Abstract
Background
Malignant neoplasms of the lymphoid or myeloid cell lines including lymphoma, leukaemia and myeloma are referred to as haematological malignancies. Complementary and alternative treatment options such as meditation practice or yoga are becoming popular by treating all aspects of the disease including physical and psychological symptoms. However, there is still unclear evidence about meditation's effectiveness, and how its practice affects the lives of haematologically‐diseased patients.
Objectives
This review aims to assess the benefits and harms of meditation practice as an additional treatment to standard care for adults with haematological malignancies.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 8, 2015), MEDLINE (1950 to August 2015), databases of ongoing trials, the metaRegister of Controlled Trials (mRCT) (http://www.controlled‐trials.com/mrct/), conference proceedings of annual meetings of: the American Society of Hematology; American Society of Clinical Oncology; European Hematology Association; European Congress for Integrative Medicine; and Global Advances in Health and Medicine (2010 to 2015).
Selection criteria
We included randomised controlled trials (RCTs) using meditation practice for adult patients with haematological malignancies.
Data collection and analysis
Two review authors independently extracted data from eligible studies and assessed the risk of bias according to predefined criteria. We evaluated quality of life and depression. The other outcomes of overall survival, anxiety, fatigue, quality of sleep and adverse events could not be evaluated, because they were not assessed in the included trial.
Main results
We included only one small trial published as an abstract article. The included study investigated the effects of meditation practice on patients newly hospitalised with acute leukaemia. Ninety‐one participants enrolled in the study, but only 42 participants remained in the trial throughout the six‐month follow‐up period and were eligible for analysis. There was no information provided about the average age and sex of the study population. We found a high risk for attrition bias and unclear risk for reporting bias, performance and detection bias because of missing data due to abstract publication only, thus we judged the overall risk of bias as high. According to the GRADE criteria, we judged the overall quality of the body of evidence for all predefined outcomes as 'very low', due to the extent of missing data on the study population, and the small sample size.
As the abstract publication did not provide numbers and results except P values, we are not able to give more details.
Meditation practice might be beneficial for the quality of life of haematologically‐diseased patients, with higher scores for participants in the mediation arms compared to the participants in the usual care control group (low quality of evidence). Levels of depression decreased for those practising meditation in both the spiritually‐framed meditation group and the secularly‐focused meditation group in comparison to the usual care control group, whose levels of depression remained constant (low quality of evidence). The influence of meditation practice on overall survival, fatigue, anxiety, quality of sleep and adverse events remained unclear, as these outcomes were not evaluated in the included trial.
Authors' conclusions
To estimate the effects of meditation practice for patients suffering from haematological malignancies, more high quality randomised controlled trials are needed. At present there is not enough information available on the effects of meditation in haematologically‐diseased patients to draw any conclusion.
Keywords: Adult, Humans, Acute Disease, Depression, Depression/therapy, Hematologic Neoplasms, Hematologic Neoplasms/psychology, Leukemia, Leukemia/psychology, Meditation, Meditation/psychology, Randomized Controlled Trials as Topic
Plain language summary
Meditation for adults with haematological malignancies
Background
Cancers of the bone marrow, lymphatic tissue and blood are considered as haematological malignancies. The most common types of haematological malignancies are lymphoma, leukaemia and myeloma but they also include myelodysplastic syndromes or myeloproliferative diseases. Within each type of disease there are various sub‐divisions. There are several treatment options depending on the type and severity of the cancer. The most common therapies are chemotherapy, radiotherapy or a combination of both. In some cases, stem cell transplantation is offered (this is where either the patient's own bone marrow cells or cells of another person are implanted in the patient's body after aggressive chemotherapy). Those suffering from haematological cancers may have serious symptoms, and treatments often cause severe and distressing side effects.
Living with cancer and undergoing aggressive treatment often leads to physical or psychological health problems such as tiredness, anxiety or depression. Even after having completed the treatment some patients are still affected by their disease and search for alternative treatment options to help them coping with their disease.
Meditation
Meditation has been practised for thousands of years by many different cultures and has it's origins in ancient eastern traditions. Meditation practice can be done in various postures and ways. For example, the person can focus on breathing in and out, on an object or repeat a word or a pair of words (a mantra). Some sorts of mediation are based on spiritual beliefs. One treatment using meditation practice is called mindfulness‐based cognitive therapy and has shown improvements on distress, quality of life and anxiety in cancer patients. However, there has not been a systematic evaluation on how meditation practice may improve and affect the lives of those with haematologically disease.
Objectives
We investigated the effects of meditation practice on adult patients with haematological malignancies. Important outcomes were quality of life, overall survival, depression, fatigue, anxiety, quality of sleep and adverse events. We reviewed the effects of meditation practice in addition to standard care for patients newly diagnosed with acute leukaemia compared to standard care only.
Findings
We included one trial with 91 adult patients, of whom only 42 were analysed. The trial involved five one‐hour meditation intervention sessions between admission and discharge of participants newly diagnosed with acute myeloid or lymphoid leukaemia. There was no information about the age of the participants included in the study. All participants in the trial were hospitalised for initial induction chemotherapy. As the abstract of the publication did not provide numbers, it is not possible to describe the results in more detail.
Participants practising meditation reported better physical health and levels of depression could be decreased.
Quality of the evidence
Most of our pre‐defined outcomes (overall survival, anxiety, fatigue, quality of sleep, and adverse events) were not reported at all. We judge the quality of the evidence for the outcomes quality of life and depression as 'very low', due to high risk of bias (only 42 out 91 participants were evaluated) and very imprecise results.
Conclusion
There were not enough data available to determine the effectiveness of meditation practice on haematologically‐diseased patients, thus the role of meditation in the treatment of haematological malignancies remains unclear. More high‐quality and larger randomised controlled trials are needed to validate possible positive effects of meditation practice for haematologically‐diseased patients.
The evidence is up‐to‐date as of August, 2015.
Summary of findings
for the main comparison.
| Meditation in addition to standard care compared with standard care only for adult patients with haematological malignancies | ||||||
|
Patient or population: adult patients with haematological malignancies Intervention: meditation in addition to standard care Comparison: standard care only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Overall survival | not reported | |||||
| Quality of life | see comment | 42 patients of 91 included patients evaluated (1 study) | ⊕⊝⊝⊝ very low 1,2 |
Minutes of meditation or relaxation practice was related to statistically significantly greater perceived social support as assessed by the MQOL (P = 0.05) at discharge. The frequency of meditation practice was associated with a statistically significant improvement in the existential sub‐scale (P = 0.05), and psychological sub‐scale (P = 0.05) at the 4‐months assessment. Participants practising spiritually‐focused meditation reported better physical health at the 6‐months follow‐up compared to the participants receiving usual care. However, there is no evidence for a difference as no numbers were provided. |
||
| Fatigue | not reported | |||||
| Anxiety | not reported | |||||
| Depression | see comment | 42 patients of 91 included patients evaluated (1 study) | ⊕⊝⊝⊝ very low 1,2 |
In the included trial the levels of depression for spiritually‐focused meditation (P = 0.04) and secularly‐framed meditation (P = 0.001) decreased significantly across assessment points while levels of depression for the usual care control remained constant. There is a correlation of minutes of meditation or relaxation practice and decreased symptoms of depression, measured by CESD (P = 0.02). | ||
| Quality of sleep | not reported | |||||
| Adverse events | not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Very small number of included patients and events leading to very high imprecision (downgraded two points)
2 High risk of bias of the included trial (high risk of performance, detection and attrition bias) (downgraded one point)
Background
Description of the condition
Haematological malignancies are neoplasms of the blood, lymph nodes and bone marrow. They can either derive from myeloid or lymphoid blood cell lines. Malignant neoplasms concerning the myeloid cells are acute and chronic myelogenous leukaemia, myelodysplastic syndrome or myeloproliferative diseases whereas lymphocytic leukemias, myelomas and lymphomas are of lymphoid origin. In 2010, the prevalence of people living with leukaemia in the United States comprised approximately 287,963 men and women. The age‐adjusted incidence rates for haematological cancer patients per 100,000 men and women per year came to 27.4 new cases of leukaemia, 45.6 new cases of lymphoma and 13 new cases of patients suffering from myeloma. In 2010 the overall five‐year survival rate for lymphoma patients was 71%, whereas leukaemia had a survival rate of 56%, and only 43.2% of myeloma patients live for more than five years after being diagnosed with cancer (Howlader 2013).
