Abstract
The objective of this study was to retrospectively evaluate the occurrence of porcine parvovirus (PPV), Aujeszky’s disease virus (ADV), transmissible gastroenteritis virus (TGEV), porcine respiratory coronavirus (PRCV), porcine reproductive and respiratory syndrome virus (PRRSV) and swine influenza virus (SIV) in selected wild boar populations in Germany (n = 1,221). Commercial enzyme linked immunosorbent assay and hemagglutination inhibition tests were used for serological monitoring. The serosurvey revealed seroprevalence rates of 64.28%, 11.26%, 7.87%, 7.84%, 3.82% and 1.59% for PPV, ADV, PRCV, SIV, PRRSV and TGEV, respectively. The seroprevalence rates differed between populations and age classes with the highest number of antibody-positive wild boars in older animals (>1 year old). No antibodies to TGEV were found in Baden–Wuerttemberg and in Mecklenburg–Western Pomerania (investigation period 1997/1998). In addition, sera collected in Mecklenburg–Western Pomerania in 1997/1998 were negative for SIV. Even though the seroprevalence rates established for these viruses, except for PPV, were relatively low, wild boars may act as a reservoir for pathogens and a source of infection for domestic pigs and humans. Based on the epidemiological situation, no risk of a spread of these viruses should emanate from wild boars, neither for wildlife nor for livestock. However, effective and science-based disease monitoring programmes should continuously be carried out in wild boar populations.
Keywords: Wild boar, Viral diseases, Serosurvey, Germany
Introduction
Wildlife diseases may represent a potential threat not only to local wildlife populations but also to domestic animals and humans. Various studies have been carried out to analyse the prevalence of pathogens in wild boar populations and the role of these populations as reservoir for pathogens or a source of infection for domestic pigs. Not only viral and bacterial agents have been diagnosed in European wild boars (e.g. Markowska-Daniel and Pejsak 1999; Albina et al. 2000; Gortázar et al. 2002; Vicente et al. 2002, 2005; Lipowski 2003; Jacobson et al. 2005; Vengust et al. 2005, 2006; Bonilauri et al. 2006; Dezorzova-Tomanova et al. 2006; Ruiz-Fons et al. 2006, 2008; Martínez et al. 2006; Kaci et al. 2008; De Deus et al. 2008) but also parasites (e.g. Takacs 1997; De-la-Muela et al. 2001; Fernandez-de-Mera et al. 2003). Normally, the prevalence rates of infections in European wild boar (Sus scrofa L.) correlate with the population density of the animals. During the last decades, populations have increased not only in Germany (Lutz and Wurm 1996; Kaden 1999; Gethöffer et al. 2007) but also in other European countries (Sáez-Royuela and Terilería 1986). This development is one of the reasons why wild boars may become a more important potential reservoir for different infectious pathogens.
In Germany, several studies have been carried out to analyse the presence of different pathogens in wild boar populations. So far, the role of infected wild boars as a potential reservoir for pathogens or a source of infection for domestic pigs has been analysed in detail for classical swine fever virus (CSFV; Dedek et al. 1989; Dahle et al. 1993; Kaden et al. 1994; Oslage et al. 1994; Lutz and Wurm 1996; Kern et al. 1999; Fritzemeier et al. 2000; Schlüter and Kramer 2001). Furthermore, serological investigations in individual wild boar populations in Germany have revealed the presence of different other viral agents, e.g. Aujeszky’s disease virus (ADV, pseudorabies virus; Dedek et al. 1989; Dahle et al. 1993; Oslage et al. 1994; Lutz and Wurm 1996; Müller et al. 1998, 2000; Kaden and Müller 2001; Lutz et al. 2003), porcine reproductive and respiratory syndrome virus (PRRSV; Oslage et al. 1994), porcine circovirus type 2 (Schulze et al. 2004; Knell et al. 2005), porcine parvovirus (PPV; Dedek et al. 1989; Liebermann et al. 1986; Lutz and Wurm 1996), swine influenza virus (SIV; Dedek et al. 1989, 1990; Polley et al. 2007; Kaden et al. 2008), bovine viral diarrhoea virus (Dahle et al. 1993; Schmitt and Wittkowski 1999) and hepatitis E virus (Kaci et al. 2008).
Within the framework of studies on the oral vaccination of wild boars against CSF in Germany (Kaden et al. 2002), sera were collected for serological investigations on the presence of CSFV. The objective of this study was to retrospectively assess the occurrence of infections with ADV, PRRSV, PPV, SIV, porcine respiratory coronavirus (PRCV), and transmissible gastroenteritis virus (TGEV) in wild boar populations of different vaccination areas.
