Abstract
Laboratory-acquired infections due to a variety of bacteria, viruses, parasites, and fungi have been described over the last century, and laboratory workers are at risk of exposure to these infectious agents. However, reporting laboratory-associated infections has been largely voluntary, and there is no way to determine the real number of people involved or to know the precise risks for workers. In this study, an international survey based on volunteering was conducted in biosafety level 3 and 4 laboratories to determine the number of laboratory-acquired infections and the possible underlying causes of these contaminations. The analysis of the survey reveals that laboratory-acquired infections have been infrequent and even rare in recent years, and human errors represent a very high percentage of the cases. Today, most risks from biological hazards can be reduced through the use of appropriate procedures and techniques, containment devices and facilities, and the training of personnel.
Keywords: Brucellosis, Personal Protective Equipment, Coccidioidomycosis, Severe Acute Respiratory Syndrome, Tularemia
Introduction
Laboratory-acquired infections (LAIs) are defined as all infections acquired through laboratories or laboratory-related activities, whether they are symptomatic or asymptomatic in nature. LAIs due to a wide variety of bacteria, viruses, fungi, and parasites are described in the literature. The largest survey of infections was reported in 1976 by Pike [1], who found that 4079 LAIs were caused by 159 biological agents, although ten agents caused infections accounting for 50 % of cases (brucellosis, Q fever, hepatitis, typhoid fever, tularemia, tuberculosis, dermatomycoses, Venezuelan equine encephalitis, psittacosis, and coccidioidomycosis). There were no distinguishable accidents or exposure events identified in more than 80 % of the reported cases. During the 20 years following the Pike and Sulkin publications, a worldwide literature review by Harding and Byers revealed 1267 cases of infections, with 22 deaths [2]. Five deaths were fetus abortions as consequences of a maternal LAI. Mycobacterium tuberculosis, Coxiella burnetii, hantaviruses, arboviruses, hepatitis B virus, Brucella spp., Salmonella spp., Shigella spp., hepatitis C virus, and Cryptosporidium spp. accounted for 1074 of the 1267 infections. Like Pike and Sulkin, Harding and Byers reported that only a small number of the LAIs involved a specific incident. These studies reported cases of old infections and refer to periods with practices and types of exposure which have since considerably evolved, especially with the introduction of high-containment laboratories.
More recently, Henkel et al. presented data reported to the Centers for Disease Control and Prevention (CDC) from 2004 to 2010 following the implementation of a nationwide program for monitoring the potential theft, loss, or release of biological select agents and toxins (BSATs) [3]. In total, 11 LAIs associated with BSAT releases were reported in an average annual population of approximately 10,000 individuals with approved access to BSATs. No cases of fatality or secondary human-to-human transmission was reported. These LAIs were associated with exposures to Brucella species (six cases), Francisella tularensis (four cases), and Coccidioides immitis/posadasii (one case) [4, 5]. They resulted from either unrecognized exposures or presumptive exposure to BSAT aerosols. These observations are consistent with Pike’s and Harding’s studies [1].
Two publications in science magazines have provided recent information about LAIs. The current Ebola crisis reveals that priority must be given to infectious diseases because of the potential consequences to individuals and society [6]. Some researchers argue for the need to increase research on Ebola virus to develop treatments, while others focus on recent incidents in biosafety facilities and the possible dissemination of these dangerous pathogens in the general population [6]. A recent example, in 2004, was the mishandling of the severe acute respiratory syndrome virus that resulted in tertiary infections and the death of an attending physician in China [7]. Lipsitch found that government data on US biosafety labs reveal accidents estimated to be between 100 and 275 potential releases of pathogens each year in labs that deal with select agents between the years 2008 and 2012; however, these reports include banal accidents like spills and record-keeping errors, and very few workers were infected [8].
However, until now, the true risk posed to laboratory workers after potential exposure to an infectious agent has been difficult to determine, in part because of the lack of systematic reporting of laboratory infections. It may vary greatly according to the pathogen and also the type of exposure considered. Currently available data are limited to retrospective and voluntary postal surveys, anecdotal case reports, and reports about selected outbreaks with specific microorganisms.
The aim of this survey was to gather information on LAIs in biosafety level 3 and 4 laboratories around the world and to assess possible underlying causes of these infections, in order to identify real current risks and to propose preventative procedures.
