Abstract
There is a widespread consensus that the earth is experiencing a mass extinction event and at the forefront are amphibians, the most threatened of all vertebrate taxa. A recent assessment found that nearly one-third (32%, 1,856 species) of the world’s amphibian species are threatened. Amphibians have existed on the earth for over 300 million years, yet in just the last two decades there have been an alarming number of extinctions, nearly 168 species are believed to have gone extinct and at least 2,469 (43%) more have populations that are declining. Infectious diseases have been recognized as one major cause of worldwide amphibian population declines. This could be the result of the appearance of novel pathogens, or it could be that exposure to environmental stressors is increasing the susceptibility of amphibians to opportunistic pathogens. Here I review the potential effects of stressors on disease susceptibility in amphibians and relate this to disease emergence in human and other wildlife populations. I will present a series of case studies that illustrate the role of stress in disease outbreaks that have resulted in amphibian declines. First, I will examine how elevated sea-surface temperatures in the tropical Pacific since the mid-1970s have affected climate over much of the world and could be setting the stage for pathogen-mediated amphibian declines in many regions. Finally, I will discuss how the apparently rapid increase in the prevalence of amphibian limb deformities is linked to the synergistic effects of trematode infection and exposure to chemical contaminants.
Keywords: Global amphibian declines, Stress-mediated infection, Climate change, Pesticide exposure
Introduction
What does the global decline in amphibians have to do with disease outbreaks in human populations and other species of wildlife? In the past two decades at least 20 major human diseases have reemerged in novel, more virulent forms (Epstein 1999). Worldwide, scientists have discovered at least 30 previously unknown human diseases, such as Ebola, AIDS, and SARS (Fauci 2001; Weinhold 2004). Moreover, the last decade has seen a number of epidemics that have caused large-scale declines in several wildlife species (Dobson and Carper 1993; Daszak et al. 2000). These changes have occurred in synchrony with the global decline of amphibian species (Blaustein and Kiesecker 2002; Collins and Storfer 2003; Semlitsch 2003). The global loss of amphibian populations was first recognized in 1989 as a phenomenon that deserved world-wide attention (Blaustein and Wake 1990; Blaustein et al. 1994). To date, approximately 168 species of frogs and salamanders are severely threatened or have gone extinct (GAA 2004). Included with many of these declines are reports of massive mortality associated with pathogenic infection and an increased incidence of developmental malformations (Daszak et al. 2001; Blaustein and Kiesecker 2002). In some populations, more than 80% of the individuals exhibit severe deformities, including extra or missing limbs (Ankley et al. 2002; Johnson et al. 2001, 2002; Johnson and Sutherland 2003; see also Hayes et al. 2002a, 2002b, 2003). The widespread nature of the declines and the apparent increase in the prevalence of deformities has lead to substantial interest from scientists and the general public.
How is the global decline of amphibians related to increased disease prevalence in human and wildlife populations? As human populations have grown, the world has dramatically changed. Between one-third and one-half of Earth’s land surface has been altered by human action (Vitousek et al. 1997). The concentration of carbon dioxide in the atmosphere has increased by approximately 30% since the late 1800s. Human actions result in the fixation of more nitrogen than all natural terrestrial sources and over half of accessible surface freshwater is utilized by human actions (Vitousek et al. 1997). I suggest that these changes have created an environment that fosters emergence of infectious diseases for both wildlife and human populations (Fig. 1).
Fig. 1.
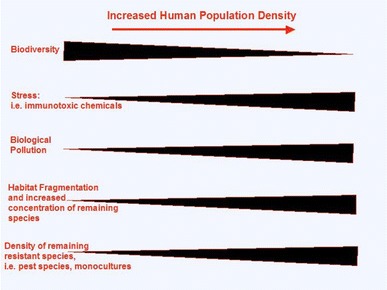
Relationship of habitat alteration often associated with increased human development that may alter disease dynamics
Emerging diseases are those that have increased in incidence, pathogenicity, geographic range, have shifted hosts or recently evolved new strains (Daszak et al. 2000). Emerging infectious diseases fall into two overarching categories. The first category includes parasitic organisms that have invaded into new populations. These diseases are characterized by an explosive spread through the naive host population after the introduction of the novel pathogen. The second category includes outbreaks where the parasite is native to a particular host and geographic range but is spreading because some external factor either promotes transmission between hosts or decreases a host’s response to infection.
Amphibians as bioindicators of environmental health
Concern about amphibians is in large part due to their value as indicators of environmental health (Blaustein and Kiesecker 2002; Storfer 2003; Kiesecker et al. 2004). Relative to other vertebrates, amphibians have unprotected permeable skin, lacking scales or hair (Blaustein and Bancroft 2007; Han et al. 2008). Their embryos develop without the benefit of shells and thus are directly exposed to environmental conditions. Their complex lifecycles expose them to both aquatic and terrestrial hazards. These characteristics make them particularly sensitive to changes in environmental conditions. The widespread nature of the disease-mediated declines and the increase in deformities could be a warning of environmental degradation (Lannoo 2008). Understanding the factors that drive increased incidence of infectious disease in amphibian populations is central to understanding increased disease prevalence in human and wildlife populations because the majority of emerging diseases are linked to human-induced environmental change (Daszak et al. 2001; Kiesecker et al. 2004). Moreover, wildlife populations have long been considered an important link to pathogen emergence, as they serve as reservoirs for zoonotic diseases (pathogens transmissible between humans and animals). Many of the emerging infectious agents of humans are zoonotic diseases (Daszak et al. 2000). Thus, increased pathogenic infection in wildlife populations could translate into increased risk of human infection. Here I consider a few cases of amphibian mortality and deformity that have been associated with increased pathogenic infection to demonstrate that the global decline in amphibians is related to a larger phenomena associated with increased disease prevalence in human and wildlife populations. I will examine cases of decline that fall under the two broad classes of emerging infectious disease, environmental stress and introduced pathogens, to illustrate the similarity of mechanisms that have promoted disease outbreaks.
Important amphibian pathogens
Although little is known about the effects of pathogens on wild amphibians, there are a number of studies showing that they may be significant contributors to declining amphibian populations. A variety of pathogens may affect wild amphibian populations. These include viruses, bacteria, trematode parasites, protozoans, oomycetes, and fungi. These pathogens can be the proximate causes of mortality or they can cause sublethal damage such as severe developmental and physiological deformities.
Three pathogens have received a great deal of attention recently with regard to amphibian population declines and deformities; a chytridomycete fungus, Batrachochytrium dendrobatidis, hereafter referred to as BD (Berger et al. 1998), a fungal-like oomycete, Saprolegnia ferax, hereafter SF (Kiesecker and Blaustein 1995; Kiesecker and Blaustein 1997a; Kiesecker et al. 2001a) and a digenetic trematode, Ribeiroia ondatrae, hereafter RO (Johnson et al. 2002). I recognize that numerous pathogens are associated with the amphibian decline/deformities phenomena (see for example Di Rosa et al. 2007; Greer and Collins 2007; Duffus et al. 2008) but here I focus on these three pathogens because of the wealth of studies examining the role of environmental change in their emergence. BD is found in several areas where population declines have occurred and is fatal to frogs under certain experimental conditions (Berger et al. 1998; Lips et al. 2003; Ron et al. 2003). Saprolegnia has long been known to affect amphibian embryos, and larvae and SF has been associated with massive embryo mortality in the Cascade Mountains of Oregon, USA. Recent experimental evidence suggests that SF may affect amphibians in several ways and its infection rate and virulence depend upon a number of factors (Kiesecker and Blaustein 1995; Kiesecker et al. 2001a, 2001b). The presence of trematode parasite, RO has been associated with the occurrence of limb deformities at natural breeding sites. Experimental studies have shown that tadpoles exposed to larval RO develop limb deformities similar to those seen at natural breeding sites (Blaustein and Johnson 2003). Below I examine the context-dependent nature of these host–pathogen interactions and compare these with representative disease outbreaks in other wildlife species and human populations.
