Abstract
Purpose of review:
Exciting translational discoveries in recent years have brought realized promise of immunotherapy for children with high-risk leukemias. This review summarizes the current immunotherapeutic landscape with a focus on key clinical trials for patients with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML).
Recent findings:
Chemotherapy resistance remains a major barrier to cure in children with high-risk leukemias. Immunotherapy approaches have potential to overcome this resistance given alternative mechanisms of action. Based upon preclinical activity and/or success in adult patients, recent clinical trials have demonstrated safety and efficacy of various monoclonal antibody, antibody-drug conjugate, bispecific T cell-engaging antibody, natural killer cell, and chimeric antigen receptor-redirected T cell immunotherapies for children with ALL or AML. Food and Drug Administration approval of several of these immunotherapies has increased the pediatric leukemia therapeutic portfolio and improved clinical outcomes for previously-incurable patients.
Summary:
Several antibody-based or cellular immunotherapy modalities have demonstrated appreciable efficacy in children with relapsed or chemotherapy-refractory leukemia via early-phase clinical trials. Some studies have also identified critical biomarkers of treatment response and resistance that merit further investigation. Continued preclinical and clinical evaluation of novel immunotherapies is imperative to improve cure rates for children with high-risk leukemias.
Keywords: antibody, cellular therapy, childhood leukemia, immunotherapy
INTRODUCTION
Though improved therapeutic regimens and supportive care for children and adolescents with B-cell acute lymphoblastic leukemia (B-ALL) now achieve relapse-free survival (RFS) of >90%, those with relapsed or chemotherapy-refractory disease remain difficult to cure. Pediatric patients with acute myeloid leukemia (AML) continue to have unacceptably lower cure rates of approximately 60% despite maximally-intensive chemotherapy and often subsequent myeloablative hematopoietic stem cell transplantation (HSCT). The advent of effective immunotherapy is now changing therapeutic paradigms, providing options for patients in whom cytotoxic chemotherapy has proven ineffective or intolerable. Immunotherapies can be exquisitely target-specific, conferring more limited toxicity to normal tissues than traditional chemotherapy drugs. Given their alternative mechanisms of action, immunotherapy agents can often be combined with chemotherapy to increase curative potential. In this review, we concisely describe major immunotherapy modalities under preclinical or current clinical study for children and adolescents/young adults (AYAs) with relapsed/refractory ALL and AML (Figure 1) and highlight the potential of these new approaches to improve outcomes.
Figure 1. Schema of immunotherapy modalities for childhood leukemia.
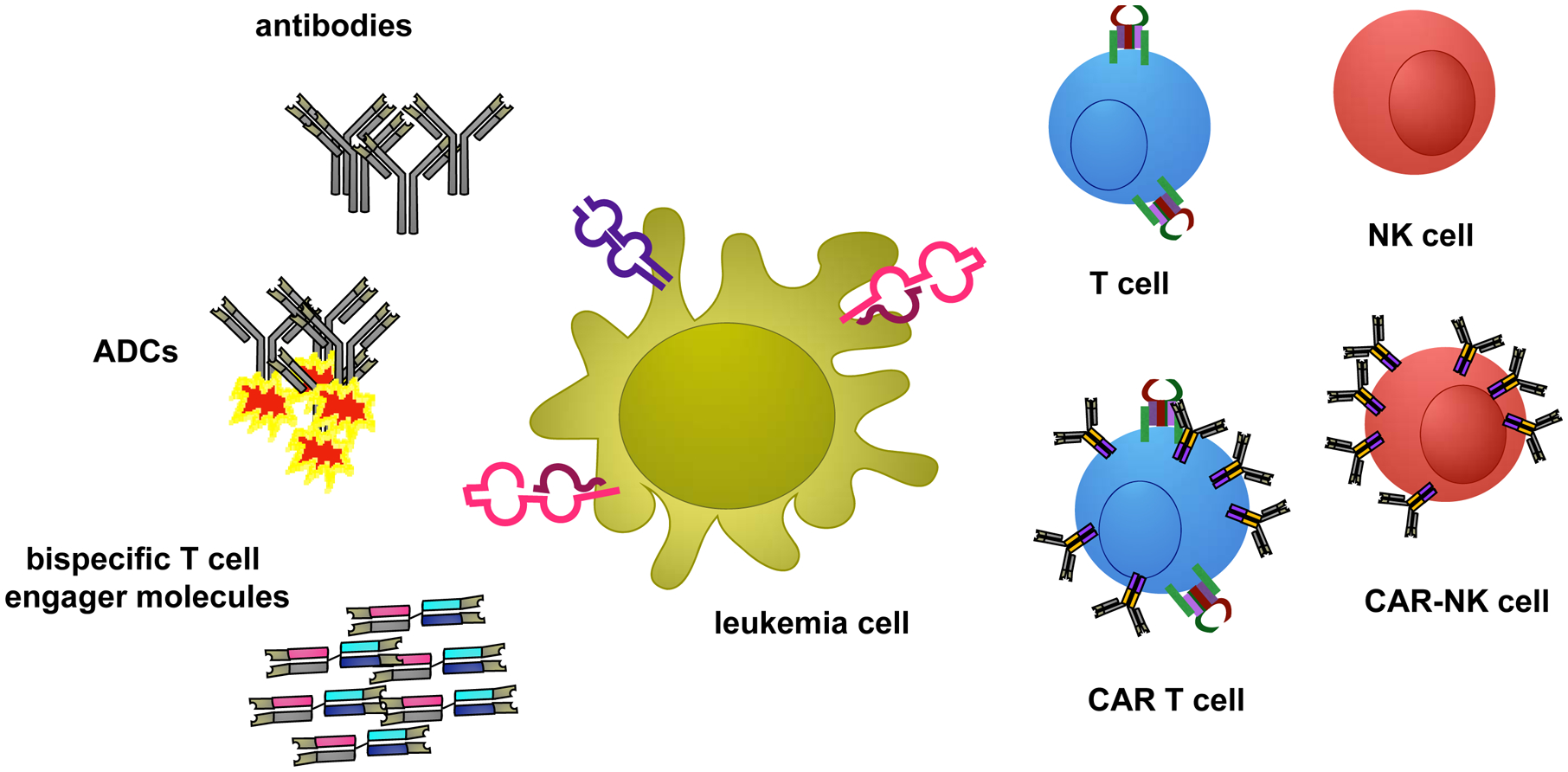
ANTIBODY-BASED IMMUNOTHERAPY
Monoclonal Antibodies
The exquisite specificity of monoclonal antibodies (moAbs) binding to target antigens can result in effective depletion of antigen-expressing malignant blasts (‘on-target/on-tumor’ effects), although depletion of antigen-expressing normal cells (‘on-target/off-tumor’ effects) can also occur. While moAb therapies are rarely used in current treatment of children with ALL and AML, they have provided an important biologic foundation for ‘next generation’ antibody-based immunotherapies described below. A current phase 1/2 clinical trial is assessing the safety and potential efficacy of the anti-CD38 moAb daratumumab with chemotherapy in pediatric patients with relapsed B-ALL or T-ALL (NCT03384654) based upon activity in preclinical models (1).
Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) are comprised of moAbs linked to cytotoxic drug ‘payloads’ and have been used in treatment of children with AML and B-ALL. The ADC gemtuzumab ozogamicin (GO) connects a CD33 moAb to the toxic antibiotic calicheamicin via an acid-labile linker, which facilitates its release upon GO internalization into CD33+ cell lysosomes (2). GO has been extensively studied in patients with AML (3–17) and is now Food and Drug Administration- (FDA) and European Medicines Agency (EMA)-approved for adults with relapsed or de novo disease and for children with relapsed/refractory disease. GO was initially studied in children with AML as monotherapy and then in combination with chemotherapy via several Children’s Oncology Group (COG) and European consortia trials (4, 5, 7–10, 14, 17, 18). Superior RFS with GO addition to chemotherapy was observed in some children with de novo AML treated on the COG phase 3 AAML0531 study (19). Based on these and other data, GO will be incorporated into frontline therapy for children and AYAs with CD33+ AML on the successor phase 3 AAML1831 trial. Other myeloid antigen-targeting ADCs (e.g., IMGN-632 targeting CD123, anetumab ravtansine targeting mesothelin) are under clinical evaluation in adults with AML (NCT03386513) and with pediatric phase 1 trials planned.