Materials and methods
In this study, 119 private or public institutions with notified containment level 3 or 4 laboratories were contacted by email to complete a survey about LAIs. The mailing list was established by investigators in Marseille. In total, 15 questions were addressed to each respondent, consisting of single-answer questions and multi-answer questions, most of the questions being mandatory (see below).
We also performed a literature analysis. To determine the worldwide number of LAIs, a systematic review of articles published during the period 1980–2015 was performed. The inclusion criterion was the presence of an accidental infection in workers or students in research laboratories working with French select agents. The following academic Internet search systems were used: PubMed, Google Scholar, and ISI Web of Knowledge. They were searched with the keywords “laboratory acquired infection”, “laboratory accident”, and with the list of the different French select agents.

Results
The experience of two skilled researchers
Prof. X, director of a laboratory dealing with rickettsial diseases in the United States, pointed out that he had not had any laboratory infections with biosafety level 3 rickettsial agents since 2000. He specified that he had personal experience with laboratory infections with rickettsial agents (particularly Rickettsia) in the USA, but these infections generally occurred before the implementation of biosafety level 3 laboratories and the exclusive handling of infectious agents in class II biosafety cabinets. He remembered one C. burnetii-related infection caused by a needle stick while the involved person was working with a previously infected mouse. However, at present, all work with C. burnetii is performed in a specific laboratory, and requires vaccination of the laboratory workers and the use of a powered air-purifying respirator in the biosafety level 3 laboratory.
Prof. Didier Raoult established the Rickettsia Unit at Aix Marseille University in 1984. Since 2008, he has been the director of the “URMITE”, the research Unit in Infectious and Tropical Emergent Diseases, and employs 450 personnel (with 80 national and international students and PhD students). The laboratory coordinates European and international networks, and serves as a leader in research on several infectious diseases, including rickettsial diseases, Q fever, and arboviral diseases, and is directly involved in defense against bioterrorism and highly contagious diseases. In the 1980s, the laboratory had three LAI cases from skin wounds caused by the manipulation of glass tubes broken after centrifugation of an infectious suspension. The involved biological agents were Rickettsia species (including R. australis, R. conorii, and R. japonica). The persons exposed received appropriate antibiotic treatment and recovered without sequelae. These incidents occurred before the publication of regulatory procedures for biosafety level 3 and 4 laboratories and good laboratory practices.
Analysis of the survey
A total of 23 of the 119 contacted laboratories accepted to participate to this survey, of which five were biosafety level 4 laboratories. As shown in Fig. 1, this survey was conducted on a global basis. Most of the questioned laboratories routinely use the following personal protective equipment: latex gloves (86 %), nitrile gloves (68 %), two pairs of gloves (77 %), FFP3 masks (64 %), goggle or face protection (81 %), waterproof coverall or gown (68 %), overboots or overshoes (90 %), and hygiene cap or hood (54 %) (Fig. 2).
Fig. 1.
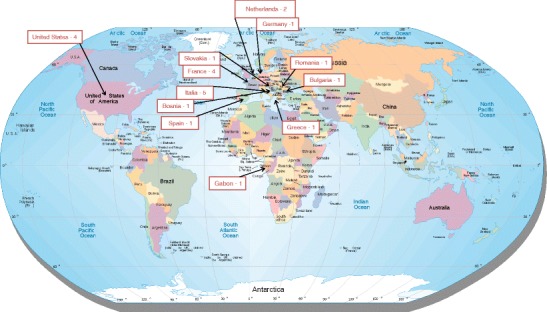
Worldwide repartition of the laboratories that responded to the survey. Retrieved from http://www.jimmymack.org/worldmap.html
Fig. 2.
