Abstract
Viral respiratory infections are the most common diseases in humans. A large range of etiologic agents challenge the development of efficient therapies. Research suggests that probiotics are able to decrease the risk or duration of respiratory infection symptoms. However, the antiviral mechanisms of probiotics are unclear. The purpose of this paper is to review the current knowledge on the effects of probiotics on respiratory virus infections and to provide insights on the possible antiviral mechanisms of probiotics. A PubMed and Scopus database search was performed up to January 2014 using appropriate search terms on probiotic and respiratory virus infections in cell models, in animal models, and in humans, and reviewed for their relevance. Altogether, thirty-three clinical trials were reviewed. The studies varied highly in study design, outcome measures, probiotics, dose, and matrices used. Twenty-eight trials reported that probiotics had beneficial effects in the outcome of respiratory tract infections (RTIs) and five showed no clear benefit. Only eight studies reported investigating viral etiology from the respiratory tract, and one of these reported a significant decrease in viral load. Based on experimental studies, probiotics may exert antiviral effects directly in probiotic–virus interaction or via stimulation of the immune system. Although probiotics seem to be beneficial in respiratory illnesses, the role of probiotics on specific viruses has not been investigated sufficiently. Due to the lack of confirmatory studies and varied data available, more randomized, double-blind, and placebo-controlled trials in different age populations investigating probiotic dose response, comparing probiotic strains/genera, and elucidating the antiviral effect mechanisms are necessary.
Keywords: Lactobacillus, Influenza Virus, Lactis, Respiratory Syncytial Virus, Acute Otitis Medium
Introduction
Respiratory tract infections (RTIs) are a major cause of morbidity and mortality worldwide. Viral pathogens are the most common etiological agents of acute respiratory disease. The social and economic impact of viral respiratory disease is substantial, due to hospitalizations, medical costs, missed work, and school and day care absences. For instance, estimates show that viral respiratory tract illnesses (mostly common colds) cost US$40 billion annually in the United States alone [1].
There are over 200 different types of viruses which cause RTIs in humans. Human rhinoviruses (HRV) are the largest group of respiratory viruses, comprising over 150 serotypes [2]. In humans, the predominant illness caused by HRV is the acute upper RTI, also known as the common cold. The second most common viruses infecting humans are the human enteroviruses (HEV), which are associated with clinical manifestations ranging from mild respiratory symptoms to serious conditions [2]. Influenza viruses, respiratory syncytial virus (RSV), and adenoviruses are also major causative agents of both upper and lower RTIs [3–5]. In addition, many other viruses or virus groups cause RTIs, e.g., parainfluenza viruses and coronaviruses can cause a broad spectrum of respiratory diseases, ranging from mild upper RTIs to pneumonia [6]. In recent years, with the rapid development of high-throughput molecular techniques, several new viruses associated with respiratory diseases, such as human bocavirus, human metapneumovirus, and the new coronaviruses HKU1 and NL63, have been identified as well [7].
The prevention of viral respiratory infections is an important challenge to public health. Currently, the only effective antivirals and vaccines for the prevention and treatment of respiratory virus infections are available against influenza viruses and adenoviruses. For the viruses causing common cold (HRV, HEV), no effective therapies are available. Large varieties of etiologic agents and increasing antibiotic and antiviral resistance challenge the development of efficient therapies. Consequently, it is of importance to find alternative and safe ways to reduce the risk of these infections. Even partially effective therapy in the treatment and prevention of viral RTIs such as the common cold could have an impact on reducing morbidity and economic losses due to this illness.
Probiotics are defined as live microorganisms that confer a health benefit on the host [8]. The most common types of microbes used as probiotics are lactobacilli and bifidobacteria, which are generally consumed as part of fermented foods, such as yoghurts or dietary supplements. Criteria for probiotic bacteria include that the bacterial strain: (1) must be able to survive in the gastrointestinal tract and to proliferate in the gut; (2) should exert benefits to the host through growth and/or activity in the human body; (3) should be non-pathogenic and non-toxic; (4) provide protection against pathogenic microorganisms by means of multiple mechanisms; and (5) should be lacking transferable antibiotic resistance [9]. Different bacterial strains of the same genus and species, verified also by genomic information, may exert completely different effects on the host.
The most promising health effects of probiotics in human intervention studies include the amelioration of acute diarrhea in children, relief of children’s milk allergy/atopic dermatitis, and relief of irritable bowel syndrome [10, 11]. Probiotics are likely to have an impact through gut mucosa by balancing the local microbiota by inhibiting the growth of pathogenic microorganisms [12], and by enhancing local and systemic immune responses [13]. They may also influence the composition and activity of microbiota in the intestinal contents. Considering the beneficial effects of probiotics in virus infections, specific probiotics have been suggested to be effective in alleviating the duration and severity of acute rotavirus gastroenteritis [14]. In addition, increasing evidence shows that probiotics are beneficial in RTIs [15], which, in most cases, are of viral origin. However, the mechanisms behind these effects are largely unknown.
Aim
The aim of this review is to present the current knowledge of the health effects of probiotics on RTIs in humans, with a focus on viral respiratory infections. In addition, possible antiviral mechanisms of probiotics are discussed in context with studies conducted in vitro and in animal models.
Methods
A PubMed and Scopus database search was performed up to January 2014 to review the relevant literature investigating the effects of probiotics on respiratory virus infections in cell culture, animal models, and clinical trials. The following search terms were used individually and in combination: ‘probiotic’, ‘Lactobacillus’, ‘Bifidobacterium’, ‘Lactococcus’, ‘respiratory infection’, ‘respiratory virus’, and ‘influenza virus’.
Health effects of probiotics in respiratory virus infections
Animal experiments
Animal experiments provide insight on the clinical effects of probiotics against respiratory virus infections (Table 1). In influenza virus infection in mice, the oral or intranasal administration of Lactobacillus pentosus strains [28–30], L. casei Shirota [16, 17], L. plantarum strains [18–20, 38], L. delbrueckii ssp. bulgaricus OLL1073R1 [39], L. rhamnosus GG [21, 23], L. gasseri TMC0356 [21, 22, 24], Lactococcus lactis ssp. cremoris FC [40], L. brevis KB [32], or B. breve YIT4064 [41] have reduced signs of infection, virus titer in the lungs or nasal washings, or increased body weight during infection and mice survival. In pneumovirus infection in mice, the virus-induced inflammation was suppressed and the mice were protected against lethal disease by L. plantarum NCIMB 8826 and L. reuteri F275 [35]. In addition, L. rhamnosus CRL1505 and L. rhamnosus CRL1506 protected mice against RSV infection [37].
Table 1.
