Abstract
Cross-sectional evidence suggests that attention bias to threat is linked to anxiety disorders and anxiety vulnerability in both children and adults. However, there is a lack of developmental evidence regarding the causal mechanisms through which attention bias to threat might convey risks for socioemotional problems, such as anxiety. Gaining insights into this question demands longitudinal research to track the complex interplay between threat-related attention and socioemotional functioning. Developing and implementing reliable and valid assessments tools is essential to this line of work. This review presents theoretical accounts and empirical evidence from behavioral, eye-tracking, and neural assessments of attention to discuss our current understanding of the development of normative threat-related attention in infancy, as well as maladaptive threat-related attention patterns that may be associated with the development of anxiety. This review highlights the importance of measuring threat-related attention using multiple attention paradigms at multiple levels of analysis. In order to understand if and how threat-related attention bias in real-life, social interactive contexts can predict socioemotional development outcomes, this review proposes that future research cannot solely rely on screen-based paradigms but needs to extend the assessment of threat-related attention to naturalistic settings. Mobile eye-tracking technology provides an effective tool for capturing threat-related attention processes in vivo as children navigate fear-eliciting environments and may help us uncover more proximal bio-psycho-behavioral markers of anxiety.
Keywords: attention bias, anxiety, behavioral inhibition, socioemotional development, eye-tracking
1. Introduction
Affect-biased attention emerges during infancy (Todd, Cunningham, Anderson, & Thompson, 2012) and may directly support individual differences in socioemotional functioning. Threat-related attention is a specific form of affect-biased attention (Morales, Fu, & Pérez-Edgar, 2016; Todd et al., 2012). Threat-related attention bias is manifested in several components, including initial facilitated engagement towards threat (i.e. threat vigilance), subsequent sustained attention to threat or difficulty in disengaging from threat, and avoidance of threat (Amso & Scerif, 2015; Cisler & Koster, 2010).
In this review, we argue that the field needs to incorporate novel experimental assessments of attention in a developmental science framework, in order to study not only if threat-related attention is linked to socioemotional functioning, but also how it influences individual differences in socioemotional functioning in development. This endeavor will benefit by leveraging a variety of measurement tools, including behavioral reaction time (RT), eye-tracking, and neural measures, to delineate threat-related attention processes in context at multiple levels of analysis and across development. Moreover, we propose that future research should take assessments of attention beyond screen-based paradigms. Measuring ambulatory attention in the context of active social interaction can shed light on how threat-related attention and socioemotional functioning may influence each other in real time.
A review of the experimental literature raises two broad concerns. First, we know that normative attention bias emerges in infancy, and it influences early socioemotional development. We also know that anxiety is a developmental disorder, in that it typically first emerges in childhood (Costello, Egger, & Angold, 2005; Egger & Angold, 2006). Correlational evidence suggests that attention bias to threat may be related to anxiety (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Dudeney, Sharpe, & Hunt, 2015; Puliafico & Kendall, 2006; Roy, Dennis, & Warner, 2015). However, we lack evidence regarding how the association between threat-related attention bias and anxiety may emerge and change during development. Experimental studies have implemented attention bias modification training (ABMT), which implicitly manipulates attention to affective stimuli in order to examine the subsequent effect on levels of anxiety. While ABMT research indicates that threat-related attention bias can influence anxiety levels, relative to placebo training (e.g. Britton, Dellarco, & Evans, 2017; Liu, Taber-Thomas, Fu, & Pérez-Edgar, 2018), it does not provide all of the necessary support for the causal relation – whether threat-related attention bias always impacts the emergence of anxiety problems (Pérez-Edgar & Hastings, 2018). Furthermore, the generated effect sizes in the child literature for threat-related attention bias and ABMT are smaller, and the patterns of threat-related attention bias associated with anxiety are more mixed (Dudeney, Sharpe, & Hunt, 2015; Roy, Dennis, & Warner, 2015), relative to the adult literature. Thus, there may be considerable individual differences in the association between attention bias and anxiety, such that not all children who display attention bias to threat will have anxiety problems. By the same token, not all anxious children show a bias to threat (Britton et al., 2012, 2013; Dudeney et al., 2015; Price et al., 2013; Roy et al., 2015). Taken together, we still need strong evidence regarding if and how threat-related attention processes lead to the development of socioemotional maladjustments, and if and how causal mechanisms change between individuals or within individuals over time.
The second broad concern lies in the methodology used to assess threat-related attention. Current experimental work largely relies on behavioral RTs to assess threat-related attention in children. Behavioral RTs lack temporal sensitivity and do not provide a continuous measure of attention functioning. Rather, they capture only the end-stage outputs of a cascade of attention processes at the time of stimulus presentation (Shechner et al., 2012; Yiend, 2010). A growing number of studies have incorporated eye-tracking (e.g. Price, et al., 2016b), event-related potentials (ERPs) (Thai, Taber-Thomas, & Pérez-Edgar, 2016), and functional magnetic resonance imaging (fMRI) (e.g. Britton et al., 2015; Fu, Taber-Thomas, & Pérez-Edgar, 2017; Monk et al., 2006) measures into standard attention tasks. Implementing multiple levels of analysis allows for enhanced temporal and spatial sensitivity and reliability in measurements of threat-related attention (Price et al., 2015; Rodebaugh et al., 2016). This is particularly beneficial for parsing out the components and time course of threat-related attention processes related to anxiety.
However, existing assessments of threat-related attention rely on screen-based paradigms, which display pre-selected, static stimuli. Thus, existing experimental studies are handicapped in capturing active attention selection or avoidance (Birmingham & Kingstone, 2009; Todd et al., 2012), as they can only assess how individuals process stimuli pre-selected for them. These paradigms do not suggest how threat-related AB in vivo may be associated with social behavior in real-life scenarios. This limitation calls for incorporating ambulatory assessment tools to examine attention processes in the context of active interactions with the social world.
We aim to review our understanding of the role of threat-related attention in socioemotional development. To address the first concern, this review argues that it is important to carry out longitudinal research to track the impact of threat-related attention bias on the developmental pathway linking early anxiety vulnerability and subsequent anxiety problems from early in development. To extend our methodology for measuring attention, this review advocates for the importance of employing multiple paradigms at multiple levels of analysis to delineate more comprehensive and fine-grained threat-related attention mechanisms. Cross-sectional studies are also important for testing and validating developmentally appropriate tools and paradigms that allow us to probe core attention mechanisms from infancy, as well as monitor how specific risk factors diverge from the development of normative threat-related attention and lead to the emergence of aberrant attention patterns.
Theoretical accounts of normative attention development and the development of attention bias associated with socioemotional problems are built on the extant understanding of the neurocognitive mechanisms subserving threat-related attention. Thus, to lay the foundation for researchers to study typical and atypical development of the varied mechanisms supporting attention, the current paper will first describe these neurocognitive substrates. It will then present theoretical frameworks supporting the argument that threat-related attention patterns at both behavioral and neural levels are associated with anxiety and its development. To illustrate the current understanding of how threat-related attention bias may impact socioemotional developmental outcomes, the review will discuss research examining threat-related attention during infancy. Building on the normative infant literature, we then review the behavioral association between pediatric anxiety and attention bias to threat. Based on the limitations of RT measures of attention bias, we highlight the importance of adopting multiple levels of analyses to study threat-related attention by incorporating eye-tracking and neural research. Table 1 provides an overview of published studies that incorporated manual RT, eye-tracking, ERPs and neuroimaging in screen-based attention paradigms to assess attention bias and the effect of ABMT. The table summarizes the key strengths of each measurement tool and the youngest age to which it can be applied. This information can facilitate future efforts for integrating multiple assessments of attention. Lastly, we point out the necessity of extending current screen-based assessments to examine threat-related attention in naturalistic contexts. We will illustrate a laboratory-controlled paradigm that incorporates a head-mounted eye-tracking device to assess children’s ambulatory attention in the context of active social interactions with threat-eliciting real-life stimuli. Multimodal and naturalistic approaches may improve the reliability and validity of attention assessment, as well as boost our ability to identify more proximal bio-psycho-behavioral markers of vulnerability to anxiety problems.
Table 1:
Studies that incorporated manual reaction time, eye-tracking, event-related potentials and neuroimaging in screen-based attention paradigms to assess attention bias and the effect of attention bias modification training
| Study Type | Measurement Method |
Main Advantages | Task Paradigm | Youngest Age Studied |
Key Findings | Representative Readings |
|---|---|---|---|---|---|---|
|
AB Assessment ABMT |
Manual RT |
• Objective, convenient index of attention task performance. • Most commonly used –facilitates cross-study comparisons |
Dot-probe, Posner, visual search Dot-probe, Posner, visual search |
4 years 6 years |
• Pediatric anxiety is associated with AB to threat, although the effect size was small. • Anxiety symptomatology and age might moderate the bias anxiety association. • Active ABMT reduces anxiety symptoms and reactivity to experimentally induced stress. • However, the anxiolytic effect of the active ABMT is not significantly greater than placebo training in some studies. Changes in AB are not consistently observed after ABMT. |
• Abend et al (2018) • Reviews: ○ Bar-Haim et al (2007) ○ Dudeney et al (2015) • Reviews: ○ Britton et al (2017) ○ Lowther and Newman (2014) |
|
AB Assessment |
Eye-tracking |
• Fine-grained, online measure of overt attention • Continuously records parameters of eye- movement (fixation location, latency, and duration) and pupil dilation • Temporally and spatially sensitive |
Free-viewing, overlap, dot-probe |
4 months |
• Normative AB to threat emerges early in infancy. Temperament and contextual factors affect individual differences in threat-related AB patterns, which in turn have an impact on infants’ socioemotional development. • Pediatric anxiety is associated with AB to threat – manifested as initial facilitated engagement to threat, threat avoidance, and difficulty in threat disengagement. |
• Infant: ○ Leppänen (2016; review) ○ Pérez-Edgar et al (2017) • Child: ○ Burris et al (2017a) ○ In-Albon et al (2010) ○ Seefeldt et al (2014) ○ Shechner et al (2013) ○ Price et al (2016b) |
|
AB Assessment |
ERPs |
• Captures brain’s electrocortical response time-locked to specific stimuli • Fine-grained temporal resolution, allowing for continuous measures of chronometry of neutral processes • Can capture covert attention |
Visual paired comparison, free-viewing, overlap, attention network test, emotional face- matching task, dot-probe, Posner |
3 months |
• Infants show augmented Nc, N290, and P400 responses toward fearful faces, indicating facilitated attention allocation toward the threatening stimuli. • Initial facilitated engagement toward threatening face, indexed by greater N170 amplitudes, and sustained attention toward threat, indexed by potentiated LPP response, may be related to pediatric anxiety and enhanced risk for anxiety. Conversely, greater deployment of attention control, indexed by greater P2 response, may mitigate anxiety vulnerability in children. |
• Infant: ○ de Haan et al (2004), ○ Jessen and Grossmann (2015), ○ Leppänen et al (2007) • Child: ○ Kujawa et al (2015), ○ O’Toole et al (2013), ○ Thai et al (2016) |
|
AB Assessment ABMT |
Neuroimaging |
fMRI: • Measures neural activities in vivo by measuring changes in oxygenated blood flow • Provides enhanced spatial resolution • Can capture activation in subcortical regions fNIRS: • Uses near-infrared light to measure oxygenated and deoxygenated hemoglobin changes associated with neural activities in the cortex • Greater temporal resolution than fMRI; motion-tolerant; can be used to study neutral activities in real-life contexts |
Dot-probe, face-attention paradigm, emotional face- matching task Dot-probe |
• fMRI: 7 years • fNIRS: 7 months 8 years |
• Infants’ individual differences in temperament are related to individual differences in prefrontal responses to faces. • Pediatric anxiety is associated with aberrant activations in the amygdala, ACC, and PFC regions in response to threatening faces, indicating that perturbations in neural functions supporting automatic and controlled attention processes are associated with anxiety. • Active ABMT alleviates anxiety symptoms, compared with the placebo group, and it can enhance the treatment effect of CBT • Active ABMT attenuates limbic activation and increases prefrontal activation in response to the threat- bias contrast. • Baseline differences in amygdala activation may moderate treatment effect. |
• Infant: ○ Ravicz et al (2015) • Child: reviews: ○ Blackford and Pine (2012), ○ Guyer et al (2013), ○ Swartz and Monk (2013) • Britton et al (2014) • Liu et al (2018) •White et al (2017b) |
Notes: published ABMT studies have implemented eye-tracking and ERP measures with adults but not with youth (< 18 years old). AB=attention bias; ABMT=attention bias modification training, RT=reaction time, ERPs=event-related potentials, fMRI=functional magnetic resonance imaging, fNIRS=functional near-infrared spectroscopy
2. Neurocognitive mechanisms underlying threat-related attention
Theoretical accounts of normative attention development and individual differences in attention development are built on the premise that attention is a multi-component processing system. While the disparate sub-components of attention are anatomically and functionally distinct, they operate interactively to support behavioral outputs, including biased attention to threat (Amso & Scerif, 2015; Petersen & Posner, 2012; Posner & Petersen, 1990; Raz & Buhle, 2006). One influential model proposed by Posner describes three distinct networks of attention: alerting, orienting, and executive attention (Petersen & Posner, 2012; Posner & Petersen, 1990). The alerting network is modulated by norepinephrine and is associated with activity in the frontal, parietal, and cingulate cortex (Posner, Rothbart, Sheese, & Voelker, 2012). Alerting encapsulates involuntary activities that serve to achieve, increase, and maintain response readiness in preparation for incoming stimuli.
Orienting is modulated by acetylcholine and activates the frontal eye fields, superior parietal lobe, temporal parietal junction (TPJ), superior temporal lobe, superior colliculus, and pulvinar (Petersen & Posner, 2012). Orienting contains three component operations: 1) disengaging attention from the current location, subserved by the TPJ and superior temporal lobe (Friedrich, Egly, Rafal, & Beck, 1998). The superior parietal lobe, frontal eye fields and superior colliculus support the operations of 2) shifting attention to a new location and 3) engaging attention (Corbetta, 1998; Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000).
Attention orienting, in turn, can be overt or covert (Posner, 2012). Overt attention involves shifting attention to a location by moving the eyes, whereas covert orienting characterizes attention shifting without moving the eyes (Posner, 2012). Orienting functions as a form of reactive control that is largely involuntary and modulated by external stimuli. As a result, attention orienting serves a regulatory role in infancy when voluntary forms of regulation are not yet developed (Rueda, 2012).
Executive attention is modulated by dopamine and engages the anterior cingulate cortex, anterior insula, prefrontal cortex and striatum (Fan, McCandliss, Sommer, Raz, & Posner, 2002; Petersen & Posner, 2012). Executive attention functions to voluntarily control attention, monitor and resolve conflicts, and override predominant responses to support a subdominant response according to task demands. Hence, executive attention serves a vital role in self-regulation as it progressively develops after the first year of life (Rothbart, Sheese, Rueda, & Posner, 2011).
A central premise of the literature is that attention orienting to threat involves dynamic interactions between bottom-up and top-down processes (Corbetta & Shulman, 2002). Bottom-up attention selection is a relatively automatic process, driven by low-level features intrinsic to the stimulus, including affective valence. At the neural level, the amygdala serves the key function of emotional salience detection and processing affective pertinence (LeDoux, 2009; Sander, Grafman, & Zalla, 2011). Top-down processes are driven by individual goals, past knowledge, and expectations. They function to allocate cognitive resources based on stimulus relevance and current goals. There is a bidirectional connection between the amygdala and orbitofrontal cortex (OFC), providing a neural basis for these reciprocal processes (Vuilleumier, 2005). While the amygdala projects directly to sensory cortices to enhance threat processing (Anderson & Phelps, 2001), it also has bidirectional connections to widespread frontal regions, including the OFC. The OFC, in turn, compliments the amygdala in assigning emotional valence and evaluating stimulus relevance based on past experiences and the current context (Anderson et al., 2003). The OFC also regulates low-level, automatic threat detection through its projections to the frontoparietal network (Bar et al., 2006). Thus, these bottom-up and top-down processes operate in parallel to modulate threat-related attention bias (Vuilleumier, 2005).
3. Theoretical frameworks of threat-related attention in anxiety
Base on Posner’s model suggesting that attention networks are present in infancy and serve a critical role for learning and the development of self-regulation (Petersen & Posner, 2012; Posner & Petersen, 1990), Morales et al (2016) propose a conceptual framework for studying the role of attention in socioemotional development. The model posits that early normative attention bias is modulated by individual differences in constitutional factors (e.g., genetics and temperament) and experiences with the environment, providing an experience-expectant foundation for socioemotional development. Maladaptive threat-related attention bias may grow out of normative attention functioning for a subgroup of children who also exhibit early risk for anxiety disorders. These perturbed threat-related attention processes further shape socioemotional functioning by biasing downstream cognitive processes and behavioral enactment, creating a cyclical relation between threat-related attention and socioemotional experiences. As the maladaptive attention bias patterns become entrenched, they may act as a tether that binds these children to a developmental trajectory towards socioemotional problems (Pérez-Edgar, Taber-Thomas, Auday, & Morales, 2014).
Much of the work examining threat-related attention has focused on anxiety. Anxiety disorders are characterized by a constellation of emotional, cognitive, and behavioral symptoms (Grills, Seligman, & Ollendick, 2014). The primary emotional symptoms are excessive fear of imminent threats or apprehension to potential threats or threatening events that is distal in time and/or space (Rosen & Schulkin, 1998). The cognitive symptoms involve negative thoughts and worries about potential threats. Finally, the behavioral symptoms are characterized by avoidance of potential threats or fear-eliciting situations (Grills et al., 2014).
Cognitive theories based on adult clinical research suggest that clinical anxiety and trait anxiety are associated with threat-related attention bias. The cognitive motivation model (Mogg & Bradley, 1998) proposes that high levels of anxiety are associated with a lowered threshold for labeling inputs as threats, resulting in the prioritized allocation of processing resources to the perceived threat. Derived from this model, the vigilance-avoidance hypothesis (Mogg & Bradley, 1998) argues that elevated anxiety is characterized by initial vigilance to threat followed by threat avoidance.
In contrast, the attention control theory (Eysenck, Derakshan, Santos, & Calvo, 2007) proposes that anxiety is related to deficiencies in top-down voluntary attention control functioning, even when dealing with “neutral” stimuli. This deficiency in attention control may underlie overt attention bias patterns. The theory argues that anxiety disrupts goal-directed attention orienting and the inhibition of task-irrelevant attention processing. This deficiency gives rise to increased involuntary engagement to threat distracters and difficulty in disengaging from them.
A dual-processing perspective (Henderson, Pine, & Fox, 2015; Henderson & Wilson, 2017) based on neural evidence provides an account of how threat-related attention bias associated with early anxiety vulnerability may contribute to the development of socioemotional maladjustments. It proposes that bottom-up hyper-reactive threat-related attention processes associated with early anxiety vulnerability may potentiate the engagement of less flexible and efficient attention control function. The bottom-up and top-down attention processes may form a positive feedback loop in which heightened deployment of attention control, in turn, amplifies automatic orienting to threat. The interplay between automatic and controlled processes and socioemotional functioning gradually calcifies attention bias to threat in at-risk children, thus further increasing their vulnerability to anxiety problems (Henderson et al., 2015; Henderson & Wilson, 2017; Morales et al., 2016; Pérez-Edgar et al., 2014).
4. Threat-related attention during infancy
4.1. Normative attention bias to threat is early-emerging
Studies of threat-related attention in infancy suggest that normative attention to threat is early emergent and might be moderated by early individual factors, such as temperament. This area of research provides an important step for tracking how early-emerging threat-related attention processes may support socioemotional development. Specifically, behavioral, eye-tracking, and neural evidence collectively indicates that normative attention bias toward fearful faces emerges between 5 and 7 months of age. In particular, 7-month-olds display greater sustained attention towards fearful faces versus simultaneously presented happy faces in visual-preference tasks (Kotsoni, Haan, & Johnson, 2001; Nelson & Dolgin, 1985).
