicate specimens from the same patient (within one week of another specimen) were identified and excluded when the results were the same.
All specimens in the data set which had not been tested against the full respiratory virus panels used by the laboratory were excluded from the analysis. Entries with unknown locations or unknown dates of birth were excluded.
Data analysis was carried out using Microsoft Excel software. Poisson regression analysis was carried out using Stata (StataCorp) software at the HPA Statistics Unit.
Results
A total of 4,821 specimens received from 21 September 2009 to 30 April 2010 met the inclusion criteria for the study. Of these, 2,374 (49.2 %) had no respiratory viruses detected and 2,447 (50.8 %) specimens had at least one respiratory virus detected.
The number of specimens received per week peaked over November and December. The percentage of received specimens that were positive averaged 47.3 %, but varied from 31.5 % (week beginning 1 March 2010) to 69.0 % (week beginning 23 November 2009).
The total numbers of specimens positive for each virus were as follows: RSV 853, rhinovirus 716, influenza A(H1N1) 469, HMPV 276, adenovirus 261, parainfluenza 236, influenza B 9.
Distribution of respiratory viruses over time
Figure 1 shows the numbers of specimens with positive virus detection results over time.
Fig. 1.
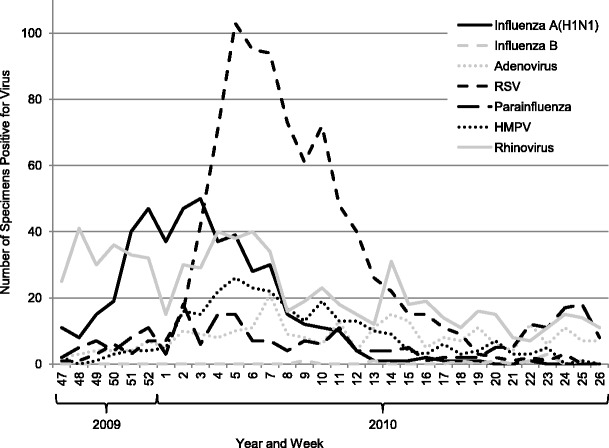
Numbers of viral detections in respiratory specimens received between 21 September 2009 and 30 April 2010 by week of receipt. RSV respiratory syncytial virus; HMPV human metapneumovirus
Influenza A(H1N1) showed a peak of increased detection from mid-October, tailing off at the end of December, with almost none from the end of January onwards. Influenza B was rarely detected and, apart from influenza A(H1N1), no other influenza A strains were detected.
Adenovirus and parainfluenza showed no temporal peak and were more or less evenly distributed across the time period.
RSV showed distinct seasonality, with a clear peak from the end of November to January, and was much less frequently detected prior to November and after February. Despite fewer detections, HMPV showed seasonality, with a pattern very similar to that of RSV.
Rhinovirus detections averaged approximately 30 positive specimens per month from the end of September to the end of December, and then from January to April, this halved to about 15 specimens per month.
Distribution of respiratory viruses by age of patient
The correlation between age and the types of viruses detected is shown in Table 1.
Table 1.
Proportion of positive specimens in each age group positive for each virus from specimens received between 21 September 2009 and 30 April 2010
| Patient age group (years) | No. of specimens | Percentage of positives | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive for at least one virus | Influenza A(H1N1) | Influenza B | Adenovirus | RSV | Parainfluenza | HMPV | Rhinovirus | |
| <5 | 808 | 1,518 | 6.0 % | 0.2 % | 13.9 % | 47.4 % | 9.3 % | 12.1 % | 30.6 % |
| 5–14 | 225 | 171 | 41.5 % | 1.2 % | 8.8 % | 9.9 % | 10.5 % | 11.1 % | 26.3 % |
| 15–24 | 197 | 145 | 46.9 % | 1.4 % | 2.8 % | 10.3 % | 10.3 % | 5.5 % | 32.4 % |
| 25–44 | 419 | 265 | 50.9 % | 0.8 % | 5.7 % | 10.2 % | 6.8 % | 5.7 % | 26.8 % |
| 45–64 | 412 | 212 | 38.2 % | 0.0 % | 5.7 % | 17.9 % | 11.3 % | 12.3 % | 25.0 % |
| 65+ | 313 | 136 | 16.9 % | 0.0 % | 2.9 % | 26.5 % | 14.7 % | 18.4 % | 25.7 % |
The under 5s group comprise by far the largest age group represented in the specimens received and also have the highest percentage of positives (65.3 %). The 15–24-year-olds are the least represented age group in the specimens received. The over 65s showed the lowest positivity rate (30.3 %).
