Abstract
Rheumatoid arthritis (RA), a chronic inflammatory disease affecting primarily the joints, is frequently characterized by the presence of autoimmune anticitrullinated protein antibodies (ACPA) during preclinical stages of disease and accumulation of hypercitrullinated proteins in arthritic joints. A strong association has been reported between RA and periodontal disease, and Porphyromonas gingivalis, a known driver of periodontitis, has been proposed as the microbial link underlying this association. We recently demonstrated P. gingivalis–mediated gut barrier breakdown and exacerbation of joint inflammation during inflammatory arthritis. In the present study, we investigated another potential role for P. gingivalis in RA etiopathogenesis, based on the generation of ACPA through the activity of a unique P. gingivalis peptidylarginine deiminase (PPAD) produced by this bacterium, which is capable of protein citrullination. Using a novel P. gingivalis W50 PPAD mutant strain, incapable of protein citrullination, and serum from disease-modifying antirheumatic drug–naïve early arthritis patients, we assessed whether autocitrullinated proteins in the P. gingivalis proteome serve as cross-activation targets in the initiation of ACPA production. We found no evidence for patient antibody activity specific to autocitrullinated P. gingivalis proteins. Moreover, deletion of PPAD did not prevent P. gingivalis–mediated intestinal barrier breakdown and exacerbation of disease during inflammatory arthritis in a murine model. Together, these findings suggest that the enzymatic activity of PPAD is not a major virulence mechanism during early stages of inflammatory arthritis.
Keywords: P. gingivalis peptidylarginine deiminase, autoimmune responses, chronic inflammation, periodontitis, microbiota, immunocrossreactivity
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects the joints and is driven by an autoimmune response. Left untreated, RA leads to progressive deterioration of the synovial lining, resulting in joint erosion, debilitating pain, and, ultimately, permanent disability. The presence of autoantibodies in the serum is characteristic for the majority of patients with RA. The most prominent among these are rheumatoid factor and IgG anticitrullinated protein antibodies (ACPA), which are used as diagnostic biomarkers and for stratification (Rantapaa-Dahlqvist et al. 2003), and are associated with poor outcomes (Kapetanovic et al. 2006). Accumulation of hypercitrullinated proteins in RA joints has been observed (Romero et al. 2013). ACPA, which target a range of citrullinated autoantigens (Sakkas et al. 2017), can be detected during the very early stages of RA (Kudo-Tanaka et al. 2007), suggesting a role in the initiation phase of the disease. The cause of RA, however, remains undefined (Maeda and Takeda 2017).
Increasing evidence is emerging for a strong association between RA and periodontal disease (Maeda and Takeda 2017). Porphyromonas gingivalis, based on its detection at periodontitis-affected sites in humans (Condorelli et al. 1998) and observed pathogenicity in animal models of periodontitis (Hajishengallis et al. 2011), is sometimes referred to as a keystone pathogen in the etiopathogenesis of periodontitis. To date, however, this keystone role has been established only in preclinical models of disease where it is able to induce oral dysbiosis, manipulate host immune responses, and drive local inflammation (Hajishengallis et al. 2011).
Another proposed etiopathogenic mechanism in RA, corroborated by the associations between RA and periodontal disease, is the mounting of a host immune response against pathogenic microbes, including P. gingivalis. Interestingly, P. gingivalis possesses a unique prokaryotic citrullinating enzyme, P. gingivalis peptidylarginine deiminase (PPAD; McGraw et al. 1999). Unlike human peptidylarginine deiminases, which preferentially citrullinate internal arginine residues on target proteins, citrullination by PPAD occurs exclusively on carboxy-terminal arginine residues, which are generated through protein cleavage by the microbe’s arginine gingipains (Goulas et al. 2015; Montgomery et al. 2016). PPAD activity has not yet been formally demonstrated at sites of periodontitis. However, as P. gingivalis has been isolated from such sites (Condorelli et al. 1998) and because PPAD is expressed mainly on the surface of this bacterium (McGraw et al. 1999; Quirke et al. 2014), PPAD activity at sites of periodontal inflammation can be inferred. Thus, assuming PPAD activity in gingiva, the discrepancy in citrullination target sites between human peptidylarginine deiminase and PPAD may enhance the antigenicity of microbial autocitrullinated proteins (Sakkas et al. 2017). In the susceptible host, as originally hypothesized by Rosenstein and colleagues (2004), this initially antimicrobial response could give rise to cross-reactive antibodies able to target citrullinated host proteins (Masson-Bessiere et al. 2001).
The putative role of autocitrullinated P. gingivalis proteins in the etiopathogenesis of RA remains of interest. Herein we report that deleting PPAD from the P. gingivalis W50 genome ablated its ability to citrullinate protein-bound arginine residues. Using sera from disease-modifying antirheumatic drug (DMARD)–naïve early RA patients, we found that the autocitrullinated proteome of P. gingivalis W50 was not specifically targeted by ACPA in RA patients. Deletion of PPAD did not reverse the ability of P. gingivalis to promote intestinal barrier disruption and exacerbation of joint disease in a model of inflammatory arthritis.
