Short abstract
Surgical sealants help achieve rapid haemostasis when applied as an adjunct to sutures in vascular surgery, but their use can lead to various side effects. This study compared the local inflammatory reaction to commercially available BioGlue and Coseal sealants in a rabbit aorta suture hole model. Twenty male New Zealand white rabbits were randomised to testing with either BioGlue or Coseal. Two weeks after sealant application to suture holes, sections of the aorta at the puncture site, and surrounding tissue, were processed for histopathological analysis. Inflammation was graded from 0 to 3 according to tissue alteration and presence of inflammatory cells. Material stiffness was measured in vitro using compression testing. From examination of the inflammatory response to the sealants, a less severe histopathological assessment score was assigned to the Coseal compared to the BioGlue group (mean ± SD: 1.56 ± 0.53 vs 2.67 ± 0.50; p = 0.002). While both materials triggered a typical foreign body reaction characterised by granulomatous inflammation, BioGlue additionally provoked eosinophilic cell infiltration. Lymphocytes, plasma cells and B cells were also more prevalent in the BioGlue compared to the Coseal specimens. Coseal residue was either absent or visible in only small quantities, while significant BioGlue deposits remained in the tissue 2 weeks after application. Coseal was much more elastic than BioGlue, with a compressive modulus an order of magnitude lower (mean ± SD: 91 ± 41 vs 1833 ± 297 kPa). Compared to BioGlue, Coseal elicited a less pronounced inflammatory response in the aortic and peri-aortic tissue in this model, and demonstrated greater elasticity.
Keywords: Anastomosis, BioGlue, Coseal, inflammation, stiffness, surgical sealant
Introduction
The adjunctive use of a surgical sealant to reinforce suture lines during surgery has been shown to significantly improve haemostasis, reduce blood loss and transfusion requirements, and shorten the duration of surgery.1 This is not only advantageous for the patient, but has the potential to provide significant economic benefits to the healthcare system.2,3
An ideal sealant for use on vascular tissue should combine elasticity and flexibility with strong tissue adhesion and favourable biocompatibility.4 A wide variety of sealants has been investigated in this setting, including those based on proteins such as fibrinogen and albumin, and those based on synthetic polymers such as polyethylene glycol (PEG) and polyurethane.4,5 Fibrin sealants have demonstrated efficacy for treatment of suture line bleeding during vascular surgery, with haemostasis achieved significantly faster compared to use of manual compression.6,7 Another protein-based sealant that has been evaluated in the setting of vascular surgery is BioGlue (CryoLife Inc., Kennesaw, GA, USA), which forms a seal composed of bovine serum albumin (BSA) crosslinked with glutaraldehyde.8 This sealant was found to be superior to standard anastomotic repair for achieving immediate haemostasis.9 Coseal (Baxter Healthcare Corp., Deerfield, IL, USA) is a sealant that contains no human or animal-derived components. It is composed of multifunctional PEG chains, which crosslink on the tissue surface to form a strongly adhered hydrogel seal.10 Suture line reinforcement with Coseal has been shown to achieve more rapid haemostasis than use of certain other sealants in vascular surgery patients.11–13
Contact between tissue and an implanted foreign substance invariably results in a reaction, the magnitude and manner of which depends on various material properties, including surface chemistry, stiffness and degradation rate.14 A significant adverse response to a tissue sealant could result in poor healing of the target lesion, leading to a requirement for re-operation.15,16 Studies have evaluated the response of a variety of different tissue types to BioGlue, with variable results reported in terms of the severity of tissue reaction.17–26 Research regarding Coseal suggests that this material may hold chemical and mechanical advantages over BioGlue26–30; however, a head-to-head comparison of the vascular tissue reactions to these two particular materials has not been performed to date. This is despite both sealants being indicated for use as an adjunct to mechanical sealing during repair and/or reconstruction of large blood vessels. The aim of the present study, therefore, was to directly compare the aortic and peri-aortic tissue response elicited by BioGlue and Coseal in a rabbit aorta suture hole model. This model was selected to indicate the efficacy of these two sealants for surgical procedures that involve suturing of endogenous vascular tissue, including prosthesis implantation and vascular repair.
Materials and methods
Materials
BioGlue consists of two components, 45% purified BSA and 10% glutaraldehyde. The two solutions mix in the applicator tip of the delivery device, where crosslinking is spontaneously initiated. Once dispensed through the tip, crosslinking also binds the material to proteins on the tissue surface, forming a seal in ∼2 min.8
Coseal consist of tetra-succinimidyl and tetra-thiol-derivatised PEG species, which are added to an applicator device as solutions in separate syringes. The two polymers combine in the applicator tip and start to crosslink. The polymer mixture is then applied to the tissue surface, either through a standard applicator tip or a spray tip, where further crosslinking results in a strongly adherent, flexible hydrogel seal within ∼1 min.2,10,27
Animal care
A total of 20 male New Zealand white rabbits from 2.3 to 3.2 kg in weight were included. The study received an animal experiment permit from the Provincial Government of Lower Austria and was performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH Publication No. 85–23; revised 2011). The rabbit is a standard model in biocompatibility testing and is typically the model of choice for implant testing (e.g. ISO 10993). Rabbits are typically used to assess short-term efficacy and safety of vascular implant materials.
The animals were housed in stainless steel cages at an approximate mean room temperature of 18°C, with a relative humidity of 55% and artificial daylight conditions alternating between night and day at 12-h intervals. The cages contained wooden gnawing blocks, muesli and hay and the animals were given ad libitum access to food (ssniff® K-H diet for rabbits; ssniff Spezialdiäten GmbH, North Rhine-Westphalia, Germany) and drinking water. The animals were manually block randomised to treatment days, with four animals undergoing the procedure on each day. On the day of surgery, four animals were randomised (1:1) to undergo testing with BioGlue (n = 2) or Coseal (n = 2).
Surgical procedure
The animals received pre-emptive analgesia (buprenorphine (0.05 mg/kg s.c.) and carprofen (4 mg/kg s.c.)) at least one hour before surgery. Anaesthesia was induced with ketamine hydrochloride (35 mg/kg s.c.) and xylazine hydrochloride (5 mg/kg s.c.). A surgical plane of anaesthesia was maintained with isoflurane (0.5–3.0%) and oxygen (97–99%) via inhalation through a face mask, as needed. Approximately 45 min after induction of anaesthesia, ketamine hydrochloride (10 mg/kg/h i.v.) was administered as needed.