Influenza B was too rarely detected to comment about its distribution by age of patients. Influenza A(H1N1) showed a distinct peak in the 25–44 years age group and a distinct trough in the over 65s. When this is considered against the total numbers of positive specimens, only 6.0 and 16.9 % of positive specimens in the under 5s and over 65s groups, respectively, were positive for influenza A(H1N1). In the 25–44 years age group, however, 50.9 % of positive specimens were positive for influenza A(H1N1).
Adenovirus, RSV, HMPV and rhinovirus were found most frequently in the under 5s. For RSV and adenovirus, this is also seen as a peak of the total percentage of positives in this age group (Table 1). HMPV was detected most often in the under 5s but, when considered as a percentage of the total number of positives in each group (Table 1), it reached its highest percentage of positives (18.4 %) in the over 65s and is lowest as a percentage of positives in the 15–24 and 25–44 years age groups. Although rhinovirus was detected most often in the under 5s, the rate of rhinovirus in each age group as a percentage of the total number of positives does not vary much (25.0 to 32.5 %).
The parainfluenza viruses were mostly detected in the under 5s, but the percentages of parainfluenza of any type in all ages groups always represented a small but fairly consistent percentage of the total number of positive samples.
Co-detections of respiratory viruses
Eighty-six percent (2,105) of positive specimens were positive for only one respiratory virus, whereas 323 (13.2 %) specimens were positive for two viruses, 22 (0.9 %) were positive for three viruses and four (0.2 %) were positive for four viruses.
Rhinovirus and RSV were the two viruses most frequently detected with other respiratory viruses (Table 2). However, parainfluenza and adenovirus had a greater proportion of their detections as co-detections with other viruses (46.7 and 33.5 %, respectively). Influenza A(H1N1) showed the lowest rate of co-detection with other viruses (10.4 %).
Table 2.
Numbers of respiratory virus detections that were single or co-detections in specimens received between 21 September 2009 and 30 April 2010
| Virus | No. of virus detections | |||||
|---|---|---|---|---|---|---|
| Total | As a single virus | With other viruses | With one other virus | With two other viruses | With three other viruses | |
| Adenovirus | 261 | 139 | 122 (46.7 %) | 102 | 17 | 3 |
| Parainfluenza | 242 | 161 | 81 (33.5 %) | 69 | 9 | 3 |
| Rhinovirus | 716 | 512 | 204 (28.5 %) | 184 | 17 | 3 |
| RSV | 853 | 650 | 203 (23.8 %) | 181 | 18 | 4 |
| Influenza B | 9 | 7 | 2 (22.2 %) | 1 | 1 | 0 |
| HMPV | 276 | 216 | 60 (21.7 %) | 54 | 4 | 2 |
| Influenza A(H1N1) | 469 | 420 | 49 (10.4 %) | 47 | 1 | 1 |
Table 3 shows the degree of positive or negative associations between different viruses. Influenza B was excluded from this analysis, as the number of detections in total was so low.
Table 3.
