Abstract
Pneumoviruses have been identified as causative agents in several respiratory disease outbreaks in habituated wild great apes. Based on phylogenetic evidence, transmission from humans is likely. However, the pathogens have never been detected in the local human population prior to or at the same time as an outbreak. Here, we report the first simultaneous detection of a human respiratory syncytial virus (HRSV) infection in western lowland gorillas (Gorilla gorilla gorilla) and in the local human population at a field program in the Central African Republic. A total of 15 gorilla and 15 human fecal samples and 80 human throat swabs were tested for HRSV, human metapneumovirus, and other respiratory viruses. We were able to obtain identical sequences for HRSV A from four gorillas and four humans. In contrast, we did not detect HRSV or any other classic human respiratory virus in gorilla fecal samples in two other outbreaks in the same field program. Enterovirus sequences were detected but the implication of these viruses in the etiology of these outbreaks remains speculative. Our findings of HRSV in wild but human-habituated gorillas underline, once again, the risk of interspecies transmission from humans to endangered great apes.
Keywords: respiratory disease, respiratory syncytial virus, enterovirus, western lowland gorillas, great apes, noninvasive detection
Introduction
Great ape habituation projects have been established in Africa since the 1950s (Gruen et al. 2013). They have contributed to our understanding of great ape behavior and societies. Together with great ape tourist sites, they have also contributed significantly to the conservation of great apes and their habitats by bringing revenue to local communities (Macfie and Williamson 2010) and by protecting animals from poaching (Pusey et al. 2007; Köndgen et al. 2008; Campbell et al. 2011).
Anecdotal reports of respiratory disease (RD) outbreaks in habituated great apes reach back to the 1960s (Lonsdorf et al. 2006). However, no systematic investigations were performed until much later, when the likely etiology of eight outbreaks in western chimpanzees (Pan troglodytes verus), eastern chimpanzees (Pan troglodytes schweinfurthii), and mountain gorillas (Gorilla beringei beringei) was clarified. These investigations identified two common human viruses, the human respiratory syncytial virus and the human metapneumovirus (HRSV and HMPV; family Paramyxoviridae, subfamily Pneumovirinae), as plausible causative agents (Kaur et al. 2008; Köndgen et al. 2008; Palacios et al. 2011). HRSV and HMPV circulate globally within human populations and are known to cause only mild respiratory symptoms in healthy adults. In infants however, they can lead to severe lower respiratory tract infections with significant mortality (Weber et al. 1998). In six outbreaks in wild-habituated great apes, these infections seem to have led to opportunistic coinfections with Streptococcus pneumoniae or Pasteurella multocida (Chi et al. 2007; Köndgen et al. 2008, 2011; Palacios et al. 2011). The impact of these outbreaks was often severe, with morbidity and fatality rates as high as 100 and 19%, respectively (Köndgen et al. 2008). Human paramyxoviruses have also been detected during great ape respiratory disease outbreaks in captive settings. In these outbreaks, morbidity and fatality rates reached up to 100 and 10%, respectively, despite treatment (Szentiks et al. 2009; Unwin et al. 2013; Slater et al. 2014).
Many recent studies identify enzootic viruses, such as enteroviruses (EV) and adenoviruses (AdV), in wild and captive great apes, some of which are closely related to human-infecting respiratory pathogens (Wevers et al. 2010, 2011; Harvala et al. 2011, 2014; Hoppe et al. 2015). In addition, serological evidence and experimental studies in captive settings show that great apes are susceptible to a number of other human RD-causing pathogens, such as parainfluenza viruses, rhinoviruses, or measles virus (Kalter et al. 1997; Bennett et al. 1998; Kooriyama et al. 2013; Buitendijk et al. 2014). This raises the possibility that not only human paramyxoviruses are responsible for RD outbreaks in wild great ape populations.
To identify whether other viruses are responsible for RD outbreaks, it is important to investigate the etiology and origin of even more great ape RD outbreaks. Ideally, strong onsite capacities will allow for immediate sample collection and diagnostics of both great apes and neighboring human populations, thereby maximizing the chances of identifying causative agents and possibly therapeutic and preventive options. Here, we report about three RD outbreaks that struck two habituated western lowland gorilla (Gorilla gorilla gorilla) groups at a research and tourism program in Dzanga Sangha Protected Areas in Central African Republic between March 2012 and November 2014. The second outbreak was investigated with a field laboratory onsite, and analyses were performed while the outbreak was active (real-time investigation). This outbreak occurred simultaneously in humans and one habituated gorilla group in August 2012. Respiratory symptoms in the local human population, including coughing, nasal discharge, sneezing, elevated breathing rate, and wheezing sounds during expiration, occurred before the first symptoms in the gorillas were noted.
Material and Methods
Field Sites
Situated in the northwestern Congo Basin, Dzanga Sangha Protected Areas (DSPA) in Central African Republic (CAR) includes the Dzanga-Ndoki National Park, which, together with Lobéké National Park in Cameroon and Nouabalé-Ndoki National Park in the Republic of Congo, form the Sangha Tri-National World Heritage Site (inscribed by UNESCO in 2012). The DSPA is managed by a partnership between the national government of CAR and Worldwide Fund for Nature (WWF). As part of WWF’s long-term habituation project, the Primate Habituation Program, where two groups of gorillas have been fully habituated (Makumba and Mayele group) at adjacent sites (Bai Hokou and Mongambe, respectively, approximately 10 km apart); another two groups were still under habituation at the time of the study. Within the National Parks, human activities such as hunting, gathering, fishing, and agriculture are prohibited and access is strictly regulated. Humans do not permanently live in the park but temporarily stay in one of the several research camps. Tourists are not allowed to stay overnight. The people working for the project, and thus entering gorilla habitat, live either in or close to the village of Bayanga, which lies within DSPA, outside of Dzanga-Ndoki National Park (Fig. 1).
Figure 1.
