Abstract
In this mini-review, we summarize the photochemical approaches for developing high-throughput carbohydrate microarray technologies. Newly established methods for photo-immobilizing unmodified monosaccharides, oligosaccharides and polysaccharides onto photoactive surfaces and coupling of photoactive carbohydrates onto polymer surfaces are reviewed.
Keywords: Photochemistry, Bioarrays, Surface chemistry
Sugar chains in living organisms are structurally diverse and characteristically suitable for storing and presenting bio-signals for specific molecular recognition [1–3]. In many physiological and pathophysiological conditions, expression of cellular glycans, in the form of either glycoproteins or glycolipids, is differentially regulated. Characteristic patterns of complex carbohydrates are frequently associated with the stages or steps of embryonic development and cell differentiation, as well as transformation of normal cells to abnormally differentiated tumor or cancer cells [3–7]. Sugar moieties are also abundantly expressed on the outer surfaces of the majority of viral, bacterial, protozoan and fungal pathogens. Many sugar structures are pathogen-specific, which makes them important molecular targets for pathogen recognition, diagnosis of infectious diseases, and vaccine development [8–11].
Developing microarray-based high-throughput technologies for structural and immunological characterization of carbohydrates has been one of the focused efforts in the areas of post genomics research and technology development [1, 11–19]. In the past few years, relatively nascent carbohydrate microarray technologies began to show their potential in biomedical applications. This is highlighted by rapid identification and characterization of immunogenic sugar moieties of SARS-CoV [20], HIV-1 [14] and anthrax spores [21], as well as fine specificity studies of carbohydrate–protein interactions of biomedical importance [22, 14, 16].
Recently, photochemical methods have shown great potential in the field of carbohydrate microarrays as they have produced some of the earliest examples of covalently bound microarrays that do not require chemical modification of the carbohydrates. Photochemical methods are an important emerging route to patterning substrates for various materials or biomaterials applications as photochemical surface modification can be spatially controlled using a photomask [23, 24]. Such photolithographic processes allow micro- and nanometer sized shapes to be patterned on a surface directly without the requirement of mechanical processes such as stamping [25]. Photochemical immobilization and patterning of biological molecules on a surface is well-known [26], although few reports have focused on carbohydrates. Aside from being amenable to standard patterning techniques, photochemistry also provides a clean method of surface derivatization in that many reactions require only photons as a reagent. In addition, the absorption of photons allows for chemicals that are normally not reactive to form new carbon–carbon bonds.
Photo-immobilization of carbohydrates onto aromatic ketones
Surfaces functionalized with aromatic ketones have been used to photoimmobilize a variety of biological molecules. Ketones are well-known chromophores that can participate in hydrogen abstraction reactions [27]. When an appropriate ketone is irradiated with UV light, the excited n − π* state of the ketone intersystem crosses to the triplet state, from which it can abstract a hydrogen atom from a nearby donor to create radicals as shown in Fig. 1. Note that other processes can compete with hydrogen abstraction including phosphorescence, internal conversion and other photochemical reactions including electron transfer, addition to a double bond, α-cleavage and β-cleavage. The photogenerated radical can recombine to form various products. When the ketone is bound to a surface, recombination of a given radical with the ketone will covalently bind the molecule to the surface. Although direct proof of covalent bond formation is difficult to prove unequivocally due to the low number of molecules on the surface and limited number of surface spectroscopic techniques for characterizing the formation of carbon–carbon bonds, substrates composed of aromatic ketones have been shown to stabilize a variety of biological and synthetic polymers [28–30].
Fig. 1.