Our findings indicate that although PPAD is capable of citrullinating the endogenous P. gingivalis proteome, these citrullinated proteins do not represent major targets for autoimmune responses in early RA patients and suggest that the role for PPAD activity in driving pathology in inflammatory arthritis is limited.
Materials and Methods
The Appendix Materials and Methods describe the methods, materials, reagents, and sources for the following: RA patient and healthy control sera; bacterial strains used and growth conditions; generation of P. gingivalis mutant strain PG1424; PPAD activity measurement by colorimetric assay and thin layer chromatography (TLC); SDS-PAGE and immunoblotting, including antimodified citrulline method; preabsorbed serum ACPA titration ELISA; induction of inflammatory arthritis by K/BxN serum transfer and inoculation with bacteria; 16S rRNA gene quantitative polymerase chain reaction (16S qPCR); and statistical analysis. Animal experiments conform to the ARRIVE guidelines (see the Appendix and ARRIVE checklist).
Results
Deletion of PPAD from P. gingivalis W50 Ablates Its Ability to Citrullinate Protein-Bound Arginine
As conflicting results have been reported pertaining to the role of autocitrullinated P. gingivalis proteins in RA, we addressed this question by generating a PPAD-mutant P. gingivalis strain lacking citrullination activity (PG1424) by homologous replacement of the ppad-encoding region PG1424 with an ermF-ermAM cassette encoding a clindamycin resistance gene (see Appendix Methods and Appendix Fig. 1 for details).
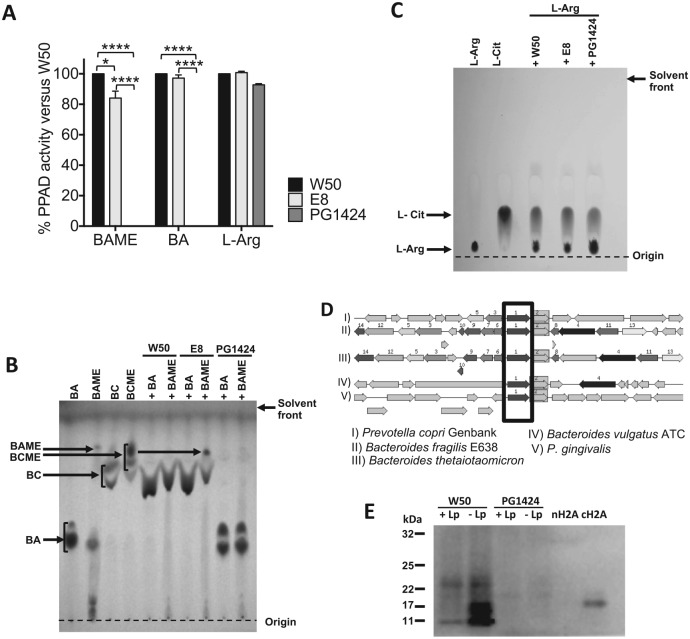
To assess the effect of PPAD deletion from P. gingivalis W50, we first compared the citrullination activity of P. gingivalis wild type (W50), a gingipain protease (rgpA, rgpB)–deficient mutant (E8; Aduse-Opoku 2006), and the PPAD-deficient mutant (PG1424). Bacterial cells (1 × 108 CFU [colony-forming units]) were incubated with 5mM N-α-benzoyl-L-arginine methyl ester (BAME), N-α-benzoyl-L-arginine (BA), or free L-arginine under anaerobic conditions at 37 ºC overnight, followed by removal of the bacterial cells. Using a colorimetric assay to measure L-citrulline levels in the supernatants, we observed complete ablation of BAME and BA citrullination when the substrate was incubated with PG1424 as compared with incubations with W50 (Fig. 1A). We also measured a 18% reduction in BAME citrullination by E8 but no significant reduction in BA citrullination, suggesting a limited role for arg-gingipain-mediated cleavage of L-arginine methyl ester substrates in facilitating citrullination.
Figure 1.
Porphyromonas gingivalis peptidylarginine deiminase (PPAD) activity can be detected in the wild-type but not the PPAD-mutant P. gingivalis strain. (A) Percentage of PPAD activity in P. gingivalis E8 (rgpA-rgpB mutant) and PG1424 (ppad mutant) in comparison with P. gingivalis W50 (wild-type strain) measured by colorimetric assay. Results are expressed as mean ± SEM. *P < 0.05. ****P < 0.0001. One-way analysis of variance, followed by Tukey’s multiple- comparisons test on the raw data. BA, N-α-benzoyl-L-arginine; BAME, N-α-benzoyl-L-arginine methyl ester; BC, N-α-benzoyl L-citrulline; BCME, N-α-benzoyl L-citrulline methyl ester. (B) BC and BCME produced by P. gingivalis strains W50, E8, and PG1424 after incubation with BA or BAME were measured by thin layer chromatography. (C) L-citrulline production from L-arginine by P. gingivalis strains W50, E8, and PG1424 was assessed by thin layer chromatography. (D) Sequence comparisons of the P. gingivalis genome with arginine deiminases (in box) of other bacterial species to identify orthologous protein sequences. (E) Comparison of autocitrullination in P. gingivalis W50 and PG1424. Immunoblotting of whole cell lysates from P. gingivalis W50 and PG1424 prepared with immediate addition of leupeptin (+Lp) or delayed addition (–Lp; after 10 min of lysis) was carried out via the antimodified citrulline method, including native histone H2A (nH2A; negative control) and citrullinated histone H2A (cH2A; positive control).