Environmental stress and increased disease prevalence
It has long been known that environmental stressors can increase host susceptibility to disease (Daszak et al. 2001; Lafferty and Holt 2003). A number of environmental stressors (i.e., habitat modification, UV-B radiation, climate change, pesticides) have been correlated with disease outbreak in amphibian populations as well as other wildlife and human populations. In this section, I will review some examples of environmental changes that affect infection risk for amphibians and relate this to similar outbreaks in other wildlife and human populations.
Global climate change and increased disease prevalence
Anthropogenic global climate change is likely to cause changes in the geographic range and incidence of many infectious diseases (Nicholls 1993; Dobson and Carper 1993; Reeves et al. 1994; Patz et al. 1996). Of particular concern is the rapid warming that has occurred of the latter half of the last century (Houghton et al. 2001). In the last century, surface temperatures have risen an average of 0.5°C, with concurrent changes in precipitation patterns and an increase in the frequency and severity of extreme weather events. In particular is the El Niño-Southern Oscillation (ENSO) phenomenon that results from air–sea interactions in the Tropical Pacific that impacts weather patterns over the entire globe (Timmermann et al. 1999). El Niño events refer to the replacement of normally cold nutrient-rich water over most of the equatorial Pacific Ocean with warm nutrient-deficient surface water every 2–7 years. La Niña, the opposite of El Niño, refers extreme cold water over the equatorial Pacific Ocean. In 1976, a large change in sea-surface temperatures in the South Pacific resulted in an increase in the frequency, duration, and intensity of ENSO events (Timmermann et al. 1999). Because ENSO events affect temperature and moisture patterns over land, amphibians and other organisms are likely to be effected.
In the Pacific Northwest of the United States, massive amphibian embryo mortality is associated with the presence of the oomycete pathogen, SF (Kiesecker and Blaustein 1997a). SF outbreaks have been linked to increased UV-B radiation (Kiesecker and Blaustein 1995). Attempts to link these disease outbreaks with environmental change have often postulated that these patterns are connected to ozone depletion. However, in some cases, climate change can be more effective than stratospheric ozone depletion in increasing the exposure of aquatic organisms to biologically effective UV-B radiation (Yan et al. 1996; Kiesecker et al. 2001a).
Amphibians, particularly those that breed in shallow montane lakes and ponds, may be quite susceptible to climate-induced changes in UV-B exposure (Blaustein et al. 1998; Blaustein and Belden 2003; Han et al. 2008). Amphibian embryos developing in montane lakes and ponds are often exposed to direct sunlight, yet the overlying water column may attenuate UV-B radiation. Where precipitation is reduced, however, associated reduction in water depth at oviposition sites may enhance UV-B exposure (Yan et al. 1996). In the Pacific Northwest of the United States, where SF outbreaks have occurred, precipitation patterns are closely tied to ENSO cycles (Redmond and Koch. 1991). For the Pacific Northwest El Niño events result in a decrease in winter precipitation. Hence, the increase in frequency and magnitude of El Niño events following the step-like warming of the tropical Pacific may have raised the incidence and severity of SF outbreaks by increasing the extent to which embryos are exposed to sunlight in shallow water (Kiesecker et al. 2001a).
To test this hypothesis, we (Kiesecker et al. 2001a) examined the relationship between pathogen-mediated embryo mortality and climate by quantifying mortality in relation to water depth at natural oviposition sites in the context of interannual variation in precipitation and the Southern Oscillation Index (SOI). We used experimentation and observation to examine how mortality associated with SF infections at natural oviposition sites is related to the water depth in which embryos develop, how water depth at an oviposition sites is a function of variability in precipitation associated with ENSO cycles, and how outbreaks of SF infection observed in shallow waters is mediated by exposure to UV-B radiation.
Observations of embryonic mortality patterns were consistent with the hypothesis that climate-induced water depth fluctuations influence the exposure of developing embryos to UV-B radiation. The percentage of mortality associated with SF infection was dependent on the water depth in which embryos developed. More than 50% of the western toad embryos that developed in relatively shallow water (<20 cm) consistently developed SF infections (Fig. 2). However, when eggs developed in water >45 cm, SF associated mortality was never more than 19%. Water depth at oviposition sites was in turn related to the amount of winter precipitation. The amount of winter precipitation in Oregon’s North Cascades Mountains during 1990–1999 was itself a function of the ENSO. Results from field experiments suggest that variation in water level influenced SF-associated mortality patterns through exposure to UV-B radiation (Fig. 2).
Fig. 2.
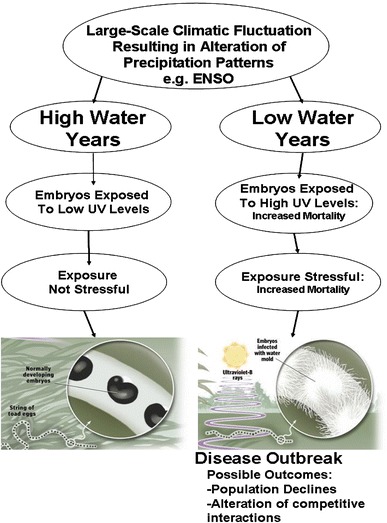
Translation of global-scale climatic change into local-scale infection patterns of developing amphibian embryos in the Cascade Mountains, USA (summarized from Kiesecker et al. 2001a)
These findings support the hypothesis that climate-induced fluctuations in water depth have caused unusually high mortality of embryos by influencing their exposure to UV-B radiation and consequently their vulnerability to SF infections. Because the survival of amphibians is closely tied to water availability, climate changes that alter hydrology may set the stage for similar mortality events that are believed to contribute to other disease-mediated population declines. These results are concordant with other studies that point to Pacific warming over recent decades as a common denominator for amphibian declines. The local manifestations of large-scale climate change, as well as their effects on living systems, are varied (Epstein 1999; Pounds 2001). Thus, the manner in which global climate change ultimately contributes to amphibian declines will likely differ among environments and species.
