Abstract
Mitochondrial permeability transition pore (mPTP) is a large channel located in the mitochondrial inner membrane. The opening of mPTP during pathological calcium overload leads to the membrane depolarization and disruption of ATP production. mPTP activation has been implicated as a central event during the process of stress-induced cell death. mPTP is a supramolecular complex composed of many proteins. Recent studies suggest that mitochondrial ATPase plays the central role in the formation of mPTP. However, the structure of the central conducting pore part of mPTP (mPTPore) remains elusive. Here we review current models proposed for the mPTPore and involvement of polyP in its formation and regulation. We discuss the underestimated role of polyP as an effector and a putative structural component of the mPTPore. We propose the hypothesis that inclusion of polyP can explain such properties of mPTP activity as calcium activation, selectivity and voltage-dependence.
Keywords: calcium, inorganic polyphosphate (polyP), mitochondria, permeability transition
Introduction
In the past few years, considerable attention has been directed towards an investigation of the multiple roles played by polyP in mammalian organisms [1–6]. Due to its molecular nature and its ubiquitous presence, polyP can be potentially involved in a plethora of biological processes. The role of polyP is particularly prominent in mitochondria where, due to the presence of phosphoanhydride bonds, similar to ones found in ATP, it participates in energy homoeostasis and is also capable to form complexes with divalent ions, including magnesium and calcium [7]. Of particular interest is the involvement of polyP in the mitochondrial response to pathological conditions. Whereas multiple possible mechanisms of polyP participation might exist, molecular details of these processes remain largely unknown. Here we will discuss the molecular mechanisms underlying the contribution of polyP towards the induction of the pathological mitochondrial membrane permeability (permeability transition) that occurs during cellular stress. We will review current experimental data that support the idea of the direct involvement of polyP in the activation and formation of the mitochondrial permeability transition pore (mPTP).
Permeability transition pore phenomenon
Mitochondria play a central role in cellular energy metabolism, as well as in Ca2+ and reactive oxygen species (ROS) signalling [8–11]. It has been established that loss of mitochondrial function is a major contributor to stress-induced necrotic and apoptotic cell death [12–15]. In terms of relevant diseases, one of the most significant and well-documented examples is the role of mitochondrial loss of function in tissue damage during the stroke [15–18]. Specifically, it has been found that ischemia occurring during stroke followed by re-oxygenation induces profound mitochondrial damage that causes cell death [19–21]. These observations lead to the paradigm in which the protection and restoration of mitochondrial function during acute stress is pivotal to cell survival. Indeed, this concept has been supported by a vast body of experimental data, ranging from cell culture models to human clinical trials [19,22–26]. Elucidation of the mechanisms responsible for the mitochondrial damage is considered a promising approach for the discovery of novel therapeutics for currently untreatable conditions resulting from apoptotic and necrotic cell death. At the molecular level, a central event responsible for the loss of mitochondrial function is the sudden and dramatic increase in the permeability of the mitochondrial inner membrane. This phenomenon, termed permeability transition, causes dissipation of the electrochemical potential across the mitochondrial inner membrane which is the essential driving force for ATP production by mitochondria and, thus, its loss immediately stops mitochondrial ATP production and, if not corrected in a timely fashion, results in cell death [27–30]. Key factors contributing to the activation of mPTP during acute stress are elevated levels of matrix Ca2+ and ROS. Prevention of mPTP opening during stress helps to maintain inner membrane integrity and ATP production. In this important way, inhibition of mPTP opening can prevent cell death [27,29,31–39]. Whereas mPTP in most cases is considered as a pathological event, its possible significance in normal physiology in the heart has recently been proposed[40].
Permeability transition pore structure: current views
Structurally, mPTP is believed to be a supra-molecular complex composed of dozens of proteins [41,42]. Genetic and pharmacological manipulations of various proteins, peripheral members of the complex, have established the proof of principle that mPTP inhibition is protective against cell death. One of the best-described proteins involved in mPTP activation is cyclophilin D. This protein is implicated as a key regulator of mPTP activity and cell death [31,43,44]. More recent studies have identified several proteins that might play a critical role in mPTP activation [45–47]. These putative components of the mPTP include SPG7, p53 and proteins from the Bcl-2 family, as Bax and Bak. SPG7 has been proposed to be a core conserved protein involved in mPTP activation and located at the contact sites between mitochondrial inner and outer membranes [45]. p53 protein involvement in triggering the mPTP opening is associated with its ability to interact directly with cyclophilin D [46]. Finally, Bax and Bak, channel-forming proteins of the outer membrane are proposed to be required for activation of mPTP opening in the inner membrane in a large pore conformation [47].