Next, P. gingivalis strains W50, E8, or PG1424 were incubated with both substrates as before; bacterial cells were removed by centrifugation; and supernatants were run on a TLC plate. Incubation of both substrates with P. gingivalis W50 or E8 yielded spots corresponding to N-α-benzoyl-L-citrulline (BC), and no BA or BAME was detected in these lanes (Fig. 1B), indicating complete conversion of the substrates. Conversely, lanes containing incubations of BA or BAME with PG1424 showed strong spots corresponding to the input substrate, but no citrullinated products were detected. Of note, incubation of E8 with BAME also produced detectable levels of N-α-benzoyl-L-citrulline methyl ester (BCME), suggesting that the absence of arginine gingipain activity may result in the further conversion of BAME to BCME.
Citrullination of free L-arginine, as assessed by colorimetric assay and TLC, was not affected by deletion of PPAD (Fig. 1A, C). Arginine deiminases (ADIs), guanidino group–modifying enzymes able to citrullinate free arginine, have been described in other bacterial taxa (Casiano-Colon and Marquis 1988; Díez et al. 2017; Cai et al. 2018). Genomic sequence comparisons with ADI proteins found in other bacterial species with the RAST database (http://rast.nmpdr.org/rast.cgi) identified orthologous areas in the P. gingivalis genome (see black box in Fig. 1D), suggesting the presence of an ADI gene in the P. gingivalis W50 genome, which could be responsible for the observed free L-arginine citrullination.
Next, using the P. gingivalis wild-type W50 and mutant PG1424, we investigated the role of PPAD in the autocitrullination of the P. gingivalis W50 proteome. Autocitrullinated P. gingivalis proteins have been proposed as triggers of autoimmune responses in RA (Wegner et al. 2010; Quirke et al. 2014). To compare citrullination of proteins in P. gingivalis W50 and PG1424, whole cell lysates were prepared in the presence of protease inhibitor leupeptin (2 mM, +Leu; Curtis et al. 2002), or leupeptin was added after 10 min of lysis (–Leu; see Appendix Methods). Citrullinated proteins were measured by immunoblot with an antimodified citrulline antibody (Shi et al. 2013). Citrullination was detected in lanes from P. gingivalis W50 colonies prepared in the presence of leupeptin, indicating the presence of citrullinated proteins in the original bacterial sample (Fig. 1E). However, we detected higher levels of citrullinated proteins if leupeptin was added to the lysis reactions after a 10-min delay, indicative of autocitrullination of C-terminal arginine residues generated by the action of arg-gingipains during the lysis period. No citrullinated proteins were detected in lanes containing any of the PG1424 lysates.
Taken together, these findings indicate that PPAD enzymatic activity is nonredundant for autocitrullination of the P. gingivalis W50 proteome as well as other substrates that have a C-terminal arginine. Moreover, P. gingivalis W50 PPAD retains its enzymatic activity after lysis of bacterial cells.
Autocitrullinated Proteins in the P. gingivalis W50 Proteome Are Not Targeted by ACPA in Serum from Early RA Patients
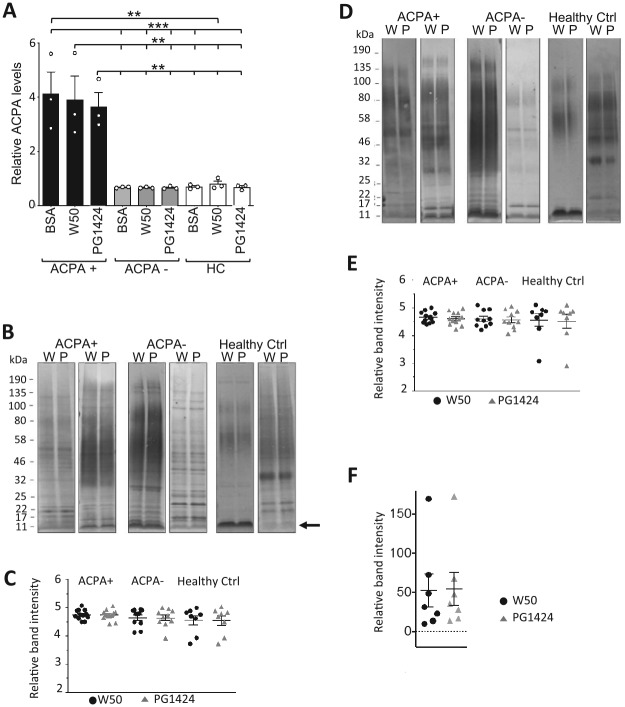
To assess potential systemic antibody responses in ACPA+ RA patients to autocitrullinated P. gingivalis proteins, we devised a preabsorbed serum ACPA titration ELISA to compare serum ACPA binding activity against the autocitrullinated and noncitrullinated P. gingivalis cellular proteome. Serum from DMARD-naïve ACPA+ and ACPA– early RA patients and from healthy controls was incubated in microtiter plate wells coated with total cell lysates collected from P. gingivalis W50 or PG1424 or bovine serum albumin (BSA)–coated control wells. Next, unbound ACPA in the preabsorbed sera were measured by anti-CCP ELISA. As expected, high levels of ACPA were detected for sera from ACPA+ RA patients that had been preabsorbed in BSA-coated control wells, while low amounts of ACPA were measured in BSA-preabsorbed sera from ACPA– RA patients and healthy controls (Fig. 2A). Of note, no differences in ACPA levels were detected between ACPA+ sera preabsorbed against lysates from W50 and PG1424 P. gingivalis strains, and levels were comparable to the BSA control, suggesting that no significant amounts of ACPA had bound to either W50 or PG1424 lysates.
