Abstract
We tested the hypothesis that the results of real-time polymerase chain reaction (PCR) analyses for respiratory viruses would reduce antibiotic treatment and length of stay in elderly patients hospitalized with respiratory infections. Within 24 h of hospital admission, a total of 922 patients aged ≥60 years were interviewed for symptoms of ongoing respiratory tract infection. Symptomatic patients were swabbed for oropharyngeal/nasopharyngeal presence of viral pathogens immediately by members of the study group. During a 2-month period, non-symptomatic volunteers among interviewed patients were swabbed as well (controls). Oropharyngeal/nasopharyngeal swabs were analyzed with real-time PCR for nine common respiratory viruses. A total of 147 out of 173 symptomatic patients and 56 non-symptomatic patients (controls) agreed to participate in the study. The patients were allocated to three cohorts: (1) symptomatic and PCR-positive (S/PCR+), (2) symptomatic and PCR-negative (S/PCR−), or (3) non-symptomatic and PCR-negative (control). There were no non-symptomatic patients with a positive PCR result. A non-significant difference in the frequency of empiric antibiotic administration was found when comparing the S/PCR+ to the S/PCR− cohort; 16/19 (84 %) vs. 99/128 (77 %) (χ2 = 0.49). Antibiotic treatment was withdrawn in only two patients in the S/PCR+ cohort after receiving a positive viral diagnosis. The length of stay did not significantly differ between the S/PCR+ and the S/PCR− groups. We conclude that, at least in our general hospital setting, access to early viral diagnosis by real-time PCR had little impact on the antimicrobial treatment or length of hospitalization of elderly patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s10096-013-1963-0) contains supplementary material, which is available to authorized users.
Keywords: Respiratory Syncytial Virus, Respiratory Virus, Polymerase Chain Reaction Result, Positive Polymerase Chain Reaction, Charlson Comorbidity Index Score
Introduction
Respiratory viral infections (RVIs) are important causes of morbidity and death in the elderly population [1–3]. Influenza viruses, respiratory syncytial virus (RSV), and human metapneumovirus (hMPV) are the major viral pathogens in the geriatric age group, and outbreaks caused by these viruses have been described in long-term care facilities [4, 5]. Following infection with influenza viruses, RSV, or hMPV, the risk of hospitalization increases with underlying heart and lung disease [6–8]. So far, vaccines and antiviral drugs are available for influenza viruses, but not for RSV and hMPV.
Both in the community and in hospitals, the burden of diseases caused by RVIs among the elderly is difficult to establish, as symptoms are often uncharacteristic [9]. Apart from the sensitivity and specificity of the involved laboratory methods, successful etiological diagnosis of RVIs depends on virus-specific factors such as the extent of shedding, as well as on the patient’s age and medical condition [10–12]. Real-time polymerase chain reaction (PCR) assays can provide a rapid etiological diagnosis more efficiently than conventional microbiological methods [13]. Therefore, real-time PCR may contribute to reducing the inappropriate use of antibiotics and to shortening the length of hospitalization. The high diagnostic sensitivity of real-time PCR may be of special value in viral respiratory infections of the elderly, who, at least in the case of RSV, are characterized by low mucosal shedding of the virus [9, 14].
In children, rapid tests for respiratory viruses have reduced the duration of hospitalization, antibiotic prescription, and the cost of hospitalization [15, 16]. Similar findings have been observed in young and middle-aged adults [17]. However, limited data are available on the effect of improved microbiological diagnostics in the elderly population [6]. We hypothesized that early results of real-time PCR analyses for respiratory viruses would reduce antibiotic treatment and length of stay in elderly patients hospitalized with respiratory infections.
The objective of this study was to test this hypothesis in a general medical ward.
Materials and methods
Ethical committee
The study design was approved by the Norwegian Regional Committee of Research Ethics.
Study design and eligibility criteria
The study took place from February 13th 2008 until February 3rd 2009 at the Department of Internal Medicine, Sorlandet Hospital Arendal, Norway. Sorlandet Hospital Arendal is the only hospital in Aust-Agder County, serving approximately 111,000 inhabitants. A total of 66 % of the population are residing in densely populated areas. At the time of the study, regional antibiotic stewardship suggested the use of intravenous penicillin G in patients with a suspected respiratory bacterial infection. A four-hour rule existed on antibiotic initiation after hospital admission, leaving the probability of antibiotic administration in patients with symptoms consistent with a respiratory infection being high.