The number of different treatment options available for haematological malignancies is probably just as high as the differences between the individual survival rates. Depending on the severity of symptoms and disease progression rate, some people may only need supportive care to reduce the symptoms that occur alongside their disease such as anaemic symptoms, osteoporosis or infections. There is a wide range of specific blood cancer therapy treatments like chemotherapy, combined drug therapy, radiation and stem cell transplantation (Longo 2011).
Description of the intervention
Meditation has its origins in ancient eastern traditions and is seen as a mind‐body practice in complementary and alternative medicine (NCCAM 2013). Meditation practice has derived from a group of techniques, like mindfulness meditation, mantra meditation, relaxation response and Zen Buddhist meditation. Throughout thousands of years, these techniques have been practised by many different cultures. To practise meditation one commonly has to consider several circumstances, such as finding a quiet location and an appropriate posture. Meditation can be practised while sitting, standing or lying down. A person can either concentrate on his or her own breath, objects, or on a mantra, which can be a chosen word, or a pair of words. It is also important to have an open attitude, which means the person practising meditation has to let distractions come and go, so that they cannot affect his or her thoughts (NCCAM 2013).
Mindfulness‐based cognitive therapy has shown improvement in terms of distress, depression and anxiety, as well as quality of life in patients diagnosed with cancer (Foley 2010). Another study using mantra‐based meditation has shown an improvement in perceived stress and negative mood; this study also suggested that the frequency of the use of meditation could affect the outcomes (Lane 2007). Spiritually‐focused meditation is another way to consider the religious and spiritual needs of leukaemia patients (Cole 2010).
Mindfulness is the source of several clinical practices and can be used in mental training or by performing different types of meditation (Carlson 2005). Mindfulness‐based stress reduction, as a clinical intervention using mindfulness as its fundament, has shown several significant positive effects for a heterogeneous group of 63 cancer patients in terms of mood and sleep disturbance, stress and fatigue (Carlson 2005). Mindfulness‐based stress reduction is a group programme, designed to lighten the symptoms of patients suffering from psychosomatic or physical disorders, using mindfulness meditation (Grossman 2004). Cancer patients often suffer from sleep disturbances, which are difficult to treat, because the causes of these disturbances can not always be detected (Carlson 2005). Spiritual and religious conceptions of cancer patients should also be respected in cancer care and may, when integrated into meditation, enhance the effects of mediation practice (Cole 2010).
How the intervention might work
The exact mechanism of how meditation can improve one's mood and have positive effects on the cognition of stress is not determined, but evidence from studies with healthy adults has shown that even brief instruction in meditation techniques can lead to health benefits that last for a long time (Lane 2007). Although derived from a relatively small number of studies, these results suggest that mindfulness‐based stress reduction may help a broad range of individuals to cope with their clinical and non‐clinical problems (Grossman 2004).
Preliminary evidence suggests that even brief instruction in a simple meditation technique can improve negative mood and perceived stress in healthy adults, which could yield long‐term health benefits. Frequency of practice does affect outcome. Those most likely to experience negative emotions may benefit the most from the intervention (Lane 2007).
One randomised controlled trial (RCT) involving more than 100 patients showed that meditation can not only have an effect on a person's mental state, but also on his physiological mechanisms of regulation by affecting the regulation of blood pressure (Carlson 2007; Schneider 1995; Schneider 2006).
Why it is important to do this review
Little is known about the prevalence of mood disorders such as anxiety or depression in haematological cancer patients. One meta‐analysis of 70 interview‐based studies concerning patients in oncological and haematological settings indicated a prevalence rate of 20.7% for depression (Mitchell 2011).
Various studies have shown positive effects on stress, fatigue and sleep quality for healthy and physically‐ or psychologically‐diseased people when they perform various meditation techniques and practise mindfulness (Grossman 2004; Kang 2012; Lane 2007; Witek‐Janusek 2008).
Several existing RCTs have shown positive effects of mindfulness‐based stress reduction for breast cancer patients (Carlson 2005: Cramer 2012; Foley 2010; Witek‐Janusek 2008). There is some evidence that this intervention enhances the psychological health of breast cancer patients (Cramer 2012). Even cortisol levels have been reduced in patients following the mindfulness‐based stress reduction programme. Beneficial effects on immune function and quality of life, as well as on coping processes of breast cancer patients, have been found (Witek‐Janusek 2008). Although those studies were not specifically designed for patients suffering from haematological malignancies, it may be assumed that meditation and mindfulness‐based stress reduction might also be advantageous for these patients.
Objectives
To assess the benefits and harms of meditation practice as an additional treatment to standard care for adults with haematological malignancies.
Methods
Criteria for considering studies for this review
Types of studies
We only included RCTs. We included both full text and abstract publications if sufficient information was available on study design, characteristics of participants, interventions and outcomes. The trials had to report at least one of the outcomes mentioned below to be included.
Types of participants
We included trials on adult (≥ 18 years) participants with confirmed diagnoses of haematological malignancies. We did not apply gender or ethnicity restrictions. We considered all subtypes and stages of haematological malignancies, including newly‐diagnosed patients and those with relapsed or drug‐resistant disease. If trials consisted of mixed populations with different conditions or types of cancers, we used data from the haematological malignancy subgroups. If subgroup data for these patients were not provided (after contacting the authors of the trial), we excluded the trial if less than 80% of patients had haematological malignancies.
Types of interventions
The intervention was meditation in addition to standard care compared with standard care only. We included any form of meditation.
Types of outcome measures
Primary outcomes
We evaluated quality of life as the primary efficacy endpoint. It had to be measured with reliable and validated instruments.
Secondary outcomes
We analysed the following outcomes as secondary outcomes.
Overall survival, defined as the time interval from random treatment assignment onto a study to death from any cause or to the last follow‐up.
Fatigue if measured with reliable and validated instruments.
Anxiety if measured with reliable and validated instruments.
Depression if measured with reliable and validated instruments.
Quality of sleep if measured with reliable and validated instruments.
Adverse events.