Figure 2.
Human serum immunoreactivity against lysates from citrullination-competent and citrullination-incompetent Porphyromonas gingivalis does not differ. (A) Preabsorbed serum anticitrullinated protein antibodies (ACPA) titration was performed by preabsorbing sera from ACPA+, ACPA– rheumatoid arthritis (RA) patients, and healthy control (HC) sera on microtiter plates coated with whole cell lysates from P. gingivalis W50, PG1424, or 10% bovine serum albumin (BSA; negative control), followed by detection of remaining serum ACPA with anti-CCP ELISA. Results are expressed as mean ± SEM. **P ≤ 0.01. ***P ≤ 0.001. One-way analysis of variance, followed by Sidak’s post hoc test. (B–E) Assessment of human serum immunoreactivity against specific components in lysates from citrullinated-competent and citrullinated-deficient P. gingivalis. Representative immunoblots are shown of (B) whole cell lysates and (D) supernatants from P. gingivalis W50 (W) and PG1424 (P) probed with serum from ACPA+ and ACPA– RA patients and healthy controls and horseradish peroxidase–conjugated anti-human IgG antibody. Arrow in panel B indicates band used for analysis in panel F. (C, E) Corresponding densitometry analysis of band intensity on immunoblots. Results in scatter-dot plots are expressed as mean ± SEM and unpaired student’s t test comparing W50 and PG1424 in each serum group. (F) Densitometry analysis of 11-kDa band (arrow in B) intensity on immunoblots of W50 or PG1424 whole cell lysates incubated with ACPA+ RA patient serum. Results are presented as mean ± SEM and unpaired Student’s t test.
We next assessed whether differences in human serum IgG antibody reactivity were discernable when immunoreactivity was compared against proteins within the entire microbial proteome. P. gingivalis W50 and PG1424 was grown for 48 h in liquid culture, and whole cell lysates and supernatants were collected, followed by immunoblotting with the sera of ACPA+ and ACPA– RA patients and healthy controls. Visual comparison of individual bands in entire lanes detected some differences in the banding patterns among different serum donors for whole cell lysates and supernatants (Fig. 2B, D; 2 representative donors per group are shown); however, we did not observe differences in band patterns between P. gingivalis W50 and PG1424 whole cell lysates or supernatants when serum from the same donor was used (Fig. 2B, D). Of note, no associations were detected between banding patterns of P. gingivalis W50 and PG1424 with sera from ACPA+ or ACPA– RA patients or healthy control groups. Densitometric quantification of total band intensities in each lane indicated that there were no differences in the immunoreactivity of patient or healthy control sera against total autocitrullinated versus noncitrullinated proteomes in whole cell lysates or supernatants (Fig. 2C, E), in line with the results from the serum preabsorption assay (Fig. 2A). Given our observation that P. gingivalis proteins preferentially citrullinated by PPAD are approximately 11 kDa (Fig. 1E), we queried whether citrullinated proteins within this band, specifically, could be targets for ACPA from early ACPA+ RA patients. Densitometric analysis indicated that binding of patient serum antibodies to proteins within the 11-kDa band did not significantly differ between citrullinated P. gingivalis W50 and noncitrullinated PG1424 lysates (Fig. 2F).
Together, these data suggest that ACPA+ or ACPA– RA patients’ serum IgG antibodies do not specifically target autocitrullinated over noncitrullinated P. gingivalis proteins.