This pattern is mirrored in the amphibian declines in the Monteverde Cloud Forest of Costa Rica, one of the most notable cases of amphibian decline (Pounds and Crump 1994). In 1999, Pounds et al. (1999) reported massive population declines in approximately 40 species of amphibian, including the apparent extinction of the Golden Toad, Bufo periglenes. They suggested that the declines were linked to climatic warming. Their evidence indicated that increased numbers of dry days and longer dry periods were caused by a climate change-mediated rise in the altitude at which cloud formation occurred, thus, impacting moisture inputs for this cloud forest. Pounds hypothesized that as drying intensified, stressful conditions may have made individuals more susceptible to infection. As the environment dried, amphibian density increased in moist areas, which facilitated transmission rates of a waterborne pathogen. Occurring in synchrony with the decline in Monteverde are the declines in harlequin frogs (Atelopus spp.) in mountainous regions of Central and South America where 67% of the 110 species endemic to this region are believed to be extinct (GAA 2004). The pathogenic chytrid fungus, BD, has been implicated in these declines (Ron et al. 2003). In 2006, Pounds et al. (2006) offered a mechanistic explanation of how climate change encourages outbreaks of BD in Central and South America leading to declines of the Atelopus spp. They present evidence to suggest that night-time temperatures in these areas are shifting towards the thermal optimum of BD. In addition, increased daytime cloudiness prevents frogs from finding thermal refuges from the pathogen. While additional studies on disease-mediated amphibian declines support the climate-linked epidemic hypothesis (Daszak et al. 2005; Alford et al. 2007; Bosch et al. 2007), several have raised issues with the mechanisms linking the climatic change and disease outbreaks in Atelopus spp. proposed by Pounds et al. (2006). For example, Alford et al. (2007) suggest that the declines in the Australian tropics agree with the climate-linked epidemic hypothesis, but that the warming trend in Australia is not consistent with the mechanism proposed by Pounds et al. (2006). In addition, several studies have questioned the existence of a link between climatic change and declines of Atelopus spp. reported by Pounds et al. (2006), For example, in their reanalysis of the patterns of Atelopus spp. declines, Lips et al. (2008) found no evidence to support the hypothesis that climate change has been driving outbreaks of BD (see also Kriger 2009). Instead, they propose a spatiotemporal-spread hypothesis that states the declines are caused by the introduction and spread of BD exhibiting a characteristic spatiotemporal signature independent of climate. In a recent rigorous independent assessment of both hypotheses, Rohr et al. (2008a) examined the link between climate and spatial structure of the declines. They demonstrated that there is a spatial structure to the timing of Atelopus declines but that the cause of this structure is unclear, emphasizing the need for additional molecular characterization of BD. They also demonstrated that the correlation between mean tropical air temperature and Atelopus declines is robust, but that evidence of a causal link is weak. Further investigation is warranted to resolve this issue.
Despite the controversy around the declines of tropical amphibians disease outbreaks associated with climatic change are not limited to amphibian diseases and underscore the concern regarding the potential influence of global warming on certain human diseases (Epstein 1999). For example, cholera, a water-borne disease caused by the bacterium Vibrio cholerae, continues to be a serious global problem for many human populations (Colwell 1996). In 1988 there were approximately 50,000 cases of cholera but by 1991 that number increased to 600,000. Moreover, deaths over the same time period rose from 2,000 to 18,000. Like the SF outbreaks discussed above, cholera outbreaks are strongly related with climatic cycles (e.g., ENSO) (Colwell 1996). Climate change in the Arctic has also altered the life cycle of the nematode parasites of musk oxen (Kutz et al. 2005). With warmer temperatures, the worms can now complete their lifecycles in 1 year rather than 2 years, and their increase in numbers impacts musk oxen survival and reproduction. Moreover, these climate-induced impacts are not limited to animal populations. For example, warmer conditions in mountainous regions of the western United States alter the mountain pine beetle (Dendroctonus ponderosae) life cycle, increasing the impact they have on pine forests (Allen and Breshears 1998). Like the chytrid fungus, these changes are altering the composition of communities over large geographic scales. These changes could ultimately shift the tree line in these mountainous areas and potentially increasing runoff and/or flooding.
Human-aided introduction of pathogens
One of the consequences of the human domination of the earth’s ecosystems is a massive biotic homogenization of the earth’s surface. This homogenization is the result of the breakdown of biogeographic boundaries that have historically maintained the distinctive flora and fauna found in different regions of the world (Mooney and Hobbs 2000). This breakdown is the direct result of increased global travel and commerce. The movement of organisms for conservation, agriculture, and hunting occurs on a global scale, which can result in the exposure of human and wildlife to exotic infectious agents. These human-aided introductions, often referred to as “biological pollution”, have caused the loss of biodiversity on a global scale.
Chytridiomycosis, the newly discovered fungal disease of amphibians, which is caused by infection with BD, has been suggested to be an introduced pathogen. This pathogen has been associated with mass die-offs of juvenile and adult frogs from Australia and Central America as well as from the western US. The pathogen was first described in 1998 from dead and dying adult amphibians collected at sites of mass die-offs in Australia and Panama (Berger et al. 1998). Signs of infection include lethargy, abnormal posture, gross lesions, and hemorrhages in the skin. Its association exclusively with keratinized tissue suggests that BD uses amphibian keratin as a nutrient. BD may cause death by both damaging the epidermis and impairing essential cutaneous respiration. The chytrids are a ubiquitous fungi found in moist soil and aquatic habitats where they act primarily as detritivores. Parasitic members of this group infect plants, protists, and invertebrates, and BD is the first chytrid known to infect a vertebrate (Berger et al. 1998).
The patterns of amphibian death and population declines associated with BD are characteristic of an introduced virulent pathogen spreading through a naive host population. Several lines of evidence suggest that BD is indeed a novel pathogen that has been recently introduced. In Central America and Australia, the population declines have been severe, occurring over the course of a few months with high rates of adult death (Ron et al. 2003; Lips et al. 2003). Such high mortality rates are often associated with introduced virulent pathogens. Genetic variation of BD isolates has also provided important information regarding the divergence time of isolates found in diverse locals. Evidence suggests that BD has recently emerged after examination of DNA sequence data from isolates that have been associated with mass mortality events (Morehouse et al. 2003). They found a maximum of 5% divergence among all isolates, which is low for fungi. Moreover, some of the Australian and Central American isolates are identical.
While the data discussed above are consistent with the idea that a novel introduced pathogen is responsible for the observed mortality, it is not possible at this time to rule out other factors that may promote outbreaks. According to the spatiotemporal-spread hypothesis, declines of highland amphibians in Central America and Australia are due solely to BD infection (Laurance et al. 1996; Rachowicz et al. 2005). This pathogen is an obvious threat, but the single-disease model is likely to be an oversimplification. As previously mentioned, Pounds and colleagues reported that population declines in amphibians of the cloud forests of Monteverde Costa Rica and declines of Atelopus spp. in Central and South America were related to climatic warming driving disease outbreaks. These declines have occurred synchronously with declines that have been attributed to BD infection. While the pathogen involved in the Monteverde declines has not been identified, BD is believed to have been involved. However, some of the mysterious declines of frog populations in Central America have been accompanied by synchronous reptile and bird declines (Pounds et al. 1999; Whitfield et al. 2007). The aquatic-borne BD is unlikely to attack reptiles and birds, so the explanation is likely to be more complicated. Although there is considerable evidence to support that BD is involved in the widespread declines in amphibians, it remains unclear whether the pathogen is novel (McCallum 2005). While it is not necessary that the pathogen be novel for it to be implicated in the declines, if a preexisting pathogen has only recently caused extinctions, cofactors must be involved. Examination of preserved museum specimens suggest that BD is widely distributed and apparently enzootic in seemingly healthy amphibians, and in certain areas appears as if infection may have existed before declines (Ouellet et al. 2005; Puschendorf et al. 2006). Further investigation will be required to resolve this issue.