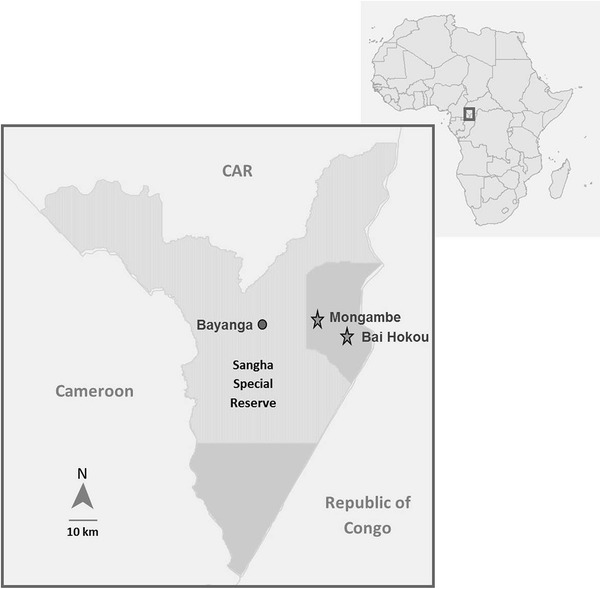
Map of field site.
Samples
Outbreak 1 (February 2012)
Seven (individually identified) gorilla fecal samples, from the seven symptomatic members of the Makumba group (group size, N = 9) were collected, put into tubes containing Ambion® RNAlater ® Solution (Invitrogen) and kept at ambient temperature. The severe symptoms observed in this outbreak (very productive cough to the point of vomiting and by day 24, 8 out of 9 group members were affected), which continued until April 2012, warranted the temporary halt of tourist visits and a veterinary intervention with antibiotics (described in Vlckova et al, submitted). Additionally, fifteen human fecal samples were collected from the corresponding research camp.
Outbreak 2 (August 2012)
Fifteen gorilla fecal samples from eight (individually identified) symptomatic individuals of the Makumba group were collected. Symptoms included cough, lethargy, and running noses. By day 14, 7 out of 8 group members were affected. Each member of the group was sampled at least once. Individuals with the most severe symptoms (highest frequency of coughing) were sampled repeatedly. Simultaneously, a total of 80 throat swabs were taken from humans who enter the gorilla habitat (or were in direct contact with those who do), divided into six demographic groups: tracker (guides) (N = 25), symptomatic tracker family members (N = 9), researcher (N = 2), symptomatic researcher family member (N = 1), project/camp assistants (N = 5), and eco-guards (park rangers) (N = 38). The samples were put into tubes containing Ambion® RNAlater ® Solution and maintained at ambient temperature for 2–3 days. Afterwards they were frozen at −14°C.
Outbreak 3 (August 2014)
Ten fecal samples from two of the most severely symptomatic gorillas (highest frequency of coughing) of the Mayele group (group size, N = 15), were taken initially with fifteen more samples from ten (individually identified) individuals collected at a later stage of the outbreak (10 weeks later). A cough persisted in the group over several months, with varying intensities and frequencies in different individuals. Sample collection was attempted from all group members, if possible, with an emphasis on those with a higher frequency or intensity of coughing. Samples were put into tubes containing Ambion® RNAlater ® Solution and kept at ambient temperature. No human samples were obtained during this outbreak. All samples were transported to Germany, where they were stored at −80°C.
Field Lab Analysis/Real-Time Investigation (Performed During Outbreak 2)
Eight gorilla fecal samples and 20 human throat swabs were tested in a field laboratory. RNA was extracted from fecal samples and cDNA synthesized as described by Köndgen et al. (2010). RNA from throat swabs was extracted using the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Samples were screened for HRSV and HMPV using generic PCR protocols as described by Reiche and Schweiger (2009) and Reiche et al. (2014), excluding the probes, targeting the N or F protein gene, respectively, with expected amplicon sizes of 142 and 161 bp. Amplification was conducted for 5 min at 95°C, followed by 45 cycles of 95°C for 15 s, and 60°C for 30 s. PCR products were analyzed using electrophoresis in a 2% agarose gel. Positive and negative controls were included in each run. Positive samples were tested with an additional confirmation PCR; for HRSV, a hemi-nested PCR was performed targeting the hypervariable region of the G protein gene as described previously by Sato et al. (2005). Amplification was carried out for 5 min at 94°C, followed by 40 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min, with a final 7 min of extension at 72°C. PCR products were analyzed by electrophoresis in a 1.5% agarose gel, with an expected amplicon size of 458 bp. Human and gorilla samples were processed separately, spaced at two weeks between with gorilla samples tested first.
Confirmation and Additional Testing at the Robert Koch-Institute
In addition to all remaining samples (seven gorilla, 60 human), the eight gorilla and 20 human samples screened in the field were retested upon their arrival in Germany. RNA was extracted as described above; gorilla and human samples were handled separately, on separate days. The samples that had been tested in the field were extracted again. Samples were tested in duplicates, for human respiratory pathogens, following the protocols summarized in Table 1, with the exception of human throat swabs, which were not tested for S. pneumoniae. PCR products from positive samples were purified using ExoSAP (USB Europe GmbH, Staufen, Germany) and sequenced using the ABI BigDye Termination Kit (Applied Biosystems, Weiterstadt, Germany). When multiple bands were present, the expected band was cut from the gel, purified, and sequenced. If no clean sequence could be obtained, the procedure was repeated and the purified PCR products were cloned with the Topo TA Cloning Kit (Invitrogen). Colonies were analyzed using colony PCR, and positive samples were purified and sequenced. When confirmation by other systems was not possible, the qPCR products were also sequenced.
Table 1.