Under anaesthesia, the abdomen was shaved and prepared using a disinfecting scrub. A median laparotomy was performed and ∼2 cm of the abdominal aorta was exposed. After heparinisation (100 IU/animal), the aorta was clamped and punctured four times in a single line with an RB-1 suture needle, evenly distributed over 2 cm. All procedures were performed by the same veterinary surgeon, who remained blinded to the test sealant until after the lesion had been created. A suitable volume of test sealant was applied for each group as per the Instructions for Use (IFU) to cover all holes while minimising excess material that was unnecessary for lesion coverage, mimicking clinical use. Each application covered the entire ‘lesion site’ but varied slightly in volumes (between 0.25 ml and 0.5 ml) due to the in situ presentation.
In the Coseal group, clamps were removed 1 min after application, as per IFU. In the Bioglue group, clamps were removed after 2 min, also as per manufacturer’s instructions. In two cases (once with Coseal and once with BioGlue), no definitive haemostasis was achieved and reapplication of the sealant was performed. Approximately 5 min after application of the test material, the incision was closed in two layers. A lidocaine (1 ml per animal) ‘splash block’ was applied after closure of the peritoneum, before closure of the skin incision.
The animals received buprenorphine (0.05 mg/kg s.c.) three times daily and carprofen (4 mg/kg s.c.) once daily for the subsequent four days. The general health and recovery of the animals was monitored by daily postoperative assessments carried out by a veterinarian, and technicians inspected the animals at least once daily for a 2-week observation period. This time length was chosen because it is late enough to observe chronic reactions and defect closure can be expected.
At the end of the 2 weeks, the animals were humanely euthanised with an overdose of thiopental under deep anaesthesia. The implantation site was visually inspected, and macroscopic signs of inflammation, including general adhesion and capsule formation, were documented by the veterinary surgeon. Tissue samples from the aorta at the puncture sites, and surrounding tissue, were then taken for histopathological analysis.
Histopathological analysis
The harvested tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin wax. Transverse sections ∼1–2 μm thick were cut at the site of injury for each lesion and subsequently stained with haematoxylin and eosin (HE). To limit bias, all HE stained slides were assessed in a blinded fashion by independent pathologists at two different institutions (Centre 1: Ludwig Boltzmann Institute for Experimental and Clinical Traumatology, Vienna; Centre 2: Institute of Pathology, University of Veterinary Medicine, Vienna). However, it should be noted that in the case of present material residues, these in theory would allow identification of the treatment group to the trained eye. Three sections from each animal were examined. Basic histological assessment was performed to characterise the magnitude, spread and type of inflammatory alterations. Differentiation of participating cell types in HE-stained slides was based on typical morphological characteristics. The degree of inflammation based on the general presence of inflammatory cells (independent of the participating cell types) was scored for the sample with the most prominent alterations as none (0), mild (1), moderate (2) or severe (3), to determine the worst-case reaction.
In addition, a secondary detailed assessment was performed at the University of Veterinary Medicine by a blinded pathologist, where the type of inflammation was categorised according to the presence and/or the predominating cell type(s) in the affected area, including granulocytes, macrophages, epithelioid cells and multinucleated giant cells. Specifically, the tissue was examined for inflammatory infiltrates (e.g. the presence of lymphocytes, macrophages and plasma cells), foreign body reaction (e.g. presence of foreign body giant cells and necrosis) and bacterial colonisation. Furthermore, the presence and quantity of sealant deposits were semiquantitatively assessed by microscopic visualisation of the HE-stained slides, and graded on a scale of 0 (none) to 3 (severe).
For evaluation of bacterial involvement Brown and Brenn staining was used in all sections. Luna staining was applied for differentiation and quantification of granulocytes in paraffin-embedded sections. Three sections from each animal were examined, and that with the most prominent alterations was selected for further analysis. The extent of eosinophilic granulocytes was visually counted per high power field (HPF) in areas of most intense cell infiltration, and was graded as follows: grade 0: <5 cells/HPF; grade 1: 5–<100 cells/HPF; grade 2: 100–500 cells/HPF; grade 3: >500 cells/HPF. To exclude the potential presence of mast cells in the HPF counts, toluidine blue staining was additionally employed.
Immunohistochemical staining
B cell tissue infiltration was evaluated by immunostaining using an anti-CD79a mouse monoclonal antibody (ab3121, Abcam, dilution 1:1000, pre-treatment pH 8) followed by an anti-mouse secondary antibody conjugated to a horseradish peroxidase (HRP)-labelled polymer (BrightVision Poly-HRP-anti-mouse; ImmunoLogic). Diaminobenzidine was subsequently employed as the substrate for visualisation, with all sections then counterstained with haematoxylin. Three sections from each animal were examined, and that with the most prominent alterations was selected for further analysis. The following grading was employed: grade 0: no positive cell in any section; grade 1: <10 positive cells interspersed in all sections; grade 2: 10–150 positive cells in circumscribed areas of most intensive infiltration; grade 3: >150 positive cells within a circumscribed inflammatory focus displaying positive expression.
In vitro mechanical testing
Cylindrical specimens of sealant (BioGlue n = 14; Coseal n = 10) with a circular cross-section of 1 cm in diameter were cast using moulds following the IFU for each product. The specimens had an approximate thickness of 4 mm. The samples were then evaluated using a uniaxial mechanical testing machine (BZ 2.5/TN1S; Zwick GmbH & Co. KG, Ulm, Germany) with a 2.5 kN load cell (Xforce HP; Zwick GmbH & Co. KG, Ulm, Germany). When a preload of 0.1 N was achieved, the distance between the plates was measured as the materials were compressed at a constant speed of 1 mm/min, up to 80% deformation. The compressive modulus was calculated by performing a regression on the segment between 15% and 20% deformation, which was considered to be linear and within the relevant range of deformation.
Statistical analysis
A Mann–Whitney U test was used to analyse the differences between inflammation grades and tissue cellular infiltration induced by the two sealants. A two-tailed t-test was used to analyse the differences between compression moduli after D’Agostino and Pearson normality testing. Statistical analysis was performed using GraphPad Prism version 5.01 (San Diego, CA, USA).
