Abstract
Viral filtration is a critical step in the purification of biologics and in the monitoring of microbiological water quality. Viral filters are also essential protection elements against airborne viral particles. The present review first focuses on cellulose-based filter media currently used for size-exclusion and/or adsorptive filtration of viruses from biopharmaceutical and environmental water samples. Data from spiking studies quantifying the viral filtration performance of cellulosic filters are detailed, i.e., first, the virus reduction capacity of regenerated cellulose hollow fiber filters in the manufacturing process of blood products and, second, the efficiency of virus recovery/concentration from water samples by the viradel (virus adsorption–elution) method using charge modified, electropositive cellulosic filters or conventional electronegative cellulose ester microfilters. Viral analysis of field water samples by the viradel technique is also surveyed. This review then describes cellulose-based filter media used in individual protection equipment against airborne viral pathogens, presenting innovative filtration media with virucidal properties. Some pros and cons of cellulosic viral filters and perspectives for cellulose-based materials in viral filtration are underlined in the review.
Keywords: Cellulose filter media, Virus filtration, Viral clearance, Adsorption–elution method, Filtering facepieces
Introduction
Virus capture/purification/concentration is critical in a number of biopharmaceutical and clinical applications. Validation of virus clearance is essential in the manufacture of therapeutic proteins, in particular blood products (Bryant and Klein 2007; Klamroth et al. 2014; de Mendoza et al. 2012; Radosevich and Burnouf 2010; Shukla et al. 2007). On the other hand, large-scale, efficient purification schemes of viruses/virus-like particles are required for the production of prophylactic vaccines and gene therapy vectors (Rodrigues et al. 2007; Segura et al. 2006, 2011; Vicente et al. 2011a, b). Virus inactivation technologies are commonly used to fulfil viral safety. They include physical (e.g., heat application, ultraviolet- and gamma irradiation) and chemical methods (e.g., solvent/detergent treatments) or their combination (e.g., exposure to photosensitizer plus UV light) (Bryant and Klein 2007; Klamroth et al. 2014; Klein and Bryant 2009; Pelletier et al. 2006; Prowse 2013; Radosevich and Burnouf 2010; Solheim 2008). Common methods for virus capture include filtration (Charcosset 2006; Grein et al. 2013; Liu et al. 2010; van Reis and Zydney 2007) and chromatography in column or membrane configurations (Charcosset 2006; Gottschalk 2008; Liu et al. 2010; Orr et al. 2013; van Reis and Zydney 2007; Segura et al. 2011). Viral filtration is usually the final purification step in the downstream processing of biopharmaceutical products, e.g., monoclonal antibodies (Fig. 1), following one or more chromatography “polishing” steps that contribute to the overall virus removal efficiency of the process before concentration of the purified product. Filtration technologies are also extensively used for the capture and concentration of waterborne viral pathogens (Gibson 2014) from drinking, environmental, recreational or waste water samples (Cashdollar and Wymer 2013; Ikner et al. 2012). In addition, particulate air filters are used in personal respiratory protective equipment (i.e., face masks and respirators) (Bunyan et al. 2013; Cohen and Birkner 2012; Rengasamy et al. 2004) to ensure short-range protection of wearers against airborne pathogens–which include a number of viruses (Tang et al. 2006)—and in air purifiers/cleaners to limit long-range aerosol transmission of infection in healthcare settings (Hyttinen et al. 2011; Tang et al. 2006).
Fig. 1.
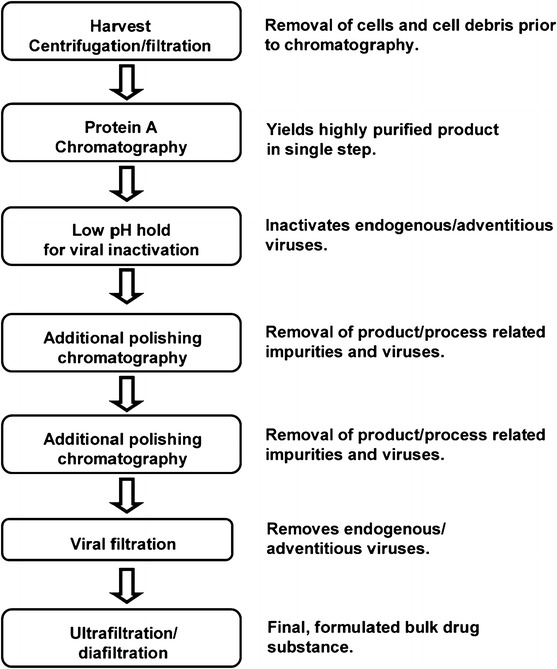
A typical monoclonal antibody recovery process.
Taken from Liu et al. (2010)
Cellulosic membrane microfilters have been used routinely for ages in laboratories to perform the so-called “sterile filtration” (cold sterilization), i.e., the absolute removal of bacteria, yeasts and molds but not viruses (Walsh and Denyer 2012) from heat-sensitive liquid media. However, many virus filtration devices currently implemented are made from cellulose and its derivatives. Some eighty years ago, Gradocol, graded collodion (cellulose nitrate) membrane filters (Elford 1931; Bauer and Hughes 1934) have been extensively used in ultrafiltration to estimate the size of several medically important viruses such as foot-and-mouth disease (Galloway and Elford 1931), vaccinia (Elford and Andrewes 1932), herpes (Elford et al. 1933), poliomyelitis (Elford et al. 1935) and influenza (Elford et al. 1936) (see also Ferry (1936) for an exhaustive review). Later on, commercial mixed cellulose ester filters with very low pore size, namely VF (virus fine), VM (virus medium) and VC (virus coarse) grade filters produced by Millipore (Billerica, MA, USA—a subsidiary of Merck KGaA, Darmstadt, Germany), have also been applied to virus ultrafiltration for grouping by size assessment (Hsiung 1965).
As part of a series of papers surveying the antiviral applications of polysaccharide-based materials, the present review focuses on viral filtration using cellulosic filter media—most of which are commercially available products that have been extensively tested over the past twenty years for the removal/concentration/purification of viral particles from liquid samples, i.e., biopharmaceutical (namely blood products) and environmental (raw or treated) water samples. The application of cellulose-based filters in individual respiratory protective devices protecting the wearer against airborne viral pathogens is also detailed. Polysaccharide-based chromatographic adsorbents for viral clearance or recovery/purification of viruses or virus-like particles will be presented elsewhere.
Viral filtration in the downstream purification processes of biopharmaceuticals
In the processing of biological products, virus removal from the product stream while providing maximum product recovery is a critical task. It is more particularly difficult to eliminate small viral particles such as parvoviruses, which may contaminate blood products and mammalian cell cultures used in the production of recombinant proteins (Charcosset 2006). Viral filters developed to answer this challenge are typically membrane (screen) filters ensuring a size-based rejection of viral particles via a sieving mechanism. Given the size of viruses, ranging roughly from 20 (Parvoviridae) to 400 nm (Poxviridae) (Segura et al. 2011), viral filtration stands between microfiltration and ultrafiltration among pressure-driven filtration processes (Fig. 2), though it is frequently but incorrectly (van Reis and Zydney 2007) classified as nanofiltration (Burnouf et al. 2005).
Fig. 2.