Materials and methods
Investigation areas and collection of blood samples
The samples were collected from wild boars bagged in five German Bundeslaender (federal states; Fig. 1) within the last decade. Altogether, 1,221 blood samples collected at different time points were investigated: in Mecklenburg–Western Pomerania, samples had been collected during the main hunting season in 1997/1998 (n = 120) and in 2000/2001 (n = 322), in Saxony–Anhalt in 2001/2002 (n = 254), in Baden–Wuerttemberg in 2001/2002 (n = 159), in Brandenburg in 2004/2005 (n = 166), and in Rhineland–Palatinate in 2005 (n = 200).
Fig. 1.
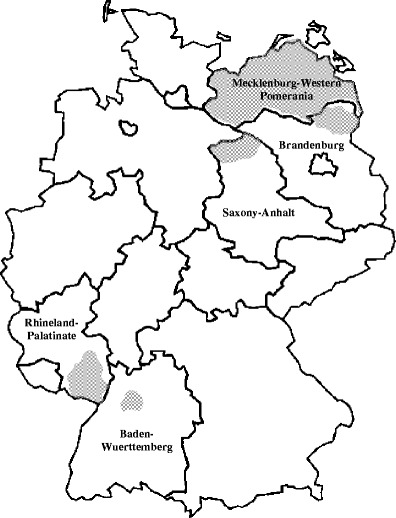
Sampling areas (grey) vs areas with oral vaccination against CSF
Blood samples for the serological survey were taken by hunters from the pericardium or the thoracic cavity immediately after the animals had been shot. The samples were sent to the local veterinary diagnostic laboratories of the Bundeslaender or to the Friedrich-Loeffler-Institut (samples from Mecklenburg–Western Pomerania) where they were centrifuged at 3,000 to 3,500 rpm, and the sera were stored at −20°C until use. All samples were collected year-round in areas where oral immunisation against CSF was carried out. However, most of them were taken during the main hunting seasons (from October to February). The samples were derived from animals of the following age classes: 35.9% from young wild boars (≤1 year old), 45.9% from sub-adults (>1 to 2 years) and 11% from adults (>2 years old); 7.2% of the collected sera could not be assigned to a specific age class.
Serological investigations
Commercially available enzyme linked immunosorbent assays were used in accordance with the manufacturers’ instructions for screening of antibodies to the following viruses: ADV (CHEKIT Aujeszky, IDEXX Laboratories/Bommeli Diagnostics, Liebefeld-Bern, Switzerland), PRCV and TGEV (INGELVAC® TGEV/PRCV-Diagnostikum, Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany), PRRSV (PRRSV Antibody Test Kit, IDEXX Laboratories, Ludwigsburg, Germany) and PPV (INGELVAC® PPV-Diagnostikum, Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany). In contrast, the sera were tested for antibodies to SIV using the hemagglutination inhibition (HI) test based on a slightly modified standard protocol of the Office International des Epizooties (OIE Diagnostic Manual, http://www.oie.int/eng/normes/mmanual/A_00137.htm). These sera were checked for antibodies to SIV H1N1, H1N2 and H3N2 as described by Kaden et al. (2008). Serum dilutions of 1:10 to 1:2,560 were tested and sera with no activity at a dilution of 1:20 were considered to be negative for antibodies in the HI test.
Statistical analysis
The statistical analysis was carried out with the statistic programme SigmaStat 3.0 (SPSS Science Software Gmb, Erkrath, Germany) using the Mann–Whitney Rank Sum Test. The significance level was set at p = 0.05.
Results
Antibodies to all investigated viruses were detected in the wild boar populations of the various Bundeslaender (Table 1). Exceptions were Mecklenburg–Western Pomerania, where the samples tested negative for antibodies to TGEV and SIV during the hunting season 1997/1998, and Baden-Wuerttemberg, where no antibodies to TGEV were found. The seroprevalence rates varied more or less among pathogens and individual populations. The highest seroprevalence rate was detected for PPV (64.28%) followed by ADV (11.26%), PRCV (7.87%), SIV (7.84%) PRRSV (3.82%) and TGEV (1.59%). The highest PPV seroprevalence rate was detected in Mecklenburg–Western Pomerania in 1997/1998 (78%), the lowest in Baden–Wuerttemberg with 17.61%. The percentage of serum samples with antibodies to ADV was relatively low to moderately high, ranging from 5.91% (Saxonay–Anhalt) to 18.01% (Mecklenburg–Western Pomerania). Table 2 indicates the number of serologically positive wild boars per age class and pathogen. In general, the proportion of seropositive animals was significantly higher in older wild boars (>1 year old) than in the juveniles (<1 year old) except for wild boars with antibodies to TGEV.
Table 1.