Research has employed visual search and overlap paradigms as means to probe the specific component of attention bias. The visual search task presents an array of stimuli (e.g. a 3×3 matrix), and measures latency to search for a target among distracters. Faster detection of the threat target among non-threat distracters indicates facilitated engagement to threat, whereas slower detection of the non-threat target suggests difficulty in disengaging from threat (Donnelly, Hadwin, Menneer, & Richards, 2010; LoBue, 2014; Öhman, Flykt, & Esteves, 2001). LoBue and DeLoache (2010) found that 8- to 14-month-old infants exhibit facilitated detection of snakes compared to frog targets, and to angry face compared to happy face targets.
Alternatively, the overlap paradigm (Aslin & Salapatek, 1975) measures the latency and/or frequency of attention orienting from a centrally presented face to a subsequent non-emotional stimulus presented in the left or right peripheral field. With this task, attention bias to fearful faces emerges between 5 and 7 months of age, displayed as lower probability of disengaging from fearful faces (Peltola, Hietanen, Forssman, & Leppänen, 2013; Peltola, Leppänen, Mäki, & Hietanen, 2009a; Peltola, Leppänen, Palokangas, & Hietanen, 2008). Together, behavioral studies indicate that infants display threat-related attention bias, evident in facilitated threat detection, sustained attention to threat, and difficulty in disengaging from threat.
The second stream of evidence comes from studies incorporating eye-tracking during the overlap paradigm. Consistent with behavioral evidence, 7-month infants show longer dwell time to fearful faces relative to happy faces (Leppänen, Cataldo, Enlow, & Nelson, 2018; Peltola, Yrttiaho, & Leppänen, 2018), and they are slower in disengaging from fearful than happy or neutral faces (Peltola, Leppänen, Vogel-Farley, Hietanen, & Nelson, 2009b). Difficulty in disengaging from fearful faces may be observed as early as 5 months of age when presenting dynamic face stimuli (Heck, Hock, White, Jubran, & Bhatt, 2016, 2017).
Neural mechanisms underlying adult-like threat-related attention bias are in place early in infancy. The ERP component, negative central (Nc), can be recorded in newborns (Nelson, 1996), and is suggested to index automatic attention orienting to salient stimuli (Nelson, 1994). Infants show larger Nc amplitude to fearful than non-fearful faces at around 7 months of age (De Haan, Belsky, Reid, Volein, & Johnson, 2004; Leppänen, Moulson, Vogel-Farley, & Nelson, 2007; Nelson & De Haan, 1996; Peltola et al., 2009a). Differential Nc responses to fearful versus non-fearful faces are preserved in 7-month-olds during subliminal face processing (Jessen & Grossmann, 2015).
The N290 and P400 components can be recorded at occipital-temporal regions. They reflect infants’ perceptual processing of structural information from faces and are considered to be the developmental precursors of the adult face-sensitive N170 (de Haan, Johnson, & Halit, 2003). Fearful faces elicit augmented N290 and P400 responses compared to non-fearful faces in 7-month-olds, indicating greater allocation of attentional resources when processing fearful faces (Jessen & Grossmann, 2015; Leppänen et al., 2007; Yrttiaho, Forssman, Kaatiala, & Leppänen, 2014). Together, neurophysiological evidence highlights that neural systems underlying the preferential processing of threatening faces are engaged in the first year of life (Leppänen & Nelson, 2009).
Threat-related attention bias in infancy is primarily supported by the alerting and orienting networks, as they are present and functioning in infancy (Posner et al., 2012). In addition, the amygdala-OFC emotion-related network, with its connection to the occipitotemporal cortex underlying face processing, may also provide a neural basis for the development of threat-related attention bias (Leppänen & Nelson, 2009). Using fNIRS recording, Minagawa-Kawai et al (2009) found that the amygdala-OFC network is functionally in place around 7 months, the age when infants typically demonstrate attention bias towards threats. Thus, infants may possess a pre-wired preparedness to attend to affectively salient stimuli.
The automatic threat-related attention orienting observed in early infancy may serve as experience-expectant mechanisms that allow the neural circuitries underlying threat-related attention processes to become more integrated and canalized through development (Leppänen & Nelson, 2009). Using the overlap paradigm, Yrttiaho et al (2014) found that infants’ behavioral attention bias towards fearful faces at 5 months predicts increased N290 amplitude towards fearful faces at 7 months. Fear-potentiated N290 activity is not consistent at 5 months. Hence, the amygdala-driven, more automatic threat-related attention bias may influence later cortical sensitivity to fearful faces.
A core research question asks if and how age-related changes in attention bias to threat may interact with individual differences to influence socioemotional development (Field & Lester, 2010; Morales et al., 2016). Below, we present existing evidence that sheds light on these overarching questions.
4.2. The stability of normative attention bias in infancy
Cross-sectional studies provide support for a stable pattern of attention bias across age groups. Using the eye-tracking overlap paradigm, Morales et al (2017a) found that 4- to 24-month infants dwell longer on the angry than the neutral faces while the distractor is present, indicating a pattern of difficulty in disengaging from threat. The eye-tracking dot-probe task is another paradigm recently adapted to assess attention bias in infants. The task presents two images side by side in each trial – one affectively salient (e.g. threat) and one neutral, or both neutral. This is followed by a probe (e.g. an asterisk) that appeared in the same location as the affective stimulus (congruent trial) or in the same location as the neutral stimulus (incongruent trial). Attention bias can be quantified by subtracting latency to fixate to the probe on congruent trials from latency to fixate to the probe on incongruent trials. A positive score indicates facilitated engagement towards the affective stimulus, whereas a negative score suggests bias away from the affective stimulus. Burris, Barry-Anwar, and Rivera (2017a) found in their cross-sectional study an increase in task speed between 9- to 48-month-old, but no accompanying age effect on patterns of bias to angry and happy faces, indicating fairly stable bias profiles.
In contrast, there is also evidence indicating that age affects attention bias patterns. Among 4- to 24-month infants, dwell time to angry faces in the Angry-Neutral trials of an eye-tracking dot-probe task increases with age (Pérez-Edgar et al., 2017). Longitudinal repeated assessments using the eye-tracking overlap task also suggested that age-related changes in normative threat-related attention bias might not be linear. Specifically, longer dwell time on fearful relative to happy faces is maintained from 7 to 12 months (Leppänen et al., 2018). Difficulty in disengaging from fearful faces reduces significantly from 7 to 24 months of age, and the difference in dwell time between fearful and happy faces is not significant at 24 months (Peltola et al., 2018). At 36 months, the bias toward fearful faces (as well as angry faces) relative to happy faces reemerges (Leppänen et al., 2018).
Age may have differential impacts on attention bias towards social versus non-social threats. Using the eye-tracking dot-probe paradigm, LoBue, Buss, Taber-Thomas, and Pérez-Edgar (2017) found that among 4- to 24-month-olds, there is an age-related increase in probe fixation latency after infants fixated on the image pair containing an emotional face. The age-related increase in difficulty in attention disengagement is greater for angry than happy faces. In contrast, age does not moderate attention bias towards non-social threats (i.e., snakes vs. frogs). The perceptual bias to snakes may be due to attention-grabbing, lower-level perceptual features that remain stable across the first 2 years of life. In contrast, the affective and motivational values of emotional faces may increase as infants gain more experience with the social world.
Considering the mixed findings regarding whether normative attention bias to threatening faces is stable from 7 months of age, more longitudinal studies are needed to depict the age-related changes in attention bias. To enhance our understanding of the developmental trajectory, these studies also need to investigate whether individual differences factors, such as temperament and rearing environment, influence normative attention bias patterns, and whether these factors moderate the developmental changes of attention bias from infancy.
4.3. Intrinsic and extrinsic factors that influence individual differences in normative attention bias in infancy
Early temperament is an intrinsic factor that may contribute to individual differences in threat-related attention. Negative affect (NA), a temperament dimension that captures biologically-rooted individual differences in negative reactivity (Rothbart & Derryberry, 1981), may affect the type of information that is perceived as salient. Nakagawa and Sukigara (2012) coded 12-month-olds’ looking behavior in the overlap task and found that higher NA is associated with more difficulty in disengaging from fearful faces. Furthermore, NA might moderate age-related differences in threat disengagement. Leveraging the enhanced temporal sensitivity of eye-tracking, Pérez-Edgar et al (2017) found that among 4- to 24-month-olds, younger infants with low NA are faster to disengage and fixate to the task-relevant probe after fixating to angry faces. This may be driven by an enhanced approach tendency associated with low NA (Hane, Fox, Henderson, & Marshall, 2008). There is a non-significant positive relation between dwell time on angry faces and subsequent disengagement latency among younger infants with high NA. For these infants, increased sustained attention to angry faces may have demanded more attentional resources and generated greater latency costs when disengaging attention from threat. NA level does not affect disengagement latency from angry faces in older infants.
Infant temperament might also impact neural activities underlying normative threat-related attention (Leppänen & Nelson, 2009). Highly fearful 7-month-olds show greater Nc amplitude to fearful than happy faces during passive viewing of faces displayed one by one (De Haan et al., 2004). Leveraging fNIRS, Ravicz, Perdue, Westerlund, Vanderwert, and Nelson (2015) found that 7-month-olds with high NA exhibit reduced prefrontal responses towards happy faces, suggesting that infants with high NA may have reduced attention processing towards happy faces versus infants with low NA.
The temperamental dimension effortful control (EC) captures individual differences in self-regulation of emotion and action (Rothbart & Derryberry, 1981). EC encompasses the functioning of top-down attention control subserved by the executive attention network (Posner & Rothbart, 1998; Rothbart et al., 2011). Martinos, Matheson, & de Hann(2012) found that 3- to 13-month-olds with high EC display shorter Nc latency and larger Nc amplitude towards fearful faces, potentially reflecting a stronger effort to regulate attention and emotional responses towards threat. However, age does not moderate the relation between EC and Nc responses to fearful faces.
The lack of age-related changes in the impact of EC on neural responses to fearful faces during infancy might be due to the prolonged development of the executive attention network. Executive attention is not fully developed in infancy. Rather, it shows increased maturation across childhood (Posner et al., 2012; Posner, Rothbart, Sheese, & Voelker, 2014) and continues to develop into adolescence and young adulthood (Pérez-Edgar, 2015; Taber-Thomas & Perez-Edgar, 2015). During infancy, there is a functional overlap between orienting and executive attention networks (Cuevas & Bell, 2014; Gao et al., 2009), and the orienting network dominates to provide the earliest form of self-regulation (Rothbart et al., 2011), modulating threat-related attention bias (Martinos et al., 2012) and negative emotion (Harman, Rothbart, & Posner, 1997). As the executive attention network becomes more integrated and efficient over childhood (Fair et al., 2009; Gao et al., 2009), it plays a more influential role in top-down regulatory control of attention and behavior (Posner et al., 2012, 2014).
In addition to temperament, extrinsic factors, such as maternal characteristics, may also contribute to individual differences in normative attention bias to threat. Among 4- to 24-month-olds, while NA is not associated with attention bias, infants of mothers with high anxiety symptoms display greater attention bias towards angry faces, quantified by longer dwell time on the angry relative to neutral faces. Maternal anxiety is not related to happy bias (Morales et al, 2017a). However, the impact of maternal anxiety on threat bias is not consistent in the literature and requires replication (Leppänen et al., 2018).
4.4. The impact of normative attention bias on socioemotional development in infancy
Factors both intrinsic to infants, such as temperament, and extrinsic to infants, such as maternal anxiety, influence the age-related differences in attention bias to threat. Since studies to date have largely been cross-sectional in nature, it remains less clear whether threat-related attention bias, in turn, predicts the development of socioemotional outcomes. Peltola, Forssman, Puura, van IJzendoorn, and Leppänen (2015) showed that normative threat-related attention bias at 7 months of age, marked by fewer attention shifts away from fearful faces relative to non-fearful faces, is related to secure attachment formation, whereas smaller threat-related attention bias predicts more attachment disorganization at 14 months. Early secure attachment facilitates the development of self-regulation and may act as a protective factor that ameliorates children’s vulnerability for internalizing (e.g. McLaughlin, Zeanah, Fox, & Nelson, 2012) and externalizing problems (e.g. Allen, Moore, Kuperminc, & Bell, 1998). Additionally, attention bias to faces at 7 months of age, not specifically to threatening faces, predict increased spontaneous helping behavior at 24 month and reduced callous-unemotional traits at 48 months (Peltola et al., 2018). Hence, normative attentional preference to faces may help children build adaptive socioemotional functions, including enhanced emotional understanding, mentalizing, and empathic abilities (Peltola et al., 2018).
Early-appearing, normative attention bias sets the foundation for socioemotional development and is further shaped and refined by individual differences in socioemotional functioning and experiences of interacting with the environment in an experience-expectant manner (Leppänen & Nelson, 2009). Large-scale longitudinal studies are needed to understand the emergence of maladaptive threat-related attention, and how it may interact with socioemotional functioning to cast risks for the development of psychopathology (Field & Lester, 2010; Morales et al., 2016). Cross-sectional studies are equally indispensable, as they could reveal more comprehensive and fine-grained attention bias patterns (Pérez-Edgar & Hastings, 2018).
4.5. Implementing multimodal and multi-task approaches to understanding threat-related attention bias in infancy
Existing research that has implemented multimodal assessment of attention in infants combined ERP measures with coded looking behavior (e.g. Leppänen et al., 2007; Peltola et al., 2009a) or ERP with eye-tracking measures (e.g. Vanderwert et al., 2015; Yrttiaho et al., 2014). One benefit of multimodal assessment is that ERPs complement behavioral or eye-tracking assessment of overt attention, and enables researchers to examine whether overt and/or covert attention processing of faces engages differential face-specific cortical activities depending on face emotion (threatening, happy, or neutral). While studies have found that infants display attention bias toward fearful faces at both neural and behavioral levels, Leppänen et al (2007) found no significant correlation between ERPs (difference in P400 or Nc amplitude in response to fearful versus happy faces) and behavioral attention bias (difference in duration of looking time toward fearful versus happy faces). Hence, neural responses and overt looking behavior toward emotional faces might be sensitive to different processes and need to be examined within-sample in order to provide a more comprehensive attention bias profile.
Secondly, simultaneous eye-tracking and ERP recording can reveal the effect of infants’ overt attention process on neural responses to emotional faces. For example, Vanderwert et al (2015) found that in 7-month-old infants, larger N290 and P400 amplitudes in response to fearful faces are associated with longer dwell time on the eye regions of fearful faces. The brain-behavior relation is not significant for happy or neutral faces. Thus, it is possible that early sensitivity to specific features of fearful faces drives enhanced neural responses to fearful faces (Farroni, Johnson, & Csibra, 2004; Vanderwert et al., 2015).
Individual studies thus far have adopted a single task paradigm to assess infant’s attention. Any particular paradigm might not be optimal for capturing all components of affect-biased attention – initial attention orienting, sustained attention, and disengagement. Additionally, these single-task studies cannot reveal trait-level attention bias that can be observed across tasks. To address this limitation, Vallorani et al (under review) implemented three eye-tracking tasks (dot-probe, overlap, and vigilance tasks) to assess attention bias toward emotional faces in a sample of 4- to 24-month infants. Adopting a person-centered approach, they categorized infants as with or without affect-biased attention based on latency and dwell time measures of attention derived from the three tasks. Specifically, NA mediates the effect of age on the probability of displaying affect-based attention toward emotional faces only among infants whose mothers have high maternal anxiety (Vallorani et al., under review).
To date, cross-sectional and longitudinal evidence indicates that normative attention bias to threat emerges between 5- and 7-month of age. However, early-appearing attention bias might not be stable for all infants as a function of trait-level and experiential differences. Multimodal assessment of attention in infants using different task paradigms may facilitate the identification of early behavioral and neurocognitive markers of risks for socioemotional problems. The review will discuss the importance of taking attention assessment out of screen-based contexts and into real-life social-interactive environments.
5. Behavioral approaches to attention bias to threat and socioemotional functioning
5.1. Behavioral evidence for the association between threat-related attention bias and anxiety problems
Anxiety problems are early emergent in childhood (Costello et al., 2005; Egger & Angold, 2006). Morales et al (2016)’s model hypothesized that aberrant attention bias to threat may develop from normative threat-related attention functioning for a subgroup of at-risk children, and the maladaptive attention bias to threat may become entrenched through development and further increase their vulnerability to anxiety disorders. On one hand, infant research has provided evidence that temperament (e.g. Pérez-Edgar et al, 2017) and parental characteristics may influence individual differences in normative threat-related attention (e.g. Morales et al., 2017a), which has an impact on socioemotional development in early childhood (e.g. Peltola et al., 2018). On the other hand, correlational evidence from studies that employed RT measures in children shows that pediatric anxiety is associated with threat-related attention bias (Table 1). However, the effect size for threat-related attention bias in youth with anxiety is small (Abend et al., 2018; Bar-Haim et al., 2007; Dudeney et al., 2015). Findings are also equivocal regarding which component of attention bias (i.e. attention vigilance, avoidance, or difficulties in disengagement) is most closely linked to pediatric anxiety (see reviews: Puliafico & Kendall, 2006; Roy et al., 2015).
Anxiety may be characterized by facilitated engagement to threatening stimuli at shorter presentation duration and threat avoidance occurs with longer stimulus presentation (Cisler & Koster, 2010; Mogg & Bradley, 1998). For example, children with clinical or trait anxiety display attention bias towards threat faces presented for 500ms (clinical anxiety: Roy et al., 2008; Waters et al., 2010; Waters, Mogg, & Bradley, 2012; trait anxiety: Watts & Weems, 2006). However, other studies suggested that pediatric anxiety disorders are associated with attention bias towards prolonged presentation of threat faces (1000–1500ms: Dalgleish, Moradi, Taghavi, Neshat-Doost, & Yule, 2001; Hankin, Gibb, Abela, & Flory, 2010; Taghavi, Neshat-Doost, Moradi, Yule, & Dalgleish, 1999; Vasey, Daleiden, Williams, & Brown, 1995; Waters, Wharton, Zimmer-Gembeck, & Craske, 2008, at pre-treatment). While some studies confirmed that children exhibit threat avoidance when faces are presented for 1000ms (clinical anxiety: Brown et al., 2013; trait anxiety: Stirling et al., 2006), other studies indicated threat avoidance in clinically anxious children at shorter presentation duration (Pine et al., 2005). Finally, there are studies suggesting that clinically-anxious children and their non-anxious counterparts do not differ in attention bias patterns (Waters, Lipp, & Spence, 2004), and clinically-anxious children show attention bias towards both angry and happy faces (Waters, Mogg, Bradley, & Pine, 2008).
Therefore, we cannot infer from correlational evidence whether threat-related attention bias contributes to the emergence of anxiety problems or whether attention bias to threat emerges as a result of anxiety.
5.2. The impact of experimental manipulation of attention bias on anxiety
One avenue to test causality is to manipulate threat-related attention and examine any resulting changes in anxiety (Roy et al., 2015). ABMT studies (Table 1) commonly use the dot-probe paradigm to implicitly train attention by manipulating the contingency of the probe. For example, training that is intended to induce attention bias away from threat presents probes in the location of the neutral stimulus for the majority of the trials. Through repeated exposure, participants are thought to implicitly learn to direct attention away from threat, as the contingency predicts the probe location (Bar-Haim, 2010). By training youth (6–17 years old) to direct attention away from threat or towards positive stimuli, active ABMT reduces anxiety symptoms relative to the placebo control group (Bar-Haim et al., 2011; De Voogd et al., 2014; Eldar et al., 2012; Liu et al., 2018; Waters et al., 2015, 2013). In addition, ABMT can augment the anxiolytic effects of cognitive-behavioral therapies (Shechner et al., 2014; White et al., 2017b; but see Britton et al., 2013).