Interestingly, the most critical question on the molecular identity of the central ‘channel’ part of mPTP remains unresolved. Here we will refer to the channel part of the complete mPTP complex as ‘mPTPore’ (pore part of mPTP). Since the discovery of the mPTP, several protein candidates have been considered to play the part of the mPTPore including adenine nucleotide translocator (ANT)[48], phosphate carrier (PiC) [49] and ‘misfolded’ proteins[50]. Although experimental data confirm the involvement of these components in mPTP, none of them appear to be essential [51–53], suggesting that they are not the integral part of the mPTPore domain. Recent studies, performed independently in three laboratories, put forward a new concept: the mPTPore is associated with mitochondrial ATPase, a supramolecular complex containing matrix (F1) and membrane (F0) parts and composed of approximately 30 individual proteins [54–56]. However, experimental data currently available do not provide a conclusive mechanism for the mPTPore formation or its molecular composition. Both of these questions are the subject of hot debate (Figure 1 for currently proposed models). Bernardi and colleagues [41,55] propose that mPTPore probably forms in between the two c-ring subdomains of the ATPase. Opposite, recent reports from Jonas and colleagues [42,54] put forward an alternative view. They showed that the mPTPore part of mPTP is probably associated with the C-subunit protein ring of the ATPase. Whereas the evidence for the involvement of C-subunit is supported by a number of experimental observations [54,56], it is also evident that the C-subunit on its own is not sufficient to explain the behaviour of the mPTP channel [41]. Indeed, the peptide sequence C-subunit consists almost entirely of hydrophobic amino acids. Highly hydrophobic peptides, such as C-subunit, even when assembled into oligomers, are not expected to form a structure that will allow passage of water and ions, essential requirement for a high-conductance low-selectivity channel-like mPTPore. Structural studies confirm that C-subunit oligomers are expected to be associated with hydrophobic molecules of lipid nature, rather than with water [57,58]. Another source of controversy regarding the mPTP activation is the nature of F0–F1 interactions during this process. Alavian et al. [54] present an evidence that that these interactions can weaken during mPTP opening. However, Bernardi et al. [41] argue that dissociation of F0 from F1 would require rather harsh conditions, which is very unlikely in vivo. It should also be noted that Halestrap [59] suggests that ATPase, ANT and PiC could all be equally important and theorizes that mPTPore exists within the complex at the interface of these proteins, without giving details; however, concerning the mechanism of pore assembly. Finally, very recently an alternative hypothesis that does not require the involvement of C-subunit has been put forward [45]. Overall, despite significant recent progress the subject of the molecular nature of the mPTPore currently is not well resolved.
Figure 1 |. Proposed mechanisms of the mPTP transition from closed to the open state.
