Abstract
Three isolectins from cultivated Eucheuma denticulatum were isolated. They were commonly monomeric proteins of about 28 kDa with a range of averaged molecular weights from 27,834 to 27,868 Da among the isolectins and shared almost the same 20 N-terminal amino acid sequences. Complementary DNA (cDNA) cloning based on the rapid amplification cDNA ends (RACE) methods elucidated the full-length sequence of EDA-2 which encodes 269 amino acids, including initiating methionine, with four tandemly repeated domains of about 67 amino acids. The primary structure of EDA-2 is highly similar to those of the high-mannose N-glycan specific lectins including Oscillatoria agardhii (OAA) and Burkholderia oklahomensis EO147 (BOA) from cyanobacteria, Myxococcus xanthus (MBHA) and Pseudomonas fluorescens Pf0-1 (PFL) from bacteria, and ESA-2 from a macro red alga. The hemagglutination activities were commonly inhibited by the glycoproteins bearing high-mannose N-glycans, but not by monosaccharides examined, including mannose. In a direct binding experiment with pyridylaminated oligosaccharides, an isolectin EDA-2 exclusively bound to high-mannose type N-glycans, but not to other glycans that include complex types and a core pentasaccharide of N-glycans, indicating that it recognized the branched oligomannoside moiety. Its binding activity was subtly different among the oligomannoside structures examined, showing that the lectin has preference affinity for high-mannose type N-glycans with an exposed (α1-3) mannose residue in the D2 arm. Interestingly, EDAs, the mixture of three isolectins inhibited the growth of shrimp pathogenic bacterium, Vibrio alginolyticus, although it did not affect the growth of V. parahaemolyticus and V. harveyi. Growth inhibition of V. alginolyticus with EDAs was not observed in the presence of yeast mannan bearing high-mannose N-glycans, suggesting that EDAs caused the activity through binding to the target receptor(s) on the surface of V. alginolyticus. These results indicate that cultivated carrageenophyte E. denticulatum is a good source of a lectin(s) that may be useful as a carbohydrate probe and an antibacterial reagent.
Keywords: cDNA cloning, Eucheuma denticulatum, Rhodophyta, Lectin, Carbohydrate-binding specificity, High-mannose type N-glycan, Antibacterial activity, Marine vibrios, Primary structure
Introduction
Lectins, or carbohydrate-binding proteins, are present in various organisms from viruses to mammals and serve as recognition molecules between cells, cell and matrix, and organisms. Owing to the capability of discriminating carbohydrate structures, not only are lectins used as valuable biochemical reagents in many research fields, including glycomics, but they are promising candidates for medicinal and clinical application (Sharon and Lis 2003). Indeed, antiviral activities have been reported for several lectins (Botos and Wlodawer 2005). Antihuman immunodeficiency virus (HIV) and/or anti-influenza virus lectins from bacteria, algae, fungi, and land plants have the common property of binding to high-mannose N-glycans, thereby blocking the entry of viruses into host cells through binding to the mannoside structures in the viral envelope glycoproteins, which are critical for the primary infection of viruses (Balzarini 2006). The anti-HIV lectins from cyanobacteria (blue-green algae) and eukaryotic macroalgae are presently marked as a new antiviral agent because their HIV-inhibiting activities are extremely strong compared to those of lectins from other biological groups (Ziólkowska and Wlodawer 2006; Balzarini 2007). Practically, a potent anti-HIV lectin, griffithsin, from the red alga Griffithsia sp., has been investigated in detail with this objective (Mori et al. 2005), as well as some anti-HIV cyanobacterial lectins (Ziólkowska and Wlodawer 2006; Sato et al. 2007). Recently, the high-mannose N-glycan-specific lectins from the red alga Kappaphycus alvarezii (KAA-2) (Sato et al. 2011a) and the green alga Boodlea coacta (BCA) (Sato et al. 2011b) showed strong anti-HIV and anti-influenza virus activities in a similar manner. Although these anti-HIV lectins share both binding specificity for high-mannose N-glycans and repeated domain structures, they differ from each another in amino acid sequences and recognizing branched mannoside structures, which may lead to subtle differences in the degree of inhibiting activities. Some of high-mannose binding lectins also inhibit the infection of other enveloped viruses such as Ebola virus (Barrientos et al. 2003), hepatitis C virus (HCV) (Helle et al. 2006), and severe acute respiratory syndrome coronavirus (SARS-CoV) (O’Keefe et al. 2010). Thus, high-mannose binding lectins may become a useful interesting target for application. Furthermore, antibacterial activities have been reported for lectins from various biological sources (Slifkin and Doyle 1990). However, little is known about the effects of marine algal lectins towards marine vibrios, except the species-specific activities against vibrios have been reported for the lectins from the red algae, Eucheuma serra and Galaxaura marginata (Liao et al. 2003). Marine vibrios are halophilic Gram-negative proteobacteria, which occupy a diverse range of ecological niches including sediments, water column, and in association with organisms either as symbionts or pathogens (Tracy et al. 2007). They cause an economically important disease of fish, marine invertebrates, and are responsible for high mortality rates in aquaculture worldwide (Marhual et al. 2010). Among the vibrios, Vibrio alginolyticus and V. parahaemolyticus are quite important, since they cause serious episodes in marine fish and shellfish including shrimp (Zorrilla et al. 2003; Marhual et al. 2010). In Vietnam, V. alginolyticus, V. parahaemolyticus, and V. harveyi have greatly reduced yields of farmed shrimps (FAO 2013).
The algae Kappaphycus alvarezii, K. striatum, and Eucheuma denticulatum are economically important species and extensively cultivated for edible purposes or as a source of carrageenophyte for industry. Those algal species have been introduced in more than 20 countries for mariculture purposes (Ask and Azanza 2002). In Vietnam, E. denticulatum has been cultivated since 2005 from algal seeds that were transported from Bohol, Philippines. Although the high-mannose N-glycan-specific lectins from carrageenophytes have been reported, including ESA-2 from Eucheuma serra (Hori et al. 2007), KAA-2 from K. alvarezii (Sato et al. 2011a), and KSA-2 from K. striatum (Hung et al. 2011), little is known about lectin from E. denticulatum, which can provide valuable information regarding lectins from this cultivated carrageenophytes, whose biological roles still remain elusive to a large extent. In a screening of hemagglutinins in Vietnamese marine algae, strong hemagglutination activity was detected in the extract of cultivated E. denticulatum (Hung et al. 2009a). The objective of this research was to evaluate the primary structure, the carbohydrate-binding specificity, and the biological properties including the antibacterial activities against the shrimp pathogenic vibrios of the lectin from cultivated E. denticulatum for future applications.
Materials and methods
The red alga Eucheuma denticulatum (Burman) Collins et Harvey was collected in a farm at Vanphong Bay, Khanhhoa Province, Vietnam, brought to the laboratory, and kept at −20 °C until use. A small portion of the alga was stored at −20 °C in RNAlater (Invitrogen) until used for the RNA extraction. Prepacked columns were purchased; Superdex R-75 HR 10/30 from GE Healthcare (England), TSKgel DEAE-5PW, TSKgel NH2-60, and TSKgel ODS80TM from Tosoh Corporation (Japan), and YMC PROTEIN-RP from YMC (Japan). A GP SENSOR kit for detection of carbohydrate was purchased from Seikagaku Corporation (Japan). Monosaccharides and yeast mannan were from Nakarai Chemical (Japan). Fucoidan, transferrin, fetuin, asialo-fetuin, porcine thyroglobulin, bovine submaxillary mucin, and porcine stomach mucin were purchased from Sigma (USA). Bovine thyroglobulin was purchased from Wako (Japan). Asialo-derivatives of bovine thyroglobulin, porcine thyroglobulin, and bovine submaxillary mucin were prepared by hydrolysis of the parent sialoglycoprotein as described previously (Hung et al. 2011). Pyridylaminated (PA)-oligosaccharides used in this study were obtained from Takara (Japan), except PA-derivatives of mannotriose (Man(α1-6)[Man(α1-3)]Man) and mannopentaose (Man(α1-6)[Man(α1-3)]Man(α1-6)[Man(α1-3)]Man). The latter two oligomannoses were purchased from Funakoshi (Japan) and pyridylaminated as described previously (Hori et al. 2007). All other chemicals used in this study were of the highest purity available. Three species of shrimp pathogenic vibrios, Vibrio alginolyticus, Vibrio parahaemolyticus, and Vibrio harveyi were obtained from Institute of Aquaculture Research No. 3, Nhatrang, Vietnam.