Primers Used in This Study
| Pathogen | Assay type | Primers | Conditions described in |
|---|---|---|---|
| Adenovirus (AdV) | TaqMan PCR |
AD-024S GAC GCY TCG GAG TAC CTG AG AD-024R RGC CAG IGT RWA ICG MRC YTT GTA AD-024 probe 6FAM-CTG GTG CAG TTY GCC CGC-TAMRA |
Chmielewicz et al. 2005 |
| Corona virus (CoV) | Conventional PCR |
CoV All F AAR TTT TAY GGY GGB TGG VAT RAY ATG TT CoV All R TGY TGD GAR CAR AAY TCR TGW GGT CC |
Nitsche, unpublished data* |
| Enterovirus (EV) | TaqMan PCR |
E-TM 1 S GCC CCT GAA TGC GGC TAA T E-TM 2 R RAT TGT CAC CAT AAG CAG YCA EV probe 6FAM AAC CGA CTA CTT TGG GTG TCC GTG TTT C-TAMRA |
Pusch et al. 2005 |
| Human metapneumovirus (HMPV) | Two-step real-time RT-PCR for screening |
hMPV F S gCTCCgTAATYTACATggTgCA hMPV F S1 gAAgCTCYgTgATTTACATggTYCA hMPV F AS gACCCTgCARTCTgACAATACCA hMPV F AS1 AgTKgATCCTgCATTTTTACAATACCA hMPV F TMGB F-CCYTgCTggATAgTAAAA-MGB hMPV F TMGB1 F-CCTTgTTggATAATCAA-MGB |
Reiche et al. 2014 |
| Conventional confirmation PCR |
MPVP 01.6 S ATG TCA TTC CCT GAA GGA MPVM 02.4 R GTC TAC TAG GTA GGA CTC CAT |
Mackay et al. 2004 | |
| Human respiratory syncytial virus (HRSV) | TaqMan PCR for screening |
RSV 1084 GAT GGC TCT TAG CAA AGT CAA GTT RSV 170 CAT CTT CWG TGA TTA ATA RCA TCR CAC ATA RSV MGB probe FAM-ACA GGA GAT ART ATT DAY ACT C-NFQ MGB |
Reiche and Schweiger 2009 |
| HRSV A | Semi-nested confirmation PCR |
GPB AAG ATG ATT ACC ATT TTG AAG T F1 CAA CTC CAT TGT TAT TTG CC nRSBG GTG GCA ACA ATC AAC TCT GC |
Sato et al. 2005 |
| HRSV B |
GPA TTG AAG TGT TCA ACT TCG TTC C F1 CAA CTC CAT TGT TAT TTG CC nRSAG TAT GCA GCA ACA ATC CAA CC |
||
| Influenza virus A (Flu A) | Real-time PCR |
M+25 AgA TgA gTC TTC TAA CCg Agg TCg M-124BB ccW gCA AAR ACA TCY TCA AgT YTC Tg M-124sw CTg CAA AgA CAC TTT CCA gTC TCT g M+64 MGB FAM-TCA ggC CCC CTC AA-MGB |
Schulze et al. 2010 |
| Influenza virus B (Flu B) | Real-time PCR |
BMP-13 gAg ACA CAA TTg CCT ACC TgC BMP-102AN TTC CCA CCR AAC CAR CAR TgT AAT BMP-72 MGB FAM-CTg CTT TgC CTT CTC-MGB |
Schulze et al. 2010 |
| Pan pneumovirus | Semi-nested PCR |
PNE-F1 GTG TAG GTA GIA TGT TYG CNA TGC ARC C PNE-F2 ACT GAT CTI AGY AAR TTY AAY CAR GC PNE-R GTC CCA CAA ITT TTG RCA CCA NCC YTC |
Tong et al. 2008 |
| Pan paramyxovirus | Semi-nested PCR |
PAR-F1 GAA GGI TAT TGT CAI AAR NTN TGG AC PAR-F2 GTT GCT TCA ATG GTT CAR GGN GAY AA PAR-R GCT GAA GTT ACI GGI TCI CCD ATR TTN C |
Tong et al. 2008 |
| Pan picornavirus | Semi-nested PCR |
OL-26 s GCA CTT CTG TTT CCC C OL-27 as CGG ACA CCC AAA GTA G JWA-1b as CAT TCA GGG GCC GGA GGA |
Jang et al. 2005 |
| S. pneumoniae | TaqMan PCR for screening |
SP_autolysin_F ACG CAA TCT AGC AGA TGA AGC SP_autolysin_R TGT TTG GTT GGT TAT TCG TGC SP_lytA_TM FAM-TTT GCC GAA AAC GCT TGA TAC AGG G–TAMRA |
MacAvin et al. 2001 |
PCR performed in a total volume of 25 µl with 0.1 µl Platinum Taq polymerase (Applied Biosystems, Darmstadt, Germany), 0.75 µM of each primer in a 300 nM concentration, a 200 µM concentration of each deoxynucleoside triphosphate (dNTP), and 2 mM MgCl2; cycling conditions: 95°C for 5 min and 45 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min, with a final 7 min of extension at 72°C and an expected amplicon size of 670 bp.nnn
All viral sequences generated were analyzed using Geneious R7.1.4 (Biomatters Limited) and blasted against the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Samples were only considered positive for a given pathogen if a sequence of sufficient quality could be obtained.
Phylogenetic Analysis
We did not perform phylogenetic analyses of the HRSV sequences, as the identical sequences found in gorilla and local human samples already substantiated a recent transmission event. In contrast, the enterovirus (EV) sequences did not find any close to perfect match in public databases. We therefore performed phylogenetic analyses on a dataset that also comprised 109 representative sequences of human and nonhuman primate EV sequences. The sequences were aligned using Muscle (Edgar 2004), and conserved blocks of the alignment were selected using Gblocks (Talavera and Castresana 2007). Both steps were performed in SeaView v4 (Gouy et al. 2010). The best model of nucleotide evolution was identified using jModelTest v2.1.4 (Guindon and Gascuel 2003; Darriba et al. 2012), applying the Bayesian information criterion. Phylogenetic analyses per se were performed in maximum likelihood (ML) and Bayesian frameworks. ML analyses were performed using PhyML v3 (Guindon et al. 2010); branch robustness was determined by nonparametric bootstrapping (250 pseudoreplicates). Bayesian analyses were run in BEAST v1.8.2 (Drummond et al. 2012) under a lognormal-relaxed clock and describing tree shape with a coalescent Bayesian SkyGrid model. Two chains were run for 50 million generations; convergence of the runs and appropriate sampling of the posterior were assessed using Tracer v1.6 (Rambaut et al. 2014). Post burn-in trees from the two chains were combined using LogCombiner v1.8.2 before being summarized onto the maximum clade credibility tree identified with TreeAnnotator v1.8.2 (both software programs are distributed with BEAST). Branch robustness was assessed through posterior probabilities.