PPAD Deletion Does Not Prevent P. gingivalis–Induced Gut Barrier Breakdown and Exacerbation of Joint Inflammation during Inflammatory Arthritis
We recently reported on the pathogenic actions of P. gingivalis in exacerbating arthritic inflammation by breaking down the intestinal barrier and promoting the breach of gut microbes into host tissues (Flak et al. 2019). These effects were not observed if animals were inoculated with a commensal microbe, suggesting that this mechanism is specific to pathobionts such as P. gingivalis.
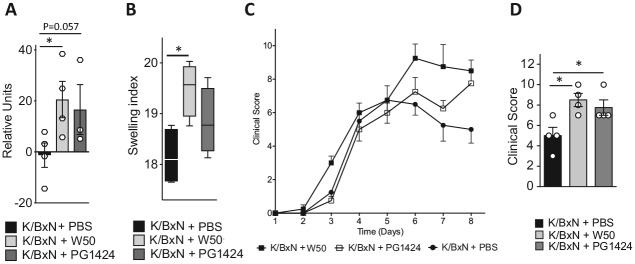
Hence, we next sought to evaluate whether PPAD contributes to the observed pathogenic actions, using our model of induced inflammatory arthritis. For this purpose, C57BL/6 mice were orally inoculated with 109 CFU of P. gingivalis W50 or PG1424 or gavaged with vehicle alone on days 0, 2, and 4. Twenty-four and 48 h after the first inoculation, mice were injected with arthritogenic K/BxN serum (50 µL, intraperitoneally) to initiate inflammatory arthritis. To assess the effects inflammatory arthritis via gut barrier function, inoculation was performed straight into the stomach of the animals. While previous studies demonstrated that inflammatory arthritis leads to periodontal inflammation (Montero-Melendez et al. 2014), we did not observe differences in the macroscopic signs of periodontal inflammation among the groups (data not shown). 16S qPCR to measure bacterial DNA in gut-draining mesenteric lymph nodes, as a readout for microbial breach of the gut barrier (Flak et al. 2019), demonstrated that PG1424 inoculation increased levels of bacterial DNA in mesenteric lymph nodes, albeit to a marginally lesser extent than the wild-type strain (Fig. 3A). Next, we assessed clinical markers of joint inflammation in terms of redness and edema formation in ankles, paws, and small joints. Here, inoculation with both P. gingivalis strains exacerbated edema formation, as determined by the swelling index representing cumulative paw and ankle width (Fig. 3B), and increased joint inflammation by day 8 after the initial inoculation when compared with vehicle-gavaged arthritic mice (Fig. 3C, D). Together, these results suggest that PPAD activity is not the virulence factor/mechanism underlying P. gingivalis–mediated gut barrier breakdown and disease exacerbation in inflammatory arthritis.
Figure 3.
Gut barrier breakdown and exacerbation of joint inflammation during inflammatory arthritis are not prevented by peptidylarginine deiminase deletion. Mice were orally inoculated with Porphyromonas gingivalis W50 or PG1424 (109 colony-forming units per mouse) or given vehicle (phosphate-buffered saline [PBS]) on days –1, 1, and 3 and injected with K/BxN serum (50 μL/mouse, intraperitoneal, days 0 and 2). Mesenteric lymph nodes were collected on day 8. (A) 16S rRNA gene levels were measured in mesenteric lymph nodes by 16S quantitative polymerase chain reaction to assess bacterial translocation across the intestinal barrier. Results are presented as mean ± SEM and representative of 3 or 4 mice per group. *P ≤ 0.05. Mann-Whitney test. (B) Swelling indices of edema formation on day 7 in paws and ankles of 4 mice per group. *P ≤ 0.05. Results are presented as median, interquartile range, and 95% CI. One-way analysis of variance, followed by Sidak’s post hoc test. (C) Daily clinical arthritis scores were recorded over time. Results are presented as mean ± SEM for 4 mice per time point per group. (D) Clinical arthritis score on day 8. Results are presented as mean ± SEM for 4 mice per group. *P ≤ 0.05. One-way analysis of variance, followed by Sidak’s post hoc test.
Discussion
In the present study, we demonstrate that autocitrullination of the P. gingivalis proteome is strictly dependent on the pathobiont’s PPAD enzymatic activity. Contrary to the frequently proposed hypothesis, our findings suggest that this autocitrullination does not generate epitopes that represent antigenic targets for ACPA autoantibodies in the serum of DMARD-naïve early RA patients.
Increased immunoreactivity of RA patient sera against P. gingivalis–derived proteins has been reported (Mikuls et al. 2009; Bender et al. 2017) and correlations with ACPA levels observed, albeit not unanimously (Okada et al. 2011; Bae and Lee 2018). Yet, the mechanistic relevance of autocitrullinated P. gingivalis proteins in autoimmune responses in RA and their contribution to the onset of the disease remain unclear (Konig, Bingham, and Andrade 2015; Olsen et al. 2018).