Extraction and purification of lectins
Extraction and purification procedures for lectins from E. denticulatum were performed by the same method as described previously for the lectins from K. striatum (Hung et al. 2011). Briefly, a fresh sample (1 kg) of cultivated E. denticulatum was homogenized with equal volume (v/w) of 40 % aqueous EtOH (v/v) and kept at 4 °C for 18 h with occasional stirring. After filtration through a cheese cloth, the filtrate was centrifuged at 3500 × g for 20 min at 4 °C. The supernatant was collected and examined for hemagglutination activity. To the supernatant, cold absolute EtOH (−20 °C) was added to attain a final concentration of about 80 % as measured by an alcoholmeter and the mixture was kept at 4 °C overnight. The resulting precipitates were collected by centrifugation at 3500 × g for 30 min at 4 °C and thoroughly dialyzed against 50-mM phosphate buffer (pH 7.0) containing 0.15-M NaCl. The non-dialyzable fraction was applied to a Superdex R 75 HR column (10 × 300 mm) equilibrated with the above buffer. The column was eluted with the same buffer at a flow rate of 1.0 mL min−1. Active fractions were pooled, concentrated by ultrafiltration, and dialyzed against 20-mM Tris–HCl buffer (pH 8.0). The concentrate was subjected to ion-exchange chromatography on a TSKgel DEAE-5PW column (7.5 × 75 mm) equilibrated with 20-mM Tris–HCl buffer (pH 8.0). This elution was performed at a flow rate of 0.4 mL min−1, first with the same buffer for 3 min, then with a linear gradient between 0 and 0.16-M NaCl in the buffer for 22 min, and finally with 1-M NaCl in the buffer for 10 min. The eluate was monitored for absorbance at 280 nm and for hemagglutination activity with trypsin-treated rabbit erythrocytes. Active fractions were pooled and dialyzed against distilled H2O.
Determination of protein content
Protein contents were determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard. Absorbance at 280 nm was also used to estimate protein contents in chromatographic fractions.
Determination of molecular mass and detection of carbohydrate
The molecular masses of purified lectins were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electron spray ionization-mass spectrometry (ESI-MS) as described previously (Hung et al. 2011). Briefly, the samples for SDS-PAGE were denatured at 100 °C for 5 min with or without 2 % 2-mercaptoethanol and then electrophored using a 10 % gel (Laemmli 1970). After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250. Prior to determination of molecular mass by ESI-MS using a LCQ (Finnigan, USA), intact lectin was further purified by high-performance liquid chromatography (HPLC) on a YMC PROTEIN-RP column (6.0 × 250 mm) which had been equilibrated with 20 % acetonitrile in 0.1 % trifluoroacetic acid (TFA). After injection of the lectin sample, the column was washed with the starting solvent and then eluted with a linear gradient of acetonitrile from 20 to 70 % in 0.1 % TFA. The eluate was monitored for absorbance at 280 nm. The peak containing lectin was recovered and dried by a Speed Vac SC100 concentrator (Sarvant, USA). The purified sample was dissolved in 50 % acetonitrile in 0.1 % TFA and subjected to mass spectrometry. The parameters of ion source were as follows: Sheath(N2) gas, 70; aus (He) gas, 10; spray voltage, 4.5; capillary temperature, 220 °C; capillary voltage, 3 kV; tube lens offset, 10. Calibration was performed using ESA-2, a lectin protein that had been analyzed before in the same assay system (Hori et al. 2007) and stocked in the laboratory. LCQ Bioworks (Finnigan, USA) was used to analyze and deconvolute the raw mass spectrum. For detection of glycoproteins, the electrophored gel was blotted to a PVDF membrane and the membrane was subjected to staining for carbohydrate using a GP SENSOR kit according to the manufacturer’s instructions.
Analysis of N-terminal amino acid sequences
The N-terminal amino acid sequences of isolectins were determined by using a Procise 492 HT protein sequencing system (Applied Biosystems, USA). Before analyses, intact lectins were further purified by reversed-phase HPLC on a YMC PROTEIN-RP column as described before.
cDNA cloning of EDA
Total RNA was extracted from 2 g of RNAlater-treated alga using a Plant RNA Purification Reagent (Invitrogen, USA). Messenger RNA (mRNA) purification from the total RNA was performed using a NucleoTrap mRNA Mini kit (TaKaRa, Japan). Full-length complementary DNAs (cDNAs) were synthesized from 150 ng of mRNA using a GeneRacer kit (Invitrogen) according to the manufacturer’s instructions.
The first polymerase chain reaction (PCR) for rapid amplification of the cDNA 3′end (3′RACE) was performed with eight aliquots of a 10-μL reaction mixture containing 1 μL of a 10 × Blend Taq buffer (Toyobo, Japan), 2 pmol of each deoxynucleotide triphosphate (dNTP), 6 pmol of the GeneRacer_3′_Primer, 2 pmol of EDA_3′RACE_F1 primer, which was designed from the N-terminal amino acid sequence of EDAs (Table 1), 0.2 μL of a 10-fold diluted synthesized cDNA, and 0.25 units of Blend Taq DNA polymerase (Toyobo). The reactions for eight aliquots were performed with a T Gradient Thermocycler (Biometra, Germany) under the following conditions: denaturation at 94 °C for 5 min, followed by 30 cycles consisting of denaturation at 94 °C for 30 s, annealing at eight different temperatures of 50–64 °C (2 °C increments) for 30 s, and extension at 72 °C for 1 min, and the final extension step at 72 °C for 5 min. The PCR products in eight aliquots were pooled, diluted to 100-fold, and then used as a template for nested PCR. The nested PCR was performed with a 50-μL reaction mixture containing 5 μL of a 10 × Blend Taq buffer, 10 pmol of each dNTP, 2 pmol of GeneRacer_3′_Nested_Primer, 50 pmol of the degenerated primer EDA_3′RACE_d_F2 which was designed from the conserved sequence among the high-mannose specific lectin family including ESA-2 (Hori et al. 2007) and Oscillatoria agardhii (OAA) (Sato et al. 2007), 1 μL of the dilution of the first PCR products, and 1.25 units of Blend Taq DNA polymerase. The nested PCR reaction was carried out by the same method of the first PCR, except for an annealing temperature of 60 °C. Nested PCR products were subcloned into pGEM-T Easy vector (Promega, USA) and transformed into Escherichia coli DH5α competent cells. Plasmids from the transformants were purified with a HiYield Plasmid Mini kit (RBC Bioscience, Taiwan) according to the manufacturer’s instructions. DNA sequencing was performed by using BigDye Terminator Cycle Sequencing kit ver. 3.1 with an ABI 3130 × L genetic analyzer (Applied Biosystems).
Table 1.