Immunomodulatory effects of probiotic bacteria in respiratory virus infections in animal experiments
| Probiotic strain/reference | Virus | Study design | Main findings |
|---|---|---|---|
|
L. casei Shirota [16] |
IFV A/PR/8/34 (H1N1) | BALB/c mice, intranasal administration 3× daily for 3 days before infection |
Mice survival rate ↑ IL-12, IFN-γ, TNF-α in MLN cells ↑ Virus titers in nasal wash ↓ |
| [17] | BALB/c mice, oral administration 5×/week for 3 weeks before infection |
Mice survival rate ↑ Pulmonary NK cell activity ↑ IL-12 production by MLN cells ↑ Viral titers in nasal wash ↓ |
|
|
L. plantarum L-137 [18] |
IFV A/FM1/47 (H1N1) | C57BL/6 mice, intragastric administration daily 7 days before and 6 days after infection |
Viral titers in the lung ↓ IFN-β in sera ↑ |
|
L. plantarum 05AM2 L. plantarum 06TCa8 L. paracasei ssp. paracasei 06TCa19 L. paracasei ssp. paracasei 06TCa22 L. paracasei ssp. tolerans 06TCa39 L. plantarum 06TCa40 L. paracasei ssp. paracasei 06TCa43 L. plantarum 06CC2 L. delbrueckii ssp. lactis 06TC3 L. plantarum 06CC9 [19] |
IFV A/PR/8/34 (H1N1) | BALB/c mice, oral administration 2× daily for 10 days starting 2 days before infection |
Effects only with L. plantarum 06CC2: Body weight loss ↓ Virus yields in lungs ↓ Mice survival ↑ No. of macrophages and neutrophils in BALF ↓ TNF-α in BALF ↓ INF-α, IL-12, IFN-γ, NK cell activity ↑ mRNA IL-12 receptor, IFN-γ in Peyer’s patches ↑ |
|
L. plantarum DK119 [20] |
BALB/c mice, oral administration daily for 10 days before infection and 14 days after infection + experiments with nasal administration |
Both administration routes: Mice survival ↑ Lung viral loads ↓ BALF IL-12, IFN-γ ↑ BALF IL-4, IL-6, TNF-α ↓ |
|
|
L. gasseri TMC0356 L. rhamnosus GG [21] |
BALB/c mice, oral administration daily for 1 day, infection on day 14 |
Effects with both bacteria: Clinical symptom scores ↓ Pulmonary virus titers ↓ |
|
| [22] |
Effects with L. gasseri: Peyer’s patches: mRNA IL-12, IL-15, IL-21 ↑ Lungs: mRNA IFN-γ, TNF, IL-12, perforin-1 ↑ |
||
| [23] | BALB/c mice, intranasal administration 3× daily for 3 days before infection |
L. gasseri TMC0356: Morbidity ↓ Mice survival ↑ mRNA IL-1β, TNF, IL-10, MCP-1 ↑ |
|
|
L. rhamnosus GG: Accumulated symptoms ↓ Mice survival ↑ mRNA IL-1β, TNF, IL-10 + MCP-1↑ | |||
| [24] | |||
|
L. rhamnosus (strain not provided) [25] |
IFV A/NWS/33 (H1N1) | BALB/c mice, sublingual administration for 10 days before infection |
Mice mortality ↓ Lung lesion scores↓ Lung anti-IFV IgA ↑ Lung IL-12 ↑, IL-6+ TNF-α ↔ Lung CD4+, CD8+, CD25 expression ↑ Splenocyte NK cell activities ↑ |
|
L. fermentum-1 L. brevis-2 [26] |
BALB/c mice, intranasal or oral administration for 21 days before infection |
Mice survival ↑ Virus titer ↓ Lung IgA + IL-12 ↑ Lung TNF-α and IL-6 ↓ Lung IFN-γ ↔ |
|
|
L. fermentum CJL-112 [27] |
BALB/c mice, intranasal administration for 21 days before infection |
Effect in lungs: IL-2, IFN-γ, IL-1β ↑ IL-4, IL-5 ↔ IL-10 ↓ Anti-influenza IgA ↑ |
|
|
L. brevis KB290 [28] |
IFV A/PR/8/34 (H1N1) IFV A/PR8/34 H1N1 |
BALB/c mice, oral administration 1× daily for 14 days before infection |
Body weight loss ↓ Clinical symptom scores ↓ BALF IFV specific IgA ↑ Serum IFN-α ↑ |
|
L. pentosus S-PT84 [29] |
BALB/c mice, intranasal administration 1× daily for 3 days before infection |
Mice survival ↑ Virus titer in BALF ↓ IL-12, IFN-γ in MLN cells ↑ BALF IL-12, IFN-α ↑ NK cell activity ↑ |
|
|
L. pentosus b240 [30] |
BALB/c mice, oral administration for 3 weeks by gavage before infection |
Mice survival ↑ Virus titers 7 days after infection ↓ Anti-IFV IgA, IgG BALF + plasma on day 7 ↑ |
|
| [31] | IFV A/California/04/2009 (H1N1) | BALB/c mice, oral administration daily for 5 weeks, IFV infection on day 21 |
Mice survival ↑ Virus proliferation ↔ Lung histopathology ↔ Cytokines/chemokines ↔ Differential regulation of antiviral gene expression |
|
L. acidophilus L-92 [32] |
IFV A/PR/8/34 (H1N1) |
BALB/c mice, oral administration daily for 21 days, infection on day 16 |
Both bacteria: - Body weight ↔ - Fatality ↔ Viable probiotic: - Symptom score ↔ - Lung virus titers ↓ - Lung NK cell activity ↑ - Lung eotaxin, M-CSF, IL-1β, RANTES, IFN-α ↑ - Lung IgG ↓, IgA ↔ Nonviable probiotic: - Symptom score ↓ - Lung virus titers ↓ - Lung NK cell activity ↑ |
|
B. longum BB536 [33] |
BALB/c mice, oral administration daily for 2 weeks before infection |
Symptom score ↓ Loss of body weight ↓ Lung virus titers ↓ Lung IL-10, IL-12 ↔ Lung IL-6, IFN-γ (↓) |
|
|
Bifidobacterium Lactobacillus Enterococcus (Bifico probiotic product) [34] |
IFV A FM1 (H1N1) | BALB/c mice were subjected to 8 days of oral neomycin administration, then infected intranasally with virus. Probiotic administration by gavage for 4 days after infection |
Lung IFN-γ, IL-17 ↑, IL-4, IL-10 ↓ Probiotic treatment significantly restored initial levels of upregulation of TLR7, MyD88, IRAK4, TRAF6, and NF-kB mRNA expression |
|
L. plantarum NCIMB 8826 L. reuteri F275 [35] |
Pneumonia virus of mice J3666 | BALB/c and C57BL/6 mice, intranasal inoculation of 2 weekly doses 2 weeks before infection |
Protection against virus infection ↑ Granulocyte recruitment ↓ CXCL10, CXCL1, CCL2,TNF↓ Virus recovery ↓ |
| [36] |
Live L. reuteri: Neutrophil recruitment ↑ CXCL1, CCL3, CCL2, CXCL10, TNF-α, IL-17A ↑ IFN-α, IFN-β, IFN-γ ↔ |
||
|
L. rhamnosus CRL1505 L. rhamnosus CRL1506 [37] |
Viral pathogen molecular pattern poly(I:C) + RSV A2 | BALB/c mice, nasal administration for 2 days before infection |
BALF + serum IL-6, IFN-α,IFN-β, TNF-α, IL-10 ↑ Lung viral loads↓ Strains differentially modulated TLR3/RIG-I-triggered antiviral respiratory immune response |
Abbreviations for columns:
Probiotic strain: L = Lactobacillus; B. = Bifidobacterium
Virus: IFV = influenza virus; RSV = respiratory syncytial virus
Main findings: IL = interleukin; IFN = interferon; TNF = tumor necrosis factor; MLN = mediastinal lymph node; NK = natural killer cell; BALF = bronchoalveolar lavage fluid
↑ = significant increase; ↓ = significant decrease; ↔ = no significant effect
Clinical trials
Children
Altogether, five clinical trials have been conducted in children using L. rhamnosus GG as a probiotic [42–46]. In healthy children attending day care, L. rhamnosus GG reduced the number of children experiencing RTIs [42, 43], the number of upper and lower RTIs [43], and the number of antibiotic treatments or absences from day care [42]. In another study, no differences were reported between the L. rhamnosus GG and the control groups in the number of antibiotic treatments or respiratory symptom episodes [47]. However, in a subgroup with L. rhamnosus GG identification in feces, L. rhamnosus GG usage reduced the duration of RTIs. In hospitalized children, L. rhamnosus GG reduced the risk of RTIs and duration of RTI episodes [42]. In preterm infants, L. rhamnosus GG reduced the incidence of RTIs [46]. In addition, a meta-analysis of four randomized controlled trials investigating the role of L. rhamnosus GG in the prevention of respiratory infections in children showed that L. rhamnosus GG has the potential to reduce the risk of upper RTIs, incidence of acute otitis media, and antibiotic use. There were no significant differences between the L. rhamnosus GG and the control groups in the incidence of lower RTIs [48].
There are seven studies conducted with probiotic bacteria other than L. rhamnosus GG. L. casei rhamnosus in children reduced the number of RTIs [49]. Also, L. casei DN114001 reduced the incidence rate for upper RTIs43 and decreased the duration (days) and incidence of only lower RTIs, but not upper RTIs [50]. L. fermentum CECT5716 with prebiotics in infants, however, reduced the incidence of both upper and lower RTIs [51]. The use of B. animalis ssp. lactis Bb12 in healthy newborns was able to reduce the number of RTIs as well, but was ineffective in reducing the occurrence of acute otitis media (AOM) or symptoms of otitis media [52]. In healthy infants, treatment with L. reuteri SD112, but not with B. animalis ssp. lactis Bb12, resulted in fewer days of absence from day care due to illness, lower number of days with fever, and clinical visits. Both strains were ineffective in reducing the incidence or duration of RTIs [53, 54]. In healthy children, L. casei CRL431 or L. reuteri DSM17938 did not reduce the incidence, number, or duration of acute RTIs or RTI episodes [55].