Search methods for identification of studies
Electronic searches
We adapted the search strategies suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We applied no language restriction to reduce the language bias.
We searched the following databases and sources.
-
Databases of medical literature:
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 8, 2015) (Appendix 1);
MEDLINE (Ovid) (1950 to Aug 2015) (Appendix 2).
-
Databases of ongoing trials:
the metaRegister of Controlled Trials (mRCT) (http://www.controlled‐trials.com/mrct/).
EU clinical trials register (https://www.clinicaltrialsregister.eu/ctr‐search/search).
Clinicaltrials.gov (https://clinicaltrials.gov/).
-
Conference proceedings of annual meetings of the following societies for abstracts, if not included in CENTRAL (2010 to 2015):
American Society of Hematology;
American Society of Clinical Oncology;
European Hematology Association;
European Congress for Integrative Medicine;
Global Advances in Health and Medicine.
Searching other resources
-
Handsearching of references
References of all identified trials, relevant review articles and current treatment guidelines for further literature.
Data collection and analysis
Selection of studies
Two review authors (IS, NS) independently screened the results of the search strategies for eligibility for this review by reading the abstracts. In the case of disagreement we obtained the full‐text publication. If no consensus was reached, we asked a third review author (Higgins 2011a).
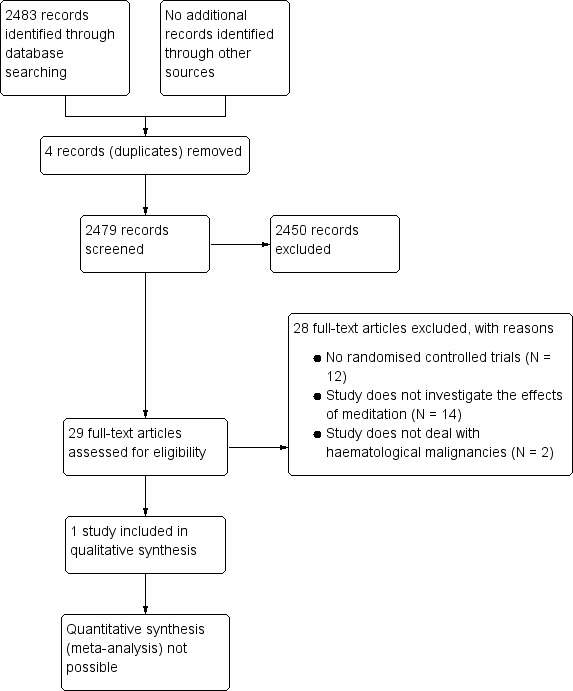
We prepared a flow chart as recommended in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (Moher 2009), showing the total numbers of retrieved references and the numbers of included and excluded studies (Figure 1).
1.
Study flow diagram.
Data extraction and management
Two review authors (IS, NS) independently extracted the data according to the guidelines proposed by The Cochrane Collaboration (Higgins 2011a). We contacted authors of individual studies for additional information, if required. We used a standardised data extraction form containing the following items.
-
General information:
author, title, source, publication date, country, language, duplicate publications.
-
Quality assessment:
allocation concealment, blinding (participants, personnel, outcome assessors), incomplete outcome data, selective outcome reporting, other sources of bias.
-
Study characteristics:
trial design, aims, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, subgroup analysis, statistical methods, power calculations, treatment cross‐overs, compliance with assigned treatment, length of follow‐up, time point of randomisation.
-
Participant characteristics:
underlying disease, stage of disease, histological subtype, additional diagnoses, age, gender, ethnicity, number of participants recruited/allocated/evaluated, participants lost to follow‐up, type of treatment (multi‐agent chemotherapy (intensity of regimen, number of cycles)), additional radiotherapy.
-
Interventions:
type, duration and intensity of meditation intervention, standard care, duration of follow‐up.
-
Outcomes:
quality of life, overall survival, fatigue, anxiety, depression, quality of sleep, adverse events.
Assessment of risk of bias in included studies
Two review authors (IS, NS) independently assessed the risk of bias for each study using the following criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b):
sequence generation;
allocation concealment;
blinding (participants, personnel, outcome assessors);
incomplete outcome data;
selective outcome reporting;
other sources of bias.
We made a judgement for every criterion, using one of the following three categories.
'Low risk': if the criterion is adequately fulfilled in the study, i.e. the study is at a low risk of bias for the given criterion.
'High risk': if the criterion is not fulfilled in the study, i.e. the study is at high risk of bias for the given criterion.
'Unclear': if the study report does not provide sufficient information to allow for a clear judgement or if the risk of bias is unknown for one of the criteria listed above.
Measures of treatment effect
We used intention‐to‐treat data. For binary outcomes, we calculated risk ratios (RRs) with 95% confidence intervals (CIs) for each trial. For time‐to‐event outcomes, we extracted extract hazard ratios (HRs) from published data according to Parmar 1998 and Tierney 2007. We calculated continuous outcomes as standardised mean differences (SMDs).
Dealing with missing data
As suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), there are many potential sources of missing data which have to be taken into account: at study level, at outcome level, and at summary data level. Firstly, it is important to distinguish between 'missing at random' and 'not missing at random'. We contacted the original investigators to request missing data, but we did not receive any reply.
In case of patients lost to follow‐up after randomisation (dichotomous data) we would have imputed missing data assuming poor outcome (worse case scenario) for missing individuals. We would have performed sensitivity analysis to assess how sensitive results are to reasonable changes in the assumptions that are made. We addressed the potential impact of missing data on the findings of the review in the discussion.
Assessment of heterogeneity
We would have assessed heterogeneity of treatment effects between trials using the Chi2 test with a significance level at P < 0.1. We would have used the I2 statistic to quantify possible heterogeneity (I2 > 30% moderate heterogeneity, I2 > 75% considerable heterogeneity) (Deeks 2011). We would have explored potential causes of heterogeneity by sensitivity and subgroup analysis.
Assessment of reporting biases
In meta‐analyses with at least 10 trials we would have investigated potential publication bias by generating a funnel plot and statistically tested for this bias by using a linear regression test (Sterne 2011). A P value of less than 0.1 would have been considered significant for this test.
Data synthesis
As we identified one trial only fitting the inclusion criteria, we could not perform data synthesis (see Differences between protocol and review).
We created a 'Summary of findings' table on absolute risks in each group according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (GRADEpro 2014; Schünemann 2011). We summarised the evidence of overall survival, quality of life, fatigue, anxiety, depression, quality of sleep and adverse effects in this table.
Subgroup analysis and investigation of heterogeneity
As we did not perform meta‐analyses, we could not investigate heterogeneity or perform subgroup analyses (see Differences between protocol and review).
Sensitivity analysis
As we did not perform meta‐analyses, we did not perform sensitivity analyses (see Differences between protocol and review).
Results
Description of studies
Results of the search
Our sensitive search strategy resulted in 2483 hits. Initially, 2454 studies out of these hits were excluded as they either did not meet the inclusion criteria for this review or were identified as duplicates. The remaining 29 references were assessed as full‐texts for eligibility. Of these articles, 28 articles were excluded, because they either did not evaluate meditation, were not randomised controlled trials or included no adults in the trial. Eventually one abstract publication with 91 participants remained and was included in this systematic review. The study flow diagram according to PRISMA shows the number of records that were screened, excluded and included (see Figure 1).