Summarised results of the serological investigations
| Bundesland (federal state) | Period of investigation | Number of animals investigated | Serologically positive samples per random sample | |||||
|---|---|---|---|---|---|---|---|---|
| PPV | ADV | TGEV | PRCV | PRRSV | SIV | |||
| Mecklenburg–Western Pomerania | 1997/98 | 120 | 78/100 | 13/99 | 0/100 | 2/100 | nt | 0/120 |
| 2000/01 | 322 | 231/322 | 58/322 | 2/321 | 1/322 | 11/322 | nt | |
| Brandenburg | 2004/05 | 166 | 96/166 | 26/166 | 5/166 | 3/166 | 3/166 | 3/120 |
| Saxony–Anhalt | 2001/02 | 254 | 156/254 | 15/254 | 6/252 | 65/252 | 25/254 | 10/120 |
| Baden–Wuerttemberg | 2001/02 | 159 | 28/159 | 11/159 | 0/156 | 17/155 | 2/159 | 21/81 |
| Rhineland–Palatinate | 2005 | 200 | 139/200 | 12/199 | 6/199 | 1/199 | 1/200 | 10/120 |
| Total | 1997–2005 | 1,221 | 772/1,201 | 135/1,199 | 19/1,194 | 94/1,194 | ne | 44/561 |
| 2000–2005 | 1,101 | 694/1,101 | 132/1,100 | 19/1,094 | 92/1,094 | 42/1,101 | 44/441 | |
nt not tested, ne not evaluable
Table 2.
Number of serologically positive wild boars by age classes
| Age | Number of animals with antibodies against | |||||
|---|---|---|---|---|---|---|
| PPV | ADV | TGEV | PRCV | PRRSV | SIV | |
| ≤1 year old | 155 | 26 | 7 | 12 | 7 | 10 |
| >1 to 2 years old | 342 | 48 | 9 | 46 | 25 | 25 |
| >2 years old | 213 | 58 | 2 | 23 | 6 | 7 |
| Unknown | 62 | 3 | 1 | 13 | 4 | 2 |
| Total | 772 (64.28%) | 135 (11.26%) | 19 (1.59%) | 94 (7.87%) | 42 (3.82%) | 44 (7.84%) |
Discussion
Consequent pathogen surveillance in wildlife may provide an effective epidemiological overview which allows to assess the risk of infection and of a spread of agents within the wild boar populations as well as from this wildlife species to domestic pigs and, in case of zoonotic agents, also to humans. Therefore, continuous serological monitoring of wild boar populations is of national and international interest. The present investigations support these intentions and were performed with the aim to retrospectively examine the epidemiological situation with regard to various pathogens (ADV, PRRSV, PPV, SIV, PRCV and TGEV) in selected wild boar populations over different years in Germany. Except for ADV, these viruses have recently caused economically important infectious viral diseases in our domestic pig herds.
As expected, antibodies to all six viruses were found in the investigated populations, however, with differences between the individual populations. It is not surprising that PPV is widespread in our wild boar populations (seroprevalence rate: 64.28%), and that its seroprevalence rate differs significantly from those of the other viruses tested. Most animals tested positive for PPV were derived from Mecklenburg–Western Pomerania. Our findings correspond to those established in this Bundesland in the 1980s (Dedek et al. 1989; Liebermann et al. 1986) as well as to data previously established in North Rhine–Westphalia (Lutz and Wurm 1996), in Lower Saxony and in Rhineland–Palatinate (Gethöffer et al. 2007). These high PPV specific seroprevalence rates in Germany also agree with findings in other European wild boar populations (Roić et al. 2005; Ruiz-Fons et al. 2006; Vengust et al. 2006). Although Vicente et al. (2002) found large differences between the PPV seroprevalence rates in local Spanish wild boar populations, our serosurvey for Germany does not generally confirm this, only in Baden-Wuerttemberg the PPV seroprevalence was lower than in the other populations tested. Despite the high PPV seroprevalence in German wild boars, this agent does not seem to have a negative influence on the reproduction rate of wild sows as determined in Mecklenburg–Western Pomerania (Kaden et al. 2005) as well as in Lower Saxony and Rhineland–Palatinate (Gethöffer et al. 2007).