Results
Of the 20 animals that were randomised, one died prior to surgery due to the anaesthesia; therefore, 19 animals underwent surgery. Of these, one animal (BioGlue group) died on postoperative day 9 due to an unrelated cause and so was excluded from the analysis. Tissue from a total of 18 animals, nine animals per sealant, was therefore evaluated.
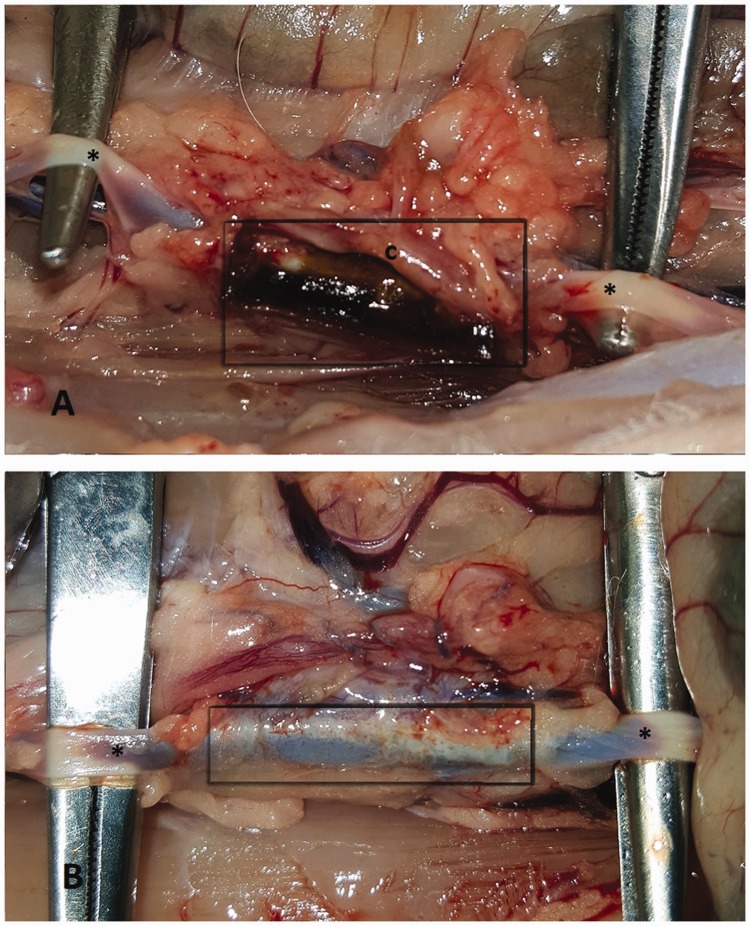
Representative macroscopic images of the lesion sites 2 weeks after sealant application are shown in Figure 1. All application sites showed signs of inflammation. BioGlue residue could be seen at the site of application, surrounded by a connective tissue capsule. In contrast, no residue from Coseal could be visualised macroscopically.
Figure 1.
Representative images of the application sites after 2 weeks: (a) BioGlue, *aorta, grey box: area of application and tissue reaction; (b) Coseal, *aorta, grey box: area of application and tissue reaction%; (c) opened tissue capsule that surrounded the BioGlue residue.
Histopathological observations
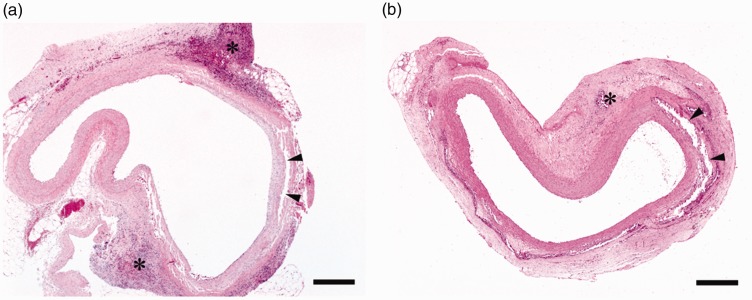
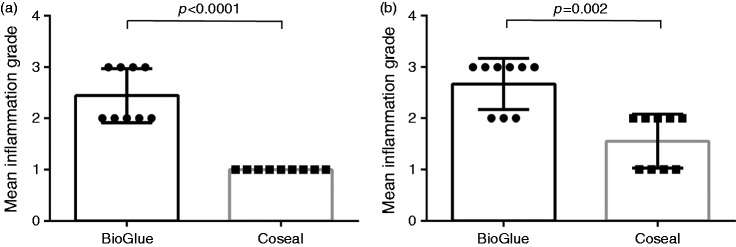
Overall, the sealant-induced inflammatory reactions, ranging from mild to severe, were primarily localised in the peri-aortic, and sometimes aortic, tissue (Figure 2). Specifically, in the BioGlue group, the grade of inflammation was moderate-to-severe (Figure 3), with mean ± SD values of 2.44 ± 0.53 as graded by the assessor at centre 1, and 2.67 ± 0.50 as graded by the assessor at centre 2. In contrast, in the Coseal group, the inflammation was graded as mild-to-moderate (Figure 3), with values of 1.00 ± 0.0 (centre 1) and 1.56 ± 0.53 (centre 2). The differences between the two sealants were found to be statistically significant both for centre 1 (p < 0.0001) and centre 2 (p = 0.002). Regardless of the sealant used, the aorta revealed a segmental circular fragmentation (Figure 2(a) and (b)), commonly accompanied by dystrophic calcification of the vessel wall, without inflammatory response. In most cases, inflammatory infiltrates were localised to the soft tissue adjacent to the aortic adventitia. In two cases in the BioGlue group, inflammation spread to the inner layers of the aortic wall, including the tunica media and tunica intima. In a small number of samples in each group, the aorta demonstrated hypertrophy of the blood vessel endothelium. In one animal in the BioGlue group, there was partial necrosis of the arterial wall, consisting of shrunken smooth myocytes with pyknotic nuclei.
Figure 2.
(a) BioGlue; (b) Coseal: in both groups inflammatory cells (*) were localised predominantly around the aorta, demonstrating segmental circular fragmentation (arrowheads) of the vessel wall, scale bar = 400 μm.
Figure 3.
(a) Mean (±standard deviation (SD)) of grades given by investigators at centre 1; (b) Mean (±SD) of grades given by investigators at centre 2. p-Values were calculated using a Mann–Whitney U test. Inflammation grade: none (0); mild (1); moderate (2); severe (3). The use of means in the figure is for the purpose of illustration.