Results
For an overview of results see Table 2, details in Table 3.
Table 2.
Overview of Findings
| Outbreak 1 (Start Feb 2012) | Outbreak 2 (Start Aug 2012) | Outbreak 3 (Start Aug 2014) | |
|---|---|---|---|
| Gorilla group | Makumba | Makumba | Mayele |
| No of individuals affected | 8 | 6 | 5 |
| Duration | Feb–Apr 2012 | Aug–Sep 2012 | Aug 2014–Feb 2015 |
| Pathogens found in gorilla fecal samples | EV (1/7) | HRSV (4/15), AdV (1/15) | EV (12/25) |
| Pathogens found in human samples (fecal samples and throat swaps) | EV (1/16), AdV (5/16) | HRSV (4/80), HMPV (1/80), RV (1/80) | N/A |
Table 3.
Sequences Obtained in Three Independent Outbreaks
| Sample type | No. of samples | Pan Pneu L gene ~240 bp | HRSV N gene ~140 bp |
HRSV G gene ~450 bp |
HMPV P gene ~350 bp |
EV 5’UTR ~320 bp |
RV 5’UTR ~390 bp |
|
|---|---|---|---|---|---|---|---|---|
| Outbreak 1 | ||||||||
| Gorillas | Feces | 7 | – | – | – | – | 1 | – |
| Humans | Feces | 15 | – | – | – | – | 1 | |
| Outbreak 2 | ||||||||
| Gorillas | Feces | 15 | 3 | 4 | 1 (poor quality) | – | – | – |
| Researcher | Throat swab | 2 | – | – | – | – | – | – |
| Researcher family member | Throat swab | 1 | 1 | 1 | 1 | – | – | – |
| Camp assistant | Throat swab | 5 | 2 | 2 | 2 | – | – | 1 |
| Tracker | Throat swab | 25 | – | – | – | – | – | – |
| Tracker family member | Throat swab | 9 | 1 | 1 | 1 | – | – | – |
| Eco-guards | Throat swab | 38 | 1 | – | – | 1 | – | – |
| Outbreak 3 | ||||||||
| Gorillas (beginning) | Feces | 10 | – | – | – | – | 6 | – |
| Gorillas (10 weeks later) | Feces | 15 | – | – | – | – | 6 | – |
Sequences identical for humans and gorillas in outbreak 2 highlighted in bold and for outbreak 1 and 3 in italics.
Outbreak 1
All gorilla fecal samples were negative for HRSV and HMPV; one tested positive for EV. The closest hit in the NCBI database, with 90% identity, was Simian agent 5 (strain B165, complete genome; Accession: AF326751.2). All gorilla samples were negative for other pathogens tested.
All simultaneously collected human fecal samples also tested negative for HRSV and HMPV; one also tested positive for EV. The closest hit in the NCBI database was swine vesicular virus (isolate ITL 2/92 5’ UTR; Accession: AY875991.1) with 89% identity and human enterovirus 71 (isolate 17001, gene for polyprotein, partial cds; Accession: AB575924.1) with 90% identity.
The gorilla EV differed in 83/234 positions from the human EV.
Outbreak 2
Field Analysis
Of the fifteen gorilla fecal samples from eight individuals, four samples (from four different individuals) tested positive for HRSV in the field laboratory with the generic screening PCR. The electrophorese gels of the confirmation PCRs showed multiple bands, which were difficult to interpret. Three of the 20 human throat swabs (one researcher, one researcher family member, and one tracker family member) tested for HRSV with the generic screening PCR were also positive on the gel.
Analyses at the Robert Koch-Institute
All collected samples were (re)tested in conventional laboratory settings. All field laboratory results from the four gorilla samples could be confirmed by real-time PCR. The sequences obtained from this assay were 100% identical with known HRSV A strains (RSVA/Homo sapiens/USA/TH_10454/2013, complete genome; Accession: KU950698.1). Unfortunately, from the confirmation assays, no sequences of satisfying quality could be obtained for phylogenetic analyses. From three of the four positive samples, sequences could be generated from the pan-pneumovirus assay (targeting the L protein gene) and were 99% identical with known HRSV A strains (human respiratory syncytial virus isolate Kilifi_10028_12_RSVA_2003, partial genome; KP317955.1). All other pathogen tests (as in Table S1) were negative.
Of the 20 retested human samples, two HRSV positive and all negative samples from the field could be confirmed. Only one sample that tested positive for HRSV in the field could not be confirmed. An additional two human throat swabs (from two camp assistants), from the 60, which had not been tested in the field, came up positive for HRSV. Sequences of relevant length could be obtained, using further PCRs targeting the G and the L genes; sequences of the G gene were 99% identical with known HRSV A strains (RSV/PUNE/NIV1063010/10/A attachment glycoprotein G gene, complete cds; and fusion protein F gene, partial cds; Accession: KF246622.1). All HRSV sequences from the different human individuals were identical, and the L and N protein gene sequences obtained from human and gorilla samples were also identical each. One eco-guard tested positive for HMPV and one camp assistant for rhinovirus. All other human throat swabs were negative for all remaining pathogens tested (note they were not tested for S. pneumoniae, because characterization of streptococci from a throat swab, only based on molecular methods, is very challenging and needs careful interpretation).