Primer sequence used in the cDNA cloning of EDA-2
| Primer | Sequence (from 5′ to 3′) |
|---|---|
| GeneRacer_3′_Primera | GCTGTCAACGATACGCTACGTAACG |
| GeneRacer_3′_Nested_Primera | CGCTACGTAACGGCATGACAGTG |
| GeneRacer_5′_Primera | CGACTGGAGCACGAGGACACTGA |
| GeneRacer_5′_Nested_Primera | GGACACTGACATGGACTGAAGGAGTA |
| EDA_3′RACE_F1 | AGAACCAGTGGGGAGGATCT |
| EDA_3′RACE_d_F1 | AYCAITAYAAYGTIGARAAYCARTGGGGb |
| EDA_5′RACE_R1 | GCAATGTTCTTGGTAGCAGC |
| EDA_5′end_F | AGAAATTCAACACCACAACT |
| EDA_3′end_R | CTGCACAAAACGTAACAATATCTAT |
aThese primers were inferred from the GeneRacer kit (Invitrogen)
bKey to symbols of the degenerated nucleotides: I represents inosine, Y represents C and T, R represents A and G
The first PCR of 5′RACE was performed in the same way as 3′RACE as described above, except that GeneRacer_5′_Primer and the primer EDA_3′end_R designed from the 3′ terminal sequence of EDA cDNA obtained by 3′RACE (Table 1). The nested PCR was performed by the same method, except for using a 100-fold dilution of the first PCR products as a template, and the GeneRacer_5′_Nested_Primer and the primer EDA_5′RACE_R1, designated from the sequence of EDA cDNA obtained by 3′RACE, as the primer pair (Table 1). Subcloning and DNA sequencing were performed as described above.
To verify the sequence accuracy, full-length cDNA of EDA was further amplified by using a high-fidelity DNA polymerase KOD Plus Neo (Toyobo), a primer pair of EDA_3′end_R and EDA_5′end_F designated from the 5′ terminal sequence of EDA cDNA obtained by 5′RACE (Table 1), and a 10-fold diluted synthesized cDNAs of E. denticulatum as a template, according to the manufacturer’s instruction. Before subcloning, the PCR products were treated with a 10 × A-attachment mix (Toyobo) according to the manufacturer’s instructions. Subcloning and DNA sequencing were then performed as described above.
Homologous sequences were identified with the basic local alignment search tool program (BLAST). The amino acid sequence comparison was performed using the CLUSTALW 2.0 program (Larkin et al. 2007).
Hemagglutination activity and hemagglutination-inhibition test
Hemagglutination activity and hemagglutination-inhibition test were determined with a 2 % (v/v) suspension of trypsin-treated rabbit erythrocytes (Hori et al. 1986a). Hemagglutination-inhibition was macroscopically observed and inhibition activity was expressed as the lowest concentration (mM or μg mL−1) of sugar or glycoprotein, at which a complete inhibition of four hemagglutination units was achieved. The sugars and glycoproteins used were D-glucose, D-mannose, D-galactose, L-rhamnose, L-fucose, D-xylose, GlcNAc, N-acetyl-D-galactosamine, N-acetyl-D-mannosamine, N-acetylneuraminic acid, lactose, and fucoidan as sugars, transferrin, fetuin, yeast mannan, porcine and bovine thyroglobulins, asialo-porcine and asialo-bovine thyroglobulins, bovine submaxillary mucin, asialo-bovine submaxillary mucin, and porcine stomach mucin as glycoproteins.
Effects of temperature, pH, and metal ions on hemagglutination activity
The effects of temperature, pH, and metal ions on hemagglutination activity of isolated lectins were determined as described previously (Hori et al. 1986a).
Oligosaccharide-binding specificity by centrifugal ultrafiltration-HPLC assay
Of the isolectins purified from E. denticulatum, EDA-2 was selected to determine oligosaccharide-binding specificity using a centrifugal ultrafiltration-HPLC method (Hori et al. 2007). Briefly, 500-nM EDA-2 (90 μL) in 50-mM Tris–HCl (pH 7.0) and 300-nM PA-oligosaccharide (10 μL) were mixed and kept at room temperature for 1 h. The reaction mixture was then ultrafiltered (10,000 × g, 30 s) using a centrifugal ultrafiltration tube (Nanospin Plus, Gelman Science, USA) having a molecular weight cut-off value of 10,000 Da to recover unreacted PA-oligosaccharides. An aliquot of filtrate was applied to a TSKgel ODS 80TM column (4.6 × 150 mm) and eluted with 10 % MeOH: 0.1-M ammonium acetate buffer (1:9, v/v) at a flow rate of 1.0 mL min−1 at 40 °C. The eluate was monitored at an excitation wavelength of 320 nm and an emission wavelength of 400 nm, and unbound PA-oligosaccharide (Ounbound) was quantified. The amount of bound PA-oligosaccharide (Obound) was obtained by the following formula: Obound = Oadded−Ounbound, where Oadded represents the amount of added PA-oligosaccharide, which was determined from the filtrate of reaction solution without a lectin. The binding activity (Obound/Oadded) was calculated as a ratio of the amount of bound PA-oligosaccharide to that of added and expressed as % binding.
Antibacterial activity and inhibition of antibacterial activity
Antibacterial activity was determined by using a dilution procedure of a lectin solution in 20-mM phosphate buffer (pH 7.0) containing 0.85 % NaCl (PBS), based on the method of Charungchitrak et al. (2011). Three species of shrimp pathogenic marine bacteria, V. harveyi, V. alginolyticus, and V. parahaemolyticus were tested. An active peak fraction obtained by gel filtration, which included three isolectins from E. denticulatum, was used as a lectin solution (EDAs). The bacteria were grown in a sterilized seawater medium containing nutrient broth and yeast malt broth and incubated overnight in a shaker 37 °C. The cell concentration of bacteria was determined by measuring the suspension turbidity at 600 nm and converted to colony forming units (105–106 CFU mL−1) using a calibration curve. Serial twofold dilutions (100 μL each) of an original solution of EDAs (1942 μg mL−1) were prepared in PBS in test tubes. To each dilution, a 50 μL of bacteria suspension (OD600 = 0.47) in a medium was added, and the mixtures were gently shaken and incubated at 37 °C for 24 h. As a positive reference, 50 μL of ampicillin solution (1000 μg mL−1) in a medium was examined in the same way. As a negative control without both lectins and ampicillin, 50 μL of the bacterial suspension (OD600 = 0.47) was added to 100 μL of PBS and incubated in the similar way. After incubation, the turbidity of reaction solutions was measured at 600 nm as the index of bacterial growth. The bacterial growth in the presence of EDAs and ampicillin was compared to that in a control. The inhibition activities of EDAs at various concentrations were determined as the ratio (%) to that of ampicillin (1000 μg mL−1) and expressed as an efficient concentration (EC50) relative to the inhibition activity of ampicillin at 1000 μg mL−1.
The antibacterial activity of EDAs was also determined in the presence of yeast mannan, an inhibitor of hemagglutination of EDAs. Briefly, to the serially twofold dilutions (50 μL each) of a EDAs (1942 μg mL−1) were prepared in PBS as described above, 50 μL each of yeast mannan (2 mg mL−1) in PBS was added. The mixtures were gently shaken and then allowed to stand at room temperature for 1 h. To them, 50 μL each of a bacterial suspension (OD600 = 0.47) was added, gently shaken, and incubated at 37 °C overnight. The bacterial growth of each reaction mixture was determined as described above. All assays were carried out in triplicate.
Results
Extraction and purification of lectins
The lectins from E. denticulatum were extracted with aqueous ethanol (about 20 % ethanol concentration) and efficiently recovered as a precipitate with cold 80 % ethanol. The precipitate gave a single active peak in gel filtration (Fig. 1a). The active fraction was further separated into three active peaks, designated as I, II, and III, by ion-exchange chromatography (Fig. 1b). The three active peaks gave a single protein band in SDS-PAGE, respectively (Fig. 2a). The purified lectins thus obtained were named as EDA-1, EDA-2, and EDA-3 in the order of elution from the column. The lectin yields were higher in the order of EDA-2 (13.4 mg), EDA-1 (6.9 mg), and EDA-3 (4.2 mg). The results of purification are summarized in Table 2.
Fig. 1.