Included studies
One randomised controlled trial (RCT), including 91 patients, met all inclusion criteria (Cole 2010). The trial was published as an abstract, from which we have extracted data. An overview of the characteristics of this trial is presented in the Characteristics of included studies table. We contacted the principal author of this trial seeking further information such as the full text article of this trial, but he did not respond.
Design of the study
The included study was performed as a three‐armed RCT. The aim of the trial was to investigate the effects of spiritually‐focused meditation on the quality of life of cancer patients. Only the participants of two arms (secularly‐focused meditation or spiritually‐focused meditation) were taught how to practise meditation.
Sample size
Ninety‐one cancer patients were randomly assigned to one of the three arms of the study.
Forty‐two patients remained throughout the follow‐up period and were assessable for analysis.Thirteen cancer patients were randomly assigned to the group who practised spiritually‐focused meditation, 12 patients were assigned to the secularly‐focused meditation practice group and 17 patients had no additional courses to usual care control. Setting
The study was conducted at the University of Pittsburgh Cancer Institute, in the United States (USA).
Patients
Included participants were older than 18 years, all newly diagnosed with acute myeloid leukaemia or acute lymphocytic leukaemia and hospitalised for initial induction chemotherapy.
Interventions
The study was designed to compare spiritually‐focused meditation and secularly‐focused meditation interventions to usual care control for patients who were newly diagnosed with acute leukaemia. Differences between these two meditation types were not clarified in the abstract of the included study. The meditation practice took place during five one‐hour intervention sessions between admission and discharge.
Outcomes
The outcome measures for the included trial were assessed by using different scales and investigated the effects of meditation on depression, quality of life, positive and negative affects, physical well‐being and pain for patients with newly diagnosed leukaemia. Outcome measures were assessed at the beginning of the study, at the moment of discharge, and in the second, the fourth and the sixth months after discharge.
Conflict of interest
The authors had no relevant conflicts of interest to declare.
Excluded studies
We screened and excluded 28 studies which seemed relevant for this review at first sight. These were excluded for the following reasons: 12 studies were not randomised controlled trials (Adamsen 2006; Bellet 1995; Broome 2001; Chen 1999; Gregory‐Addesa 1986; Kelly 2009; Kopp 2001; LaBaw 1975; Lin 2001; Walter 1989; Williams 2006; Yeh 2006); 14 studies did not investigate the effects of meditation (Burns 2008; Stringer 2008) of which 12 were also in children (Collipp 1969; Jacknow 1994; Kazak 1996; Kazak 1998; Lindwall 2014; Liossi 1999; Liossi 2003; Liossi 2006; Maurice‐Stam 2009; Pederson 1996; Schurman 2010; Suderman 1990). Moreover, two studies did not deal with haematological malignancies (Lichstein 1985; Loening‐Baucke 1988).
Risk of bias in included studies
The risk of bias for the included study has been considered as unclear, mostly because of missing information due to abstract publication only. Also see Figure 2 for more information.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged risk of allocation bias for random sequence generation and allocation concealment as unclear due to insufficient information.
Blinding
We judged risk of bias as high, as blinding is not mentioned, and usually not possible in studies evaluating meditation compared to standard care (high risk of performance bias). As the reported outcomes are all patient‐reported outcomes we judge risk of potential detection bias as high.
Incomplete outcome data
Out of 91 acute leukaemia patients who enrolled into the study, only 42 remained throughout the 6‐months follow‐up period and were assessable for analysis. Therefore, we judged the risk of attrition bias as high.
Selective reporting
We judged risk of selective reporting bias as unclear as we did not receive any additional data other than the abstract.
Other potential sources of bias
No information was provided so we judged the risk of other potential bias as unclear.
Effects of interventions
See: Table 1
The only included trial (Cole 2010) in this review investigated the effects of meditation on the quality of life of patients who were newly diagnosed and hospitalised with acute leukaemia. The aim of the study was to investigate whether the patients' spiritual or religious beliefs implemented into meditation practice could affect their quality of life.
Primary outcomes
Quality of life
The McGill Quality of Life Questionnaire (MQOL) is a clinical care tool to evaluate quality of life and was developed by S. Robin Cohen at the McGill University (Cohen 1995). It is a questionnaire to assess patients' physical and emotional health symptoms. The participants have to evaluate questions concerning several topics about their feelings in the past 2 days by using a scale ranging from 0 to 10, with 0 meaning that it does not apply to the participants feelings at all and 10 that it fully applies.
Cole 2010 et al. reported that the length of meditation or relaxation practice was related to statistically significantly greater perceived social support as assessed by the MQOL (P = 0.05) at discharge. The frequency of meditation practice was associated with a statistically significant improvement at existential sub‐scale (P = 0.05), and psychological sub‐scale (P = 0.05) at the four‐month assessment.
The Functional Assessment of Cancer Therapy ‐ Physical Well‐being sub‐scale (FACT‐ph, Cella 1993), was also used as a tool to evaluate the patients' quality of life in the included trial. It is a validated and brief yet sensitive 33‐item general cancer quality‐of‐life measure for evaluating patients receiving cancer treatment. In addition to a total score, there are sub‐scale scores for physical, functional, social and emotional well‐being, as well as satisfaction with the treatment relationship.
In the included trial, the authors reported that there was an interaction of time, but did not provide the direction of the effect and for which intervention arms. Participants practising spiritually‐focused meditation reported better physical health at the 6‐month follow‐up compared to the participants receiving usual care. However, there is no evidence for a difference as no data were provided.
Secondary outcomes
Overall survival
This outcome was not evaluated in the included trial.
Fatigue
This outcome was not evaluated in the included trial.
Anxiety
This outcome was not evaluated in the included trial.
Depression
The Center for Epidemiologic Studies Depression‐Revised (CESD‐R) is a screening test for depression and depressive disorder (van Dam 2011). The CESD‐R measures symptoms defined by the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) for a major depressive episode. There are several statements in the test and patients have to indicate how often they have recently felt this way by selecting the option they most agree with.
In the included trial the levels of depression for spiritually‐focused meditation (P = 0.04) and secularly‐framed meditation (P = 0.001) decreased significantly across assessment points while levels of depression for the usual care control remained constant. There is a correlation of minutes of meditation or relaxation practice and decreased symptoms of depression, measured by CESD (P = 0.02).
Quality of sleep
This outcome was not evaluated in the included trial.
Adverse events
No information regarding adverse events was reported in the included trial.
Discussion
Summary of main results
The results listed below derive from this systematic review which investigates the effects of meditation practice for patients newly diagnosed with haematological malignancies, compared to standard care only. We identified only one trial which fulfilled our inclusion criteria and investigated the effects of different meditation practices on the quality of life and depression rates of 91 patients hospitalised with acute leukaemia. The study was conducted as a three‐armed trial and only 42 patients remained in the study throughout the follow‐up period and were eligible for analysis.
Studies investigating the use of any kind of meditation practice for haematologically‐diseased people are very rare.
There is very low quality evidence that meditation practice might improve the quality of life of people with acute leukaemia.
There is very low quality evidence that meditation practice might improve levels of depression in participants with acute leukaemia compared to participants only receiving usual care control.