Inotuzumab ozogamicin (InO) is a similar ADC that links a CD22 moAb to calicheamicin and is now FDA- and EMA-approved for adults and children with relapsed/refractory B-ALL after demonstration of safety and efficacy (20–25). In the pediatric realm, InO was initially trialed in five children with multiply-relapsed B-ALL with responses reported in three patients (22). These encouraging results were followed by extended pediatric access to InO. A retrospective analysis of 51 children with relapsed/refractory B-ALL treated with InO reported complete morphologic responses (CR) in 67% of patients, 71% of whom achieved minimal residual disease (MRD)-negative remission (24). In this study, CD22 downregulation on leukemia cells was suggested as a mechanism of therapeutic evasion, although antigen expression levels were not systematically quantified (24). The potential safety and efficacy of InO treatment of children and AYAs with relapsed/refractory B-ALL is under current evaluation in the COG phase 2 AALL11621 trial (NCT02981628).
Due to their calicheamicin payloads, GO and InO have potential for hepatic toxicity with sinusoidal obstructive syndrome (SOS), particularly when administered in close proximity to HSCT. Although high rates of SOS were initially reported in adult patients with AML treated with GO (26), multiple pediatric clinical trials have not demonstrated increased SOS incidence in GO-treated children with relapsed or de novo AML (9, 10, 12, 14, 16, 18). The AALL1621 study is carefully monitoring rates and severity of SOS in children with relapsed/refractory B-ALL treated with InO.
Bispecific T cell-engager molecules
Technological advances in protein engineering have led to development of immunotherapeutic agents capable of binding tumor-specific targets while simultaneously activating endogenous immune effector T cells. Bispecific T-cell engager (BiTE) molecules consist of single-chain variable fragments (scFvs) derived from linked heavy and light chains of moAbs specific for tumor-associated antigens that are fused to a second scFv specific for the T-cell receptor (TCR) CD3ε component and promote formation of an effective immunological synapse (27). Given their extremely short half-lives (28), blinatumomab and other BiTEs require administration via continuous intravenous infusion, which may pose quality-of-life issues for some patients. The safety and activity of the CD19xCD3 BiTE blinatumomab has been demonstrated in adults and children with relapsed/refractory B-ALL in several studies (29–33), which led to FDA and EMA approval in 2018. An international phase 1/2 trial of blinatumomab in children with relapsed/refractory B-ALL determined recommended pediatric dosing of blinatumomab and reported a CR rate of 37% after up to two cycles (33). Ongoing studies are assessing potential biomarkers of treatment response and failure (34). Blinatumomab is under additional pediatric evaluation in the relapsed and newly-diagnosed settings in the COG AALL1331 (NCT02101853) and AALL1731 (NCT03914625) trials, respectively.
BiTEs targeting myeloid antigens (e.g., CD33, CD123, FLT3R [CD135]) are also under early-phase clinical evaluation for adults with AML (NCT02520427, NCT03224819, NCT03541369, NCT03739606, NCT02152956). The PEPN1812 phase 1 trial of the CD123xCD3 dual affinity-retargeting antibody flotetuzumab for children and AYAs with relapsed/refractory AML will open soon via the Pediatric Early Phase Clinical Trials Network.
NON-ENGINEERED CELLULAR THERAPIES
T cell therapies
Pioneering studies reported that T cells with specific activity against cells infected with virus (viral-specific T cells [VSTs]) can be identified in peripheral blood and used to treat infection and virus-associated malignancy even when host immunity is ineffective (35–38). Similar to VSTs, circulating leukemia-specific T cells have been identified, can be isolated ex vivo, and have shown activity against ALL (39). Several groups have now proven safety and feasibility of this approach with single and multiple-antigen specific cells generated and infused into patients for treatment or prevention of leukemia relapse (40–42), although responses against high-burden disease have not been observed. Combinatorial therapy with leukemia-specific cells and immunosuppressive checkpoint inhibitors may ultimately be most effective, and such strategies are being actively explored.
NK cell therapies
Patients with leukemia have known deficiencies in NK functionality, which include lack or downregulation of NK cytotoxicity receptors (43–45), diminished killing capacity (46, 47), and altered microRNA expression (48, 49). Complete replacement of the dysfunctional NK lineage is possible with HSCT, which confers significant toxicity risk. Alternatively, ex vivo activation, expansion, and infusion of patient-derived NK cells (50) has proven safe, albeit with limited anti-tumor efficacy to date (51–59). Achieving effective in vivo NK cell doses remains a challenge in delivering NK cell therapy to patients with acute leukemias, and improved expansion and proliferation techniques are needed.
ENGINEERED CELLULAR THERAPIES
Chimeric antigen receptor (CAR) T cell therapies
CD19 and CD22 CAR T cells for B-ALL
Technological advances in cellular engineering and improved biologic understanding (60) have contributed to the rapid development and clinical success of autologous CD19-redirected chimeric antigen receptor (CAR) T cell for patients with multiply-relapsed/chemorefractory B-ALL (61–65). CARs are synthetic receptors comprised of an extracellular scFv-based domain that binds a tumor-specific surface antigen, a hinge, a transmembrane domain, and one or more intracellular signaling domains that confer activation, proliferation, and/or survival signals to (usually virally-transduced) T cells. Though first developed as a tool to divorce HLA restriction from inherent T-cell biology (66), autologous CAR T cells have quickly demonstrated potent and paradigm-changing therapeutic efficacy, as reflected by accelerated clinical development and FDA and EMA approval of some products (67). The first CAR T cells were designed against solid tumors and contained a single T-cell activation domain (CD3ζ) (68). It was subsequently realized that incorporation of a second intracellular T cell signaling domain (e.g., CD28, CD137) to provide co-stimulation resulted in greater proliferative capacity, anti-tumor efficacy, and in vivo persistence (69–71). These ‘second-generation’ CAR T cells targeting CD19 and/or CD22 have now shown robust efficacy in children with relapsed/refractory B-ALL (61–64, 72, 73).
Clinical experience and challenges with CD19 CAR T cell (CD19CART) testing in children with B-ALL have been comprehensively reviewed elsewhere (74, 75). Recently, CD22CART approaches have been developed to overcome CD19-negative leukemia relapses now known to occur at high frequency after CD19CART or blinatumomab therapy (described in detail below) (75–79). Initial data from an ongoing pediatric phase 1 study (NCT02315612) reported a 73% CR rate at 28 days post-CD22CART, although 14 of 17 patients subsequently relapsed (73). Allogeneic ‘off-the-shelf’ CD19CART approaches (80) are also under early-phase evaluation in adults and children to circumvent issues of suboptimal autologous T cell pheresis in heavily chemotherapy-myelosuppressed patients and to decrease timing to infusion (NCT03190278). Because of the clinical efficacy of CD19CART cells, rapid US and international development has occurred. This widespread interest is evident in the extensive number and location of active clinical trials (Table 1, Figure 2).
Table 1.