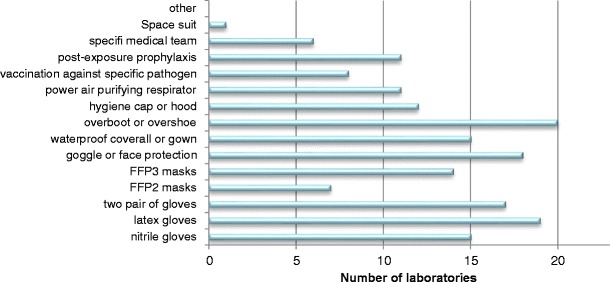
Personal protective equipment in the laboratories
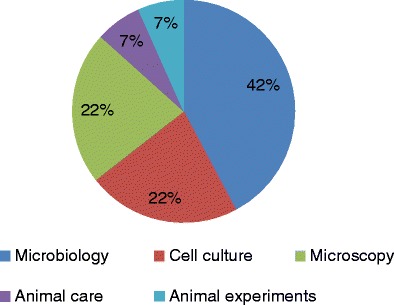
Only four of the 23 surveyed laboratories reported 15 LAIs caused by four different pathogenic organisms. Bacterial infections predominated, particularly biosafety level 3 bacteria belonging to the following species: Mycobacterium tuberculosis (ten cases), Coxiella burnetii (two cases), and Brucella melitensis (two cases) (Table 1). The remaining case was caused by a biosafety level 2 virus (foamy virus). The majority of the LAIs (73 %) occurred in a biosafety level 3 laboratory in the context of microbiology activities (42 %), followed by microscopy (22 %) and cell culture (22 %) (Fig. 3).
Table 1.
Biological agents involved in laboratory-acquired infections (LAIs)
| Species | Biosafety level | Number of LAIs |
|---|---|---|
| Coxiella burnetii | 3 | 2 |
| Foamy virus | 2 | 1 |
| Brucella melitensis | 3 | 2 |
| Mycobacterium tuberculosis | 2 | 10 |
Fig. 3.
In which context did the infection happen?
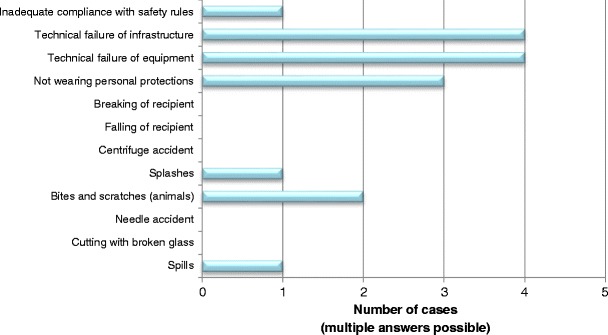
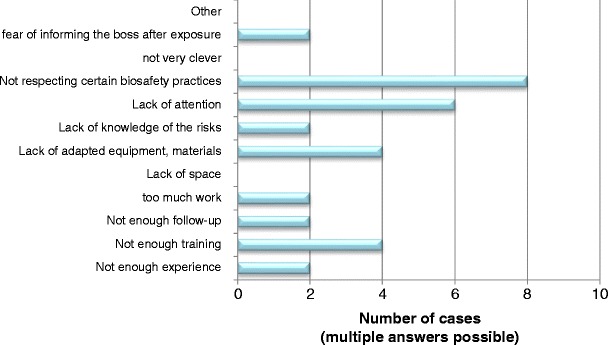
Laboratory technicians were most commonly infected (87 % of the cases), while in only 7 and 6 % of the cases, respectively, the infected person was an animal caretaker or a researcher. It should be noted that laboratory technicians are more numerous than researchers worldwide, and also probably more often exposed to biological agents. All 15 LAI cases recovered from their infection, without sequelae in 13 persons (87 %), but with sequelae in two patients. Fortunately, no deaths were reported. Notably, for 93 % of the cases, post-exposure treatment was prescribed. A small percentage of exposed persons had only reinforced medical surveillance (13 %), and only 40 % were vaccinated against the involved pathogen prior to exposure. Regarding the potential transmission routes involved in LAIs, 87 % of the cases were airborne infections, while the others were percutaneous infections. In none of the LAIs was the infection transmitted to another person. Half of the cases were related to technical failures in equipment and infrastructure. However, these cases occurred in a single laboratory where the environment was not safe. Consequently, the laboratory was closed after this incident. For the remaining cases, three contaminations occurred because of not wearing personal protective equipment. Other incidents leading to LAIs were animal bites and scratches (two cases), splashes (one case), inadequate compliance with safety rules (one case), and spills (one case) (Fig. 4). Not respecting certain biosafety practices (eight cases), lack of attention (six cases), lack of appropriate equipment and materials (four cases), and insufficient training (four cases) seem to be the principal causes of LAIs (Fig. 5). Therefore, human error accounted for 78 % of the underlying causes of LAIs.
Fig. 4.
Type of incident involved in the infection
Fig. 5.