Several other studies have suggested that the pathogens responsible for recent mass mortality events of amphibians may have been introduced as a result of human activity (Kiesecker 2003). SF, discussed above, is a common disease of fishes, particularly those fish reared in hatcheries (Kiesecker and Blaustein 1997a). Many of the species introduced into Pacific Northwest lakes (Salmo spp., Salvelinus spp., and Oncorhynchus spp.) are common carriers of SF (Kiesecker et al. 2001b). Experiments have shown that hatchery-reared rainbow trout (Oncorhynchus mykiss) can potentially transmit SF to developing amphibians and/or amphibian breeding habitats (Kiesecker et al. 2001b). In the San Rafael Valley in southern Arizona, populations of the Sonora tiger salamander Ambystoma tigrinum stebbinsi have experienced decimating epizootics. An iridovirus (Ambystoma tigrinum virus, ATV) was isolated from diseased tiger salamanders and determined to be the causative agent involved in these epizootics (Collins et al. 2004). They speculated that the origin of ATV at their sites may have been introduced salamanders that are used as fish bait. Research in Venezuela implicates introduced bullfrogs Rana catesbeiana as a potential vector and reservoir for BD (Hanselmann et al. 2004). The direct impacts of bullfrog introductions can be significant on native amphibians (Kiesecker and Blaustein 1997b, 1998; Kiesecker et al. 2001c). Samples from apparently healthy bullfrogs introduced into the Venezuelan Andes found that 96% of the animals tested positive for BD. However, none exhibited clinical signs of the disease, suggesting that they may be a good reservoir and vector for the pathogen. Given the extensive nature of introducing hatchery-reared fish and the release and spread of many other introduced species (IS) that interact with amphibians, I suggest that IS could be a major vector for diseases that are contributing to amphibian losses. It is interesting to note that IS (e.g., introduced fish and bullfrogs) are found at sites where disease outbreaks and catastrophic declines of amphibians have been observed (e.g., the rainforest of eastern Australia and montane streams of Central and South America). Clearly more work is needed to assess the role of IS as a vector for amphibian disease.
There are numerous examples that parallel the larger paradigm of introduction, spread, and impact of novel pathogens on wildlife and human populations (see Daszak et al. 2000, 2001 for a review). Examples extend from the early human colonization of the globe (i.e., Spanish conquistadors introduced smallpox and measles to the New World) to today’s headlines (Desowitz 1997). For example, in recent times, chronic wasting disease (CWD), or transmissible spongiform encephalopathy, appears to be spreading through wild ungulates populations when infected elk are transported among game ranches (Prusiner 1997; Williams and Miller 2002). Duck plague, one of the most important emerging diseases of North American wildfowl was introduced to the continent through the importation of infected waterfowl (Leibovitz and Hwang 1968). Raccoon rabies was also introduced into the eastern USA when infected raccoons were released for hunting purposes (Dobson 2000). Another example taken right from recent headlines, West Nile virus (WNV) is also believed to have been introduced into North America as a direct result of human activity (LaDeau et al. 2007). WNV is an arthropod-borne flavivirus transmitted by mosquitoes, Culex spp., infecting humans, horses, as well as birds. The virus has a widespread distribution in Africa, Asia, and the Middle East. The recent appearance of this virus in the northeastern United States was associated with a large outbreak (epizootic) in wild and captive zoo birds. The close phylogenetic relationship between the American isolate and an isolate collected from Israel, suggests that WNV was imported from the Mid-East. The WNV could have entered through a number of avenues, including travel by infected humans, importation of infected domestic pets, or unintentional introduction of infected mosquitoes (LaDeau et al. 2007). Since its introduction, the virus has spread across much of North America, causing upwards of 5,000 confirmed cases, including ~500 human fatalities. WNV illustrates the rapid degree in which a novel path can spread once it has encountered new naive hosts populations.
Amphibian deformities
Amphibian deformities, in particular those related to limb development, have now been reported in 46 US states and in five Canadian provinces, as well as in several other countries around the world (Blaustein and Johnson 2003; Ouellet et al. 1997). The occurrence of amphibian limb deformities is not a new phenomenon, in fact, amphibian limb deformities can be found in reports dating back to the early 1700s, suggesting that the processes that are responsible for deformities have been present for centuries (Ouellet et al. 1997; Kiesecker et al. 2004). While a small number of deformities can be expected in any amphibian population, their occurrence is typically not more than 5%. Historical reports describe one or two affected frogs in a population, while contemporary reports document high frequencies (15–90%), which often impact several species at a given site (Blaustein and Johnson 2003). Hypotheses proposed to explain the deformities fall into two broad camps: chemical contaminants, and trematode infection (see Blaustein and Johnson 2003 for a review). While previous studies have linked deformities to either infection by some species of trematode or exposure to certain contaminants alone, here we focus on studies that have examined how environmental change has promoted increased infection and in turn increased limb deformities and not an exhaustive review of the factors surrounding amphibian limb deformities.
Habitat modifications that promote trematode infection
A great variety of parasites are dependent on freshwater environments. The digenetic trematodes, or flukes, are a prominent example, whose members usually have free-swimming aquatic forms and whose intermediate hosts are often freshwater organisms. Digeneans are the agents of several well-known human diseases including schistosomiasis, echinostomiasis, and cercarial dermatitis. Collectively, these diseases are known to impact hundreds of millions of people around the world. However, digeneans have received close attention recently because of their suspected role in outbreaks of amphibian deformities (Sessions and Ruth 1990). In fact, there is a broad perception among epidemiologists, parasitologists, and health professionals that the incidence of several trematode diseases is greatly affected by human-mediated changes in freshwater environments (Lardans and Dissous 1998). As humans develop the landscape, bodies of freshwater are often modified (Blaustein and Johnson 2003). There are numerous studies reporting correlations between human modifications of fresh waters and associated changes in the incidence of human disease (Alemayehu et al. 1998). In a number of these cases, scientists have been able to link disease outbreaks with increases in snail abundance (Molyneux 1998). A commonly invoked hypothesis holds that humans often modify freshwater environments in ways that increase the abundance of a critical host for digenetic trematodes. Thus, humans may inadvertently alter the environment in ways that increase human health risks. Recent evidence suggests that similar changes may positively impact the occurrence of RO, the trematode associated with amphibian limb deformities (Kiesecker 2002).
Work in the western United States suggests that RO occurrence and limb deformities were associated with highly productive artificial ponds often associated agricultural areas (Blaustein and Johnson 2003; Johnson et al. 2001). These systems are highly productive because of fertilizer use and the presence of cattle manure. This leads to increased algal growth and increased snail density. These environments are also readily used by both amphibians and birds, the latter necessary hosts for RO. The number of these types of artificial wetlands has increased dramatically since the 1940s (Blaustein and Johnson 2003).
Schistosomiasis, a trematode parasite that infects human and causes an estimated 1 million deaths a year, is expanding worldwide. This increase is due to a rise in suitable habitat for the snail intermediate host, resulting from various human activities, including dam construction, deforestation, and eutrophication (Molyneux 1998). Human habitat modifications have not only influenced water-borne diseases. Complex interactions among the ecology of hosts, vectors, and human-habitat alterations can have surprising consequences for disease emergence. The abandonment of agriculture and the resulting reforestation of former farmland in the northeastern USA likely fostered the emergence of Lyme disease (Ostfeld and Keesing 2000a, b).
Pesticide stress and decreased host immunocompetency
The same kinds of human induced environmental degradation that would result in increased snail densities could also result in increased exposure to immunotoxic pollutants such as pesticides. Many deformed frogs have been found in agricultural areas where insecticides are applied extensively (Ouellet et al. 1997; Storrs and Kiesecker 2004). Since the first use of pesticides for crop protection in the mid-1940s, the global use of pesticides has continued to expand. In the mid-1940s, approximately 50 million kg/year of pesticides were applied; today approximately, 2.5 billion kg/year are applied globally (Colborn and Thayer 2000). However, the potential hazard is greater than the increase in applied amounts alone since many modern pesticides are considerably more toxic to organisms.