Co-detection of virus pairs in specimens received between 21 September 2009 and 30 April 2010
| Influenza A(H1N1) | Adenovirus | RSV | Para-influenza | HMPV | Rhinovirus | |
|---|---|---|---|---|---|---|
| Influenza A(H1N1) | – | 7 | 20 | 6 | 2 | 17 |
| Prevalence in influenza A-positive population | – | 1.5 % | 4.3 % | 1.3 % | 0.4 % | 3.6 % |
| Statistically significant association? | – | No | No | No | p = 0.013 | p = 0.026 |
| Adenovirus | 7 | – | 55 | 17 | 13 | 53 |
| Prevalence in adenovirus-positive population | 2.7 % | – | 21.1 % | 6.5 % | 5.0 % | 20.3 % |
| Statistically significant association? | No | – | p < 0.000 | p < 0.000 | No | p < 0.000 |
| RSV | 20 | 55 | – | 25 | 25 | 104 |
| Prevalence in RSV-positive population | 2.3 % | 6.4 % | – | 2.9 % | 2.9 % | 12.2 % |
| Statistically significant association? | No | p < 0.000 | – | No | No | p = 0.002 |
| Parainfluenza | 6 | 17 | 25 | – | 8 | 32 |
| Prevalence in parainfluenza-positive population | 2.5 % | 7.2 % | 10.6 % | – | 3.4 % | 13.6 % |
| Statistically significant association? | No | p < 0.000 | No | – | No | p = 0.003 |
| HMPV | 2 | 13 | 25 | 8 | – | 20 |
| Prevalence in HMPV-positive population | 0.7 % | 4.7 % | 9.1 % | 2.9 % | – | 7.2 % |
| Statistically significant association? | p = 0.013 | No | No | No | – | No |
| Rhinovirus | 17 | 53 | 104 | 32 | 20 | – |
| Prevalence in rhinovirus-positive population | 2.4 % | 7.4 % | 14.5 % | 4.5 % | 2.8 % | – |
| Statistically significant association? | p = 0.026 | p < 0.000 | p = 0.002 | p = 0.003 | No | – |
| Prevalence in all specimens | 9.7 % | 5.4 % | 17.7 % | 4.9 % | 5.7 % | 14.9 % |
The numbers in the grid show the total number of specimens in which both viruses were detected for each pair
For each virus, the prevalence within the sub-population of specimens positive for the co-detected virus is shown as a percentage
Where the observed prevalence is noticeably below the expected value, it has been highlighted in italics. Where the observed prevalence is noticeably above the expected value, it has been highlighted in bold
p-values are given for statistically significant associations and are highlighted in italics for negative associations and bold for positive associations
All patterns of association, positive or negative, whether statistically significant or not, were found to be reciprocal (Table 3).
The most common co-detection was of RSV and rhinovirus. Poisson regression analysis also showed a statistically significant association between these two viruses (p-value 0.002).
All non-influenza viruses detected had a lower prevalence in the influenza A(H1N1)-positive population than that in the total number of specimens. Influenza A(H1N1) also had a lower prevalence in all samples positive for other viruses than would be expected from random associations. Poisson regression analysis confirmed the statistical significance of the negative associations between influenza A and HMPV (p-value 0.013), and influenza A and rhinovirus (p-value 0.026).
Statistically significant positive associations were found between adenovirus and RSV, parainfluenza and rhinovirus (p-value <0.000 for all three pairs).
Statistical analysis using Poisson regression also suggested significant positive associations between parainfluenza and rhinovirus (p-value 0.003) and rhinovirus and RSV (p-value 0.002), despite the fact that each of these viruses actually has a slightly lower prevalence in the population positive for the associated virus than in all specimens (Table 3).
Discussion
The first notable feature of the analysis of the 2009–2010 winter season routine specimen data is that nearly half of all the specimens tested had no virus detected. Assuming that specimens were taken from symptomatic patients, it is evident that many cases of respiratory tract infection are not due to the viruses investigated in this study. These undiagnosed infections could be due to bacteria; other respiratory viruses such as bocaviruses, enteroviruses or coronaviruses or even as-yet-unknown respiratory pathogens.