Probable causes of the incident
Discussion
LAIs represent an occupational hazard unique to laboratory workers, especially those working in microbiology laboratories. Before the introduction of regulations concerning biosafety levels in laboratories and good laboratory practices, laboratory manipulations, including the handling of cultures of human pathogens, took place on the bench, without any specific protection. For example, it was permissible to smoke, eat, or drink in such laboratories, to conduct an olfactory examination of the cultures, or to perform mouth pipetting of infectious suspensions, all practices that are now well known to be associated with a high risk of laboratory infections. Therefore, many LAIs occurred, as described by Pike [1].
The actual risk of LAIs is difficult to quantify because there is no systematic reporting system. Because of this lack of information, control measures are proposed and implemented by competent authorities, and regulations are increasing dramatically, which, in turn, profoundly affect research [9]. The additional, sometimes draconian, measures for laboratories working with highly pathogenic microorganisms have been implemented without solid evidence that they will provide additional public or laboratory safety.
We performed a comparison of three different sources on the number of LAIs due to biological select agents: this survey, the reference [3] dealing with LAIs due to select agents in United States between 2004 and 2010, and a literature review of LAIs over the last 35 years (Table 2). It is evident that 23/119 (19 %) laboratories responding to our questionnaire present a limited proportion of laboratories and we understand that it is a limitation of this work. However, with the strengthening of regulations, we believe that some laboratories are reluctant to expose their accidents. However, due to the long period of survey for several investigators, we believe that the obtained results are a good reflection of the frequency and cause of accidents. Overall, this analysis allows us to conclude that LAIs have been infrequent with highly pathogenic microorganisms (two in our survey, ten in reference [3], and 220 during the last 35 years), and even rare in recent years. This phenomenon is almost certainly due to the improvement of working conditions, particularly the biosecurity measures implemented during this time. This is especially true with biosafety level 4 laboratories, in which no accident was observed in the five laboratories participating in this work. Moreover, with the exception of a case of a technician accidentally inoculated with Ebola virus who did not develop hemorrhagic fever [10], we could not find any LAI reports in biosafety level 4 laboratories (Table 2). As accidents in this kind of laboratories are likely to become publicized, we do not believe we missed any information. Recent incidents in the USA involving BSATs have focused attention on the need to improve and maintain a culture of biosafety and biosecurity in the life sciences. Notable incidents included the discovery of vials labeled “variola”, the virus responsible for smallpox, in a storage room in a Food and Drug Administration laboratory located on the Bethesda campus of the National Institutes of Health, the potential exposure of staff members at the CDC to Bacillus anthracis, and the inadvertent cross-contamination of a low pathogenic avian influenza A (H9N2) virus sample with a highly pathogenic avian influenza A (H5N1) virus and subsequent shipment of the contaminated culture to an external high-containment laboratory. None of these events resulted in human contamination, but suggested an inadequate compliance with existing regulations, policies, and procedures [11, 12]. After these events, the White House published a report dealing with national biosafety and biosecurity in order to protect the nation’s health and in order to prevent, detect, and respond to infectious threats around the world, resulting in a set of recommendations in terms of biosafety and associated biosecurity [11]. Fortunately, none of these events resulted in a casualty.