However, several issues limit our ability to conclude that there is a causal link between attention bias to threat and anxiety. First, the mechanisms through which ABMT produces anxiety reduction effects are unclear, since some studies did not find significant reduction in attention bias to threat after active ABMT relative to the placebo condition (Britton et al., 2013, experiment one; Ollendick et al., 2018; Shechner et al., 2014; Waters et al., 2015). It is possible that the treatment effect on anxiety may be attributed to nonspecific factors, such as ABMT-induced increases in attention control and flexibility and/or repeated exposure to threat stimuli (Pergamin-Hight, Pine, Fox, & Bar-Haim, 2016). Second, even though ABMT may reduce both attention bias to threat and anxiety symptoms, we lack evidence suggesting whether changes in attention bias longitudinally predict anxiety reduction. Third, individual differences at pre-training may moderate ABMT effect on anxiety. Larger pre-training attention bias to threat, indexed by RT measures (Waters et al., 2015), and poorer attention control ability reported by parents (Pergamin-Hight et al., 2016; but see Ollendick et al., 2018) are associated with greater anxiolytic effects post-training. Individual differences in neural connectivity and functioning during the baseline dot-probe task also differentiate children’s treatment response (Britton et al., 2015; White et al., 2017b).
Hence, the ABMT literature does not provide strong support for the causal link between attention bias and anxiety. Considerable individual differences at baseline moderate the training effect, and directing attention away from threat does not lead to anxiety reduction for all youth who received active versus placebo training. Moreover, the anxiolytic effects of ABMT may not be specifically attributed to reduced attention bias to threat. Longitudinal research is needed to capture if attention bias towards threat actually plays a causal role in the development of anxiety problems.
5.3. The role of threat-related attention in the development of socioemotional problems
Another avenue to study the causal role of threat-related attention in socioemotional development is to assess attention bias to threat early in development before the onset of clinical disorders and examine the association between attention bias to threat and early risk factors for developing anxiety or other socioemotional problems. Additionally, longitudinal studies are essential for investigating the impact of attention bias in the developmental pathway between early vulnerability and later symptoms. Findings would allow researchers to infer whether attention bias to threat is a precursor of socioemotional problems and whether it potentiates the risks for socioemotional maladjustments.
Early risks factors for socioemotional problems can be intrinsic, such as temperamental risks, or extrinsic to the child, such as familial risks and exposures to adverse environments. In 5-to 7-year-old children, Kujawa and colleagues (2011) found that daughters of mothers with depressive disorders show greater attention bias to sad faces presented in a dot-probe task after negative mood induction, compare to girls without the familial risk. The group difference in attention bias is not significant for boys. Moreover, attention bias to threat moderates the concurrent link between the experience of abuse and anxiety in 3- to 5-year-olds, such that children who are victims of family abuse and also have attention bias towards angry faces in a dot-probe task display greater anxiety than exposed children without attention bias (Briggs-Gowan et al., 2015). These studies collectively suggest that threat-related attention bias, indexed by RTs, is observable in young children, and it is related to vulnerability to anxiety and depression prior to the onset of clinical disorders.
Another line of research examines attention bias in children with temperamental risks of anxiety. Fearful temperament is a dimensional construct, capturing the extent to which children display an early disposition towards fearful reactions to novelty. Behavioral inhibition (BI) describes the most-studied category of temperament in children, reflecting the top 10–15% of these fearful temperament traits (Kagan, Reznick, & Snidman, 1988). In the review, we use “fearful temperament” as an inclusive term when describing literatures that have adopted a dimensional measure or studies that identified either BI or an alternative fearful temperament category, such as dysregulated fear (Buss, 2011).
BI is biologically-based, early-emerging, and characterized by heightened vigilance and reactivity to novelty in infancy (Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984) and social reticence in childhood (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). BI is the strongest individual-difference predictor of anxiety problems (Pérez-Edgar & Guyer, 2014). Indeed, early stable BI predicts an increased likelihood for developing lifetime anxiety disorders (Chronis-Tuscano et al., 2009) and a sevenfold increase of risk for developing SAD (Clauss & Blackford, 2012). However, not all BI children develop anxiety problems (Degnan & Fox, 2007). Threat-related attention bias may potentiate the vulnerability to anxiety seen in children with BI.
Early BI is linked to a biologically-based tendency to display heightened vigilance and negative reactivity to novelty in infancy (Kagan et al., 1984). Individual variation in motor activities and distress towards novel stimuli are observable at 4 months of age. These reactive tendencies are predictive of BI characterization at age 2 (Fox et al., 2001; Fox, Snidman, Haas, Degnan, & Kagan, 2015). In toddlerhood, BI is traditionally characterized by observing a child’s behavior in novel and fear-eliciting situations in the laboratory. The child is identified as BI when s/he exhibits high fear and behavioral avoidance in response to unfamiliar objects and people (Fox et al., 2001; Garcia-Coll, Kagan, & Reznick, 1984). If BI characterization is maintained across childhood, the biological disposition will often manifest as social withdrawal (SW) (Fox et al., 2001; Kagan, Reznick, & Snidman, 1988; Rubin, Burgess, & Hastings, 2002). SW, in turn, is marked by the behavioral prototype of isolating oneself from the peer group through the consistent display of solitary behavior in front of familiar and/or unfamiliar peers (Rubin, Burgess, & Coplan, 2002; Rubin, Coplan, & Bowker, 2009). SW increases the risk for BI children to develop internalizing problems (Boivin, Hymel, & Bukowski, 1995; Prior, Smart, Sanson, & Oberklaid, 2000; Rubin, Chen, McDougall, Bowker, & McKinnon, 1995).
BI shares overlapping neural and behavioral patterns with anxiety symptoms (Pérez-Edgar & Guyer, 2014). However, longitudinal evidence indicates that the majority of BI children never develop SW and anxiety problems (Biederman et al., 2001; Degnan et al., 2014; Stifter, Putnam, & Jahromi, 2008). Threat-related attention bias may be a risk mechanism that binds children with stable BI to a developmental pathway towards anxiety (Morales, et al., 2016; Pérez-Edgar, et al., 2014).
BI is associated with a biologically-based tendency to show quick and automatic attention orienting to novel and potentially threat stimuli (Kagan, 2012; Sylvester et al., 2017). Lower levels of sustained attention in infancy, manifested as more attentional shifts to a novel distracter stimulus, predicts greater stability of BI throughout early childhood. Moreover, BI at 14 months predicts greater social difficulties during adolescents only for participants who displayed reduced sustained attention in infancy (Pérez-Edgar et al., 2010). Hyperreactive bottom-up attention processes may influence the development and implementation of voluntary attention control processes. The interplay of automatic and controlled processes with socioemotional functioning may gradually calcify attention bias to threat in BI children. Once entrenched, attention bias may bind these children to a developmental trajectory towards anxiety problems (Henderson et al., 2015; Henderson & Wilson, 2017; Morales et al., 2016; Pérez-Edgar et al., 2014).
Most studies have relied on the RT scores derived from the dot-probe paradigm to index attention bias in children with fearful temperament. Similar to the correlational evidence presented above, these studies have yielded mixed results regarding the pattern of attention bias associated with fearful temperament. While some studies found that children with fearful temperament show attention bias towards threat (Pérez-Edgar et al., 2010; Szpunar & Young, 2012), others found that they display attention bias away from threat (Morales et al., 2014), or found no significant relation between fearful temperament and threat-related attention bias (Broeren, Muris, Bouwmeester, Field, & Voerman, 2011; Cole, Zapp, Fettig, & Pérez-Edgar, 2016; Pérez-Edgar et al., 2011; Vervoort et al., 2011).
Longitudinal evidence supports the premise that temperamentally fearful children develop SW (Morales et al., 2014; Pérez-Edgar et al., 2010, 2011) and anxiety problems (Nozadi et al., 2016; White et al., 2017a) if they also exhibit threat-related attention bias. Only two studies have examined the prospective relation among toddlerhood BI, childhood attention bias and anxiety across multiple time points. White et al (2017a) found that attention bias to either threat or positive faces at age 5 does not predict anxiety symptoms at age 7. Age-five attention bias also does not moderate the link between BI and age-seven anxiety. However, attention bias to threat and positive stimuli assessed at age 7 moderates the relation between BI and age-seven anxiety. Specifically, BI predicts anxiety in children who display attention bias towards threat or attention bias away from happy faces. Conversely, the BI-anxiety link is not significant for children showing attention bias away from threat or towards happy faces.
Within the same sample, Nozadi et al (2016) identified an anxiety class based on parent-report, children’s self-report and clinical interviews at age 10. They found that BI in toddlerhood predicts the probability of being in the anxiety class for children who display attention bias towards threat at age 5. However, consistent with other longitudinal studies, there is no significant zero-order correlation between attention bias to threat and concurrent or subsequent anxiety levels (Morales et al., 2014; Pérez-Edgar et al., 2010; 2011; White et al., 2017a).
The existing findings derived from RT measures are inconsistent regarding whether fearful temperament and anxiety are related to aberrant threat-related attention patterns. Based on longitudinal evidence, it is possible that threat-related attention bias on its own may not directly predict the onset of anxiety. Rather, it may act as a developmental tether that maintains the trajectory from early vulnerability to later anxiety problems (Morales et al., 2016; Pérez-Edgar et al., 2014). A better understanding of the relation between early temperamental risk, attention bias, and anxiety across development is dependent on implementing robust assessments of attention bias.
6. Theoretical and methodological issues with using RTs to measure threat-related attention
While the dot-probe paradigm is widely used to assess attention bias, studies indicate that the traditional RT index of attention bias to threat derived from the dot-probe task has poor test-retest reliability (Youth: Britton et al., 2013a; Brown et al., 2014; Adults: Rodebaugh et al., 2016; Schmukle, 2005; Staugaard, 2009) and internal consistency (Youth: Brown et al., 2014; Adults: Kappenman, Farrens, Luck, & Proudfit, 2014a; Rodebaugh et al., 2016; Schmukle, 2005; Staugaard, 2009; Waechter, Nelson, Wright, Hyatt, & Oakman, 2014). Moreover, White et al (2017a) indicated that there is a lack of attention bias stability from age 5 to age 7.
Manual RTs may not provide a direct measure of threat-related attention processes, hence, compromising the measurement reliability. Multiple attention shifts may occur during and after stimulus presentation and before the motor response to the probe. Therefore, RT measures may only tap into related attentional behavior at the snapshot of time (Yiend, 2010), and cannot capture dynamic attention processes with sufficient temporal sensitivity. Moreover, dot-probe RT measures may be too noisy to reliably capture the “core” threat-related attention processes, given that individual differences in threat vigilance, threat avoidance, and manual response speed may affect RT outcomes (Mogg, Holmes, Garner, & Bradley, 2008).
6.1. Improvement in RT assessment 1: Use multiple paradigms to assess attention
One avenue to improve attention bias assessment is to employ multiple attention paradigms. This strategy could allow researchers to assess multiple components of attention bias within the same sample, as well as examine whether there is a consistent pattern of attention bias that is most predictive of anxiety or anxiety risk. As an alternative attention paradigm, the Posner task (Posner, 1980) presents one cue (e.g. a face) at one side of the visual field, followed by a target probe appearing either at the cued location (valid cue) or the alternative location (invalid cue). The validity effect is quantified by subtracting RTs on the valid trials from invalid trials. Positive scores reflect greater effort needed to disengage from invalid cues. Studies consistently find a larger validity effect for threat cues in anxious than non-anxious adults (Bar-Haim et al., 2007).
Using both dot-probe and Posner tasks, Sylvester Hudziak, Gaffrey, Barch, & Luby (2015) found that children (average age 12.9 years) with a history of anxiety or depression, but not healthy controls, exhibit attention bias towards threat cues presented for 150ms in a Posner task and later attention bias away from threat cues presented for 500ms in a dot-probe task. Hence, threat-related attention bias in the clinical group is characterized by both early threat vigilance and later threat avoidance.
In a sample of 9- to 12-year-olds characterized for BI via maternal report, Morales, Taber-Thomas, and Pérez-Edgar (2017b) found that the correlation between dot-probe attention bias scores and validity effect scores obtained from an affective Posner task is significant only for BI children, but not across the whole sample. Furthermore, consistent attention bias across tasks is associated with increased anxiety symptoms. Hence, the implementation of multiple paradigms can potentially reveal a more comprehensive attention bias profile associated with symptomology (e.g. Sylvester et al., 2015). Moreover, it offers a person-centered approach to assess whether stronger attention bias stability across paradigms is associated with clinical anxiety and/or anxiety vulnerability (e.g. Morales et al., 2017b).
In contrast to the dot-probe and the Posner tasks that present simplified visual displays at each trial (e.g. a pair of faces and a single face, respectively), the visual search paradigm presents an array of stimuli (e.g. a 3×3 matrix). This complexity may better capture threat-related selective attention, rather than orienting to pre-selected stimuli (Todd et al., 2012). The visual search task is designed to isolate components of attention bias: faster RTs to detect the threat target among non-threat distracters indicate facilitated engagement to threat, whereas slower RTs to detect the non-threat target or to determine threat absence is inferred as difficulty in disengaging from threat (Donnelly et al., 2010; LoBue, 2014; Öhman, Lundqvist, & Esteves, 2001). LoBue and Pérez-Edgar (2014) showed that fearful temperament in 4- to 7-year olds is related to facilitated detection of angry faces, but not faster detection of non-social threat, compared to non-fearful children.
Elevated bias scores obtained from the dot-probe may be attributed to either facilitated engagement to threat or difficulty in threat disengagement or both. The visual search paradigm complements the affective Posner task by requiring participants to inhibit attention processing of simultaneously presented threat distracters (Hadwin & Field, 2010). As a future direction, conducting within-sample comparisons between the validity effect scores obtained from the affective Posner task and RT in detecting non-threat target among threat distracters in the visual search task may shed light on whether anxiety or anxiety vulnerability (e.g. fearful temperament) is associated with a consistent pattern of slower disengagement of task-irrelevant threat in order to support goal-directed performance. Thus, findings could provide potential support for the ACT (Eysenck et al., 2007). Furthermore, multiple attention paradigms administered at multiple assessment points may tap stability in attention bias across development that cannot be revealed using a single attention task (Britton et al., 2013; White et al., 2017a). Studies need reliable measures of attention bias, to then investigate whether and when in development attention bias to threat leads to the onset of socioemotional adjustment problems.
6.2. Improvement in RT assessment 2: Compute additional attention bias indices
Another reason for the poor reliability of attention bias assessment based on RTs may lie in the computation of global attention bias indices (Price et al., 2015; Rodebaugh et al., 2016). Attention bias scores are commonly computed by subtracting mean RTs of two task conditions from one another. One limitation of the global difference scores is that they reflect multiple attentional and non-attentional processes (Yiend, 2010). Thus, they may lack the sensitivity and specificity needed to identify threat-related attention patterns associated with individual differences in fearful temperament and anxiety.
The diffusion model (Ratcliff, 1978) provides an alternative attention bias index. It uses both RT and accuracy data obtained from two-choice tasks (including dot-probe, Posner, and visual search tasks). The model estimates four parameters, drift rate, boundary separation, start point, and nondecision processing, which respectively index the latent psychological processes of cognitive processing efficiency (i.e. facilitated processing of stimuli), speed-accuracy trade-off settings (i.e. levels of cautiousness in making a response), response bias (i.e. increased likelihood to make one type of response over the other) and the speed to make the required motor response (Ratcliff & Tuerlinckx, 2002). Applying the diffusion model to analyze manual response data enables researchers to examine facilitated attention engagement in isolation, uncontaminated by individual differences in non-attentional factors (Yiend, 2010).
An initial analysis of threat-related attention bias in children (9- to 12-year-olds) using the dot-probe paradigm found that children characterized as BI have faster drift rates to the probe, averaged across conditions (threat-neutral vs. neutral-neutral face pairs), suggesting that BI children are more attentive to the task than non-behaviorally inhibited children (Wise, Huang-Pollock, and Pérez-Edgar, in prep). The group-difference in task performance is not observed when using traditional RT indices. The findings further underscore that drift rates offer enhanced sensitivity for detecting individual differences in task performance.
The second limitation of the global difference scores is that they mask possible moment-by-moment variations in the expression of threat-related attention bias over time (Price et al., 2015; Zvielli, Bernstein, & Koster, 2015). Intraindividual variability would impair the reliability of attention bias scores (Rodebaugh et al., 2016). Studies have therefore captured within-subject, within-task variability of attention bias using an Attention Bias Variability (ABV) index (Iacoviello et al., 2014; Naim et al., 2015) or a trial-level bias score (TL-BS) method (Amir, Zvielli, & Bernstein, 2016; Zvielli, Bernstein, & Koster, 2015).
Studies with adults indicate that there is temporal variability in participants’ attention bias expression within a testing session, ranging from attention bias towards to away from threat. Moreover, greater within-task temporal variability is associated with more severe anxiety symptoms (Iacoviello et al., 2014; Naim et al., 2015; Zvielli et al., 2015). ABV and TL-BS methods demonstrate modest to good reliability, significantly higher than the traditional attention bias scores (Amir et al., 2016; Price et al., 2015; Rodebaugh et al., 2016; Zvielli et al., 2015). However, it is not clear if the variability indices are reliable in children, and whether anxiety vulnerability is associated with altered temporal variability of attention bias. White et al (2016) showed that the ABV index obtained from RT measures of fMRI dot-probe tasks has low test-retest reliability in 10- to 17-year-olds. In a large-scale study that uses dot-probe RT data collected from 6 studies (participants’ mean age ranging from 5.3 to 21.7 years), Yang, Ram, Perez-Edgar and Buss (in prep) found that BI levels are not significantly related to the traditional attention bias score or the TL-BS indices. Hence, caution needs to be taken when implementing this computation in child research.
A recent simulation study argues that it is unclear if higher ABV indices or TL-BS’s reflect greater frequency in switching bias directions, greater bias magnitude or random effects (i.e. measurement errors), or a combination of these factors (Kruijt, Field, & Fox, 2016). Moreover, large numbers of trials are needed to reach satisfactory reliability with variability measures. This may raise practical concerns for developmental research. Price et al (2015) found that the ABV index is the most reliable in adults who completed 320 trials (256 threat-neutral trials), and becomes less reliable in 9–13 year-olds who completed a 48-trial dot-probe task (32 threat-neutral trials). Zvielli et al (2015) recommend that at least 40 threat-neutral trials are needed to optimally compute TL-BS indices. Tasks containing larger trial numbers likely increase fatigue, movement, and impair performance in children, which may create artificial effects on attention bias variability. The trial-number requirement is particularly difficult to satisfy if the task is designed to test the specificity of attention bias (e.g. including happy-neutral trials) and/or incorporate biological measures that require participants to remain still during the task.
Alternative approaches to analyzing RT data have given us an additional tool in the attempt to reveal core attention bias processes that are associated with anxiety and anxiety vulnerability. However, RT measures, with their limited temporal resolution, still suffer from the essential limitation of capturing only the end-stage outputs of a cascade of cognitive and neural processes (Shechner et al., 2012). Multiple levels of analyses are crucial for compensating the limited temporal resolution of RT measures, in order to capture the temporal dynamics of threat-related attention.
7. Implementing stationary eye-tracking measures to study threat-related attention in relation to anxiety
Relative to RT measures, eye-tracking provides a more proximal, continuous and temporally sensitive tool for measuring attention processes (Table 1). Hence, eye-tracking is especially suited to test the hypothesis that anxiety is associated with initial threat vigilance and subsequent threat avoidance (Mogg & Bradley, 1998) or difficulty in disengaging from threat (Eysenck et al., 2007). Moreover, eye-tracking dot-probe measures demonstrate satisfactory internal consistency reliability in adults (indexed by Cronbach’s alpha, Waechter et al., 2014) and youth (indexed by split-half correlation in 9–48-month-olds, Burris et al., 2017a; index by intraclass correlation coefficient in 9–13-year-olds, Price et al., 2015).