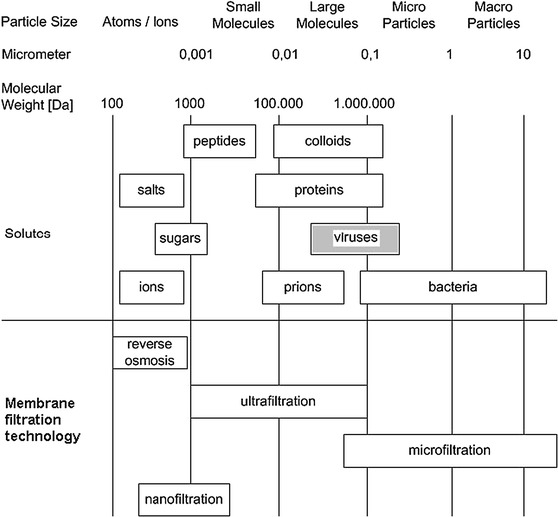
The separation spectrum for filtration membranes.
Adapted from Fröhlich et al. (2012)
Regenerated cellulose hollow fiber (HF) membrane filters
Common virus filtration membranes are made from poly(ethersulfone) (PES), poly(vinylidene fluoride) (PVDF), and regenerated cellulose (Burnouf and Radosevich 2003; Carter and Lutz 2002; Liu et al. 2010; van Reis and Zydney 2007). Among the latter, the commercial Planova™ filters (Asahi Kasei Medical, Tokyo, Japan) have been widely used to clear viruses from biologically produced pharmaceuticals, in particular blood products. Planova filters are composed of cuprammonium regenerated cellulose HF (Bemberg™ cupro fibers), prepared from cellulose cuprammonium spinning solution via microphase separation under precise spinning conditions (Tsurumi et al. 1990b, c). The wall of each HF has a three-dimensional web structure of pores consisting of large, bulky void pores interconnected by fine capillaries (Tsurumi et al. 1990a, b, c) (Fig. 3). During filtration, as the feed solution containing the product of interest is circulated through the HF bore, viruses accumulate in the large, bulky void pores of the fiber network while the product solution passes through the capillary pores. Since the HF wall is several tens of micrometers thick (Fig. 4), viruses are captured gradually inside the porous structure that can be considered multi-layered (100–200 layers) (Hongo-Hirasaki et al. 2006). Hence, Planova filters behave like “membrane depth filters” (Walsh and Denyer 2012) operating on the basis of size exclusion. They offer a choice of 4 nominal mean pore sizes, namely 15 nm (15N), 19 nm (20N), 35 nm (35N) and 72 nm (75N)—the large pore 75N model being essentially used as a prefilter to remove impurities or aggregates prior to final virus filtration—and can be operated in normal (dead-end/flow through) or tangential flow (cross flow) filtration mode (Phillips et al. 2007).
Fig. 3.
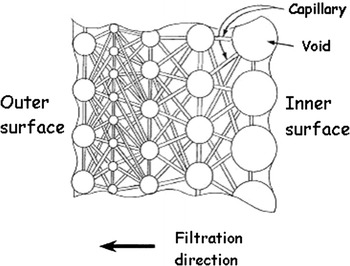
Schematic representation of the void pore structure of regenerated cellulose hollow fibers used in Planova filters.
Adapted from Makino et al. (1994)
Fig. 4.
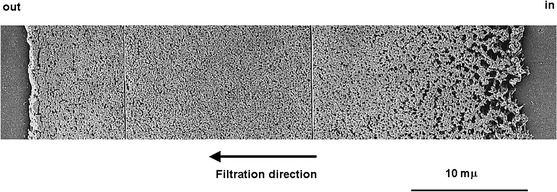
Cross sectional micrograph of Planova (20N model) hollow fiber wall.
Taken from Hongo-Hirasaki (2006)
Planova filters have emerged to answer a public-health problem of worldwide magnitude, i.e., blood contamination by HIV (Hamamoto et al. 1989; Manabe et al. 1989) and hepatitis viruses (Yuasa et al. 1991; Sekiguchi et al. 1989, 1990). Table 1 gives examples of viral clearance studies assessing the capacity of Planova filters to remove viruses from blood products. In these viral clearance assays, product streams resulting from successive, scaled-down purification steps representative of the manufacturing process of the product (Kundu and Reindel 2007), were artificially contaminated (“spiked”) with model viruses of different sizes (Table 2) (at much higher concentrations than what might be commonly found in the product intermediate) before being submitted to filtration. The virus removal efficiency of the filtration step was expressed as LRV (log reduction value, i.e., log ratio of the viral load in the spiked product feed stream to that recovered in the product filtrate), which implies that residual virus infectivity can never be reduced to zero but may be greatly reduced mathematically (ICH 1999). Minimum LRV values were most frequently given. They were estimated when no viruses could be detected in filtered samples taking into account the detection limit of the assay (viral titer estimated as 1 infectious unit per sample volume). From the measured LRVs, the filtration operation is usually classified as effective (LRV > 4), moderately effective (1 < LRV < 4) or ineffective (LRV < 1) (Phillips et al. 2007). Since filtration complements other viral clearance methods in the downstream processing of biotherapeutics (Brorson 2007; Klamroth et al. 2014; Shukla et al. 2007), several studies quoted in Table 1 also report LRVs for common virus reduction steps such as inactivation by pasteurization (Gröner 2014; Gröner et al. 2012; Terpstra et al. 2007) or solvent/detergent treatment (Dichtelmüller et al. 2012; Terpstra et al. 2006), and chromatographic capture (Gröner 2014; Gröner et al. 2012). Virus removal during the plasma fractionation process (Bryant and Klein 2007) leading from the crude plasma pool to the product (immunoglobulin here) stream has also been quantified (Dichtelmüller et al. 2012; Koenderman et al. 2012; Terpstra et al. 2006). Overall virus reduction factors obtained by addition of successive LRVs are given in these works, as illustrated by Table 3.
Table 1.