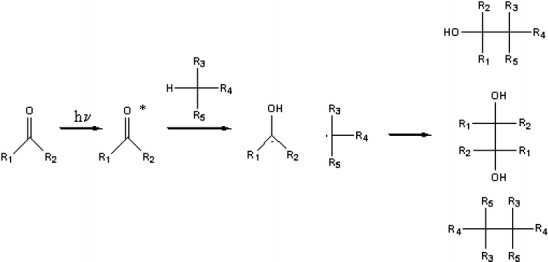
Upon absorption of a photon, ketones can abstract a hydrogen atom from an appropriate donor, forming radicals that can recombine to form carbon–carbon bonds
Recently, photoactive surfaces that incorporate surface-bound phthalimide chromophores have been applied to fabricate carbohydrate microarrays. Phthalimide derivatives are a class of aromatic ketones that are known to undergo all the major photochemical reactions of ketones [31]. Their salts can be easily incorporated into halogenated molecules and the commercial availability of halogenated silanes allows for a one-step synthesis of an appropriate heterobifunctional molecule for self-assembly onto an appropriate substrate as shown in Fig. 2a. Self-assembled phthalimide monolayers have been prepared on glass, Si and quartz substrates [32]. Irradiation of spin-coated films of polysaccharides, disaccharides and monosaccharides on the phthalimide monolayer results in a stable binding of the carbohydrates presumably by a covalent bond formed by hydrogen abstraction followed by radical recombination.
Fig. 2.
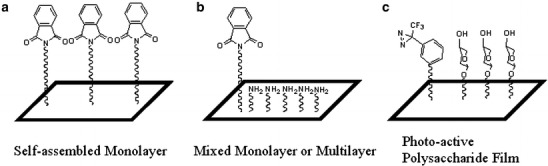
Photoactive surfaces allow for adsorbed carbohydrates to bind to the surface. a A phthalimide self-assembled monolayer can bind underivatized carbohydrates to the surface most likely by hydrogen abstraction followed by radical recombination. b A phthalimide–amine mixed surface enhances carbohydrate adsorption to the surface when a robotic spotter is used. Subsequent irradiation binds the sugars to the surface. c An aziridine derivatized polysaccharide film can bind spotted carbohydrates to the surface after irradiation by forming a carbene that reacts with adsorbed sugars. Carbohydrates immobilized on surfaces (b and c) have been shown to recognize appropriate antibodies and lectins
Microarrays can be constructed by using a spotter to deliver carbohydrates to the photoactive surface followed by UV illumination. The propensity for physisorption and subsequently the yield of the photo-immobilization reaction can be increased substantially by modifying the surface energy of the substrate such that the capillary interactions between the spotter containing aqueous carbohydrate solution and the photoactive surface favor wetting of the solution. Incorporation of aminopropyltrimethoxy silane into a phthalimide-derivatized surface (Fig. 2b) provides a more favorable substrate for spotting carbohydrates and results in a stable photo-immobilization of the carbohydrates onto the chip after irradiation. Photo-immobilized α1 6 dextran polysaccharides were shown to retain their antigenic reactivity toward an anti-dextran monoclonal antibody (16. 4. 12E), which is specific for the terminal non-reducing ends of the polysaccharide [33]. However, spotted mono- and oligosaccharide arrays showed differential activities with the lectin Concanavalin A (Con A). This lectin is Man- and/or Glc-specific and requires the C-3, C-4, and C-5 hydroxyl groups of the Man or Glc ring for binding. Photo-coupled oligosaccharides with three (IM3), five (IM5), and seven glucoses (IM7) are reactive to Con A on the photoactive surface but not on a nitrocellulose-coated slide. By contrast, none of the spotted monosaccharides were reactive to the lectin on these surfaces.
Given that this method of photocoupling can target any CH– group on the sugar rings with varying specificity depending on the structure of the ring [34, 35], there is a possibility that the site of covalent attachment may interfere significantly with lectin binding to the monosaccharides Man and Glc. The limited specificity of the reaction and the lesser amount of saccharide epitopes present for smaller carbohydrates reduces the probability that a biologically active epitope presents itself at the air–monolayer interface. It is interesting to note that another method that allows for underivatized carbohydrates to be covalently immobilized via a chemical reaction with a hydrazide monolayer similarly was unable to properly display some monosaccharides for lectin recognition [36]. For example, Con A was not able to recognize mannose, glucose and N-acetylglucosamine. The lack of biological activity was attributed to an improper β-configuration at the anomeric position.