The second highest seroprevalence rate (averaged 11.26%) was found for ADV. Generally, the differences in the seroprevalence rates between the Bundeslaender were lower than for PPV. The proportion of wild boars with antibodies to ADV (15.66%), which were shot in the eastern part of Brandenburg during the hunting season 2004/2005, corresponds well with previous findings from 1995 (Müller et al. 1997). As the investigation areas were largely identical, the findings suggest a stable epidemiological situation. In contrast to these results in Eastern Brandenburg, Oslage et al. (1994) found a very low seroprevelance (0.9%) in Western Brandenburg. This underlines the differences in the epidemiological situation in this Bundesland. Whereas the seroprevalence rates for ADV generally were on the same level in Mecklenburg–Western Pomerania and Brandenburg, the proportion of antibody-positive wild boars in Saxony–Anhalt (5.91%), Baden–Wuerttemberg (6.92%) and Rhineland–Palatinate (6.03%) was significantly lower (p > 0.05). In the 1990s, a similar seroprevalence rate (7%) was found in North Rhine–Westphalia (Lutz and Wurm 1996), whereas investigations in Lower Saxony showed a very low ADV prevalence of between 1.7% and 0% in wild boars (Dahle et al. 1993; Gethöffer et al. 2007). The latter authors found 26% of the wild boars in the Eifel region, Rhineland–Palatinate, to be antibody-positive to ADV, the investigations in the Pfalz region (Palatine Forest) showed a negative result (n = 27). These findings do not correspond with our experiences in the Pfalz region. We suppose that the higher sampling rate in our study better reflects the epidemiological situation in this population. Considerably higher percentages of seropositive wild boars were detected in other European wild boar populations, e.g. between 36% (Vicente et al. 2002) and 60.6% (Ruiz-Fons et al. 2006) in Spain, 26–31% in Slovenia (Vengust et al. 2005, 2006), 54.54% in Croatia (Župančič et al. 2002) and 55% in Macedonia (Gagrčin et al. 1989). In contrast, the proportion of wild boars seropositive to ADV seems to be relatively low in The Netherlands (Cromwijk 1995; Elbers et al. 2000).
Although coronaviruses, especially TGEV and PRCV, are important pathogens in commercial pig farms worldwide, only little is known on the epidemiological situation in European wild boars. In Slovenia, Vengust et al. (2006) found no antibodies against TGEV in wild boars; however, 3% of the investigated samples were seropositive to PRCV. A sero-surveillance study in feral pigs in the USA (Saliki et al. 1998) also was negative for TGEV. Our serosurvey underlines that the occurrence of TGEV (1.59% antibody-positive animals) was generally lower than that of PRCV (7.87%) in the investigated wild boar populations. None of the animals derived from Baden–Wuerttemberg (investigation period 2001/2002) and Mecklenburg–Western Pomerania (bagged in 1997/1998) had antibodies against TGEV. These findings in Mecklenburg–Western Pomerania are inconsistent with those established in 2000/01 which showed a very low seroprevalence (0.6% animals with antibodies). The PRCV seroprevalence rates in Saxony–Anhalt (25.8%) and in Baden–Wuerttemberg (11%) were significantly higher than the TGEV seroprevalences in both Bundeslaender. At present, it is not possible to assess the reason for this relatively high seroprevalence rate for PRCV in Saxony–Anhalt. As PRCV infections in pig farms do not play an important role in this region (Tyrpe, pers. communication), livestock does not seem to be the source of infection for the wild boar population of Saxony–Anhalt. Hence, further investigations should be carried out with the aim to evaluate the development of the epidemiological situation regarding PRCV in the population including a risk assessment for wild boars and domestic pig farms.
The low proportion of wild boars with antibodies against PRRSV in this study generally corresponds with previous serological investigations in Brandenburg, Saxony–Anhalt, North Rhine–Westphalia, Lower Saxony and Rhineland–Palatinate (Oslage et al. 1994; Lutz and Wurm 1996; Gethöffer et al. 2007). Interestingly, a relative high proportion (9.84%) of wild boars with antibodies against PRRSV was found in Saxony–Anhalt. As PRRSV is widespread in pig herds, a spread of this virus from domestic pigs to wild boar cannot be excluded. Virus transmission from livestock to wild boar can take place by different routes. The main sources presumably are infected manure and slurry as well as kitchen waste (feeding of animal waste is banned in the EU) as the un-enveloped PRRSV shows a high tenacity and survives in the environment for a relative long time. Direct contacts between infected domestic pigs and wildlife are supposed to be very rare. In contrast to our findings, wild boars were found to be free from antibodies to PRRSV in some European countries, e.g. in Spain (Vicente et al. 2002; Ruiz-Fons et al. 2006), in Croatia (Župančič et al. 2002) and in Slovenia (Vengust et al. 2006). Likewise low seroprevalence rates were reported by Albina et al. (2000) in French wild boars. Infections with this Arterivirus also occur in Italy (Bonilauri et al. 2006).