Current immunotherapy trials for patients with relapsed/refractory ALL.
| Title | Registration Number | Eligible Ages | Sponsor |
|---|---|---|---|
| Study Evaluating KTE-C19 in Pediatric and Adolescent Subjects with Relapsed/Refractory B-Precursor Acute Lymphoblastic Leukemia (ZUMA-4) | NCT026525480 | 2–21 years | KITE/Gilead, Los Angeles, CA, USA |
| Transposon-Manipulated Allogeneic CARCIK-CD19 Cells in Pediatric and Adult Patients with r/r ALL Post HSCT (CARCIK) | NCT03389035 | 1–75 year | Fondazione Matilde Tettamanti Menotti De Marchi Onlus, Monza MI, Italy |
| Evaluation of CD19-specific CAR Engineered Autologous T-cells for Treatment of Relapsed/Refractory CD19+ Acute Lymphoblastic Leukemia | NCT03573700 | ≤21 years | St. Jude Children’s Research Hospital, Memphis, TN, USA |
| CD19 T-CAR for Treatment of Children and Young Adults with r/r B-ALL | NCT03467256 | 3 months to 25 years | Federal Research Institute of Pediatric Hematology, Oncology and Immunology, Moscow, Russia |
| Study of UCART19 in Pediatric Patients with Relapsed/Refractory B Acute Lymphoblastic Leukemia (PALL) | NCT02808442 | 6 months to 17 years | Insitut de Recherches Internationales Servier, Paris, France |
| CAR-20/19-T cells in Pediatric Patients with Relapsed/Refractory B cell ALL (CAR-20/19-T) | NCT04049383 | 1–39.99 years | Medical College of Wisconsin, Milwaukee, WI, USA |
| Study of efficacy and safety of tisagenlecleucel in HR B-ALL EOC MRD Positive Patients (CASSIOPEIA) | NCT03876769 | 1–25 years | Novartis, Basel, Switzerland |
| Study of huCART19 for Very High-Risk (VHR) Subsets of Pediatric B-ALL | NCT03792622 | 1–29 years | University of Pennsylvania, Philadephia, PA, USA |
| T-cells Expressing Anti-CD19 CAR in Pediatric and Young Adults with B-Cell Malignancies | NCT02772198 | 1–50 years | Sheba Medical Center, Ramat Gan, Israel |
| Anti-CD19 CAR T cells in Pediatric Patients Affected by Relapsed/Refractory CD19+ALL and NHL | NCT03373071 | 6 months-25 years | Bambino Gesu Hospital and Research Institute, Rome, Italy |
| Anti-CD19, Dual Co-Stimulatory (4–1BB, CD3ζ) Chimeric Antigen Receptor T-cells in Patients with relapsed/refractory aggressive lymphoma or acute lymphoblastic leukemia (ALL) (ACIT001/EXC002) | NCT03938987 | 2–70 years | University of Alberta, Edmonton, Alberta, Canada |
| CD22-Redirected Autologous T cells for ALL | NCT02650414 | 1–24 years | University of Pennsylvania, Philadephia, PA, USA |
| Administration of Autologous CAR-T CD19 Antigen with inducible Safety Switch in Patients with Relapsed/Refractory ALL | NCT03016377 | 3–70 years | UNC Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA |
| MB-CART19.1 in Patients with R/R ALL | NCT03321123 | 2 months - 18 years | Shanghai Children’s Medical Center, Shanghai, China |
| Pilot Study of Redirected Autologous T Cells Engineered to Contain Humanized Anti-CD19 In Patients with Relapsed or Refractory CD19+ Leukemia and Lymphoma Previously Treated with Cell Therapy | NCT02374333 | 1–24 years | University of Pennsylvania, Philadephia, PA, USA |
| Patient-Individualized Peptide Vaccination Based On Tumor-specific Mutations in Children and Young Adults with Primary/Relapsed ALL | NCT035559413 | 1–30 years | University Children’s Hospital Tuebingen, Tuebingen, Germany |
| MB-CART19.1 r/r CD19+ B-cell Malignancies (BCM) | NCT03853616 | ≥1 year | Miltenyi Biotec GmbH, Bergisch Gladbach, Germany |
| Treatment of Patients with Relapsed or Refractory CD19+ Lymphoid Disease with T cells Expressing a Third- Generation CAR | NCT03676504 | ≥3 years | University Hospital Heidelberg, Heidelberg, Germany |
| CTL019 Ourt of Specification MAP for ALL or DLBCL Patients | NCT03601442 | Novartis, Basel, Switzerland | |
| A Pediatric and Young Adult Trial of Genetically Modified T cells Directed Against CD19 for Relapsed/Refractory CD19+ Leukemia | NCT02028455 | 1–26 years | Seattle Children’s Hospital, Seattle, WA, USA |
| Pilot Study of T-APCs Following CAR T cell Immunotherapy for CD19 Leukemia | NCT03186118 | 1–26 years | Seattle Children’s Hospital, Seattle, WA, USA |
| CAR-T Therapy for Central Nervous System B-cell Acute Lymphocytic Leukemia | NCT03064269 | 10–60 years | Shanghai Unicar-Therapy Bio-medicine Technology Co., Ltd., Shanghai, China |
| CART-19 cells for R/R B-ALL (CCFRRBA) | NCT03391739 | 1–60 years | Fujian Medical University, Fuzhou, Fujian, China |
| CART-19 cells for MRD Positive CD19+ ALL (CCFMPCA) | NCT03027739 | 1–60 years | Fujian Medical University, Fuzhou, Fujian, China |
| CD19-specific CAR T cells with a fully human binding domain for CD19+ Leukemia or lymphoma | NCT03684889 | 1–28 years | Seattle Children’s Hospital, Seattle, WA, USA |
| CARPALL: Immunotherapy with CD19 CAR T-cells for CD19+ Haematological Malignancies | NCT02443831 | ≤24 years | University College, London, England |
| CD19/22 CAR T cells (AUTO3) for the treatment of B cell ALL (AMELIA) | NCT03289455 | 1–24 years | Autolus Limited, White City, London, England |
| A feasibility and safety study of CD38 CAR-T cell immunotherapy for relapsed B-cell acute lymphoblastic leukemia after CD19 CAR-T adoptive cellular immunotherapy | NCT03754764 | 12–70 years | Chinese PLA General Hospital, Beijing, China |
| Safety and efficacy evaluation of IM19 CAR-T cells (IM19CAR-T) | NCT03142646 | 4–65 years | Beijing Immunochina Medical Science and Technology Co., Ltd., Beijing, China |
| CD19 CAR-T cells for Patients with Relapse and Refractory CD19+ B-ALL | NCT03671460 | ≥1 year | Tianjin Mycure Medical Technology Co., Ltd., Tianjin, China |
| Anti-CD19 CAR-T Therapy Combien with HSCT to Treat MRD+ B-cell Malignancies | NCT03366324 | ≤70 years | Wuhan Sian Medical Technology Co., Ltd., Wuhan, Hubei, China |
| CD19-CAR-T Cells in Patients with R/R B-ALL | NCT03574168 | 3–70 years | Bioceltech Therapeutics, Ltd., Beijing, China |
| CART-19 for Relapsed/refractory acute Lymphoblastic Leukemia (ALL) | NCT03544021 | 14–75 years | The Affiliated Hospital of the Chinese Academy of Military Medical Sciences, Shanghai, China |