Twice a week, all patients born in 1948 or earlier and admitted to the department during the previous day were interviewed by one out of two specially trained team members of the study group (one doctor or one nurse) for any symptoms of ongoing respiratory tract infection and for details of possible ongoing antimicrobial therapy, regardless of findings and diagnosis upon admission. Out of a total of 922 interviewed patients, 173 (19 %) were eligible according to the pre-set inclusion criteria (Table 1) and 147/173 patients agreed to be tested according to the study protocol. Of the 26 patients who declined to participate, 14 were males and 12 females; their mean age was 74.8 [standard deviation (SD) 7.9] years. As a control group for possible mucosal carriers of respiratory viruses among non-symptomatic individuals, 23 patients without symptoms of a respiratory tract infection were swabbed once during the first 4 weeks and 33 similar patients once during the last 4 weeks of the study (Table 1).
Table 1.
Inclusion criteria
| Inclusion criteria | |
|---|---|
| Symptomatic patients |
Patients born in 1948 or earlier AND at least one of the following current symptoms with debut less than 3 weeks prior: • Nasal congestion or runny nose • Throat pain • Fever (>38 °C) • Malaise • Muscle pain • Self-diagnosis of “the common cold” • Diarrhea or eye infection combined with laboratory values supporting an infection |
| Non-symptomatic patients | Patients born in 1948 or earlier |
Two nasopharyngeal swabs, two oropharyngeal swabs, and two serum samples were collected per patient immediately after the interviews, as previously described [18]. The swabbed material was analyzed by real-time PCR for influenza virus A and B, parainfluenza-virus 1–4, RSV A and B, hMPV, and adenovirus. Data for analysis in this paper were obtained during a study examining the sampling efficacy of rayon and nylon flocked swabs for the diagnosis of RVIs in the elderly [18].
PCR results were communicated to the attending physician within 24–48 h after sampling. A change in antibiotic treatment within 48 h after communication of the PCR results were viewed as a direct response to the PCR results.
We defined three cohorts of patients: symptomatic subjects with a positive real-time PCR (S/PCR+); symptomatic subjects with negative PCR (S/PCR−); non-symptomatic control subjects with negative PCR (control). Additionally, two subgroups of patients were identified for further analysis: (1) patients admitted by general practitioners to the hospital with a tentative diagnosis of pneumonia and (2) patients with chest X-ray at admittance consistent with pneumonia.
Additional examinations
To compare their respective comorbidities at the time of admission, the patients in these three groups were scored with the Modified Cumulative Illness Rating Scale for Geriatrics (CIRS-G) by Miller et al. [19], with subsequent modifications [20, 21] and the Charlson comorbidity index (CCI), as described by Extermann [22]. Also, C-reactive protein (CRP) analysis, leukocyte counts, and assessments of chest X-rays were routinely performed on all patients at the time of admission. Leukocyte counts above 10.5 G/L and CRP values above 100 mg/L were considered signs of a bacterial infection and, when combined with an X-ray finding consistent with pneumonia, an indication of bacterial pneumonia [23]. The CRP value upon admission as well as the maximum CRP level during hospitalization were noted, whereas leukocytes were registered at the time of admission only.
Statistics
Continuous data analysis was performed by non-parametric methods using the Kruskal–Wallis test for skewed data, as well as Student’s t-test for normally distributed data. Categorical data analysis was performed by the use of Pearson’s Chi-squared test. Length of stay analysis was performed by the use of Cox regression. One-way analysis of variance (ANOVA) with Bonferroni corrections were performed when comparing groups. Two-tailed p-values less than 0.05 were considered significant. All statistical analyses were performed using PASW 18 (SPSS Inc., Quarry Bay, Hong Kong).
Results
The results of the PCR analyses are published elsewhere [18]. Briefly, a positive real-time PCR test was obtained with at least one swab in 19 out of 147 patients: seven tested positive for influenza A virus, three for RSV, three for hMPV, two for adenovirus, two for parainfluenza virus type 3, one for influenza B virus, and one for parainfluenza virus type 4 [18]. None of the controls had a positive PCR.