Although the degree of selectivity of the surface photo-immobilization reaction is unknown, studies of hydrogen abstraction towards carbohydrates in solution have been performed and provide insight into what may occur on the surface. Care should be exercised in applying such information to surface reactions however, since surface effects such as the orientation of reactants can affect reactions. Using model compounds, such as tetrahydrofuran (THF), it has been shown that hydrogen abstraction reactions occur preferentially at the anomeric center [37]. The selectivity of the reaction is due to stabilization of the resulting radical by the electron donating oxygen atom. When actual carbohydrates are used, the degree of selectivity has in some cases been found to be dependent on substituents. Time resolved EPR experiments have shown that the photo-excited triplet of acetone attacks hydrogens in the C1–C4 positions preferentially on glucose, galactose, xylose and maltose [34]. The reaction of hydroxyl radicals with carbohydrates has also been studied using EPR [35]. Although hydroxyl radicals may show different reactivity than photogenerated triplets in a given system, they do provide insight into radical reactivity towards the various carbohydrate C–H groups. For example, hydrogen abstraction from carbons in the pyranose ring and linked by glycosidic bonds in dextran polysaccharides was found to be inhibited. Additionally, abstraction from galacturonan and d-galacturonic acid were found to occur dominantly on the carbon atom adjacent to the carboxyl group. Based on these studies the excited phthalimide is expected to attack the accessible C–H groups of an adsorbed carbohydrate indiscriminately, if the only other substituents are OH groups. Addition of other substituents may favor selectivity. The type of reaction occurring may also change when electron-donating heteroatoms are introduced into the carbohydrate. For example, amine-containing glycans may undergo an electron transfer reaction with a phthalimide. Note that phthalimide electron transfer reactions have been shown to render regioselective product formation for some systems [38]. The affect of the surface on reaction products and selectivity is currently unknown. Additionally, the aminopropyl silanes mixed into the surface may also affect the reaction. For example, a competing primary process may be electron abstraction from an amine. In any case, the phthalimide–amine derivatized surface was found to immobilize carbohydrates on the surface after irradiation with UV light and the larger immobilized carbohydrates retained their biorecognition properties.
The phthalimide-derivatized surface has been used to characterize the immunogenic sugar moieties of Bacillus anthracis [21]. A tetrasaccharide composed of a trisaccharide of rhamnopyranosyl units attached to a terminal residue given the name anthrose is O-linked to the glycoprotein BclA expressed on the surface of anthrax spores [39]. The tetrasaccharide and components of the tetrasaccharide were synthesized and photoimmobilized on the phthalimide–amine-coated substrates. The surface-bound tetrasaccharide was found to be specifically reactive with antibodies elicited by anthrax spore immunization indicating that the tetrasaccharide forms an antigenic determinant on the glycoprotein. Inhibition studies showed that an anthrose monosaccharide inhibits reactivity of the antibody towards the tetrasaccharide and other rhamnopyranosyl oligosaccharides containing anthrose, showing that the anthrose residue contributes significantly to the antigenic determinant of the tetrasaccharide. This study illustrates the feasibility of using photogenerated microarrays for studying the immunogenic properties of carbohydrates.
Photogeneration of carbenes and nitrenes
Other carbohydrate microarrays have been prepared by photogenerating carbenes and nitrenes. Carbenes and nitrenes can be generated from diazo compounds and azides by photo-elimination of N2 as shown in Fig. 3 [27]. The kinds of reactions these intermediates undergo will depend on whether the reaction is initiated from the singlet or triplet state, however both can result in carbon–carbon bond formation. Singlets, which contain more zwitterionic character, can undergo 1,2 sigmatropic shifts, stereospecific insertion into sigma bonds, stereospecific insertion into pi bonds and addition of a nucleophile or electrophile. Triplets contain more diradicaloid character and can undergo atom abstraction reactions, nonstereospecific addition to pi bonds and addition of radicals. Often compounds incorporating caged carbenes and nitrenes contain fluorine substituents which help prevent autoreactivity. An added benefit in terms of surface reactions is that C–F substituents are low energy functional groups that tend to migrate to the solid–air interface in order to decrease interfacial tension, inadvertently making the photoactive group more accessible to an adsorbate [40]. In general this is more of a potential problem for photoactive polymer films rather than more constrained end-functionalized self-assembled monolayers.