The effect of meditation on physical health symptoms remains unclear, as the authors did not provide numbers for the difference between the participants receiving usual care and those practicing meditation.
The outcomes overall survival, anxiety, fatigue, quality of sleep, and adverse events were not evaluated in the trial.
Overall completeness and applicability of evidence
Although we searched several medical databases and relevant conference proceedings, only one small study could be identified. The included study is a randomised controlled trial (RCT), with 91 adult patients suffering from acute leukaemia who were randomly assigned to one of the three arms of the trial (spiritually‐focused meditation, secularly‐focused meditation and usual care). As only 42 patients out of 91 remained in the study and were evaluated, no firm conclusions are possible. No reasons for the high dropout rate was reported.
Unfortunately, there was no information concerning the average age, gender or ethnicity of the patients included in the study, because the study was an abstract publication only. The principal author of the study did not provide further information after being contacted. More detailed information about the study population would be useful for comparing future studies dealing with meditation and haematological malignancies.
Quality of the evidence
The included trial is considered to be at high risk of bias, as only 42 out of 91 randomised patients were analysed (high risk of attrition bias). There is no information provided concerning blinding of participants or personnel supervising the meditation interventions, furthermore there is no information on blinding of the outcome assessors (high risk of performance and detection bias).
The overall quality of the evidence for the reported outcomes quality of life and depression is considered to be very low, because of a high risk of bias of the included trial (high risk of performance, detection and attrition bias) and very imprecise results (only 42 patients evaluated, no results reported).
Potential biases in the review process
The biggest restriction for this systematic review is that despite the broad search for literature, we found only one RCT fulfilling our inclusion criteria. It is possible that unpublished or still ongoing studies have been missed, especially Asian or Indian RCTs that were not published in English or not accessible via MEDLINE or CENTRAL. Therefore, publication bias and language bias cannot be excluded.
Agreements and disagreements with other studies or reviews
As far as we know, this systematic review is the first ever providing an overview of meditation for adult patients with haematological malignancies. Other studies investigating the effects of meditation mainly focus on solid cancer patients (Carlson 2005; Carlson 2007; Cramer 2012; Foley 2010). Most of these studies dealt with breast cancer patients.
Carlson 2005 investigated the effects of mindfulness‐based stress reduction (MBSR) on sleep quality, stress, fatigue, and mood in outpatients with cancer. Patients reported that their sleep quality had improved and the study showed evidence for a reduction in sleep disturbance and fatigue. Positive effects of MBSR on anxiety and depression of breast cancer patients has been shown by a meta‐analysis by Cramer 2012. It provided some evidence that MBSR is favourable for psychological health in breast cancer patients. Similar results have been shown by Foley 2010, stating significant improvements due to the intervention in terms of anxiety, depression and quality of life of cancer patients. Witek‐Janusek 2008 examined the effects of MBSR on quality of life and coping for women newly diagnosed with early stage breast cancer. Unfortunately this study was not an RCT. Overall, the results of various studies showed that cancer patients could benefit from the use of MBSR in oncology settings, especially in terms of quality of life, which is in line with our findings.
Unfortunately, most studies do not provide information about adverse events. As meditation is an active therapy that may have beneficial effects, it may also have negative effects, but there is no good evidence about the potential harms of this intervention.
Authors' conclusions
Implications for practice.
For the efficacy of meditation in addition to standard care being a treatment for patients with haematological malignancies, no credible conclusions can be drawn at present. The findings of this review derive from only a single trial with 91 patients of whom only 42 have been evaluated. There is some very low quality evidence that meditation practice, either secularly‐ or spiritually‐focused, might improve levels of depression and might relate to greater reported physical well‐being for patients with acute leukaemia. Adverse effects of meditation practice have not been reported. All the results should be considered cautiously as there is a lack of randomised controlled trials related to meditation for adults diagnosed with haematological malignancies.
Implications for research.
For evaluating the effects of meditation practice on haematologically‐diseased patients, more high‐quality randomised controlled trials, including a larger number of patients, are needed. These studies need to focus on patient‐relevant outcomes such as quality of life, overall survival and potential harms with longer follow‐up periods.
The physical and psychological effects of meditation practice are not yet fully scientifically understood. Therefore, a detailed description of meditation techniques and intervention procedures being used in studies relating to meditation for adults with haematological malignancies is advisable, to make future studies more comparable. To reduce bias, information should be provided concerning blinding of participants and personnel. A few studies state that meditation practice is feasible and safe for cancer patients, and that there were no adverse events observed. Nevertheless, most studies do not provide information about adverse events. Therefore, forthcoming studies should place greater emphasis on the reporting of adverse events and the reasons for study drop‐outs or withdrawals.
Notes
Parts of the methods section are from the standard Cochrane Haematological Malignancies Group template for protocols and reviews.
Acknowledgements
We are grateful to the following people for their comments and for improving the protocol and review:
Dr Guido Schwarzer and Dr Bastian von Tresckow (editors of the Cochrane Haematological Malignancies Group), Dr Susan Wieland (peer referee, Cochrane Complementary Medicine Field), Oliver Blank (member of the editorial team), Faiza Coleman‐Salako (consumer editor of the Cochrane Haematological Malignancies Group), Joey Kwong and Megan Prictor (Wiley Copy Edit Support) for copy editing.