The effectiveness of several combinations of probiotics on RTIs has been investigated in four clinical trials. A combination of L. rhamnosus GG, L. rhamnosus Lc705, B. breve Bb99, and P. freudenreichii ssp. shermanii JS in otitis-prone children [56] or a combination of L. rhamnosus GG and B. animalis ssp. lactis Bb12 in healthy newborns [57] both reduced the occurrence of recurrent RTIs, but not the incidence of AOM. A combination of L. acidophilus and B. bifidum in healthy children reduced the duration of acute RTI symptoms, school absence, and the risk of upper RTI symptoms as well [58]. However, a combination of 12 bacteria including species of Lactobacillus, Bifidobacterium, Streptococcus, and Enterococcus was not able to reduce the number of RTIs [49].
The viral etiologies of RTIs were investigated in only five studies. In preterm infants, L. rhamnosus GG decreased the incidence of rhinovirus-induced episodes, but not rhinovirus load [46]. In otitis-prone children, a combination of L. rhamnosus GG, L. rhamnosus Lc705, B. breve Bb99, and P. freudenreichii ssp. shermanii JS reduced human bocavirus load in the nasopharynx [59], but not picornaviruses [60]. In healthy children attending day care, L. rhamnosus GG was not able to decrease significantly respiratory viruses (HRV, HEV, influenza viruses, parainfluenza viruses, RSV, adenovirus, and human bocavirus) in the upper respiratory tract [47]. Healthy children receiving L. casei rhamnosus had significantly lower odds of viral infection diagnosed by a doctor and a significant difference in doctor-diagnosed RTI. However, specific viruses were not reported in that study [49].
Adults
Probiotics’ effectiveness in RTIs has been addressed in 13 studies in healthy adults, in athletes, and in individuals under stressful conditions. In healthy adults, L. fermentum CETC5716 reduced the number of RTIs and increased antigen-specific IgA formation after influenza virus vaccination [61]. In addition, a combination of L. gasseri PA16/8, B. longum SP07/3, and B. bifidum MF20/5 reduced the duration of RTI symptoms [62], duration of RTI episodes [63, 64], but not the severity of RTI symptoms [63, 64]. None of these trials reported the effects of combinations on respiratory virus load, although their viral etiology was studied. B. animalis ssp. lactis Bl-04 reduced the risk of an upper RTI episode [65]. A combination of L. rhamnosus GG and B. animalis ssp. lactis Bb12 reduced both the duration of upper RTI and the severity of RTI symptoms [66].
Altogether, seven trials have been conducted among athletes or stressed individuals, but they did not report studying the viral etiology. In male elite distance runners, L. fermentum VRI003 reduced the duration of RTI symptoms, but not the incidence of RTIs or the severity of symptoms [67]. In competitive cyclists, L. fermentum (PCC) had some decreasing effects on the symptoms of upper RTI in males, but not in females [68]. In rugby union players [69], a combination of L. gasseri, B. bifidum, and B. longum reduced the incidence of upper RTIs, but not the severity of symptoms. However, in marathon runners, L. rhamnosus GG did not decrease the number of RTI episodes or the severity or the duration of RTI symptoms [70]. In addition, in commando trainers, L. casei DN114001 was ineffective in reducing the incidence of RTIs or RTI symptoms [62–64, 71]. Similarly, L. salivarius did not lower the number of RTI episodes or reduce the severity or the duration of RTI symptoms in trainers [72]. However, in shift workers, L. casei DN114001 reduced the number of RTIs and increased the function of immune cell activity [73].
The elderly
Only five studies have investigated the effects of probiotics on RTIs, but not on the occurrence of specific viruses, in the elderly. L. casei DN114001 decreased the duration of RTIs [74, 75], but had no effect on the incidence of RTIs [74]. L. casei Shirota did not have an effect on the number of upper RTIs or the severity of upper RTI symptoms, but probiotics decreased the duration of upper RTIs [76]. However, in another study, L. casei Shirota had no effect on the duration of RTI symptoms [77]. A combination of L. rhamnosus GG, L. rhamnosus Lc705, B. breve Bb99, and P. freudenreichii ssp. shermanii JS was ineffective in lowering the number of RTIs and reducing the duration of RTI symptoms. However, the combination reduced the duration of RTI episodes [60].
The clinical trials in children, adults, and the elderly presented in this review are summarized in Table 2. A variety of probiotic strains have been used in these clinical trials, most of them belonging to the genus Lactobacillus. In addition, various combinations of probiotics have been used. Of 33 studies, altogether, 28 studies reported that probiotics had beneficial effects in the outcome of RTIs and five showed no clear benefit. Only eight studies, however, reported investigating the viral etiology. Of these, only one study showed a statistically significant reduction in the virus load in the probiotic group. A Cochrane systematic review by Hao et al. concluded that probiotics were better than placebo in terms of reducing the number of upper RTI episodes, the incidence of acute upper RTI episodes, and antibiotics used [15]. Although clinical trials show that the use of specific probiotics and probiotic combinations are beneficial in RTIs, there are also studies that report no clear advantage. In addition, several viruses can cause respiratory illnesses, but only a few studies have investigated probiotics’ effectiveness on viral agents. The lack of consistent evidence between probiotic strains/genera and even within strains may be due to variation in study designs and reported outcome measures, the length of intervention, study populations used (children vs. adults) or bacterial doses (106–1010 cfu), and matrices (milk, yoghurt, capsule) used. In addition, in the elderly, decreased immunity due to aging may partly explain the conflicting results [79].
Table 2.
Reported effects of probiotics in respiratory tract infections (RTIs) in clinical settings in children, healthy adults, and the elderly
| Study design | Subjects | Probiotics used | Main findings: probiotic vs. placebo |
|---|---|---|---|
| Children | |||
|
R DB PC 7 months [42] |
571 healthy children at day care centers (1–6 years) | L. rhamnosus GG in milk (on average, 108 cfu) 3× daily |
- Days with respiratory symptoms ↔ - No. of children with RTIs ↓ - Antibiotic treatments ↓ - Days of absence from day care ↓ - Age-adjusted results ↔ |
|
R DB PC 7 months [45] |
523 healthy children at day care centers (2–6 years) | L. rhamnosus GG in milk (on average, 108 cfu) 3× daily |
- Days with respiratory symptoms/month ↔ (subgroup of completed cases:↓) - Respiratory symptom episodes/month ↔ - Antibiotic treatments ↔ |
| [47] |
Subgroup of children visiting study physician: - Days with respiratory symptoms/month ↓ - Occurrence of respiratory viruses in the nasopharynx ↔ - RTI symptoms associated with viral findings ↔ |
||
|
R DB PC 3 months [43] |
281 healthy children at day care centers (2–6 years) | L. rhamnosus GG (109 cfu) in milk daily |
- No. of children with RTIs ↓ - No. of URTIs ↓ - No. of lower RTIs ↔ - No. of RTIs lasting >3 days ↓ |
|
R DB PC during hospital stay [44] |
742 hospitalized children (≥12 months) | L. rhamnosus GG (109 cfu) in milk administered daily for duration of hospitalization |
- Risk for RTIs ↓ - Risk for duration of RTI episodes lasting >3 days ↓ - Duration of hospitalization ↔ |
|
R DB PC 57 days (3 days from birth) [46] |
94 preterm infants (gestational age >32 + 0 and <36 + 6 weeks) | Prebiotic GOS and polydextrose mixture or L. rhamnosus GG 1 × 109 cfu/day for 1–30 days and 2 × 109 cfu/day for 31 to 60 days stirred in 10 ml of liquid |
Prebiotic and L. rhamnosus group: - Incidence of RTIs ↓ - Incidence of HRV-induced episodes ↓ - HRV RNA load during infections ↔ - Duration of HRV RNA shedding ↔ - Duration/severity of HRV infections ↔ |
|
R DB PC 6 months [56] |
309 otitis-prone children (10 months to 6 years) | Combination of L. rhamnosus GG, L. rhamnosus LC705, B. breve 99, P. freudenreichii JS in capsules 8–9 × 109 cfu/capsule of each strain on 1 capsule daily |
- Occurrence of AOM ↔ - Occurrence of recurrent (≥4) RTIs ↓ - Moraxella catarrhalis in the nasopharynx ↑ |
| [59] | - HBoV DNA in the nasopharynx after 3–6 months (studied in 152 children) ↓ | ||
|
R DB PC 10–12 months [57] |