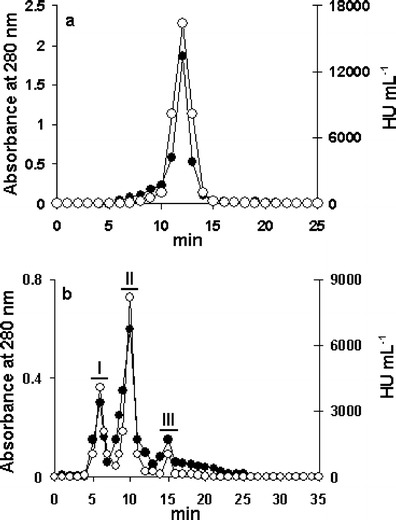
a Gel filtration of an 80 % ethanol precipitate on a Superdex R 75 HR column (10 × 300 mm). The column was eluted at a flow rate of 1.0 mL min−1. Fractions of 1 mL were collected and measured for their absorbance at 280 nm (closed circles) and for their hemagglutination activity (open circles). b Ion-exchange chromatography on a TSKgel DEAE-5PW column (7.5 × 75 mm) of the active peak obtained by gel filtration. The elution was performed at a flow rate of 0.4 mL min−1. Fractions of 0.4 mL were collected and measured for their absorbance at 280 nm (closed circles) and for their hemagglutination activity (open circles). HU hemagglutination unit
Fig. 2.
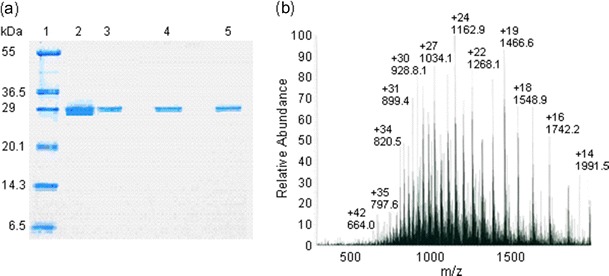
Molecular weight determination of the lectins isolated from E. denticulatum. a Non-reducing SDS-PAGE for isolated EDAs. Lane 1, a mixture of reference proteins; lane 2, an active fraction obtained by gel filtration; lane 3, EDA-1; lane 4, EDA-2; and lane 5, EDA-3. From lane 3 to lane 5, active fractions obtained from ion-exchange chromatography. The relative migration of EDAs was not changed in a reducing SDS-PAGE. b ESI-MS spectrum of the isolectin EDA-1. An ESI-MS multiply charged spectrum for EDA-1 with average molecular weight of 27,868.0 Da was represented for an example
Table 2.
Summary of purification of the isolectins from E. denticulatum. Fresh alga (1 kg) was used as a starting material
| Extraction and purification step | Protein (mg) | Total activity (× 103 HUa mL−1) | Specific activity (× 103 HU mg−1) | Yield (%) |
|---|---|---|---|---|
| Extraction | 614.4 | 865.3 | 1.4 | 100.0 |
| Ethanol precipitation | 85.1 | 654.6 | 7.7 | 75.6 |
| Gel filtration | ||||
| EDAs | 33.2 | 510.7 | 15.4 | 59.0 |
| Ion exchange | ||||
| EDA-1 | 6.9 | 115.0 | 16.7 | 13.3 |
| EDA-2 | 13.4 | 248.2 | 18.5 | 28.7 |
| EDA-3 | 4.2 | 78.3 | 18.8 | 9.1 |
aHemagglutination units (hemagglutination titer)
Molecular mass and N-terminal amino acid sequence
The relative molecular masses of the purified lectins were estimated to be about 28 kDa by both non-reducing and reducing SDS-PAGE (Fig. 2a). They were also determined to be 27,868.0 Da (EDA-1) (Fig. 2b), 27,834.2 Da (EDA-2), and 27,861.0 Da (EDA-3) by ESI-MS, indicating that their molecular weights are similar to one another but not identical. The purified lectins have no carbohydrate as they did not stain for carbohydrate (data not shown). Thus, the lectins from E. denticulatum were commonly monomeric proteins of about 28 kDa. The 20 N-terminal amino acid sequences were also identical among them, except EDA-1 and EDA-3 which have Val in place of Trp at position 16 (Fig. 3).
Fig. 3.

N-terminal amino acid sequences of the lectins isolated from E. denticulatum and the related lectins: E. serra (ESA-1 and ESA-2) (Kawakubo et al. 1997); K. alvarezii (KAA-1, KAA-2 and KAA-3) (Hung et al. 2009b); K. striatum (KSA-1, KSA-2 and KSA-3) (Hung et al. 2011). Underlines indicate different amino acid residues
Primary structure of EDA-2
The isolated full-length cDNA of EDA isolectin consisted of 1158 bp containing 103 bp of a 5′untranslated region (5′UTR), 807 bp of an open reading frame (ORF), and 248 bp of 3′UTR (Fig. 4). ORF coded a polypeptide of 269 amino acids including an initiating methionine. The calculated molecular mass of the deduce amino acid sequence in EDA isolectin cDNA was 27.834.19 Da, which was consistent with that of EDA-2, 27,834.2 Da, which was determined by ESI-MS. Thus, we concluded the isolated cDNA encoding EDA-2. The 20 N-terminal amino acid sequence of EDA-2, which had been determined by Edman degradation, was found in the deduced amino acid sequence of EDA-2 cDNA. The primary structure of EDA-2 has four tandemly repeated domains, each consisting of 67 amino acids and sharing 47 % sequence identity (Fig. 5). The homologous sequences were found in database search, including lectins from cyanobacteria Oscillatoria agardhii (OAA) (132 aa) (P84330), proteobacteria, Burkholderia oklahomensis EO147 (BOA) (276 aa) (ZP_02360833), Pseudomonas fluorescens Pf0-1 (PFL) (133 aa) (YP_346241), and Myxococcus xanthus (MBHA) (267 aa) (M13831), and from marine red alga Eucheuma serra (ESA-2) (268 aa) (P84331). All of them contain four tandemly repeated homologous domains of about 67 amino acids, except OAA and PFL, which are composed of only two tandemly repeated homologous domains. The identity of ESA-2, MBHA, and BOA with EDA-2 in amino acid sequences were 95.2, 59.0, and 57.1 %, respectively, whereas OAA and PFL showed a considerable sequence identity, 61.4 and 63.6 %, to the N-terminal half of EDA-2, respectively (Fig. 6).
Fig. 4.
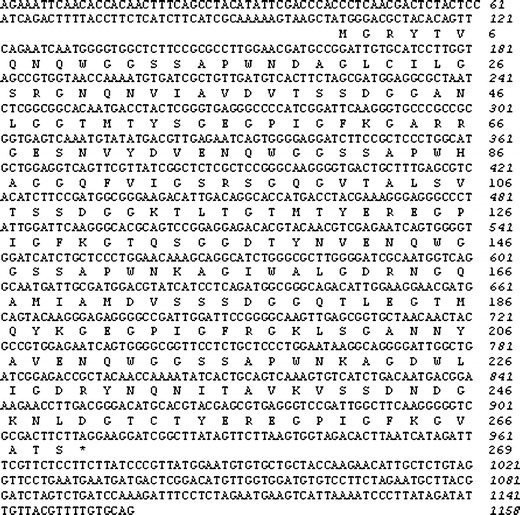
Nucleotide and deduced amino acid sequences of EDA-2, a lectin from E. denticulatum. The stop codon TAG is shown as an asterisk. The italicized and non-italicized numbers represent the positions of nucleotides and amino acids, respectively
Fig. 5.

Comparison of tandem-repeated sequences of EDA-2. Identical amino acids among the four repeated domains in the lectin molecule are shaded
Fig. 6.