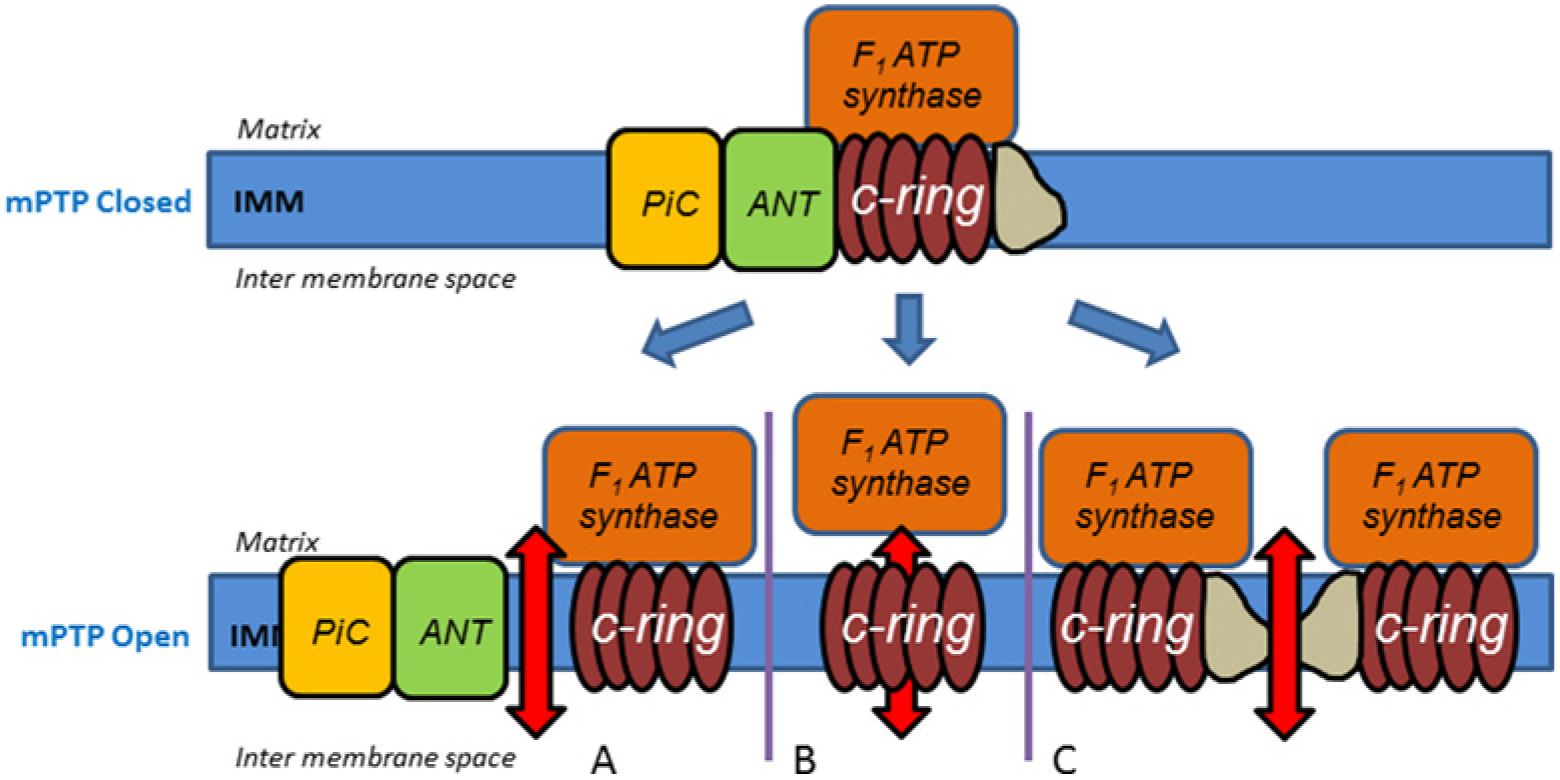
(A) mPTPore is formed at the intersection between ANT, PiC and ATPase [49]. (B) mPTPore is formed within the C-ring of ATPase [45]. (C) mPTP is formed between two monomers of the ATPase [46].
Permeability transition pore activation by polyP
Most of the recent efforts in the investigation of the mPTP have been focused on identification of its protein components. Interestingly, even with recent additions of the protein components of ATPase to the picture, key the properties of the mPTPpore remain unexplained. For example, it is well established that mPTP activation is sensitive to the concentration of free matrix calcium, the condition that is expected to occur during stress related to ischaemic injury [60–62]. However, at the level of the intact mitochondria, mPTP activation can be achieved by the accumulation of calcium inside the matrix without the increase in the concentration of free calcium [63]. These experiments suggest that, contrary to general belief, mPTP is not directly activated by calcium. Electrophysiological recordings demonstrate that mPTP is a channel with well-defined conductance states, selectivity pattern and voltage-dependence [64,65]. This indicates the presence of the core structure that can form stable conducting pathway and presumably contains a number of functional groups responsible for characteristic channel properties. Although the ‘ring’ formed by C-subunit oligomers cannot form a complete channel on its own, considering its structure it can provide the backbone for a conducting pore.