Across various eye-tracking paradigms, early facilitated engagement or vigilance is measured using parameters of initial fixations, such as the latency of first fixations and the proportion of trials in which the initial fixation was directed to the target stimulus (e.g. Gamble & Rapee, 2009; Price et al., 2015, 2016; Shechner et al., 2013). Overall attention maintenance is assessed using stimulus dwell time (e.g. Price et al., 2015, 2016b; Shechner et al., 2013). Late attention components, including disengagement and avoidance, can be indexed by event-related fixation measures. For example, in a dot-probe task, disengagement difficulty is indexed by the latency to orient away from the face stimulus just prior to the onset of the probe that appears in the opposite location from the participant’s fixation location (e.g. Price et al., 2015). Late attention components can also be assessed using epoch-related measures. For example, studies using free viewing paradigms divide the stimulus presentation period into equal time intervals. During later time intervals, higher fixation durations or proportion of dwell time indicates greater difficulty in disengagement, whereas lower durations/proportion of dwell time on threat suggests attention avoidance (e.g. In-Albon, Kossowsky, & Schneider, 2009; Seefeldt, Krämer, Tuschen-Caffier, & Heinrichs, 2014).
The majority of eye-tracking studies with anxious children have employed variations of free viewing paradigms that present pairs of threatening and benign images. Studies suggest that pediatric anxiety is characterized by heterogeneous patterns of attention bias. Shechner et al (2013) found that, compared to their non-anxious group, 8- to 17-year-olds with anxiety disorders show initial facilitated engagement to angry faces. In contrast, other studies in children between 7 to 17 years of age showed that anxiety disorders are associated with threat avoidance but not initial facilitated engagement towards threat (Gamble & Rapee, 2009; with 500ms face presentation; In-Albon, Kossowsky, & Schneider, 2009; Shechner et al., 2017), or no anxiety-group differences in attention bias patterns (Dodd et al., 2014; Gamble & Rapee, 2009, with 3000ms face presentation). Moreover, Seefeldt et al (2014) indicated that 8- to 12-year-olds with SAD show difficulty in threat disengagement, indexed by greater dwell time on threat relative to non-threat faces during the last time interval of stimulus presentation (2501ms-3000ms). Together, existing free-viewing eye-tracking paradigms incorporated various stimulus presentation duration, ranging from 500ms (Gamble & Rapee, 2009) to 4000ms (In-Albon et al., 2009). Based on continuous measures of attention, studies showed that pediatric anxiety might be associated with threat-related attention that takes place at both early and late stages of visual information processing.
A limited number of studies have implemented dot-probe eye-tracking tasks with children (Burris et al., 2017a; Burris et al., 2017b; Hilt, Leitzke, & Pollak, 2017; Price et al., 2013; Price et al., 2016b; Tsypes, Owens, & Gibb, 2017). In the only study that examined the relation between attention bias and anxiety symptoms, Price et al (2016b) assessed threat-related attention using the dot-probe paradigm in 9- to 13-year-olds with anxiety disorders just prior to receiving psychotherapy. Initial vigilance towards threat faces and the percentage of dwell time on threat versus neutral faces do not correlate with anxiety levels assessed at baseline and two years after treatment. However, a pattern of sustained threat avoidance, indexed by both dwell time and pupil dilation, but not initial vigilance, predicts the transition from anxiety to later depression symptoms post-treatment. Threat avoidance may hinder the use of effective emotional regulation strategies, thus heightening the vulnerability to develop depression despite anxiety treatment.
Interestingly, in two studies that compared eye-tracking and RT measures, Hilt et al (2017) found that greater self-reported rumination is associated with longer dwell time on emotional faces in 9- to 14-year-olds. Tsypes et al (2017) found that children (7–11 years old) with suicidal ideation display greater dwell time to fearful faces, but show no bias in dwell time towards happy faces. Neither study suggested that that RT attention bias significantly differentiates between symptomatic vs. non-symptomatic groups, supporting eye-tracking measures as a more sensitive index of symptom-related attention bias than RT measures (Price et al., 2015).
No study has implemented eye-tracking paradigms to investigate the concurrent or longitudinal association between fearful temperament and anxiety problems in children. Hence, there is a gap in our understanding regarding how the specific pattern of normative attention bias to threat (e.g. difficulty in disengaging from threatening faces) is maintained to childhood, and how it may give rise to vulnerability to anxiety problems. Before pursuing this direction, a few methodological issues relating to eye-tracking paradigms need to be considered.
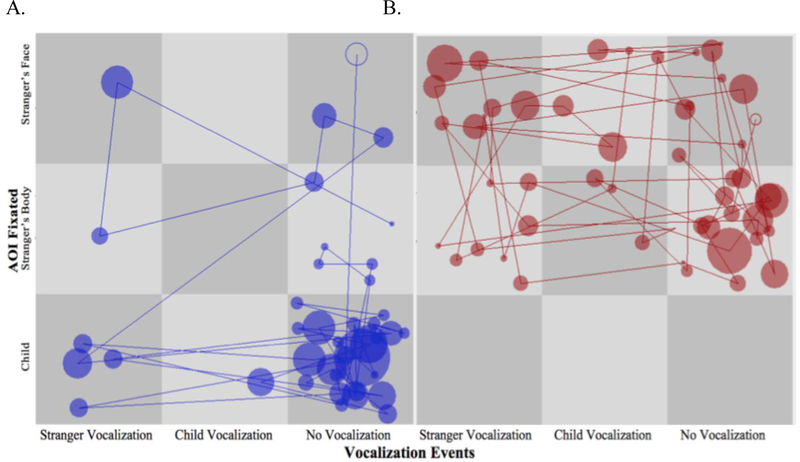
First, existing free-viewing and dot-probe paradigms commonly present stimuli ranging from relatively simple constellations, such as face pairs (e.g. Dodd et al., 2014; Gamble & Rapee, 2009; Shechner et al., 2013), to more complex pictorial scenes (In-Albon et al., 2009; Shechner et al., 2017). Variations in stimulus complexity may contribute to the mixed attention bias patterns found in these studies, considering the argument that the overt attention bias components associated with anxiety might be stimulus-dependent (Armstrong & Olatunji, 2012; Gibb, McGeary, & Beevers, 2015; Richards, Benson, Donnelly, & Hadwin, 2014). The adult literature indicates that studies adopting relatively simple stimulus constellations might be biased to find facilitated initial engagement to threat, since participants are restricted to direct their initial gaze to threat (Armstrong & Olatunji, 2012; Richards et al., 2014).
Indeed, the two studies that used more complex visual displays found threat avoidance in anxious youth (In-Albon et al., 2009; Shechner et al., 2017), echoing the pattern of attention avoidance found in anxious adults using more naturalistic displays (Chen, Thomas, Clarke, Hickie, & Guastella, 2015; Huijding, Mayer, Koster, & Muris, 2011). Incorporating eye-tracking in visual search paradigms, which present an array of competing stimuli, provides an effective tool for separately examining facilitated threat detection of threat targets versus difficulty in threat disengagement from threat distracters (Armstrong & Olatunji, 2012). The potential effect of stimulus complexity on threat-related attention also calls for the use of more ecologically valid stimulus constellations that may better tap into the functioning of threat-related attention in real-life contexts.
Second, eye-tracking indices of attention bias derived from a single task may have different levels of reliability. Using the dot-probe paradigm, Price et al (2015) found that only eye-tracking indices of early threat vigilance and overall threat preference, but not measures of difficulty in threat disengagement, demonstrate satisfactory test-retest reliability in youth (age 9–13 years). Individual paradigms may not be optimal for assessing all components of attention bias. For example, the eye-tracking dot-probe tasks can capture latency in threat disengagement only in trials where the participant fixated on both the face stimulus and the probe that subsequently appeared in the opposite side of the face. In comparison, the overlap task might be more effective for measuring threat disengagement. To facilitate interpretation of eye-tracking findings, it is important to assess and report the reliability of eye-tracking attention bias indices (Burris et al., 2017a; Price et al., 2015). Research can also benefit from administering multiple eye-tracking tasks in the same sample to examine whether anxiety is characterized by a convergent pattern of attention bias.
8. Implementing neural measures to study threat-related attention in relation to anxiety
Findings from both behavioral RT and eye-tracking paradigms do not rule out the possibility that anxiety and/or anxiety vulnerability may be linked to a constellation of attention bias patterns rather than a single attention mechanism. Neural measures help to reveal underlying mechanisms contributing to the overt motor behavior or eye movement outputs (Table 1). As reviewed above, neural circuits subserving automatic orienting are in place from infancy (Leppänen & Nelson, 2009). Biologically-based and environmental factors may jointly influence threat-related attention through their impact on brain functions (Caouette & Guyer, 2014; Monk, 2008; Pine, 2007). Hence, measuring neural activity facilitates the identification of biomarkers of anxiety and anxiety vulnerability even when behavioral measures do not reveal anxiety-based group differences (Perez-Edgar & Bar-Haim, 2010).
Specifically, studies that use ERPs and fMRI to track threat-related attention processes have advanced theory and boost our understanding of the role of threat-related attention bias in the development of socioemotional problems. Based on the neural evidence, the dual-processing perspective highlights that bottom-up automatic and top-down controlled processes jointly contribute to the development and maintenance of anxiety symptoms (Bishop, 2008; Eysenck et al., 2007; Henderson et al., 2015; Henderson & Wilson, 2017). BI is rooted in a neural profile characterized by amygdala hyper-reactivity (Kagan, 2012) and decreased connectivity in the ventral attention network (Sylvester et al., 2017), which predispose children with BI to display hypervigilance towards novel, potentially threatening stimuli (Davis & Whalen, 2001; Shackman et al., 2016). The automatic information processing style associated with fearful temperament impedes the development and implementation of efficient and flexible attention control functioning, possibly by potentiating executive attention processes that are employed to entrench, rather than ameliorate, maladaptive behavior (Henderson et al., 2015; Henderson & Wilson, 2017).
8.1. ERP studies of threat-related attention processes associated with anxiety
ERPs provide an online, continuous, and covert measurement of neurophysiological responses time-locked to stimulus presentation (Table 1). Thus, it is an ideal tool for capturing bottom-up automatic attention processes that take place in early time windows and top-down goal-directed processes that have a relatively late onset.
Studies measuring the amplitude of ERP components time-locked to face presentations suggest that early facilitated engagement towards threat may contribute to the maintenance of anxiety in childhood. O’Toole, DeCicco, Berthod, and Dennis (2013) administered a modified version of the Attention Network Test (Fan et al., 2002) in 5-to-7-year-olds. They found that children with higher anxiety levels maintained their anxiety symptoms when assessed two years later if they also exhibited facilitated engagement towards task-irrelevant angry relative to happy faces, indexed by enhanced face-locked N170 amplitudes. Hence, initial vigilance towards task-irrelevant threat might be a moderating factor that influences the development of anxiety.
With superior temporal resolution compared to RT measures, ERPs may be more sensitive in detecting anxiety group differences in threat-related attention processes. Indeed, studies have found that anxiety moderates ERPs indices of attention bias toward emotional faces, but not parallel RT measures (e.g. Bar-Haim, Lamy, & Glickman, 2005; Rossignol, Campanella, Bissot, & Philippot, 2013). In particular, healthy adults display initial facilitated attention allocation towards threat images, indexed by significant N2pc potentiation observed in 175–225ms following the onset of the threat-neutral stimulus pairs in dot-probe tasks. In contrast, RT scores do not show a significant attention bias to threat (Kappenman et al., 2014a; Kappenman, MacNamara, & Proudfit, 2014b). Behavioral responses cannot capture rapid covert attention shifts. It is possible that participants have already shifted attention away from the threat location by the time they behaviorally responded to the probe (Kappenman et al., 2014a; Kappenman et al., 2014b).
To examine the association between anxiety vulnerability and electrophysiological correlates of attention bias, Thai, Taber-Thomas, and Perez-Edgar (2016) recorded ERPs during a dot-probe task in 9- to 12-year-olds with varying levels of BI. They found that P2 time-locked to face displays moderates the link between BI and social anxiety. That is, BI is negatively related to social anxiety in children displaying greater P2 response. The deployment of greater attention control may serve as a compensatory mechanism that reduces social anxiety symptoms in children with high BI.
In addition, face-locked N2 moderates the relation between BI and RT attention bias scores, such that larger N2 predicts bias away from threat only in children with high BI. Threat avoidance may hinder the use of effective emotional regulation strategies, thus heightening the vulnerability for developing anxiety problems in temperamentally fearful children (Morales et al., 2014). Together, the study indicates that voluntary attention control processes modulate the behavioral expression of attention bias and social anxiety. Children with temperamental risk for anxiety may allocate greater attentional resources to processing threat information compared to healthy children. This may be a less efficient, compensatory strategy to offset their initial heightened reactivity to threat (Thai et al., 2016).
The late positive potential (LPP) reflects sustained attention towards affectively and motivationally salient stimuli (Hajcak, MacNamara, & Foti, 2012). The LPP has demonstrated good test-retest reliability in 8- to 13-year-old children for measuring attentional processing towards emotional stimuli over two years of development (Kujawa, Kline, & Proudfit, 2012). In youth (7 to 19 years of age), enhanced LPP amplitudes time-locked to the presentation of symptom-relevant threat stimuli are associated with anxiety disorders (Kujawa, MacNamara, Fitzgerald, Monk, & Phan, 2015) and pediatric spider phobia (Leutgeb, Schäfer, Köchel, Scharmüller, & Schienle, 2010). Moreover, 5- to 7-year-olds who show increased LPP response to unpleasant relative to neutral pictures during passive viewing also display elevated fearful behavior observed in the laboratory (Solomon, DeCicco, & Dennis, 2012). Collectively, pediatric anxiety is associated with sustained attention processing towards threat, indexed by increased LPP potentiation in response to threat stimuli. LPP potentiation might be a biomarker for threat-related attention bias towards affectively and motivationally salient stimuli (Hajcak et al., 2012).
ERP research suggests that enhanced neural engagement supporting both initial facilitated threat detection and voluntary attention processes towards threat may be associated with pediatric anxiety, and contribute to the development of anxiety. Neuroimaging can complement ERPs to help to identify perturbed neural networks that may underlie the link between threat-related attention bias and anxiety.
8.2. fMRI studies of threat-related attention processes in anxious and at-risk youth
Theoretical accounts and empirical evidence support the proposition that neural networks implicated in anxiety overlap with circuitries underlying threat-related attention bias (LeDoux & Pine, 2016; Shin & Liberzon, 2011). Dot-probe studies examining neural correlates of threat-related attention processing in youth (between the ages of 7 to 17 years) with high trait anxiety or anxiety disorders find that anxiety is associated with aberrant activation in the amygdala, the ACC in the cingulo-opercular network, and various PFC regions, including the vlPFC in the ventral attention network and the dlPFC in the fronto-parietal network (Table 1; for reviews, see Blackford & Pine, 2012; Guyer, Masten, & Pine, 2013; Swartz & Monk, 2013).
Monk et al (2006), for example, found that youth (9–17 years old) with GAD display greater vlPFC responses to angry faces presented for 500ms compared to healthy controls. Other studies have focused on attention disengagement in the task by comparing incongruent versus congruent neutral-threat trials. They found that youth with higher trait anxiety engage greater dlPFC function when required to shift attention to the opposite location of threat compared to the same location (9–12 years old, Fu et al., 2017; 11–18 years old; Telzer et al., 2008). Clinically-anxious youth also exhibit reduced functional connectivity between the limbic regions and the rostrodorsal ACC than non-anxious youth (9–13 years old; Price et al., 2014).
Studies have also examined adolescents’ neural response to threat faces by manipulating their attention state. Participants were asked to either passively view the face stimuli or rate the faces based on their emotional (e.g. “how afraid are you of this face?”) or non-emotional features (“how wide is the nose?”). Compared to healthy controls, adolescents with anxiety disorders exhibit elevated amygdala response and increased connectivity between the amygdala, ventral prefrontal cortex, and ACC when attending to their own subjective fear relative to passive viewing (Beesdo et al., 2009; McClure et al., 2007). Taken together, the neural underpinnings of pediatric anxiety is characterized by a combination of amygdala hyper-reactivity and aberrant compensatory regulation of amygdala reactivity by the ACC and PFC regions (Blackford & Pine, 2012; Guyer et al., 2013; Pine, 2007; Swartz & Monk, 2013).
Neural correlates of threat-related attention bias may have greater reliability than RT measures of attention bias. Using the dot-probe paradigm, Britton et al (2013) found that while RT bias scores in healthy youth (8–17 years old) are not stable across two assessment points, participants reliably recruit greater vlPFC function in the threat-incongruent relative to congruent trials across testing sessions. In another sample of 10- to 17-year-olds, White et al (2016) replicated the earlier findings, indicating that dot-probe RT measures have low reliability. However, vlPFC activation to the incongruent-versus-congruent contrast and the amygdala-dlPFC connectivity to the threat-versus-neutral contrast are stable across the nine-week period. In contrast to RT measures that reduce complex processes to one score, neuroimaging might be an effective tool to identify more stable attention processes (Britton et al., 2012, 2013; White et al., 2016).
Research examining neural activities that support threat-related attention processing in individuals with temperamental risk for anxiety suggests that concurrent and/or a history of early BI is associated with aberrant responding in the frontolimbic circuitries that overlap with networks implicated in threat-related attention processing and anxiety. The BI-related neural perturbations are apparent even in the absence of full-fledged anxiety disorders among BI individuals (Pérez-Edgar & Guyer, 2014). Thus, the neutral perturbation might in and of itself be a risk marker of anxiety problems.
Young adults characterized as BI show atypical amygdala in response to novel faces (faster response latency: Blackford, Avery, Shelton, & Zald, 2009; failure to habituate responses: Blackford, Allen, Cowan, & Avery, 2012; Schwartz et al., 2012; greater magnitude: Pérez-Edgar et al., 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003). Individuals characterized with BI also exhibit aberrant frontal functioning underlying attention control processes, even when they do not show greater behavioral attention bias compared to non-BI individuals. Hardee et al (2013) found that young adults with early BI display stronger negative amygdala-dlPFC connectivity than individuals without childhood BI for the angry-versus-neutral face contrast in a dot-probe task. Similarly, Fu et al (2017) found that BI children (9–12 years old) deploy greater dlPFC activation than non-BI children in response to incongruent relative congruent trials. BI children might have recruited greater compensatory attention processes in order to carry out goal-directed behavior (i.e. respond to the probe appeared in the opposite location of threat). Furthermore, elevated dlPFC activation predicted greater anxiety symptoms through BI level.
It is possible that supraliminal presentation of threat may elicit more controlled processes subserved by frontal regions and less limbic activities that support early, automatic attention engagement (Monk et al., 2006). It is challenging to parse out the two processing in standard dot-probe paradigms, due to fMRI’s limited temporal resolution. Studies have addressed this issue by presenting subliminal faces (17ms) in the dot-probe task, in order to capture bottom-up automatic attention processing. Monk et al (2008) found that adolescents (mean age: 14 years) with GAD show greater amygdala responses to angry faces. Auday, Taber-Thomas, and Pérez-Edgar (2018) showed that non-BI children (mean age: 11 years) display greater activation in prefrontal and temporal regions that might function to modulate responses toward threat. When exposed to subliminal threat, age-matched BI children may be less efficient in engaging cognitive control to down-regulate initial threat responses.