Virus reduction factors (LRVs) achieved by filtration with Planova filters in the manufacturing process of different blood products (confidence intervals not shown)
| Product | Filtera | Virusb | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B19 V | CPV | PPV | HAV | EMCV | WNV | BVDV | HIV-1 | PRV | |||
| Coagulation factor VIII (cross eight M®) | 35N (DE) | <1.0 | – | – | – | 1.5 | – | >5.3 | – | >4.9 | Furuya et al. (2006) |
| 20N (DE) | 4.9 | – | >5.1 | >3.4 | >5.8 | – | >4.6 | – | >4.0 | ||
| Coagulation factor VIII (Beriate®) | 20N | – | 3.4 | – | ≥5.5 | – | – | ≥5.8 | ≥6.0 | ≥7.2 | Gröner (2014) |
| Coagulation factor VIII (Factane®) | 35N + 15N (DE) | – | – | ≥6.1 | ≥3.6 | – | – | ≥4.1 | ≥3.8 | ≥4.9 | Chtourou et al. (2007) |
| Coagulation factor IX (Replenine®-VF) | 15N (DE) | – | – | – | >6.0 | 5.7 | – | – | >6.6 | – | Roberts et al. (2010) |
| Human normal immunoglobulin (Nanogam®) | 15N (T)c | >6.1 | >3.4 | – | – | >5.8 | – | >6.3 | >5.4 | >6.1 | Terpstra et al. (2006) |
| 15N + 15N (T)d | >6.1 | >3.9; ≤4.1 | – | – | >5.8 | – | >6.3 | >5.4 | >6.1 | Koenderman et al. (2012) | |
| 20N + 20N (DE)d | >4.3; ≤5.1 | >5.8 | >7.2 | >6. | >6.4 | ||||||
| Human intravenous IgG (IVIG) | 35N (DE) | – | – | <1.0 | – | – | – | >4.9 | >5.2 | >4.6 | Dichtelmüller et al. (2012) |
| C1 esterase inhibitor (C1-INH) (Cetor®) | 15N (DE) | – | >4.5 | – | >4.9 | – | – | >5.5 | >5.6 | >6.4 | Terpstra et al. (2007) |
| C1-INH | 20N + 15N (DE) | – | ≥7.2 | ≥5.3 | – | – | ≥8.0 | ≥5.3 | ≥5.1 | ≥7.1 | Gröner et al. (2012) |
| Holotransferrin | 15N + 15N (DE) | – | >6.9 | – | >5.6 | – | – | >7.5 | >6.3 | >6.8 | Koenderman et al. (2012) |
| Plasma-derived proteinse | 15/20N | – | – | 4.9 | – | 5.3 | ≥5.5 | 4.7 | ≥5.5 | ≥5.3 | Caballero et al. (2014) |
a DE dead-end (normal) mode, T tangential mode. Filters were associated in series in DE and in parallel in T
bSee Table 2
cFiltration step combined with pepsin treatment at pH 4·4
dExperiments were performed in duplicate; the lowest LRV is given. The filtration step was combined with low pH (4.4) inactivation for enveloped viruses (BVDV, HIV, PRV)
eSeven plasma proteins (and 2 types of Planova filters) were involved (mean LRV values are given)
Table 2.
Main characteristics of viruses used to evaluate the viral clearance efficiency of Planova filters
| Familya | Acronym | Name | Relevantb/model forc | References |
|---|---|---|---|---|
|
Parvoviridae (ssDNA, NE) 18–26 nm |
B19 V | Human parvovirus B19 | Relevant | Furuya et al. (2006) and Terpstra et al. (2006) |
| CPV | Canine parvovirus | B19 V | Gröner (2014), Gröner et al. (2012), Koenderman et al. (2012) and Terpstra et al. (2006, 2007) | |
| PPV | Porcine parvovirus | B19 V | Caballero et al. (2014), Chtourou et al. (2007), Dichtelmüller et al. (2012), Furuya et al. (2006) and Gröner et al. (2012) | |
|
Picornaviridae (ssRNA, NE) 22–30 nm |
HAV | Hepatitis A virus | Relevant | Chtourou et al. (2007), Furuya et al. (2006), Gröner (2014), Roberts et al. (2010) and Terpstra et al. (2007) |
| EMCV | Encephalomyocarditis virus | HAV | Furuya et al. (2006, Roberts et al. (2010), Terpstra et al. (2006), Koenderman et al. (2012), Caballero et al. (2014) | |
|
Flaviviridae (ssRNA, E) 40–60 nm |
WNV | West Nile virus | Relevant/HCV | Caballero et al. (2014) and Gröner et al. (2012) |
| BVDV | Bovine viral diarrhea virus | HCV | Caballero et al. (2014); Chtourou et al. (2007), Dichtelmüller et al. (2012), Furuya et al. (2006), Gröner (2014), Gröner et al. (2012), Koenderman et al. (2012) and Terpstra et al. (2006, 2007) | |
|
Retroviridae (ssRNA, E) 80–120 nm |
HIV-1 | Human immunodeficiency virus 1 | Relevant | Caballero et al. (2014, Chtourou et al. (2007), Dichtelmüller et al. (2012), Gröner 2014 (2012), Koenderman et al. (2012), Roberts et al. (2010) and Terpstra et al. (2006, 2007) |
|
Herpesviridae (dsDNA, E) 180–250 nm |
SuHV-1 (PRV) |
Suid herpes virus 1 (pseudorabies virus) |
HSV, CMV | Caballero et al. (2014), Chtourou et al. (2007), Dichtelmüller et al. (2012), Furuya et al. (2006), Gröner (2014), Gröner et al. (2012), Koenderman et al. (2012) and Terpstra et al. (2006, 2007) |
a ss single stranded, ds double stranded, E enveloped, NE nonenveloped
bKnown to be a frequent contaminant of blood products
c HCV Hepatitis C virus, HSV herpes simplex virus, CMV cytomegalovirus
Table 3.
Virus reduction capacity of the manufacturing process of Beriate®.
Adapted from Gröner (2014)
| Manufacturing process | Virus reduction factors (log10) | ||||
|---|---|---|---|---|---|
| HIV | BVDV | PRV | HAV | Parvovirusa | |
| Pasteurization | ≥6.8 | ≥9.3 | 4.7 | 3.9 | ≥3.8b |
| Ion exchange chromatography | 3.3 | 3.0 | 2.1 | 1.3 | 3.4c |
| 20 N virus filtration | ≥6.0 | ≥5.8 | ≥7.2 | ≥5.5 | 3.4c |
| Overall virus reduction factor | ≥16.1 | ≥18.1 | ≥14.0 | ≥10.7 | ≥10.6 |
aVirus reduction factor demonstrated for
bB19 V (relevant human parvovirus)
cCPV (model virus for B19V)
In the virus spiking studies quoted in Table 1, the actual viral filtration step was preceded by pre-filtration to eliminate viral and/or protein aggregates that might be present in the spiked product solution. The presence of aggregated viruses may lead to overestimation of size exclusion effectiveness while contributing with protein aggregates to the fouling of viral filters, which reduces the membrane hydraulic permeability and may affect virus retention (Phillips et al. 2007). Pre-filtration was performed on the spike-virus stock suspension (Caballero et al. 2014; Gröner et al. 2012; Roberts et al. 2010) on the product intermediate before virus inoculation (Gröner et al. 2012; Koenderman et al. 2012), and/or on the virus spiked material (Chtourou et al. 2007; Dichtelmüller et al. 2012; Koenderman et al. 2012; Terpstra et al. 2006, 2007). For pre-filtration of the virus preparation, micro filters with a pore size adapted to the spike virus size, i.e., slightly lower than the virus size, were used to remove cell aggregates but not single viral particles—usual pore sizes ranging between 0.1 and 0.45 μm. Micro filters with 0.1–0.2 μm pore size (Koenderman et al. 2012; Terpstra et al. 2007; 2006), but also 75N (Dichtelmüller et al. 2012) and 35N (Chtourou et al. 2007). Planova viral filters were used to pre-filtrate the spiked product solution. In the latter work, the association in series of 35N and 15N filters led to efficient removal of small viruses such as PPV (Chtourou et al. 2007). This was not the case when 35N was used as the main viral filter after pre-filtration with 75N (Dichtelmüller et al. 2012), which confirms previous date showing that the 35 nm pore size was too large to retain the smallest viral particles (Furuya et al. 2006; Hongo-Hirasaki et al. 2006).