It is possible that the above mentioned apparent controversies can be resolved if non-protein components of mPTP complex are taken into consideration. In the past, some of the non-protein components have been implicated in mPTP activity, including non-esterified fatty acids [66–68] as well as mPTP activation through interactions that involve phospholipid bilayers [69]. However, lipid-based pores are not expected to form stable and well-defined channels as in the case of mPTP (reviewed recently in [70]). On the other hand, polyP is known to be an integral and essential part of fully synthetic ion channels in lipid bilayers [71]. In living organisms, polyP is known to be a central participant in calcium signalling [4], as well as being a structural component of biological ion channels [72–74]. In mammalian mitochondria, polyP is found to be associated with highly purified channel fraction that resembles native mPTP when reconstituted into lipid bilayers (BLM). Involvement of polyP in mPTP has also been confirmed in experiments with living cells. When mitochondria are depleted of polyP, they either do not undergo calcium-induced mPTP or its opening is significantly delayed. In these experiments in cultured cells, polyP was hydrolysed by targeted mitochondrial overexpression of the specific polyphosphatase. Under these conditions, despite active calcium uptake, mitochondria did not develop mPTP. Importantly, similar results were found for different cell types, including stable cultured cells as well as neuronal and cardiac primary cultures [75–78].
Taking into account the multiple roles played by polyP in cell physiology, it is most likely that PolyP regulation of mPTP occurs via several independent mechanisms. PolyP is involved in mitochondrial energy metabolism and activity of the respiratory chain [75,79]. Thus, polyP can modulate the mPTP activation through indirect effects on mitochondrial bioenergetics. More direct effects of polyP might involve its potential ability to contribute towards calcium–phosphate interactions [63]. This property of polyP has not been investigated in the context of mitochondria but has been demonstrated in tissue calcification [80]. mPTP activation requires accumulation of significant amounts of calcium inside mitochondrial matrix. This accumulation does not change the free calcium concentration but leads to the increase in the amounts of calcium-phosphate precipitates of unknown forms [81]. Thus, it is possible that the lack of polymerized form of phosphate might shift the nature and amounts of the calcium precipitates and, by doing so, change the amount of calcium required for mPTP activation.
Can polyP be a structural part of mPTPpore?
In addition to the above-mentioned indirect roles of polyP in mPTP activation, polyP can be directly involved as an essential structural component of mPTPore. It has been demonstrated before in experiments with synthetic polymers, as well as with polymers of bacterial origin that polyP in combination with polyhydroxybutyrate (PHB) can form stable ion channels [71,73]. In mammalian mitochondria, PHB has been implicated as an endogenous ionophore that can be involved in calcium transport [82–84]. Interestingly, in bacteria dramatic increase in this channel assembly is stimulated by the addition of calcium [73,85]. In mammalian organisms, the essential participation of polyP was demonstrated for protein-based TRPM8 channel [72]. Channels formed by the complex of polyP and PHB have also been purified from mammalian mitochondria that were pre-treated with calcium. Importantly, this highly purified mitochondrial extract was essentially protein-free, except of C-subunit of the ATP-synthase. The channel activity of this highly purified complex was not sensitive to the cyclosporine A due to the absence of cyclophilin D but resembled all the key electrophysiological features of the mPTPore channel as seen in native mitochondrial membranes [86]. It is conceivable that in the case of mammalian mitochondria, the formation of mPTP might occur through a calcium-induced assembly of the channel forming complex made of C-subunit, PHB and polyP. Putative model illustrating of how these assemblies might occur during calcium overload is presented in Figure 2. It should be noted that dissociation of F1 from F0 proposed by Alavian et al. [54] and adapted for Figure 2 might not necessarily take place [41]. However, during stress conditions, morphology of the F0–F1 complex could undergo significant changes. Involvement of polyP can explain the phenomenon of calcium activation of mPTP, through the involvement of calcium–phosphate interactions. Further, the presence of polyP and PHB provides an explanation on how a highly hydrophobic protein such as C-subunit can participate in the formation of ion water filled ion-conducting pore. In this scenario, the amphipathic PHB polymer can modify C-subunit in the inside part of the ‘ring’ and, by folding, provide a hydrophilic centre that can interact with calcium and polyP providing a microenvironment that allows ion passage by the pore mechanism (Figure 2). Finally, as a highly charged polymer, polyP is capable of playing a role as the voltage sensor and selectivity filter of mPTP. It is noteworthy that removal of polyP from TRPM8 channel dramatically changes voltage-sensitivity of this channel [72].