Antibodies against SIV (on average 7.84%) were found in wild boars of all investigated areas except for Mecklenburg–Western Pomerania in 1997/1998. Unfortunately, no investigations could be carried out with blood samples collected in this Bundesland in 2000/2001 as no sufficient material was available. However, an SIV serosurvey performed in Mecklenburg–Western Pomerania in 2005/2006 (Kaden et al. 2008) revealed a low infection rate of wild boars (3.1%). In our investigation areas, only antibodies to SIV subtypes H1N1 and H3N2 were detected. The results obtained in Mecklenburg–Western Pomerania in 2005/2006 correspond to those previously reported by Dedek et al. (1990). The seroprevalence rates in the other Bundeslaender presented here showed large differences, ranging between 2.5% in Brandenburg and 25.93% in Baden–Wuerttemberg. The high proportion of seropositive animals in Baden–Wuerttemberg cannot be explained at the moment. Our investigations indicate that especially SIV subtypes H1N1 and H3N2 are circulating in the population with a dominance of subtype H1N1 which is also predominant in domestic pigs in Europe (Van Reeth 2007). However, antibodies against all three SIV subtypes may occur in European wild boars (summarised by Ruiz-Fons et al. 2008).
Antibodies against the tested viruses were present in animals of all age classes. As expected, the seroprevalences generally were higher in the older animals (>1 year old) except those for TGEV. We can only speculate on the origin of antibodies in the juveniles (≤1 year old) as we do not have any information on the precise age of these wild boar piglets. Based on the experiences with other diseases, it must be assumed that the antibodies of serologically positive juveniles (wild boar piglets) are of maternal origin during the first 3 to 4 months of life; later on, they are induced by natural infection. However, maternal antibodies to PPV may obviously persist longer, i.e. for up to 6 months (Johnson et al. 1976).
In conclusion, our study shows that PPV, ADV, TGEV, PRCV, PRRSV and SIV are present in German wild boar populations. The detected seroprevalence rates of most pathogens were relatively low, except that of PPV. The high seroprevalence of PPV suggests an endemic status of this virus in our wild boar populations. As PPV is widespread in European wild boars and domestic pigs, no risk emanates from wild boars at present. Independently of this assessment, wild boars may generally act as a reservoir for pathogens and as a source of infection for domestic pigs. However, it is important to recognise that virus transmission between wildlife and domestic animals is not a one-way street. For example, wild boars may also become infected indirectly or directly through infected pigs. To assess the real epidemiological situation in wildlife and the risk of disease for wild boars and livestock, effective and science-based disease monitoring programmes in wild boars must be carried out, especially for economically important and notifiable agents that may affect wild boars and domestic pigs as well as humans. These surveillance programmes should not only analyse prevalences of pathogens but also include wildlife-biological parameters and management strategies as well as the molecular–biological characterization of pathogens circulating in wild boars and domestic pigs. The latter might become important for the evaluation of the epidemiological process. Based on the experiences with CSF control in wild boar populations (Kaden et al. 2006) and the risk of an introduction of new or re-emerging diseases to Europe, as e.g. African swine fever which just now circulates in domestic pigs, free-range backyard pigs and/or in wild boars in Armenia, Georgia and Russia (www.oie.int), surveillance should include the whole territory of a country and should be carried out continuously, that is year-round.
Acknowledgements
We thank Silvia Schuparis and Sybilla Welsch for technical assistance as well as R. Dürrwald (Impfstoffwerk Dessau-Tornau) and J. Süß (formerly Federal Institute for Risk Assessment, Berlin) for providing SIV strains used for HI tests. Furthermore, we gratefully acknowledge Anette Beidler, Th. Vahlenkamp and W. Boehle for critical review of the manuscript and hunters for the collection of blood samples.