The characteristics of the three groups are described in Table 2. The mean age and proportion living in a nursing home differed between the groups, being highest in the S/PCR+ group and lowest in the control group. Diagnosis at admittance is described in Online resource 1.
Table 2.
Characteristics of the study population
| S/PCR+ | S/PCR− | Control | p-Value/χ2 | |
|---|---|---|---|---|
| Number of patients | 19 | 128 | 56 | |
| Male gender, % | 42.1 | 60.2 | 66.1 | 0.183a |
| Age, mean (SD), years | 79 (8) | 76 (9) | 71 (9) | 0.002b |
| Smoking, % | 27.8 | 28.1 | 28.6 | 0.997a |
| Living in a nursing home, % | 21.1 | 11.3 | 1.8 | 0.027a |
| Leukocyte count at admission, mean (SD), G/L | 10.3 (6.0) | 12.4 (5.8) | 8.22 (2.6) | 0.000b |
| Maximum CRP, median (25th/75th percentile) | 60.0 (39/140) | 119.0 (56.5/227) | 11.5 (2/44) | <0.001b |
| Pneumonia at admission, % | 37 | 31 | 2 | <0.001a |
| In-hospital antibiotic treatment, % | 84 | 77 | 14 | <0.001a |
| Total CIRS-G score, median, (25th/75th percentile) | 11 (8/14) | 12 (9/16) | 11 (8/15) | 0.739a |
| CIRS-G respiratory, median (25th/75th percentile) | 2 (0/4) | 3 (0/4) | 0 (0/2) | <0.001a |
| CIRS-G cardiac, median, (25th/75th percentile) | 2 (1/2) | 2 (0/3) | 3 (0/3) | 0.010a |
| CCI, median (25th/75th percentile) | 1 (0/2) | 2 (1/2) | 1 (1/2) | 0.525a |
| Length of hospitalization, median (25th/75th percentile) | 3.9 (2.7/7.2) | 3.9 (2.3/6.8) | 2.2 (1.2/3.8) | 0.001c |
Characteristics of the study population divided into three groups: symptomatic and PCR-positive (S/PCR+), symptomatic and PCR-negative (S/PCR−), and non-symptomatic control (control)
CIRS-G Modified Cumulative Illness Rating Scale for Geriatrics; CCI Charlson comorbidity index
aPearson’s Chi-squared test
bOne-way analysis of variance (ANOVA)
cCox regression
The frequency of diagnosed pneumonia by chest X-ray and the frequency of antibiotic treatment during hospitalization did not differ between the S/PCR+ and S/PCR− symptomatic cohorts (Table 2), while pneumonia and antibiotic treatment was significantly lower in the control group. A total of 19/37 (51 %) of the patients receiving a tentative diagnosis of pneumonia at admission by the general practitioners had their pneumonia confirmed by chest X-ray. With no exception, the patients with a tentative diagnosis of pneumonia at admission and/or patients with a chest X-ray consistent with pneumonia were administered antibiotics immediately after hospitalization.
When the PCR results became available, only two of the 16 patients (12.5 %) in the S/PCR+ cohort had their antimicrobial treatment discontinued. In both cases, the PCR results were stated as the main reason for antibiotic discontinuation in the patients’ charts. Both patients were admitted by general practitioners with a diagnosis of pneumonia, and one of these had chest X-rays consistent with pneumonia.
Four further cases had CRP values below 100 mg/L and negative chest X-rays and, hence, in these cases, the likelihood of an ongoing bacterial infection was relatively low. In the remaining 10 patients, four had CRP values above 100 mg/L, two were diagnosed with chest X-ray findings consistent with pneumonia, and four patients presented with both of the latter findings. In those 10 patients, the possibility of an ongoing bacterial infection was relatively high.
In the length-of-stay analysis, a significant difference was found between the two symptomatic cohorts and the controls (p < 0.001), but no difference was observed between the S/PCR− and S/PCR+ cohorts (p = 0.39) (Fig. 1). Subgroup analysis of patients admitted by general practitioners to the hospital with a tentative diagnosis of pneumonia or patients with chest X-ray at admittance consistent with pneumonia revealed no significant difference between the two symptomatic groups.
Fig. 1.