Appendices
Appendix 1. CENTRAL search strategy
| 1 | MeSH descriptor: [Mind‐Body Therapies] explode all trees |
| 2 | body‐mind* |
| 3 | mind‐body* |
| 4 | (mind‐body near/3 (program* or therap* or medicin*)) |
| 5 | #1 or #2 or #3 or #4 |
| 6 | mindfulness based stress reduction* |
| 7 | mindfulness based* |
| 8 | mbsr* or mbct* |
| 9 | MeSH descriptor: [Meditation] explode all trees |
| 10 | meditation* |
| 11 | MeSH descriptor: [Relaxation Therapy] explode all trees |
| 12 | (relaxation* near/2 (technique* or therap*)) |
| 13 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 |
| 14 | MeSH descriptor: [Hematologic Diseases] explode all trees |
| 15 | MeSH descriptor: [Hematologic Neoplasms] explode all trees |
| 16 | (hematolog* near/1 malignan*) OR (hematolog* near/1 neoplas*) OR (haematolog* near/1 malignan*) OR (haematolog* near/1 neoplas*) |
| 17 | MeSH descriptor: [Bone Marrow Diseases] explode all trees |
| 18 | MeSH descriptor: [Lymphoma] explode all trees |
| 19 | MeSH descriptor: [Leukemia] explode all trees |
| 20 | (hogkin* or hodkin* or hodgin*):ti,ab,kw |
| 21 | lymphogranulomato* |
| 22 | lymphom* |
| 23 | histiocy* |
| 24 | granulom* |
| 25 | non‐hodgkin* |
| 26 | nonhodgkin* |
| 27 | Reticulosis |
| 28 | reticulosarcom* |
| 29 | (burkitt* NEAR/ (lymph* or tumo*)) |
| 30 | brill‐symmer* |
| 31 | plasm**ytom* |
| 32 | myelom* |
| 33 | Sezary |
| 34 | leuk*em* |
| 35 | myelodysplas* |
| 36 | aplast* an*em* |
| 37 | #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 |
| 38 | #13 and #37 in Trials |
Appendix 2. MEDLINE search strategy
| # | Searches |
| 1 | MIND‐BODY THERAPIES/ |
| 2 | body‐mind$.tw,kf,ot. |
| 3 | mind‐body$.tw,kf,ot. |
| 4 | (mind‐body near/3 (program* or therap* or medicin*)) |
| 5 | #1 or #2 or #3 or #4 |
| 6 | mindfulness based stress reduction$.tw,kf,ot. |
| 7 | mindfulness based$.tw,kf,ot. |
| 8 | (mbsr$ or mbct$).tw,kf,ot. |
| 9 | MEDITATION/ |
| 10 | meditation$.tw,kf,ot. |
| 11 | RELAXATION THERAPY/ |
| 12 | (relaxation$ adj2 (technique$ or therap$)).tw,kf,ot. |
| 13 | or/6‐12 |
| 14 | 5 or 13 |
| 15 | HEMATOLOGIC DISEASES/ |
| 16 | exp HEMATOLOGIC NEOPLASMS/ |
| 17 | (hematolog$ adj1 malignan$).tw,kf,ot. |
| 18 | (hematolog$ adj1 neoplas$).tw,kf,ot. |
| 19 | (haematolog$ adj1 malignan$).tw,kf,ot. |
| 20 | (haematolog$ adj1 neoplas$).tw,kf,ot. |
| 21 | exp BONE MARROW DISEASES/ |
| 22 | exp LYMPHOMA/ |
| 23 | exp LEUKEMIA/ |
| 24 | hodgkin$.tw,kf,ot. |
| 25 | lymphogranulomato$.tw,kf,ot. |
| 26 | lymphom$.tw,kf,ot. |
| 27 | histiocy$.tw,kf,ot. |
| 28 | granulom$.tw,kf,ot. |
| 29 | non‐hodgkin$.tw,kf,ot. |
| 30 | nonhodgkin$.tw,kf,ot. |
| 31 | reticulosis.tw,kf,ot. |
| 32 | reticulosarcom$.tw,kf,ot. |
| 33 | (burkitt$ adj (lymph$ or tumo?r$)).tw,kf,ot. |
| 34 | lymphosarcom$.tw,kf,ot. |
| 35 | brill‐symmer$.tw,kf,ot. |
| 36 | plasm##ytom$.tw,kf,ot. |
| 37 | myelom$.tw,kf,ot. |
| 38 | sezary.tw,kf,ot. |
| 39 | leuk?em$.tw,kf,ot. |
| 40 | myelodysplas$.tw,kf,ot. |
| 41 | aplast$ an?em$.ti,kf,ot. |
| 42 | or/15‐41 |
| 43 | randomized controlled trial.pt. |
| 44 | controlled clinical trial.pt. |
| 45 | randomi?ed.ab. |
| 46 | placebo.ab. |
| 47 | drug therapy.fs. |
| 48 | randomly.ab. |
| 49 | trial.ab. |
| 50 | groups.ab. |
| 51 | or/43‐50 |
| 52 | humans.sh. |
| 53 | 51 and 52 |
| 54 | 14 and 42 |
| 55 | 54 and 53 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cole 2010.
| Methods | Randomisation: 3 arms
Recruitment period: not reported Median follow‐up time: six months (outcome measures were completed two, four and six months after discharge from the clinic). |
|
| Participants | Patients randomised: (N = 91) N = 42 patients remained during the follow‐up period and were assessable for analysis.
Eligibility criteria: Patients newly diagnosed with acute myeloid leukaemia or acute lymphocytic leukaemia. |
|
| Interventions | Ninety‐one participants with acute leukaemia were enrolled in the study and randomly assigned to:
During five 1‐hour intervention sessions delivered between admission and discharge, participants were taught secular meditation (SM condition) or spiritually‐framed meditation (SpM condition). |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Not reported: Adverse events |
|
| Notes | No relevant conflicts of interest were stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Ninety‐one acute leukaemia patients enrolled in the study and were randomly assigned to: usual care control (UCC), spiritually‐focused meditation (SpM), or secularly‐focused meditation (SM)." No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As blinding is not mentioned, we judge that the trial is not blinded, due to the various interventions applied. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | As blinding is not mentioned, we judge that the trial is not blinded, due to the various interventions applied. As the reported outcomes were all patient‐reported outcomes we judge that patients were aware of the assigned intervention, leading to high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Forty‐two participants (17 UCC, 13 SpM, 12 SM) who remained in the study through the follow‐up period were assessable for analysis." |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available, no information provided. |
| Other bias | Unclear risk | No information provided. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamsen 2006 | Not a randomised controlled trial. |
| Bellet 1995 | Not a randomised controlled trial. |
| Broome 2001 | Not a randomised controlled trial. |
| Burns 2008 | This publication has been excluded because it investigates the effects of music imagery, not meditation. |
| Chen 1999 | Not a randomised controlled trial. |
| Collipp 1969 | This publication has been excluded because there were no adults included in the study. Moreover, prayer rather than meditation was evaluated. |
| Gregory‐Addesa 1986 | Not a randomised controlled trial. |
| Jacknow 1994 | This publication has been excluded because there were no adults included in the study. Moreover, hypnosis rather than meditation was evaluated. |
| Kazak 1996 | This publication has been excluded because there were no adults included in the study. Moreover, psychological and pharmacological interventions rather than meditation were evaluated. |
| Kazak 1998 | This publication has been excluded because there were no adults included in the study. Moreover, psychological and pharmacological interventions rather than meditation were evaluated. |
| Kelly 2009 | Not a randomised controlled trial. |
| Kopp 2001 | Not a randomised controlled trial |
| LaBaw 1975 | Not a randomised controlled trial |
| Lichstein 1985 | Study does not deal with haematological malignancies. |
| Lin 2001 | Not a randomised controlled trial. |
| Lindwall 2014 | This publication has been excluded because there were no adults included in the study. Moreover, non‐pharmacological interventions for parents were evaluated, rather than mediation. |
| Liossi 1999 | This publication has been excluded because there were no adults included in the study. Moreover, hypnosis rather than meditation was evaluated. |
| Liossi 2003 | This publication has been excluded because there were no adults included in the study. Moreover, hypnosis rather than meditation was evaluated. |
| Liossi 2006 | Study does not apply to meditation. |
| Loening‐Baucke 1988 | Study does not apply to haematological malignancies. |
| Maurice‐Stam 2009 | This publication has been excluded because there were no adults included in the study. Moreover, psycho‐educational interventions rather than meditation were evaluated. |
| Pederson 1996 | This publication has been excluded because there were no adults included in the study. Moreover, non‐pharmacological interventions for parents, rather than meditation, were evaluated. |
| Schurman 2010 | This publication has been excluded because there were no adults included in the study. Moreover, bio‐feedback rather than meditation was evaluated. |
| Stringer 2008 | Study does not apply to meditation. |
| Suderman 1990 | Study does not apply to meditation. |
| Walter 1989 | Not a randomised controlled trial. |
| Williams 2006 | Not a randomised controlled trial. |
| Yeh 2006 | Not a randomised controlled trial. |
Differences between protocol and review
In accordance with Methodological Expectations of Cochrane Intervention Reviews (MECIR), we additionally searched the following clinical trial registers which were not listed in the protocol:
EU clinical trials register: https://www.clinicaltrialsregister.eu/ctr‐search/search
Clinicaltrials.gov: https://clinicaltrials.gov/
We were not able to perform meta‐analysis as we had planned to do in the protocol (Salhofer 2014) because we identified only one trial. If the data were similar enough to be combined, we would have merged the results of the studies by applying meta‐analyses. In case of meta‐analyses, we would have assessed heterogeneity of treatment effects between trials by using the Chi² test and to quantify heterogeneity we would have used the I² statistic. We would have performed analyses according to the recommendations of The Cochrane Collaboration (Deeks 2011) using The Cochrane Collaboration's statistical software, Review Manager 2014, for analysis. We would have also investigated possible causes of heterogeneity by using subgroup analyses for the following characteristics:
entity (indolent non‐Hodgkin lymphoma, aggressive non‐Hodgkin lymphoma, acute leukaemia, chronic myeloid leukaemia, multiple myeloma, chronic lymphocytic leukaemia, Hodgkin lymphoma) of underlying disease;
anti‐cancer therapy (first‐line therapy, relapse therapy);
type/duration/intensity of meditation.