Complementing ERP findings, neuroimaging studies support the argument that enhanced threat vigilance and aberrant attention control functioning are attributed to perturbations in the frontolimbic circuitry encompassing the amygdala, the prefrontal and ACC regions (Blackford & Pine, 2012; Guyer et al., 2013; Swartz & Monk, 2013; Sylvester et al., 2012). The neural network subserving automatic attention orienting is early appearing during infancy (Leppänen & Nelson, 2009). Disruption in the network is associated with the development of BI and may predispose temperamentally at-risk toddlers to develop habitual biased attention to threat (Sylvester et al., 2017).
Cross-sectional ERP and neuroimaging studies thus far have advanced our understanding of the attention mechanisms that fuel the relation between early temperamental risk and anxiety problems. Strong support for the dual-processing perspective will come from longitudinal neural research, which tracks intraindividual changes in neural functions supporting automatic and controlled attention processes, and examine how they interact with concurrent socioemotional functioning to predict subsequent anxiety problems (Guyer, Pérez-Edgar, & Crone, 2018).
9. Future directions: Investigating threat-related attention processes and socioemotional behavior in social-interactive contexts
Thus far, our understanding of the threat-related attention mechanisms that contribute to the onset and development of anxiety relies on using well-controlled experiments that present relatively artificial screen-based stimuli. Hence, attention processes that are delineated using the computerized attention paradigms cannot be generalized to active, real-life, situations. This review aims to underscore the importance of incorporating components of real-life social interactions in the assessment of threat-related attention. There is a recent push to examine the relevance of threat-related attention bias to every-day life (Shackman et al., 2016). For example, Price et al (2016a) found that among youth (9 to 14 years old) with anxiety disorders, greater attention bias toward threat indexed by dot-probe RT measures is linked to reduced connectivity between the right amygdala and right dlPFC regions during the task. Connectivity, in turn, predicts greater employment of attention avoidance strategies when participants encountered negative life events, as assessed through repeated experience-sampling.
However, measuring threat-related attention using the computerized paradigms reviewed up to this point cannot capture the functioning of active attention selection in processing complex stimulus constellations that exist in everyday environments. Evidence suggests that individuals display more nuanced attention patterns towards naturalistic stimuli than computerized presentations (Risko, Laidlaw, Freeth, Foulsham, & Kingstone, 2012; Risko, Richardson, & Kingstone, 2016; Schilbach, 2014). More specifically, fearful temperament and anxiety disorders share the core feature of displaying social difficulties. It is still unclear how threat-related attention assessed in the context of online social interaction may impact fear- and/or anxiety-related phenotypes. Perturbed threat-related attention patterns observed in vivo might be a more proximal risk marker of disorders characterized by social impairments (Chawarska, Macari, & Shic, 2012; Chevallier et al., 2015; Speer, Cook, McMahon, & Clark, 2007).
9.1. Why do we need to study threat-related attention in naturalistic contexts?
Computerized attention paradigms commonly present static and relatively simple visual displays (e.g. face pairs). As a result, these tasks primarily measure orienting to preselected stimuli, rather than active attention selection (Birmingham & Kingstone, 2009; Todd et al., 2012). Mobile eye-tracking offers a measurement tool for capturing person-centered attention processes in real-life situations. A mobile eye-tracking device contains both scene and eye cameras, allowing it to capture the visual field and parameters of eye movements (Franchak, 2017). Additionally, the continuous recording allows for studying real-time within-person changes in attention processes as the individual interacts with the environment. Compared to screen-based attention tasks, paradigms that allow participants to selectively attend to and behaviorally navigate through competing stimuli may yield enhanced ecological validity, and reveal different, more nuanced, attention patterns.
Using a mobile eye-tracking paradigm, Isaacowitz, Livingstone, Harris, and Marcotte (2015) found that younger and older adults do not show differences in the valence of the stimuli attended when they have the discretion to select objects to attend. The findings contradict prior stationary eye tracking studies suggesting that older adults have a greater tendency to fixate on positive versus negative stimuli, which in turn, leads to more positive mood (Isaacowitz, 2012). Lange, Tierney, Reinhardt-Rutland, and Vivekananda-Schmidt (2004) monitored looking behaviors of adults with subclinical spider phobia when they are in a room with a live tarantula. They found that the participants make more scans of the room than non-phobic individuals, possibly reflecting a state of hypervigilance towards threat (Richards et al., 2014) or increased tendency to seek safety (Lange et al., 2004).
One critical advantage of examining attention in real-life contexts lies in the enhanced opportunity for studying the mutual influences of attention processes and social interaction, a fundamental aspect of social cognition (Redcay & Warnell, 2018; Risko et al., 2012; Schilbach et al., 2013). Paradigms that do not contain or model the reciprocity of social interaction cannot capture to the dual function of gaze – both obtaining information from the environment and communicating information to other people (Nasiopoulos, Risko, & Kingstone, 2015; Risko et al., 2016).
A series of mobile eye-tracking studies (Foulsham, Walker, & Kingstone, 2011; Freeth, Foulsham, & Kingstone, 2013; Kretch & Adolph, 2015; Laidlaw, Foulsham, Kuhn, & Kingstone, 2011) investigated the effect of social interaction on attention patterns by allowing the opportunity for social contact during the tasks. They collectively indicate that adults tend to avoid directly looking at strangers, possibly driven by their implicit understanding of social customs. Foulsham, Walker, and Kingstone (2011) found that when participants are walking on a university campus or watching video clips taken from the perspective of a walker, they are equally likely to fixate on pedestrians who are at a distance. However, when pedestrians are in close proximity to the walkers, the participants are less likely to look at the pedestrians when they are walking (the potential for social interactions is present) than when they are watching people walking (the potential for social interactions is absent).
Consistent with the findings, Kretch and Adolph (2015) asked mothers to carry their infants in a forward-facing carrier and walk through hallways. Mothers fixate less on other people than their infants do. Moreover, Laidlaw, Foulsham, Kuhn, and Kingstone (2011) found that participants initiate fewer fixations to an experimenter physically sitting in the room (the potential for social interactions is present) than participants who view the videotaped experimenter (no opportunity for social interactions). In a subsequent study (Freeth et al., 2013), participants fixate more to the live experimenter’s face during a conversation when the experimenter initiates eye contact than when the experimenter averts his gaze away. The manipulation of the experimenter’s eye gaze direction does not affect viewing behavior in adults who interact with a videotaped experimenter. Hence, during real-life social interactions, adults’ practical knowledge of social etiquette and expectations of others’ potential reactions may guide their gaze behavior (Klin, Jones, Schultz, & Volkmar, 2003).
To investigate whether different neural processes underlie social interaction versus social observation, Redcay et al (2010) asked adults to interact with an experimenter via a live video feed or watch video recordings of a previous experiment session with a different participant. During live interactions, participants communicated their intent using eye gazes. Thus, relative to the passive viewing condition, the live interaction condition allows for self-relevance and contingency between participants’ and the experimenter’s behavior. They found that live interactions elicit greater activations in regions associated with mentalizing, attention, and reward processing. Further studies revealed that when adults (Rice & Redcay, 2016) and children (Rice, Moraczewski, & Redcay, 2016) simply believe that they are communicating with a live partner they exhibit enhanced activation in neural systems implicated in social cognition. Taken together, mobile eye-tracking and fMRI evidence show that screen-based attention paradigms that do not permit real or perceived social interactions may not be as valid for delineating attention functioning in real-world social contexts (Birmingham & Kingstone, 2009; Redcay & Warnell, 2018; Risko et al., 2012; Schilbach, 2014).
More specifically, a growing number of studies on attention processes associated with early socioemotional maladjustments have adopted naturalistic stimuli and task paradigms to assess social attention in children with autism spectrum disorder (ASD). Anxiety disorders and ASD are both marked by deficiencies in social cognition and functioning, albeit manifested in a disorder-specific manner (Monk, 2008). ASD is characterized by impaired social attention, manifested as reduced fixations and dwell time on social stimuli, compared to typically developing (TD) controls (Chita-Tegmark, 2016). Compared to using static images, more realistic, dynamic stimuli that represent social interactions are more effective in eliciting social attention patterns that can differentiate ASD from typically TD controls (Chawarska et al., 2012; Chevallier et al., 2015; Speer et al., 2007). For example, Chevallier et al (2015) found that, while ASD and TD children do not differ in fixation durations towards static and dynamic displays of faces and objects, children with ASDs display more attention avoidance from video clips of children playing together, compared to their TD peers. Naturalistic stimuli that are more socially relevant might be more affectively and motivationally potent to invoke disorder-related attention disturbances, and thus more sensitive for detecting differences between the symptomatic and non-symptomatic control group.
9.2. How can we study threat-related attention in social interactive contexts?
Mobile eye-tracking technology has been implemented to study ambulatory looking behavior in naturalistic environments. Research on normative motor and cognitive development has used mobile eye-tracking to examine infants’ gaze behaviors in locomotion (Franchak & Adolph, 2010; Franchak, Kretch, & Adolph, 2017; Franchak, Kretch, Soska, & Adolph, 2011; Kretch & Adolph, 2015; Kretch, Franchak, & Adolph, 2014) and joint attention between infant-parent dyads (Yu & Smith, 2013, 2016a, 2016b, 2017). In addition, an emerging literature has demonstrated that head-mounted eye-trackers can be implemented to assess social attention in young children with ASD. These studies highlighted that mobile eye tracking provides a mean to study visual attention within a dynamic system that encompasses on-line social interactions and sensory-motor co-ordinations between the body, hands, and eyes (Smith, Yu, Yoshida, & Fausey, 2015; Thelen & Smith, 1994). Thus, the technology facilitates researchers to delineate the dynamic changes in attention processes as individuals’ behaviors unfold in real time.
One approach to capturing ambulatory threat-related attention processes in children is to examine children’s gaze behavior as they experience fear-eliciting scenarios that contain novel and potentially threatening objects or events. Two published studies used video recordings during a fear episode taken from the Lab-TAB (Buss & Goldsmith, 2000), a gold-standard protocol designed for assessing temperament, to examine toddlers’ attention towards a putative threat stimulus (i.e. a gorilla mask). Kiel and Buss (2011) found that sustained attention towards the scary mask at 24 months of age predicts increased social inhibition in the kindergarten year. Moreover, toddlers’ behavioral avoidance moderates this relation, such that those toddlers with attention bias to threat who also remain at a distance from the threat stimulus develop greater social inhibition. A subsequent study (Hummel, Premo, & Kiel, 2017) found that sustained attention towards the gorilla mask at 24 months predicts reduced shyness a year later only for those toddlers with high externalizing symptoms. These findings highlight that threat-related attention patterns in the context of approach/withdrawal behavioral tendencies predict subsequent socioemotional adjustment outcomes. However, traditional video recordings cannot allow for fine-grained assessment of attention.
Mobile eye-tracking recording can be incorporated in standardized, developmentally appropriate scenarios that elicit fear and withdrawal behavior. For example, Figure 1A shows snapshots from videos of a 6-year-old boy and girl, each in the Stranger Approach episode, taken from the Preschool version of the Lab-TAB (Buss & Goldsmith, 2000; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1994). In the episode, a male experimenter that the child has never met walks toward the child, sits down, and initiates a conversation. Figure 1B is a part of the recording taken from the Storytelling episode (Fox et al., 1995) of a standard social dyad paradigm. Here, each 6-year-old boy was asked to stand up and give a speech about his recent birthday party. Mobile eye-tracking paradigms enable researchers to measure gaze behavior with superior spatial resolution compare to traditional video recordings of the testing room. As shown in Figure 1A, the room recording (left) suggests that both children are looking at the stranger. However, the mobile eye-tracking recording (right) differentiates the girl who was looking directly at the stranger’s face versus the boy who was looking at the stranger’s body.
Fig 1. Eye-gaze Behavior during Social Interaction.
A) Recording of a girl and a boy (both 6 years old) in the Stranger Approach Episode, taken from the Preschool Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith et al., 1994). During the episode, a male stranger walks toward the child, sits down and initiates a conversion. The room recording (left) and the mobile eye-tracking recording (right) were synchronized offline for data coding. In the mobile eye-tracking recording, recording from the eye cameras was overlaid onto the recording from the scene camera. B) Recording of an age-matched boy dyad (both 6 years old) in the Storytelling episode, which has been used to assess social reticence (e.g. Fox et al., 1995). The dyad plays a game of show-and-tell during which each child is asked to stand up and give a speech about his recent birthday party. The room recording and the mobile eye-tracking recording for each child were synchronized. As Participant #7092 was giving the speech, he was looking at the experimenter, while Participant #7078 was looking at the speaker. The dyad paradigm allows researchers to gauge the level of attention synchrony between the dyad.
9.3. How can the proposed paradigm advance our understanding of threat-related attention and its role in the development of socioemotional problems?
By continuously recording children’s gaze locations from a first-person perspective, the proposed mobile eye-tracking paradigm allows us to assess how children’s threat-related gaze patterns evolve temporally as social interaction unfolds. As an illustration, Figure 2 presents gaze data collected from two children during the Stranger Approach episode. It shows gaze on three areas of interests (AOIs: child him/herself, stranger’s body, and stranger’s face during three types of vocalization events in the episode (stranger talking to the child, child talking to the stranger, and no one is talking).
Fig 2. State space grids depicting gazes during the Stranger Approach episode.
The state space grids were produced using GridWare (Lamey, Hollenstein, Lewis, & Granic, 2004; Lewis, Lamey, & Douglas, 1999). The areas of interest are child him/herself, stranger’s body, and stranger’s face. The vocalization events of interest are stranger vocalization, child vocalization and no vocalization. Data from two children are presented in Panel A and B. The hollow circle represents the starting point for each child. The lines depict the transition of AOI gazes across vocalization events. The size of the circle is proportional to the duration of a continuous gaze on an AOI. Bigger circles indicate longer continuous gaze on the AOI.
The prolonged, continuous, online recording of gaze offers three key advantages. First, researchers can test the vigilance-avoidance hypothesis (Mogg & Bradley, 1998) – for example, whether children with anxiety or anxiety vulnerability initially look more toward the stranger, but gradually show gaze avoidance form the stranger. Second, data can reveal both inter- and intra-individual differences in children’s attention patterns. For example, as shown in Figure 2, while 93.96% of child one’s AOI gazes were on himself (Figure 2A), child two (Figure 2B) never looked at himself during the episode. In terms of intra-individual differences, children’s dwell times on different AOIs differed. In addition, the differences in dwell times between AOIs differed across vocalization events. Existing theories and research have primarily focused on studying anxiety-related inter-individual differences in attention bias. Continuous and sensitive recording of gaze could uncover whether there are anxiety-related inter-individual differences in the temporal dynamics of attention processes during an online social interaction.
Third, incorporating ambulatory gaze measures in Lab-TAB fear-eliciting episodes enables researchers to investigate whether trait level BI (indexed by aggregated fear scores across episodes and/or parent-report) interacts with state, in-the-moment, fearful behavior to influence threat-related attention patterns. Moreover, in cross-sectional studies, investigating the relation among trait level BI, state fearful behavior and threat-related attention could provide an informative snapshot of how the dynamic interplay between socioemotional behavior and online attention processes predicts socioemotional outcomes (Morales, et al., 2016; Pérez-Edgar et al., 2014). Thus, findings may facilitate the identification of more proximal bio-psycho-behavioral risk markers of anxiety or broader socioemotional problems.
Consistent with the recent call for measuring threat-related attention using multiple paradigms at multiple levels of analysis (Price et al., 2015; Rodebaugh et al., 2016), studies will also benefit from assessing threat-related attention using both naturalistic and screen-based assessments within the same sample. Comparing threat-related attention patterns captured by mobile eye-tracking with those revealed in computerized stationary eye-tracking paradigms could help to delineate context-dependent and context-independent attention processes. Such within-sample comparison could also reveal if threat-related attention bias in vivo serves as a better risk marker of anxiety than traditional, static measures of attention bias.
10. Concluding remarks
Threat-related attention bias is characterized by initial facilitated engagement towards threat, followed by sustained attention to threat or difficulty in disengaging from threat, and avoidance of threat (Amso & Scerif, 2015; Cisler & Koster, 2010). Over time, attentional prioritization to threat becomes an automatic, default processing style that drives individuals to orient to threat (Todd et al., 2012). We reviewed behavioral, eye-tracking, and neural evidence in an attempt to address the question of if and how threat-related attention impacts the development of socioemotional maladjustments, specifically anxiety problems. We also discussed attention assessment approaches that could advance our understanding of these questions.
Normative threat-related attention is early appearing, largely supported by bottom-up neural mechanisms that are functioning in infancy (Leppänen & Nelson, 2009, 2012). Individual differences in infant temperament are associated with variations in threat-related attention processes (Martinos et al., 2012; Nakagawa & Sukigara, 2012; Pérez-Edgar et al., 2017; Ravicz et al., 2015). Early normative threat-related attention serves as an experience-expectant foundation for the development of both attention and down-stream socioemotional behavior through increasing exposure to social experiences (Leppänen & Nelson, 2009).
Longitudinal studies suggest that children with fearful temperament develop social withdrawal and anxiety problems if they also show attention bias to threat, indexed by dot-probe RT scores. Relative to behavioral RT measures, eye-tracking and ERP measures provide a continuous recording of attention processes with superior temporal resolution. Eye-tracking and ERP evidence indicates that pediatric anxiety is associated with altered processing at both early (initial facilitated engagement to threat: e.g. O’Toole et al., 2013; Shechner et al., 2013) and later attention components (sustained attention to threat: e.g. Kujawa et al., 2015; Seefeldt et al., 2014; threat avoidance: e.g. Shechner et al., 2017). Neuroimaging evidence reveals that perturbed frontolimbic activities are linked to anxiety and anxiety vulnerability. Importantly, neural evidence collectively highlights that the interplay between hyperactive automatic threat-related attention processing and less effective, less flexible attention control functioning might be the risk mechanism that binds temperamentally fearful children to the developmental trajectory towards anxiety problems (Henderson et al., 2015; Henderson & Wilson, 2017).
We advocate for the implementation of multiple attention paradigms at multiple levels of analysis (Rodebaugh et al., 2016). Obtaining behavioral, eye-tracking, electrophysiological, and neuroimaging measures of threat-related attention from the same individuals can improve assessment reliability and validity, as well as enhance our ability to delineate fine-grained, multidimensional threat-related attention profiles that convey risks for developing socioemotional problems. Understanding the behavioral and neural signatures of aberrant threat-related attention facilitates early identification of at-risk children and helps us to pinpoint neurocognitive mechanisms that need to be targeted for intervention.
We propose that future research needs to take the assessment of threat-related attention to naturalistic social interactive settings (Redcay & Warnell, 2018; Risko et al., 2012; Schilbach et al., 2013) that afford opportunities for self-directed explorations and social interactions. One strategy to achieve this is to leverage emerging mobile eye-tracking technologies to continuously capture attention processes in real-time as children navigate a variety of environments. Examining the fine-grained interplay between attention and online behavior may enable us to identify more proximal risk markers of anxiety. As novel methodologies are refined from cross-sectional studies, they can then be implemented in longitudinal studies to investigate how threat-related attention bias gives rise to anxiety and other socioemotional maladjustments.
Highlights:
Threat-related attention plays a role in shaping socioemotional development.
Testing potential causal effects of attention relies on methodological advancements.
Attention mechanisms must be measured at multiple levels of analysis.
Mobile eye-tracking offers fine-grained sampling of attention in real-life settings.