Fig. 3.
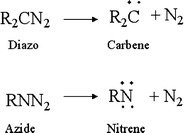
Irradiation of diazo compounds and azides results in the loss of N2 and formation of carbene and nitrene intermediates
Sigrist and colleagues [41] developed aziridine derivatized polysaccharide films for photoimmobilizing underivatized carbohydrates as shown in Fig. 2c [41]. Upon absorption of a photon, the aziridine group loses N2 to form a highly reactive carbene that can react with the spotted sugars to form a covalent bond. Bacterial exopolysaccharides were photoimmobilized and positively stained by appropriate lectins. Similarly, glycoproteins, neoglycoproteins and cell extracts were immobilized. Oligosaccharide immobilization required conjugation to a protein prior to deposition. The authors point out that the hydrophilic polysaccharide surface resists non-specific protein binding. In addition the polymer film may provide a larger amount of surface area for adsorption of greater amounts of carbohydrates relative to the often-utilized “two-dimensional” self-assembled monolayer.
The above methods allow for underivatized carbohydrates or carbohydrates lacking an appropriate functional group to be immobilized on a chip. However, the photocoupling sites on the carbohydrates are not specifically defined. For polysaccharides this is less of a concern since they can contain many epitopes. As a sugar decreases in size, the probability of inactivating the functional part of the carbohydrate increases. Statistically one may expect to get a mixture of inactivated and activated sugars in a single spot. For very small sugars that are susceptible to secondary processes that degrade the sugar ring all activity may be annihilated. As has been described above, the photoactive surfaces have been shown to be functional for many carbohydrates, suggesting that at least some of the photoimmobilized carbohydrates within a given microspot retain their activity. Regardless of the percentage of biologically active carbohydrates, a discernable fluorescence signal can be detected after staining with antibodies.
Photoactive carbohydrates
By chemically derivatizing a sugar with photoactive groups, carbohydrates can be site-specifically photo-immobilized. For example, diazirine derivatized mono- and disaccharides (Fig. 4) have been photochemically immobilized on diamond [42] and poly(styrene) [43]. Similarly, mono- and disaccharides derivatized with perfluorophenylazide (PFPA) have been photo-immobilized on PEO films that were photo-immobilized on PFPA monolayers [44]. PFPA loses N2 upon irradiation to produce a nitrene, which is isoelectronic with carbenes and undergoes similar reactions. Mono- and disaccharides immobilized in this fashion are able to distinguish between different lectins. Although this approach allows a variety of monosaccharides to be immobilized that have discernable signals after staining with lectins, one of the disadvantages of this approach is the need for derivatizing the carbohydrates prior to immobilization. One could imagine combining this approach with one of the above by incorporating photoactive molecules into a polymer. In this way monosaccharides derivatized with photoactive groups could be spotted along with underivatized sugars, resulting in the photogeneration of a large array of active carbohydrates using only photons as reagents. In principle a photomask could be used in conjunction with a spotter to break an individual spot into many very small spots.
Fig. 4.
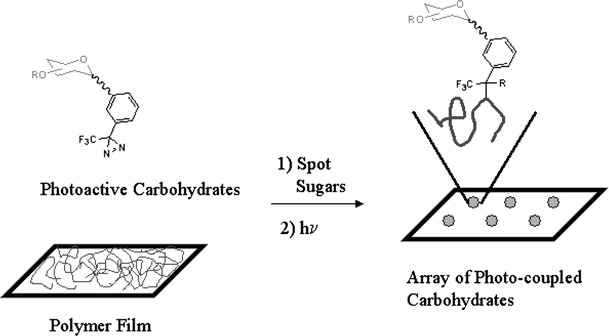
Adsorption of photoactive monosaccharides onto polymer films followed by irradiation produces a stable linkage to the surface
In summary, photochemical methods for immobilizing carbohydrate microarrays on a surface allow for a clean and stable binding that can be patterned using either a photomask or robotic spotter. Methods that have been demonstrated include the spotting and immobilization of underivatized sugars and the use of derivatized, photoactive carbohydrates for polymer-coated substrates. The latter has the advantage of site-selective immobilization, but has the disadvantage in that each carbohydrate in the array must be chemically modified prior to deposition. Regardless of the method used to construct the arrays, it is critical to validate whether the biological reactivities of the carbohydrates are preserved on the array substrates.