References
- Albina E, Mesplède A, Chenut G, Le Portier MF, Bourbao G, Le Gal S, Leforban Y. A serological survey on classical swine fever (CSF), Aujeszky’s disease (AD) and procine reproductive and respiratory syndrome (PRRS) virus infections in French wild boars from 1991 to 1998. Vet Microbiol. 2000;77:43–57. doi: 10.1016/S0378-1135(00)00255-8. [DOI] [PubMed] [Google Scholar]
- Bonilauri P, Merialdi G, Dottori M, Barbieri I. Presence of PRRSV in wild boar in Italy. Vet Rec. 2006;158:107–108. doi: 10.1136/vr.158.3.107-a. [DOI] [PubMed] [Google Scholar]
- Cromwijk WAJ. Serologisch onderzoek bij wilde zwijnen op de Veluwe (Serological investigations on wild swine in the Veluwe region) Tijdschr Diergeneeskd. 1995;120:364–365. [PubMed] [Google Scholar]
- Dahle J, Patzelt T, Schagemann G, Liess B. Antibody prevalence of hog cholera, bovine viral diarrhoea and Aujeszky’s disease virus in wild boar in Northern Germany. Dtsch tierärztl Wschr. 1993;100:330–333. [PubMed] [Google Scholar]
- De Deus N, Peralta B, Pina S, Allepuz A, Mateu E, Vidal D, Ruiz-Fons F, Martín M, Gortázar C, Selegés J. Epidemiological study of hepatitis E virus infection in European wild boars (Sus scrofa) in Spain. Vet Microbiol. 2008;129:163–170. doi: 10.1016/j.vetmic.2007.11.002. [DOI] [PubMed] [Google Scholar]
- De-la-Muela N, Hernández de Luján S, Ferre I. Helminths of wild boar in Spain. J Wildl Dis. 2001;37:840–843. doi: 10.7589/0090-3558-37.4.840. [DOI] [PubMed] [Google Scholar]
- Dedek J, Loepelmann H, Kokles R. Ergebnisse flächendeckender serologischer Untersuchungen beim Schwarzwild (Sus scrofa) in einem Bezirk der DDR. Verhandlungsbericht des 31. Internationalen Symposiums über die Erkrankungen der Zoo- und Wildtiere. Berlin: Akademie; 1989. pp. 309–314. [Google Scholar]
- Dedek J, Lange E, Pohle V, Kokles R, Loepelmann H, Klähn J. Zum Vorkommen von Antikörpern gegen Influenza-A-Virus beim Schwarzwild. Mh Vet-Med. 1990;45:201–203. [Google Scholar]
- Dezorzova-Tomanova K, Smola J, Trcka I, Lamka J, Pavlik I. Detection of Lawsonia intracellularis in wild boar and fallow deer bred in one game enclosure in the Czech Republic. J Vet Med B. 2006;53:42–44. doi: 10.1111/j.1439-0450.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Elbers ARW, Dekkers LJM, van der Giessen JWB. Sero-surveillance of wild boar in The Netherlands, 1996–1999. Rev sci tech Off int Epiz. 2000;19:848–854. doi: 10.20506/rst.19.3.1254. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-Mera IG, Gortazar C, Vicente J, Höfle U, Fierro Y. Wild boar helminths: risk in animal translocations. Vet Parasitol. 2003;115:335–341. doi: 10.1016/S0304-4017(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Fritzemeier J, Teuffert J, Greiser-Wilke I, Staubach C, Schlüter H, Moennig V. Epidemiology of classical swine fever in Germany in the 1990s. Vet Microbiol. 2000;77:29–41. doi: 10.1016/S0378-1135(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Gagrčin M, Ćirković D, Orlić D. Wild pigs reservoirs of Aujeszky’s disease. Mikrobiologija. 1989;26:149–152. [Google Scholar]
- Gethöffer F, Sodeikat G, Pohlmeyer K. Reproductive parameters of wild boar (Sus scrofa) in three different parts of Germany. Eur J Wildl Res. 2007;53:287–297. doi: 10.1007/s10344-007-0097-z. [DOI] [Google Scholar]
- Gortázar C, Vicente J, Fierro Y, León L, Cubero MJ, González M. Natural Aujeszky’s Disease in a Spanish Wild Boar Population. Ann N Y Acad Sci. 2002;969:210–212. doi: 10.1111/j.1749-6632.2002.tb04380.x. [DOI] [PubMed] [Google Scholar]
- Jacobson M, Löfstedt MG, Holmgren N, Lundeheim N, Fellström C. The prevalences of Brachyspira spp. and Lawsonia intracellularis in Swedish piglet producing herds and wild boar population. J Vet Med B. 2005;52:386–391. doi: 10.1111/j.1439-0450.2005.00865.x. [DOI] [PubMed] [Google Scholar]
- Johnson RH, Donaldson-Wood C, Joo HS, Allender U. Observations on the epidemiology of porcine parvovirus. Aust Vet J. 1976;52:80–84. doi: 10.1111/j.1751-0813.1976.tb13862.x. [DOI] [PubMed] [Google Scholar]