Acknowledgement:
This work was supported by a grant from the National Institutes of Health [R21 MH111980] to KPE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abend R., Voogd L. de, Salemink E., Wiers RW., Pérez-Edgar K., Fitzgerald A., … Bar-Haim Y. (2018). Association between attention bias to threat and anxiety symptoms in children and adolescents. Depression and Anxiety, 35(3), 229–238. 10.1002/da.22706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP., Moore C., Kuperminc G., & Bell K. (1998). Attachment and Adolescent Psychosocial Functioning. Child Development, 69(5), 1406–1419. 10.1111/j.1467-8624.1998.tb06220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir I., Zvielli A., & Bernstein A. (2016). (De)coupling of our eyes and our mind’s eye: A dynamic process perspective on attentional bias. Emotion, 16(7), 978–986. 10.1037/emo0000172 [DOI] [PubMed] [Google Scholar]
- Amso D., & Scerif G. (2015). The attentive brain: insights from developmental cognitive neuroscience. Nature Reviews Neuroscience, 16(10), 606–619. 10.1038/nrn4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK., Christoff K., Stappen I., Panitz D., Ghahremani DG., Glover G., … Sobel N. (2003). Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience, 6(2), 196–202. 10.1038/nn1001 [DOI] [PubMed] [Google Scholar]
- Armstrong T., & Olatunji BO. (2012). Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review, 32(8), 704–723. 10.1016/j.cpr.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin RN., & Salapatek P. (1975). Saccadic localization of visual targets by the very young human infant. Perception & Psychophysics, 17(3), 293–302. 10.3758/BF03203214 [DOI] [Google Scholar]
- Auday ES., Taber-Thomas BC., & Pérez-Edgar KE. (2018). Neural correlates of attention bias to masked facial threat cues: Examining children at-risk for social anxiety disorder. NeuroImage: Clinical, 19, 202–212. 10.1016/j.nicl.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M., Kassam KS., Ghuman AS., Boshyan J., Schmid AM., Dale AM., … Halgren E. (2006). Top-down facilitation of visual recognition. Proceedings of the National Academy of Sciences of the United States of America, 103(2), 449–454. 10.1073/pnas.0507062103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y. (2010). Research Review: attention bias modification (ABM): a novel treatment for anxiety disorders. Journal of Child Psychology and Psychiatry, 51(8), 859–870. 10.1111/j.1469-7610.2010.02251.x [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., & Glickman S. (2005). Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition, 59(1), 11–22. 10.1016/j.bandc.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg MJ., & van IJzendoorn MH. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133(1), 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Morag I., & Glickman S. (2011). Training anxious children to disengage attention from threat: a randomized controlled trial. Journal of Child Psychology and Psychiatry, 52(8), 861–869. 10.1111/j.1469-7610.2011.02368.x [DOI] [PubMed] [Google Scholar]
- Beesdo K., Lau JYF., Guyer AE., McClure-Tone EB., Monk CS., Nelson EE., … Pine DS. (2009). Common and Distinct Amygdala-Function Perturbations in Depressed vs Anxious Adolescents. Archives of General Psychiatry, 66(3), 275–285. 10.1001/archgenpsychiatry.2008.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J., Hirshfeld-Becker DR., Rosenbaum JF., Hérot C., Friedman D., Snidman N., … Faraone SV. (2001). Further Evidence of Association Between Behavioral Inhibition and Social Anxiety in Children. American Journal of Psychiatry, 158(10), 1673–1679. 10.1176/appi.ajp.158.10.1673 [DOI] [PubMed] [Google Scholar]
- Birmingham E., & Kingstone A. (2009). Human Social Attention. Annals of the New York Academy of Sciences, 1156(1), 118–140. 10.1111/j.1749-6632.2009.04468.x [DOI] [PubMed] [Google Scholar]
- Bishop SJ. (2008). Neural Mechanisms Underlying Selective Attention to Threat. Annals of the New York Academy of Sciences, 1129(1), 141–152. 10.1196/annals.1417.016 [DOI] [PubMed] [Google Scholar]
- Blackford JU., Avery SN., Shelton RC., & Zald DH. (2009). Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience, 10, 145 10.1186/1471-2202-10-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU., Allen AH., Cowan RL., & Avery SN. (2012). Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience, nsr078 10.1093/scan/nsr078 [DOI] [PMC free article] [PubMed]
- Blackford JU., & Pine DS. (2012). Neural Substrates of Childhood Anxiety Disorders A Review of Neuroimaging Findings. Child and Adolescent Psychiatric Clinics of North America, 21(3), 501–525. 10.1016/j.chc.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ., Pollak SD., Grasso D., Voss J., Mian ND., Zobel E., … Pine DS. (2015). Attention bias and anxiety in young children exposed to family violence. Journal of Child Psychology and Psychiatry, 56(11), 1194–1201. 10.1111/jcpp.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC., Bar-Haim Y., Carver FW., Holroyd T., Norcross MA., Detloff A., … Pine DS. (2012). Isolating neural components of threat bias in pediatric anxiety. Journal of Child Psychology and Psychiatry, 53(6), 678–686. 10.1111/j.1469-7610.2011.02503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC., Bar-Haim Y., Clementi MA., Sankin LS., Chen G., Shechner T., … Pine DS. (2013). Training-associated changes and stability of attention bias in youth: Implications for Attention Bias Modification Treatment for pediatric anxiety. Developmental Cognitive Neuroscience, 4, 52–64. 10.1016/j.dcn.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC., Dellarco DV., & Evans TC. (2017). Reducing Pediatric Anxiety through Training: an Integrative Neurocognitive Approach. Current Behavioral Neuroscience Reports, 4(3), 231–253. 10.1007/s40473-017-0118-5 [DOI] [Google Scholar]
- Britton JC., Suway JG., Clementi MA., Fox NA., Pine DS., & Bar-Haim Y. (2015). Neural changes with attention bias modification for anxiety: a randomized trial. Social Cognitive and Affective Neuroscience, 10(7), 913–920. 10.1093/scan/nsu141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeren S., Muris P., Bouwmeester S., Field AP., & Voerman JS. (2011). Processing biases for emotional faces in 4-to 12-year-old non-clinical children: An exploratory study of developmental patterns and relationships with social anxiety and behavioral inhibition. Journal of Experimental Psychopathology, 2(4), 454–474. [Google Scholar]
- Brown HM., Eley TC., Broeren S., Macleod C., Rinck M., Hadwin JA., & Lester KJ. (2014). Psychometric properties of reaction time based experimental paradigms measuring anxiety-related information-processing biases in children. Journal of Anxiety Disorders, 28(1), 97–107. 10.1016/j.janxdis.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Brown Hannah M., McAdams TA., Lester KJ., Goodman R., Clark DM., & Eley TC. (2013). Attentional threat avoidance and familial risk are independently associated with childhood anxiety disorders. Journal of Child Psychology and Psychiatry, 54(6), 678–685. 10.1111/jcpp.12024 [DOI] [PubMed] [Google Scholar]
- Burris JL., Barry-Anwar RA., & Rivera SM. (2017a). An eye tracking investigation of attentional biases towards affect in young children. Developmental Psychology, 53(8), 1418–1427. 10.1037/dev0000345 [DOI] [PubMed] [Google Scholar]
- Burris JL., Barry-Anwar RA., Sims RN., Hagerman RJ., Tassone F., & Rivera SM. (2017b). Children With Fragile X Syndrome Display Threat-Specific Biases Toward Emotion. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(6), 487–492. 10.1016/j.bpsc.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Buss KA. (2011). Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology, 47(3), 804–819. 10.1037/a0023227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA., & Goldsmith HH. (2000). Manual and normative data for the Laboratory Temperament Assessment Battery - Toddler Version. Madison, Wisconsin: University of Wisconsin. [Google Scholar]
- Caouette JD., & Guyer AE. (2014). Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Developmental Cognitive Neuroscience, 8, 65–76. 10.1016/j.dcn.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K., Macari S., & Shic F. (2012). Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(8). 10.1111/j.1469-7610.2012.02538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NTM., Thomas LM., Clarke PJF., Hickie IB., & Guastella AJ. (2015). Hyperscanning and avoidance in social anxiety disorder: The visual scanpath during public speaking. Psychiatry Research, 225(3), 667–672. 10.1016/j.psychres.2014.11.025 [DOI] [PubMed] [Google Scholar]
- Chevallier C., Parish-Morris J., McVey A., Rump KM., Sasson NJ., Herrington JD., & Schultz RT. (2015). Measuring social attention and motivation in autism spectrum disorder using eye-tracking: Stimulus type matters. Autism Research, 8(5), 620–628. 10.1002/aur.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chita-Tegmark M. (2016). Social attention in ASD: A review and meta-analysis of eye-tracking studies. Research in Developmental Disabilities, 48, 79–93. 10.1016/j.ridd.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Cisler JM., & Koster EHW. (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30(2), 203–216. 10.1016/j.cpr.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CE., Zapp DJ., Fettig NB., & Pérez-Edgar K. (2016). Impact of attention biases to threat and effortful control on individual variations in negative affect and social withdrawal in very young children. Journal of Experimental Child Psychology, 141, 210–221. 10.1016/j.jecp.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. (1998). Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences, 95(3), 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Kincade JM., Ollinger JM., McAvoy MP., & Shulman GL. (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3(3), 292–297. 10.1038/73009 [DOI] [PubMed] [Google Scholar]
- Costello EJ., Egger HL., & Angold A. (2005). The Developmental Epidemiology of Anxiety Disorders: Phenomenology, Prevalence, and Comorbidity. Child and Adolescent Psychiatric Clinics, 14(4), 631–648. 10.1016/j.chc.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Cuevas K., & Bell MA. (2014). Infant Attention and Early Childhood Executive Function. Child Development, 85(2), 397–404. 10.1111/cdev.12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T., Moradi AR., Taghavi MR., Neshat-Doost HT., & Yule W. (2001). An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychological Medicine, 31(03), 541–547. 10.1017/S0033291701003567 [DOI] [PubMed] [Google Scholar]
- Davis M., & Whalen PJ. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. 10.1038/sj.mp.4000812 [DOI] [PubMed] [Google Scholar]
- De Haan M., Belsky J., Reid V., Volein A., & Johnson MH. (2004). Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. Journal of Child Psychology and Psychiatry, 45(7), 1209–1218. 10.1111/j.1469-7610.2004.00320.x [DOI] [PubMed] [Google Scholar]
- De Haan M., Johnson MH., & Halit H. (2003). Development of face-sensitive event-related potentials during infancy: a review. International Journal of Psychophysiology, 51(1), 45–58. 10.1016/S0167-8760(03)00152-1 [DOI] [PubMed] [Google Scholar]
- De Voogd EL., Wiers RW., Prins PJM., & Salemink E. (2014). Visual search attentional bias modification reduced social phobia in adolescents. Journal of Behavior Therapy and Experimental Psychiatry, 45(2), 252–259. 10.1016/j.jbtep.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Degnan KA., Almas AN., Henderson HA., Hane AA., Walker OL., & Fox NA. (2014). Longitudinal trajectories of social reticence with unfamiliar peers across early childhood. Developmental Psychology, 50(10), 2311–2323. 10.1037/a0037751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan KA., & Fox NA. (2007). Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology, 19(03), 729–746. 10.1017/S0954579407000363 [DOI] [PubMed] [Google Scholar]
- Dodd HF., Hudson JL., Williams T., Morris T., Lazarus RS., & Byrow Y. (2014). Anxiety and Attentional Bias in Preschool-Aged Children: An Eyetracking Study. Journal of Abnormal Child Psychology, 43(6), 1055–1065. 10.1007/s10802-014-9962-x [DOI] [PubMed] [Google Scholar]
- Donnelly N., Hadwin JA., Menneer T., & Richards HJ. (2010). The use of visual search paradigms to understand attentional biases in childhood anxiety In Hadwin JA. & Field AP. (Eds.), Information processing biases and anxiety: A developmental perspective (pp. 109–127). John Wiley & Sons Ltd. [Google Scholar]
- Dudeney J., Sharpe L., & Hunt C. (2015). Attentional bias towards threatening stimuli in children with anxiety: A meta-analysis. Clinical Psychology Review, 40, 66–75. 10.1016/j.cpr.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Egger HL., & Angold A. (2006). Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. Journal of Child Psychology and Psychiatry, 47(3–4), 313–337. 10.1111/j.1469-7610.2006.01618.x [DOI] [PubMed] [Google Scholar]
- Eldar S., Apter A., Lotan D., Edgar KP., Naim R., Fox NA., … Bar-Haim Y. (2012). Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. The American Journal of Psychiatry, 169(2), 213–220. 10.1176/appi.ajp.2011.11060886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW., Derakshan N., Santos R., & Calvo MG. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336–353. 10.1037/1528-3542.7.2.336 [DOI] [PubMed] [Google Scholar]
- Fair DA., Cohen AL., Power JD., Dosenbach NUF., Church JA., Miezin FM., … Petersen SE. (2009). Functional Brain Networks Develop from a “Local to Distributed” Organization. PLOS Computational Biology, 5(5), e1000381 10.1371/journal.pcbi.1000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., McCandliss BD., Sommer T., Raz A., & Posner MI. (2002). Testing the Efficiency and Independence of Attentional Networks. Journal of Cognitive Neuroscience, 14(3), 340–347. 10.1162/089892902317361886 [DOI] [PubMed] [Google Scholar]
- Farroni T., Johnson MH., & Csibra G. (2004). Mechanisms of Eye Gaze Perception during Infancy. Journal of Cognitive Neuroscience, 16(8), 1320–1326. 10.1162/0898929042304787 [DOI] [PubMed] [Google Scholar]
- Field AP., & Lester KJ. (2010). Is There Room for ‘Development’ in Developmental Models of Information Processing Biases to Threat in Children and Adolescents? Clinical Child and Family Psychology Review, 13(4), 315–332. 10.1007/s10567-010-0078-8 [DOI] [PubMed] [Google Scholar]
- Foulsham T., Walker E., & Kingstone A. (2011). The where, what and when of gaze allocation in the lab and the natural environment. Vision Research, 51(17), 1920–1931. 10.1016/j.visres.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Fox NA., Henderson HA., Rubin KH., Calkins SD., & Schmidt LA. (2001). Continuity and Discontinuity of Behavioral Inhibition and Exuberance: Psychophysiological and Behavioral Influences across the First Four Years of Life. Child Development, 72(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Fox NA., Rubin KH., Calkins SD., Marshall TR., Coplan RJ., Porges S., W, … Stewart S. (1995). Frontal Activation Asymmetry and Social Competence at Four Years of Age. Child Development, 66(6), 1770–1784. 10.1111/1467-8624.ep9601152101 [DOI] [PubMed] [Google Scholar]
- Franchak JM. (2017). Using head-mounted eye tracking to study development In Hopkins B., Geangu E., & Linkenauger S. (Eds.), The Cambridge Encyclopedia of Child Development (Second). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Franchak JM., & Adolph KE. (2010). Visually guided navigation: Head-mounted eye-tracking of natural locomotion in children and adults. Vision Research, 50(24), 2766–2774. 10.1016/j.visres.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchak JM., Kretch KS., & Adolph KE. (2017). See and be seen: Infant–caregiver social looking during locomotor free play. Developmental Science, (0), e12626 10.1111/desc.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchak JM., Kretch KS., Soska KC., & Adolph KE. (2011). Head-mounted eye-tracking: A new method to describe infant looking. Child Development, 82(6), 1738–1750. 10.1111/j.1467-8624.2011.01670.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeth M., Foulsham T., & Kingstone A. (2013). What Affects Social Attention? Social Presence, Eye Contact and Autistic Traits. PLoS ONE, 8(1), e53286 10.1371/journal.pone.0053286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich FJ., Egly R., Rafal RD., & Beck D. (1998). Spatial attention deficits in humans: A comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology, 12(2), 193–207. 10.1037/0894-4105.12.2.193 [DOI] [PubMed] [Google Scholar]
- Fu X., Taber-Thomas BC., & Pérez-Edgar K. (2017). Frontolimbic functioning during threat-related attention: Relations to early behavioral inhibition and anxiety in children. Biological Psychology, 98–109. 10.1016/j.biopsycho.2015.08.010 [DOI] [PMC free article] [PubMed]
- Gamble AL., & Rapee RM. (2009). The time-course of attentional bias in anxious children and adolescents. Journal of Anxiety Disorders, 23(7), 841–847. 10.1016/j.janxdis.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello KS., Smith JK., Shen D., Gilmore JH., & Lin W. (2009). Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences, 106(16), 6790–6795. 10.1073/pnas.0811221106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Coll C., Kagan J., & Reznick JS. (1984). Behavioral Inhibition in Young Children. Child Development, 55(3), 1005–1019. 10.2307/1130152 [DOI] [Google Scholar]
- Gibb BE., McGeary JE., & Beevers CG. (2015). Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, n/a-n/a. 10.1002/ajmg.b.32383 [DOI] [PMC free article] [PubMed]
- Goldsmith HH., Reilly HH., Lemery KS., Longley S., & Prescott A. (1994). Manual for the Preschool Laboratory Temperament Assessment Battery (Lab-TAB). University of Wisconsin, Madison, Unpublished manuscript. [Google Scholar]
- Guyer AE., Masten CL., & Pine DS. (2013). Neurobiology of pediatric anxiety disorders In Vasa RA. & Roy AK. (Eds.), Pediatric Anxiety Disorders: A Clinical Guide. (pp. 23–46). Springer; New York. [Google Scholar]
- Guyer AE., Pérez-Edgar K., & Crone EA. (2018). Opportunities for Neurodevelopmental Plasticity From Infancy Through Early Adulthood. Child Development, 89(3), 687–697. 10.1111/cdev.13073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwin JA., & Field AP. (Eds.). (2010). Information processing biases and anxiety: A developmental perspective. John Wiley & Sons Ltd. [Google Scholar]
- Hajcak G., MacNamara A., & Foti. (2012). ERPs and the study of emotion In Luck SJ. & Kappenman ES. (Eds.), The Oxford handbook of event-related potential components (pp. 441–474). New York, NY: Oxford University Press. [Google Scholar]
- Hane AA., Fox NA., Henderson HA., & Marshall PJ. (2008). Behavioral Reactivity and Approach-Withdrawal Bias in Infancy. Developmental Psychology, 44(5), 1491–1496. 10.1037/a0012855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL., Gibb BE., Abela JRZ., & Flory K. (2010). Selective attention to affective stimuli and clinical depression among youths: Role of anxiety and specificity of emotion. Journal of Abnormal Psychology, 119(3), 491–501. 10.1037/a0019609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE., Benson BE., Bar-Haim Y., Mogg K., Bradley BP., Chen G., … Pérez-Edgar K. (2013). Patterns of Neural Connectivity During an Attention Bias Task Moderate Associations Between Early Childhood Temperament and Internalizing Symptoms in Young Adulthood. Biological Psychiatry, 74(4), 273–279. 10.1016/j.biopsych.2013.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman C., Rothbart MK., & Posner MI. (1997). Distress and attention interactions in early infancy. Motivation and Emotion, 21(1), 27–43. [Google Scholar]
- Heck A., Hock A., White H., Jubran R., & Bhatt RS. (2016). The development of attention to dynamic facial emotions. Journal of Experimental Child Psychology, 147, 100–110. 10.1016/j.jecp.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck A., Hock A., White H., Jubran R., & Bhatt RS. (2017). Further evidence of early development of attention to dynamic facial emotions: Reply to Grossmann and Jessen. Journal of Experimental Child Psychology, 153, 155–162. 10.1016/j.jecp.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA., Pine DS., & Fox NA. (2015). Behavioral Inhibition and Developmental Risk: A Dual-Processing Perspective. Neuropsychopharmacology, 40(1), 207–224. 10.1038/npp.2014.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA., & Wilson MJG. (2017). Attention Processes Underlying Risk and Resilience in Behaviorally Inhibited Children. Current Behavioral Neuroscience Reports, 4(2), 99–106. 10.1007/s40473-017-0111-z [DOI] [Google Scholar]