Both 20N (Caballero et al. 2014; Furuya et al. 2006; Gröner 2014) and 15N (Caballero et al. 2014; Roberts et al. 2010; Terpstra et al. 2007; 2006) Planova filters and their combination (Gröner et al. 2012; Koenderman et al. 2012) showed effective virus removal over a wide range of viral particle sizes (Table 1). As a general rule, the removal efficiency of the filters increased with the ratio of pore size to virus size, as illustrated by Gröner (2014) and Terpstra et al. (2007). This was also demonstrated by Roberts et al. (2010) by filtrating poliovirus type 1 (a picorvanirus) through Planova filters manufactured with different pore sizes, ranging between 15 (LRV = 6.9) and 35 nm (LRV = 0.2). The two-filter combinations were globally more efficient than single filters at removing small viruses from spiked protein intermediates, though two 15N filters connected in parallel for tangential filtration (Koenderman et al. 2012) yielded LRVs quite similar to those obtained using 15N alone (Terpstra et al. 2006). Care should be taken when comparing vertically the data collected in Table 1, however. In addition to the sizes of viral particles and filter pores, the results of such virus-spiking studies are dependent on a number of process parameters such as the filtration operating conditions (filtration mode, transmembrane pressure, volume per filter area, temperature…), the characteristics of the product feed stream (pH, conductivity, nature and concentration of the product…), or else the purity level and concentration of the virus spike. Moreover, several studies include pre-filtration (Dichtelmüller et al. 2012) or viral inactivation (Koenderman et al. 2012; Terpstra et al. 2006) in the calculation of LRVs. This variability in test conditions for filtration-based virus removal makes difficult any comparison between LRVs collected from multiple sources as in Table 1, even though some results (Caballero et al. 2014; Hongo-Hirasaki et al. 2006; Roberts et al. 2010) contradict its influence on filtration performance, underlining the robustness of the filtration process. Also, some variations in virus removal capacity may occur between commercial filter modules owing to their manufacture from different batches of HF, as illustrated by Roberts et al. (2010) for 15N filters.
Cellulose-fiber depth filters with inorganic filter aid
Like membrane microfiltration, depth filtration is widely used as a clarification step in biopharmaceutical purification to ensure removal of cell debris, large aggregates and contaminants from the product stream prior to purification processes such as column/membrane chromatography and viral filtration/inactivation steps (Liu et al. 2010; van Reis and Zydney 2007; Vicente et al. Vicente et al. 2011a, b). Commercially available depth filters currently employed in bioprocessing are composed of cellulose fibers with a porous inorganic filter aid and a resin binder, generally cationic. For instance, the Zeta Plus™ filter media (3 M Purification Inc.– formerly Cuno, Meriden, CT, USA) is composed of a cellulose fiber depth matrix containing silica-based filter-aid material and positively charged by chemical bounding of a cationic charge modifier (Ostreicher 1991). Varying retention ratings are available (0.1–1.0 μm). The triple layered A1HC filter media from EMD Millipore include two depth filtration layers (cellulose + diatomaceous earth) of different grades and a 0.1 μm-pore-size microporous membrane pre-filter made of mixed cellulose esters (van Reis and Zydney 2007). Hence, these cellulosic depth filters rely on both size exclusion and electrostatic adsorptive binding to effect separation (Liu et al. 2010). In viral spiking studies (Barnette et al. 2012; Dichtelmüller et al. 2012; Zhou et al. 2008), they were found to retain viral particles whose surface is negatively charged over a wide range of pH as their isoelectric points (pI) range most frequently between 3.5 and 7.0, with a mean value of 5.0 ± 1.3 (Michen and Graule 2010).
Viral monitoring of environmental waters
Viruses are recognized as a major cause of water-related disease (Bosch et al. 2008; Fong and Lipp 2005; Gibson 2014; Hamza et al. 2011). Enteric viruses, more particularly, which are associated primarily with gastrointestinal illness (Bosch et al. 2008; Fong and Lipp 2005; Gibson 2014; Hamza et al. 2011), are implied in most waterborne viral outbreaks (Gibson 2014; Hamza et al. 2011). All types of water, including waters used for drinking (surface or ground supplies), recreational waters (fresh, marine, and swimming pool), agricultural waters for irrigation (rivers and groundwater) and waste waters (sewage or industrial effluents), have been shown to be potential vehicles for virus transmission (Bosch et al. 2008). Contamination is most frequently of fecal origin (Bosch et al. 2008; Wong et al. 2012). Although viral levels may be high at the contamination source, e.g., concentrations ranging between 105and 1011 virus particles per gram of feces are referred to in the literature (Michen and Graule 2010), viral concentrations as low as 1–10 viral particles per liter may be found in environmental water (Julian and Schwab 2012). Since enteric viruses display high infectivity (Fong and Lipp 2005; Julian and Schwab 2012), such titers may constitute a health risk and should be detected for reliable surveillance of viral pathogens in water. As a consequence, efficient concentration methods are needed to capture viruses in large-volume water samples and release the retained viral material in concentrated form. Most common methods are based on filtration processes (Cashdollar and Wymer 2013; Ikner et al. 2012). In particular, virus adsorption–elution (viradel) filter methods have been widely implemented since the 1970s (Cashdollar and Wymer 2013). Briefly, in a viradel method, viruses from aqueous samples are reversibly adsorbed to microporous filters and then eluted from the filters in a small liquid volume (APHA, AWWA and WEF 1998). Adsorbent filters carry electrical charges and virus retention occurs via electrostatic interaction rather than by size exclusion. In other words, viradel filters act as depth filters rather than sieves. Contrary to viral filters that retain viruses by a sieving mechanism to achieve complete viral clearance, their pore size lies in the microporous range, which allows the high flow filtration necessary for virus capture in large water samples. Positively or negatively charged filters can be used in a viradel procedure, among which those made of cellulosic materials have been dominant over the last few decades.
Electropositive filters
Since their pI is below the pH of natural water (i.e., around neutrality) (Michen and Graule 2010), the surface charge of most waterborne viruses is usually negative under normal environmental conditions. Hence, electropositive filters have been logically developed to concentrate viruses in water through electrostatic adsorption. Recently used as virus-retentive prefilters in IgG manufacturing processes (Barnette et al. 2012; Dichtelmüller et al. 2012) (see above), the Zeta Plus™ S Series depth filter disks commercialized by 3 M Purification had also been tested earlier for concentration of viruses in water samples of different origins (Table 4). More recent studies made use of Zeta Plus™ 1 MDS microfilters, however (Table 5). In these works, pure (e.g., distilled), tap, environmental or sewage water samples were seeded with varying enteric viruses that are common waterborne pathogens, i.e., members of the families Picornaviridae (polioviruses, coxsakieviruses, teschoviruses), Adenoviridae (adenoviruses), Caliciviridae (noroviruses, caliciviruses) and Reoviridae (rotaviruses) (Fong and Lipp 2005), or bacteriophages (enterobacteria phages) that are considered as alternative indicators of fecal contamination and as index organisms for the presence of enteric viruses in waters (Goodridge and Steiner 2012). Naturally contaminated waters containing sufficient levels of indigenous viruses, more particularly bacteriophages, were also used in addition to spiking studies sensu stricto (Table 4). Following sample filtration, viral particles adsorbed to the filter were eluted in concentrated form using a variety of eluting solutions, the most common eluent consisting of a slightly alkaline (pH 9.0–9.5) protein solution (i.e., beef extract), frequently buffered with glycine–NaOH or another amino acid solution (sometimes supplemented with salt to aid disruption of electrostatic interactions between viruses and filters (Shields and Farrah 1983), a chaotropic agent (e.g., urea) or a surfactant (e.g., Tween 80) to affect virus-filter hydrophobic interactions (Farrah et al. 1981). Eluted viruses were quantified—using plaque titer assays, 50% tissue culture infectious dose (TCID50), quantitative real-time PCR (qPCR) and reverse-transcription PCR (RT-qPCR) (Hamza et al. 2011)—to assess the recovery efficiency of the filtration step by comparing to input titers. This efficiency depends on a number of process parameters including the filter type and filtration conditions (e.g., filtration rate and pressure), the elution buffer composition and eluting conditions, the nature, input titer and titration method of the tested virus—in addition to the water matrix characteristics that may affect virus quantification and filter performance (Borchardt et al. 2013). Hence, Tables 4 and 5 display a wide range of recovery yields.