- Kaci S, Nöckler K, Johne R. Detection of hepatitis E virus in archived German wild boar samples. Vet Microbiol. 2008;128:380–385. doi: 10.1016/j.vetmic.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kaden V. Bekämpfung der Klassischen Schweinepest beim Schwarzwild. Z Jagdwiss. 1999;45:45–59. doi: 10.1007/BF02240719. [DOI] [Google Scholar]
- Kaden V, Müller T. Gefährliche Verwandtschaften: Schwarzwild—ein natürliches Reservoir für Infektionserreger und Ansteckungsquelle für Hausschweine? ForschungsReport Nr. 1. Bonn: Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz; 2001. pp. 24–28. [Google Scholar]
- Kaden V, Kosmidou A, Kramer M, Moennig V, Thiel H-J, Weiland E, Ahl R. Die Schweinepest in Deutschland in den Jahren 1992 und 1993. ForschungsReport Nr. 9. Bonn: Bundesministerium für Ernährung, Landwirtschaft und Forsten; 1994. pp. 25–28. [Google Scholar]
- Kaden V, Heyne H, Kiupel H, Letz W, Kern B, Lemmer U, Gossger K, Rothe A, Böhm H, Tyrpe P. Oral immunisation of wild boar against classical swine fever: concluding analysis of the recent field trials in Germany. Berl Münch Tierärztl Wschr. 2002;115:179–185. [PubMed] [Google Scholar]
- Kaden V, Steyer H, Schnabel J, Bruer W. Classical swine fever (CSF) in wild boar: the role of the transplacental infection in the perpetuation of CSF. J Vet Med B. 2005;52:161–164. doi: 10.1111/j.1439-0450.2005.00838.x. [DOI] [PubMed] [Google Scholar]
- Kaden V, Kramer M, Kern B, Hlinak A, Mewes L, Hänel A, Renner Ch, Dedek J, Bruer W. Diagnostic procedure after completion of oral immunization against classical swine fevere in wild boar. Rev sci tech Off int Epiz. 2006;25:989–997. [PubMed] [Google Scholar]
- Kaden V, Lange E, Starick E, Bruer W, Krakowski W, Klopries M. Epidemiological survey of swine influenza A virus in selected wild boar populations in Germany. Vet Microbiol. 2008;131:123–132. doi: 10.1016/j.vetmic.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kern B, Depner KR, Letz W, Rott M, Thalheim S, Nitschke B, Plagemann R, Liess B. Incidence of classical swine fever (CSF) in wild boar in a densely populated area indicating CSF virus persistence as a mechanism from virus perpetuation. Zbl Vet Med B. 1999;46:63–67. doi: 10.1046/j.1439-0450.1999.00214.x. [DOI] [PubMed] [Google Scholar]
- Knell S, Willems H, Hertrampf B, Reimer G. Comparative genetic characterization of porcine circovirus type 2 samples from German wild boar populations. Vet Microbiol. 2005;109:169–177. doi: 10.1016/j.vetmic.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Liebermann H, Dedek J, Loepelmann H, Hille G. Serologische Untersuchungen auf porcine Parvovirus beim Schwarzwild. Mh Vet-Med. 1986;41:410–412. [Google Scholar]
- Lipowski A. European wild boar (Sus scrofa L.) as reservoir of infectious diseases for domestic pigs. Med Weter. 2003;59:861–863. [Google Scholar]
- Lutz W, Wurm R. Serologische Untersuchungen zum Nachweis von Antikörpern gegen Viren des Seuchenhaften Spätaborts, der Aujeszkyschen Krankheit, der Europäischen Schweinepest und Porzine Parvoviren beim Wildschwein (Sus scrofa, L., 1758) in Nordrhein-Westfalen. Z Jagdwiss. 1996;42:123–133. doi: 10.1007/BF02240507. [DOI] [Google Scholar]
- Lutz W, Junghans D, Schmitz D, Müller T. A long-term survey of pseudorabies virus infections in European wild boar of western Germany. Z Jagdwiss. 2003;49:130–140. doi: 10.1007/BF02190453. [DOI] [Google Scholar]
- Markowska-Daniel I, Pejsak Z. The sero-prevalence of influenza virus amongst pigs and wild boar in Poland. Med Weter. 1999;55:302–305. [Google Scholar]
- Martínez L, Kekarainen T, Sibila M, Ruiz-Fons F, Vidal D, Gortázar C, Segalés J. Torque teno virus (TTV) is highly prevalent in the European wild boar (Sus scrofa) Vet Microbiol. 2006;118:223–229. doi: 10.1016/j.vetmic.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Müller T, Teuffert J, Zellmer R, Staubach C, Klupp B, Otte MJ, Conraths FJ (1997) Pseudorabies virus infections in European wild boar—a potential danger for domestic pigs? Proceedings of the 8th International Symposium of Veterinary Epidemiology and Economics, July 8–11, Paris, Epidémiol santé anim: pp 31–33, 01.08
- Müller T, Teuffert J, Ziedler K, Possardt C, Kramer M, Staubach C, Conraths FJ. Pseudorabies in the European wild boar from Eastern Germany. J Wildl Dis. 1998;34:251–258. doi: 10.7589/0090-3558-34.2.251. [DOI] [PubMed] [Google Scholar]