Cellular infiltration
Irrespective of the sealant, inflammatory infiltrates typically contained macrophages, individually scattered either in the form of epithelioid cells and/or multinucleated giant cells, sometimes arranged around residual tissue sealants (Table 1). However, there was no clear difference in the distribution of macrophages between the two groups. Lymphocyte infiltration was also present, but varied depending on the sealant used; being moderate-to-severe in the BioGlue group and mild-to-moderate in the Coseal group (Table 1). Plasma cells were found in varying numbers in all BioGlue specimens. In contrast, mild plasma cell infiltration was evident in only two Coseal specimens. In addition, moderate-to-severe infiltration by intensively eosinophilic-stained, distinctly granulated granulocytes was found in all BioGlue specimens. Conversely, eosinophilic-stained cells were found in only three Coseal specimens, with this infiltration classed as mild-to-moderate.
Table 1.
Inflammation pattern A: granulomatous with eosinophil-like cell infiltration; pattern B: granulomatous with few/no eosinophil-like cells.
| Inflammation pattern |
Eosinophils |
Epithelioid/giant cells | Lymphocytes | Plasma cells | B cells | Residual sealant | ||
|---|---|---|---|---|---|---|---|---|
| Test item | HE | HE | Lunaa | HE | HE | HE | Anti-CD79ab | HEc |
| BioGlue | A | 2 | 2 | 1 | 2 | 2 | 1 | 3 |
| BioGlue | A | 3 | 2 | 1 | 2 | 2 | 1 | 3 |
| BioGlue | A | 2 | 2 | 1 | 2 | 1 | 3 | 2 |
| BioGlue | A | 3 | 3 | 1 | 2 | 2 | 3 | 2 |
| BioGlue | A | 2 | 2 | 1 | 2 | 1 | 2 | 3 |
| BioGlue | A | 2 | 2 | 2 | 2 | 3 | 3 | 2 |
| BioGlue | A | 2 | 3 | 1 | 2 | 1 | 3 | 2 |
| BioGlue | A | 3 | 3 | 2 | 3 | 2 | 3 | 3 |
| BioGlue | A | 2 | 2 | 2 | 3 | 2 | 3 | 3 |
| Median | – | 2 | 2 | 1 | 2 | 2 | 3 | 3 |
| Mean | 2.33 | 2.33 | 1.33 | 2.22 | 1.77 | 2.44 | 2.56 | |
| SD | 0.5 | 0.5 | 0.5 | 0.44 | 0.67 | 0.88 | 0.53 | |
| Coseal | B | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Coseal | B | 0 | 0 | 2 | 1 | 0 | 1 | 1 |
| Coseal | B | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Coseal | B | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Coseal | A | 2 | 1 | 2 | 2 | 0 | 2 | 1 |
| Coseal | B | 0 | 0 | 1 | 1 | 0 | 0 | 2 |
| Coseal | B | 1 | 1 | 2 | 1 | 1 | 2 | 2 |
| Coseal | – | 1 | 0 | 0 | 1 | 0 | 2 | 0 |
| Coseal | B | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Median | – | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Mean | – | 0.44 | 0.22 | 1 | 1.11 | 0.22 | 0.89 | 0.89 |
| SD | 0.73 | 0.44 | 0.87 | 0.33 | 0.44 | 0.93 | 0.78 | |
| p-value | – | 0.0003 | <0.0001 | 0.5 | 0.0003 | 0.0004 | 0.003 | 0.0006 |
Grading defined qualitatively on a scale of 0 (no cells) to 3 (high infiltration of cells).
aGrading defined as cells per high-power field as follows: grade 0, 5 cells; grade 1, 5–<100 cells; grade 2, 100–500 cells; grade 3, >500 cells.
bGrading defined as follows: grade 0, no positive cells in any area; grade 1, <10 positive cells in each area; grade 2, 10–150 positive cells in areas of most intense infiltration; grade 3, >150 positive cells within a set area.
cGrading defined qualitatively as extent of residual sealant as follows: grade 0, no residual sealant; grade 1, minimal residual sealant; grade 2, moderate quantity of residual sealant; grade 3, large quantity of residual sealant. p-Values calculated using a Mann–Whitney U test.
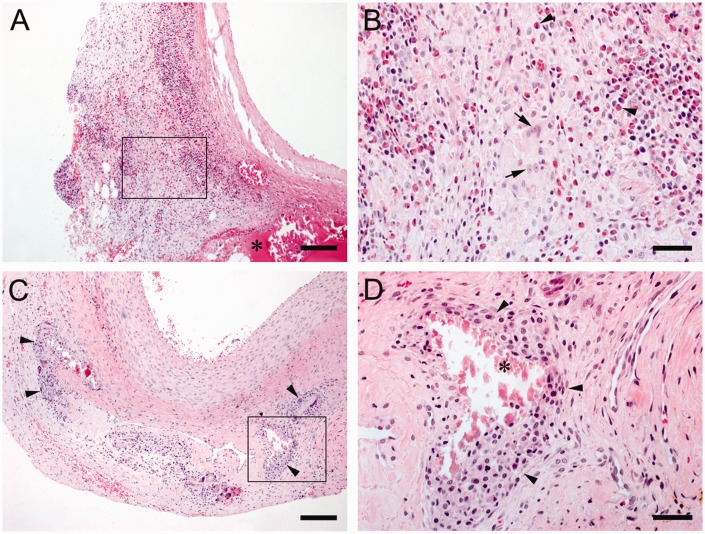
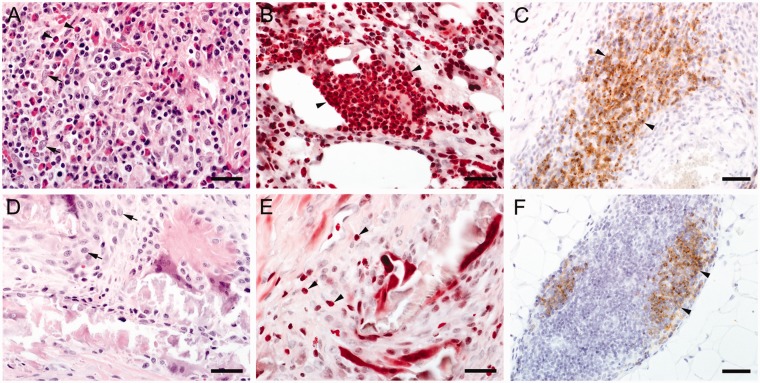
When the specimens were classified according to the predominating cells present, two distinct patterns of granulomatous reactions were identified; pattern A was characterised by a robust presence of eosinophilic granulocytes (Figures 4(a) and (b) and 5(a) and (b)), while pattern B was devoid of them (Figures 4(c) and (d) and 5(d) and (e)). Pattern A was found in all samples of the BioGlue group (9/9) and one Coseal sample, whereas pattern B clearly predominated in Coseal samples (7/9).