Multiple alignment of EDA-2 with related proteins. Multiple sequence alignments were carried out using the CLUSTALW 2.0 program (Larkin et al. 2007). The following sequences were obtained from GenBank database: ESA-2 from Eucheuma serra (P84331); MBHA from Myxococcus xanthus (YP_635174); BOA from Burkholderia oklahomensis EO147 (ZP_02360833); OAA from Oscillatoria agardhii (P84330); PFL from Pseudomonas fluorescens Pf0-1 (YP_346241). The identical amino acids were indicated by shade
Effects of temperature, pH, and metal ions on hemagglutination activity
The purified lectins were relatively thermostable because their hemagglutination activities were unchanged after incubation at 50 °C for 30 min; however, the activities gradually decreased as incubation temperature exceeded 50 °C. Their activities were stable in a wide range of pH from 3 to 10 (Fig. 7) and were not affected by either presence of EDTA or addition of divalent cations such as Ca2+ and Mg2+ (data not shown).
Fig. 7.
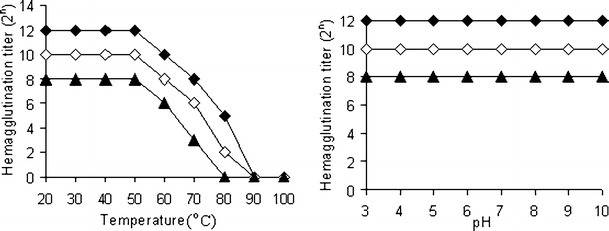
The effects of temperature and pH on hemagglutination activities of isolectins from E. denticulatum. Hemagglutination titer of EDA-1( ), EDA-2(
), EDA-2( ) and EDA-3(
) and EDA-3( )
)
Hemagglutination-inhibition test
The purified isolectins showed the same hemagglutination-inhibition profiles with a series of sugars and glycoproteins (Table 3). The monosaccharides, lactose, and fucoidan did not inhibit their hemagglutination. Strong inhibition was observed with glycoproteins including yeast mannan, porcine and bovine thyroglobulins, and their asialo-derivatives, all of which have high-mannose type N-glycans in the molecules, although both thyroglobulins contain complex type N-glycans, too. Transferrin bearing only complex type N-glycans was no inhibitory. However, fetuin bearing both complex type N-glycans and O-glycans and bovine submaxillary mucin bearing O-glycans were also moderately inhibitory, although porcine stomach mucin bearing O-glycans were no inhibitory. These results suggested that the isolectins are commonly specific for high-mannose N-glycans.
Table 3.
Hemagglutination-inhibition test of the three isolectins isolated from E. denticulatum. The hemagglutination-inhibition test was carried out as described in the Experimental
| Sugar and glycoprotein | EDA-1 | EDA-2 | EDA-3 |
|---|---|---|---|
| Sugar (mM) | |||
| Monosaccharidesa | –b | – | – |
| Lactose | – | – | – |
| Fucoidan | – | – | – |
| Glycoprotein (μg mL−1) | |||
| Transferrin | – | – | – |
| Fetuin | 62.5 | 62.5 | 62.5 |
| Porcine thyroglobulin | 1.9 | 1.9 | 1.9 |
| Asialo-porcine thyroglobulin | 1.9 | 1.9 | 1.9 |
| Bovine thyroglobulin | 7.8 | 7.8 | 7.8 |
| Asialo-bovine thyroglobulin | 7.8 | 7.8 | 7.8 |
| Yeast mannan | 3.9 | 3.9 | 3.9 |
| Bovine submaxillary mucin | 31.2 | 31.2 | 31.2 |
| Asialo-bovine submaxillary mucin | 31.2 | 31.2 | 31.2 |
| Porcine stomach mucin | – | – | – |
Values indicate the lowest concentration of sugar (mM) and glycoprotein (μg mL−1) at which complete inhibition of hemagglutination (titer 4) was achieved
aThe monosaccharides examined are described in Experimental
bIndicates no inhibition at 100 mM for monosaccharides and at 2000 μg mL−1 for glycoproteins
Oligosaccharide-binding specificity of EDA-2
An isolectin, EDA-2 was examined for oligosaccharide-binding specificity by a centrifugal ultrafiltration-HPLC assay with 17 pyridylaminated (PA)-oligosaccharides. The structures of the oligosaccharides used are shown in Fig. 8. The lectin exclusively bound to high-mannose type N-glycans (3–13 in Fig. 8), but did not bind to other oligosaccharides, including the complex type N-glycans (1–2), oligomannoses (15, 16), and a core pentasaccharide (17) (Table 4). The binding activity of EDA-2 was more than 90 % with the PA-oligosaccharides, 4, 6, 8, and 11, all of which have an exposed (α1-3) Man residue at non-reducing terminus in the D2 arm, while the activity significantly decreased with PA-oligosaccharides, 5, 7, 9, 10, and 12 (less than 50 %), where the (α1-3) Man residue in the D2 arm was masked by the addition of a non-reducing terminal (α1-2) Man residue. The addition of (α1-2) Man at the terminus of D1 or D3 arm had little effect, as the binding activity was unchangeable with the PA-oligosaccharides 6, 8, or 11. On the other hand, the addition of one (α1-2) Man residue to Man(α1-3)Manβ1-unit in the D1 arm significantly enhanced the binding activity of EDA-2 to high-mannose glycans (Table 4). For instance, the comparison of binding activity between a PA-oligosaccharide 4 (binding activity of 100 %) having one (α1-2) Man residue attached to the Man(α1-3)Manβ1-unit in the D1 arm and a PA-oligosaccharide 3 (55.1 %) being devoid the (α1-2) Man residue in the D1 arm. The similar enhancing effect of the (α1-2) Man residue was also found from the comparison of binding activity between the PA-oligosaccharides having the same residue, 7 (44.5 %) and 9 (43.4 %) and a PA-oligosaccharide without the residue, 12 (21.4 %)
Fig. 8.

The structures of 17 PA-oligosaccharides used in this study. R, GlcNAcβ1-4GlcNAc-PA; Gal, galactose; GlcNAc, N-acetylglucosamine; Man, mannose; PA, pyridylaminated
Table 4.
Oligosaccharide-binding activity of EDA-2
| Oligosaccharides | Binding activity (%) | |||
|---|---|---|---|---|
| EDA-2 | ESA-2a | KSA-2b | KAA-2c | |
| Complex type | ||||
| 1 | 0.0 | 0.0 | 10.3 | 0.0 |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 |
| High-mannose type | ||||
| 3 | 55.1 | 79.0 | 42.5 | 95.8 |
| 4 | 100.0 | 91.5 | 100.0 | 100.0 |
| 5 | 40.1 | 68.5 | 56.6 | 17.2 |
| 6 | 100.0 | 93.4 | 100.0 | 100.0 |
| 7 | 44.5 | 66.4 | 46.7 | 34.6 |
| 8 | 98.7 | 84.9 | 94.6 | 76.7 |
| 9 | 43.3 | 69.9 | 51.4 | 65.0 |
| High-mannose type | ||||
| 10 | 54.1 | 68.2 | 53.7 | 49.1 |
| 11 | 98.6 | 88.2 | 100.0 | 74.2 |
| 12 | 21.4 | 52.8 | 28.4 | 61.5 |
| 13 | 37.1 | 79.0 | 37.1 | ND |
| 14 | 0.0 | 0.0 | 0.0 | ND |
| Oligomannose | ||||
| 15 | 0.0 | 0.0 | 0.0 | 0.0 |
| 16 | 0.0 | 0.0 | 0.0 | 0.0 |
| Core pentasaccharide | ||||
| 17 | 0.0 | 0.0 | 0.0 | 0.0 |
Binding activity was determined by the centrifugal ultrafiltration-HPLC method as described in the Experimental and expressed as a ratio (%) of the amount of a bound oligosaccharide to that of the added oligosaccharide. The structures of PA-oligosaccharides are presented in Fig. 8
ND not determined
aCited from Hori et al. (2007)
bCited from Hung et al. (2011)
cCited from Sato et al. (2011a)
Antibacterial assay and inhibition of antibacterial activity
The lectin fraction (EDAs) significantly inhibited the growth of V. alginolyticus at more than 60.6 μg mL−1, whereas it did not affect the growth of V. parahaemolyticus and V. harveyi even at 1942 μg mL−1. The highest concentration of EDAs (1942 μg mL−1) suppressed the growth of V. alginolyticus to 69 % in comparison to that in the control without EDAs, whereas ampicillin did to 60 % at 1000 μg mL−1. The degrees of inhibition at various concentrations of EDAs to that of ampicillin at 1000 μg mL−1 were plotted as the inhibition ratios relative to ampicillin (Fig. 9). The antibacterial activity of EDAs was comparable to that of ampicillin with a relative EC50 of 343 μg mL−1, although the chemical structures of both compounds are clearly distinct. Interestingly, the growth-inhibition activity of EDAs toward V. alginolyticus was lost in the presence of yeast mannan bearing high-mannose N-glycans (Fig. 9) which was the strongest inhibitory glycoprotein toward hemagglutination with EDAs (Table 3).