Table 4.
Studies of the eighties assessing the virus recovery efficiency of viradel concentration methods using electropositive depth filters of cellulosic nature (Zeta Plus™ S Series)
| Filter gradea | Elution bufferb | Secondary concentrationc | Water nature | Virusesh | Recovery yieldj (%) | Concentration factork | References |
|---|---|---|---|---|---|---|---|
| 50S (47 mm) | EB1 (15 ml) | – |
Tap waterg (3.8 l) pH adjusted to 7.5 |
PV-1 | 56 (±8, n = 17) | 142 | Sobsey and glass (1980) |
| 50S (90 mm) | EB2 (72 ml) | – |
Tap waterg (9.4–17.5 l) pH adjusted to 7.0–7.5 |
MS-2 ØX174/T2/T4 |
89 (n = 2) 40 (n = 3)/34 (n = 2)/56 (n = 2) |
170 96/80/113 |
Goyal et al. (1980) |
|
EB2 (60-130 ml) EB2 (30-50 ml) |
– |
Secondary sewage effluent (5.0–10.0 l) Raw sewage (500 ml) |
Indigenous coliphages |
56 (n = 3) 57 (±16, n = 8) |
41 7 |
||
| 60S (142 mm) | EB3, pH 9.0 (75 ml) | Organic flocculationd (×20) |
Tap waterg (2 l) pH adjusted to 6.5 Tap waterg (33–64.5 l) pH adjusted to 6.5 |
SiRV-A/SA11 |
62 (±16, n = 8) Combined 47 (n = 2) 75 (± 40, n = 8) (low viral input) 23 (±8, n = 3) Combined 16 (± 7, n = 3) |
17 251 400 135 1800 |
Guttman-Bass and Armon (1983) |
|
30S (47 mm) 30S (142 mm) |
EB4 (10 ml) EB4 (50 ml) + EB5 (50 ml) |
Hydroextractione (×10) |
River water (sewage-polluted) 250 ml 20 l |
RV-A |
44 (±6, n = 3) Combined 16 (±3, n = 3) |
11 320 |
Raphael et al. (1985) |
|
60S (273 mm) |
EB6 (500 ml) |
Hollow fiber ultrafiltration (×14) |
Surface water (pond) (65 l) Clarified by prefiltration pH adjusted to 5.5–6.0 Surface water (river) (65 l) |
MS-2/PR4 ØX174/T2/P22 Ø6 Indigenous enteric bacteriophages |
93/80 24/87/82 79 Combined 50–60 |
121/104 31/113/107 103 929/1114 |
Logan et al. (1980) |
| 30S (273 mm) | EB7 (400 ml) |
Inorganicf + organicd flocculation (×330–500) |
Activated sludge effluent (19 l) |
PV-1 CVB3 |
Combined 49 (± 7, n = 3) 44 (n = 2) |
9027 7504 |
Chang et al. (1981) |
| 50S (142 mm) | EB3, pH 9.5 (50 ml) | – |
Sewage effluent: - Primary (1 l) - Secondary (5 l/10 l) |
Indigenous coliphages |
14 100/50 (n = 2) |
3 100 |
Rose et al. (1984) |
| 60S (142 mm) | EB3, pH 9.0 (50–75 ml) | – |
Wastewater: - Untreated (300–450 ml) - Primary effluent (550–850 ml) |
Indigenous enteroviruses |
24 (±4, n = 3) 43 (±38, n = 3) |
4 1.4 |
Guttman-Bass et al. (1987) |
aZeta Plus™ S Series depth filters are electropositive filter disks made of a charge-modified cellulose-diatomaceous earth matrix with varying diameters (given in parentheses) and nominal retention ratings: (approximately) 0.6–2.0 μm (30S), 0.4–0.8 μm (50S), 0.2–0.6 μm (60S)
bEB1: 0.05 M glycine–NaOH, pH 11.0; EB2: 1:1 ratio of 1 M NaCl + 8% beef extract, pH 9.0; EB3: 3% beef extract; EB4: 1% tryptose phosphate broth, pH 9.0; EB5: 6% arginine, 6% glycine; EB6: 1% (w/v) beef extract, 0.05 M arginine, pH 9.0; EB7: 4 M urea, 0.05 M lysine, pH 9.0
cFigures in parentheses are volume reduction factors (vol. filter eluate/vol. final viral concentrate)
dObtained by lowering the pH of the protein solution (e.g. beef extract eluent) to 3.5 (Katzenelson et al. 1976)
eDialysis against PEG (Ramia and Sattar 1979)
fPrecipitation with aluminium hydroxide (Wallis and Melnick 1967)
d,e,fSee also APHA, AWWA and WEF Standard Methods for the Examination of Water and Wastewater, 20th edition, 1998, Parts 9510 C (Virus concentration from large sample volumes by adsorption to and elution from microporous filters)g, 9510 D (Virus concentration by aluminum hydroxide adsorption–precipitation)j, and 9510 E (Hydroextraction-dialysis with polyethylene glycol)h
gTap water was dechlorinated by addition of sodium thiosulfate
hCVB3, coxsackievirus B3; PV-1, poliovirus type 1; RV-A, rotavirus A; SiRV-A/SA11, simian rotavirus A/SA11; MS-2, PR4, enterobacteria phages (sex-specific); ØX174, T2, T4, P22, enterobacteria phages (somatic coliphages); Ø6, pseudomonas phage
jAverage values are given (± , standard deviation; n, sample size); combined recovery yields were obtained by combining viradel filtration with a secondary concentration step
kCalculated as (recovery yield) × (volume reduction factor)
Table 5.