Table 2.
Number of LAIs caused by French select agents according to three different sources (this survey, reference [3], and literature reviews)
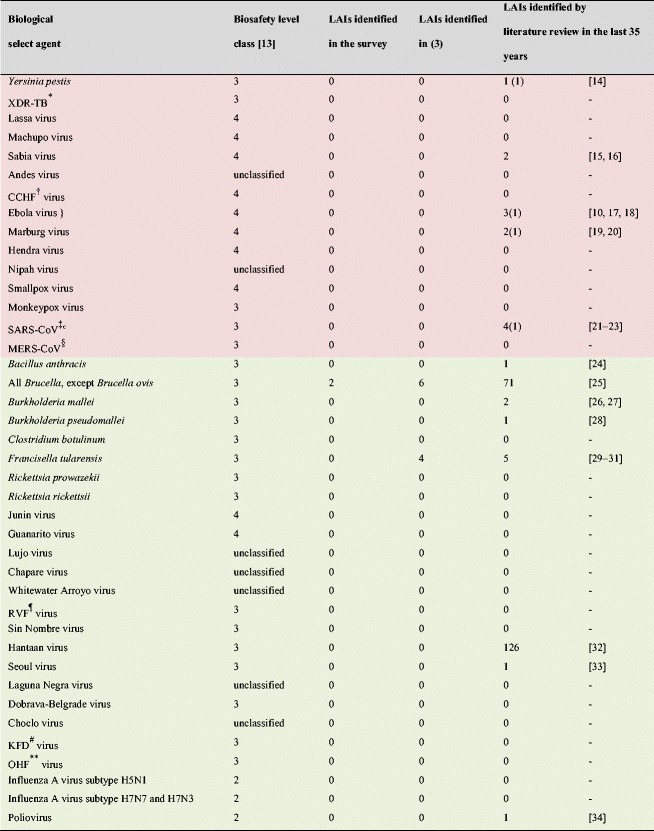
The biological agents colored in red belong to annex 1 of the French regulation concerning select agents [35] (Highly pathogenic microorganisms presenting the highest risk to public health)
The biological agents colored in green belong to annex 2 of the French regulation concerning select agents [35]
(): number of deaths
*XDR-TB: extensively drug-resistant tuberculosis
†CCHF: Crimean-Congo hemorrhagic fever
‡SARS-CoV: severe acute respiratory syndrome coronavirus
§MERS-CoV: Middle East respiratory Syndrome coronavirus
¶RVF: Rift Valley fever
#KFD: Kyasanur forest disease
**OHF: Omsk hemorrhagic fever virus
} unique case of class 4 pathogen contamination in a biosafety level 4 laboratory
One important point that could improve safety in laboratories working with highly pathogenic agents is the training of personnel to reduce LAIs caused by human error, and, in particular, to avoid the involvement of people with psychological problems in research on highly pathogenic agents by means of a regular medical follow-up. An example of a failure in medical monitoring was provided during the anthrax attacks that occurred in the USA in 2001 [36, 37]. Dr. Ivins was working in a military laboratory at Fort Detrick, Maryland on the development of anthrax vaccines. He had mental health and professional problems because his work on anthrax vaccine was unsuccessful. An apparent lack of careful oversight allowed him to send letters containing anthrax spores to various offices and senators, resulting in the deaths of five people and infections in 17 others [38].
When examining actual biological hazards, human errors represent a very high percentage of LAIs. If in the past, as observed in laboratory number 3 of the present work, cases could be related to technical failures in equipment and infrastructure, today, most risks from biological hazards for humans can be reduced through the use of appropriate procedures and techniques, containment devices, and facilities. It should be noticed that humans are not the unique possible victims of biological hazards; cattle were affected by an outbreak of foot-and-mouth disease in the UK in 2007 that was later suspected to be due to a cracked pipe from two laboratories working on this virus in their vicinity [39]. The control measures used in the laboratories questioned are designed to protect employees and the public from exposure to infectious agents, and these measures seem to be sufficient. The consequence of not respecting such measures is that many of the laboratories will simply abandon the study of critically important biohazardous agents. The biosafety procedures adopted so far have greatly contributed to reducing the burden of LAIs in recent years, and data from this survey contribute to improving the balance between the need to facilitate research activities and assuring appropriate biosafety and biosecurity procedures. Tim Trevan recommended that scientists working with hazardous materials take lessons from the nuclear industry, hospitals, and other sectors that have established a safety culture [40]. In conclusion, there is still a need to implement a culture of biosafety in the life sciences, rather than strengthen regulations.
Acknowledgments
We thank Robert A. Heinzen and Jean-Marc Reynes for their participation in the survey.
This study was supported by the projects APVV-0280-12 and VEGA 2/0005/15.