To deal with missing data, we would have performed sensitivity analyses such as quality components with regard to low and high risk of bias or fixed‐effect modelling.
Contributions of authors
Ines Salhofer (IS): conception and writing of the review.
Andrea Will (AW): advice and proof reading.
Ina Monsef (IM): search strategy development.
Nicole Skoetz (NS): methodological and clinical expertise and advice, proof reading.
All authors have read and accepted the final version of the review.
Sources of support
Internal sources
-
University Hospital of Cologne, Germany.
Cochrane Haematological Malignancies Group, Department I of Internal Medicine
External sources
No sources of support supplied
Declarations of interest
Ines Salhofer (IS): none known.
Andrea Will (AW): none known.
Ina Monsef (IM): none known.
Nicole Skoetz (NS): none known.
New
References
References to studies included in this review
Cole 2010 {published data only}
- Cole B, Broer K, Hopkins C, Tisak J, Hunt R, McNally C, et al. A randomized controlled trial of spiritually‐focused meditation in patients newly diagnosed with acute leukemia. Blood 2010;116:1519. [Google Scholar]
References to studies excluded from this review
Adamsen 2006 {published data only}
- Adamsen L, Quist M, Midtgaard J, Andersen C, Moller T, Knutsen L, et al. The effect of a multidimensional exercise intervention on physical capacity, well‐being and quality of life in cancer patients undergoing chemotherapy. Supportive Care in Cancer 2006;14:116‐27. [DOI] [PubMed] [Google Scholar]
Bellet 1995 {published data only}
- Bellet PS, Kalinyak KA, Shukla R, Gelfand MJ, Rucknagel DL. Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. New England Journal of Medicine 1995;333(11):699‐703. [DOI] [PubMed] [Google Scholar]
Broome 2001 {published data only}
- Broome ME, Maikler V, Kelber S, Bailey P, Lea G. An intervention to increase coping and reduce health care utilization for school‐age children and adolescents with sickle cell disease. Journal of National Black Nurses Association 2001;12(2):6‐14. [PubMed] [Google Scholar]
Burns 2008 {published data only}
- Burns DS, Azzouz F, Sledge R, Rutledge C, Hincher K, Monahan PO, et al. Music imagery for adults with acute leukemia in protective environments: a feasibility study. Supportive Care in Cancer 2008;16(5):507‐13. [DOI] [PubMed] [Google Scholar]
Chen 1999 {published data only}
- Chen E, Zeltzer LK, Craske MG, Katz ER. Alteration of memory in the reduction of children's distress during repeated aversive medical procedures. Journal of Consulting and Clinical Psychology 1999;67(4):481‐90. [DOI] [PubMed] [Google Scholar]
Collipp 1969 {published data only}
- Collipp PJ. The efficacy of prayer: a triple‐blind study. Medical Times 1969;97(5):201‐4. [PubMed] [Google Scholar]
Gregory‐Addesa 1986 {published data only}
- Gregory‐Addesa G. Helping your patient when nausea goes with the treatment. RN. Journal of Nursing 1986;49:43‐4. [PubMed] [Google Scholar]
Jacknow 1994 {published data only}
- Jacknow DS, Tschann JM, Link MP, Boyce WT. Hypnosis in the prevention of chemotherapy‐related nausea and vomiting in children: a prospective study. Journal of Developmental and Behavioral Pediatrics 1994;15(4):258‐64. [PubMed] [Google Scholar]
Kazak 1996 {published data only}
- Kazak AE, Penati B, Boyer BA, Himelstein B, Brophy P, Waibel MK, et al. A randomized controlled prospective outcome study of a psychological and pharmacological intervention protocol for procedural distress in pediatric leukemia. Journal of Pediatric Psychology 1996;21:615‐31. [DOI] [PubMed] [Google Scholar]
Kazak 1998 {published data only}
- Kazak AE, Penati B, Brophy P, Himelstein B. Pharmacologic and psychologic interventions for procedural pain. Pediatrics 1998;102(1):59‐66. [DOI] [PubMed] [Google Scholar]
Kelly 2009 {published data only}
- Kelly KM. Integrative therapies for children with hematological malignancies. Hematology / the Education Program of the American Society of Hematology 2009:307‐12. [DOI] [PubMed] [Google Scholar]
Kopp 2001 {published data only}
- Kopp M, Holzner B, Brugger A, Nachbaur D. Successful management of claustrophobia and depression during allogeneic SCT. European Journal of Haematology 2001;67:54‐5. [DOI] [PubMed] [Google Scholar]
LaBaw 1975 {published data only}
- LaBaw WL. Auto‐hypnosis in haemophilia. Haematologia 1975;9(1‐2):103‐10. [PubMed] [Google Scholar]
Lichstein 1985 {published data only}
- Lichstein KL, Eakin TL. Progressive versus self‐control relaxation to reduce spontaneous bleeding in hemophiliacs. Journal of Behavioral Medicine 1985;8(2):149‐62. [DOI] [PubMed] [Google Scholar]
Lin 2001 {published data only}
- Lin MC, Nahin R, Gershwin ME, Longhurst JC, Wu KK. State of complementary and alternative medicine in cardiovascular, lung, and blood research: executive summary of a workshop. Circulation 2001;103(16):2038‐41. [DOI] [PubMed] [Google Scholar]
Lindwall 2014 {published data only}
- Lindwall JJ, Russell K, Huang Q, Zhang H, Vannatta K, Barrera M, et al. Adjustment in parents of children undergoing stem cell transplantation. Biology of Blood and Marrow Transplantation 2014;20(4):543‐8. [PUBMED: 24434783] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liossi 1999 {published data only}
- Liossi C, Hatira P. Clinical hypnosis versus cognitive behavioral training for pain management with pediatric cancer patients undergoing bone marrow aspirations. The International Journal of Clinical and Experimental Hypnosis 1999;47(2):104‐16. [DOI] [PubMed] [Google Scholar]
Liossi 2003 {published data only}
- Liossi C, Hatira P. Clinical hypnosis in the alleviation of procedure‐related pain in pediatric oncology patients. The International Journal of Clinical and Experimental Hypnosis 2003;51(1):4‐28. [DOI] [PubMed] [Google Scholar]
Liossi 2006 {published data only}
- Liossi C, White P, Hatira P. Randomized clinical trial of local anesthetic versus a combination of local anesthetic with self‐hypnosis in the management of pediatric procedure‐related pain. Health Psychology 2006;25(3):307‐15. [DOI] [PubMed] [Google Scholar]
Loening‐Baucke 1988 {published data only}
- Loening‐Baucke V, Desch L, Wolraich M. Biofeedback training for patients with myelomeningocele and fecal incontinence. Developmental Medicine and Child Neurology 1988;30(6):781‐90. [DOI] [PubMed] [Google Scholar]
Maurice‐Stam 2009 {published data only}