To examine the relationship between trematode-mediated limb deformities and chemical contaminants, I quantified limb deformities in relation to trematode infection at natural breeding sites in the context of variation in exposure to pesticides (Kiesecker 2002). These findings supported the hypothesis that parasite infection explains the development of limb deformities observed in wood frog populations in nature. When cercariae were prevented from accessing the developing amphibians, developmental abnormalities were prevented (Fig. 3). The results suggest that the occurrence of limb deformities at natural oviposition sites is directly related to trematode infection of developing amphibians. The occurrence of trematode-mediated limb deformities was, however, dependent upon the context of the interaction. Frogs in ponds adjacent to agricultural fields developed a significant number of limb deformities. In contrast, the impact of trematode exposure was reduced in ponds not receiving agricultural runoff (Fig. 3). Stress in the form of pesticide exposure may have decreased the host tadpoles’ ability to resist infection, resulting in higher parasite loads and a higher risk of limb deformities. Laboratory experiments revealed association between pesticide (atrazine, malathion, and esfenvalerate) exposure and increased infection. Pesticide levels used in laboratory experiments reflected EPA maximum contaminant levels for drinking water and thus likely reflected realistic exposure levels. Exposure to low concentrations of contaminants had dramatic effects on the immunocompetency of wood frogs. For each of the contaminants tested, exposure increased the proportion of cercariae that successfully encysted (Fig. 4). Likewise, exposure also altered the number of wood frogs’ circulating eosinophils, which are believed to play a role in controlling macroparasite infections (Fig. 4). Further support for this pattern has been provided through field surveys and mesocosm experiments examining trematode infection patterns and exposure to agrochemicals in the northern leopard frog, Rana pipiens (Rohr et al. 2008b). Field surveys demonstrated that the combination of atrazine and phosphate accounts for 74% of the variation in the abundance of larval trematodes in amphibian hosts. A mesocosm experiment demonstrated that exposure to atrazine resulted in immunosuppressed tadpoles with elevated trematode loads and, more attached algae and snails, further supporting a causal link between pesticide exposure and elevated trematode infection (Rohr et al. 2008b). There is also evidence to suggest that exposure to pesticides results in increased infection risk for amphibians beyond trematode infections. For example, low-level pesticide exposure can result in immunosuppression in ranid frog species (Gendron et al. 2003) and ambystomatid salamanders (Forson and Storfer 2006) resulting in increased development of lungworms and susceptibility to viral infections, respectively.
Fig. 3.
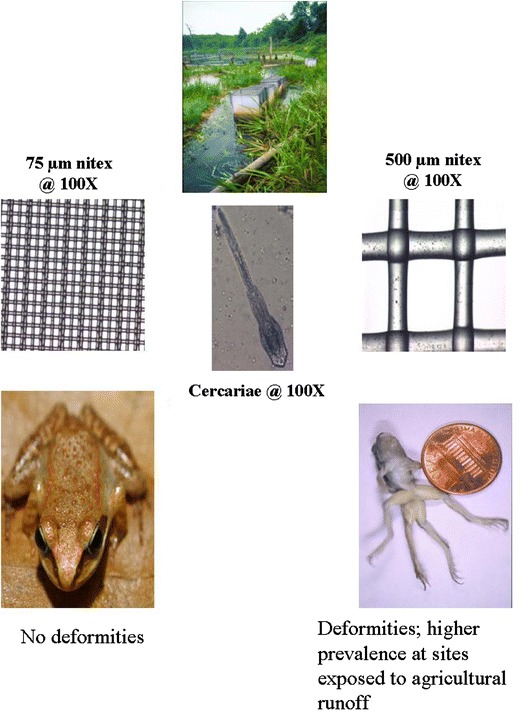
Summary of field experiments examining how exposure to trematode infection (exposed or protected) affects limb deformities in wood frogs at natural breeding sites. Developing wood frog larvae exposed to natural levels of infection developed limb deformities, while counterparts in cages with a screen that prevented access by cercariae did not. Levels of deformities were also higher in ponds contaminated by runoff from agricultural pesticides. (Inset) Schematic of Nitex screens used in field enclosure arrays (data summarized from Kiesecker 2002)
Fig. 4.
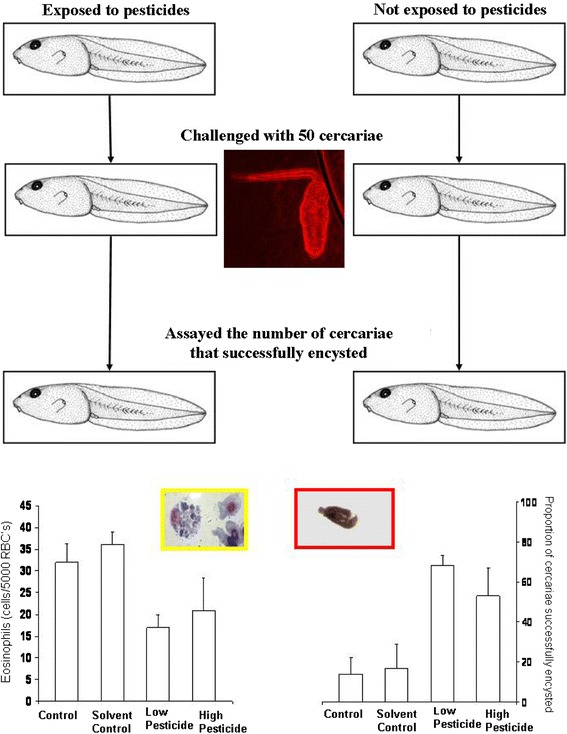
Summary of the effects of pesticide esfenvalerate exposure at low (80 μg/l) or high (1,800 μg/l) levels and exposure to trematode infection on the number of eosinophils (left) and the proportion of cercariae (out of exposure to 50 cercariae) that successfully encysted (right). (Inset) Typical wood frog eosinophil with RBCs, cercariae, and metacercariae, for reference (data summarized from Kiesecker 2002)
This work parallels a number of other disease outbreaks, including situations where pathogens have invaded a host population that is immunocompromised because of human-induced environmental stress. In a recent example, phocine distemper virus (PDV) invaded the North Sea pinniped populations after the harp seal, its native host shifted its range southward. Evidence suggests that the North Sea pinnipeds might also have been particularly sensitive because exposure to pollutants had left them immunocompromised (Kendall et al. 1992). Work on aquatic mammals indicates that pollutants, in particular pesticides, have immunotoxic properties, impairing the ability of hosts to illicit an effective immune response.