Spiking studies assessing the virus recovery efficiency of viradel concentration methods using electropositive microfilters of cellulosic nature (Zeta Plus™ 1 MDS)
| Filter typea | Elution bufferb | Secondary concentrationc | Water nature | Virusesh | Recovery yieldj(%) |
Concentra-tion factork | References | |
|---|---|---|---|---|---|---|---|---|
| 47-mm disk |
EB1, pH 9.5 (5 ml) EB1, pH 9.5 (5 ml) EB2 (5 ml) |
– |
Pure water (40 ml) Seawater (50 ml) Pure water (1 l) |
PV-1 |
50 6 62 |
4 0.6 124 |
Katayama et al. (Katayama et al. 2002) | |
| 47-mm disk | EB1, pH 9.0 (5 ml) | – |
Pure water (500 ml) Tap waterg (500 ml) |
PV-1 |
34 (n = 2) 15 (n = 2) |
34 15 |
Haramoto et al. (2007a) | |
| 47-mm disk |
EB3, pH 10.0 (100 ml) |
Organic flocculationd (×20) Celite adsorption/ elutione (×20) |
Tap waterg (100ml) |
HAdV-40 HAdV-41 HAdV-40 HAdV-41 |
Combined 40 (±10, n = 3) 23 (±8, n = 3) 52 (±22, n = 3) 64 (±4, n = 3) |
8 1.5 10 13 |
McMinn (2013) | |
| 2 × 90-mm disks | EB4 (n.g.) |
PEG precipitationf (n.g.) |
Pure water (200 ml) Tap waterg (200 ml) Ground water (200 ml) Surface water (200 ml) Tap waterg (40 l) Ground water (40 l) Surface water (1.9-2.3 l) |
Pan-1 Pan-1/PV-1 Pan-1 Pan-1 |
94 (n = 2) 73 (n = 2) 67 (n = 2) 64 (n = 2) Combined 38 (n = 2)/53 (n = 2) 19 (n = 2) 14 (n = 2) |
Huang et al. (2000) | ||
| 142-mm disk | EB5 (500 ml) | – | Pure water (1 l) |
MNV-1 HuNV GII.4 |
54.4 (±8.9, n = 3) 67.5 (±40.3, n = 3) |
1 1.4 |
Lee et al. (2011) | |
| 2 × 142-mm disks | EB6 (25 ml) | – | Tap waterg (1 l) |
MS-2 ØX174 |
89 (±10, n = 5) 78 (±17, n = 4) |
36 31 |
Polaczyk et al. (2007) | |
| Cartridge | EB6 (1 l) | – | Tap waterg (20 l) |
MS-2 ØX174 |
32 (±13, n = 4) 37 (±26, n = 4) |
6 7 |
||
| Cartridge | EB3, PH 9.0 (2 × 500 ml) | Celite adsorption/elutione (×12.5) |
Tap waterg River water |
10 l 100 l 10 l 100 l |
PV-1 |
Combined 44 (±9, n = 3) 67 (±6, n = 3) 30 (±11, n = 3) 36 (±21, n = 3) |
55 838 38 450 |
Karim et al. (2009) |
aZeta Plus™ 1 MDS microfilters (pore size: 0.2 μm) are composed of an electropositive, charge-modified, fiberglass and cellulose medium. Flat filter discs are available in varying diameters (e.g. 47 and 142 mm). Cartridges are made of two layers of pleated medium and are available in double open-ended configurations (c. 25-cm length, 0.39-m2 filtration surface)
bEB1: 3% (w/v) beef extract; EB2: 1 mM NaOH, pH 10.5 to 10.8; EB3: 1.5% beef extract, 0.05 M glycine; EB4, 3% beef extract, 0.4 M NaCl, 0.05 M glycine, pH 9.0; EB5: 1.5% beef extract, 0.05 M glycine, 0.01% Tween 80; EB6: 1.5% beef extract, 0.05 M glycine, 0.1% (w/v) sodium polyphosphate, 0.01% (v/v) Tween 80, 0.001% (v/v) Antifoam Y-30 Emulsion, pH 8.0
c,dSee notes to Table 4
eCelite® (Imerys Filtration Minerals, San Jose, CA, USA) diatomaceous earth powder was added to the filter eluent. Viruses adsorbed onto the diatomite particles were released in phosphate buffer (Dahling and Wright 1986)
fAddition of a simple aqueous solution of PEG to the (salt-containing) filter eluate (Lewis and Metcalf 1988; and references therein)
gTap water was dechlorinated by addition of sodium thiosulfate
hHAdV-40, human adenovirus species F, type 40; HAdV-41, human adenovirus species F, type 41; MNV-1, murine norovirus type 1; HuNV GII.4, human norovirus genogroup II; genotype 4; Pan-1, primate calicivirus; PV-1, poliovirus type 1; MS-2, F-RNA coliphage; ØX174, somatic coliphage
j,kSee notes to Table 4
In practical tests aimed at the detection of naturally-occurring viruses in field, large-volume water samples, the filtration (adsorption–elution) step is usually a primary concentration step which is followed by a secondary one to reach the virus detection threshold, e.g., organic flocculation (Katzenelson et al. 1976) or PEG precipitation (Lewis and Metcalf 1988). The recovery yield of such two-step concentration processes was evaluated in several studies using seeded or naturally contaminated water samples (Tables 4, 5). As a general rule, the viral loss due to secondary concentration was balanced by a huge increase in concentration factor allowing virus detection in water concentrates from large-volume samples, as reported by Chang et al. (1981) and Raphael et al. (1985) for indigenous enteroviruses in wastewater and rotaviruses in sewage-polluted surface water, respectively. After determining the optimal elution conditions for 1 MDS disk-adsorbed noroviruses following filtration of spiked distilled water samples, Lee et al. (2011) (Table 5) applied the optimized procedure to the detection of noroviruses in environmental water. They combined adsorptive filtration using 1 MDS cartridge with organic flocculation to enrich viruses from large-volume surface (200 l) and ground (500 l) water samples. This integrated two-step process led to high volume reduction factors, i.e., 10,000 and 25,000 for surface and ground water samples, respectively (200–500× for filtration, 50× for organic flocculation), but its recovery efficiency was not evaluated by spiking studies. In the same way, beside spiking studies like those detailed in Table 5, 1 MDS filter cartridges have been widely applied to concentrate enteric viruses in water samples of different origins (Table 6), ranging from large-volume samples of weakly contaminated water intended for drinking or drinking water production (Borchardt et al. 2003, 2004; Lee et al. 2014; Sedmak et al. 2005; Verheyen et al. 2009) to wastewater samples with high viral content—to assess the virus removal efficiency of wastewater treatment plants (Kuo et al. 2010; Simmons et al. 2011). In these tests of field water samples for enteric viruses, 1 MDS filtration was mainly combined with second-stage concentration by organic flocculation as detailed by Fout et al. (2001) in the USEPA (United States Environmental Protection Agency) manual of methods for virology. The elution of filter-adsorbed viral particles was mostly performed with an alkaline beef extract solution supplemented or not with glycine (buffers EB3 and EB1 in Table 5). The work by Verheyen et al. (2009) is an exception to these common procedures. To concentrate viruses in small-volume samples from drinking water sources, these authors used two filter cartridges in series with no additional concentration step and an alkaline powdered milk solution as elution buffer. They found that 20% (3/15) and 13% (32/247) of surface and ground (well) water sources, respectively, were contaminated with adenoviruses, with very few samples positive to rotavirus (0/15 surface water samples, 6/247 well water sources). Despite the differences in virus concentration methods, these results compare with those obtained by Xagoraraki et al. (2007) and Cheong et al. (2009) for adenovirus detection in surface (14/58 samples, 24%) and ground water (4/29 samples, 14%), respectively. They also agree with data reported by Borchardt et al. (2003) and Cheong et al. (2009) showing the low contamination level of ground water by rotavirus. It is worth noting here, however, that the number of positive samples reported in these viral analyses of water (and the others quoted in Table 6) is dependent on the sensitivity of the detection techniques used to assess the presence of viruses, i.e., nucleic acid-based amplification methods such as conventional PCR, reverse-transcription PCR (RT-PCR), nested PCR, real-time PCR/RT-PCR or integrated cell culture PCR (ICC/PCR) to determine virus infectivity (Fong and Lipp 2005; Hamza et al. 2011, Mattison and Bidawid 2009; Watzinger et al. 2006) with different detection thresholds.