72 healthy newborns (<2 months) | Combination of L. rhamnosus GG, B. animalis ssp. lactis Bb12 1010 cfu in capsules supplemented to infant formula once a day |
During first 7 months of life: - Incidence of AOM ↓ - Antibiotic treatments ↓ - No. of RTIs ↔ During first 12 months of life: - Incidence of AOM ↔ - No. of recurrent RTIs ↓ |
|
R DB PC 3 months [58] |
80 healthy children (8–13 years) | Combination of L. acidophilus (min. 109/capsule) and B. bifidum (min. 109/capsule) (strain information not provided) in capsules 2× daily |
- Median duration of cold symptoms + school absence ↓ - Risk of fever, cough, rhinorrhea, school absence, and school absence related to common cold ↓ |
|
R DB PC 6–7 months [52] |
109 healthy newborns (1 month old) | B. animalis ssp. lactis Bb12 (109 cfu/day) in tablet, 2× daily |
- No. of RTIs ↓ - Occurrence of AOM ↔ - Symptoms of otitis media ↔ |
|
R DB PC 3 months [53] |
201 healthy infants (4–10 months) | L. reuteri SD 112 (107 cfu/g) or B. animalis ssp. lactis Bb12 (107 cfu/g) in milk formula daily |
L. reuteri vs. B. Bb12/control: - No. of days with fever, clinic visits, child care absences, and antibiotic prescriptions ↓ Both bacteria: - Rate and duration of RTIs ↔ |
|
R DB PC 5 months [50] |
251 healthy school children (3–12 years) | L. casei DN 114001 2× daily in fermented yoghurt |
- Incidence and duration (days) of RTI ↔ - Duration of lower RTIs ↓ - Incidence of lower RTI and fatigue ↓ |
|
CR DB PC 3 months [78] |
638 healthy children (3–6 years) | L. casei DN 114001 (1 × 108 cfu/g ) in fermented dairy yoghurt drink: 1× bottle daily |
- Incidence rate for CIDs ↓ - Incidence rate for URTIs ↓ - Missed day care/school or parental missed work ↔ |
|
R DB C 6 months [55] |
494 healthy children (1–6 years) | L. casei CRL431(5 × 108 cfu/day) or L. reuteri DSM17938 (5 × 108 cfu/day) in milk (low or regular calcium) |
- Incidence of acute RTIs ↔ - No. of RTI episodes ↔ - Duration of acute RTIs↔ |
|
R DB PC 6 months [51] |
215 healthy infants (6 months) | L. fermentum CECT5716 (2 × 108 cfu/day) + GOS in formula daily |
- Incidence ratio of URTIs ↓ - Incidence ratio of upper and lower RTIs↓ |
|
DBRC 3–7 months [49] |
986 children (<5 years) |
L. casei rhamnosus: 2 sachets (2× 108 cfu) daily or L. rhamnosus T cell-1: 3 tablets (1 × 1010 cfu) daily or combination of 12 bacteria (7× Lactobacilli, 3× Bifidobacteria, 1× Streptococcus, 1× Enterococcus 5 capsules daily (109 cfu/strain) 5 days a week |
L. casei rhamnosus: - Incidence of bacterial infections ↓ - Doctor-diagnosed viral infection in 3 months ↓ - Doctor-diagnosed RTI in 3 and 7 months ↓ L. rhamnosus T cell-1: - Incidence of bacterial infections in 7 months ↓ Combination: - No. of RTIs ↔ |
| Adults | |||
|
R DB PC 1 month + 5 months follow-up (intramuscular anti-influenza vaccine) [61] |
50 healthy adults (22–56 years) | L. fermentum CECT5716 in capsule (1010 cfu/day): 2 weeks before and 2 weeks after vaccination |
- No. of RTIs ↓ - Antigen-specific IgA ↑ |
|
R DB PC C-O 1 month [67] |
20 healthy elite male distance runners | L. fermentum VR1003 (1.3 × 1010 cfu/day), 3× capsules 2× daily |
- Incidence of RTIs ↔ - No. of days with respiratory symptoms ↓ - Severity of symptoms ↔ |
|
R DB PC 11 weeks [68] |
99 competitive cyclists (26–45 years) | L. fermentum PCC® (minimum 109 cfu/day) in capsules: 1× daily |
- URTI illness load ↔ - Self-reported symptoms of lower RTI ↔ (↓ in men) |
|
R DB PC 4 months [73] |
1,000 shift workers (18–65 years) | L. casei DN 114001 (1010 cfu/g) in yoghurt drink, 2× 100-g bottle daily |
- Cumulated number of CIDs ↓ - Proportion of volunteers experiencing at least 1 CID ↓ -No. of CIDs in the subgroup of smokers ↓ - Leukocyte, neutrophil, and natural killer cell counts and activity ↑ |
|
R DB PC 1 month [71] |
47 healthy men in French commando training | L. casei DN 114001 in milk, 3× 100 ml/day during training |
- Incidence of RTIs ↔ - Proportion of rhinopharyngitis ↑ - Symptoms of infection ↔ |
|
R DB PC 3 months [70] |
141 marathon runners (22–69 years) | L. rhamnosus GG in milk 2× bottles daily (4 × 1010 cfu) or capsules 2× daily (1010 cfu) |
- No. of RTI episodes (during training or 2 weeks after marathon) ↔ - No. of healthy days ↔ |
|
R DB PC 4 months [72] |
66 healthy training adults (18–35 years) | L. salivarius(2 × 1010 cfu) powder in water daily for 16 weeks |
- No. of RTI episodes ↔ - Severity and duration of URTI symptoms ↔ |
|
R DB PC 3 months [63] |
479 healthy adults (18–67 years) | Combination of L. gasseri PA16/8 (4 × 107 cfu/tablet), B. longum SP07/3 (5 × 106 cfu/tablet), B. bifidum MF20/5 (5 × 106 cfu/tablet), vitamins, minerals, 1 tablet daily |
- Duration of RTI episode ↓ - Severity of RTI symptoms ↔ - Duration of fever ↓ |
|
R DB PC 3–5 months [64] |
- Number of RTI episodes ↔ - Duration of RTI episodes ↓ - Severity of RTI symptoms ↔ |
||
|
R DB PC 3–5.5 months [62] |
477 healthy adults (23–49 years) |
- Viral-induced incidence and duration of RTI ↔ - Days with fever ↓ - Duration of RTIs ↔ |
|
|
R DB PC over 150 days [65] |
460 physically active adults (18 to 60 years) | B. animalis ssp. lactis Bl-04 2 × 109 cfu in sachet per day or L. acidophilus NCFM + B. animalis ssp. lactis Bi-07 5 × 109 cfu in sachet per day |
Both bacteria groups: - 0.7 + 0.9 month delay in the median time to an illness episode - Duration of RTIs ↔ Only B. animalis: - Risk of URTI episode ↓ |
|
R DB PC 1 month [69] |
30 rugby union players | Combination of L. gasseri (2.6 × 109 cfu), B. bifidum (0.2 × 109 cfu), and B. longum (0.2 × 109 cfu) 1× capsule daily |
- Incidence of URTI ↔ - Incidence of any symptoms ↓ - Severity of symptoms ↔ |
|
R DB PC 3 months [66] |
198 healthy college students (18–25 years) | Combination of L. rhamnosus GG + B. animalis ssp. lactis Bb12, 1× powder/stick (2 × 109 cfu) daily |
- Median duration of URTI ↓ - Severity of URTI ↓ - No. of missed work days ↔ - Missed school days ↓ |
| Elderly | |||
|
R C (pilot) 3 weeks [74] |
260 healthy elderly (>60 years) | L. casei DN 114001 in fermented yoghurt drink |
- Incidence of RTI ↔ - Duration of RTI ↓ |
|
R DB PC 3 months [75] |
1,072 elderly (≤70 years) | L. casei DN 114001 (≤1010 cfu/g) in yoghurt drink, 2 × 100 g daily |
- Cumulative number or severity of CID ↔ - Average duration per episode of CID ↓ - Cumulative duration of CID ↓ - Average duration per episode of URTI ↓ - Cumulative duration of URTI ↓ |
|
R DB PC 5 months [76] |
154 elderly (74–92 years) | L. casei Shirota (4 × 1010 cfu) in milk 1 × 80 ml daily |
- No. of persons diagnosed with acute URTIs ↔ - No. of acute URTI events ↔ - Severity of URTIs ↔ - No. of acute URTI events/total days of observation ↔ - Mean duration of URTI per infection event ↓ |
|
R DB PC 176 days [77] |
737 healthy people aged >65 years in nursing homes | L. casei Shirota (>6.5 × 109 live bacteria/bottle) in milk, 2× daily |
- Duration of RTIs ↔ - No. of participants with RTI symptoms ↔ - Influenza vaccination immune response ↔ |
|
R DB PC 5 months [60] |
265 institutionalized elderly (>65 years) | Combination of L. rhamnosus GG, L. rhamnosus LC705, B. breve 99, P. freudenreichii JS (8-9 × 109 cfu/capsule of each strain), 2× daily |
- No. of RTIs ↔ - Duration of RTI episodes ↓ - Duration of RTI symptoms ↔ |
Abbreviations for columns:
Study design and duration: R DB PC = randomized double-blind placebo-controlled; CR = cluster-randomized; C-O = cross-over
Probiotics used: L = Lactobacillus; B = Bifidobacterium; P = Propionibacterium; cfu = colony-forming units; GOS = galactooligosaccharides
Main findings: probiotic vs. placebo: RTI = respiratory tract infection; URTI = upper respiratory tract infection, AOM = acute otitis media; CID = common infectious disease; Ig = immunoglobulin; HBoV = human bocavirus; HRV = human rhinovirus
↑ = significant increase; ↓ = significant decrease; ↔ = no significant effect
Possible mechanisms of actions of probiotics in respiratory virus infections
Clinical and animal studies have demonstrated that specific probiotics have antiviral effects, but the underlying mechanisms are unclear. Additionally, the strain-to-strain variation may be relatively large concerning strain properties and efficacy. Possible antiviral mechanisms of probiotics include: (1) hindering the adsorption and (2) cell internalization of the virus; (3) production of metabolites and substances with a direct antiviral effect; and 4) crosstalk (immunomodulation) with the cells in establishing the antiviral protection. The possible mechanisms of probiotics against respiratory viruses are presented in Fig. 1.