Decreased biodiversity and increased disease risk
Proponents of biodiversity conservation have often attempted to place value on animals, plants, and microbes as sources of medicines or other products useful for society (Daily 1997). However, species diversity may play a more tangible role regarding the influence of disease risk. The dilution effect hypothesis suggests that diversity per se may influence risk of exposure to disease (Ostfeld and Keesing 2000a, b). The concept was developed to understand how changes in the diversity of terrestrial vertebrates in North America could alter the risk of human exposure to Borrelia burgdoferi, the tick-transmitted spirochete that causes Lyme disease. In a diverse community, many ticks do not become infected because some vertebrate hosts are inefficient at transmitting the spirochete infections to feeding ticks. As habitats are degraded by human influences, many species of the vertebrate community disappear. The most effective reservoir, the white-footed mouse, Peromyscus leucopus, is one of the species that remains in disturbed habitats. Increased species diversity may dilute the power of the white-footed mouse to infect ticks by causing ticks to feed on hosts that are less effective at transmitting the spirochete. Thus, higher species diversity should result in lower prevalence of infection in ticks, and in turn, lower the local risk of infection for humans. The role of habitat disturbance and diversity loss in disease emergence on a global scale are in need of serious assessment. Changes in diversity are likely to co-vary with a number of factors that influence infection patterns (Fig. 1). For example, the remaining species that persist in disturbed habitats may be released from competition or predation that keeps them in check in undisturbed settings. Alternatively, the same factors (e.g., fragmentation, eutrophication, and pesticides) that influence diversity may stress organisms, decreasing their ability to resist infection. Any factor that concentrates wildlife can ultimately increase transmission rates between hosts. For example, habitat fragmentation may increase the contact between wildlife that remain in undisturbed areas and increase contact between other host species living in the disturbed areas, which facilitates cross-species transmission. Furthermore, species that are able to persist with habitat disturbance may reach high densities because factors that regulate their populations may be altered. The high densities that species may be able to reach in disturbed habitats could facilitate transmission rates between hosts. Whether differences in diversity directly influence infection risk will need to be examined in greater detail. If the relationship between species diversity and disease risk is a general one, then the debate about the benefits of biodiversity to human welfare will become more tangible and more pressing.
Conclusions
Global biodiversity loss and increased prevalence of infectious disease are two of the most serious global environmental concerns facing humanity. The dynamics of biodiversity loss and disease emergence are tightly intertwined, with emerging infectious disease acting as both a cause for (and consequence of) biodiversity loss. The impact of human environmental change on biodiversity loss is clear. There is no doubt that human population growth is accompanied by biodiversity loss. In fact, the past 50 years has seen an unprecedented loss of habitat and species. However, not all organisms are negatively impacted by human environmental change. Indeed, some organisms respond in a positive way to these changes. Many pathogens and parasites are among the organisms that appear to be included in this benefited group. Thus, if human habitat disturbance and biodiversity loss is left unchecked, it is likely we will see a continued increase in disease causing organisms for both wildlife and human populations.
The global decline of amphibians has been considered by some to be separate from the overall biodiversity crisis. It is now clear that the global decline in amphibians is related to a larger phenomena associated with increased disease prevalence in human and wildlife populations. Like other wildlife diseases, emerging infectious diseases of amphibians appear to be both a cause for amphibian loss and a consequence of biodiversity loss. The key for the future will be to understand and predict future disease outbreaks. Given their life-history characteristics, amphibians may be particularly sensitive to changes in environmental conditions. This sensitivity may make them an early warning system of environmental degradation. Amphibians are also amenable to experimental manipulations. This will allow experimental regimes that simulate key aspects of human environmental change (e.g., habitat destruction, fragmentation, and pollution) and the impacts of disease emergence assessed. Thus, amphibians can also serve as a model system to understand disease emergence.
Acknowledgments
Thanks to Cheri Kiesecker, Mike Rubbo, Ryan Peterson, Gia Vigiano, Sara Storrs, Julian Avery, and Andrew Blaustein for providing technical assistance; and thanks to Mike Rubbo, Lisa Belden, Kat Shea, Billy Costigan, Colin Sullivan, and Frank Costello for discussions that shaped this concept. I thank Z. Kawabata for the opportunity to present our thoughts at the 2008 Kyoto symposium on ‘Environmental Change, Pathogens and Human Linkages. Funding was provided by National Institutes of Health/National Science Foundation Ecology of Infectious Diseases Grant (1R01ES11067-01), The Nature Conservancy, and The Research Institute for Humanity and Nature.
References
- Alemayehu T, Ye-ebiyo Y, Ghebreyesus TA, Witten KH, Bosman A, Teklehaimanot A. Malaria, schistosomiasis, and intestinal helminths in relation to microdams in Tigray, northern Ethiopia. Parassitologia. 1998;40:259–267. [PubMed] [Google Scholar]
- Alford RA, Bradfield KS, Richards SJ. Global warming and amphibian losses. Nature. 2007;447:E3–E4. doi: 10.1038/nature05940. [DOI] [PubMed] [Google Scholar]
- Allen CD, Breshears DD. Drought-induced shift of a forest–woodland ecotone: rapid landscape response to climate variation. Proc Natl Acad Sci USA. 1998;95:14839–14842. doi: 10.1073/pnas.95.25.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Diamond SA, Tietge JE, Holcombe GW, Jensen KM, Defoe DL, et al. Assessment of the risk of solar ultraviolet radiation to amphibians. I. Dose-dependent induction of hindlimb malformations in the northern leopard frog (Rana pipiens) Environ Sci Technol. 2002;36:2853–2858. doi: 10.1021/es011195t. [DOI] [PubMed] [Google Scholar]
- Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein AR, Bancroft BA. Amphibian population declines: evolutionary considerations. Bioscience. 2007;57:437–444. doi: 10.1641/B570517. [DOI] [Google Scholar]
- Blaustein AR, Belden LK. Amphibian defenses against ultraviolet-B radiation. Evol Dev. 2003;5:89–97. doi: 10.1046/j.1525-142X.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Johnson PT. Explaining frog deformities. Sci Am. 2003;288:60–65. doi: 10.1038/scientificamerican0203-60. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Kiesecker JM. Complexity in conservation: lessons from the global decline of amphibian populations. Ecol Lett. 2002;5:597–608. doi: 10.1046/j.1461-0248.2002.00352.x. [DOI] [Google Scholar]
- Blaustein AR, Wake DB. Declining amphibian populations: a global phenomenon? Trends Ecol Evol. 1990;5:203–204. doi: 10.1016/0169-5347(90)90129-2. [DOI] [Google Scholar]