Figure 4.
(a and b) BioGlue, Pattern A: (a) Intense granulomatous inflammation, scale bar = 150 μm, material residues are marked with *; (b) Note lymphocytes, plasma cells, multinucleated giant cells (arrows) and many intensively eosinophilic-stained granulocytes (arrowheads), scale bar = 40 μm; (c and d), Coseal, Pattern B: (c) Moderate granulomatous inflammation (arrowheads), scale bar = 150 μm; (d) note the presence of many macrophages (arrowheads) and few lymphocytes, scale bar = 40 μm. Localisation of high magnification images are highlighted in the low-power images.
Figure 5.
(a to c) BioGlue, Pattern A: (a) HE staining demonstrating a mixed pattern of epithelioid cells (arrowheads), eosinophilic granulocytes (arrows) and a few plasma cells and lymphocytes, scale bar = 30 μm; (b) Luna staining showing numerous intensively stained (red) eosinophilic granulocytes (arrowheads), scale bar = 30 μm; note that erythrocytes reveal a similar staining pattern, but can be differentiated in high power images as they do not contain nuclei; (c) CD79a immunostaining indicate that many cells of the infiltrate comprise B-lymphocytes (arrowheads), scale bar = 40 μm; (d to f) Coseal, Pattern B: (d) HE staining demonstrating granulomatous inflammation comprising epitheloid cells (arrows), scale bar = 30 μm; (e) Just single eosinophilic granulocytes can be found by Luna staining (arrowheads), scale bar = 30 μm; (f) Some immunopositive B-cells can be demonstrated by CD79a immunohistochemistry (arrowheads), scale bar = 40 μm.
In the BioGlue specimens, Luna staining demonstrated predominantly moderate-to-severe infiltration by eosinophilic granulocytes (Figure 5(b)). In contrast, these cells were typically absent in Coseal specimens; if present, they were observed in minimal numbers and were individually interspersed (Figure 5(e)). Irrespective of the sealant, no mast cells were detected in any of the HE- and toluidine blue-stained sections.
Brown and Brenn staining revealed that bacteria were not present in all samples.
Immunohistochemical staining
In six BioGlue specimens, CD79a immunohistochemical staining revealed a severe infiltration of B cells (Figure 5(c); Table 1), whereas mild to moderate B-cell infiltrations were found in three BioGlue cases. In the Coseal group, mild infiltration of these cells was observed in two specimens, whereas moderate B-cell infiltration was evident in three cases (Figure 5(f)). B cells were predominantly arranged in a follicular pattern. In the remaining four Coseal specimens, B cells were not found.
Sealant residues
Notable quantities of homogeneous, eosinophilic sealant material were consistently observed in the BioGlue samples 2 weeks after application (Figure 4(a)). When residual sealant was quantified using a scale of 0 (no residual sealant) to 3 (large quantity of residual sealant), a mean ± SD score of 2.60 ± 0.52 was calculated for the BioGlue specimens, while Coseal residues were graded as 0.89 ± 0.78. Intraluminal sealant was not present in all specimens.
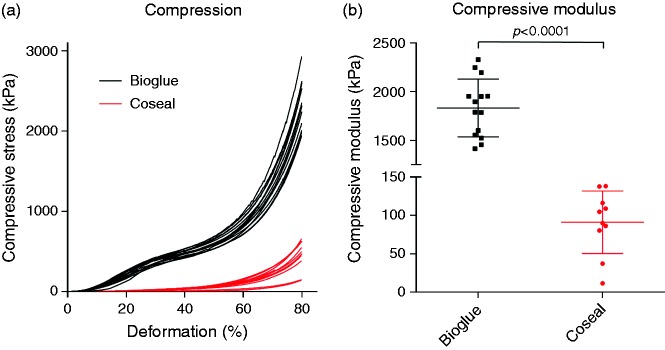
Mechanical properties
The two sealants presented very different stress–strain profiles (Figure 6(a)), with BioGlue displaying the profile of a highly reticulated polymer, while Coseal displayed the exponential behaviour of a hydrogel. The compressive moduli were found to differ by an order of magnitude (p < 0.0001), (Figure 6(b)); the mean ± SD compressive modulus for BioGlue was 1833 ± 297 kPa, while it was 91 ± 41 kPa for Coseal.
Figure 6.
(a) Stress–strain curve resulting from compression of sealant samples; (b) Comparison of compressive moduli for sealant samples showing mean (central line), SD (bars) and individual sample values (squares/dots).
Discussion
The pathological examination of the tissue response to BioGlue and Coseal revealed marked quantitative and qualitative differences between the two tested products. In all but two samples in the Coseal group, the unspecific HE staining demonstrated a typical foreign body reaction characterised by granulomatous inflammation. However, blinded grading of the magnitude of inflammation demonstrated that compared to BioGlue, Coseal elicited a markedly milder tissue response.
The granulomatous character and the distribution of inflammatory response are thought to be due to sealant eliciting foreign body reaction. In our study 2 weeks after application the amount of sealant residues was clearly higher in the BioGlue group. A longer persistence of sealant might elicit a greater immune response. Contrary to BioGlue, in most cases little or no sealant was evident in the Coseal group, indicating an early active degradation and removal in most cases.
Coseal was designed to hydrolytically degrade within 1–2 weeks. Murdock et al.31 has shown an in vitro hydrolytic degradation time in a standardised ASTM in vitro test of 6 days for Coseal, while BioGlue did not degrade over 30 days. Hill et al.27 has shown that ‘small amounts of CoSeal were visible grossly or histologically at day 7’. Similarly, Wallace et al.10 reported that ‘the in vivo data suggested a tissue lifetime of ∼1 week for Coseal’.