Figure 2 |. Putative mechanism of the polyP participation in mPTPore.

(A) Under normal conditions (mPTP closed) ATPase complex is fully assembled with PHB associated with the C-subunit and F1 complex. (B). During pathological conditions, Ca2+ and polyP induce dissociation of F1 from C-ring and formation of the mPTPore complex from C-subunit, PHB, Ca2+ and polyP.
Conclusions
In conclusion, we propose the hypothesis that essential role of polyP in mPTP activation might be explained by its direct participation in the formation of mPTPore. Participation of polyP will involve calcium-induced interactions with protein parts of ATPase, most probably its C-subunit. As a highly charged structural component of mPTP, polyP could hypothetically function as the voltage sensor of mPTPore and be responsible for its selectivity. Further studies are needed to confirm the molecular details of polyP involvement in mPTP ion channel function.
Funding
NYU University Research Challenge Fund to EP.
Abbreviations:
- ANT
adenine nucleotide translocator
- mPTP
mitochondrial permeability transition pore
- mPTPore
pore part of mPTP
- PHB
polyhydroxybutyrate
- PiC
phosphate carrier
- ROS
reactive oxygen species
References
- 1.Smith SA, Choi SH, Collins JN, Travers RJ, Cooley BC and Morrissey JH (2012) Inhibition of polyphosphate as a novel strategy for preventing thrombosis and inflammation. Blood 120, 5103–5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA and Docampo R (2012) Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J. Biol. Chem 287, 28435–28444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiba T, Nishimura D, Kawazoe Y, Onodera Y, Tsutsumi K, Nakamura R and Ohshiro M (2003) Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J. Biol. Chem 278, 26788–26792 [DOI] [PubMed] [Google Scholar]
- 4.Holmstrom KM, Marina N, Baev AY, Wood NW, Gourine AV and Abramov AY (2013) Signalling properties of inorganic polyphosphate in the mammalian brain. Nat. Commun 4, 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller WE, Wang X, Diehl-Seifert B, Kropf K, Schlossmacher U, Lieberwirth I, Glasser G, Wiens M and Schroder HC (2011) Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 7, 2661–2671 [DOI] [PubMed] [Google Scholar]
- 6.Stotz SC, Scott LO, Drummond-Main C, Avchalumov Y, Girotto F, Davidsen J, Gomez-Garcia MR, Rho JM, Pavlov EV and Colicos MA (2014) Inorganic polyphosphate regulates neuronal excitability through modulation of voltage-gated channels. Mol. Brain 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulakovskaya TV, Lichko LP, Vagabov VM and Kulaev IS (2010) Inorganic polyphosphates in mitochondria. Biochemistry 75, 825–831 [DOI] [PubMed] [Google Scholar]
- 8.David G, Nicholls and Stuart J. Ferguson (2013), Bioenergetics 4, Academic Press, Amsterdam [Google Scholar]
- 9.Brookes PS, Yoon Y, Robotham JL, Anders MW and Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol 287, C817–C833 [DOI] [PubMed] [Google Scholar]
- 10.Duchen MR (2004) Roles of mitochondria in health and disease. Diabetes 53 (Suppl 1), S96–S102 [DOI] [PubMed] [Google Scholar]
- 11.Duchen MR (2000) Mitochondria and Ca(2 +)in cell physiology and pathophysiology. Cell Calcium 28, 339–348 [DOI] [PubMed] [Google Scholar]
- 12.Green DR and Reed JC (1998) Mitochondria and apoptosis. Science 281, 1309–1312 [DOI] [PubMed] [Google Scholar]
- 13.Danial NN and Korsmeyer SJ (2004) Cell death: critical control points. Cell 116, 205–219 [DOI] [PubMed] [Google Scholar]
- 14.Kroemer G, Dallaporta B and Resche-Rigon M (1998) The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol 60, 619–642 [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP and Kroemer G (2003) Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol. Med 9, 196–205 [DOI] [PubMed] [Google Scholar]
- 16.Sims NR and Muyderman H (2010) Mitochondria, oxidative metabolism and cell death in stroke. Biochim. Biophys. Acta 1802, 80–91 [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6, 337–350 [DOI] [PubMed] [Google Scholar]
- 18.Sluijter JP, Condorelli G, Davidson SM, Engel FB, Ferdinandy P, Hausenloy DJ, Lecour S, Madonna R, Ovize M, Ruiz-Meana M et al. (2014) Novel therapeutic strategies for cardioprotection. Pharmacol. Ther 144, 60–70 [DOI] [PubMed] [Google Scholar]
- 19.Christophe M and Nicolas S (2006) Mitochondria: a target for neuroprotective interventions in cerebral ischemia-reperfusion. Curr. Pharm. Des 12, 739–757 [DOI] [PubMed] [Google Scholar]