- Blaustein AR, Wake DB, Sousa WP. Amphibian declines: judging stability, persistence, and susceptibility of populations to local and global extinctions. Conserv Biol. 1994;8:60–71. doi: 10.1046/j.1523-1739.1994.08010060.x. [DOI] [Google Scholar]
- Blaustein AR, Kiesecker JM, Chivers DP, Anthony RG. Ambient UV-B radiation causes deformities in amphibian embryos. Proc Natl Acad Sci USA. 1998;94:13735–13737. doi: 10.1073/pnas.94.25.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J, Carrascal LM, Durán L, Walker S, Fisher MC. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of central Spain: is there a link? Proc R Soc Lond B. 2007;274:253–260. doi: 10.1098/rspb.2006.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, Thayer K. Aquatic ecosystems: harbingers of endocrine disruption. Ecol Appl. 2000;10:949–957. doi: 10.1890/1051-0761(2000)010[0949:AEHOED]2.0.CO;2. [DOI] [Google Scholar]
- Collins JP, Storfer A. Global amphibian declines: sorting the hypotheses. Divers Distrib. 2003;9:89–98. doi: 10.1046/j.1472-4642.2003.00012.x. [DOI] [Google Scholar]
- Collins JP, Brunner JL, Jancovich JK, Schock DM. A model host-pathogen system for studying infectious disease dynamics in amphibians: tiger salamanders (Ambystoma tigrinum) and Ambystoma tigrinum virus. Herpetol J. 2004;14:195–200. [Google Scholar]
- Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- Daily GC. Nature’s services: societal dependence on natural ecosystems. Washington, DC: Island Press; 1997. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78:103–116. doi: 10.1016/S0001-706X(00)00179-0. [DOI] [PubMed] [Google Scholar]
- Daszak P, Scott DE, Kilpatrick AM, Faggoni C, Gibbons JW, Porter D. Amphibian population declines at Savannah River site are linked to climate, not chytridiomycosis. Ecology. 2005;86:3232–3237. doi: 10.1890/05-0598. [DOI] [Google Scholar]
- Desowitz RS. Who gave pinta to the Santa Maria? New York: Harcourt Brace & Company; 1997. p. 256. [Google Scholar]
- Di Rosa I, Simoncelli F, Fagotti A, Pascolini R. The proximate cause of frog declines? Nature. 2007;447:E4–E5. doi: 10.1038/nature05941. [DOI] [PubMed] [Google Scholar]
- Dobson AP. Raccoon rabies in space and time. Proc Natl Acad Sci USA. 2000;97:14014–14063. doi: 10.1073/pnas.97.26.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AP, Carper ER. Biodiversity. Lancet. 1993;342:1096–1099. doi: 10.1016/0140-6736(93)92069-6. [DOI] [PubMed] [Google Scholar]
- Duffus ALJ, Pauil BD, Wozney K, Brunetti CR, Berrill M. Frog virus 3-like infections in aquatic amphibian communities J. Wildl Dis. 2008;44:109–120. doi: 10.7589/0090-3558-44.1.109. [DOI] [PubMed] [Google Scholar]
- Epstein PR. Perspectives: medicine, climate and health. Science. 1999;285:347–348. doi: 10.1126/science.285.5426.347. [DOI] [PubMed] [Google Scholar]
- Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32:675–685. doi: 10.1086/319235. [DOI] [PubMed] [Google Scholar]
- Forson D, Storfer A. Effects of atrazine and iridovirus infection on survival and life-history traits of the long-toed Salamander (Ambystoma macrodactylum) Environ Toxicol Chem. 2006;25:168–173. doi: 10.1897/05-260R.1. [DOI] [PubMed] [Google Scholar]
- Gendron AD, Marcogliese DJ, Barbeau S, Christin MS, Brousseau P, Ruby S, Cyr D, Fournier M. Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia. 2003;135:469–476. doi: 10.1007/s00442-003-1210-y. [DOI] [PubMed] [Google Scholar]
- Global Amphibian Assessment (GAA) (2004) http://www.globalamphibians.org
- Greer AL, Collins JP. Sensitivity of a diagnostic test for amphibian Ranavirus varies with sampling protocol. J Wildl Dis. 2007;43:525–532. doi: 10.7589/0090-3558-43.3.525. [DOI] [PubMed] [Google Scholar]
- Han BA, Bradley BW, Blaustein AR (2008) Ancient behaviors of larval amphibians in response to an emerging fungal pathogen, Batrachochytrium dendrobatidis. Behav Ecol Sociobiol. doi:10.1007/s00265-008-0655-8
- Hanselmann R, Rodrıguez I, Lampo M, Fajardo-Ramos L, Aguirre AA, Marm Kilpatrick A, Rodrıguez JP, Daszak P. Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biol Conserv. 2004;120:115–119. doi: 10.1016/j.biocon.2004.02.013. [DOI] [Google Scholar]
- Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Herbicides: feminization of male frogs in the wild. Nature. 2002;419:895–896. doi: 10.1038/419895a. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci USA. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes T, Haston K, Tsui M, Hoang A, Haefelle C, Vonk A. Atrazine-induced hermaphrodism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environ Health Perspect. 2003;111:568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton JT et al (eds) (2001) Climate change 2001, the scientific basis. Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
- Johnson P, Sutherland D. Amphibian deformities and Ribeiroia infection: an emerging helminthiasis. Trends Parasitol. 2003;19:332–335. doi: 10.1016/S1471-4922(03)00148-X. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Lunde KB, Haight RW, Bowerman J, Blaustein AR. Ribeiroia ondatrae (Trematoda: Digenea) infection induces severe limb malformations in western toads (Bufo boreas) Can J Zool. 2001;79:370–379. [Google Scholar]
- Johnson PTJ, Lunde KB, Thurman EM, Ritchie EG, Wray SN, Sutherland DR, et al. Parasite (Ribeiroia ondatrae) infection linked to amphibian malformations in the western United States. Ecol Monogr. 2002;72:151–168. doi: 10.1890/0012-9615(2002)072[0151:PROILT]2.0.CO;2. [DOI] [Google Scholar]
- Kendall MD, Safieh B, Harwood J, Pomeroy PP. Plasma thymulin concentrations, the thymus and organochlorine contaminant levels in seals infected with phocine distemper virus. Sci Total Environ. 1992;115:133–144. doi: 10.1016/0048-9697(92)90038-T. [DOI] [PubMed] [Google Scholar]
- Kiesecker JM. Synergism between trematode infection and pesticide exposure: a link to amphibian limb deformities in nature? Proc Natl Acad Sci USA. 2002;99:9900–9904. doi: 10.1073/pnas.152098899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesecker JM. Invasive species: defining their role in amphibian declines. In: Semlitsch RD, editor. Amphibian conservation. Washington, DC: Smithsonian Institution Press; 2003. pp. 113–126. [Google Scholar]
- Kiesecker JM, Blaustein AR. Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature. Proc Natl Acad Sci USA. 1995;92:11049–11052. doi: 10.1073/pnas.92.24.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesecker JM, Blaustein AR. Egg laying behavior influences pathogenic infection of amphibian embryos. Conserv Biol. 1997;11:214–220. doi: 10.1046/j.1523-1739.1997.95509.x. [DOI] [Google Scholar]
- Kiesecker JM, Blaustein AR. Population differences in responses of red-legged frogs (Rana aurora) to introduced bullfrogs (Rana catesbeiana) Ecology. 1997;78:1752–1760. [Google Scholar]
- Kiesecker JM, Blaustein AR. Effects of introduced bullfrogs and smallmouth bass on the microhabitat use, growth and survival of native red-legged frogs. Conserv Biol. 1998;12:776–787. doi: 10.1046/j.1523-1739.1998.97125.x. [DOI] [Google Scholar]
- Kiesecker JM, Blaustein AR, Belden LK. Complex causes of amphibian population declines. Nature. 2001;410:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- Kiesecker JM, Blaustein AR, Miller CL. The transfer of a pathogen from fish to amphibians. Conserv Biol. 2001;15:1064–1070. doi: 10.1046/j.1523-1739.2001.0150041064.x. [DOI] [Google Scholar]
- Kiesecker JM, Miller CL, Blaustein AR. Potential mechanisms underlying the displacement of native red-legged frogs by introduced bullfrogs. Ecology. 2001;82:1964–1970. doi: 10.1890/0012-9658(2001)082[1964:PMUTDO]2.0.CO;2. [DOI] [Google Scholar]