- Müller T, Conraths FJ, Hahn EC. Pseudorabies virus infection (Aujeszky’s disease) in wild swine. Infect Dis Rev. 2000;2:27–34. [Google Scholar]
- Oslage U, Dahle J, Müller T, Kramer M, Beier D, Liess B. Prävalenz von Antikörpern gegen die Viren der Europäischen Schweinepest, Aujeszkyschen Krankheit, und des ”Porcinen reproductiven and respiratory syndrome“(PRRS) bei Wildschweinen in den Bundesländern Sachsen-Anhalt und Brandenburg. Dtsch tierärzt Wschr. 1994;101:33–38. [PubMed] [Google Scholar]
- Polley B, Akimkin V, Hänel A, Hoferer M, Sting R. Zum Vorkommen von Antikörpern gegen verschiedene Influenza-A-Subtypen bei Haus- und Wildschweinen in Baden-Württemberg. Tierärztl Umsch. 2007;62:34–140. [Google Scholar]
- Roić B, Čajavec S, Tončić J, Madić J, Lipej Z, Jemeršić L, Lojkić M, Mihaljević Ž, Čač Ž, Šoštarić B. Prevalence of Antibodies to Procine Parvovirus in Wild Boars (Sus scrofa) in Croatia. J Wildl Dis. 2005;41:796–799. doi: 10.7589/0090-3558-41.4.796. [DOI] [PubMed] [Google Scholar]
- Ruiz-Fons F, Vicente J, Vidal D, Höfle U, Villanúa D, Gauss C, Segalés J, Almería S, Montoro V, Gortázar C. Seroprevalence of six reproductive pathogens in European wild boar (Sus scrofa) from Spain: The effect on wild boar female reproductive performance. Theriogenology. 2006;65:731–743. doi: 10.1016/j.theriogenology.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ruiz-Fons F, Segalés J, Gortázar C. A review of viral diseases of the European wild boar: Effects of population dynamics and reservoir rôle. Vet J. 2008;176:158–169. doi: 10.1016/j.tvjl.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliki JT, Rogers SJ, Eskew G. Serosurvey of selected viral and bacterial diseases in wild swine from Oklahoma. J Wildl Dis. 1998;34:834–838. doi: 10.7589/0090-3558-34.4.834. [DOI] [PubMed] [Google Scholar]
- Sáez-Royuela C, Terilería JL. The increased population of the wild boar (Sus scrofa L.) in Europe. Mammal Rev. 1986;16:97–101. doi: 10.1111/j.1365-2907.1986.tb00027.x. [DOI] [Google Scholar]
- Schlüter H, Kramer M. Epidemiologische Beispiele zur Seuchenausbreitung. Dtsch Tierärztl Wschr. 2001;108:338–343. [PubMed] [Google Scholar]
- Schmitt D, Wittkowski G. Untersuchungen zur Verbreitung von BVD-Virusinfektionen bei Schalenwild in Bayern. Tierarztl Umsch. 1999;54:284–288. [Google Scholar]
- Schulze C, Segalés J, Neumann G, Hlinak A, Calsamiglia M, Domingo M. Identification of postweaning multisystemic wasting syndrome in European wild boar (Sus scrofa) Vet Rec. 2004;154:694–697. doi: 10.1136/vr.154.22.694. [DOI] [PubMed] [Google Scholar]
- Takacs A. Contribution to the helminth infestation in wild boar (Sus scrofa L.) in Hungary. Wien Tierärztl Mschr. 1997;84:314–316. [Google Scholar]
- Van Reeth K. Avian and swine influenza viruses: our current understanding of the zoonotic risk. Vet Res. 2007;38:243–260. doi: 10.1051/vetres:2006062. [DOI] [PubMed] [Google Scholar]
- Vengust G, Valencak Z, Bidovec A. Presence of Antibodies Against Aujeszky’s Disease Virus in Wild Boar (Sus scrofa) in Slovenia. J Wildl Dis. 2005;41:800–802. doi: 10.7589/0090-3558-41.4.800. [DOI] [PubMed] [Google Scholar]
- Vengust G, Valencak Z, Bidovec A. A Serological survey of selected pathogens in wild boar in Slovenia. J Vet Med B. 2006;53:24–27. doi: 10.1111/j.1439-0450.2006.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente J, León-Vizcaíno L, Gortázar C, Cubero MJ, González M, Martín-Atance P. Antibodies to selected viral and bacterial pathogens in european wild boars from Southcentral Spain. J Wildl Dis. 2002;38:649–652. doi: 10.7589/0090-3558-38.3.649. [DOI] [PubMed] [Google Scholar]
- Vicente J, Ruiz-Fons F, Vidal D, Höfle U, Acevedo P, Villanúa D, Fernández-de-Mera IG, Martín MP, Gortázar C. Serosurvey of Aujeszky’s disease virus infection in European wild boar in Spain. Vet Rec. 2005;156:408–412. doi: 10.1136/vr.156.13.408. [DOI] [PubMed] [Google Scholar]
- Župančič Ž, Jukić B, Lojkić M, Čač Ž, Jemeršić L, Starešina V. Prevalence of antibodies to classical swine fever, Aujeszky’s Disease, porcine reproductive and respiratory syndrome, and bovine diarrhoea viruses in wild boars in Croatia. J Vet Med B. 2002;49:253–256. doi: 10.1046/j.1439-0450.2002.00562.x. [DOI] [PubMed] [Google Scholar]