| A study of C-CAR066 in subjects with r/r B cell Lymphoma who received CD19 CAR-T Therapy | NCT04036019 | 14–70 years | Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China |
| A clinical research of CD20-Targeted CAR-T in B Cell Malignancies | NCT02710149 | 14–75 years | Southwest Hospital China, Chongqing, China |
| A clinical research of CD22-Targeted CAR-T in B Cell Malignancies | NCT02935153 | 14–75 years | Southwest Hospital China, Chongqing, China |
| Anti-CD22 Chimeric Antigen Receptor (CAR)-Modified T cell Therapy for Relapsed Refractory B-cell Malignancies | NCT04007978 | 14–70 years | Wuhan Union Hospital, China, Jianghan, Wuhan, China |
| Anti-CD19 CAR-T Therapy Bridging to HSCT for CD19+ B-Cell Malignancies | NCT03366350 | ≤70 years | Wuhan Sian Medical Technology Co., Ltd., Wuhan, Hubei, China |
| Humanized CD19 CAR-T Cells with RS Suppression Technology for R/R CD19+ Acute Lymphoblastic Leukemia | NCT03275493 | 6–65 years | Shanghai Unicar-Therapy Bio-medicine Technology Co., Ltd., Shanghai, China |
| A feasibility and safety study of Universal Dual Specificity CD19 and CD20 or CD22 CAR-T Cell Immunotherapy for Relapsed or refractory Leukemia and Lymphoma | NCT03398967 | 12–70 years | Chinese PLA General Hospital, Beijing, China |
| CD19-CAR Treatment for ALL | NCT03232619 | 6–70 years | Shanghai Bioray Laboratory Inc., Shanghai, China |
| CD19 Chimeric Antigen receptor (CAR)-modified T cell therapy in Treating Patients with B-cell Malignancies | NCT02965092 | ≤70 years | Wuhan Sian Medical Technology Co., Ltd., Whuan, Hubei, China |
| Phase I CD19/CD22 Chimeric Antigen Receptor T cells in Peds Recurrent/Refractory B cell Malignancies | NCT03241940 | 1–30 years | Stanford University, Palo Alto, CA, USA |
| Intravenous Autologou CD19-CAR-T Cells for R/R B-ALL | NCT03937544 | 13–65 years | National University of Malaysia, Bangi, Malaysia |
| Senl_1904A and Senl_1904B Chimeric Antigen Receptor (CAR) T-Cell in the Treatment of r/r Acute B Lymphocytic Leukemia | NCT03840317 | 3–65 years | Henei Senang Biotechnology Inc., Ltd., Shijiazhuang, Hebei, China |
| CART19 Cells Treatment of MRD of B cell Malignancies and then Auto-HSCT | NCT03685786 | 14–75 years | Shenzhen Second People’s Hospital, Shenzhen, China |
| CAR-T Immunotherapy Targeting CD19-ALL | NCT04016129 | 6 months to 75 years | Shenzhen Second People’s Hospital, Shenzhen, China |
| CD19-Directed CAR T cells Therapy in Relapsed/Refractory B cell Malignancy | NCT02537977 | 6–85 years | Shanghai Tongji Hospital, Tongji University School of Medicine |
| CAR-T Therapy Targeting to CD19 for R/R ALL | NCT03919240 | No age posted | The First Affiliated Hospital of Soochow University |
| CD19-targeting, 3rd Generation CAR T Cells for Refractory B cells Malignancy | NCT03068416 | ≤100 years | Uppsala University, Uppsala, Sweden |
| A study of GC007F CAR-T Cell Immunotherapy for Relapsed or Refractory B-ALL | NCT03825718 | 2–70 yrs | Hebei Yanda Ludaopei Hospital, Yanjiao, Hebei, Chin |
| Humanized CD19 Chimeric Antigen Receptor (CAR)-Modified T cell Therapy in Treating Patients with B-cell Malignancies | NCT04008251 | 14–70 yrs | Wuhan Sian Medical Technology Co., Ltd., Wuhan, Hubei, China |
| Immunotherapy for High Risk/Relapsed CD19+ Acute Lymphoblastic Leukemia Using CAR T-cells to Target CD19 (ALLCAR19) | NCT02935257 | 16– 65 yrs | University College, London, England, UK |
| A Study of GC022 CAR-T Cell Immunotherapy for Relapsed or Refractory B-ALL | NCT03825731 | 2–70 yrs | Hebei Yanda Ludaopei Hospital, Yanjiao, Hebei, Chin |
| CD19/CD22-targeted Chimeric Antigen Recptor Engineered T cell (CART) in B-cell Acute Lymphblastic Leukemia | NCT03614858 | 6–65 yrs | Shanghai Unicar-Therapy Bio-medicine Technology Co., Ltd, Shanghai, China |
| Multi-CAR T Cell Therapy Targeting CD7-positive Malignancies | NCT04033302 | 6 months to 75 yrs | Shenzhen Geno-Immune Medical Institute, Shenzhen, China |
| A Clinical Research of CAR T cells Targeting CD19 Positive Malignant B-ell Derived Leukemia and Lymphoma | NCT02349698 | 4 –75 years | Southwest Hospital China, Chongqing, China |
| MT2017–45: CAR-T Cell Therapy for Heme Malignancies | NCT03642626 | ≤75 years | Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA |
| A Phase I/II Multiple Center Trial of 4SCAR19 Cells in the Treatment of Relapsed and Refractory B cell Malignancies | NCT03050190 | ≥6 months | Shenzhen Geno-Immune Medical Institute, Shenzhen, China |
| Production of Clinical-grade Anti-CD19 Chimeric antigen Receptor T cells for Refractory B-cell Malignancies | NCT03624686 | National Taiwan University Hospital, Taipei, Taiwan | |
| CD19/CD22 Chimeric Antigen Receptor (CAR) T cells in Children and Young Adults with Recurrent or Refractory CD19/CD22-expressing B cell Malignancies | NCT03448393 | 3–30 years | National Cancer Institute, Bethesda, MD |
| Autologous T cell expressing a Second Generation CAR for Traemtnet of T-Cell Malignancies Expressing CD5 Antigen (MAGENTA) | NCT03081910 | ≤75 yrs | Baylor College of Medicine, Houston, TX, USA |
| Study of TBI-1501 for Relapsed or Refractory Acute Lymphblastic Leukemia (TBI-1501) | NCT03155191 | ≥16 years | Takara Bio, Inc., Kusatsu, Shiga Prefecture, Japan |
| Activated T-Cells Expressing 2nd or 3rd Generation CD19-Specific CAR, Advanced B-Cell NHL, ALL, and CLL (SAGAN) | NCT01853631 | ≤75 years | Baylor College of Medicine, Houston, TX, USA |
| CD19-hsCAR-T for Refractory/Relapsed CD19+ B-ALL Patients | NCT03902197 | 1–75 yrs | Xuanwu Hospital, Beijing, China |
| A Clinical Study Evaluating the Safety and Efficacy of BinD10 Treatment in Childhood R/R ALL and Lymphoma Subjects | NCT03265106 | 1–18 years | ShenZhen BinDeBio Ltd., Shenzhen, China |
| Safety and Efficacy Evaluation of CD19-UCART | NCT03229876 | 6–65 years | Shanghai Bioray Laboratory Inc., Shanghai, China |
| Treatment of Children CD19+ Leukemia and Non-Hodgkin Lymphoma With CD19-TriCAR-T/SILK Cell Therapy | NCT03910842 | ≤18 years | Timmune Biotech Inc., Hunan, China |
Figure 2. Geographic locations of current CAR T cell trials allowing pediatric patient participation.
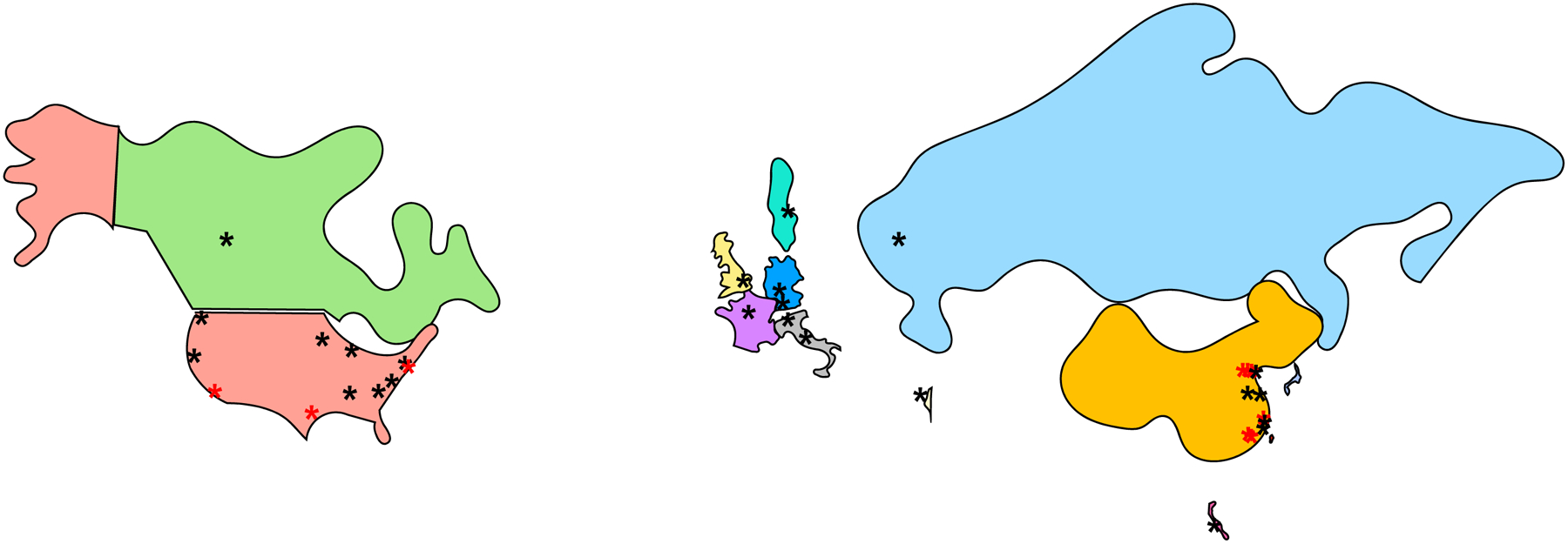
Black = ALL trials, red = AML trials.