- 20.Starkov AA, Chinopoulos C and Fiskum G (2004) Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium 36, 257–264 [DOI] [PubMed] [Google Scholar]
- 21.Baines CP (2010) The cardiac mitochondrion: nexus of stress. Annu. Rev. Physiol 72, 61–80 [DOI] [PubMed] [Google Scholar]
- 22.Hirakawa A, Takeyama N, Nakatani T and Tanaka T (2003) Mitochondrial permeability transition and cytochrome c release in ischemia-reperfusion injury of the rat liver. J. Surg. Res 111, 240–247 [DOI] [PubMed] [Google Scholar]
- 23.Singh D, Chander V and Chopra K (2005) Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology 207, 339–347 [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto T and Siesjo BK (1999) Posttreatment with the immunosuppressant cyclosporin A in transient focal ischemia. Brain Res. 839, 283–291 [DOI] [PubMed] [Google Scholar]
- 25.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D and Ovize M (2005) Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J. Mol. Cell. Cardiol 38, 367–374 [DOI] [PubMed] [Google Scholar]
- 26.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D et al. (2008) Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med 359, 473–481 [DOI] [PubMed] [Google Scholar]
- 27.Baines CP (2009) The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res. Cardiol 104, 181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79, 1127–1155 [DOI] [PubMed] [Google Scholar]
- 29.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di LF and Forte MA (2006) The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 273, 2077–2099 [DOI] [PubMed] [Google Scholar]
- 30.Gunter TE and Sheu SS (2009) Characteristics and possible functions of mitochondrial Ca(2 +) transport mechanisms. Biochim. Biophys. Acta 1787, 1291–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW et al. (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 [DOI] [PubMed] [Google Scholar]
- 32.Das DK and Maulik N (2005) Mitochondrial function in cardiomyocytes: target for cardioprotection. Curr. Opin. Anaesthesiol 18, 77–82 [DOI] [PubMed] [Google Scholar]
- 33.Hausenloy DJ, Ong SB and Yellon DM (2009) The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res. Cardiol 104, 189–202 [DOI] [PubMed] [Google Scholar]
- 34.Kinnally KW, Peixoto PM, Ryu SY and Dejean LM (2011) Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim. Biophys. Acta 1813, 616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristal BS, Stavrovskaya MV, Narayanan BF, Krasnikov AM, Brown MF, Beal IG and Friedlander RM (2004) The mitochondrial permeability transition as a target for neuroprotection. J. Bioenerg. Biomembr 36, 309–312 [DOI] [PubMed] [Google Scholar]
- 36.Lemasters JJ, Theruvath TP, Zhong Z and Nieminen AL (2009) Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta 1787, 1395–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morin D, Assaly R, Paradis S and Berdeaux A (2009) Inhibition of mitochondrial membrane permeability as a putative pharmacological target for cardioprotection. Curr. Med. Chem 16, 4382–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norenberg MD and Rao KV (2007) The mitochondrial permeability transition in neurologic disease. Neurochem. Int 50, 983–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stavrovskaya IG and Kristal BS (2005) The powerhouse takes control of the cell: is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic. Biol. Med 38, 687–697 [DOI] [PubMed] [Google Scholar]
- 40.Kwong JQ and Molkentin JD (2015) Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 21, 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernardi P, Rasola A, Forte M and Lippe G (2015) The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol. Rev 95, 1111–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jonas EA, Porter GA Jr, Beutner G, Mnatsakanyan N and Alavian KN (2015) Cell death disguised: the mitochondrial permeability transition pore as the c-subunit of the FF ATP synthase. Pharmacol. Res 99, 382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffiths EJ and Halestrap AP (1991) Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. Biochem. J 274(Pt 2), 611–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halestrap AP, Connern CP, Griffiths EJ and Kerr PM (1997) Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol. Cell Biochem 174, 167–172 [PubMed] [Google Scholar]