- Kiesecker JM, Belden LK, Shea K, Rubbo MJ. Amphibian declines and emerging disease. Am Sci. 2004;92:138–147. [Google Scholar]
- Kriger KM. Lack of evidence for the drought-linked chytridiomycosis hypothesis. J Wildl Dis. 2009;45:537–541. doi: 10.7589/0090-3558-45.2.537. [DOI] [PubMed] [Google Scholar]
- Kutz SJ, Hoberg EP, Nagy J, Polley L, Elkin B (2005) “Emerging” parasitic infections in Arctic ungulates. Proc R Soc Lond B. doi:10.1098/rspb.2005.3285 [DOI] [PubMed]
- LaDeau LD, Kilpatrick MD, Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710–713. doi: 10.1038/nature05829. [DOI] [PubMed] [Google Scholar]
- Lafferty K, Holt RD. How should environmental stress affect the population dynamics of disease? Ecol Lett. 2003;6:654–664. doi: 10.1046/j.1461-0248.2003.00480.x. [DOI] [Google Scholar]
- Lannoo MJ. Malformed frogs: the collapse of aquatic ecosystems. Berkeley, CA: University of California Press; 2008. [Google Scholar]
- Lardans V, Dissous C. Snail control strategies for reduction of schistosomiasis transmission. Parasitol Today. 1998;14:413–417. doi: 10.1016/S0169-4758(98)01320-9. [DOI] [PubMed] [Google Scholar]
- Laurance WF, McDonald KR, Speare R. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conserv Biol. 1996;10:1–9. doi: 10.1046/j.1523-1739.1996.10020406.x. [DOI] [Google Scholar]
- Leibovitz L, Hwang J. Duck plague on the American continent. Avian Dis. 1968;12:361–378. doi: 10.2307/1588237. [DOI] [PubMed] [Google Scholar]
- Lips K, Green DE, Pappendick R. Chytridiomycosis in wild frogs from southern Costa Rica. J Herpetol. 2003;37:215–218. doi: 10.1670/0022-1511(2003)037[0215:CIWFFS]2.0.CO;2. [DOI] [Google Scholar]
- Lips KR, Diffendorfer JE, Mendelson JR, Sears MW. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:441–454. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H. Inconclusiveness of chytridiomycosis as the agent in widespread frog declines. Conserv Biol. 2005;19:1421–1430. doi: 10.1111/j.1523-1739.2005.00217.x. [DOI] [Google Scholar]
- Molyneux DH. Vectorborne parasitic diseases—an overview of recent changes. Int J Parasitol. 1998;28:927–934. doi: 10.1016/S0020-7519(98)00067-8. [DOI] [PubMed] [Google Scholar]
- Mooney HA, Hobbs RJ, editors. Invasive species in a changing world. Washington, DC: Island Press; 2000. [Google Scholar]
- Morehouse EA, James TY, Ganley ARD, Vilgalys R, Berger L, Murphy PJ, Longcore JE. Multilocus sequence typing suggests that the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol. 2003;12:395–403. doi: 10.1046/j.1365-294X.2003.01732.x. [DOI] [PubMed] [Google Scholar]
- Nicholls N. El Niño southern oscillation and vector-borne disease. Lancet. 1993;342:1284–1285. doi: 10.1016/0140-6736(93)92368-4. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv Biol. 2000;14:722–728. doi: 10.1046/j.1523-1739.2000.99014.x. [DOI] [Google Scholar]
- Ostfeld RS, Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can J Zool. 2000;78:2061–2078. doi: 10.1139/z00-172. [DOI] [Google Scholar]
- Ouellet M, Bonin J, Rodrigue J, DesGranges J, Lair S. Hindlimb deformities (ectromelia, ectrodactyly) in free living anurans from agricultural habitats. J Wildl Dis. 1997;33:95–104. doi: 10.7589/0090-3558-33.1.95. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Mikaelian I, Pauli BD, Rodrigue J, Green DM. Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv Biol. 2005;19:1431–1440. doi: 10.1111/j.1523-1739.2005.00108.x. [DOI] [Google Scholar]
- Patz JA, Epstein PR, Burke TA, Balbus JM. Global climate change and emerging infectious diseases. JAMA. 1996;275:217–223. doi: 10.1001/jama.275.3.217. [DOI] [PubMed] [Google Scholar]
- Pounds JA. Climate and amphibian declines. Nature. 2001;410:639–640. doi: 10.1038/35070683. [DOI] [PubMed] [Google Scholar]
- Pounds JA, Crump ML. Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conserv Biol. 1994;8:72–85. doi: 10.1046/j.1523-1739.1994.08010072.x. [DOI] [Google Scholar]
- Pounds JA, Fogden MPL, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398:611–615. doi: 10.1038/19297. [DOI] [Google Scholar]
- Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sanchez-Azofeifa GA, Still CJ, Young BE. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prion diseases and the BSE crisis. Science. 1997;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Puschendorf R, Bolanos F, Chaves G. The amphibian chytrid fungus along an altitudinal transect before the first reported declines in Costa Rica. Biol Conserv. 2006;132:136–142. doi: 10.1016/j.biocon.2006.03.010. [DOI] [Google Scholar]
- Rachowicz LJ, Hero JM, Alford RA, Taylor JW, Morgan JAT, Vredenburg VT, Collins JP, Briggs CJ. The novel and endemic pathogen hypotheses: competing explanations for the origin of emerging infectious diseases of wildlife. Conserv Biol. 2005;19:1441–1448. doi: 10.1111/j.1523-1739.2005.00255.x. [DOI] [Google Scholar]
- Redmond KT, Koch RW. Surface climate and stream flow variability in the western United States and their relationship to large-scale circulation indexes. Water Resour Res. 1991;27:2381–2399. doi: 10.1029/91WR00690. [DOI] [Google Scholar]
- Reeves WC, Hardy JL, Reisen WK, Milby MM. The potential effect of global warming on mosquito-borne arboviruses. J Med Entomol. 1994;31:323–332. doi: 10.1093/jmedent/31.3.323. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. Evaluating the links between climate, disease spread, and amphibian declines. Proc Natl Acad Sci USA. 2008;105:17436–17441. doi: 10.1073/pnas.0806368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455:1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- Ron SR, Duellman WE, Coloma LA, Bustamante M. Population decline of the Jambato toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. J Herpetol. 2003;37:116–126. doi: 10.1670/0022-1511(2003)037[0116:PDOTJT]2.0.CO;2. [DOI] [Google Scholar]
- Semlitsch RD (ed) (2003) Amphibian conservation. Smithsonian Press, Washington, DC
- Sessions SK, Ruth SB. Explanation for naturally occurring supernumerary limbs in amphibians. J Exp Zool. 1990;254:38–47. doi: 10.1002/jez.1402540107. [DOI] [PubMed] [Google Scholar]
- Storfer A. Amphibian declines: future directions. Divers Distrib. 2003;9:151–163. doi: 10.1046/j.1472-4642.2003.00014.x. [DOI] [Google Scholar]
- Storrs SI, Kiesecker JM. Survivorship patterns of larval amphibians exposed to low concentrations of atrazine. Environ Health Perspect. 2004;112:1054–1057. doi: 10.1289/ehp.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann A, Oberhuber J, Bacher A, Esch M, Latif M, Roeckner E. Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature. 1999;398:694–697. doi: 10.1038/19505. [DOI] [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277:494–499. doi: 10.1126/science.277.5325.494. [DOI] [Google Scholar]
- Weinhold R. Infectious disease: the human costs of our environmental errors. Environ Health Perspect. 2004;112:A32–A39. doi: 10.1289/ehp.112-a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield SM, Bell KE, Philippi T, Sasa M, Bolan F, Chaves G, Savage JM, Donnelly MA. Amphibian and reptile declines over 35 years at La Selva, Costa Rica. Proc Natl Acad Sci USA. 2007;104:8352–8356. doi: 10.1073/pnas.0611256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ES, Miller MW. Chronic wasting disease in deer and elk in North America. Rev Sci Tech Off Int Epizoot. 2002;21:305–316. doi: 10.20506/rst.21.2.1340. [DOI] [PubMed] [Google Scholar]
- Yan ND, Keller W, Scully NM, Lean DRS, Dillon PJ. Increased UV-B penetration in a lake owing to drought-induced acidification. Nature. 1996;381:141–143. doi: 10.1038/381141a0. [DOI] [Google Scholar]