Autocitrullinated PPAD has recently been investigated for its potential role in triggering loss of self-tolerance and induction of cross-reactive ACPA in RA (Quirke et al. 2014). Interestingly, studies by several groups have reported conflicting results with respect to the ability of PPAD to autocitrullinate and the antigenicity of such autocitrullinated PPAD in RA (Quirke et al. 2014; Konig, Bingham, and Andrade 2015). Using a colorimetric assay for citrullination, TLC, and immunoblotting, we found that autocitrullination of the P. gingivalis proteome occurs only in the presence of a functioning PPAD and was not detected on the proteome of a PPAD-deleted mutant P. gingivalis PG1424.
Our findings also indicate clear antibody reactivity against P. gingivalis W50 in serum from DMARD-naïve early RA patients and healthy individuals. Yet, despite the inability of mutant strain PG1424 to autocitrullinate its proteins, no differences were found in the pattern of reactivity of DMARD-naïve RA patient serum against lysates from wild-type P. gingivalis W50 or the PPAD-deleted mutant PG1424, regardless of patients’ ACPA status. This suggests that while patient serum antibodies are likely to target proteins of the pathobiont that are autocitrullinated, the autocitrullinated residues themselves do not serve as epitopes that are specifically targeted by ACPA. Although severity of arthritic disease is reportedly linked to the presence of periodontitis in susceptible individuals and, subsequently, RA symptoms may decrease following successful treatment of periodontal disease (Bingham and Moni 2013; Chou et al. 2015), our findings indicate that the effect of periodontitis on joint disease is not dependent on generation of epitopes within the autocitrullinated P. gingivalis proteome. However, while our data support the notion that this autocitrullinome does not act as a trigger of initial ACPA production as occurs during early stages of RA, we cannot rule out that autocitrullinated P. gingivalis proteins may contribute to autoimmune responses during later stages of disease (Kudo-Tanaka et al. 2007). Moreover, host-derived inflammatory cues, as may arise during periodontitis that is driven by P. gingivalis or other oral pathobionts but also nonmicrobial factors (Curtis et al. 2011), may induce posttranslational protein modifications, including citrullination and carbamylation (Bright et al. 2018), and thus could be a mechanism linking periodontitis and RA.
It is also possible that the absence of detectable RA patient antibody binding specifically to citrullinated epitopes within the autocitrullinome of P. gingivalis may be due to the levels of ACPA targeting these bacterial epitopes being extremely low and below the detection levels of our assays. This would imply that PPAD autocitrullination is not a trigger of strong (auto)immune responses, at least not during the early stages of RA. Moreover, epitopes generated by PPAD citrullination of P. gingivalis proteins may be structurally different to other types of citrullinated residues that are bound by ACPA or that the modified epitope may not be accessible to the antibody.
It is also possible that, rather than constituting an epitope for (auto)antibodies, citrullination and thus PPAD activity may instead contribute to the triggering of RA through other mechanisms, for example, by uncovering cryptic noncitrullinated epitopes by altering the secondary or tertiary structures of proteins (Koziel et al. 2014)—thus not through molecular mimicry but through what perhaps might be considered biofunctional mimicry. Alternatively, it has recently been reported that P. gingivalis PPAD may influence biofilm formation (Vermilyea et al. 2019) and gene expression in keratinocytes, although it is unclear whether citrullination is required for the latter (Aliko et al. 2018). Our recent discovery that impaired gut barrier function in the arthritic host facilitates pathogenic behavior of P. gingivalis, including intestinal barrier breakdown, promotion of gut microbial invasion, and exacerbation of joint inflammation, provides insights into the mechanisms underlying the connection between RA and periodontitis/P. gingivalis. Two previous studies with another model of inflammatory arthritis reported reduced exacerbation of joint inflammation after inoculation with PPAD-deleted P. gingivalis (Maresz et al. 2013; Gully et al. 2014). However, the first study, due to colonization resistance of the mouse strain used, required exposure of the immune system to P. gingivalis inside a subcutaneously implanted chamber, not via mucosal surfaces (Maresz et al. 2013). The second study involved antibiotic pretreatment prior to P. gingivalis inoculation, and induction of inflammatory arthritis took place after completion of a long inoculation period over 4 wk (Gully et al. 2014). Results from the present study, where inoculation occurred in the gastrointestinal tract without antibiotic pretreatment and disease was initiated shortly after, suggest that the pathogenic actions of P. gingivalis on the gut barrier and joint inflammation are essentially independent of PPAD activity. This is in line with our previous studies demonstrating that P. gingivalis disrupts the gut barrier via downregulating epithelial barrier junctional proteins and those by Katz et al. (2002) demonstrating that in the oral mucosa this pathobiont can directly breakdown epithelial barrier proteins. Although there may be an initial delay, by day 8 after arthritis initiation, the PPAD mutant, like the wild-type strain, promotes microbial gut barrier breach and significantly exacerbates clinical symptoms of joint inflammation
To conclude, our findings support the hypothesis (Konig, Paracha, et al. 2015) that the autoctrullinated P. gingivalis proteome does not contain strongly immunogenic epitopes targeted by serum ACPA during the early stages of RA and that PPAD activity is not required for the gut barrier breakdown underlying P. gingivalis–driven inflammatory arthritis.