Compliance with ethical standards
Funding
None.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Pike RM. Laboratory-associated infections: summary and analysis of 3921 cases. Health Lab Sci. 1976;13:105–114. [PubMed] [Google Scholar]
- 2.Harding LH, Byers KB. Epidemiology of laboratory-acquired infections. In: Fleming DO, Hunt DL, editors. Biological safety: principles and practices. 4. Washington, DC: ASM Press; 2006. [Google Scholar]
- 3.Henkel RD, Miller T, Weyant RS. Monitoring select agent theft, loss and release reports in the United States—2004–2010. Appl Biosafety. 2012;17:171–180. doi: 10.1177/153567601201700402. [DOI] [Google Scholar]
- 4.Mattar ZR, Abugiazya A, Torok N, Assaly R. Laboratory-acquired coccodioidmycosis. Chest. 2009;136:28S. doi: 10.1378/chest.136.4_MeetingAbstracts.28S-d. [DOI] [Google Scholar]
- 5.Kaye D. Tularemia, laboratory acquired. Clin Infect Dis. 2005;40:3–4. [Google Scholar]
- 6.Berkelman RL, Le Duc JW. Culture of responsibility. Science. 2014;345:1101. doi: 10.1126/science.1260424. [DOI] [PubMed] [Google Scholar]
- 7.Normile D. Infectious diseases. Mounting lab accidents raise SARS fears. Science. 2004;304:659–661. doi: 10.1126/science.304.5671.659. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser J. The catalyst. Science. 2014;345:1112–1115. doi: 10.1126/science.345.6201.1112. [DOI] [PubMed] [Google Scholar]
- 9.Wurtz N, Grobusch MP, Raoult D. Negative impact of laws regarding biosecurity and bioterrorism on real diseases. Clin Microbiol Infect. 2014;20:507–515. doi: 10.1111/1469-0691.12709. [DOI] [PubMed] [Google Scholar]
- 10.Günther S, Feldmann H, Geisbert TW, Hensley LE, Rollin PE, Nichol ST et al (2011) Management of accidental exposure to Ebola virus in the biosafety level 4 laboratory, Hamburg, Germany. J Infect Dis 204:S785–S790 [DOI] [PubMed]
- 11.Next steps to enhance biosafety and biosecurity in the United States. October 29, 2015. Available online at: https://www.whitehouse.gov/sites/default/files/docs/10-2015_biosafety_and_biosecurity_memo.pdf
- 12.Centers for Disease Control and Prevention (CDC) (2014) Report on the potential exposure to anthrax. Available online at: http://www.cdc.gov/about/pdf/lab-safety/Final_Anthrax_Report.pdf
- 13.Arrêté du 30 juin 1998 modifiant l’arrêté du 18 juillet 1994 modifié fixant la liste des agents biologiques pathogènes (1998) Available online at: http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000573056
- 14.Centers for Disease Control and Prevention (CDC) Fatal laboratory-acquired infection with an attenuated Yersinia pestis strain—Chicago, Illinois, 2009. MMWR Morb Mortal Wkly Rep. 2011;60:201–205. [PubMed] [Google Scholar]
- 15.Barry M, Russi M, Armstrong L, Geller D, Tesh R, Dembry L, et al. Brief report: treatment of a laboratory-acquired Sabiá virus infection. N Engl J Med. 1995;333:294–296. doi: 10.1056/NEJM199508033330505. [DOI] [PubMed] [Google Scholar]
- 16.Vasconcelos PF, Travassos da Rosa AP, Rodrigues SG, Tesh R, Travassos da Rosa JF, Travassos da Rosa ES. Laboratory-acquired human infection with SP H 114202 virus (Arenavirus: Arenaviridae family): clinical and laboratory aspects. Rev Inst Med Trop Sao Paulo. 1993;35:521–525. doi: 10.1590/s0036-46651993000600008. [DOI] [PubMed] [Google Scholar]
- 17.Formenty P, Hatz C, Le Guenno B, Stoll A, Rogenmoser P, Widmer A. Human infection due to Ebola virus, subtype Côte d’Ivoire: clinical and biologic presentation. J Infect Dis. 1999;179:S48–S53. doi: 10.1086/514285. [DOI] [PubMed] [Google Scholar]
- 18.Feldmann H. Are we any closer to combating Ebola infections? Lancet. 2010;375:1850–1852. doi: 10.1016/S0140-6736(10)60597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beer B, Kurth R, Bukreyev A. Characteristics of Filoviridae: Marburg and Ebola viruses. Naturwissenschaften. 1999;86:8–17. doi: 10.1007/s001140050562. [DOI] [PubMed] [Google Scholar]
- 20.Nikiforov VV, Turovskiĭ I, Kalinin PP, Akinfeeva LA, Katkova LR, Barmin VS, et al. A case of a laboratory infection with Marburg fever. Zh Mikrobiol Epidemiol Immunobiol. 1994;3:104–106. [PubMed] [Google Scholar]