Fig. 1.
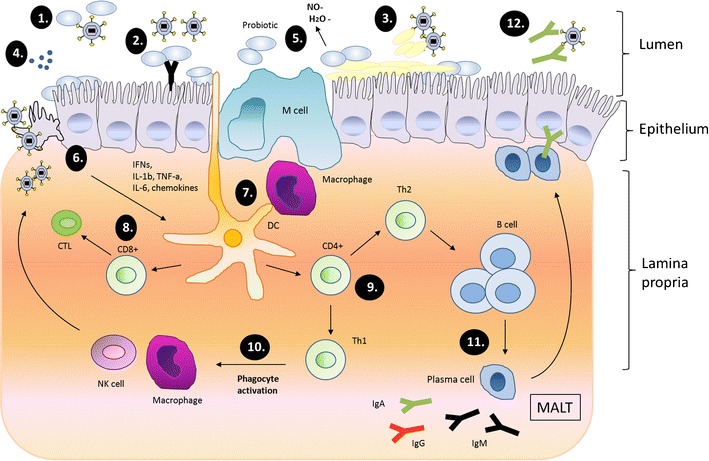
Schematic presentation of possible antiviral effect mechanisms of probiotics in respiratory virus infections (adapted from Lehtoranta [80]). 1 Probiotic bacteria may bind directly to the virus and inhibit virus attachment to the host cell receptor. 2 Adhesion of probiotics on the epithelial surface may block viral attachment by steric hindrance, cover receptor sites in a non-specific manner, or by competing for specific carbohydrate receptors. 3 Probiotics may induce mucosal regeneration: intestinal mucins may bind to viruses, and inhibit their adherence to epithelial cells and inhibit virus replication. 4 Probiotics also show direct antimicrobial activity against pathogens by producing antimicrobial substances. 5 Induction of low-grade nitric oxide (NO) production and dehydrogenase production may have antiviral activities. 6 Modulation of immune response through epithelial cells. 7 Modulation and activation of immune responses through macrophages and dendritic cells (DCs). 8 Upon activation, CD8+ T lymphocytes differentiate into cytotoxic T lymphocytes (CTLs), which destroy virus-infected cells. 9 CD4+ T lymphocytes differentiate into Th1 and Th2 cells. 10 T-helper cells type 1 (Th1) activates phagocytes, promoting virus killing. 11 Th2-cells induce proliferation of B-cells, which travel to secondary lymphatic organs in mucosa-associated lymphoid tissue (MALT) and differentiate into immunoglobulin (Ig)-producing plasma cells, which may migrate back to the infection site. 12 Secretory antibodies neutralize the virus
Antagonism to respiratory viruses
The respiratory tract is covered by mucosal epithelial surfaces, which are constantly exposed to numerous microorganisms and serve as primary ports of entry for respiratory viruses. Virus attachment to a host cell is the first essential step in the disease process, and, therefore, interruption of this attachment could be beneficial to the host. Probiotic bacteria may bind directly to the virus and inhibit virus attachment to the host cell receptor. For instance, there is evidence that specific strains of lactobacilli are able to bind and inactivate vesicular stomatitis virus (flu-like virus) in vitro [81]. Probiotics may also show direct antimicrobial activity against pathogens by producing antimicrobial substances such as organic acids, hydrogen peroxide, biosurfactants, and bacteriocins [12]. In experimental studies in epithelial cells and macrophages, metabolic products of specific lactobacilli and bifidobacteria prevented vesicular stomatitis virus infection in a strain-specific manner [81]. In addition, metabolites of bacteria in yoghurts showed antiviral activity, inhibiting influenza virus replication [82]. The induction of low-level synthesis of nitric oxide may also be involved in the protective actions of probiotics against viruses in the respiratory cells, as shown in alveolar macrophages in vitro [27, 83, 84]. However, it should be noted that respiratory viruses infect cells with different mechanisms by using various receptors and, also, the antiviral effects of probiotics are strain-specific.
Immunomodulation
Cell-mediated immunity
The induction of antiviral cytokines such as interferons (IFNs), as well as proinflammatory cytokines and chemokines, upon antigen recognition in epithelial cells or underlying effector cells [macrophages, dendritic cells (DCs), neutrophils] play a key role in virus infections by initiating cell-mediated viral elimination and adaptive immune responses. Probiotics may mediate their antiviral effects against respiratory viruses possibly by eliciting systemic immune responses via gut or enhancing cellular immunity in the airways with increased activity of natural killer cells and macrophages. In the gut epithelial cells and/or antigen-presenting cells, probiotics are recognized by toll-like receptors (TLRs) [85–88]. Probiotics may, therefore, modulate cytokine expression patterns through epithelial cells [89] and through underlying professional antigen-presenting cells, such as macrophages and dendritic cells [90–95].
Many experimental studies in vitro and in animals show that specific strains of probiotics are capable of providing protection against virus infections by stimulating antiviral, cytokine, and chemokine responses in the respiratory and gastrointestinal epithelial cells or immune cells. In murine DCs, L. acidophilus NCFM and L. acidophilus X37 induced the expression of viral defense genes (IFN-β, IL-12, IL-10) [96]. In human macrophages, L. rhamnosus Lc705 induced type I interferon-dependent gene activation, which correlated with the prevention of influenza A virus replication and the production of viral proteins [97]. In influenza infection in mice, orally administered probiotic product containing Bifidobacterium, Lactobacillus, and Enterococcus regulated the TRL7 signaling pathway [34] and L. pentosus b240 regulated antiviral gene expression against the infection [31]. In addition, orally ingested probiotics strains of Lactobacillus [17, 19, 20, 22, 26, 28, 32] and Bifidobacterium [33] have enhanced cytokine production in the lungs or serum against viruses. There is also evidence that intranasally administrated probiotics protect against respiratory virus infection in mice by stimulating innate immune responses directly in the respiratory epithelium [20, 23, 24, 26, 27, 29, 35–37, 98]. Additionally, sublingual administration of L. rhamnosus protected against influenza virus infection by enhancing mucosal secretory IgA production, T and NK cell activity, and lung IL-12 levels [25]. Table 1 summarizes the effects of probiotic bacteria on cell-mediated immunity upon respiratory virus challenge in animal models.
Humoral immunity
Data from animal studies indicate that strains of lactobacilli and bifidobacteria provide protection against respiratory virus infections also by inducing the synthesis of virus-specific immunoglobulins in the respiratory secretions and in serum [25, 30, 39, 41]. In addition, studies in healthy human subjects suggest that specific probiotics may enhance the immunogenicity of viral vaccines. L. rhamnosus GG was effective in inducing protective immune response against the H3N2 strain in influenza virus vaccine [99]. Moreover, L. fermentum CECT5716 ingestion in adults resulted in lower influenza-like illness, increased proportion of NK cells in blood, significantly higher TNF-α, and increased anti-influenza-specific IgA and IgM after influenza vaccination [61]. The consumption of B. animalis ssp. lactis Bb12 or L. paracasei ssp. paracasei L. casei 431431 also showed significantly greater increase in influenza virus vaccine-specific IgG antibodies in plasma and secretory IgA in saliva [100]. In the elderly, the consumption of fermented yoghurt with L. casei DN-114 001 increased significantly influenza-specific antibody titers after influenza vaccination, especially against influenza B virus [101]. These studies suggest that orally ingested lactobacilli and bifidobacteria have an adjuvant-like effect on the humoral responses.