Outbreak 3
Ten fecal samples from two gorillas tested negative for HRSV and HMPV. EV was detected in both individuals. The sequences were identical with each other and the human sequence obtained from outbreak 1 but clearly distinct from the gorilla sequence from outbreak 1 (Fig. 2). Fifteen samples from 10 individuals of the same group, including the two initially tested individuals, were tested two months after the onset of the outbreak when symptoms were continuing or increasing in some individuals. The same EV was found in three gorillas that had the most severe symptoms (i.e., highest frequency and intensity of coughing). All sequences were compared to the nonredundant nucleotide sequence database of the National Center for Biotechnology Information (NCBI), using BLAST. The closest hits were swine vesicular virus and human enterovirus 71 (isolates as above in outbreak 1). In line with BLAST results, the new gorilla EV sequence did not appear in close sistership with any EV sequence published to date (Fig. 2). Furthermore, it did not cluster with any sequences identified from wild chimpanzees, or other nonhuman primates that could be included in these analyses. All samples were negative for the remaining pathogens tested.
Figure 2.
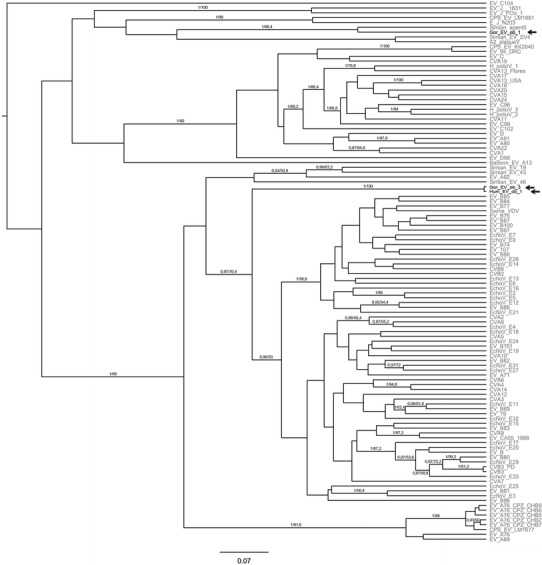
Phylogenetic positions of enteroviruses found in a human and a gorilla and two years later in another gorilla group during respiratory disease outbreaks. EVs were named as follows: ‘Gor_EV_ob_1’ Gorilla Enterovirus outbreak 1, ‘Hum_EV_ob_1’ human enterovirus outbreak 1, ‘Gor_EV_ob_3’ Gorilla Enterovirus outbreak 3. This tree is a maximum clade credibility tree generated from the output of Bayesian Markov chain Monte Carlo (BMCMC) analyses. We also ran maximum likelihood (ML) analyses; the ML tree was topologically very similar. Branch robustness was assessed through posterior probabilities (BMCMC) and nonparametric bootstrapping (ML); posterior probabilities/bootstrap values are plotted above branches.
Discussion
Until recently, diagnostics of respiratory pathogens were often only made after the outbreaks had taken place. Yet, timely onsite diagnostics allow for better disease management, ranging from targeted interventions (e.g., treatments by blow pipe using selected antibiotics as in our Outbreak 1) to management decisions (quarantine or similar for humans). Importantly, this approach also greatly enhances the likelihood of tracing back the origin of a given outbreak. The first simultaneous detection of HRSV in humans and wild-habituated great apes, as described for our outbreak 2, represents compelling evidence of a cross-species transmission from humans. That said, a spillback of the virus into the human population also remains possible.
No fatalities occurred in any of the outbreaks. All investigations were performed noninvasively. Successful detection of viruses in great ape feces can vary, however, depending on the viral load excreted, which will change over the time of the outbreak (Köndgen et al. 2010). Unfortunately, RNA degrades quickly in the environment and successful detection might also be influenced by variables such as food intake. This could explain why additional samples from individuals that tested positive for HRSV during outbreak 2 tested negative.
Real-time investigations can be challenging. For this study, during outbreak 2, throat swabs from humans were taken when the gorillas had already started showing symptoms and not at the exact time when the transmission would have occurred. Thus, the infectious pressure that can be determined from the samples, taken after the onset of the outbreak, does not represent the infectious pressure at the time of presumed transmission. However, the virus was circulating in the human population in the same time period as in the gorilla group, and likely before. This, along with the finding of other respiratory viruses circulating in the human population, also demonstrates the importance of an adequate onsite field laboratory to continuously monitor the health of habituation project staff, the surrounding human populations, and potentially any visitors who are brought into close contact with the apes.
Another reason that makes a human-to-gorilla-transmission plausible is that the sporadic contact between gorilla groups makes it highly unlikely that HRSV circulated within the ape population. In the described cases, group composition did not change shortly before the outbreaks started and no encounter with other groups was observed. However, once a pathogen enters a group, there are some distinct ape species differences with a marked effect on the potential outcome. In a habituated western lowland gorilla group, habituation success and group stability largely depend on the silverback. In contrast to chimpanzee communities, if the silverback dies during the course of an outbreak the remaining group members will disperse, which can lead to pathogen-mediated dispersal (Nunn et al. 2008). This would not only bear considerable economic losses for the habituation project but comes with the risk of infected group members joining other groups and thereby spreading the infection.
The habituation project described here, has employed a range of measures to prevent disease transmission from humans, including controlled human health status, no sick people allowed with the gorillas (although this does not mitigate people being nonsymptomatic carriers), minimum viewing distance, small observer groups, no eating, spitting, or leaving anything behind in the forest. Yet, facemasks had not been included in the prevention program, relying instead on behavioral mechanism such as, if provoked, turning away and coughing or sneezing into clothing, not hands. The use of facemasks, as a preventive measure, is widely recommended (Gilardi et al. 2015), based on assumptions made from human hygiene and respiratory disease prevention. For influenza viruses, which show similar modes of aerosol transmission as pneumoviruses, wearing of surgical masks can reduce the overall viral copy numbers of exhaled aerosols by 3.4-fold; however, it cannot completely avoid the shedding of infectious virus beyond the mask (Milton et al. 2013). Commonly used surgical facemasks and N95 respirators have been shown to be equally effective in filtering influenza virus (Johnson et al. 2009). Unfortunately, compliance seems to be a major limitation resulting in lower efficacy of face mask use (Radonovich et al. 2009). In a hot and humid environment under physical strain, wearing a N95 facemask is uncomfortable and it is unlikely that recommendations will be followed at all times. Due to the incomplete protection through face masks, the additional use of a field lab, which detects virus shedding, and a quarantine system could be an effective asset to existing prevention programs, as it does not rely as much on human compliance—although this would present logistical difficulties for tourism programs. An in-situ field lab could also be used for real-time and onsite diagnostics in other wildlife disease outbreaks, which would allow for timely management decisions. Furthermore it is useful in distinguishing humans infected with (and shedding) a respiratory pathogen from those with, for instance, a noninfectious smoker´s cough. Importantly, 96% (23/24) of the results from the field were confirmed at the Robert Koch-Institute in Germany, which supports the efficacy of a field laboratory.