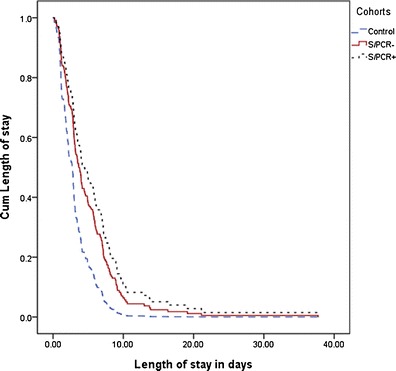
Cumulative length of stay in the three cohorts. Length-of-stay analysis was performed by Cox regression. The length of stay differed significantly between the three cohorts (p < 0.001), but not when comparing the two symptomatic cohorts S/PCR+ and S/PCR− (p = 0.39)
No difference between the three cohorts was found with regard to the total CIRS-G score (p = 0.29) and CCI score (p = 0.15) (Table 2). A significantly higher proportion of category four CIRS-G domain scores were found in the S/PCR− cohort (p = 0.032) compared to the S/PCR+ cohort.
When comparing the self-reported symptoms between the two symptomatic cohorts, a significant difference was found only for coughing (p = 0.004), which was reported more often in the S/PCR+ than in the S/PCR− cohort (Table 3). No significant difference between the cohorts was found when comparing the number of days spent in intensive care or deaths during the next 12 months.
Table 3.
The patients’ self-reported symptoms at the time of study inclusion
| S/PCR+ | S/PCR− | p-Value | |
|---|---|---|---|
| Cough, % | 100 (19/19)a | 71.5 (89/125)a | 0.004 |
| Fever, anamnestic, % | 73.7 (14/19)a | 60.8 (76/125)a | 0.321 |
| Phlegm, % | 52.9 (9/17)a | 50.4 (59/117)a | 1.000 |
| Nasal discharge, % | 52.6 (10/19)a | 31.2 (39/125)a | 0.075 |
| Sore throat, % | 42.1 (8/19)a | 21.6 (27/125)a | 0.081 |
| No. of symptomatic days, mean (SD) | 5.8 (3.8) | 5.7 (5.3) | 0.940 |
Persistent, self-reported symptoms at the time of study inclusion, lasting less than 3 weeks. A comparison between the two groups was performed by the use of Fisher’s exact two-sided test, with significant p-values at < 0.05
aNumber of patients reporting symptoms/total number of patients
Discussion
The main conclusions of this study of elderly patients hospitalized with symptoms of respiratory tract infections were: (1) a positive viral diagnosis by PCR resulted in the discontinuation of antibacterial treatment in only a minority of the cases; (2) symptoms of RVI, regardless of the real-time PCR results, predicted a longer hospital stay; (3) as all our non-symptomatic controls were negative for these common virus types by real-time PCR, the detection of influenza virus or RSV in the nasopharynx of an elderly person with symptoms of a respiratory tract infection was likely to be etiologically relevant.
In the S/PCR+ cohort, 6/19 patients were prescribed antibiotics prior to hospitalization and16/19 patients were administered antibiotics at the time of hospitalization. Only 2/16 patients had their antimicrobial treatment discontinued following viral PCR diagnostics. These results are comparable to other studies, where antibiotics were discontinued in 9.1–28.6 % of the patients after receiving a positive rapid viral test [6, 17, 24]. The impact of rapid viral screening on antibiotic administration in children is not easily transferable to the elderly. First, a negative result of PCR in elderly adults is less reliable due to the reduced viral shedding in this age group [9]. Second, even with a positive viral PCR result, there is an understandable reluctance to discontinue antibiotic treatment in the elderly, as immunosenescence and comorbidities render these patients at increased risk for a bacterial co-infection [25]. Unfortunately, the elderly may be particularly vulnerable to the consequences of inappropriate use of antibiotics, such as adverse drug events, super-infection by Clostridium difficile, and, ultimately, selection of resistant bacteria [26]. Due to their reduced physiological reserves, residents in long-term care facilities seem to be at particular risk in this regard [27, 28].