Safety
Probiotics are frequently part of the normal gastrointestinal microbiota, and, therefore, probiotic therapy is generally considered as safe [102]. However, probiotic therapy has raised potential safety concerns, including systemic infections, toxic or metabolic effects on the gastrointestinal tract, and the transfer of antibiotic resistance in the gastrointestinal microbiota [103]. In rare cases, some studies have reported Lactobacillus septicemia in children [104], in immunocompromised subjects [105], and detrimental effects in subjects with hepatitis [106]. However, the European Food Safety Authority (EFSA) has concluded that there are no specific safety concerns regarding Lactobacillus, Bifidobacterium, or Propionibacterium strains, as they have a long history of safe use in food [107]. In addition, for instance in Finland, increased consumption of probiotic products containing L. rhamnosus GG has not resulted in a significant increase in Lactobacillus bacteremia [108] and L. rhamnosus GG consumption is regarded as safe in immunocompromised human immunodeficiency virus (HIV)-infected patients [108]. It should be taken into consideration that the safety of probiotics has not been as systematically investigated as in drugs, and the safety evaluation is partly based on long-term experience.
Summary and conclusions
The aim of this review was to summarize the current literature investigating the effects of probiotics in respiratory virus infections in cell models, in animal models, and in humans. In addition, possible antiviral mechanisms of probiotics in respiratory virus infections were discussed. Probiotic therapy may offer an interesting alternative in the alleviation or prevention of viral respiratory tract infections (RTIs), which cause a significant health and economic burden to humans. Based on this review, clinical trials in human subjects show promising data demonstrating that specific probiotics are able to shorten the duration or reduce the risk of respiratory infections. However, only a few clinical studies have actually investigated the effects of probiotics on specific viruses, which are the most common agents causing RTIs. Thus, more clinical research should be targeted to revealing which probiotics or their combinations would be the most effective ones against RTI viruses.
There are also contradictory data on probiotic use in the prevention of RTIs. The variability in the outcomes between clinical trials studying probiotics’ role in RTIs may be explained by the use of different probiotic strains, bacterial dose, and matrices. In addition, it should be noted that the effects of probiotics are highly strain-specific and the adequate amount of bacteria transferred into the effector sites in the gut may be crucial. Due to the lack of confirmatory studies and varied data available, more randomized, double-blind, and placebo-controlled clinical trials in different age populations investigating probiotic dose response, comparing probiotic strains, and elucidating the mechanisms of effects are necessary.
As many animal studies show that probiotic administration through the nose is able to reduce viral titers and relieve clinical symptoms, nasal bacteriotherapy for viral RTIs in humans could be worthy approach for consideration in the future. Probiotics’ ability to enhance local and systemic innate immunity during virus infection in animal experiments is a likely, yet unverified, effect mechanism behind beneficial effects, and an interesting area of future research. The inclusion of serological and immunological diagnostics, such as the identification of virus-specific immunoglobulins and cytokines, in clinical research would have clear benefits in providing valuable information on the effects of probiotics in respiratory virus infections.
Acknowledgments
Conflict of interest
None.
References
- 1.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 2.Tapparel C, Siegrist F, Petty TJ, Kaiser L. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol. 2013;14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Zambon MC. Epidemiology and pathogenesis of influenza. J Antimicrob Chemother. 1999;44:3–9. doi: 10.1093/jac/44.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 4.Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30:510–517. doi: 10.1097/INF.0b013e3182184ae7. [DOI] [PubMed] [Google Scholar]
- 5.Robinson CM, Seto D, Jones MS, Dyer DW, Chodosh J. Molecular evolution of human species D adenoviruses. Infect Genet Evol. 2011;11:1208–1217. doi: 10.1016/j.meegid.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols WG, Peck Campbell AJ, Boeckh M. Respiratory viruses other than influenza virus: impact and therapeutic advances. Clin Microbiol Rev. 2008;21:274–290. doi: 10.1128/CMR.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jartti T, Jartti L, Ruuskanen O, Söderlund-Venermo M. New respiratory viral infections. Curr Opin Pulm Med. 2012;18:271–278. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- 8.Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) (2002) Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food
- 9.Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria
- 10.Wolvers D, Antoine JM, Myllyluoma E, Schrezenmeir J, Szajewska H, Rijkers GT. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of infections by probiotics. J Nutr. 2010;140:698S–712S. doi: 10.3945/jn.109.113753. [DOI] [PubMed] [Google Scholar]
- 11.Aureli P, Capurso L, Castellazzi AM, Clerici M, Giovannini M, Morelli L, et al. Probiotics and health: an evidence-based review. Pharmacol Res. 2011;63:366–376. doi: 10.1016/j.phrs.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Bodera P, Chcialowski A. Immunomodulatory effect of probiotic bacteria. Recent Pat Inflamm Allergy Drug Discov. 2009;3:58–64. doi: 10.2174/187221309787158461. [DOI] [PubMed] [Google Scholar]
- 14.Guarino A, Lo Vecchio A, Canani RB. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol. 2009;25:18–23. doi: 10.1097/MOG.0b013e32831b4455. [DOI] [PubMed] [Google Scholar]
- 15.Hao Q, Lu Z, Dong BR, Huang CQ, Wu T (2011) Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev (9):CD006895 [DOI] [PubMed]
- 16.Hori T, Kiyoshima J, Shida K, Yasui H. Effect of intranasal administration of Lactobacillus casei Shirota on influenza virus infection of upper respiratory tract in mice. Clin Diagn Lab Immunol. 2001;8:593–597. doi: 10.1128/CDLI.8.3.593-597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasui H, Kiyoshima J, Hori T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei shirota. Clin Diagn Lab Immunol. 2004;11:675–679. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeda N, Nakamura R, Hirose Y, Murosaki S, Yamamoto Y, Kase T, et al. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int Immunopharmacol. 2009;9:1122–1125. doi: 10.1016/j.intimp.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Takeda S, Takeshita M, Kikuchi Y, Dashnyam B, Kawahara S, Yoshida H, et al. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int Immunopharmacol. 2011;11:1976–1983. doi: 10.1016/j.intimp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Park MK, Ngo V, Kwon YM, Lee YT, Yoo S, Cho YH, et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One. 2013;8:e75368. doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawase M, He F, Kubota A, Harata G, Hiramatsu M. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett Appl Microbiol. 2010;51:6–10. doi: 10.1111/j.1472-765X.2010.02849.x. [DOI] [PubMed] [Google Scholar]
- 22.Kawase M, He F, Kubota A, Yoda K, Miyazawa K, Hiramatsu M. Heat-killed Lactobacillus gasseri TMC0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses. FEMS Immunol Med Microbiol. 2012;64:280–288. doi: 10.1111/j.1574-695X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- 23.Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol. 2010;50:597–602. doi: 10.1111/j.1472-765X.2010.02844.x. [DOI] [PubMed] [Google Scholar]
- 24.Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, et al. Intranasally administered Lactobacillus gasseri TMC0356 protects mice from H1N1 influenza virus infection by stimulating respiratory immune responses. World J Microbiol Biotechnol. 2011;27:411–416. doi: 10.1007/s11274-010-0472-x. [DOI] [Google Scholar]
- 25.Lee YN, Youn HN, Kwon JH, Lee DH, Park JK, Yuk SS, et al. Sublingual administration of Lactobacillus rhamnosus affects respiratory immune responses and facilitates protection against influenza virus infection in mice. Antiviral Res. 2013;98:284–290. doi: 10.1016/j.antiviral.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Youn HN, Lee DH, Lee YN, Park JK, Yuk SS, Yang SY, et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res. 2012;93:138–143. doi: 10.1016/j.antiviral.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Yeo JM, Lee HJ, Kim JW, Lee JB, Park SY, Choi IS, et al. Lactobacillus fermentum CJL-112 protects mice against influenza virus infection by activating T-helper 1 and eliciting a protective immune response. Int Immunopharmacol. 2014;18:50–54. doi: 10.1016/j.intimp.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Waki N, Yajima N, Suganuma H, Buddle BM, Luo D, Heiser A, et al. Oral administration of Lactobacillus brevis KB290 to mice alleviates clinical symptoms following influenza virus infection. Lett Appl Microbiol. 2014;58:87–93. doi: 10.1111/lam.12160. [DOI] [PubMed] [Google Scholar]