- Hilt LM., Leitzke BT., & Pollak SD. (2017). Can’t Take My Eyes Off of You: Eye Tracking Reveals How Ruminating Young Adolescents Get Stuck. Journal of Clinical Child & Adolescent Psychology, 46(6), 858–867. 10.1080/15374416.2015.1121824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijding J., Mayer B., Koster EHW., & Muris P. (2011). To look or not to look: An eye movement study of hypervigilance during change detection in high and low spider fearful students. Emotion, 11(3), 666–674. 10.1037/a0022996 [DOI] [PubMed] [Google Scholar]
- Hummel AC., Premo JE., & Kiel EJ. (2017). Attention to Threat as a Predictor of Shyness in the Context of Internalizing and Externalizing Behavior. Infancy, 22(2), 240–255. 10.1111/infa.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoviello BM., Wu G., Abend R., Murrough JW., Feder A., Fruchter E., … Charney DS. (2014). Attention Bias Variability and Symptoms of Posttraumatic Stress Disorder. Journal of Traumatic Stress, 27(2), 232–239. 10.1002/jts.21899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- In-Albon T., Kossowsky J., & Schneider S. (2009). Vigilance and Avoidance of Threat in the Eye Movements of Children with Separation Anxiety Disorder. Journal of Abnormal Child Psychology, 38(2), 225–235. 10.1007/s10802-009-9359-4 [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM. (2012). Mood Regulation in Real-Time: Age Differences in the Role of Looking. Current Directions in Psychological Science, 21(4), 237–242. 10.1177/0963721412448651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM., Livingstone KM., Harris JA., & Marcotte SL. (2015). Mobile eye tracking reveals little evidence for age differences in attentional selection for mood regulation. Emotion, 15(2), 151–161. 10.1037/emo0000037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen S., & Grossmann T. (2015). Neural signatures of conscious and unconscious emotional face processing in human infants. Cortex, 64, 260–270. 10.1016/j.cortex.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Kagan J., Reznick JS., & Snidman N. (1988). Biological bases of childhood shyness. Science, 240(4849), 167–171. 10.1126/science.3353713 [DOI] [PubMed] [Google Scholar]
- Kagan Jerome. (2012). The Biography of Behavioral Inhibition In Zentner M. & Shiner RL. (Eds.), The Handbook of Temperament (pp. 69–82). In M. Zentner & R. Shiner (Eds.): New York, NY: Guilford Press. [Google Scholar]
- Kagan Jerome, Reznick JS., Clarke C., Snidman N., & Garcia-Coll C. (1984). Behavioral Inhibition to the Unfamiliar. Child Development, 55(6), 2212–2225. 10.2307/1129793 [DOI] [Google Scholar]
- Kappenman ES., Farrens JL., Luck SJ., & Proudfit GH. (2014a). Behavioral and ERP measures of attentional bias to threat in the dot-probe task: poor reliability and lack of correlation with anxiety. Personality and Social Psychology, 5, 1368 10.3389/fpsyg.2014.01368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES., MacNamara A., & Proudfit GH. (2014b). Electrocortical evidence for rapid allocation of attention to threat in the dot-probe task. Social Cognitive and Affective Neuroscience, nsu098 10.1093/scan/nsu098 [DOI] [PMC free article] [PubMed]
- Kiel EJ., & Buss KA. (2011). Toddlers’ Duration of Attention towards Putative Threat. Infancy : The Official Journal of the International Society on Infant Studies, 16(2), 198–210. 10.1111/j.1532-7078.2010.00036.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A., Jones W., Schultz R., & Volkmar F. (2003). The enactive mind, or from actions to cognition: lessons from autism. Philosophical Transactions of the Royal Society B: Biological Sciences, 358(1430), 345–360. 10.1098/rstb.2002.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsoni E., Haan M. de, & Johnson MH. (2001). Categorical Perception of Facial Expressions by 7-Month-Old Infants. Perception, 30(9), 1115–1125. 10.1068/p3155 [DOI] [PubMed] [Google Scholar]
- Kretch KS., & Adolph KE. (2015). Active vision in passive locomotion: real-world free viewing in infants and adults. Developmental Science, 18(5), 736–750. 10.1111/desc.12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretch KS., Franchak JM., & Adolph KE. (2014). Crawling and Walking Infants See the World Differently. Child Development, 85(4), 1503–1518. 10.1111/cdev.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijt A-W., Field AP., & Fox E. (2016). Capturing Dynamics of Biased Attention: Are New Attention Variability Measures the Way Forward? PLOS ONE, 11(11), e0166600 10.1371/journal.pone.0166600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa AJ., Torpey D., Kim J., Hajcak G., Rose S., Gotlib IH., & Klein DN. (2011). Attentional Biases for Emotional Faces in Young Children of Mothers with Chronic or Recurrent Depression. Journal of Abnormal Child Psychology, 39(1), 125–135. 10.1007/s10802-010-9438-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A., Klein DN., & Hajcak G. (2012). Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience, 2(4), 458–467. 10.1016/j.dcn.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A., MacNamara A., Fitzgerald KD., Monk CS., & Phan KL. (2015). Enhanced Neural Reactivity to Threatening Faces in Anxious Youth: Evidence from Event-Related Potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. 10.1007/s10802-015-0029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw KEW., Foulsham T., Kuhn G., & Kingstone A. (2011). Potential social interactions are important to social attention. Proceedings of the National Academy of Sciences, 108(14), 5548–5553. 10.1073/pnas.1017022108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamey A., Hollenstein T., Lewis MD., & Granic I. (2004). GridWare (Version 1.1). [Computer software]. Retrieved from http://www.statespacegrids.org
- Lange WGT., Tierney KJ., Reinhardt-Rutland AH., & Vivekananda-Schmidt P. (2004). Viewing behaviour of spider phobics and non-phobics in the presence of threat and safety stimuli. British Journal of Clinical Psychology, 43(3), 235–243. 10.1348/0144665031752989 [DOI] [PubMed] [Google Scholar]
- LeDoux JE., & Pine DS. (2016). Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. American Journal of Psychiatry, 173(11), 1083–1093. 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Leppänen JM., Cataldo JK., Enlow MB., & Nelson CA. (2018). Early development of attention to threat-related facial expressions. PLOS ONE, 13(5), e0197424 10.1371/journal.pone.0197424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM., Moulson MC., Vogel-Farley VK., & Nelson CA. (2007). An ERP Study of Emotional Face Processing in the Adult and Infant Brain. Child Development, 78(1), 232–245. 10.1111/j.1467-8624.2007.00994.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM., & Nelson CA. (2009). Tuning the developing brain to social signals of emotions. Nature Reviews Neuroscience, 10(1), 37–47. 10.1038/nrn2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM., & Nelson CA. (2012). Early Development of Fear Processing. Current Directions in Psychological Science, 21(3), 200–204. 10.1177/0963721411435841 [DOI] [Google Scholar]
- Leutgeb V., Schäfer A., Köchel A., Scharmüller W., & Schienle A. (2010). Psychophysiology of spider phobia in 8- to 12-year-old girls. Biological Psychology, 85(3), 424–431. 10.1016/j.biopsycho.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Lewis MD., Lamey AV., & Douglas L. (1999). A new dynamic systems method for the analysis of early socioemotional development. Developmental Science, 2(4), 457–475. 10.1111/1467-7687.00090 [DOI] [Google Scholar]
- Liu P., Taber-Thomas BC., Fu X., & Pérez-Edgar KE. (2018). Biobehavioral Markers of Attention Bias Modification in Temperamental Risk for Anxiety: A Randomized Control Trial. Journal of the American Academy of Child & Adolescent Psychiatry, 57(2), 103–110. 10.1016/j.jaac.2017.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue V. (2014). Measuring Attentional Biases for Threat in Children and Adults. Journal of Visualized Experiments, (92). 10.3791/52190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue V., Buss KA., Taber-Thomas BC., & Pérez-Edgar K. (2017). Developmental Differences in Infants’ Attention to Social and Nonsocial Threats. Infancy, 22(3), 403–415. 10.1111/infa.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue V., & DeLoache JS. (2010). Superior detection of threat-relevant stimuli in infancy. Developmental Science, 13(1), 221–228. 10.1111/j.1467-7687.2009.00872.x [DOI] [PubMed] [Google Scholar]
- LoBue V., & Pérez-Edgar K. (2014). Sensitivity to social and non-social threats in temperamentally shy children at-risk for anxiety. Developmental Science, 17(2), 239–247. 10.1111/desc.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinos M., Matheson A., & de Haan M. (2012). Links between infant temperament and neurophysiological measures of attention to happy and fearful faces. Journal of Child Psychology and Psychiatry, 53(11), 1118–1127. 10.1111/j.1469-7610.2012.02599.x [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, & et al. (2007). Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry, 64(1), 97–106. 10.1001/archpsyc.64.1.97 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA., Zeanah CH., Fox NA., & Nelson CA. (2012). Attachment security as a mechanism linking foster care placement to improved mental health outcomes in previously institutionalized children. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(1), 46–55. 10.1111/j.1469-7610.2011.02437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Matsuoka S., Dan I., Naoi N., Nakamura K., & Kojima S. (2009). Prefrontal Activation Associated with Social Attachment: Facial-Emotion Recognition in Mothers and Infants. Cerebral Cortex, 19(2), 284–292. 10.1093/cercor/bhn081 [DOI] [PubMed] [Google Scholar]
- Mogg K., & Bradley BP. (1998). A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy, 36(9), 809–848. 10.1016/S0005-7967(98)00063-1 [DOI] [PubMed] [Google Scholar]
- Mogg K., Holmes A., Garner M., & Bradley BP. (2008). Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behaviour Research and Therapy, 46(5), 656–667. 10.1016/j.brat.2008.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS. (2008). The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology, 20(4), 1231–1250. 10.1017/S095457940800059X [DOI] [PubMed] [Google Scholar]
- Monk CS., Nelson EE., McClure EB., Mogg K., Bradley BP., Leibenluft E., … Pine DS. (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. The American Journal of Psychiatry, 163(6), 1091–1097. 10.1176/ajp.2006.163.6.1091 [DOI] [PubMed] [Google Scholar]
- Monk CS., Telzer EH., Mogg K., Bradley BP., Mai X., Louro HMC., … Pine DS. (2008). Amygdala and Ventrolateral Prefrontal Cortex Activation to Masked Angry Faces in Children and Adolescents With Generalized Anxiety Disorder. Archives of General Psychiatry, 65(5), 568–576. 10.1001/archpsyc.65.5.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S., Brown KM., Taber-Thomas BC., LoBue V., Buss KA., & Pérez-Edgar KE. (2017a). Maternal anxiety predicts attentional bias towards threat in infancy. Emotion (Washington, D.C.), 17(5), 874–883. 10.1037/emo0000275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S., Fu X., & Pérez-Edgar KE. (2016). A developmental neuroscience perspective on affect-biased attention. Developmental Cognitive Neuroscience, 21, 26–41. 10.1016/j.dcn.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S., Pérez-Edgar KE., & Buss KA. (2014). Attention Biases Towards and Away from Threat Mark the Relation between Early Dysregulated Fear and the Later Emergence of Social Withdrawal. Journal of Abnormal Child Psychology, 43(6), 1067–1078. 10.1007/s10802-014-9963-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S., Taber-Thomas BC., & Pérez-Edgar KE. (2017b). Patterns of attention to threat across tasks in behaviorally inhibited children at risk for anxiety. Developmental Science, 20(2), n/a-n/a. 10.1111/desc.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim R., Abend R., Wald I., Eldar S., Levi O., Fruchter E., … Bar-Haim Y. (2015). Threat-Related Attention Bias Variability and Posttraumatic Stress. American Journal of Psychiatry, 172(12), 1242–1250. 10.1176/appi.ajp.2015.14121579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A., & Sukigara M. (2012). Difficulty in disengaging from threat and temperamental negative affectivity in early life: A longitudinal study of infants aged 12–36 months. Behavioral & Brain Functions, 8(1), 40–47. 10.1186/1744-9081-8-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiopoulos E., Risko EF., & Kingstone A. (2015). Social attention, social presence, and the dual function of gaze In Puce A. & Bertenthal BI. (Eds.), The many faces of social attention: Behavioral and neural measures (pp. 129–155). New York, NY: Springer. [Google Scholar]
- Nelson CA. (1994). Neural correlates of recognition memory in the first postnatal year of life In Dawson G. & Fischer K. (Eds.), Human behavior and the developing brain (pp. 269–313). New York: Guilford Press. [Google Scholar]
- Nelson CA. (1996). Electrophysiological correlates of memory development in the first year of life In Reese H. & Franzen M. (Eds.), Biological and neuropsychological mechanisms: Life span developmental psychology (pp. 95–131). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Nelson CA., & De Haan M. (1996). Neural correlates of infants’ visual responsiveness to facial expressions of emotion. Developmental Psychobiology, 29(7), 577–595. [DOI] [PubMed] [Google Scholar]
- Nelson CA., & Dolgin KG. (1985). The Generalized Discrimination of Facial Expressions by Seven-Month-Old Infants. Child Development, 56(1), 58–61. 10.2307/1130173 [DOI] [PubMed] [Google Scholar]
- Nozadi S., Troller-Renfree S., White LK., Frenkel T., Degnan KA., Bar-Haim Y., … Fox NA. (2016). The Moderating Role of Attention Biases to Threat on the Link between Behavioral Inhibition and Anxiety in Children. Journal of Experimental Psychopathology, 7(3), 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A., Flykt A., & Esteves F. (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130(3), 466–478. 10.1037/0096-3445.130.3.466 [DOI] [PubMed] [Google Scholar]
- Öhman A., Lundqvist D., & Esteves F. (2001). The face in the crowd revisited: A threat advantage with schematic stimuli. Journal of Personality and Social Psychology, 80(3), 381–396. 10.1037/0022-3514.80.3.381 [DOI] [PubMed] [Google Scholar]
- Ollendick TH., White SW., Richey J., Kim-Spoon J., Ryan SM., Wieckowski AT., … Smith M. (2018). Attention Bias Modification Treatment for Adolescents With Social Anxiety Disorder. Behavior Therapy. 10.1016/j.beth.2018.04.002 [DOI] [PMC free article] [PubMed]
- O’Toole LJ., DeCicco JM., Berthod S., & Dennis TA. (2013). The N170 to Angry Faces Predicts Anxiety in Typically Developing Children Over a Two-Year Period. Developmental Neuropsychology, 38(5), 352–363. 10.1080/87565641.2013.802321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola MJ., Forssman L., Puura K., van IJzendoorn MH., & Leppänen JM. (2015). Attention to Faces Expressing Negative Emotion at 7 Months Predicts Attachment Security at 14 Months. Child Development, 86(5), 1321–1332. 10.1111/cdev.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola MJ., Hietanen JK., Forssman L., & Leppänen JM. (2013). The Emergence and Stability of the Attentional Bias to Fearful Faces in Infancy. Infancy, 18(6), 905–926. 10.1111/infa.12013 [DOI] [Google Scholar]
- Peltola MJ., Leppänen JM., Mäki S., & Hietanen JK. (2009a). Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Social Cognitive and Affective Neuroscience, nsn046 10.1093/scan/nsn046 [DOI] [PMC free article] [PubMed]
- Peltola MJ., Leppänen JM., Palokangas T., & Hietanen JK. (2008). Fearful faces modulate looking duration and attention disengagement in 7-month-old infants. Developmental Science, 11(1), 60–68. 10.1111/j.1467-7687.2007.00659.x [DOI] [PubMed] [Google Scholar]
- Peltola MJ., Leppänen JM., Vogel-Farley VK., Hietanen JK., & Nelson CA. (2009b). Fearful Faces But Not Fearful Eyes Alone Delay Attention Disengagement in 7-Month-Old Infants. Emotion (Washington, D.C.), 9(4), 560–565. 10.1037/a0015806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola MJ., Yrttiaho S., & Leppänen JM. (2018). Infants’ attention bias to faces as an early marker of social development. Developmental Science, 0(0), e12687 10.1111/desc.12687 [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K. (2015). Effortful control in adolescence: Individual differences within a unique developmental window In Oettingen G. & Gollwitzer P. (Eds.), Self-Regulation in Adolescence (pp. 78–102). Cambridge: Cambridge University Press. [Google Scholar]
- Pérez-Edgar K., & Bar-Haim Y. (2010). Application of cognitiveneuroscience techniques to the study of anxiety-related processing biases in children In Hadwin JA. & Field AP. (Eds.), Information Processing Biases and Anxiety: A Developmental Perspective (pp. 183–206). John Wiley & Sons Inc. [Google Scholar]
- Pérez-Edgar K., Bar-Haim Y., McDermott JM., Chronis-Tuscano A., Pine DS., & Fox NA. (2010). Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion (Washington, D.C.), 10(3), 349–357. 10.1037/a0018486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar KE., & Guyer AE. (2014). Behavioral Inhibition: Temperament or Prodrome? Current Behavioral Neuroscience Reports, 1(3), 182–190. 10.1007/s40473-014-0019-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., & Hastings PD. (2018). Emotion Development from an Experimental and Individual Difference Lens In Wixted JT. (Ed.), The Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience (Fouth, Vol. 4, pp. 289–322). [Google Scholar]
- Pérez-Edgar K., Martin McDermott JN., Korelitz K., Degnan KA., Curby TW., Pine DS., & Fox NA. (2010). Patterns of Sustained Attention in Infancy Shape the Developmental Trajectory of Social Behavior From Toddlerhood Through Adolescence. Developmental Psychology, 46(6), 1723–1730. 10.1037/a0021064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Morales S., LoBue V., Taber-Thomas BC., Allen EK., Brown KM., & Buss KA. (2017). The impact of negative affect on attention patterns to threat across the first 2 years of life. Developmental Psychology, 53(12), 2219–2232. http://dx.doi.org.ezaccess.libraries.psu.edu/10.1037/dev0000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Reeb-Sutherland BC., McDermott JM., White LK., Henderson HA., Degnan KA., … Fox NA. (2011). Attention Biases to Threat Link Behavioral Inhibition to Social Withdrawal over Time in Very Young Children. Journal of Abnormal Child Psychology, 39(6), 885–895. 10.1007/s10802-011-9495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Roberson-Nay R., Hardin MG., Poeth K., Guyer AE., Nelson EE., … Ernst M. (2007). Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage, 35(4), 1538–1546. 10.1016/j.neuroimage.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Taber-Thomas B., Auday E., & Morales S. (2014). Temperament and Attention as Core Mechanisms in the Early Emergence of Anxiety In Lagattuta KH. (Ed.), Contributions to Human Development (Vol. 26, pp. 42–56). Basel: S. KARGER AG; Retrieved from http://www.karger.com?doi=10.1159/000354350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergamin-Hight L., Pine DS., Fox NA., & Bar-Haim Y. (2016). Attention bias modification for youth with social anxiety disorder. Journal of Child Psychology and Psychiatry, 57(11), 1317–1325. 10.1111/jcpp.12599 [DOI] [PubMed] [Google Scholar]
- Petersen SE., & Posner MI. (2012). The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience, 35, 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. (2007). Research Review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry, 48(7), 631–648. 10.1111/j.1469-7610.2007.01751.x [DOI] [PubMed] [Google Scholar]
- Pine DS., Mogg K., Bradley BP., Montgomery L., Monk CS., McClure E., … Kaufman J. (2005). Attention Bias to Threat in Maltreated Children: Implications for Vulnerability to Stress-Related Psychopathology. American Journal of Psychiatry, 162(2), 291–296. 10.1176/appi.ajp.162.2.291 [DOI] [PubMed] [Google Scholar]