It is conceivable that the subgroup of elderly patients needing hospitalization for a respiratory tract infection is more frail than the average elderly population. To our knowledge, no frailty assessments using validated scales in combination with real-time viral PCR have been performed in this patient group. In the present study, no difference in comorbidity measured by the total CIRS-G and CCI scores was found between the three hospitalized groups, but no comparison could be made in this regard with a representative group of non-hospitalized elderly individuals. The present control group was younger and less likely to reside in a nursing home than the symptomatic groups. With the often atypical clinical presentation of an RVI in elderly patients, adherence to clinical guidelines might be difficult, often due to difficulties with the initial diagnosis [9, 23]. In the present study, a tentative pneumonia diagnosis at admission was confirmed by chest X-ray in only half of the patient population. In one study, a local educational program for the treatment of respiratory infections in the elderly significantly reduced the prescription rate and influenced the types of antibiotics prescribed [29], thus indicating the effectiveness of age-adjusted guidelines of antibiotic treatment also in elderly patients.
No difference in the length of stay was found between the two symptomatic cohorts. These results are in accordance with previous studies in the elderly, where no difference in length of stay was observed between influenza-positive and influenza-negative patients hospitalized due to respiratory symptoms [6]. Studies performed by Lee et al. have shown a decrease in the average length of stay with antiviral treatment [30]. None of our seven influenza-positive patients received antiviral treatment, which, in an early phase of the disease, might have decreased the average length of stay in the S/PCR+ cohort.
Nineteen percent of the patients in our study reported at least one symptom consistent with an acute respiratory infection. As in most viral infections of the respiratory tract the shedding declines rapidly, there is a high risk of obtaining a swabbed sample with a virus concentration below the detection threshold, especially a few days after the start of symptoms [8, 9].
The strengths of this study are the prospective design, the relatively high number of patients included, and the use of real-time PCR for virological analysis. Also, since our hospital is the only general hospital in the county of Aust-Agder, our patients are representative for the elderly hospitalized population in our region. In addition, the low number of specially trained staff harvesting the viral samples minimized the risk of poor-quality samples. A limitation to this study is the low number of patients with positive viral swabs. Economical considerations prohibited PCR analysis for rhinovirus and coronavirus; an inclusion of these viruses in the PCR panel could have improved the number of virus-positive swabs. Although final conclusions regarding antibiotic use and length of stay in this patient group are not possible based on this study alone, we believe that our results contribute to improved knowledge of the impact of real-time PCR for respiratory viruses in the elderly.
In conclusion, this study of elderly patients reporting symptoms of an RVI has shown that access to an early viral diagnosis had limited impact on the antimicrobial treatment. The challenge related to unnecessary antibiotic treatment in the elderly needs to be addressed, both in research and in guidelines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Diagnosis of admittance for the three cohorts. (DOCX 12 kb)
Acknowledgments
Thanks go to Anne Liv Lyngroth for her contribution to the study planning and data acquisition.
Conflict of interest
The nylon flocked swabs and UTM transport tubes were donated by Copan Italia, Bresica, Italy, and the rayon swabs and transport medium were donated by Chemi-teknik A/S, Oslo, Norway. Both suppliers delivered the swabs free of charge. The suppliers had no role in the planning, running, evaluation, or reporting of this trial. The authors declare that they have no conflict of interest related to this report.
Sponsor’s role
This project was financed with the aid of EXTRA funds from the Norwegian Foundation for Health and Rehabilitation in association with the Norwegian Heart and Lung Patient Organization, from the South-Eastern Norway Regional Health Authority, and from Sorlandet Hospital HF.