Acknowledgements
This material is based upon work supported by, or in part by, the U.S. Army Research Laboratory and the U.S. Army Research Office under contract/grant number DA W911NF-04-1-0282 and in part by the National Science Foundation under grant numbers DMR-02-14263, IGERT-02-21589, and CHE-04-15516 to N.J. Turro and J.T. Koberstein at Columbia University and supported in part by the Phil N. Allen Trust and NIH grant AI064104 to D. Wang at Stanford University. G.T. Carroll acknowledges an IGERT fellowship. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
References
- 1.Wang R., Trummer B.J., Gluzman E., Deng C., Wang D. Probing the antigenic diversity of sugar chains. ACS Symposium Series. 2004;873:39–52. doi: 10.1021/bk-2004-0873.ch004. [DOI] [Google Scholar]
- 2.Wang D., Kabat E.A. Carbohydrate antigens (polysaccharides) In: van Regenmortal M.H.V., editor. Structure of Antigens, vol. 3. Boca Raton: CRC Press; 1996. pp. 247–276. [Google Scholar]
- 3.Crocker P.R., Feizi T. Carbohydrate recognition systems: functional triads in cell–cell interactions. Curr. Opin. Struck. Biol. 1996;6:679–691. doi: 10.1016/S0959-440X(96)80036-4. [DOI] [PubMed] [Google Scholar]
- 4.Feizi T. The antigens Ii, SSEA-1 and ABH are in interrelated system of carbohydrate differentiation antigens expressed on glycosphingolipids and glycoproteins. Adv. Exp. Med. Biol. 1982;152:167–177. [PubMed] [Google Scholar]
- 5.Feizi T. Progress in deciphering the information content of the 'glycome'—a crescendo in the closing years of the millennium. Glycoconj. J. 2000;17:553–565. doi: 10.1023/A:1011022509500. [DOI] [PubMed] [Google Scholar]
- 6.Focarelli R., La Sala G.B., Balasini M., Rosati F. Carbohydrate-mediated sperm–egg interaction and species specificity: a clue from the Unio elongatulus model. Cells Tissues Organs. 2001;168:76–81. doi: 10.1159/000016808. [DOI] [PubMed] [Google Scholar]
- 7.Hakomori S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer Res. 1985;45:2405–2414. [PubMed] [Google Scholar]
- 8.Dochez A.R., Avery O.T. The elaboration of specific soluble substance by pneumococcus during growth. J. Exp. Med. 1917;26:477–493. doi: 10.1084/jem.26.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidelberger M., Avery O.T. The soluble specific substance of pneumococcus. J. Exp. Med. 1923;38:73–80. doi: 10.1084/jem.38.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins J.B., Schneerson R. Polysaccharide–protein conjugates: a new generation of vaccines. J. Infect. Dis. 1990;161:821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 11.Mond J.J., Lees A., Snapper C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 12.Willats W.G., Rasmussen S.E., Kristensen T., Mikkelsen J.D., Knox J.P. Sugar-coated microarrays: A novel slide surface for the high-throughput analysis of glycans. Proteomics. 2002;2:1666–1671. doi: 10.1002/1615-9861(200212)2:12<1666::AID-PROT1666>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Wang D. Carbohydrate microarrays. Proteomics. 2003;3:2167–2175. doi: 10.1002/pmic.200300601. [DOI] [PubMed] [Google Scholar]
- 14.Adams E.W., Ratner D.M., Bokesch H.R., McMahon J.B., O’Keefe B.R., Seeberger P.H. Oligosaccharide and glycoprotein microarrays as tools in HIV glycobiology glycan-dependent gp120/protein interactions. Chem. Biol. 2004;11:875–881. doi: 10.1016/j.chembiol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Fazio F., Bryan M.C., Blixt O., Paulson J.C., Wong C.-H. Synthesis of sugar arrays in microtiter plate. J. Am. Chem. Soc. 2002;124:14397–14402. doi: 10.1021/ja020887u. [DOI] [PubMed] [Google Scholar]