- Maurice‐Stam H, Silberbusch LM, Last BF, Grootenhuis MA. Evaluation of a psycho‐educational group intervention for children treated for cancer: a descriptive pilot study. Psychooncology 2009;18:762‐6. [DOI] [PubMed] [Google Scholar]
Pederson 1996 {published data only}
- Pederson C. Promoting parental use of nonpharmacologic techniques with children during lumbar punctures. Journal of Pediatric Oncology Nursing 1996;13:21‐30. [DOI] [PubMed] [Google Scholar]
Schurman 2010 {published data only}
- Schurman JV, Wu YP, Grayson P, Friesen CA. A pilot study to assess the efficacy of biofeedback‐assisted relaxation training as an adjunct treatment for pediatric functional dyspepsia associated with duodenal eosinophilia. Journal of Pediatric Psychology 2010;35(8):837‐47. [DOI] [PubMed] [Google Scholar]
Stringer 2008 {published data only}
- Stringer J, Swindell R, Dennis M. Massage in patients undergoing intensive chemotherapy reduces serum cortisol and prolactin. Psychooncology 2008;17(10):1024‐31. [DOI] [PubMed] [Google Scholar]
Suderman 1990 {published data only}
- Suderman JR. Pain relief during routine procedures for children with leukemia. MCN The American Journal of Maternal Child Nursing 1990;15:163‐6. [PubMed] [Google Scholar]
Walter 1989 {published data only}
- Walter T, Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics 1989;84(1):7‐17. [PubMed] [Google Scholar]
Williams 2006 {published data only}
- Williams PD, Piamjariyakul U, Ducey K, Badura J, Boltz KD, Olberding K, et al. Cancer treatment, symptom monitoring, and self‐care in adults: pilot study. Cancer Nursing 2006;29:347‐55. [DOI] [PubMed] [Google Scholar]
Yeh 2006 {published data only}
- Yeh ML, Lee TI, Chen HH, Chao TY. The influences of Chan‐Chuang qi‐gong therapy on complete blood cell counts in breast cancer patients treated with chemotherapy. Cancer Nursing 2006;29(2):149‐55. [DOI] [PubMed] [Google Scholar]
Additional references
Carlson 2005
- Carlson LE, Garland SN. Impact of mindfulness‐based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. International Journal of Behavioral Medicine 2005;12(4):278‐85. [PUBMED: 16262547] [DOI] [PubMed] [Google Scholar]
Carlson 2007
- Carlson LE, Speca M, Faris P, Patel KD. One year pre‐post intervention follow‐up of psychological, immune, endocrine and blood pressure outcomes of mindfulness‐based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, Behavior, and Immunity 2007;21(8):1038‐49. [PUBMED: 17521871] [DOI] [PubMed] [Google Scholar]
Cella 1993
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11(3):570‐9. [PUBMED: 8445433] [DOI] [PubMed] [Google Scholar]
Cohen 1995
- Cohen SR, Mount BM, Strobel MG, Bui F. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliative Medicine 1995;9(3):207‐19. [DOI] [PubMed] [Google Scholar]
Cramer 2012
- Cramer H, Lauche R, Paul A, Dobos G. Mindfulness‐based stress reduction for breast cancer‐ a systematic review and meta‐analysis. Current Oncology 2012;19(5):e343‐52. [PUBMED: 23144582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Foley 2010
- Foley E, Baillie A, Huxter M, Price M, Sinclair E. Mindfulness‐based cognitive therapy for individuals whose lives have been affected by cancer: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2010;78(1):72‐9. [PUBMED: 20099952] [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. Version 01.11.2015. www.gradepro.org. McMaster University, 2014.
Grossman 2004
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness‐based stress reduction and health benefits. A meta‐analysis. Journal of Psychosomatic Research 2004;57(1):35‐43. [PUBMED: 15256293] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howlader 2013
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975‐2010. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2010/ based on November 2012 SEER data submission, posted to the SEER web site, April 2013 (accessed 23 June 2013).
Kang 2012
- Kang G, Oh S. Effects of mindfulness meditation program on perceived stress, ways of coping, and stress response in breast cancer patients. Journal of Korean Academy of Nursing 2012;42(2):161‐70. [PUBMED: 22699165] [DOI] [PubMed] [Google Scholar]
Lane 2007
- Lane JD, Seskevich JE, Pieper CF. Brief meditation training can improve perceived stress and negative mood. Alternative Therapies in Health and Medicine 2007;13(1):38‐44. [PUBMED: 17283740] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J (editors). Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Longo 2011
- Longo D, Fauci A, Kasper D, Hauser S, Jameson JL, Loscalzo J. Harrison's Principles of Internal Medicine. 18th Edition. Bethesda, MD: National Institute of Health, 2011. [Google Scholar]
Mitchell 2011
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncology 2011;12(2):160‐74. [PUBMED: 21251875] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
NCCAM 2013
- National Center for Complementary and Alternative Medicine (NCCAM). Meditation: an introduction. http://nccam.nih.gov/health/meditation/overview.htm (accessed 24 June 2013).
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schneider 1995
- Schneider RH, Staggers F, Alxander CN, Sheppard W, Rainforth M, Kondwani K, et al. A randomised controlled trial of stress reduction for hypertension in older African Americans. Hypertension 1995;26(5):820‐7. [PUBMED: 7591024] [DOI] [PubMed] [Google Scholar]
Schneider 2006
- Schneider RH, Walton KG, Salerno JW, Nidich SI. Cardiovascular disease prevention and health promotion with the transcendental meditation program and Maharishi consciousness‐based health care. Ethnicity & Disease 2006;16(3 Suppl 4):S4‐15‐26. [PUBMED: 16938913] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH (editors). Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials Journal 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dam 2011
- Dam NT, Earleywine M. Validation of the Center for Epidemiologic Studies Depression Scale‐‐Revised (CESD‐R): pragmatic depression assessment in the general population. Psychiatry Research 2011;186(1):128‐32. [DOI] [PubMed] [Google Scholar]
Witek‐Janusek 2008
- Witek‐Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo‐Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain, Behavior, and Immunity 2008;22(6):969‐81. [PUBMED: 18359186] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Salhofer 2014
- Salhofer I, Rancea M, Will A, Monsef I, Engert A, Skoetz N. Meditation for adult patients with haematological malignancies. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD011157] [DOI] [Google Scholar]