CAR T cells for AML
Successful CAR T cell development for children and AYAs with myeloid leukemias has proven challenging given lack of identified ‘universal’ AML antigens and appreciable risk of both hematologic and non-hematologic on-target/off-tumor toxicity (81, 82). Several phase 1 clinical trials of CD33CART or CD123CART are underway in adults with AML after promising preclinical data (83–98) (NCT03126864, NCT03904069, NCT02159495, NCT03766126); some of these trials now allow older pediatric patient participation (Table 2). Additional pediatric-specific institutional phase 1 trials of CAR T cells targeting CD33 (NCT03971799), CD123, CD135 (FLT3R), and CD371 (CLEC12A) are in development with other preclinical studies ongoing.
Table 2.
Current immunotherapy trials for patients with relapsed/refractory AML.
| Title | Registration Number | Eligible Ages | Sponsor |
|---|---|---|---|
| Evaluating QTc, PK, safety of gemtuzumab ozogamicin (GO) in patents with CD33+ R/R AML | NCT037227750 | 12 years | Pfizer, New York, NY, USA |
| Study of Adoptive Cellular Therapy Using Autologous T Cells Transduce with Lentivirus To Express A CD33 Specific Chimeric Antigen Receptor in Patients with Relapsed or Refractory CD33-positive Acute Myeloid Leukemia | NCT03126864 | 1–80 years | MD Anderson Cancer Center, Houston, TX, USA |
| Study of Anti-CD33 Chimeric Antigen Receptor-Expressing T Cells (CD33CART) in Children and Young Adults with Relapsed/Refractory Acute Myeloid Leukemia | NCT03971799 | 1–30 years | CIBMTR: National Cancer Institute, Bethesda, MD and Children’s Hospital of Philadelphia, PA, USA |
| Study Evaluating the Safety, Tolerability, and Efficacy of FLT3 CAR-T AMG 553 in FLT3-Positive Relapsed/Refractory AML | NCT03904069 | ≥12 years | Amgen, Thousand Oaks, CA, USA |
| Genetically Modified T-cell Immunotherapy in Treating Patients with Relapsed/Refractory Acute Myeloid Leukemia and Persistent/Recurrent Plastic Plasmacytoid Dendritic Cell Neoplasm | NCT02159495 | ≥12 years | City of Hope Medical Center, Duarte, CA, USA |
| CD123/CLL1 CAR-T Cells for R/R AML | NCT03631576 | ≤70 years | Fujian Medical University, Fuzhou, China |
| CAR-T cells Therapy in Relapsed/Refractory Acute Myeloid Leukemia (AML) | NCT03473457 | ≥6 months | Zhujiang Hospital, Guangzhou, China |
| Multiple CAR-T Cell Therapy Targeting AML | NCT04010877 | ≥6 months | Shenzhen Geno-Immune Medical Institute, Shenzhen, China |
| Safety and efficacy evaluation of IM23 CAR-T cells (IM23CAR-T) | NCT03585517 | 3–80 years | Beijing Immunochina Medical Science & Technology Co., Ltd., Beijing, China |
| Study Evaluating Safety and Efficacy of CAR-T cells targeting CD123 in Patients with Acute Myelocytic Leukemia | NCT03796390 | 2–65 years | Hebei Senang Biotechnology Inc., Ltd., Shijiazhuang, China |
| CART-123 for Relapsed/Refractory Acute Myelocytic Leukemia | NCT03556982 | 14–75 years | The Affiliated Hospital fo the Chinese Academy of Military Medical Sciences, Beijing, China |
| CAR-T Cells Therapy in Relapsed/Refractory Acute Myeloid Leukemia | NCT03473457 | ≥6 months | Guangzhou, China |
CAR T cell resistance: antigenic modulation
Data from pediatric and adult phase 1 trials have now highlighted major potential mechanisms of immunotherapeutic resistance in children with B-ALL (99). One study reported B-ALL cell CD19 splice variants (either de novo or after CD19CART therapeutic pressure) that result in loss of the CAR binding epitope and surface antigen expression (76, 100). Loss of CD81, a CD19 chaperone, also results in cytoplasmic retention of CD19 that prevents CD19-targeted immunotherapy activity (101, 102). Immune escape via CD22 surface protein downregulation after CD22CART can also occur (73). Finally, lymphoid-to-myeloid lineage switch after CAR T cell or BiTE immunotherapy has been reported (78, 79).
Dual antigen-targeting CAR T cells developed to combat immunotherapeutic resistance are under active exploration; studies of CD19xCD20CART or CD19xCD22CARThave shown preliminary safety and efficacy in a small number of patients with relapsed/refractory B-cell malignancies (NCT03241940, NCT03330691) (72). Alternative ‘syn-notch’ approaches coupling target recognition by a constitutively expressed CAR to transcriptional expression of a second chimeric receptor require surface expression of both antigens for effective T-cell mediated cytotoxicity (103). In this system, initial target recognition induces cleavage of a transcription factor, which then drives expression of another CAR that binds a distinct tumor-associated target. Such strategies remain under preclinical evaluation and have not yet been tested in patients.
CAR-NK cell therapies
While CAR-T cell production from patient- and donor-derived peripheral blood mononuclear cells is becoming more accessible, engineering of other immune effector cell types is also evolving. The use of artificial antigen- and cytokine-presenting cells (104, 105) to stimulate NK cell activation and expansion has allowed for ex vivo culture and genetic modification of innate effector cells. In contrast to adoptively-transferred CAR T cells that can persist for years in patients (106), NK cells with their shorter in vivo persistence induce far more limited on-target/off-tumor toxicity, which may allow targeting of a greater number of potential tumor antigens. Another benefit of NK cell-based therapies is their inability to cause graft-versus-host disease given lack of cell surface TCRs (107). As such, the therapeutic potential of CAR-NK cells could be far greater than autologous CAR T cell approaches (97, 108–111), as multiple cell doses could be generated from each allogeneic donor independent of recipient host health factors. Bulk NK cell production and storage could also dramatically decrease therapeutic cost and timing between recognized patient need and NK cell infusion.
An acknowledged limitation of CAR-NK cells to date has been the observed lack of robust in vivo expansion in non-engineered adoptive NK cell transfer trials (54). Cytokine (e.g., IL-15, IL-21) NK cell stimulation has improved expansion and anti-tumor activity in preclinical models (112, 113). A phase 1 clinical trial is currently assessing the safety and potential efficacy of CD19CAR-NK cells co-expressing membrane-bound IL-15 in adults with relapsed/refractory B-cell malignancies (NCT03579927), but does not yet allow pediatric participation. Similar CD33CAR-NK strategies have demonstrated activity in preclinical AML models and have proven safe in patients, although durable responses have not been observed (114). Additional non-CAR cytokine-primed allogeneic NK cell immunotherapy trials are ongoing in adults and children with relapsed myelodysplastic syndrome or AML (NCT04024761, NCT03068819) (58, 115).