In our outbreak 2, eight of the nine human tracker family members tested were infants <5 years of age. 50% of the HRSV positive samples stem from these infants, who made up only 10% of the human sample pool collected during the outbreak. However, in addition to small sample size, this analysis is biased, because only infants with respiratory symptoms were sampled. In contrast, only 30 of the 72 adults showed mild respiratory symptoms, of which most can likely be attributed to smoker´s cough. Even though only opportunistic sampling of symptomatic family members was performed, infants were the only individuals with severe respiratory symptoms (including wet cough, sneezing, nasal discharge, wheezing). HRSV is believed to be the most important viral pathogen causing acute lower respiratory disease (ALRI) in young children, with ALRI being the leading cause of global child mortality, with 99% of RSV-caused deaths occurring in developing countries (Nair et al. 2010). Thus, the contact of project staff and researchers with young children may represent a risk factor for the transmission of pneumoviruses to wild-habituated great apes. A One Health approach, improving children´s health around great ape habitat, could help to reduce infectious pressure on wild great apes.
In recent years, several enzootic viruses have been identified in wild great apes, some of which might cause clinical disease, such as EV and AdV. EV are among the most common human viruses, with high rates of subclinical infections (Palacios and Oberste 2005). Yet, they can be the cause of several, sometimes severe diseases and syndromes, such as acute hemorrhagic conjunctivitis, aseptic meningitis, acute flaccid paralysis, myopericarditis, hand-foot-and-mouth disease, and respiratory disease. Harvala et al. (2014) and Sadeuh-Mba et al. (2014) showed that EV are cocirculating in human and wild great ape populations; some are shared virus types and some genetically divergent EV variants. Unfortunately, studies investigating EV occurrence in wild great apes do not reveal information on the health status of the investigated animals; therefore, it is difficult to determine whether the pathogens had caused any illness. In captive settings, some human EV strains have been shown to cause RD in different nonhuman primates, including great apes (Kelly et al. 1977; Zhang et al. 2011; Nielsen et al. 2012). In our study, we found EV in both gorillas, and a human. The presence of an EV in the human population or wild great apes, respectively, is per se not surprising. However, the finding of the same virus in a human, and two years later in a gorilla group, is an indicator for yet another transmission, even if the directionality cannot be determined. Unlike HRSV, which is rather unstable in the environment, EV infection can occur, for instance, through contaminated water (Harvala et al. 2014). Thus a cocirculation in ape and human populations does not require the same physical proximity. The viruses found are unknown strains, which could be enzootic great ape strains or human EV strains unknown due to underreporting in the region. The fact that the shared EV was detected in the most symptomatic gorilla individuals could point toward its involvement as a causative agent.
In conclusion, our study adds to the growing body of evidence substantiating the early concerns expressed by Woodford et al. (2002). It further demonstrates that onsite real-time health monitoring offers excellent potential to untangle the causes of RD in habituated great apes. Once more, it pinpoints the involvement of human respiratory pathogens as likely causative agents.
Despite the risk of disease transmission that human presence in great ape habitat poses to the animals, research and tourism sites have also been demonstrated to have protective effects (Pusey et al. 2007; Köndgen et al. 2008; Campbell et al. 2011). To minimize the negative effects of human presence, disease surveillance and development of effective prevention strategies are crucial for the conservation of these endangered species.
Acknowledgments
We would like to thank the government of the Central African Republic for long-term support, especially the Ministère d’Eaux et Fôret, Chasse et Peche and the Ministère de l’Education Nationale, de l’Alphabetisation, de l’Enseignement Superieur, et de la Recherche. In particular, we thank Jean-Baptiste Mamang-Kanga, Guian Zokoe, and Christian Ndadet. We thank the staff of DSPA and especially of the Primate Habituation Programme, for logistical support in the field, and WWF for their support at DSPA and in Bangui. We would also like to thank the Hans Böckler Stiftung, the EAZA Ape Conservation Fund, Zoo Leipzig, and WWF Germany for support and funding. KJP worked under institutional support of the Institute of Vertebrate Biology Academy of Sciences of the Czech Republic (RVO: 68081766).