- 45.Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, Nemani N, Hines KJ, Smith DJ, Eguchi A, Vallem S et al. (2015) SPG7 is an essential and conserved component of the mitochondrial permeability transition pore. Mol. Cell 60, 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S and Moll UM (2012) p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149, 1536–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J et al. (2013) Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2, e00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brustovetsky N and Klingenberg M (1996) Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+. Biochemistry 35, 8483–8488 [DOI] [PubMed] [Google Scholar]
- 49.Leung AW, Varanyuwatana P and Halestrap AP (2008) The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem 283, 26312–26323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA and Herman B (1998) The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1366, 177–196 [DOI] [PubMed] [Google Scholar]
- 51.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR and Wallace DC (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427, 461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baines CP, Kaiser RA, Sheiko T, Craigen WJ and Molkentin JD (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol 9, 550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, Wang X, Huang T and Molkentin JD (2014) Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ 21, 1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr and Jonas EA (2014) An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. U.S.A 111, 10580–10585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giorgio V, von SS, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I et al. (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. U.S.A 110, 5887–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonora M, Bononi A, De ME, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A et al. (2013) Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12, 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker JE (2013) The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans 41, 1–16 [DOI] [PubMed] [Google Scholar]
- 58.Dmitriev OY, Jones PC and Fillingame RH (1999) Structure of the subunit c oligomer in the F1Fo ATP synthase: model derived from solution structure of the monomer and cross-linking in the native enzyme. Proc. Natl. Acad. Sci. U.S.A 96, 7785–7790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halestrap AP (2014) The C ring of the F1Fo ATP synthase forms the mitochondrial permeability transition pore: a critical appraisal. Front Oncol 4, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halestrap AP (2006) Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans 34, 232–237 [DOI] [PubMed] [Google Scholar]
- 61.McStay GP, Clarke SJ and Halestrap AP (2002) Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. J 367, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Connern CP and Halestrap AP (1996) Chaotropic agents and increased matrix volume enhance binding of mitochondrial cyclophilin to the inner mitochondrial membrane and sensitize the mitochondrial permeability transition to [Ca2 +]. Biochemistry 35, 8172–8180 [DOI] [PubMed] [Google Scholar]
- 63.Chalmers S and Nicholls DG (2003) The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem 278, 19062–19070 [DOI] [PubMed] [Google Scholar]
- 64.Kinnally KW, Zorov D, Antonenko Y and Perini S (1991) Calcium modulation of mitochondrial inner membrane channel activity. Biochem. Biophys. Res. Commun 176, 1183–1188 [DOI] [PubMed] [Google Scholar]
- 65.Szabo I and Zoratti M (1992) The mitochondrial megachannel is the permeability transition pore. J. Bioenerg. Biomembr 24, 111–117 [DOI] [PubMed] [Google Scholar]
- 66.Mironova GD, Lazareva A, Gateau-Roesch O, Tyynela J, Pavlov Y, Vanier M and Saris NE (1997) Oscillating Ca2+ -induced channel activity obtained in BLM with a mitochondrial membrane component. J. Bioenerg. Biomembr 29, 561–569 [DOI] [PubMed] [Google Scholar]
- 67.Gateau-Roesch O, Pavlov E, Lazareva AV, Limarenko EA, Levrat C, Saris NE, Louisot P and Mironova GD (2000) Calcium-binding properties of the mitochondrial channel-forming hydrophobic component. J. Bioenerg. Biomembr 32, 105–110 [DOI] [PubMed] [Google Scholar]