Fig. 9.
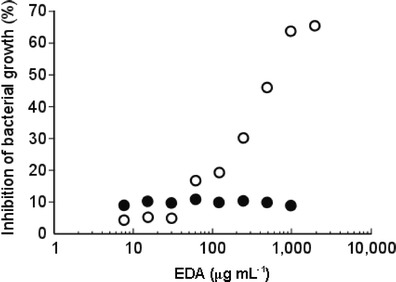
Antibacterial activity and inhibition of antibacterial activity. The growth of V. alginolyticus after incubated at 37 °C for 24 h was measured at absorbance of 600 nm. The inhibitory effect of EDAs against the bacteria growth was expressed in percentage where the inhibition effect of 1 mg mL−1 ampicillin (a positive control) was hundred percent and that of non-lectin or -ampicillin supplemented solution (a negative control) was zero percent. White and black circles indicate EDAs treatment (○) and EDAs treatment with yeast mannan (●), respectively
Discussion
The red alga E. denticulatum contained three isolectins which were purified by a combination of extraction with aqueous ethanol, ethanol precipitation, and ion-exchange chromatography. The use of aqueous ethanol was effective to remove viscous algal polysaccharides as previously reported (Hori et al. 1986b, 1988; Kawakubo et al. 1997). The sum of the yields of the isolated lectins (Table 2) is much lower than those reported for genus Eucheuma (Kawakubo et al. 1997, 1999) and Kappaphycus (Hung et al. 2009b, 2011).
The lectins isolated from E. denticulatum showed similar biochemical properties not only to one another but also to those of lectins from the genera Eucheuma (Kawakubo et al. 1997, 1999) and Kappaphycus (Hung et al. 2009b, 2011). All of them were monomeric proteins of about 28 kDa and shared almost the same N-terminal amino acid sequences. Their hemagglutination activities were commonly inhibited by glycoproteins bearing high-mannose N-glycans (Table 3). The inhibition with O-glycans may be caused by non-specific interaction between the lectins and O-glycan-linked glycoproteins, which was often observed for many lectins including the Eucheuma and Kappaphycus lectins (Kawakubo et al. 1997, 1999; Hung et al. 2009b, 2011).
The binding activity of EDA-2 differed among high-mannose N-glycans examined, being attributed to subtle differences in the structures of branched oligomannosides (Fig. 8, Table 4). The binding activity of EDA-2 was considerably decreased when (α1-2)-linked Man is attached at the terminus of D2 arm (5, 7, 9, 10, and 12), whereas it was not affected but rather enhanced by the presence of (α1-2)-linked Man at the terminus of D1 or D3 arm (4, 6, 8, and 11). In other words, the attachment of Man (α1-2) at the non-reducing terminus of D2 arm blocked the carbohydrate interaction of EDA-2. This suggests that a C2-OH of the Man (α1-3) residue in the D2 arm may interact directly with the EDA-2 molecule. The Man (α1-3) in the D2 arm may be the primary target for EDA-2 binding because EDA-2 did not interact to the oligosaccharide 14 (0 %) lacking the Man (α1-3) in the D2 arm, whereas it bound to the oligosaccharide 13 (37.1 %) containing this residue, indicating that EDA-2 recognized preferentially the exposed (α1-3) Man in the D2 arm. The similar binding specificity has also been reported for high-mannose binding lectins from the algae such as ESA-2 (Hori et al. 2007), KSA-2 (Hung et al. 2011) and OAA (Sato et al. 2007). On the other hand, the addition of one (α1-2) Man residue to Man(α1-3)Manβ1-unit in the D1 arm notably enhanced the binding activity (binding activity of EDA-2 with PA-oligosaccharides 4 compared to that with 3), indicating that the (α1-2) Man residue attached at Man(α1-3)Manβ1-unit in the D1 arm serves as another target for enhancing the binding affinity of EDA-2 to oligomannosides. The similar cases have also been reported for the algal lectin ESA-2 (Hori et al. 2007), KSA-2 (Hung et al. 2011), while the binding activity of OAA was not affected by the (α1-2) Man residue(s) in the D1 arm (Sato et al. 2007). In contrast, a leaf lectin CRLL from the land plant Cycas revoluta, which belongs to the Jacalin-like superfamily, loses the binding to high-mannose glycans in which a (α1-2) Man residue was attached at Man(α1-3)Manβ1-unit in the D1 arm, indicating that the addition of (α1-2) Man to the core Man(α1-3)Manβ had rather a destructive effect on CRLL recognition (Nakamura et al. 2005).
The high-mannose N-glycan recognition profiles basically resemble other lectins from carrageenophytes, including EDA-2, ESA-2 (Hori et al. 2007), KSA-2 (Hung et al. 2011), and KAA-2 (Sato et al. 2011a), whose glycan recognition profiles were also similar to that of OAA, a lectin from the cyanobacterium O. agardhii (Sato et al. 2007), but clearly differed from those of other high-mannose binding lectins such as griffithsin from the red alga Griffithsia sp. (Moulaei et al. 2010), BCA from the green alga B. coacta (Sato et al. 2011b), cyanovirin-N from the cyanobacterium Nostoc ellipsosporum (Boyd et al. 1997), MVL from the cyanobacterium M. viridis (Williams et al. 2005), SVN from the cyanobacterium Scytonema varium (Bokesch et al. 2003), ASAs from the garlic A. sativum (Dam et al. 1998; Bachyhawat et al. 2001), and HRL from the mushroom Hygrophorus russula (Suzuki et al. 2012). The high-mannose binding affinities of EDA-2, ESA-2, KSA-2, KAA-2, and OAA were impaired by the addition of the terminal (α1-2) Man in D2 arm, while griffithsin, BCA, cyanovirin-N, SVN, and ASAs preferentially recognized the non-reducing terminal (α1-2) Man of the mannosides such as Man8GlcNAc2 and Man9GlcNAc2 (Moulaei et al. 2010; Sato et al. 2011b; Botos et al. 2002; Bokesch et al. 2003; Dam et al. 1998). MVL recognized the core tetra- and pentasaccharides of high-mannose N-glycans (Williams et al. 2005), and HRL showed the highest affinity for N-glycan core structure, Man(α1-6)[Man(α1-3)]Man(β1-4)GlcNAc(β1-4)GlcNAc, but had much lower affinity for Man8GlcNAc2 and Man9GlcNAc2 (Suzuki et al. 2012). Thus, there seems to be subtle difference in the recognition modes among high-mannose N-glycan-binding lectins.