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- Bennett BT, Abee CR, Henrickson R. Nonhuman primates in biomedical research: diseases. Cambridge: Academic Press; 1998. [Google Scholar]
- Buitendijk H, Fagrouch Z, Niphuis H, Bogers WM, Warren KS, Verschoor EJ. Retrospective serology study of respiratory virus infections in captive great apes. Viruses. 2014;6(3):1442–1453. doi: 10.3390/v6031442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G, Kuehl H, Diarrassouba A, N’Goran PK, Boesch C (2011) Long-term research sites as refugia for threatened and over-harvested species. Biology Letters rsbl20110155 [DOI] [PMC free article] [PubMed]
- Chi F, Leider M, Leendertz F, Bergmann C, Boesch C, Schenk S, Pauli G, Ellerbrok H, Hakenbeck R. New Streptococcus pneumoniae clones in deceased wild chimpanzees. Journal of Bacteriology. 2007;189:6085–6088. doi: 10.1128/JB.00468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewicz B, Nitsche A, Schweiger B, Ellerbrok H. Development of a PCR-based assay for detection, quantification, and genotyping of human adenoviruses. Clinical Chemistry. 2005;51:1365–1373. doi: 10.1373/clinchem.2004.045088. [DOI] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9(8):772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KV, Gillespie TR, Leendertz FH, Macfie EJ, Travis DA, Whittier CA, Williamson EA. Best Practice Guidelines for Health Monitoring and Disease Control in Great Ape Populations. Gland: IUCN SSC Primate Specialist Group; 2015. p. 56. [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution. 2010;27(2):221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Systematic Biology. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Gruen L, Fultz A, Pruetz J. Ethical issues in African great ape field studies. Institute for Laboratory Animal Research Journal. 2013;54(1):24–32. doi: 10.1093/ilar/ilt016. [DOI] [PubMed] [Google Scholar]
- Harvala H, Sharp CP, Ngole EM, Delaporte E, Peeters M, Simmonds P. Detection and genetic characterization of enteroviruses circulating among wild populations of chimpanzees in Cameroon: relationship with human and simian enteroviruses. Journal of Virology. 2011;85(9):4480–4486. doi: 10.1128/JVI.02285-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvala H, Van Nguyen D, McIntyre C, Ahuka-Mundeke S, Ngole EM, Delaporte E, Peeters M, Simmonds P. Co-circulation of enteroviruses between apes and humans. Journal of General Virology. 2014;95(2):403–407. doi: 10.1099/vir.0.059048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe E, Pauly M, Gillespie TR, Akoua-Koffi C, Hohmann G, Fruth B, Karhemere S, Madinda NF, Mugisha L, Muyembe JJ, Todd A, Petrzelkova KJ, Gray M, Robbins M, Bergl RA, Wittig RM, Zuberbühler K, Boesch C, Schubert G, Leendertz FH, Ehlers B, Calvignac-Spencer S (2015) Multiple cross-species transmission events of human adenoviruses (HAdV) during hominine evolution. Molecular Biology and Evolution, msv090 [DOI] [PMC free article] [PubMed]
- Jang YJ, Lee SH, Kwon HJ, Chung YS, Lee BJ. Development of rhinovirus study model using organ culture of turbinate mucosa. Journal of Virological Methods. 2005;125:41–47. doi: 10.1016/j.jviromet.2004.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clinical Infectious Diseases. 2009;49:275–277. doi: 10.1086/600041. [DOI] [PubMed] [Google Scholar]
- Kalter SS, Heberling RL, Cooke AW, Barry JD, Tian PY, Northam WJ. Viral infections of nonhuman primates. Comparative Medicine. 1997;47(5):461–467. [PubMed] [Google Scholar]
- Kaur T, Singh J, Tong S, Humphrey C, Clevenger D, Tan W, Szekely B, Wang Y, Li Y, Muse EA, Kiyono M, Hanamura S, Inoue E, Nakamura M, Huffman MA, Jiang B, Nishida T. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. American Journal of Primatology. 2008;70:755–765. doi: 10.1002/ajp.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ME, Soike K, Ahmed K, Iatropoulos MJ. Coxsackievirus in an infant chimpanzee. Journal of Medical Primatology. 1977;7(2):119–121. doi: 10.1159/000459795. [DOI] [PubMed] [Google Scholar]
- Köndgen S, Kühl H, N’Goran P, Walsh P, Schenk S, Ernst N, Biek R, Formenty P, Mätz-Rensing K, Schweiger B, Junglen S, Ellerbrok H, Nitsche A, Briese T, Lipkin I, Pauli G, Boesch C, Leendertz F. Pandemic human viruses cause decline of endangered great apes. Current Biology. 2008;18:1–5. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Köndgen S, Schenk S, Pauli G, Boesch C, Leendertz FH. Noninvasive monitoring of respiratory viruses in wild chimpanzees. Ecohealth. 2010;7(3):332–341. doi: 10.1007/s10393-010-0340-z. [DOI] [PubMed] [Google Scholar]
- Köndgen S, Leider M, Lankester F, Bethe A, Lübke-Becker A, Leendertz FH, Ewers C. Pasteurella multocida involved in respiratory disease of wild chimpanzees. PloS one. 2011;6(9):e24236–e24236. doi: 10.1371/journal.pone.0024236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooriyama T, Okamoto M, Yoshida T, Nishida T, Tsubota T, Saito A, Tomonaga M, Matsuzawa T, Akari H, Nishimura H, Miyabe-Nishiwaki T. Epidemiological study of zoonoses derived from humans in captive chimpanzees. Primates. 2013;54(1):89–98. doi: 10.1007/s10329-012-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf EV, Travis D, Pusey AE, Goodall J. Using retrospective health data from the Gombe chimpanzee study to inform future monitoring efforts. Journal of Medical Primatology. 2006;68:897–908. doi: 10.1002/ajp.20296. [DOI] [PubMed] [Google Scholar]
- Macfie EJ and Williamson EA (2010) Best Practice Guidelines for Great Ape Tourism. Gland: IUCN/SSC Primate Specialist Group (PSG). 78 pp
- Mackay IM, Bialasiewicz S, Waliuzzaman Z, Chidlow GR, Fegredo DC, Laingam S, Adamson P, Harnett GB, Rawlinson W, Nissen MD, Sloots TP. Use of the P gene to genotype human metapneumovirus identifies 4 viral subtypes. The Journal of Infectious Diseases. 2004;190:1913–1918. doi: 10.1086/425013. [DOI] [PubMed] [Google Scholar]