CONCLUSION
The enthusiasm for immunotherapy for children with high-risk leukemia is clearly warranted. However, appreciable therapeutic challenges remain, both in maximization of treatment efficacy and minimization of normal tissue toxicity. Future studies will continue to optimize ADC and BiTE immunotherapies (some in combination with chemotherapy) and identify the most optimal disease state and patient population(s) for treatment. For cellular therapies, the ‘tunability’ of engineered cell activity, cost of cell manufacturing, and time to patient infusion are of particular importance. Given the potential life-year gains in children with leukemia treated with immunotherapy, consideration of on-target/on-tumor activity and on-target/off-tumor bystander toxicity must also be carefully balanced. Long-term follow-up of treated patients with continued CAR T cell persistence will likely provide greater insight regarding potential effects upon fertility and embryologic toxicity.
The efficacy of leukemia-targeted immunotherapy in the absence of the common toxicities associated with cytotoxic chemotherapy is exciting. Even so, administration of bispecific T cell-engaging antibodies and engineered T cells are associated with risks of cytokine release syndrome (CRS) and immune effector cell neurotoxicity syndrome (ICANS), which can be life-threatening (116). T-cell activation, expansion, and resultant inflammation from these immunotherapies can manifest clinically as hypotension, tachycardia, hypoxia, and end-organ toxicity that require maximal supportive care, often in the intensive care unit setting when of high-grade (117). Though reversible in the majority of patients, CRS and ICANS benefit from early recognition and treatment or supportive care. Ongoing studies seek to identify associated biomarkers that may predict patients at particular risk for these toxicities (118). Interleukin-6 (IL-6) has been recognized as a critical mediator of systemic inflammatory toxicity resulting from both antibody-based and cellular immunotherapies. Severe immune hyperactivation can be successfully ameliorated in most patients with tociluzimab, an anti-IL-6 receptor monoclonal antibody now FDA-approved for treatment of CRS (119).
Ongoing and future studies will continue to optimize next-generation CAR T cell strategies for children with leukemia. Several groups have invented mechanisms for pharmacologic control of CAR expression or CAR-T activation (120–125), while others have designed infusible components to direct and control binding of the CAR to surface antigen targets (126, 127). CAR degradation, stimulated by small molecule blockade of proteasome-mediated degron cleavage, is another approach under current study (128). Ultimately, assessment of safety and toxicity will require bench-to-bedside translation and clinical trial testing, as animal models cannot perfectly predict human toxicity. The plasticity inherent in leukemias resistant to standard treatment regimens are likely drivers of both chemotherapeutic and immunotherapeutic resistance. Antigenic downregulation after immunotherapy has been identified as a major contributor to relapse. Strategies to either prevent or preemptively treat this immune escape are needed. For gene-modified therapies, there is a risk of insertional mutagenesis, a concern that has been reinforced now with identification of clonal CAR T cell expansion in two treated patients (129, 130). Nevertheless, the promise of cure in what was recently considered incurable leukemia is exhilarating and provides hope to stimulate continued scientific discovery.
KEY POINTS.
Immunotherapies have shown promising efficacy in treatment of children with highly chemotherapy-refractory leukemias.
Antibody-based therapeutics targeting leukemia-associated antigens appear safe and may overcome chemoresistance.
The success of cellular therapies, particularly engineered CAR-T and CAR-NK cells, has been transformative and has cured previously-incurable children.
ACKNOWLEDGEMENTS
CLB and SKT wrote and edited the manuscript and approved the final version.
This work was supported by the Damon Runyon Sohn Cancer Research Foundation (CLB), the American Society for Transplantation and Cellular Therapy (CLB), Gabrielle’s Angel Cancer Research Foundation (SKT), Rally Foundation for Childhood Cancer Research (SKT), St Baldrick’s Foundation/Stand Up to Cancer Pediatric Dream Team (SKT), and NIH/NCI K08CA184418 and U01CA232486 (SKT).
CLB has pending patent applications in field of engineered cellular therapies. SKT is a member of the scientific advisory board for Aleta Biotherapeutics.
REFERENCES WITH ANNOTATION
- 1.Bride KL, Vincent TL, Im SY, Aplenc R, Barrett DM, Carroll WL, et al. Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood. 2018;131(9):995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13(1):47–58. [DOI] [PubMed] [Google Scholar]
- 3.Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7(6):1490–6. Summary of clinical data leading to first FDA approval of gemtuzumab ozogamicin.
- 4.Zwaan CM, Reinhardt D, Corbacioglu S, van Wering ER, Bokkerink JP, Tissing WJ, et al. Gemtuzumab ozogamicin: first clinical experiences in children with relapsed/refractory acute myeloid leukemia treated on compassionate-use basis. Blood. 2003;101(10):3868–71. [DOI] [PubMed] [Google Scholar]
- 5.Arceci RJ, Sande J, Lange B, Shannon K, Franklin J, Hutchinson R, et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood. 2005;106(4):1183–8. [DOI] [PubMed] [Google Scholar]
- 6.Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66–71. [DOI] [PubMed] [Google Scholar]
- 7.Aplenc R, Alonzo TA, Gerbing RB, Lange BJ, Hurwitz CA, Wells RJ, et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(14):2390–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brethon B, Yakouben K, Oudot C, Boutard P, Bruno B, Jerome C, et al. Efficacy of fractionated gemtuzumab ozogamicin combined with cytarabine in advanced childhood myeloid leukaemia. Br J Haematol. 2008;143(4):541–7. [DOI] [PubMed] [Google Scholar]
- 9.Zwaan CM, Reinhardt D, Zimmerman M, Hasle H, Stary J, Stark B, et al. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: results of a phase II study. Br J Haematol. 2010;148(5):768–76. [DOI] [PubMed] [Google Scholar]
- 10.Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118(3):761–9. [DOI] [PubMed] [Google Scholar]
- 11.Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–16. [DOI] [PubMed] [Google Scholar]
- 12.Satwani P, Bhatia M, Garvin JH Jr., George D, Dela Cruz F, Le Gall J, et al. A Phase I study of gemtuzumab ozogamicin (GO) in combination with busulfan and cyclophosphamide (Bu/Cy) and allogeneic stem cell transplantation in children with poor-risk CD33+ AML: a new targeted immunochemotherapy myeloablative conditioning (MAC) regimen. Biol Blood Marrow Transplant. 2012;18(2):324–9. [DOI] [PubMed] [Google Scholar]
- 13.Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021–32. Phase 3 clinical trial data demonstrating improved disease-free survival with gemtuzumab addition to chemotherapy in some children with newly-diagnosed AML.
- 15.Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M, et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J Clin Oncol. 2016;34(9):972–9. [DOI] [PubMed] [Google Scholar]
- 16.Zahler S, Bhatia M, Ricci A, Roy S, Morris E, Harrison L, et al. A Phase I Study of Reduced-Intensity Conditioning and Allogeneic Stem Cell Transplantation Followed by Dose Escalation of Targeted Consolidation Immunotherapy with Gemtuzumab Ozogamicin in Children and Adolescents with CD33+ Acute Myeloid Leukemia. Biol Blood Marrow Transplant. 2016;22(4):698–704. [DOI] [PubMed] [Google Scholar]
- 17.Niktoreh N, Lerius B, Zimmermann M, Gruhn B, Escherich G, Bourquin JP, et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Munster study group. Haematologica. 2019;104(1):120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasle H, Abrahamsson J, Forestier E, Ha SY, Heldrup J, Jahnukainen K, et al. Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: results from NOPHO-AML 2004. Blood. 2012;120(5):978–84. [DOI] [PubMed] [Google Scholar]
- 19.Pollard JA, Loken M, Gerbing RB, Raimondi SC, Hirsch BA, Aplenc R, et al. CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children’s Oncology Group Trial AAML0531. J Clin Oncol. 2016;34(7):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103(5):1807–14. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, Thomas D, Jorgensen J, Kebriaei P, Jabbour E, Rytting M, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rytting M, Triche L, Thomas D, O’Brien S, Kantarjian H. Initial experience with CMC-544 (inotuzumab ozogamicin) in pediatric patients with relapsed B-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(2):369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740–53. Phase 3 clinical trial data demonstrating improved clinical outcomes of inotuzumab immunotherapy for adults with relapsed/refractory B-ALL.