- 29.Izumo T, Maekawa T, Ida M, Noguchi A, Kitagawa Y, Shibata H, et al. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int Immunopharmacol. 2010;10:1101–1106. doi: 10.1016/j.intimp.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi N, Saito T, Uematsu T, Kishi K, Toba M, Kohda N, et al. Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int Immunopharmacol. 2011;11:199–203. doi: 10.1016/j.intimp.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Kiso M, Takano R, Sakabe S, Katsura H, Shinya K, Uraki R, et al. Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against influenza A virus. Sci Rep. 2013;3:1–8. doi: 10.1038/srep01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto H, Sagitani A, Ashida N, Kato S, Hirota T, Shinoda T, et al. Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity. Br J Nutr. 2013;110:1810–1818. doi: 10.1017/S0007114513001104. [DOI] [PubMed] [Google Scholar]
- 33.Iwabuchi N, Xiao JZ, Yaeshima T, Iwatsuki K. Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol Pharm Bull. 2011;34:1352–1355. doi: 10.1248/bpb.34.1352. [DOI] [PubMed] [Google Scholar]
- 34.Wu S, Jiang ZY, Sun YF, Yu B, Chen J, Dai CQ, et al. Microbiota regulates the TLR7 signaling pathway against respiratory tract influenza a virus infection. Curr Microbiol. 2013;67:414–422. doi: 10.1007/s00284-013-0380-z. [DOI] [PubMed] [Google Scholar]
- 35.Gabryszewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB, et al. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J Immunol. 2011;186:1151–1161. doi: 10.4049/jimmunol.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Crespo KE, Chan CC, Gabryszewski SJ, Percopo CM, Rigaux P, Dyer KD, et al. Lactobacillus priming of the respiratory tract: heterologous immunity and protection against lethal pneumovirus infection. Antiviral Res. 2013;97:270–279. doi: 10.1016/j.antiviral.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomosada Y, Chiba E, Zelaya H, Takahashi T, Tsukida K, Kitazawa H, et al. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013;14:40. doi: 10.1186/1471-2172-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kechaou N, Chain F, Gratadoux JJ, Blugeon S, Bertho N, Chevalier C, et al. Identification of one novel candidate probiotic lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl Environ Microbiol. 2013;79:1491–1499. doi: 10.1128/AEM.03075-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai T, Makino S, Ikegami S, Itoh H, Yamada H. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int Immunopharmacol. 2011;11:2246–2250. doi: 10.1016/j.intimp.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Maruo T, Gotoh Y, Nishimura H, Ohashi S, Toda T, Takahashi K. Oral administration of milk fermented with Lactococcus lactis subsp. cremoris FC protects mice against influenza virus infection. Lett Appl Microbiol. 2012;55:135–140. doi: 10.1111/j.1472-765X.2012.03270.x. [DOI] [PubMed] [Google Scholar]
- 41.Yasui H, Kiyoshima J, Hori T, Shida K. Protection against influenza virus infection of mice fed Bifidobacterium breve YIT4064. Clin Diagn Lab Immunol. 1999;6:186–192. doi: 10.1128/cdli.6.2.186-192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatakka K, Savilahti E, Pönkä A, Meurman JH, Poussa T, Näse L, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. Br Med J. 2001;322:1327–1329. doi: 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hojsak I, Snovak N, Abdović S, Szajewska H, Misak Z, Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2010;29:312–316. doi: 10.1016/j.clnu.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Hojsak I, Abdović S, Szajewska H, Milošević M, Krznarić Ž, Kolaček S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics. 2010;125:e1171–e1177. doi: 10.1542/peds.2009-2568. [DOI] [PubMed] [Google Scholar]
- 45.Kumpu M, Kekkonen RA, Kautiainen H, Järvenpää S, Kristo A, Huovinen P, et al. Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2012;66:1020–1023. doi: 10.1038/ejcn.2012.62. [DOI] [PubMed] [Google Scholar]
- 46.Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E (2013) Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol pii: S0091-6749(13)01307-9 [DOI] [PMC free article] [PubMed]
- 47.Kumpu M, Lehtoranta L, Roivainen M, Rönkkö E, Ziegler T, Söderlund-Venermo M, et al. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. J Med Virol. 2013;85:1652–1658. doi: 10.1002/jmv.23623. [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Hu P, Du X, Zhou T, Pei X. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377–381. doi: 10.1007/s13312-013-0123-z. [DOI] [PubMed] [Google Scholar]
- 49.Lin JS, Chiu YH, Lin NT, Chu CH, Huang KC, Liao KW, et al. Different effects of probiotic species/strains on infections in preschool children: a double-blind, randomized, controlled study. Vaccine. 2009;27:1073–1079. doi: 10.1016/j.vaccine.2008.11.114. [DOI] [PubMed] [Google Scholar]
- 50.Cobo Sanz JM, Mateos JA, Muñoz Conejo A. Effect of Lactobacillus casei on the incidence of infectious conditions in children. Nutr Hosp. 2006;21:547–551. [PubMed] [Google Scholar]
- 51.Maldonado J, Cañabate F, Sempere L, Vela F, Sánchez AR, Narbona E, et al. Human milk probiotic lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J Pediatr Gastroenterol Nutr. 2012;54:55–61. doi: 10.1097/MPG.0b013e3182333f18. [DOI] [PubMed] [Google Scholar]
- 52.Taipale T, Pienihäkkinen K, Isolauri E, Larsen C, Brockmann E, Alanen P, et al. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr. 2011;105:409–416. doi: 10.1017/S0007114510003685. [DOI] [PubMed] [Google Scholar]
- 53.Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]
- 54.Niittynen L, Pitkäranta A, Korpela R. Probiotics and otitis media in children. Int J Pediatr Otorhinolaryngol. 2012;76:465–470. doi: 10.1016/j.ijporl.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Agustina R, Kok FJ, Van De Rest O, Fahmida U, Firmansyah A, Lukito W, et al. Randomized trial of probiotics and calcium on diarrhea and respiratory tract infections in Indonesian children. Pediatrics. 2012;129(5):e1155–e1164. doi: 10.1542/peds.2011-1379. [DOI] [PubMed] [Google Scholar]
- 56.Hatakka K, Blomgren K, Pohjavuori S, Kaijalainen T, Poussa T, Leinonen M, et al. Treatment of acute otitis media with probiotics in otitis-prone children—a double-blind, placebo-controlled randomised study. Clin Nutr. 2007;26:314–321. doi: 10.1016/j.clnu.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy—a randomised, double-blind, placebo-controlled study. Br J Nutr. 2009;101:1722–1726. doi: 10.1017/S0007114508116282. [DOI] [PubMed] [Google Scholar]
- 58.Rerksuppaphol S, Rerksuppaphol L. Randomized controlled trial of probiotics to reduce common cold in schoolchildren. Pediatr Int. 2012;54:682–687. doi: 10.1111/j.1442-200X.2012.03647.x. [DOI] [PubMed] [Google Scholar]
- 59.Lehtoranta L, Söderlund-Venermo M, Nokso-Koivisto J, Toivola H, Blomgren K, Hatakka K, et al. Human bocavirus in the nasopharynx of otitis-prone children. Int J Pediatr Otorhinolaryngol. 2012;76:206–211. doi: 10.1016/j.ijporl.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatakka K (2007) Probiotics in the prevention of clinical manifestations of common infectious diseases in children and in the elderly. Dissertation, University of Helsinki
- 61.Olivares M, Díaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonollá J, Navas M, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23:254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Winkler P, de Vrese M, Laue Ch, Schrezenmeir J. Effect of a dietary supplement containing probiotic bacteria plus vitamins and minerals on common cold infections and cellular immune parameters. Int J Clin Pharmacol Ther. 2005;43:318–326. doi: 10.5414/CPP43318. [DOI] [PubMed] [Google Scholar]
- 63.de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr. 2005;24:481–491. doi: 10.1016/j.clnu.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 64.de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine. 2006;24:6670–6674. doi: 10.1016/j.vaccine.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 65.West NP, Horn PL, Pyne DB, Gebski VJ, Lahtinen SJ, Fricker PA et al (2013) Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr pii: S0261-5614(13)00261-6 [DOI] [PubMed]
- 66.Smith TJ, Rigassio-Radler D, Denmark R, Haley T, Touger-Decker R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013;109:1999–2007. doi: 10.1017/S0007114512004138. [DOI] [PubMed] [Google Scholar]
- 67.Cox AJ, Pyne DB, Saunders PU, Fricker PA. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br J Sports Med. 2010;44:222–226. doi: 10.1136/bjsm.2007.044628. [DOI] [PubMed] [Google Scholar]