References
- 1.Ellis SE, Coffey CS, Mitchel EF, Jr, Dittus RS, Griffin MR. Influenza- and respiratory syncytial virus-associated morbidity and mortality in the nursing home population. J Am Geriatr Soc. 2003;51(6):761–767. doi: 10.1046/j.1365-2389.2003.51254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Kaye M, Skidmore S, Osman H, Weinbren M, Warren R. Surveillance of respiratory virus infections in adult hospital admissions using rapid methods. Epidemiol Infect. 2006;134(4):792–798. doi: 10.1017/S0950268805005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boivin G, de Serres G, Hamelin ME, Côté S, Argouin M, Tremblay G, Maranda-Aubut R, Sauvageau C, Ouakki M, Boulianne N, Couture C. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44(9):1152–1158. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 6.Falsey AR, Murata Y, Walsh EE. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167(4):354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- 7.Duncan CB, Walsh EE, Peterson DR, Lee FE, Falsey AR. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J Infect Dis. 2009;200(8):1242–1246. doi: 10.1086/605948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168(22):2489–2496. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talbot HK, Falsey AR. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis. 2010;50(5):747–751. doi: 10.1086/650486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, Valleron AJ. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 11.Lee N, Chan PK, Hui DS, Rainer TH, Wong E, Choi KW, Lui GC, Wong BC, Wong RY, Lam WY, Chu IM, Lai RW, Cockram CS, Sung JJ. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulrennan S, Tempone SS, Ling IT, Williams SH, Gan GC, Murray RJ, Speers DJ. Pandemic influenza (H1N1) 2009 pneumonia: CURB-65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS One. 2010;5(9):e12849. doi: 10.1371/journal.pone.0012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carman WF, Elder AG, Wallace LA, McAulay K, Walker A, Murray GD, Stott DJ. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet. 2000;355(9198):93–97. doi: 10.1016/S0140-6736(99)05190-9. [DOI] [PubMed] [Google Scholar]
- 14.Falsey AR, McCann RM, Hall WJ, Criddle MM. Evaluation of four methods for the diagnosis of respiratory syncytial virus infection in older adults. J Am Geriatr Soc. 1996;44(1):71–73. doi: 10.1111/j.1532-5415.1996.tb05641.x. [DOI] [PubMed] [Google Scholar]
- 15.Mahony JB, Blackhouse G, Babwah J, Smieja M, Buracond S, Chong S, Ciccotelli W, O’Shea T, Alnakhli D, Griffiths-Turner M, Goeree R. Cost analysis of multiplex PCR testing for diagnosing respiratory virus infections. J Clin Microbiol. 2009;47(9):2812–2817. doi: 10.1128/JCM.00556-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112(2):363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 17.Barenfanger J, Drake C, Leon N, Mueller T, Troutt T. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J Clin Microbiol. 2000;38(8):2824–2828. doi: 10.1128/jcm.38.8.2824-2828.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernes SS, Quarsten H, Hagen E, Lyngroth AL, Pripp AH, Bjorvatn B, Bakke PS. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur J Clin Microbiol Infect Dis. 2011;30(2):159–165. doi: 10.1007/s10096-010-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-N. [DOI] [PubMed] [Google Scholar]
- 20.Salvi F, Miller MD, Grilli A, Giorgi R, Towers AL, Morichi V, Spazzafumo L, Mancinelli L, Espinosa E, Rappelli A, Dessì-Fulgheri P. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008;56(10):1926–1931. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 21.Rognstad GPR (2009) The modified cumulative illness rating scale, Norwegian version
- 22.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453–471. doi: 10.1016/S0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 23.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Read R, Verheij TJ, Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oosterheert JJ, van Loon AM, Schuurman R, Hoepelman AI, Hak E, Thijsen S, Nossent G, Schneider MM, Hustinx WM, Bonten MJ. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41(10):1438–1444. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beadling C, Slifka MK. How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dis. 2004;17(3):185–191. doi: 10.1097/00001432-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Niederman MS. Principles of appropriate antibiotic use. Int J Antimicrob Agents. 2005;26(Suppl 3):S170–S175. doi: 10.1016/S0924-8579(05)80324-3. [DOI] [PubMed] [Google Scholar]
- 27.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012;81(1):1–14. doi: 10.1016/j.jhin.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Lautenbach E, Lee I, Shiley KT. Treating viral respiratory tract infections with antibiotics in hospitals: no longer a case of mistaken identity. LDI Issue Brief. 2010;16(3):1–4. [PubMed] [Google Scholar]
- 29.Lutters M, Harbarth S, Janssens JP, Freudiger H, Herrmann F, Michel JP, Vogt N. Effect of a comprehensive, multidisciplinary, educational program on the use of antibiotics in a geriatric university hospital. J Am Geriatr Soc. 2004;52(1):112–116. doi: 10.1111/j.1532-5415.2004.52019.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee N, Chan PK, Choi KW, Lui G, Wong B, Cockram CS, Hui DS, Lai R, Tang JW, Sung JJ. Factors associated with early hospital discharge of adult influenza patients. Antivir Ther. 2007;12(4):501–508. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnosis of admittance for the three cohorts. (DOCX 12 kb)