- Posner MI., & Rothbart MK. (1998). Attention, self–regulation and consciousness. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 353(1377), 1915–1927. 10.1098/rstb.1998.0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner Michael I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32(1), 3–25. [DOI] [PubMed] [Google Scholar]
- Posner Michael I. (2012). Attention in a social world. New York: Oxford University Press. [Google Scholar]
- Posner Michael I., & Petersen SE. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42. 10.1146/annurev.ne.13.030190.000325 [DOI] [PubMed] [Google Scholar]
- Posner Michael I., Rothbart MK., Sheese BE., & Voelker P. (2012). Control networks and neuromodulators of early development. Developmental Psychology, 48(3), 827–835. 10.1037/a0025530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner Michael I., Rothbart MK., Sheese BE., & Voelker P. (2014). Developing Attention: Behavioral and Brain Mechanisms. Advances in Neuroscience, 2014, e405094 10.1155/2014/405094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB., Allen KB., Silk JS., Ladouceur CD., Ryan ND., Dahl RE., … Siegle GJ. (2016a). Vigilance in the laboratory predicts avoidance in the real world: A dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Developmental Cognitive Neuroscience, 19, 128–136. 10.1016/j.dcn.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB., Kuckertz JM., Siegle GJ., Ladouceur CD., Silk JS., Ryan ND., … Amir N. (2015). Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment, 27(2), 365–376. 10.1037/pas0000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB., Rosen D., Siegle GJ., Ladouceur CD., Tang K., Allen KB., … Silk JS. (2016b). From anxious youth to depressed adolescents: Prospective prediction of 2-year depression symptoms via attentional bias measures. Journal of Abnormal Psychology, 125(2), 267–278. 10.1037/abn0000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB., Siegle GJ., Silk JS., Ladouceur CD., McFarland A., Dahl RE., & Ryan ND. (2014). Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depression and Anxiety, 31(3), 178–187. 10.1002/da.22255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB., Siegle GJ., Silk JS., Ladouceur C., McFarland A., Dahl RE., & Ryan ND. (2013). Sustained Neural Alterations in Anxious Youth Performing an Attentional Bias Task: A Pupilometry Study. Depression and Anxiety, 30(1), 22–30. 10.1002/da.21966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliafico AC., & Kendall PC. (2006). Threat-Related Attentional Bias in Anxious Youth: A Review. Clinical Child and Family Psychology Review, 9(3–4), 162–180. 10.1007/s10567-006-0009-x [DOI] [PubMed] [Google Scholar]
- Ratcliff R. (1978). A theory of memory retrieval. Psychological Review, 85(2), 59–108. 10.1037/0033-295X.85.2.59 [DOI] [Google Scholar]
- Ratcliff R., & Tuerlinckx F. (2002). Estimating parameters of the diffusion model: Approaches to dealing with contaminant reaction times and parameter variability. Psychonomic Bulletin & Review, 9(3), 438–481. 10.3758/BF03196302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz MM., Perdue KL., Westerlund A., Vanderwert RE., & Nelson CA. (2015). Infants’ neural responses to facial emotion in the prefrontal cortex are correlated with temperament: a functional near-infrared spectroscopy study. Frontiers in Psychology, 6 10.3389/fpsyg.2015.00922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., & Buhle J. (2006). Typologies of attentional networks. Nature Reviews Neuroscience, 7(5), 367–379. 10.1038/nrn1903 [DOI] [PubMed] [Google Scholar]
- Redcay E., Dodell-Feder D., Pearrow MJ., Mavros PL., Kleiner M., Gabrieli JDE., & Saxe R. (2010). Live face-to-face interaction during fMRI: A new tool for social cognitive neuroscience. NeuroImage, 50(4), 1639–1647. 10.1016/j.neuroimage.2010.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., & Warnell KR. (2018). Chapter One - A Social-Interactive Neuroscience Approach to Understanding the Developing Brain In Benson JB. (Ed.), Advances in Child Development and Behavior (Vol. 54, pp. 1–44). JAI; 10.1016/bs.acdb.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Rice K., Moraczewski D., & Redcay E. (2016). Perceived live interaction modulates the developing social brain. Social Cognitive and Affective Neuroscience, 11(9), 1354–1362. 10.1093/scan/nsw060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K., & Redcay E. (2016). Interaction matters: A perceived social partner alters the neural processing of human speech. NeuroImage, 129, 480–488. 10.1016/j.neuroimage.2015.11.041 [DOI] [PubMed] [Google Scholar]
- Richards HJ., Benson V., Donnelly N., & Hadwin JA. (2014). Exploring the function of selective attention and hypervigilance for threat in anxiety. Clinical Psychology Review, 34(1), 1–13. 10.1016/j.cpr.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Risko EF., Laidlaw KEW., Freeth M., Foulsham T., & Kingstone A. (2012). Social attention with real versus reel stimuli: toward an empirical approach to concerns about ecological validity. Frontiers in Human Neuroscience, 6 10.3389/fnhum.2012.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risko EF., Richardson DC., & Kingstone A. (2016). Breaking the Fourth Wall of Cognitive Science: Real-World Social Attention and the Dual Function of Gaze. Current Directions in Psychological Science, 25(1), 70–74. 10.1177/0963721415617806 [DOI] [Google Scholar]
- Rodebaugh TL., Scullin RB., Langer JK., Dixon DJ., Huppert JD., Bernstein A., … Lenze EJ. (2016). Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. Journal of Abnormal Psychology, 125(6), 840–851. 10.1037/abn0000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol M., Campanella S., Bissot C., & Philippot P. (2013). Fear of negative evaluation and attentional bias for facial expressions: An event-related study. Brain and Cognition, 82(3), 344–352. 10.1016/j.bandc.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Rothbart MK., & Derryberry D. (1981). Development of individual differences in temperament In Lamb ME. & Brown AL. (Eds.), Advances in Developmental Psychology (Vol. 1, pp. 37–86). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Rothbart MK., Sheese BE., Rueda MR., & Posner MI. (2011). Developing Mechanisms of Self-Regulation in Early Life. Emotion Review, 3(2), 207–213. 10.1177/1754073910387943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK., Dennis TA., & Warner CM. (2015). A Critical Review of Attentional Threat Bias and Its Role in the Treatment of Pediatric Anxiety Disorders. Journal of Cognitive Psychotherapy, 29(3), 171–184. 10.1891/0889-8391.29.3.171 [DOI] [PubMed] [Google Scholar]
- Roy AK., Vasa RA., Bruck M., Mogg K., Bradley BP., Sweeney M., … Pine DS. (2008). Attention Bias Toward Threat in Pediatric Anxiety Disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 47(10), 1189–1196. 10.1097/CHI.0b013e3181825ace [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR. (2012). Effortful control In Zentner M. & Shiner RL. (Eds.), The handbook of temperament (pp. 145–167). New York, NY: Guilford Press. [Google Scholar]
- Schilbach L. (2014). On the relationship of online and offline social cognition. Frontiers in Human Neuroscience, 8 10.3389/fnhum.2014.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Timmermans B., Reddy V., Costall A., Bente G., Schlicht T., & Vogeley K. (2013). Toward a second-person neuroscience. Behavioral and Brain Sciences, 36(04), 393–414. 10.1017/S0140525X12000660 [DOI] [PubMed] [Google Scholar]
- Schmukle SC. (2005). Unreliability of the dot probe task. European Journal of Personality, 19(7), 595–605. 10.1002/per.554 [DOI] [Google Scholar]
- Schwartz CE., Kunwar PS., Greve DN., Kagan J., Snidman NC., & Bloch RB. (2012). A phenotype of early infancy predicts reactivity of the amygdala in male adults. Molecular Psychiatry, 17(10), 1042–1050. 10.1038/mp.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Carl E., Wright CI., Shin LM., Kagan J., & Rauch SL. (2003). Inhibited and Uninhibited Infants “Grown Up”: Adult Amygdalar Response to Novelty. Science, 300(5627), 1952–1953. 10.1126/science.1083703 [DOI] [PubMed] [Google Scholar]
- Seefeldt WL., Krämer M., Tuschen-Caffier B., & Heinrichs N. (2014). Hypervigilance and avoidance in visual attention in children with social phobia. Journal of Behavior Therapy and Experimental Psychiatry, 45(1), 105–112. 10.1016/j.jbtep.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Shackman AJ., Stockbridge MD., Tillman RM., Kaplan CM., Tromp DPM., Fox AS., & Gamer M. (2016). The neurobiology of dispositional negativity and attentional biases to threat: Implications for understanding anxiety disorders in adults and youth. Journal of Experimental Psychopathology, 7(3), 311–342. 10.5127/jep.054015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Britton JC., Pérez-Edgar K., Bar-Haim Y., Ernst M., Fox NA., … Pine DS. (2012). Attention biases, anxiety, and development: toward or away from threats or rewards? Depression & Anxiety (1091–4269), 29(4), 282–294. 10.1002/da.20914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Jarcho JM., Britton JC., Leibenluft E., Pine DS., & Nelson EE. (2013). Attention Bias of Anxious Youth During Extended Exposure of Emotional Face Pairs: An Eye-Tracking Study. Depression and Anxiety, 30(1), 14–21. 10.1002/da.21986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Jarcho JM., Wong S., Leibenluft E., Pine DS., & Nelson EE. (2017). Threats, rewards, and attention deployment in anxious youth and adults: An eye tracking study. Biological Psychology, 122(Supplement C), 121–129. 10.1016/j.biopsycho.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Rimon-Chakir A., Britton JC., Lotan D., Apter A., Bliese PD., … Bar-Haim Y. (2014). Attention bias modification treatment augmenting effects on cognitive behavioral therapy in children with anxiety: randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry, 53(1), 61–71. 10.1016/j.jaac.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM., & Liberzon I. (2011). The Neurocircuitry of Fear, Stress, and Anxiety Disorders. FOCUS, 9(3), 311–334. 10.1176/foc.9.3.foc311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB., Yu C., Yoshida H., & Fausey CM. (2015). Contributions of Head-Mounted Cameras to Studying the Visual Environments of Infants and Young Children. Journal of Cognition and Development, 16(3), 407–419. 10.1080/15248372.2014.933430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B., DeCicco JM., & Dennis TA. (2012). Emotional picture processing in children: An ERP study. Developmental Cognitive Neuroscience, 2(1), 110–119. 10.1016/j.dcn.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer LL., Cook AE., McMahon WM., & Clark E. (2007). Face processing in children with autism Effects of stimulus contents and type. Autism, 11(3), 265–277. 10.1177/1362361307076925 [DOI] [PubMed] [Google Scholar]
- Staugaard SR. (2009). Reliability of Two Versions of the Dot-Probe Task Using Photographic Faces. Psychology Science, 51(3), 339. [Google Scholar]
- Stifter CA., Putnam S., & Jahromi L. (2008). Exuberant and inhibited toddlers: Stability of temperament and risk for problem behavior. Development and Psychopathology, 20(02), 401–421. 10.1017/S0954579408000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling LJ., Eley TC., & Clark DM. (2006). Preliminary Evidence for an Association Between Social Anxiety Symptoms and Avoidance of Negative Faces in School-Age Children. Journal of Clinical Child & Adolescent Psychology, 35(3), 431–439. 10.1207/s15374424jccp3503_9 [DOI] [PubMed] [Google Scholar]
- Swartz JR., & Monk CS. (2013). The Role of Corticolimbic Circuitry in the Development of Anxiety Disorders in Children and Adolescents In Andersen SL. & Pine DS. (Eds.), The Neurobiology of Childhood (pp. 133–148). Springer; Berlin Heidelberg: 10.1007/7854_2013_242 [DOI] [PubMed] [Google Scholar]
- Sylvester CM., Corbetta M., Raichle ME., Rodebaugh TL., Schlaggar BL., Sheline YI., … Lenze EJ. (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences, 35(9), 527–535. 10.1016/j.tins.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM., Hudziak JJ., Gaffrey MS., Barch DM., & Luby JL. (2015). Stimulus-Driven Attention, Threat Bias, and Sad Bias in Youth with a History of an Anxiety Disorder or Depression. Journal of Abnormal Child Psychology, 1–13. 10.1007/s10802-015-9988-8 [DOI] [PMC free article] [PubMed]
- Sylvester CM., Smyser CD., Smyser T., Kenley J., Ackerman JJ., Shimony JS., … Rogers CE. (2017). Cortical Functional Connectivity Evident After Birth and Behavioral Inhibition at Age 2. American Journal of Psychiatry, appi.ajp.2017.17010018. 10.1176/appi.ajp.2017.17010018 [DOI] [PMC free article] [PubMed]
- Taber-Thomas BC., & Perez-Edgar K. (2015). Emerging adulthood brain development. In Arnett JJ. (Ed.), The Oxford Handbook of Emerging Adulthood (pp. 126–141).
- Taghavi MR., Neshat-Doost HT., Moradi AR., Yule W., & Dalgleish T. (1999). Biases in Visual Attention in Children and Adolescents With Clinical Anxiety and Mixed Anxiety-Depression. Journal of Abnormal Child Psychology, 27(3), 215–223. 10.1023/A:1021952407074 [DOI] [PubMed] [Google Scholar]
- Telzer EH., Mogg K., Bradley BP., Mai X., Ernst M., Pine DS., & Monk CS. (2008). Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biological Psychology, 79(2), 216–222. 10.1016/j.biopsycho.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai N., Taber-Thomas BC., & Pérez-Edgar KE. (2016). Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: An ERP study. Developmental Cognitive Neuroscience, 19, 200–210. 10.1016/j.dcn.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E., & Smith LB. (1994). A dynamic systems approach to the development of cognition and action. (Vol. 10). Cambridge, MA: MIT Press. [Google Scholar]
- Todd RM., Cunningham WA., Anderson AK., & Thompson E. (2012). Affect-biased attention as emotion regulation. Trends in Cognitive Sciences, 16(7), 365–372. 10.1016/j.tics.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Tsypes A., Owens M., & Gibb BE. (2017). Suicidal ideation and attentional biases in children: An eye-tracking study. Journal of Affective Disorders, 222, 133–137. 10.1016/j.jad.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallorani A., Fu X., Morales S., LoBue V., Buss KA., & Pérez-Edgar K. (under review). Person-centered profiles of infant affect-biased attention are associated with maternal anxiety and infant negative affect.
- Vanderwert RE., Westerlund A., Montoya L., McCormick SA., Miguel HO., & Nelson CA. (2015). Looking to the eyes influences the processing of emotion on face-sensitive event-related potentials in 7-month-old infants. Developmental Neurobiology, 75(10), 1154–1163. 10.1002/dneu.22204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey MW., Daleiden EL., Williams LL., & Brown LM. (1995). Biased attention in childhood Anxiety disorders: A preliminary study. Journal of Abnormal Child Psychology, 23(2), 267–279. 10.1007/BF01447092 [DOI] [PubMed] [Google Scholar]
- Vervoort L., Wolters LH., Hogendoorn SM., Prins PJ., Haan E. de, Boer F., & Hartman CA. (2011). Temperament, Attentional Processes, and Anxiety: Diverging Links Between Adolescents With and Without Anxiety Disorders? Journal of Clinical Child & Adolescent Psychology, 40(1), 144–155. 10.1080/15374416.2011.533412 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9(12), 585–594. 10.1016/j.tics.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Waechter S., Nelson AL., Wright C., Hyatt A., & Oakman J. (2014). Measuring Attentional Bias to Threat: Reliability of Dot Probe and Eye Movement Indices. Cognitive Therapy and Research, 38(3), 313–333. 10.1007/s10608-013-9588-2 [DOI] [Google Scholar]
- Waters AM., Henry J., Mogg K., Bradley BP., & Pine DS. (2010). Attentional bias towards angry faces in childhood anxiety disorders. Journal of Behavior Therapy and Experimental Psychiatry, 41(2), 158–164. 10.1016/j.jbtep.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Waters AM., Lipp OV., & Spence SH. (2004). Attentional bias toward fear-related stimuli: An investigation with nonselected children and adults and children with anxiety disorders. Journal of Experimental Child Psychology, 89(4), 320–337. 10.1016/j.jecp.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Waters AM., Mogg K., & Bradley BP. (2012). Direction of threat attention bias predicts treatment outcome in anxious children receiving cognitive-behavioural therapy. Behaviour Research and Therapy, 50(6), 428–434. 10.1016/j.brat.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Waters AM., Mogg K., Bradley BP., & Pine DS. (2008). Attentional Bias for Emotional Faces in Children With Generalized Anxiety Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 47(4), 435–442. 10.1097/CHI.0b013e3181642992 [DOI] [PubMed] [Google Scholar]
- Waters AM., Pittaway M., Mogg K., Bradley BP., & Pine DS. (2013). Attention training towards positive stimuli in clinically anxious children. Developmental Cognitive Neuroscience, 4, 77–84. 10.1016/j.dcn.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM., Wharton TA., Zimmer-Gembeck MJ., & Craske MG. (2008). Threat-based cognitive biases in anxious children: Comparison with non-anxious children before and after cognitive behavioural treatment. Behaviour Research and Therapy, 46(3), 358–374. 10.1016/j.brat.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Waters AM., Zimmer-Gembeck MJ., Craske MG., Pine DS., Bradley BP., & Mogg K. (2015). Look for good and never give up: A novel attention training treatment for childhood anxiety disorders. Behaviour Research and Therapy, 73, 111–123. 10.1016/j.brat.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Watts SE., & Weems CF. (2006). Associations Among Selective Attention, Memory Bias, Cognitive Errors and Symptoms of Anxiety in Youth. Journal of Abnormal Child Psychology, 34(6), 838–849. 10.1007/s10802-006-9066-3 [DOI] [PubMed] [Google Scholar]
- White LK., Britton JC., Sequeira S., Ronkin EG., Chen G., Bar-Haim Y., … Pine DS. (2016). Behavioral and neural stability of attention bias to threat in healthy adolescents. NeuroImage. 10.1016/j.neuroimage.2016.04.058 [DOI] [PMC free article] [PubMed]
- White LK., Degnan KA., Henderson HA., Pérez-Edgar K., Walker OL., Shechner T., … Fox NA. (2017a). Developmental relations among behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Development, 88(1), 141–155. 10.1111/cdev.12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK., Sequeira S., Britton JC., Brotman MA., Gold AL., Berman E., … Pine DS. (2017b). Complementary Features of Attention Bias Modification Therapy and Cognitive-Behavioral Therapy in Pediatric Anxiety Disorders. American Journal of Psychiatry, 174(8), 775–784. 10.1176/appi.ajp.2017.16070847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Ram N., Perez-Edgar K., & Buss KA. (in prep). Eight Measures of Attention Bias from Dot Probe Paradigm.
- Yiend J. (2010). The effects of emotion on attention: A review of attentional processing of emotional information. Cognition & Emotion, 24(1), 3–47. 10.1080/02699930903205698 [DOI] [Google Scholar]
- Yrttiaho S., Forssman L., Kaatiala J., & Leppänen JM. (2014). Developmental Precursors of Social Brain Networks: The Emergence of Attentional and Cortical Sensitivity to Facial Expressions in 5 to 7 Months Old Infants. PLOS ONE, 9(6), e100811 10.1371/journal.pone.0100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., & Smith LB. (2013). Joint Attention without Gaze Following: Human Infants and Their Parents Coordinate Visual Attention to Objects through Eye-Hand Coordination. PLOS ONE, 8(11), e79659 10.1371/journal.pone.0079659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., & Smith LB. (2016a). Multiple Sensory-Motor Pathways Lead to Coordinated Visual Attention. Cognitive Science, n/a-n/a. 10.1111/cogs.12366 [DOI] [PMC free article] [PubMed]
- Yu C., & Smith LB. (2016b). The Social Origins of Sustained Attention in One-Year-Old Human Infants. Current Biology, 26(9), 1235–1240. 10.1016/j.cub.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., & Smith LB. (2017). Hand–Eye Coordination Predicts Joint Attention. Child Development, 88(6), 2060–2078. 10.1111/cdev.12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvielli A., Bernstein A., & Koster EHW. (2015). Temporal Dynamics of Attentional Bias. Clinical Psychological Science, 3(5), 772–788. 10.1177/2167702614551572 [DOI] [Google Scholar]