- 68.West NP, Pyne DB, Cripps AW, Hopkins WG, Eskesen DC, Jairath A, et al. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10:30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haywood BA, Black KE, Baker D, McGarvey J, Healey P, Brown RC (2013) Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J Sci Med Sport pii: S1440-2440(13)00190-4 [DOI] [PubMed]
- 70.Kekkonen RA, Vasankari TJ, Vuorimaa T, Haahtela T, Julkunen I, Korpela R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int J Sport Nutr Exerc Metab. 2007;17:352–363. doi: 10.1123/ijsnem.17.4.352. [DOI] [PubMed] [Google Scholar]
- 71.Tiollier E, Chennaoui M, Gomez-Merino D, Drogou C, Filaire E, Guezennec CY. Effect of a probiotics supplementation on respiratory infections and immune and hormonal parameters during intense military training. Mil Med. 2007;172:1006–1011. doi: 10.7205/milmed.172.9.1006. [DOI] [PubMed] [Google Scholar]
- 72.Gleeson M, Bishop NC, Oliveira M, McCauley T, Tauler P, Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int J Sport Nutr Exerc Metab. 2012;22:235–242. doi: 10.1123/ijsnem.22.4.235. [DOI] [PubMed] [Google Scholar]
- 73.Guillemard E, Tanguy J, Flavigny A, de la Motte S, Schrezenmeir J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J Am Coll Nutr. 2010;29:455–468. doi: 10.1080/07315724.2010.10719882. [DOI] [PubMed] [Google Scholar]
- 74.Turchet P, Laurenzano M, Auboiron S, Antoine JM. Effect of fermented milk containing the probiotic Lactobacillus casei DN-114001 on winter infections in free-living elderly subjects: a randomised, controlled pilot study. J Nutr Health Aging. 2003;7:75–77. [PubMed] [Google Scholar]
- 75.Guillemard E, Tondu F, Lacoin F, Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br J Nutr. 2010;103:58–68. doi: 10.1017/S0007114509991395. [DOI] [PubMed] [Google Scholar]
- 76.Fujita R, Iimuro S, Shinozaki T, Sakamaki K, Uemura Y, Takeuchi A, et al. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: a multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am J Infect Control. 2013;41:1231–1235. doi: 10.1016/j.ajic.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Van Puyenbroeck K, Hens N, Coenen S, Michiels B, Beunckens C, Molenberghs G, et al. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am J Clin Nutr. 2012;95:1165–1171. doi: 10.3945/ajcn.111.026831. [DOI] [PubMed] [Google Scholar]
- 78.Merenstein D, Murphy M, Fokar A, Hernandez RK, Park H, Nsouli H, et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: the DRINK study. A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur J Clin Nutr. 2010;64:669–677. doi: 10.1038/ejcn.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol. 2010;104:183–190. doi: 10.1016/j.anai.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Lehtoranta L (2012) Probiotics and virus infections: the effects of Lactobacillus rhamnosus GG on respiratory and gastrointestinal virus infections. Dissertation, University of Helsinki
- 81.Botić T, Klingberg TD, Weingartl H, Cencic A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int J Food Microbiol. 2007;115:227–234. doi: 10.1016/j.ijfoodmicro.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 82.Choi H-J, Song J-H, Ahn Y-J, Baek S-H, Kwon D-H. Antiviral activities of cell-free supernatants of yogurts metabolites against some RNA viruses. Eur Food Res Technol. 2009;228:945–950. doi: 10.1007/s00217-009-1009-0. [DOI] [Google Scholar]
- 83.Ivec M, Botić T, Koren S, Jakobsen M, Weingartl H, Cencic A. Interactions of macrophages with probiotic bacteria lead to increased antiviral response against vesicular stomatitis virus. Antiviral Res. 2007;75:266–274. doi: 10.1016/j.antiviral.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Pipenbaher N, Moeller PL, Dolinšek J, Jakobsen M, Weingartl H, Cencič A. Nitric oxide (NO) production in mammalian non-tumorigenic epithelial cells of the small intestine and macrophages induced by individual strains of lactobacilli and bifidobacteria. Int Dairy J. 2009;19:166–171. doi: 10.1016/j.idairyj.2008.09.003. [DOI] [Google Scholar]
- 85.Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto M, et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–6062. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vinderola G, Matar C, Perdigon G. Role of intestinal epithelial cells in immune effects mediated by gram-positive probiotic bacteria: Involvement of Toll-like receptors. Clin Diagn Lab Immunol. 2005;12:1075–1084. doi: 10.1128/CDLI.12.9.1075-1084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236–243. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miettinen M, Veckman V, Latvala S, Sareneva T, Matikainen S, Julkunen I. Live Lactobacillus rhamnosus and Streptococcus pyogenes differentially regulate Toll-like receptor (TLR) gene expression in human primary macrophages. J Leukoc Biol. 2008;84:1092–1100. doi: 10.1189/jlb.1206737. [DOI] [PubMed] [Google Scholar]
- 89.O’Hara AM, O’Regan P, Fanning A, O’Mahony C, MacSharry J, Lyons A, et al. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology. 2006;118:202–215. doi: 10.1111/j.1365-2567.2006.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Latvala S, Miettinen M, Kekkonen R, Korpela R, Julkunen I. Potentially probiotic bacteria induce cytokine production and suppressor of cytokine signaling 3 gene expression in human monocyte-derived macrophages. Cytokine. 2009;48:100–101. doi: 10.1016/j.cyto.2009.07.423. [DOI] [Google Scholar]
- 91.Miettinen M, Lehtonen A, Julkunen I, Matikainen S. Lactobacilli and streptococci activate NF-kappa B and STAT signaling pathways in human macrophages. J Immunol. 2000;164:3733–3740. doi: 10.4049/jimmunol.164.7.3733. [DOI] [PubMed] [Google Scholar]
- 92.Veckman V, Miettinen M, Matikainen S, Lande R, Giacomini E, Coccia EM, et al. Lactobacilli and streptococci induce inflammatory chemokine production in human macrophages that stimulates Th1 cell chemotaxis. J Leukoc Biol. 2003;74:395–402. doi: 10.1189/jlb.0402212. [DOI] [PubMed] [Google Scholar]
- 93.Veckman V, Miettinen M, Pirhonen J, Sirén J, Matikainen S, Julkunen I. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J Leukoc Biol. 2004;75:764–771. doi: 10.1189/jlb.1003461. [DOI] [PubMed] [Google Scholar]
- 94.Latvala S, Miettinen M, Kekkonen RA, Korpela R, Julkunen I. Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin Exp Immunol. 2011;165:94–103. doi: 10.1111/j.1365-2249.2011.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weiss G, Rasmussen S, Zeuthen LH, Nielsen BN, Jarmer H, Jespersen L, et al. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology. 2010;131:268–281. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiss G, Christensen HR, Zeuthen LH, Vogensen FK, Jakobsen M, Frøkiær H. Lactobacilli and bifidobacteria induce differential interferon-beta profiles in dendritic cells. Cytokine. 2011;56:520–530. doi: 10.1016/j.cyto.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 97.Miettinen M, Pietilä TE, Kekkonen RA, Kankainen M, Latvala S, Pirhonen J, et al. Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages. Gut Microbes. 2012;3:510–522. doi: 10.4161/gmic.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hori T, Kiyoshima J, Shida K, Yasui H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacillus casei strain Shirota. Clin Diagn Lab Immunol. 2002;9:105–108. doi: 10.1128/CDLI.9.1.105-108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davidson LE, Fiorino AM, Snydman DR, Hibberd PL. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 2011;65:501–507. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rizzardini G, Eskesen D, Calder PC, Capetti A, Jespersen L, Clerici M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2011;107:876–884. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 101.Boge T, Rémigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27:5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 102.Boyle RJ, Robins-Browne RM, Tang MLK. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–1264. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]
- 103.Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hörmannsperger G, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–185. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115:178–181. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 105.Kalima P, Masterton RG, Roddie PH, Thomas AE. Lactobacillus rhamnosus infection in a child following bone marrow transplant. J Infect. 1996;32:165–167. doi: 10.1016/S0163-4453(96)91622-9. [DOI] [PubMed] [Google Scholar]
- 106.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 107.European Food Safety Authority (EFSA) Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2011 update) EFSA J. 2011;9:2497. [Google Scholar]
- 108.Salminen MK, Tynkkynen S, Rautelin H, Saxelin M, Vaara M, Ruutu P, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis. 2002;35:1155–1160. doi: 10.1086/342912. [DOI] [PubMed] [Google Scholar]