- 21.Normile D. Infectious diseases. Second lab accident fuels fears about SARS. Science. 2004;303:26. doi: 10.1126/science.303.5654.26. [DOI] [PubMed] [Google Scholar]
- 22.Orellana C. Laboratory-acquired SARS raises worries on biosafety. Lancet Infect Dis. 2004;4:64. doi: 10.1016/S1473-3099(04)00911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim PL, Kurup A, Gopalakrishna G, Chan KP, Wong CW, Ng LC, et al. Laboratory-acquired severe acute respiratory syndrome. N Engl J Med. 2004;350:1740–1745. doi: 10.1056/NEJMoa032565. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Suspected cutaneous anthrax in a laboratory worker—Texas, 2002. JAMA. 2002;287:2356–2358. [PubMed] [Google Scholar]
- 25.Traxler RM, Lehman MW, Bosserman EA, Guerra MA, Smith TL. A literature review of laboratory-acquired brucellosis. J Clin Microbiol. 2013;51:3055–3062. doi: 10.1128/JCM.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) Laboratory-acquired human glanders—Maryland, May 2000. MMWR Morb Mortal Wkly Rep. 2000;49:532–535. [PubMed] [Google Scholar]
- 27.Srinivasan A, Kraus CN, DeShazer D, Becker PM, Dick JD, Spacek L, et al. Glanders in a military research microbiologist. N Engl J Med. 2001;345:256–258. doi: 10.1056/NEJM200107263450404. [DOI] [PubMed] [Google Scholar]
- 28.Schlech WF, 3rd, Turchik JB, Westlake RE, Jr, Klein GC, Band JD, Weaver RE. Laboratory-acquired infection with Pseudomonas pseudomallei (melioidosis) N Engl J Med. 1981;305:1133–1135. doi: 10.1056/NEJM198111053051907. [DOI] [PubMed] [Google Scholar]
- 29.Janovská S, Pávková I, Reichelová M, Hubáleka M, Stulík J, Macela A. Proteomic analysis of antibody response in a case of laboratory-acquired infection with Francisella tularensis subsp. tularensis. Folia Microbiol (Praha) 2007;52:194–198. doi: 10.1007/BF02932159. [DOI] [PubMed] [Google Scholar]
- 30.Barry MA (2005) Report of pneumonic tularemia in three Boston University researchers. Boston Public Health Commission
- 31.Lam ST, Sammons-Jackson W, Sherwood J, Ressner R. Laboratory-acquired tularemia successfully treated with ciprofloxacin: a case report. Infect Dis Clin Pract. 2012;20:204–207. doi: 10.1097/IPC.0b013e318234c383. [DOI] [Google Scholar]
- 32.Kawamata J, Yamanouchi T, Dohmae K, Miyamoto H, Takahaski M, Yamanishi K, et al. Control of laboratory acquired hemorrhagic fever with renal syndrome (HFRS) in Japan. Lab Anim Sci. 1987;37:431–436. [PubMed] [Google Scholar]
- 33.Nielsen CF, Sethi V, Petroll AE, Kazmierczak J, Erickson BR, Nichol ST, et al. Seoul virus infection in a Wisconsin patient with recent travel to China, March 2009: first documented case in the Midwestern United States. Am J Trop Med Hyg. 2010;83:1266–1268. doi: 10.4269/ajtmh.2010.10-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulders MN, Reimerink JH, Koopmans MP, van Loon AM, van der Avoort HG. Genetic analysis of wild-type poliovirus importation into The Netherlands (1979–1995) J Infect Dis. 1997;176:617–624. doi: 10.1086/514081. [DOI] [PubMed] [Google Scholar]
- 35.Arrêté du 30 avril 2012 fixant la liste des micro-organismes et toxines prévue à l’article L. 5139-1 du code de la santé publique (2012) Available online at: http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000025837146&dateTexte=&categorieLien=id
- 36.Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 37.Klietmann WF, Ruoff KL. Bioterrorism: implications for the clinical microbiologist. Clin Microbiol Rev. 2001;14:364–381. doi: 10.1128/CMR.14.2.364-381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharjee Y. Anthrax investigation. Army missed warning signs about alleged anthrax mailer. Science. 2011;332:27. doi: 10.1126/science.332.6025.27. [DOI] [PubMed] [Google Scholar]
- 39.Independent review of the safety of UK facilities handling foot-and-mouth disease virus (2007) Available online at: http://webarchive.nationalarchives.gov.uk/20130822084033/http://www.defra.gov.uk/footandmouth/investigations/pdf/spratt_final.pdf
- 40.Trevan T. Biological research: rethink biosafety. Nature. 2015;527:155–158. doi: 10.1038/527155a. [DOI] [PubMed] [Google Scholar]