- 24.Bhojwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. 2019;33(4):884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gokbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102(5):1578–82. [DOI] [PubMed] [Google Scholar]
- 27.Velasquez MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131(1):30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119(26):6226–33. [DOI] [PubMed] [Google Scholar]
- 29.Gokbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–31. Clinical trial data demonstrating benefit of blinatumomab immunotherapy for adults with MRD-level relapsed/refractory B-ALL.
- 30.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. Phase 2 clinical trial data demonstrating efficacy of blinatumomab immunotherapy for adults with MRD-level relapsed/refractory B-ALL.
- 31.Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–40. [DOI] [PubMed] [Google Scholar]
- 32.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–8. [DOI] [PubMed] [Google Scholar]
- 33.von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2016;34(36):4381–9. Phase 1/2 clinical data data demonstrating safety and efficacy of blinatumomab in children with relapsed/refractory ALL.
- 34.Nagele V, Kratzer A, Zugmaier G, Holland C, Hijazi Y, Topp MS, et al. Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL. Exp Hematol Oncol. 2017;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–41. First report of allogeneic viral-specific T cell transfer showing safety in 3 patients.
- 36.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–44. [DOI] [PubMed] [Google Scholar]
- 37.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330(17):1185–91. [DOI] [PubMed] [Google Scholar]
- 38.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–55. [PubMed] [Google Scholar]
- 39.Weber G, Caruana I, Rouce RH, Barrett AJ, Gerdemann U, Leen AM, et al. Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia--implications for immunotherapy. Clin Cancer Res. 2013;19(18):5079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YJ, Cho SG, Lee S, Kim MS, Kim EK, Cho BS, et al. Potential role of adoptively transferred allogeneic WT1-specific CD4+ and CD8+ T lymphocytes for the sustained remission of refractory AML. Bone Marrow Transplant. 2010;45(3):597–9. [DOI] [PubMed] [Google Scholar]
- 41.Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5(174):174ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber G, Gerdemann U, Caruana I, Savoldo B, Hensel NF, Rabin KR, et al. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia. 2013;27(7):1538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–7. [DOI] [PubMed] [Google Scholar]
- 44.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109(1):323–30. [DOI] [PubMed] [Google Scholar]
- 45.Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26(9):2019–26. [DOI] [PubMed] [Google Scholar]
- 46.Tajima F, Kawatani T, Endo A, Kawasaki H. Natural killer cell activity and cytokine production as prognostic factors in adult acute leukemia. Leukemia. 1996;10(3):478–82. [PubMed] [Google Scholar]
- 47.Rouce RH, Shaim H, Sekine T, Weber G, Ballard B, Ku S, et al. The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia. 2016;30(4):800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saultz JN, Freud AG, Mundy-Bosse BL. MicroRNA regulation of natural killer cell development and function in leukemia. Mol Immunol. 2018. [DOI] [PubMed] [Google Scholar]
- 49.Scoville SD, Nalin AP, Chen L, Chen L, Zhang MH, McConnell K, et al. Human AML activates the aryl hydrocarbon receptor pathway to impair NK cell development and function. Blood. 2018;132(17):1792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venton G, Labiad Y, Colle J, Fino A, Afridi S, Torres M, et al. Natural killer cells in acute myeloid leukemia patients: from phenotype to transcriptomic analysis. Immunol Res. 2016;64(5–6):1225–36. [DOI] [PubMed] [Google Scholar]
- 51.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. [DOI] [PubMed] [Google Scholar]
- 52.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brehm C, Huenecke S, Quaiser A, Esser R, Bremm M, Kloess S, et al. IL-2 stimulated but not unstimulated NK cells induce selective disappearance of peripheral blood cells: concomitant results to a phase I/II study. PLoS One. 2011;6(11):e27351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi I, Yoon SR, Park SY, Kim H, Jung SJ, Jang YJ, et al. Donor-derived natural killer cells infused after human leukocyte antigen-haploidentical hematopoietic cell transplantation: a dose-escalation study. Biol Blood Marrow Transplant. 2014;20(5):696–704. [DOI] [PubMed] [Google Scholar]
- 56.Choi I, Yoon SR, Park SY, Kim H, Jung SJ, Kang YL, et al. Donor-Derived Natural Killer Cell Infusion after Human Leukocyte Antigen-Haploidentical Hematopoietic Cell Transplantation in Patients with Refractory Acute Leukemia. Biol Blood Marrow Transplant. 2016;22(11):2065–76. [DOI] [PubMed] [Google Scholar]
- 57.Lee DA, Denman CJ, Rondon G, Woodworth G, Chen J, Fisher T, et al. Haploidentical Natural Killer Cells Infused before Allogeneic Stem Cell Transplantation for Myeloid Malignancies: A Phase I Trial. Biol Blood Marrow Transplant. 2016;22(7):1290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357ra123. Preclinical and clinical data demonstrating activity of NK cells against human AML.
- 59.Ciurea SO, Schafer JR, Bassett R, Denman CJ, Cao K, Willis D, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130(16):1857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. First intent-to-treat National Cancer Institute phase 1 trial experience with CD19CART immunotherapy in children and AYAs with relapsed/refractory B-ALL.
- 62.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–31. First intent-to-treat Seattle Children’s Hospital phase 1 trial experience with CD19CART immunotherapy in children and AYAs with relapsed/refractory B-ALL.
- 63.Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449–59. Long-term follow up of phase 1 trial experience with CD19CART immunotherapy in adults with relapsed/refractory B-ALL.
- 64.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. Multi-institutional international phase 1 trial experience with CD19CART immunotherapy in children and AYAs with relapsed/refractory B-ALL.
- 65.Ghorashian S, Kramer AM, Onuoha S, Wright G, Bartram J, Richardson R, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019. First Great Ormond Street Hospital phase 1 trial experience of low-affinity CD19CART immunotherapy in children with relapsed/refractory B-ALL.
- 66.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86(24):10024–8. First preclinical report of T cell engineered to express functional chimeric antigen receptors.
- 67.Eshhar Z, Waks T, Gross G. The emergence of T-bodies/CAR T cells. Cancer J. 2014;20(2):123–6. [DOI] [PubMed] [Google Scholar]
- 68.Hwu P, Shafer GE, Treisman J, Schindler DG, Gross G, Cowherd R, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993;178(1):361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20(1):70–5. [DOI] [PubMed] [Google Scholar]
- 70.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–6. Clinical trial proving importance of inclusion of co-stimulatory domains for in vivo CAR T cell persistence.
- 72.Qin H, Ramakrishna S, Nguyen S, Fountaine TJ, Ponduri A, Stetler-Stevenson M, et al. Preclinical Development of Bivalent Chimeric Antigen Receptors Targeting Both CD19 and CD22. Mol Ther Oncolytics. 2018;11:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacoby E, Shahani SA, Shah NN. Updates on CAR T-cell therapy in B-cell malignancies. Immunol Rev. 2019;290(1):39–59. [DOI] [PubMed] [Google Scholar]
- 75.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5(12):1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504–6. [DOI] [PubMed] [Google Scholar]
- 78.Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406–10. Initial description of lymphoid-to-myeloid leukemia lineage switch after CD19-directed immunotherapy in a child with B-ALL.