Table 6.
Viral analysis of environmental water samples following virus concentration by the viradel procedure using 1MDS filter cartridges
| Secondary concentrationa | Water nature | Samplesc | Detected virusese | References | ||
|---|---|---|---|---|---|---|
| Vol (l) | No. | >0 | ||||
| OF | Groundwater (private household wells) | 1234 | 194 | 5 | HAV, PV-3, RV-A, NLV GII | Borchardt et al. (2003) |
| OF or PEGP | Groundwater (municipal drinking-water wells) | 1611 | 48 | 24 | EVsf, RV-A, HAV, NoV GI | Borchardt et al. (2004) |
| Surface (river) water | 431 | 12 | 10 | EVsf, RV-A, NoV GI | ||
| PEGP | Groundwater (confined aquifer) | 1448 | 30 | 7 | EVsf | Borchardt et al. (2007) |
| PEGP + CU | Groundwater (irrigation water) | 300 | 29 | 5 | PV-3, HAdVs | Cheong et al. (2009) |
| Celite A/E | Groundwater (wells and spring in quartz aquifers) | 1570 | 14 | 9 | EVs, ReoVs | Johnson et al. (2011) |
| (Two cartridges in series) | Drinking water sources (wells, pumps, surface water) | 10 | 541 | 43 | RVs, HAdVs | Verheyen et al. (2009 |
| OF | Groundwater | 4464 | 5 | 2 | NoV GI/GII | Lee et al. (2011) |
| Surface water | 186 | 5 | 4 | NoV GI | ||
| OF | Surface water | – | 29 | 27 | EVs, HAstVs, HAdV-40/-41 | Chapron et al. (2000) |
| OF | Surface (lake) water (source water for drinking water production) | 200 | 204 | 18 | ReoVs | Sedmak et al. (2005) |
| OF | Surface (lake) water (recreational beach water) | 250–300 | 58 | 14 | HAdVs | Xagoraraki et al. (2007 |
| OF | Surface (river, lake) water (source water for tap water production) | 200 | 265 | 9 | HAV | Lee et al. (2014) |
| OF | Wastewater (raw sewage, primary effluent, membrane influent, membrane effluent)b | 20–400 | 32 | 32 | HAdV-A/-C/-F | Kuo et al. (2010) |
| 32 | 32 | EVs, NoV GII | Simmons et al. (2011) | |||
| OF | Wastewater (membrane bioreactor effluents) | – | 26 | 21 | HAdVs | Hirani et al. 2013 |
| OF | Receiving waters | 120–280 | 125 | 92d | HAdVs, NoVs | Rodriguez et al. Rodríguez et al. 2012 |
aOF, organic flocculation; PEGP, polyethylene glycol precipitation; CU, centrifugal ultrafiltration; Celite A/E, Celite adsorption/elution
bWastewater samples taken at different treatment stages of a full-scale municipal membrane bioreactor
cThe average volume, total number and number of virus-positive (for at least one virus type) samples are given
dPositive samples according to total culturable virus assays (HAdVs, 53/125; NoVs, 25/125)
eEV, human enterovirus; HAdV, human adenovirus; HAdV-A/-C/-F, human adenovirus species A/C/F; HAdV-40/-41, human adenovirus species F, type 40/41; HAstV, human astrovirus; HAV, hepatitis A virus; NLV GII, Norwalk-like virus (norovirus) genogroup II; NoV, norovirus; NoV GI/GII, norovirus genogroup I/II; PV-3, human poliovirus type 3; ReoV, reovirus (respiratory enteric orphan virus); RV, rotavirus; RV-A, rotavirus A
fHuman coxsackieviruses, echoviruses (enteric cytopathogenic human orphan viruses) and polioviruses
It emerges from this glance at literature that cellulose-based electropositive filters are still commonly used for viral monitoring of water. These filters are expensive, however, and face competition with cheaper products, in particular nanoalumina fiber filters and glass wool filters (Cashdollar and Wymer 2013; Wong et al. 2012). The former, NanoCeram™ filters manufactured by Argonide Corporation (Sanford, Fla., USA), are composed of nanosized (2 nm diameter), alumina-based {mainly boehmite, γ-AlO(OH)} fibrilles dispersed in a microglass fiber matrix, resulting in an electropositive filter media with 2–3 μm average pore size (Tepper and Kaledin 2005, 2006). The latter consist of commercial sodocalcic glass wool coated with mineral oil (type Bourre 725 QN/TECH Loose Wool, Isover Saint-Gobain, Courbevois, France), hand packed into columns or filter holders in the laboratory (Vilaginès et al. 1993). These glass wool filters harbor electropositive sites while presenting hydrophobic surface characteristics. Efficient enrichment of (seeded or/and) indigenous viruses from various water samples using NanoCeram (Gibbons et al. 2010; Ikner et al. 2011; Pang et al. 2012), and glass wool (Deboosere et al. 2011; Lambertini et al. 2008; Wyn-Jones et al. 2011) filters has been reported in recent years (see also Cashdollar and Wymer 2013; Wong et al. 2012). Compared with 1 MDS filters (Table 5), NanoCeram filters showed similar (Lee et al. 2011), slightly lower (McMinn 2013) or higher (Karim et al. 2009) virus concentration efficiency.
Electronegative filters
Commercially available under different pore sizes (0.025–8.0 μm), mixed cellulose ester membrane filters are negatively charged over a wide range of pH values, their overall negative charge increasing with pH (Kessick and Wagner 1978). Microporous filters in the 0.1–0.45 μm pore size range have been used for ages in laboratory and industry for size-based filtration of bacterial particles and cell debris (surface filters). These and larger pore size filters have been shown to retain enteroviruses, however, despite the much smaller size of viral particles compared to the nominal mean diameter of filter pores (Cliver 1968). The presence of salts enhanced virus adsorption to cellulose ester membranes, this effect increasing with the cation valence (i.e., Al3+ > Mg2+ > Na+) (Wallis et al. 1972). Acidification of the viral suspension also improved virus adsorption efficiency, even in the absence of exogenously added salts (Sobsey et al. 1973). These early results were later confirmed by Lukasik et al. (2000), who investigated the influence of mono-, di-, and trivalent salts (NaCl, MgCl2, and AlCl3) on the adsorption of poliovirus and enterobacteria phages to the MF-Millipore™ membrane filter type HA (0.45 μm pore size) and the electropositive 3M™ Zeta Plus™ 1 MDS microfilter at neutral or acidic pH. At pH 7, salts promoted virus adsorption to HA filter while affecting adsorption to 1 MDS filter. At pH 3.5, more than 95% of the viruses tested adsorbed to HA filter with or without salt added to the viral suspension—the salts interfering again with viral adsorption to 1MDS filter. Furthermore, the addition of urea or Tween 80 to the salt solution affected virus adsorption to both filters at pH 3.5 and HA filter at pH 7. In agreement with previous studies by the same group (Haramoto et al. 2004, 2005; Lukasik et al. (2000) explained these results by the antichaotropic effect of salts that increased hydrophobic interactions between filters and viruses and was impaired by the chaotropic agent or the detergent. At neutral pH, charge screeening by salt addition reduced electrostatic attractive and repulsive forces between viruses and 1 MDS or HA filter, respectively. Cation-mediated cross-complexation between negative groups on virus and filter surfaces (Kessick and Wagner 1978) and, more particularly, strengthened hydrophobic virus-filter interactions contributed to improving the adsorption efficiency of HA filter. At acidic pH, viruses displayed a positive surface charge and their electrostatic interactions with HA filter switched from repulsive to attractive—and inversely for electropositive 1 MDS filter. The presence of salt probably affected these interactions but was balanced by the promoting effect of salt on hydrophobic interactions.