- 16.Fukui S., Feizi T., Galustian C., Lawson Alexander M., Chai W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate–protein interactions. Nat. Biotechnol. 2002;20:1011–1017. doi: 10.1038/nbt735. [DOI] [PubMed] [Google Scholar]
- 17.Houseman B.T., Mrksich M. Carbohydrate arrays for the evaluation of protein binding and enzymatic modification. Chem. Biol. 2002;9:443–454. doi: 10.1016/S1074-5521(02)00124-2. [DOI] [PubMed] [Google Scholar]
- 18.Park S., Shin I. Fabrication of carbohydrate chips for studying protein–carbohydrate interactions. Angew. Chem. Int. Ed. 2002;41:3180–3182. doi: 10.1002/1521-3773(20020902)41:17<3180::AID-ANIE3180>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Wang D., Liu S., Trummer B.J., Deng C., Wang A. Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat. Biotechnol. 2002;20:275–281. doi: 10.1038/nbt0302-275. [DOI] [PubMed] [Google Scholar]
- 20.Wang D., Lu J. Glycan arrays lead to the discovery of autoimmunogenic activity of SARS-CoV. Physiol. Genomics. 2004;18:245–248. doi: 10.1152/physiolgenomics.00102.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Carroll G.T., Turro N.J., Koberstein J.T., Kovac P., Saksena R., Adamo R., Herzenberg L.A., Herzenberg L.A., Steinman L. Photogenerated glycan arrays identify immunogenic sugar moieties of Bacillus anthracis exosporium. Proteomics. 2007;7:180–184. doi: 10.1002/pmic.200600478. [DOI] [PubMed] [Google Scholar]
- 22.Stevens J., Blixt O., Glaser L., Taubenberger J.K., Palese P., Paulson J.C., Wilson I.A. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J. Mol. Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Fodor S.P.A., Read J.L., Pirrung M.C., Stryer L., Lu A.T., Solas D. Light-directed, spatially addressable parallel chemical synthesis. Science. 1991;251:767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 24.Lee K., Pan F., Carroll G.T., Turro N.J., Koberstein J.T. Photolithographic technique for direct photochemical modification and chemical micropatterning of surfaces. Langmuir. 2004;20:1812–1818. doi: 10.1021/la0358163. [DOI] [Google Scholar]
- 25.Wallraff G.M., Hinsberg W.D. Lithographic imaging techniques for the formation of nanoscopic features. Chem. Rev. 1999;99:1801–1821. doi: 10.1021/cr980003i. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y. Photoimmobilization for microarrays. Biotechnol. Prog. 2006;22:924–932. doi: 10.1021/bp060143a. [DOI] [PubMed] [Google Scholar]
- 27.Turro N.J. Modern Molecular Photochemistry. Sausalito, CA: University Science Books; 1991. [Google Scholar]
- 28.Delamarche E., Sundarababu G., Biebuyck H., Michel B., Gerber C., Sigrist H., Wolf H., Ringsdorf H., Xanthopoulos N., Mathieu H.J. Immobilization of antibodies on a photoactive self-assembled monolayer on gold. Langmuir. 1996;12:1997–2006. doi: 10.1021/la950836t. [DOI] [Google Scholar]
- 29.Rozsnyai L.F., Fodor S.P.A., Schultz P.G., Benson D.R. Photolithographic immobilization of biopolymers on solid supports. Angew. Chem. 1992;104:801–802. doi: 10.1002/ange.19921040638. [DOI] [Google Scholar]