- 79.Jacoby E, Nguyen SM, Fountaine TJ, Welp K, Gryder B, Qin H, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. 2016;7:12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qasim W Allogeneic CAR T cell therapies for leukemia. Am J Hematol. 2019;94(S1):S50–S4. [DOI] [PubMed] [Google Scholar]
- 81.Perna F, Berman SH, Soni RK, Mansilla-Soto J, Eyquem J, Hamieh M, et al. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell. 2017;32(4):506–19 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haubner S, Perna F, Kohnke T, Schmidt C, Berman S, Augsberger C, et al. Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leukemia. 2019;33(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GM, Cribioli E, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161(3):389–401. [DOI] [PubMed] [Google Scholar]
- 84.Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122(18):3138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014;28(8):1596–605. [DOI] [PubMed] [Google Scholar]
- 86.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonifant CL, Szoor A, Torres D, Joseph N, Velasquez MP, Iwahori K, et al. CD123-Engager T Cells as a Novel Immunotherapeutic for Acute Myeloid Leukemia. Mol Ther. 2016;24(9):1615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qin H, Edwards JP, Zaritskaya L, Gupta A, Mu CJ, Fry TJ, et al. Chimeric Antigen Receptors Incorporating D Domains Targeting CD123 Direct Potent Mono- and Bi-specific Antitumor Activity of T Cells. Mol Ther. 2019;27(7):1262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dutour A, Marin V, Pizzitola I, Valsesia-Wittmann S, Lee D, Yvon E, et al. In Vitro and In Vivo Antitumor Effect of Anti-CD33 Chimeric Receptor-Expressing EBV-CTL against CD33 Acute Myeloid Leukemia. Adv Hematol. 2012;2012:683065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29(8):1637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Minagawa K, Jamil MO, Al-Obaidi M, Pereboeva L, Salzman D, Erba HP, et al. In Vitro Pre-Clinical Validation of Suicide Gene Modified Anti-CD33 Redirected Chimeric Antigen Receptor T-Cells for Acute Myeloid Leukemia. PLoS One. 2016;11(12):e0166891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tashiro H, Sauer T, Shum T, Parikh K, Mamonkin M, Omer B, et al. Treatment of Acute Myeloid Leukemia with T Cells Expressing Chimeric Antigen Receptors Directed to C-type Lectin-like Molecule 1. Mol Ther. 2017;25(9):2202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laborda E, Mazagova M, Shao S, Wang X, Quirino H, Woods AK, et al. Development of A Chimeric Antigen Receptor Targeting C-Type Lectin-Like Molecule-1 for Human Acute Myeloid Leukemia. Int J Mol Sci. 2017;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol. 2018;11(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krawczyk E, Zolov SN, Huang K, Bonifant CL. T-cell Activity against AML Improved by Dual-Targeted T Cells Stimulated through T-cell and IL7 Receptors. Cancer Immunol Res. 2019;7(4):683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jetani H, Garcia-Cadenas I, Nerreter T, Thomas S, Rydzek J, Meijide JB, et al. CAR T-cells targeting FLT3 have potent activity against FLT3(−)ITD(+) AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018;32(5):1168–79. [DOI] [PubMed] [Google Scholar]
- 97.Oelsner S, Waldmann A, Billmeier A, Roder J, Lindner A, Ullrich E, et al. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive B-ALL and inhibit in vivo leukemia growth. Int J Cancer. 2019;145(7):1935–45. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y, Xu Y, Li S, Liu J, Xing Y, Xing H, et al. Targeting FLT3 in acute myeloid leukemia using ligand-based chimeric antigen receptor-engineered T cells. J Hematol Oncol. 2018;11(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fischer J, Paret C, El Malki K, Alt F, Wingerter A, Neu MA, et al. CD19 Isoforms Enabling Resistance to CART-19 Immunotherapy Are Expressed in B-ALL Patients at Initial Diagnosis. J Immunother. 2017;40(5):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Braig F, Brandt A, Goebeler M, Tony HP, Kurze AK, Nollau P, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129(1):100–4. [DOI] [PubMed] [Google Scholar]
- 102.Bagashev A, Sotillo E, Tang CH, Black KL, Perazzelli J, Seeholzer SH, et al. CD19 Alterations Emerging after CD19-Directed Immunotherapy Cause Retention of the Misfolded Protein in the Endoplasmic Reticulum. Mol Cell Biol. 2018;38(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell. 2016;164(4):770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–83. First description of CAR-NK cell generation and anti-tumor activity.
- 105.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7(1):e30264. Correlative study demonstrating decades-long persistence of adoptively transferred T cells.
- 106.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol Ther. 2019;27(6):1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kloss S, Oberschmidt O, Morgan M, Dahlke J, Arseniev L, Huppert V, et al. Optimization of Human NK Cell Manufacturing: Fully Automated Separation, Improved Ex Vivo Expansion Using IL-21 with Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells. Hum Gene Ther. 2017;28(10):897–913. [DOI] [PubMed] [Google Scholar]
- 110.Oberschmidt O, Morgan M, Huppert V, Kessler J, Gardlowski T, Matthies N, et al. Development of Automated Separation, Expansion, and Quality Control Protocols for Clinical-Scale Manufacturing of Primary Human NK Cells and Alpharetroviral Chimeric Antigen Receptor Engineering. Hum Gene Ther Methods. 2019;30(3):102–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mehta RS, Rezvani K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front Immunol. 2018;9:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4(4):329–36. [DOI] [PubMed] [Google Scholar]
- 113.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083–9. [PMC free article] [PubMed] [Google Scholar]
- 115.Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica (Cairo). 2014;2014:205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. American Society for Transplantation and Cellular Therapy Grading Scale for Immune Effector Associated Toxicities.
- 117.Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden. Am Soc Clin Oncol Educ Book. 2019;39:433–44. [DOI] [PubMed] [Google Scholar]
- 118.Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6(6):664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350(6258):aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Juillerat A, Marechal A, Filhol JM, Valton J, Duclert A, Poirot L, et al. Design of chimeric antigen receptors with integrated controllable transient functions. Sci Rep. 2016;6:18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sakemura R, Terakura S, Watanabe K, Julamanee J, Takagi E, Miyao K, et al. A Tet-On Inducible System for Controlling CD19-Chimeric Antigen Receptor Expression upon Drug Administration. Cancer Immunol Res. 2016;4(8):658–68. [DOI] [PubMed] [Google Scholar]
- 123.Mata M, Gerken C, Nguyen P, Krenciute G, Spencer DM, Gottschalk S. Inducible Activation of MyD88 and CD40 in CAR T Cells Results in Controllable and Potent Antitumor Activity in Preclinical Solid Tumor Models. Cancer Discov. 2017;7(11):1306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Drent E, Poels R, Mulders MJ, van de Donk N, Themeli M, Lokhorst HM, et al. Feasibility of controlling CD38-CAR T cell activity with a Tet-on inducible CAR design. PLoS One. 2018;13(5):e0197349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duong MT, Collinson-Pautz MR, Morschl E, Lu A, Szymanski SP, Zhang M, et al. Two-Dimensional Regulation of CAR-T Cell Therapy with Orthogonal Switches. Mol Ther Oncolytics. 2019;12:124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viaud S, Ma JSY, Hardy IR, Hampton EN, Benish B, Sherwood L, et al. Switchable control over in vivo CAR T expansion, B cell depletion, and induction of memory. Proc Natl Acad Sci U S A. 2018;115(46):E10898–E906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A. 2016;113(4):E459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Juillerat A, Tkach D, Busser BW, Temburni S, Valton J, Duclert A, et al. Modulation of chimeric antigen receptor surface expression by a small molecule switch. BMC Biotechnol. 2019;19(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558(7709):307–12. Description of clonal expansion secondary to site-specific integration of chimeric antigen receptor transgene.
- 130.Shah NN, Qin H, Yates B, Su L, Shalabi H, Raffeld M, et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019;3(15):2317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