- McAvin JC, Reilly PA, Roudabush RM, Barnes WJ, Salmen A, Jackson GW, Beninga KK, Astorga A, McCleskey FK, Huff WB, Niemeyer D, Lohman KL. Sensitive and specific method for rapid identification of Streptococcus pneumoniae using real-time fluorescence PCR. Journal of Clinical Microbiology. 2001;39:3446–3451. doi: 10.1128/JCM.39.10.3446-3451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza Virus Aerosols in Human Exhaled Breath: Particle Size, Culturability, and Effect of Surgical Masks. PLoS Pathogens. 2013;9(3):e1003205. doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EAF, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SCA, Mourier T, Baandrup U, Søland TM, Bertelsen MF, Gilbert MTP, Nielsen LP. Probable transmission of coxsackie B3 virus from human to chimpanzee. Denmark. Emerging Infectious Diseases. 2012;18(7):1163. doi: 10.3201/eid1807.111689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn CL, Thrall PH, Stewart K, Harcourt AH. Emerging infectious diseases and animal social systems. Evolutionary Ecology. 2008;22(4):519–543. doi: 10.1007/s10682-007-9180-x. [DOI] [Google Scholar]
- Palacios G, Oberste MS. Enteroviruses as agents of emerging infectious diseases. Journal of Neurovirology. 2005;11(5):424–433. doi: 10.1080/13550280591002531. [DOI] [PubMed] [Google Scholar]
- Palacios G, Lowenstine LJ, Cranfield MR, Gilardi KV, Spelman L, Lukasik-Braum M, Kinani JF, Mudakikwa A, Nyirakaragire E, Bussetti AV, Savji N, Hutchison S, Egholm M, Lipkin WI. Human metapneumovirus infection in wild mountain gorillas. Rwanda. Emerging Infectious Diseases. 2011;17(4):711. doi: 10.3201/eid1704.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch D, Oh DY, Wolf S, Dumke R, Schröter-Bobsin U, Höhne M, Röske I, Schreier E. Detection of enteric viruses and bacterial indicators in German environmental waters. Archives of Virology. 2005;150:929–947. doi: 10.1007/s00705-004-0467-8. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J. The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conservation Biology. 2007;21(3):623–634. doi: 10.1111/j.1523-1739.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- Radonovich LJ, Jr, Cheng J, Shenal BV, Hodgson M, Bender BS. Respirator tolerance in health care workers. Journal of the American Medical Association. 2009;301:36–38. doi: 10.1001/jama.2008.894. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer v1.6. Available from http://beast.bio.ed.ac.uk/Tracer
- Reiche J, Schweiger B. Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. Journal of Clinical Microbiology. 2009;47:1800–1810. doi: 10.1128/JCM.02286-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiche J, Jacobsen S, Neubauer K, Hafemann S, Nitsche A, Milde J, Wolff T, Schweiger B. Human Metapneumovirus: insights from a ten-year molecular and epidemiological analysis in Germany. PloS One. 2014;9:2. doi: 10.1371/journal.pone.0088342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeuh-Mba SA, Bessaud M, Joffret ML, Endegue Zanga MC, Balanant J, Mpoudi Ngole E, Njouom R, Reynes JM, Delpeyroux F, Rousset D. Characterization of Enteroviruses from Non-Human Primates in Cameroon Revealed Virus Types Widespread in Humans along with Candidate New Types and Species. PLoS Neglected Tropical Diseases. 2014;8(7):e3052. doi: 10.1371/journal.pntd.0003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze M, Nitsche A, Schweiger B, Biere B. Diagnostic approach for the differentiation of the pandemic influenza A (H1N1) v virus from recent human influenza viruses by real-time PCR. PloS one. 2010;5(4):e9966. doi: 10.1371/journal.pone.0009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Saito R, Sakai T, Sano Y, Nishikawa M, Sasaki A, Sano Y, Nishikawa M, Sasaki A, Shobugawa Y, Gejyo F, Suzuki H. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. Journal of Clinical Microbiology. 2005;43:36–40. doi: 10.1128/JCM.43.1.36-40.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater OM, Terio KA, Zhang Y, Erdman DD, Schneider E, Kuypers JM, Wolinsky SM, Kunstman KJ, Kunstman J, Kinsel MJ, Gamble KC. Human Metapneumovirus Infection in Chimpanzees. United States. Emerging Infectious Diseases. 2014;20(12):2115. doi: 10.3201/eid2012.140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentiks CA, Köndgen S, Silinski S, Speck S, Leendertz FH. Lethal pneumonia in a captive juvenile chimpanzee (Pan troglodytes) due to human-transmitted human respiratory syncytial virus (HRSV) and infection with Streptococcus pneumoniae. Journal of Medical Primatology. 2009;38(4):236–240. doi: 10.1111/j.1600-0684.2009.00346.x. [DOI] [PubMed] [Google Scholar]
- Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- Tong S, Chern SWW, Li Y, Pallansch MA, Anderson LJ. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. Journal of Clinical Microbiology. 2008;46(8):2652–2658. doi: 10.1128/JCM.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin S, Chatterton J, Chantrey J. Management of severe respiratory tract disease caused by human respiratory syncytial virus and Streptococcus pneumoniae in captive chimpanzees (Pan troglodytes) Journal of Zoo and Wildlife Medicine. 2013;44(1):105–115. doi: 10.1638/1042-7260-44.1.105. [DOI] [PubMed] [Google Scholar]
- Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Tropical Medicine & International Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Wevers D, Leendertz FH, Scuda N, Boesch C, Robbins MM, Head J, Ludwig C, Kühn J, Ehlers B. A novel adenovirus of Western lowland gorillas (Gorilla gorilla gorilla) Virology Journal. 2010;7(1):303. doi: 10.1186/1743-422X-7-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers D, Metzger S, Babweteera F, Bieberbach M, Boesch C, Cameron K, Couacy-Hymann E, Cranfield M, Gray M, Harris LA, Head J, Jeffery K, Knauf S, Lankester F, Leendertz SAJ, Lonsdorf E, Mugisha L, Nitsche A, Reed P, Robbins M, Travis DA, Zommers Z, Leendertz Fabian H, Ehlers B. Novel adenoviruses in wild primates: a high level of genetic diversity and evidence of zoonotic transmissions. Journal of Virology. 2011;85(20):10774–10784. doi: 10.1128/JVI.00810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford MH, Butynski TM, Karesh WB. Habituating the great apes: the disease risks. Oryx. 2002;36(02):153–160. doi: 10.1017/S0030605302000224. [DOI] [Google Scholar]
- Zhang Y, Cui W, Liu L, Wang J, Zhao H, Liao Y, Na R, Dong C, Wang L, Xie Z, Gao J, Cui P, Zhang X, Li Q. Pathogenesis study of enterovirus 71 infection in rhesus monkeys. Laboratory Investigation. 2011;91(9):1337–1350. doi: 10.1038/labinvest.2011.82. [DOI] [PubMed] [Google Scholar]