The pattern of specimen receipt and positivity in the West Midlands over the 2009–2010 winter season showed the classic Northern Hemisphere midwinter peak of respiratory infection. The pattern of virus detection throughout this period also corresponded with established patterns of seasonality [8].
HPA data from England and Wales (http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1247728930779) shows a double peak of influenza in the summer of 2009 and relatively early in the winter when compared with previous years. This was a highly unusual feature of the most recent influenza A pandemic, as influenza A usually has a defined peak from December to March. The fact that no other strain of influenza A was detected either in the summer or the winter demonstrates how completely this new strain has replaced the previous circulating seasonal influenza.
Analysis of the winter respiratory virus data by patient age showed that the under 5s form an epidemiological group distinct from older children and adults. Their rate of specimen positivity is highest, their frequency of infection with viruses is different (e.g. higher RSV and lower influenza A) from other age groups and the percentage of multiple virus detections is about twice that of the older age groups.
The co-detection of different respiratory viruses is well documented [1, 4–6, 9–15]. The results of this study’s analysis suggest that co-detections are not totally random and most associations appear to be reciprocal—observations also made by Brunstein et al. [10] and Greer et al. [12] in their surveys of acute respiratory tract infections.
The most striking association observed between respiratory viruses in this study was the lower prevalence of influenza A(H1N1) in patients positive for adenovirus, RSV, parainfluenza, HMPV and rhinovirus and vice versa, which reached statistical significance with HMPV and rhinovirus (Table 3).
From seasonal patterns of incidence, it has been suggested that interference between different respiratory viruses may contribute to their seasonal patterns and that RSV and rhinovirus outbreaks may delay influenza outbreaks [16, 17]. The patterns observed in the West Midlands during the 2009–2010 winter season showed the rhinovirus and influenza peaks to be concurrent (Fig. 1). If associations were random, these co-incident peaks would lead us to expect an increased incidence of co-detection. Interestingly, despite these overlapping peaks, the rate of positivity for rhinovirus in the influenza A(H1N1)-positive population was observed to be lower than would be expected from random associations.
Greer et al.’s study also noted negative associations of influenza A with rhinovirus and RSV, but concluded that rhinovirus, RSV and HMPV were most likely to be associated with reduced co-detection of other viruses [12]. Brunstein et al.’s study also suggested decreased incidence of influenza A in backgrounds of RSV, parainfluenza, HMPV and rhinovirus, and decreased incidence of RSV, HMPV and rhinovirus in an influenza A-positive background, but none of these reached statistical significance in their study [10].
The potential negative associations observed between RSV and parainfluenza and between HMPV and RSV have also been noted by both Greer et al. [12] and Brunstein et al. [10], which suggests that these observations are not co-incidental, despite not reaching statistical significance in the study.
The positive associations we observed with adenovirus and RSV, parainfluenza and rhinovirus, and rhinovirus with parainfluenza and RSV have not been observed in previous studies.
This study did not analyse the clinical significance of multiple viral detection/infection. The clinical significance of respiratory co-infections is debatable and requires more study. Studies of respiratory viral infections in children reviewed by Tregoning and Schwarze [18] and Paranhos-Baccalà et al. [5] showed differences in clinical severity between single and dual infections, but the impact of such dual infections appears to depend on which viruses co-infect.
The question of whether the co-detection of viruses really indicates co-infection is also one that has not been fully answered. It is known that PCR can detect genomic material after clinical symptoms have ended [3, 19] and in the absence of respiratory symptoms [20]. Semi-quantitative reverse transcription (RT)-PCR on nasopharyngeal aspirates has been proposed as a means of differentiating true infections from low-level shedding [6]. Whether this method would still be valid for less standardised specimens like nose and throat swabs and whether a correlation between viral load and severity of disease can be determined remain to be seen.
This analysis of viruses detected during the West Midlands 2009–2010 winter season has some limitations. The sample is drawn from a somewhat skewed population. Most of the specimens were from hospitalised patients and those needing medical care, whilst the majority of respiratory infections won’t present to healthcare, especially in adults, due to the self-limiting nature of the infections [3, 21]. This sample is not, therefore, strictly representative of the wider population, although it is drawn from it.