Similar to our results, some studies demonstrate a granulomatous inflammatory reaction following BioGlue application in human patients up to two years after cardiovascular surgery.20,32 Experimental studies in rabbits evaluating effects of PEG sealants demonstrate the potential to induce granulomatous inflammation 11 weeks after application in 50% of the specimens.33
Upon detailed examination of the cellular infiltrates at the lesion sites, further differences between the two materials were apparent. Most striking was a greater presence of eosinophilic granulocytes in the HE-stained BioGlue samples, which was rarely observed in the Coseal samples, a finding corroborated by the eosinophil-targeted Luna staining. It has been previously reported that the infiltration of such cells can be elicited in rabbits in response to trauma.34 Therefore, the response of the aortic tissue in the present model could be indicative of an adverse reaction to the BioGlue. This finding is in agreement with a study reported by Cerezal-Garrido et al., who evaluated various sealants applied to rat tracheal lesions.26 They reported that 8 weeks after application, severe inflammation with presence of eosinophilic granulocytes was found in the BioGlue samples, with the presence of this particular cell type suggesting an ongoing active inflammatory response. In contrast, no inflammation was observed in the Coseal samples at this time point.
The second most notable difference between the two sealants was the more prevalent infiltration of lymphocytes and plasma cells in the BioGlue specimens (compared to Coseal), which is indicative of more persistent immunostimulation in response to the tissue sealant.35 Schiller et al. also reported a long-term inflammatory response to BioGlue in rabbits, with moderate-to-severe inflammation evident 60 days after its application to the carotid arteries.22 In a porcine coronary anastomosis model, a severe inflammatory response was observed three months after application of BioGlue, while the responses to gelatin–resorcinol–formaldehyde and cyanoacrylate sealants were classed as minimal and moderate, respectively.36 A comparison of the effects of a number of different tissue sealants on rat colon also demonstrated a severe inflammatory response to BioGlue; however, the PEG-based Duraseal Xact (Covidien, MA, USA) elicited a much milder reaction.23 At 28 days after sealant application, the tissue exposed to BioGlue displayed signs of necrosis, while there were no signs of an ongoing response to Duraseal. In a canine iliac model, a mild-to-moderate inflammatory response to Coseal was observed at 30 and 60 days after surgery, which was similar to that of the control anastomoses (no sealant).27 Clinical evidence further supports the above findings: in three patients requiring surgical wound revision after transcatheter aortic valve implantation utilising BioGlue, a foreign body reaction was evident in all cases.15
In most cases, inflammatory cells were localised to the aortic wall at the presumed site of the aortic lesion. However, in a limited number of specimens, endothelial cell hypertrophy was evident. Furthermore, in two cases in the BioGlue group, the inflammatory cells appeared to spread into the inner layers of the vessel. Injury to the vessel endothelium is of significant concern, as this could cause postoperative complications such as thromboembolism, potentially resulting in increased morbidity and mortality due to myocardial infarction, stroke or pulmonary embolism. In addition, one animal in the BioGlue group displayed partial necrosis of the vessel wall. Impacts on vascular structures, following application of BioGlue are reported in humans and rabbits.32,37,38 Experimental studies on circumferential application of BioGlue around aortoaortic anastomoses in piglets demonstrate impaired vascular growth and anastomotic strictures.38 Pseudoaneurysm formation was thought to be caused by an inflammatory reaction due to Bioglue application in two cases two years after cardiovascular surgery.32 Furst and Banerjee reported that BioGlue applied to rabbit lung and liver tissue resulted in necrosis, although a less severe inflammatory reaction was observed for aortic tissue.37 Fragmentation and dystrophic calcification of the vessel wall, which was commonly observed in both groups are thought to be the consequence of mechanical manipulation rather than tissue response to tissue sealants. Comparative studies evaluating various topical haemostatic agents demonstrate that Coseal is a very effective sealant for vascular and cardiac surgery applications with the capability inducing rapid haemostasis. However, it should not be used to surround anatomic structures that could be harmed by compression.39
While this study investigated the inflammatory response at a single time point, comparisons with previous published literature provide an indication of the dynamic progression of the inflammatory response, and its dominant features. The tissue reaction to Coseal was investigated by Hill et al. in a canine iliac polytetrafluoroethylene graft model.27 At day 7, marked-to-moderate inflammation was seen at the sites of Coseal application, with moderate inflammation also seen around the suture lines without sealant. There appears to be large variation in the reported responses of vascular tissue to BioGlue, with some studies demonstrating little inflammation at 3 months20 or a year,40 while another noted an adverse reaction at 3 months.41
It has been suggested that the glutaraldehyde component of BioGlue may be responsible for eliciting an adverse tissue response.37,42 Furst and Banerjee incubated a sample of crosslinked BioGlue in saline solution for 1 min in vitro, with analysis of the resulting supernatant demonstrating a glutaraldehyde concentration of up to 200 μg/ml.37 Furthermore, the addition of small amounts of supernatant to cell culture medium resulted in almost complete loss of cell viability after just 100 min of culture.37A further study evaluated the cytotoxicity of the degradation products of BioGlue and Coseal formed after incubation of the materials in cell culture medium for 7 days.31 When cells were cultured in the resulting solutions for 24 h, no significant differences in cytotoxicity were observed. However, it should be noted that such a system is not representative of the in vivo situation, as it does not take into consideration diffusion of the degradation products away from the site of application. The rate of degradation of Coseal would have resulted in a solution with high osmolality after the 7-day incubation, which could be cytotoxic.
The mechanical properties of the sealant would likely have a notable effect on tissue response and efficacy, which is particularly significant for elastic tissue such as the aorta. A large difference in stiffness between the tissue and the sealant could result in excess stress being applied to the blood vessel.30 In the present study, measurement of the compressive modulus of the two materials investigated showed clear differences, with BioGlue demonstrated to be much stiffer than Coseal. This is in agreement with a study by Azadani et al. who reported Young’s moduli of 3122 and 100 kPa for BioGlue and Coseal, respectively.30 Furthermore, Vakalopoulos et al. reported correlation between tensile strength and markers of inflammation when comparing a variety of different tissue sealants (including BioGlue, but not Coseal).23 The high compressive modulus of BioGlue indicates that the sealant may limit changes in vessel diameter in response to pulsatile blood flow, whereas the low compressive modulus of Coseal indicates a pliable material allowing natural vascular compliance and rebound.