- 30.Jeyaprakash J.D., Samuel S., Ruehe J. A facile photochemical surface modification technique for the generation of microstructured fluorinated surfaces. Langmuir. 2004;20:10080–10085. doi: 10.1021/la049428s. [DOI] [PubMed] [Google Scholar]
- 31.Kanaoka Y. Photoreactions of cyclic imides. Examples of synthetic organic photochemistry. Acc. Chem. Res. 1978;11:407–413. doi: 10.1021/ar50131a002. [DOI] [Google Scholar]
- 32.Carroll G.T., Wang D., Turro N.J., Koberstein J.T. Photochemical micropatterning of carbohydrates on a surface. Langmuir. 2006;22:2899–2905. doi: 10.1021/la0531042. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda T., Kabat E.A. Variable region cDNA sequences and antigen binding specificity of mouse monoclonal antibodies to isomaltosyl oligosaccharides coupled to proteins. T-dependent analogues of alpha(1,6)dextran. J. Immunol. 1989;142:863–870. [PubMed] [Google Scholar]
- 34.Shkrob I.A., Depew M.C., Wan J.K.S. Time-resolved, electron-spin resonance study of radical species derived from naturally occurring carbohydrates. Chem. Phys. Lett. 1993;202:133–140. doi: 10.1016/0009-2614(93)85362-R. [DOI] [Google Scholar]
- 35.Gilbert B.C., King D.M., Thomas C.B. The oxidation of some polysaccharides by the hydroxyl radical: an e.s.r. investigation. Carbohydr. Res. 1984;125:217–235. doi: 10.1016/0008-6215(84)85158-7. [DOI] [PubMed] [Google Scholar]
- 36.Lee M.R., Shin I. Facile preparation of carbohydrate microarrays by site-specific, covalent immobilization of unmodified carbohydrates on hydrazide-coated glass slides. Org. Lett. 2005;7:4269–4272. doi: 10.1021/ol051753z. [DOI] [PubMed] [Google Scholar]
- 37.Binkley E.R., Binkley R.W. Carbohydrate Photochemistry. Washington, D.C.: American Chemical Society; 1998. [Google Scholar]
- 38.Yoon U.C., Mariano P.S. The synthetic potential of phthalimide SET photochemistry. Acc. Chem. Res. 2001;34:523–533. doi: 10.1021/ar010004o. [DOI] [PubMed] [Google Scholar]
- 39.Daubenspeck J.M., Zeng H., Chen P., Dong S., Steichen C.T., Krishna N.R., Pritchard D.G., Turnbough C.L., Jr. Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J. Biol. Chem. 2004;279:30945–30953. doi: 10.1074/jbc.M401613200. [DOI] [PubMed] [Google Scholar]
- 40.Koberstein J.T. Molecular design of functional polymer surfaces. J. Polym. Sci. B. 2004;42:2942–2956. doi: 10.1002/polb.20157. [DOI] [Google Scholar]
- 41.Angeloni S., Ridet J.L., Kusy N., Gao H., Crevoisier F., Guinchard S., Kochhar S., Sigrist H., Sprenger N. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology. 2005;15:31–41. doi: 10.1093/glycob/cwh143. [DOI] [PubMed] [Google Scholar]
- 42.Chevolot Y., Bucher O., Leonard D., Mathieu H.J., Sigrist H. Synthesis and characterization of a photoactivatable glycoaryldiazirine for surface glycoengineering. Bioconjug. Chem. 1999;10:169–175. doi: 10.1021/bc980050h. [DOI] [PubMed] [Google Scholar]
- 43.Chevolot Y., Martins J., Milosevic N., Leonard D., Zeng S., Malissard M., Berger E.G., Maier P., Mathieu H.J., Crout D.H., Sigrist H. Immobilisation on polystyrene of diazirine derivatives of mono- and disaccharides: biological activities of modified surfaces. Bioorg. Med. Chem. 2001;9:2943–2953. doi: 10.1016/S0968-0896(01)00172-9. [DOI] [PubMed] [Google Scholar]
- 44.Pei Z., Yu H., Theurer M., Walden A., Nilsson P., Yan M., Ramstroem O. Photogenerated carbohydrate microarrays. ChemBioChem. 2007;8:166–168. doi: 10.1002/cbic.200600447. [DOI] [PMC free article] [PubMed] [Google Scholar]