In conclusion, this study found fairly classical age and seasonal distributions of respiratory viruses during the Northern Hemisphere 2009–2010 winter season in the West Midlands region of the UK. Patterns of co-detection of different respiratory viruses were found not to be random and most associations were reciprocal, either positively or negatively. The pandemic strain of influenza A(H1N1) was notable in that it was the least likely to be co-detected with another respiratory virus.
Acknowledgements
We acknowledge the work of the staff at the HPA PHL Birmingham in carrying out the PCR respiratory virus testing and also the help of Andrew Grant at the HPA Statistics Unit, Colindale, UK.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Data from this study were presented, in part, as a poster (P954) at the 22nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), London, March/April 2012.
References
- 1.Mahony JB. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev. 2008;21:716–747. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112:4S–12S. doi: 10.1016/S0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 3.Heikkinen T, Järvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruohola A, Waris M, Allander T, Ziegler T, Heikkinen T, Ruuskanen O. Viral etiology of common cold in children, Finland. Emerg Infect Dis. 2009;15:344–346. doi: 10.3201/eid1502.081468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paranhos-Baccalà G, Komurian-Pradel F, Richard N, Vernet G, Lina B, Floret D. Mixed respiratory virus infections. J Clin Virol. 2008;43:407–410. doi: 10.1016/j.jcv.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lassaunière R, Kresfelder T, Venter M. A novel multiplex real-time RT-PCR assay with FRET hybridization probes for the detection and quantitation of 13 respiratory viruses. J Virol Methods. 2010;165:254–260. doi: 10.1016/j.jviromet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanner HE, Curran MD, Boxall EH, Osman H. Viral respiratory infections during the 2009 influenza A(H1N1) outbreak in the West Midlands Region, UK. Epidemiol Infect. 2011;11:1–6. doi: 10.1017/S0950268811002251. [DOI] [PubMed] [Google Scholar]
- 8.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–160. doi: 10.1017/S0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605–610. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 10.Brunstein JD, Cline CL, McKinney S, Thomas E. Evidence from multiplex molecular assays for complex multipathogen interactions in acute respiratory infections. J Clin Microbiol. 2008;46:97–102. doi: 10.1128/JCM.01117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo C, García-García ML, Blanco C, Vázquez MC, Frías ME, Pérez-Breña P, Casas I. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol. 2008;42:268–272. doi: 10.1016/j.jcv.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer RM, McErlean P, Arden KE, Faux CE, Nitsche A, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol. 2009;45:10–15. doi: 10.1016/j.jcv.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng D, Zhao DC, Liu JT, Wang X, Yang K, Xicheng H, Li Y, Wang F. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J. 2009;6:115. doi: 10.1186/1743-422X-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richard N, Komurian-Pradel F, Javouhey E, Perret M, Rajoharison A, Bagnaud A, Billaud G, Vernet G, Lina B, Floret D, Paranhos-Baccalà G. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J. 2008;27:213–217. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 15.Stempel HE, Martin ET, Kuypers J, Englund JA, Zerr DM. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 2009;98:123–126. doi: 10.1111/j.1651-2227.2008.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anestad G. Interference between outbreaks of respiratory syncytial virus and influenza virus infection. Lancet. 1982;1:502. doi: 10.1016/S0140-6736(82)91466-0. [DOI] [PubMed] [Google Scholar]
- 17.Anestad G, Nordbo SA. Interference between outbreaks of respiratory viruses. Euro Surveill. 2009;14:19359. [PubMed] [Google Scholar]
- 18.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nokso-Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkäranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol. 2002;66:417–420. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winther B, Alper CM, Mandel EM, Doyle WJ, Hendley JO. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119:1069–1075. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- 21.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Bautista B, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, Kumar A, Lim M, Shindo N, Penn C, Nicholson KG KG. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]