The low level of inflammatory response of vascular tissue to Coseal, taken together with its mechanical properties, suggests that it is a superior sealant to BioGlue for use with elastic tissues. These data are not only relevant for arteries, but could also be applicable to other highly compliant tissues, such as lung and intestine. These tissues have previously displayed adverse reactions to BioGlue.17,23,43
One limitation of this study was that a single time point was evaluated; therefore, dynamic progression of the inflammatory response, and its dominant features (e.g. eosinophil infiltration), could not be assessed. A further limitation is that a control group where manual compression was used to achieve haemostasis was not included in the study. This was deemed unnecessary, as the aim of the study was to evaluate the tissue reaction to the two sealants. A further control group where the sealants were applied in the absence of suture holes could have provided additional information. However, in order to minimise the number of animals used, we decided to exclude such a group from the present study. Finally, use of additional time points could have provided information on the progression of inflammation over time.
Conclusions
This detailed head-to-head histopathological comparison of two tissue sealants, BioGlue and Coseal, demonstrated notable differences in type and severity of the resulting tissue reaction. While both materials elicited granulomatous inflammation, there was significant infiltration of eosinophil-like cells around the BioGlue-treated lesions, indicating a more severe response. When taken together with its greater elasticity, Coseal appears to be superior to BioGlue for use in surgical procedures involving the aorta.
Acknowledgements
We thank Petra Kodajova for excellent technical assistance, Klaus Bittermann for his skilled photographic work, Alexander Tichy for statistical assistance and Sonja Lebel for the superb animal care.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Paul Slezak and Heinz Redl are CEOs of Trauma Care Consult GmbH, the company that received the funding to conduct the study. Daniel Spazierer and Heinz Gulle are full time employees of Baxter Medical Products GmbH. All other authors have no conflicts of interest to declare. The work reported here was designed and performed with established scientific methods using impartial data collection and analysis. Baxter Healthcare Corporation paid for writing assistance provided by Meridian HealthComms Ltd. The authors are solely responsible for the content of the work.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Baxter Healthcare Corporation is the manufacturer of Coseal and provided financial support for the conduct of the research and for preparation of the article. Employees of Baxter contributed to study design, reviewed the manuscript and provided intellectual input. No Baxter employees were involved in data collection or analysis.
ORCID iD
Paul Slezak https://orcid.org/0000-0002-1000-4393
References
- 1.Rogers AC, Turley LP, Cross KS, et al. Meta-analysis of the use of surgical sealants for suture-hole bleeding in arterial anastomoses. Br J Surg 2016; 103: 1758–1767. [DOI] [PubMed] [Google Scholar]
- 2.Natour E, Suedkamp M, Dapunt OE. Assessment of the effect on blood loss and transfusion requirements when adding a polyethylene glycol sealant to the anastomotic closure of aortic procedures: a case-control analysis of 102 patients undergoing Bentall procedures. J Cardiothorac Surg 2012; 7: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buskens E, Meijboom MJ, Kooijman H, et al. The use of a surgical sealant (CoSeal) in cardiac and vascular reconstructive surgery: an economic analysis. J Cardiovasc Surg 2006; 47: 161–170. [PubMed] [Google Scholar]
- 4.Annabi N, Yue K, Tamayol A, et al. Elastic sealants for surgical applications. Eur J Pharm Biopharm 2015; 95: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vyas KS, Saha SP. Comparison of hemostatic agents used in vascular surgery. Expert Opin Biol Ther 2013; 13: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalmers RT, Darling Iii RC, Wingard JT, et al. Randomized clinical trial of tranexamic acid-free fibrin sealant during vascular surgical procedures. Br J Surg. 2010; 97: 1784–1789. [DOI] [PubMed] [Google Scholar]
- 7.Chetter I, Stansby G, Sarralde JA, et al. A prospective, randomized, multicenter clinical trial on the safety and efficacy of a ready-to-use fibrin sealant as an adjunct to hemostasis during vascular surgery. Ann Vasc Surg 2017; 45: 127–137. [DOI] [PubMed] [Google Scholar]
- 8.Bhamidipati CM, Coselli JS, LeMaire SA. BioGlue in 2011: what is its role in cardiac surgery? J Extra Corpor Technol 2012; 44: P6–12. [PMC free article] [PubMed] [Google Scholar]
- 9.Coselli JS, Bavaria JE, Fehrenbacher J, Stowe CL, et al. Prospective randomized study of a protein-based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J Am Coll Surg 2003; 197: 243–252. [DOI] [PubMed] [Google Scholar]
- 10.Wallace DG, Cruise GM, Rhee WM, et al. A tissue sealant based on reactive multifunctional polyethylene glycol. J Biomed Mater Res 2001; 58: 545–555. [DOI] [PubMed] [Google Scholar]
- 11.Glickman M, Gheissari A, Money S, et al. A polymeric sealant inhibits anastomotic suture hole bleeding more rapidly than gelfoam/thrombin: results of a randomized controlled trial. Arch Surg 2002; 137: 326–331. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg RC, Safi HJ, Sabik J, et al. Improved intraoperative management of anastomotic bleeding during aortic reconstruction: results of a randomized controlled trial. Am Surg 2004; 70: 307–311. [PubMed] [Google Scholar]
- 13.Fonouni H, Kashfi A, Majlesara A, et al. Analysis of the hemostatic potential of modern topical sealants on arterial and venous anastomoses: an experimental porcine study. J Mater Sci Mater Med 2017; 28: 134. [DOI] [PubMed] [Google Scholar]