EDAs, the mixture of three isolectins inhibited the growth of the shrimp pathogenic bacterium, Vibrio alginolyticus, although it did not affect the growth of V. parahaemolyticus and V. harveyi. The growth inhibition of V. alginolyticus with EDAs was not observed in the presence of yeast mannan bearing high-mannose N-glycans, indicating that V. alginolyticus have mannoside or mannoside-like structure(s) on the cell surface, which might respond as a receptor(s) for EDAs, while V. parahaemolyticus and V. harveyi do not have such structure on their cell surfaces. The species-specific antibacterial activities have been reported for the lectins from the red algae, E. serra and Galaxaura marginata, in which both lectins displayed antibacterial activity toward the fish pathogen V. vulnificus, but not toward V. neresis and V. pelagius (Liao et al. 2003). Although antibacterial activities have been reported for lectins from various biological sources (Santi-Gadelha et al. 2006; Charungchitrak et al. 2011; Riera et al. 2003), there seems to be not reported for the growth-inhibiting activity against shrimp and fish pathogens, except the high-mannose N-glycan specific lectins from carrageenophytes mentioned above. These algal lectins may be useful to not only clarify the infection mechanism of their marine pathogens but also protect fishes and shellfishes from the infection.
In this study, we have determined the primary structure of EDA-2 (Fig. 4) and confirmed that it strongly resembles that of ESA-2 (Hori et al. 2007) and MBHA (Romeo et al. 1986; Koharudin et al. 2012) (Fig. 6), including its molecular size and the presence of four tandemly repeated motifs. Clusters of identical amino acids of EDA-2 were located on both N- and C-terminal regions of each repeated domain, portions of which also correspond to the conserved regions among the four repeated domains of the lectin. Recently, genes coding the lectins belonging to the same lectin family including OAA and ESA-2 have been discovered in several other prokaryotic microorganisms, including cyanobacteria, proteobacteria, chlorobacteria, and in an eukaryotic marine red alga (Sato and Hori 2009; Koharudin et al. 2012). Similar to members of this lectin family, EDA-2 shared a considerable structure similarity and high-mannose N-glycan binding specificity with ESA-2 (Hori et al. 2007), BOA (Whitley et al. 2013), OAA (Sato et al. 2007; Sato and Hori 2009), MBHA (Romeo et al. 1986; Koharudin et al. 2012) and PFL (Sato et al. 2012). The lectins in this family are commonly monomeric proteins composed of either two or four tandem repeats of homologous domains, depending on different family members (Sato and Hori 2009; Koharudin et al. 2012). From a comparison of the sequences, it is also speculated that the highly conserved segments in both the N-terminal and C-terminal regions may be involved in the carbohydrate binding (Fig. 6). Although the high-mannose N-glycan recognition profile of EDA-2 was slightly differed from those of the high-mannose binding cyanobacterial lectins such as CV-N (Botos et al. 2002), MVL (Williams et al. 2005) and SVN (Bokesch et al. 2003), EDA-2 has no sequence similarity with these cyanobacterial lectins, except for OAA (Sato et al. 2007). The strict specificity for high-mannose oligosaccharides with very high binding affinities seems to be a common feature among the lectins from lower organisms such as cyanobacteria and algae.
The cultivated E. denticulatum from which three isolectins (EDA-1 ~ 3) were isolated is edible. EDAs also inhibited the growth of a marine pathogenic bacterium, V. alginolyticus in dose-dependent manner. The oligosaccharide-binding properties and the primary structure of EDA-2 well resemble those of the antiviral lectins which belong to the high-mannose N-glycan specific lectin family in lower organisms (Sato et al. 2007, 2011a), suggesting that EDA-2 is also predicted to have antiviral and other applicable activities. This study indicates that E. denticulatum might become a candidate for a functional food that can prevent virus infection in the future. Clarifying the structural basis of the recognition mode of the carrageenophyte lectins, including E. denticulatum lectins, should contribute to demonstrate the biological function(s) as well as application of this lectin group.
Acknowledgments
This research was supported by JSPS-RONPAKU Program-Japan, Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.05–2011.35, and VAST.HTQT.JAPAN.03/2012–2013, in addition to Grant-in-Aid for Scientific Research (B) from Japan Society of the Promotion of Science.
Contributor Information
Le Dinh Hung, Phone: +84 58 3521133, Email: ledinhhungims@yahoo.co.uk.
Makoto Hirayama, Email: hirayama@hiroshima-u.ac.jp.
Kanji Hori, Email: kanhori@hiroshima-u.ac.jp.
References
- Ask EI, Azanza RV. Advances in cultivation technology of commercial eucheumatoid species: a review with suggestions for future research. Aquaculture. 2002;206:257–277. doi: 10.1016/S0044-8486(01)00724-4. [DOI] [Google Scholar]
- Bachyhawat K, Thomas CJ, Amutha B, Krishnasastry MV, Khan MI, Surolia A. On the stringent requirement of mannosyl substitution in manno-oligosaccharides or the recognition by garlic (Allium sativum) lectin. J Biol Chem. 2001;276:5541–5546. doi: 10.1074/jbc.M009533200. [DOI] [PubMed] [Google Scholar]
- Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. Antivir Res. 2006;71:237–247. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Balzarini J. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antivir Chem Chemother. 2007;18:1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- Barrientos LG, O’Keefe BR, Bray M, Sanchez A, Gronenborn AM, Boyd MR. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antivir Res. 2003;58:47–56. doi: 10.1016/S0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Bokesch HR, O’Keefe BR, McKee TC, Pannell LK, Patterson GM, Gardella RS, Sowder RC, Turpin J, Watson K, Buckheit RW, Boyd MR. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42:2578–2584. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- Botos I, Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. Prog Biophys Mol Biol. 2005;88:233–282. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Botos I, O’Keefe BR, Shenoy SR, Cartner LK, Ratner DM, Seeberger PH, Boyd MR, Wlodawer A. Structures of the complexes of a potent anti-HIV protein cyanovirin-N and high mannose oligosaccharides. J Biol Chem. 2002;277:34336–34342. doi: 10.1074/jbc.M205909200. [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, Mcmahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, II, Buckheit RW, Jr, Nara PL, Pannell LK, Sowder RC, II, Henderson LE. Discovery of cyanovirin-N, a novel human immunodeficiency virus inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charungchitrak S, Petsom A, Sangvanich P, Karnchanatat A. Antifungal and antibacterial activities of lectin from the seeds of Archidendron jiringa Nielsen. Food Chem. 2011;126:1025–1032. doi: 10.1016/j.foodchem.2010.11.114. [DOI] [Google Scholar]
- Dam TK, Bachyhawat K, Rani PG, Surolia A. Garlic (Allium sativum) lectins bind to high mannose oligosaccharide chains. J Biol Chem. 1998;273:5528–5535. doi: 10.1074/jbc.273.10.5528. [DOI] [PubMed] [Google Scholar]
- FAO (2013) Report of the FAO/MARD Technical Workshop on Early Mortality Syndrome (EMS) or Acute Hepatopancreatic Necrosis Syndrome (AHPNS) of Cultured Shrimp (under TCP/VIE/3304). Hanoi, Vietnam, on 25–27 June 2013. FAO Fisheries and Aquaculture Report No. 1053. Rome. 54 pp