Author Contributions
E. Muñoz-Atienza, M.B. Flak, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; J. Sirr, contributed to data acquisition and analysis, critically revised the manuscript; N.A. Paramonov, J. Aduse-Opoku, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; C. Pitzalis, M.A. Curtis, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519898144 for The P. gingivalis Autocitrullinome Is Not a Target for ACPA in Early Rheumatoid Arthritis by E. Muñoz-Atienza, M.B. Flak, J. Sirr, N.A. Paramonov, J. Aduse-Opoku, C. Pitzalis and M.A. Curtis in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This work was supported by funding from Versus Arthritis (formerly Arthritis Research UK) to C.P. and M.A.C. (grant 21134) and to C.P. (grant 20022), from the Medical Research Council to M.A.C. (grants MR/P012175/1 and MR/P012175/2), and a Marie Skłodowska-Curie Individual Fellowship under the European Union’s Horizon 2020 research and innovation program (grant 746183) to M.B.F.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: E. Muñoz-Atienza  https://orcid.org/0000-0001-6218-1511
https://orcid.org/0000-0001-6218-1511
M.B. Flak  https://orcid.org/0000-0002-8238-9835
https://orcid.org/0000-0002-8238-9835
References
- Aduse-Opoku J, Slaney JM, Hashim A, Gallagher A, Gallagher RP, Rangarajan M, Boutaga K, Laine ML, Van Winkelhoff AJ, Curtis MA. 2006. Identification and characterization of the capsular polysaccharide (K-antigen) locus of Porphyromonas gingivalis. Infect Immun. 74(1):449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliko A, Kaminska M, Bergum B, Gawron K, Benedyk M, Lamont RJ, Malicki S, Delaleu N, Potempa J, Mydel P. 2018. Impact of Porphyromonas gingivalis peptidylarginine deiminase on bacterial biofilm formation, epithelial cell invasion, and epithelial cell transcriptional landscape. Sci Rep. 8(1):14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SC, Lee YH. 2018. Association between anti–Porphyromonas gingivalis antibody, anti-citrullinated protein antibodies, and rheumatoid arthritis: a meta-analysis. Z Rheumatol. 77(6):522–532. [DOI] [PubMed] [Google Scholar]
- Bender P, Burgin WB, Sculean A, Eick S. 2017. Serum antibody levels against Porphyromonas gingivalis in patients with and without rheumatoid arthritis—a systematic review and meta-analysis. Clin Oral Investig. 21(1):33–42. [DOI] [PubMed] [Google Scholar]
- Bingham CO, 3rd, Moni M. 2013. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol. 25(3):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Thiele GM, Manavis J, Mikuls TR, Payne JB, Bartold PM. 2018. Gingival tissue, an extrasynovial source of malondialdehyde-acetaldehyde adducts, citrullinated and carbamylated proteins. J Periodontal Res. 53(1):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Jiang H, Zhang T, Jiang B, Mu W, Miao M. 2018. Thermostability and specific-activity enhancement of an arginine deiminase from Enterococcus faecalis SK23.001 via semirational design for l-citrulline production. J Agric Food Chem. 66(33):8841–8850. [DOI] [PubMed] [Google Scholar]
- Casiano-Colon A, Marquis RE. 1988. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol. 54(6):1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Lai KL, Chen DY, Lin CH, Chen HH. 2015. Rheumatoid arthritis risk associated with periodontitis exposure: a nationwide, population-based cohort study. PLoS One. 10(10):e0139693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli F, Scalia G, Cali G, Rossetti B, Nicoletti G, Lo Bue AM. 1998. Isolation of Porphyromonas gingivalis and detection of immunoglobulin a specific to fimbrial antigen in gingival crevicular fluid. J Clin Microbiol. 36(8):2322–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Aduse Opoku J, Rangarajan M, Gallagher A, Sterne JA, Reid CR, Evans HE, Samuelsson B. 2002. Attenuation of the virulence of Porphyromonas gingivalis by using a specific synthetic Kgp protease inhibitor. Infect Immun. 70(12):6968–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Zenobia C, Darveau RP. 2011. The relationship of the oral microbiotia to periodontal health and disease. Cell Host Microbe. 10(4):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez L, Solopova A, Fernández-Pérez R, González M, Tenorio C, Kuipers OP, Ruiz-Larrea F. 2017. Transcriptome analysis shows activation of the arginine deiminase pathway in Lactococcus lactis as a response to ethanol stress. Int J Food Microbiol. 257:41–48. [DOI] [PubMed] [Google Scholar]
- Flak MB, Colas RA, Muñoz-Atienza E, Curtis MA, Dalli J, Pitzalis C. 2019. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI insight. 4(13):125191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas T, Mizgalska D, Garcia-Ferrer I, Kantyka T, Guevara T, Szmigielski B, Sroka A, Millán C, Usón I, Veillard F, et al. 2015. Structure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci Rep. 5:11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gully N, Bright R, Marino V, Marchant C, Cantley M, Haynes D, Butler C, Dashper S, Reynolds E, Bartold M. 2014. Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLoS One. 9(6):e100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, et al. 2011. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 10(5):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic MC, Saxne T, Sjoholm A, Truedsson L, Jonsson G, Geborek P. 2006. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology. 45(1):106–111. [DOI] [PubMed] [Google Scholar]