- 68.Mironova GD, Gateau-Roesch O, Levrat C, Gritsenko E, Pavlov E, Lazareva AV, Limarenko E, Rey C, Louisot P and Saris NE (2001) Palmitic and stearic acids bind Ca2+ with high affinity and form nonspecific channels in black-lipid membranes. Possible relation to Ca2+ -activated mitochondrial pores. J. Bioenerg. Biomembr 33, 319–331 [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto T, Ito M, Kageyama K, Kuwahara K, Yamashita K, Takiguchi Y, Kitamura S, Terada H and Shinohara Y (2014) Mastoparan peptide causes mitochondrial permeability transition not by interacting with specific membrane proteins but by interacting with the phospholipid phase. FEBS J. 281, 3933–3944 [DOI] [PubMed] [Google Scholar]
- 70.Szabo I and Zoratti M (2014) Mitochondrial channels: ion fluxes and more. Physiol Rev. 94, 519–608 [DOI] [PubMed] [Google Scholar]
- 71.Das S, Lengweiler UD, Seebach D and Reusch RN (1997) Proof for a nonproteinaceous calcium-selective channel in Escherichia coli by total synthesis from (R)-3-hydroxybutanoic acid and inorganic polyphosphate. Proc. Natl. Acad. Sci. U.S.A 94, 9075–9079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zakharian E, Thyagarajan B, French RJ, Pavlov E and Rohacs T (2009) Inorganic polyphosphate modulates TRPM8 channels. PLoS. One 4, e5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reusch RN and Sadoff HL (1988) Putative structure and functions of a poly-beta-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc. Natl. Acad. Sci. U.S.A 85, 4176–4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Negoda A, Xian M and Reusch RN (2007) Insight into the selectivity and gating functions of Streptomyces lividans KcsA. Proc. Natl. Acad. Sci. U.S.A 104, 4342–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abramov AY, Fraley C, Diao CT, Winkfein R, Colicos MA, Duchen MR, French RJ and Pavlov E (2007) Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc. Natl. Acad. Sci. U.S.A 104, 18091–18096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seidlmayer LK, Blatter LA, Pavlov E and Dedkova EN (2012) Inorganic polyphosphate-an unusual suspect of the mitochondrial permeability transition mystery. Channels 6, 463–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seidlmayer LK, Gomez-Garcia MR, Blatter LA, Pavlov E and Dedkova EN (2012) Inorganic polyphosphate is a potent activator of the mitochondrial permeability transition pore in cardiac myocytes. J. Gen. Physiol 139, 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seidlmayer LK, Juettner VV, Kettlewell S, Pavlov EV, Blatter LA and Dedkova EN (2015) Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2 +, ROS, pH, and inorganic polyphosphate. Cardiovasc. Res 106, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pavlov E, Aschar-Sobbi R, Campanella M, Turner RJ, Gomez-Garcia MR and Abramov AY (2010) Inorganic polyphosphate and energy metabolism in mammalian cells. J. Biol. Chem 285, 9420–9428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omelon S, Georgiou J, Henneman ZJ, Wise LM, Sukhu B, Hunt T, Wynnyckyj C, Holmyard D, Bielecki R and Grynpas MD (2009) Control of vertebrate skeletal mineralization by polyphosphates. PLoS One 4, e5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nicholls DG and Chalmers S (2004) The integration of mitochondrial calcium transport and storage. J. Bioenerg. Biomembr 36, 277–281 [DOI] [PubMed] [Google Scholar]
- 82.Smithen M, Elustondo PA, Winkfein R, Zakharian E, Abramov AY and Pavlov E (2013) Role of polyhydroxybutyrate in mitochondrial calcium uptake. Cell Calcium 54, 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elustondo PA, Angelova PR, Kawalec M, Michalak M, Kurcok P, Abramov AY and Pavlov EV (2013) Polyhydroxybutyrate targets mammalian mitochondria and increases permeability of plasmalemmal and mitochondrial membranes. PLoS One 8, e75812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kane DA and Pavlov EV (2013) Calculation of ion currents across the inner membrane of functionally intact mitochondria. Channels 7, 426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reusch RN, Hiske TW and Sadoff HL (1986) Poly-beta-hydroxybutyrate membrane structure and its relationship to genetic transformability in Escherichia coli. J. Bacteriol 168, 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pavlov E, Zakharian E, Bladen C, Diao CT, Grimbly C, Reusch RN and French RJ (2005) A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys. J 88, 2614–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]