- Helle F, Wychowski C, Vu-Dac N, Gustafson KR, Voisset C, Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J Biol Chem. 2006;281:25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- Hori K, Miyazawa K, Ito K. Preliminary characterization of agglutinins from seven marine algal species. Bull Jpn Soc Sci Fish. 1986;52:323–331. doi: 10.2331/suisan.52.323. [DOI] [Google Scholar]
- Hori K, Miyazawa K, Fusetani N, Hashimoto K, Ito K. Hypnins, low-molecular weight peptidic agglutinins isolated from a marine red alga Hypnea japonica. Biochim Biophys Acta. 1986;873:228–236. doi: 10.1016/0167-4838(86)90049-X. [DOI] [Google Scholar]
- Hori K, Ikegami S, Miyazawa K, Ito K. Mitogenic and antineoplastic isoagglutinins from the red alga Solieria robusta. Phytochemistry. 1988;27:2063–2067. doi: 10.1016/0031-9422(88)80097-9. [DOI] [Google Scholar]
- Hori K, Sato Y, Ito K, Fujiwara Y, Iwamoto Y, Makino H, Kawakubo A. Strict specificity for high-mannose type N-glycans and primary structure of a red alga Eucheuma serra lectin. Glycobiology. 2007;17:479–491. doi: 10.1093/glycob/cwm007. [DOI] [PubMed] [Google Scholar]
- Hung LD, Hori K, Nang HQ. Screening and preliminary characterization of hemagglutinins in Vietnamese marine algae. J Appl Phycol. 2009;21:89–97. doi: 10.1007/s10811-008-9360-2. [DOI] [Google Scholar]
- Hung LD, Sato T, Shibata H, Hori K. Biochemical comparison of lectins among three different color strains of the red alga Kappaphycus alvarezii. Fish Sci. 2009;75:723–730. doi: 10.1007/s12562-009-0088-y. [DOI] [Google Scholar]
- Hung LD, Sato Y, Hori K. High-mannose N-glycan-specific lectins from the red alga Kappaphycus striatum (Carrageenophyte) Phytochemistry. 2011;72:855–861. doi: 10.1016/j.phytochem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Kawakubo A, Makino H, Ohnishi J, Hirohara H, Hori K. The marine red alga Eucheuma serra J Agardh, a high yielding source of two isolectins. J Appl Phycol. 1997;9:331–338. doi: 10.1023/A:1007915006334. [DOI] [Google Scholar]
- Kawakubo A, Makino H, Ohnishi J, Hirohara H, Hori K. Occurrence of highly yielded lectins homologous within the genus Eucheuma. J Appl Phycol. 1999;11:149–156. doi: 10.1023/A:1008062127564. [DOI] [Google Scholar]
- Koharudin LMI, Kollipara S, Aiken C, Gronenborn AM. Structural insights into the Anti-HIV activity of the Oscillatoria agardhii agglutinin homolog lectin family. J Biol Chem. 2012;287:33796–33811. doi: 10.1074/jbc.M112.388579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Liao WR, Lin JY, Shieh WY, Jeng WL, Huang R. Antibiotic activity of lectins from marine algae against marine vibrios. J Ind Microbiol Biotechnol. 2003;30:433–439. doi: 10.1007/s10295-003-0068-7. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marhual NP, Das BK, Sadique M, Swain AK, Mishra BK, Maiti NK, Eknath AE. Molecular identification and typing of Vibrio alginolyticus and Vibrio parahaemolyticus strains isolated from black tiger shrimp Penaeus monodon. J Aquac Trop. 2010;25:25–33. [Google Scholar]
- Mori T, O’Keefe BR, Sowder RC, Bringans S, Gardella RS, Berg S, Cochran P, Turpin JA, Buckheit RW, McMahon JB, Jr, Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- Moulaei T, Shenoy SR, Giomarelli B, Thomas C, McMahon JB, Zbigniew DZ, O’Keefe BR, Wlodawer A. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure. 2010;18:1104–1115. doi: 10.1016/j.str.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Yagi F, Totani K, Ito Y, Hirabayashi J. Comparative analysis of carbohydrate-binding properties of two tandem repeat-type Jacalin-related lectins, Castanea crenata agglutinin and Cycas revoluta leaf lectin. FEBS J. 2005;272:2784–2799. doi: 10.1111/j.1742-4658.2005.04698.x. [DOI] [PubMed] [Google Scholar]
- O’Keefe BR, Giomarelli B, Barnard DL, Shenoy SR, Chan PK, McMahon JB, Palmer KE, Barnett BW, Meyerholz DK, Wohlford-Lenane CL, McCray PB., Jr Broad spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family coronaviridae. J Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera AS, Daud A, Gallo A, Genta S, Aybar M, Sanchez S. Antibacterial activity of lactose-binding lectins from Bufo arenarum skin. Biocell. 2003;27:37–46. [PubMed] [Google Scholar]
- Romeo JM, Esmon B, Zusman DR. Nucleotide sequence of the myxobacterium hemagglutinin gene contains four homologous domains. Proc Natl Acad Sci U S A. 1986;83:6332–6336. doi: 10.1073/pnas.83.17.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi-Gadelha T, Gadelha CAA, Aragão KS, Oliveira CC, Mota MRL, Gomes RC. Purification and biological effects of Araucaria angustifolia (Araucariaceae) seed lectin. Biochem Biophys Res Commun. 2006;350:1050–1055. doi: 10.1016/j.bbrc.2006.09.149. [DOI] [PubMed] [Google Scholar]
- Sato T, Hori K. Cloning, expression, and characterization of a novel anti-HIV lectin from the cultured cyanobacterium, Oscillatoria agardhii. Fish Sci. 2009;75:743–753. doi: 10.1007/s12562-009-0074-4. [DOI] [Google Scholar]
- Sato Y, Okuyama S, Hori K. Primary structure and carbohydrate-binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium, Oscillatoria agardhii. J Biol Chem. 2007;282:11021–11029. doi: 10.1074/jbc.M701252200. [DOI] [PubMed] [Google Scholar]
- Sato Y, Morimoto K, Hirayama M, Hori K. High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem Biophys Res Commun. 2011;405:291–296. doi: 10.1016/j.bbrc.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Hirayama M, Morimoto K, Yamamoto N, Okuyama S, Hori K. High mannose-binding lectin with preference for the cluster of α1–2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J Biol Chem. 2011;286:19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Morimoto K, Kubo T, Yanagihara K, Seyama T. High mannose-binding antiviral lectin PFL from Pseudomonas fluorescens Pf0-1 promotes cell death of gastric cancer cell MKN28 via interaction with α2-integrin. PLoS One. 2012;7:e45922. doi: 10.1371/journal.pone.0045922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N, Lis H. Lectins. 2. Dordrecht: Kluwer; 2003. [Google Scholar]
- Slifkin M, Doyle RJ. Lectins and their application to clinical microbiology. Clin Microbiol Rev. 1990;3:197–218. doi: 10.1128/cmr.3.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sugiyama K, Hirai H, Ito H, Morita T, Dohra H, Murata T, Usui T, Tateno H, Hirabayashi J, Kobayashi Y, Kawagishi H. Mannose-specific lectin from the mushroom Hygrophorus russula. Glycobiology. 2012;22:616–629. doi: 10.1093/glycob/cwr187. [DOI] [PubMed] [Google Scholar]
- Tracy HH, Dongying W, Jonathan AE, Patricia AS. Sequence characterization and comparative analysis of three plasmids isolated from environmental Vibrio spp. Appl Environ Microbiol. 2007;73:7703–7710. doi: 10.1128/AEM.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley MJ, Furey W, Kollipara S, Gronenborn AM. Burkholderia oklahomensis agglutinin is a canonical two-domain OAA-family lectin: structures, carbohydrate binding, and anti-HIV activity. FEBS J. 2013;280:2056–2067. doi: 10.1111/febs.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DC, Jr, Lee JY, Cai M, Bewley CA, Clore GM. Crystal structures of the HIV-1 inhibitory cyanobacterial protein MVL free and bound to Man3GlcNAc2: structural basis for specificity and high-affinity binding to the core pentasaccharide from N-linked oligomannoside. J Biol Chem. 2005;280:29269–29276. doi: 10.1074/jbc.M504642200. [DOI] [PubMed] [Google Scholar]
- Ziólkowska NE, Wlodawer A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim Pol. 2006;53:617–626. [PubMed] [Google Scholar]
- Zorrilla I, Arijo S, Chabrillon M, Diaz P. Vibrio species isolated from diseased farmed sole Solea senegalensis (Kaup) and evaluation of the potential virulence role of their extracellular products. J Fish Dis. 2003;26:103–108. doi: 10.1046/j.1365-2761.2003.00437.x. [DOI] [PubMed] [Google Scholar]