- Katz J, Yang QB, Zhang P, Potempa J, Travis J, Michalek SM, Balkovetz DF. 2002. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect Immun. 70(5):2512–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig MF, Bingham CO, 3rd, Andrade F. 2015. PPAD is not targeted as a citrullinated protein in rheumatoid arthritis, but remains a candidate for inducing autoimmunity. Ann Rheum Dis. 74(1):e8. [DOI] [PubMed] [Google Scholar]
- Konig MF, Paracha AS, Moni M, Bingham CO, 3rd, Andrade F. 2015. Defining the role of Porphyromonas gingivalis peptidylarginine deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Ann Rheum Dis. 74(11):2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel J, Mydel P, Potempa J. 2014. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. 16(3):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo-Tanaka E, Ohshima S, Ishii M, Mima T, Matsushita M, Azuma N, Harada Y, Katada Y, Ikeue H, Umeshita-Sasai M, et al. 2007. Autoantibodies to cyclic citrullinated peptide 2 (CCP2) are superior to other potential diagnostic biomarkers for predicting rheumatoid arthritis in early undifferentiated arthritis. Clin Rheumatol. 26(10):1627–1633. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Takeda K. 2017. Role of gut microbiota in rheumatoid arthritis. J Clin Med. 6(6):E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz KJ, Hellvard A, Sroka A, Adamowicz K, Bielecka E, Koziel J, Gawron K, Mizgalska D, Marcinska KA, Benedyk M, et al. 2013. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 9(9):e1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, Serre G. 2001. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 166(6):4177–4184. [DOI] [PubMed] [Google Scholar]
- McGraw WT, Potempa J, Farley D, Travis J. 1999. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun. 67(7):3248–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, Holers VM, Kuhn KA, O’Dell JR. 2009. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 9(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Melendez T, Madeira MFM, Norling LV, Alsam A, Curtis MA, da Silva TA, Perretti M. 2014. Association between periodontal disease and inflammatory arthritis reveals modulatory functions by melanocortin receptor type 3. Am J Pathol. 184(8):2333–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AB, Kopec J, Shrestha L, Thezenas ML, Burgess-Brown NA, Fischer R, Yue WW, Venables PJ. 2016. Crystal structure of Porphyromonas gingivalis peptidylarginine deiminase: implications for autoimmunity in rheumatoid arthritis. Ann Rheum Dis. 75(6):1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Kobayashi T, Ito S, Yokoyama T, Komatsu Y, Abe A, Murasawa A, Yoshie H. 2011. Antibody responses to periodontopathic bacteria in relation to rheumatoid arthritis in Japanese adults. J Periodontol. 82(10):1433–1441. [DOI] [PubMed] [Google Scholar]
- Olsen I, Singhrao SK, Potempa J. 2018. Citrullination as a plausible link to periodontitis, rheumatoid arthritis, atherosclerosis and Alzheimer’s disease. J Oral Microbiol. 10(1):1487742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirke AM, Lugli EB, Wegner N, Hamilton BC, Charles P, Chowdhury M, Ytterberg AJ, Zubarev RA, Potempa J, Culshaw S, et al. 2014. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis. 73(1):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. 2003. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48(10):2741–2749. [DOI] [PubMed] [Google Scholar]
- Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM, van Eyk J, Rosen A, Andrade F. 2013. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. 5(209):209ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. 2004. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 28(6):311–318. [DOI] [PubMed] [Google Scholar]
- Sakkas LI, Daoussis D, Liossis SN, Bogdanos DP. 2017. The infectious basis of ACPA-positive rheumatoid arthritis. Front Microbiol. 8:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Willemze A, Janssen GM, van Veelen PA, Drijfhout JW, Cerami A, Huizinga TW, Trouw LA, Toes RE. 2013. Recognition of citrullinated and carbamylated proteins by human antibodies: specificity, cross-reactivity and the “AMC-Senshu” method. Ann Rheum Dis. 72(1):148–150. [DOI] [PubMed] [Google Scholar]
- Vermilyea DM, Ottenberg GK, Davey ME. 2019. Citrullination mediated by PPAD constrains biofilm formation in P. gingivalis strain 381. NPJ Biofilms Microbiomes. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, Kinloch A, Culshaw S, Potempa J, Venables PJ. 2010. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 62(9):2662–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519898144 for The P. gingivalis Autocitrullinome Is Not a Target for ACPA in Early Rheumatoid Arthritis by E. Muñoz-Atienza, M.B. Flak, J. Sirr, N.A. Paramonov, J. Aduse-Opoku, C. Pitzalis and M.A. Curtis in Journal of Dental Research