- 14.Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J 2010; 12: 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasic M, Unbehaun A, Drews T, et al. Late wound healing problems after use of BioGlue for apical hemostasis during transapical aortic valve implantation. Interact Cardiovasc Thorac Surg 2011; 13: 532–534. [DOI] [PubMed] [Google Scholar]
- 16.Klimo P, Jr., Khalil A, Slotkin JR, et al. Wound complications associated with the use of bovine serum albumin-glutaraldehyde surgical adhesive in pediatric patients. Neurosurgery 2007; 60: 305–309. [DOI] [PubMed] [Google Scholar]
- 17.Box GN, Lee HJ, Abraham JB, et al. Evaluation of the outcomes of electrosurgical induced bowel injury treated with tissue glue/sealant versus sutured repair in a rabbit model. J Endourol 2009; 23: 535–540. [DOI] [PubMed] [Google Scholar]
- 18.Despoudi K, Mantzoros I, Ioannidis O, et al. Effects of albumin/glutaraldehyde glue on healing of colonic anastomosis in rats. World J Gastroenterol 2017; 23: 5680–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber-Blum S, Petter-Puchner AH, Mika K, et al. A comparison of a bovine albumin/glutaraldehyde glue versus fibrin sealant for hernia mesh fixation in experimental onlay and IPOM repair in rats. Surg Endosc 2010; 24: 3086–3094. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt CW, Marra SW, Kann BR, et al. BioGlue surgical adhesive for thoracic aortic repair during coagulopathy: efficacy and histopathology. Ann Thorac Surg 2001; 71: 1609–1612. [DOI] [PubMed] [Google Scholar]
- 21.Schiller W, Rudorf H, Kiderlen MJ, et al. Short-term tissue response of lapine carotid artery microanastomoses to BioGlue. Thorac Cardiovasc Surg 2007; 55: 298–303. [DOI] [PubMed] [Google Scholar]
- 22.Schiller W, Rudorf H, Welzel CB, et al. Sutureless anastomoses of rabbit carotid arteries with BioGlue. J Thorac Cardiovasc Surg 2007; 134: 1513–1518. [DOI] [PubMed] [Google Scholar]
- 23.Vakalopoulos KA, Wu Z, Kroese LF, et al. Clinical, mechanical, and immunohistopathological effects of tissue adhesives on the colon: an in-vivo study. J Biomed Mater Res B Appl Biomater 2017; 105: 846–854. [DOI] [PubMed] [Google Scholar]
- 24.Witter K, Tonar Z, Matejka VM, et al. Tissue reaction to three different types of tissue glues in an experimental aorta dissection model: a quantitative approach. Histochem Cell Biol 2010; 133: 241–259. [DOI] [PubMed] [Google Scholar]
- 25.Kalsi P, Thom M, Choi D. Histological effects of fibrin glue and synthetic tissue glues on the spinal cord: are they safe to use? Br J Neurosurg 2017; 31: 695–700. [DOI] [PubMed] [Google Scholar]
- 26.Cerezal-Garrido LJ, Agudo-Bernal J, Vaquero-Puerta C. Histological benefits of sealants in tracheal lesions in Wistar rats. Surg Technol Int 2016; 28: 29–35. [PubMed] [Google Scholar]
- 27.Hill A, Estridge TD, Maroney M, et al. Treatment of suture line bleeding with a novel synthetic surgical sealant in a canine iliac PTFE graft model. J Biomed Mater Res 2001; 58: 308–312. [DOI] [PubMed] [Google Scholar]
- 28.Giuratrabocchetta S, Rinaldi M, Cuccia F, et al. Protection of intestinal anastomosis with biological glues: an experimental randomized controlled trial. Tech Coloproctol 2011; 15: 153–158. [DOI] [PubMed] [Google Scholar]
- 29.Gruber-Blum S, Fortelny RH, Keibl C, et al. Liquid antiadhesive agents for intraperitoneal hernia repair procedures: Artiss((R)) compared to CoSeal((R)) and Adept((R)) in an IPOM rat model. Surg Endosc 2017; 31: 4973–4980. [DOI] [PubMed] [Google Scholar]
- 30.Azadani AN, Matthews PB, Ge L, et al. Mechanical properties of surgical glues used in aortic root replacement. Ann Thorac Surg 2009; 87: 1154–1160. [DOI] [PubMed] [Google Scholar]
- 31.Murdock MH, Chang JT, Luketich SK, et al. Cytocompatibility and mechanical properties of surgical sealants for cardiovascular applications. J Thorac Cardiovasc Surg 2019; 157: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luk A, David ED, Butany J. Complications of Bioglue postsurgery for aortic dissections and aortic valve replacement. J Clin Pathol 2012; 65: 1008–1012. doi:10.1136/jclinpath-2012-200809 [DOI] [PubMed] [Google Scholar]
- 33.Beer GM, Schneller M, Schmitz HC, et al. Mihic-Probst D. fibrin versus polyethylene glycol sealant: an experimental study in rabbits. Eur J Plast Surg 2008; 31: 243–248. DOI: 10.1007/s00238-008-0265-8 [DOI] [Google Scholar]
- 34.Fudge AA. Rabbit hematology. Laboratory medicine: avian and exotic pets. Philadelphia, USA: WB Saunders Company, 2000, pp. 273–275. [Google Scholar]
- 35.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008; 20: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wippermann J, Konstas C, Breuer M, et al. Long-term effects in distal coronary anastomoses using different adhesives in a porcine off-pump model. J Thorac Cardiovasc Surg 2006; 132: 325–331. [DOI] [PubMed] [Google Scholar]
- 37.Furst W, Banerjee A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann Thorac Surg 2005; 79: 1522–1528. [DOI] [PubMed] [Google Scholar]
- 38.LeMare SA, Schmittling ZC, Coselli JS, et al. BioGlue surgical adhesive impairs aortic growth and causes anastomotic strictures. Ann Thorac Surg 2002; 73: 1500–1505; discussion 1506. DOI: 10.1016/S0003-4975(02)03512-9 [DOI] [PubMed] [Google Scholar]
- 39.Achneck HE, Sileshi B, Jamiolkowski RM, et al. Comprehensive review of topical hemostatic agents efficacy and recommendations for use. Ann Surg 2010; 251: 217–228. [DOI] [PubMed] [Google Scholar]
- 40.Gundry SR, Black K, Izutani H. Sutureless coronary artery bypass with biologic glued anastomoses: preliminary in vivo and in vitro results. J Thorac Cardiovasc Surg 2000; 120: 473–477. [DOI] [PubMed] [Google Scholar]
- 41.Erasmi AW, Sievers HH, Wolschlager C. Inflammatory response after BioGlue application. Ann Thorac Surg 2002; 73: 1025–1026. [DOI] [PubMed] [Google Scholar]
- 42.LeMaire SA, Ochoa LN, Conklin LD, et al. Nerve and conduction tissue injury caused by contact with BioGlue. J Surg Res 2007; 143: 286–293. [DOI] [PubMed] [Google Scholar]
- 43.Bures M, Hoffler HK, Friedel G, et al. Albumin-glutaraldehyde glue for repair of superficial lung defect: an in vitro experiment. J Cardiothorac Surg 2016; 11: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]