Hence, most tests reported so far for virus concentration from water samples using cellulose ester filters (essentially HA filters) have been performed after addition of multivalent cations (mainly Mg2+) to the water samples with or without pH adjustment to an acidic level. According to Katayama et al. (2002), the virus-loaded filters were rinsed with an acidic solution to eliminate remaining cations before elution with NaOH or other alkaline buffers. Haramoto et al. (2004, 2005, 2007a) proposed a variation in the method that consisted in pre-conditioning the filter with Al3+ ions. Aluminum chloride was passed through the filter, making an electropositive ion coating, which avoided cation addition to the water sample. The recovery efficiency of both protocols has been evaluated by seeding water samples with enteric viruses and bacteriophages and quantifying eluted viruses (Table 7). Similarly to spiking studies (Tables 4, 5) and viral analyses of field water samples (Table 6) with electropositive filters, the HA-based filtration/elution process was frequently associated with a secondary concentration step to increase virus concentration factors. Here, centrifugal ultrafiltration (CU) using commercial Millipore (Centriprep®, Centricon® or Amicon®) concentrators was the elective concentration method. These CU units contain a low adsorptive regenerated cellulose membrane (Ultracel®) whose nominal molecular-weight cutoff ranges between 3 and 100 kDa—a MWCO value of 50 kDa being most frequently selected for secondary concentration of eluted viruses. They are routinely used in laboratories to purify and concentrate biomacromolecules such as peptides, proteins, and nucleic acids from small-volume biological samples (e.g., 2–15 ml for the Centriprep filter unit). Tested or not for virus recovery by spiking experiments, the combined concentration procedures have been extended to the detection of enteric viruses in field water samples including tap (Haramoto et al. 2004), sea (Katayama et al. 2002), river (surface) (Fong et al. 2010; Hamza et al. 2009; Haramoto et al. 2005) and waste (Fong et al. 2010; Katayama et al. 2008) water (Table 8). Samples with different volumes were collected according to the water source, i.e., sample volumes increased as the expected contamination level decreased. For instance, Katayama et al. (2002) applied HA filtration, followed by acid rinse of the filter, viral elution with NaOH and secondary concentration of the eluate by CU, to concentrate naturally occurring viruses (noroviruses, enteroviruses and HAV—hepatitis A virus) in seawater samples. Based on the volume reduction factor (2-l sample/2 ml final concentrate, i.e., 1000) and the recovery yield of the two-step process (Table 7), a virus concentration factor of 670 was reached. Only HAV virus was not detected in any sample tested. Later on, Katayama et al. (2008) followed the same protocol to concentrate enteric viruses in the raw influent of a wastewater treatment plant. Samples of 100-ml volume were collected and submitted to the two-step concentration process, yielding a volume reduction factor of c. 140. The four tested kinds of enteric viruses were detected in all 72 wastewater samples but one lacking norovirus GI (NoV GI). Using PEG precipitation as the secondary concentration step, Hamza et al. (2009) tested river water samples for contamination by enteric viruses and bacteriophages. A volume reduction factor of 5000 (10-l sample/2 ml final concentrate) and virus concentration factors ranging between 1000 (norovirus) and 5000 (adenovirus) (see Table 7 for recovery yields) were obtained. All 41 analyzed samples were found positive for enteric viruses. Human adenovirus and norovirus were detected in 97.5 and 32% of the samples, respectively. Haramoto et al. (2004) illustrated the ability of Al3+-coated filters to concentrate viruses from large-volume freshwater samples without salt addition by detecting noroviruses in tap water from Tokyo University. Tap water samples (303-l average volume) underwent two successive filtration/elution steps using 293-mm and 47-mm diameter HA filters prior to concentration by CU (final volume: 0.9 ml), which ensured a volume reduction factor higher than 300,000. However, the virus recovery efficiency of the 3-step concentration process was not evaluated. Ten of the 98 tested samples were found positive for noroviruses. These results compare to those reported by Lee et al. (2011) (Table 5) using 1 MDS cartridge with organic flocculation to enrich noroviruses from large surface and ground water samples.
Table 7.
Spiking studies assessing the virus recovery efficiency of viradel concentration methods by using: electronegative microporous membrane filters of cellulosic nature
| Filter typea Conditioningb | Sample pretreatmentc | Elution bufferd | Secondary concentratione | Water nature | Virusesf | Recovery yieldg (%) | Concentration factork | References |
|---|---|---|---|---|---|---|---|---|
|
HA (47 mm) Acid rinse |
MgCl2 (25 mM) (not for seawater) | EB1 (5 ml) | CU (×5) |
Pure water (40 ml) Seawater (50 ml) Artificial seawater (1 l) Seawater (1 l) |
PV-1 |
95 89 (n = 2) 62 (±10, n = 8) Combined 67 (n = 3) |
8 9 124 670 |
Katayama et al. (2002) |
|
HA (47 mm) Acid rinse |
MgCl2 (25 mM) | EB1 (2.5 ml) | – |
Influent wastewater (25 ml) Secondary effluent (250 ml) Final effluent (250 ml) |
PV-1 |
23 (±19, n = 20) 80 (±42, n = 18) 65 (±28, n = 24) |
2 80 65 |
Katayama et al. (2008) |
|
HA (47 mm) Acid rinse |
MgCl2 (25 mM) (not for seawater) | EB1 (5 ml) | – |
Pure/tap water (500 ml) Secondary effluent (250 ml) Seawater (250 ml) |
PV-1 |
83/79 (n = 3) 50 (id.) 115 (id.) |
83/79 25 58 |
Haramoto et al. (2007a) |
|
HA Acid rinse |
MgCl2 (25 mM), pH adjusted to 5.0 | EB1 (15 ml) | CU (×7.5) | Diluted wastewater (2 l) | RV-A |
Combined 3.5 (n = 6) |
35 | Fumian et al. (2010) |
|
HA (142 mm) Acid rince |
MgCl2 (5(1) or 25(2) mM) |
EB1 (12 ml) | CU (×6) |
(2-l sample volume) Tap water(1) Mineral water(2) Seawater(2) River water(1) |
NoV GII/ HAstV-1 |
Combined 3/2 (n = 3) 23/64 (id.) 5/14 (id.) 18/43 (id.) |
30/20 230/640 50/140 180/430 |
Victoria et al. (2009) |
|
HA (47 mm) Acid rinse |
MgCl2(50 mM), pH adjusted to 3.5 | EB2 (6 ml) | CU (n.g.) | Diluted river water(100 ml) | MS-2/PAdV/ PTV |
Combined h 6/7/0.5 (n = 3) |
– | Jones et al. (2014) |
|
HA (142 mm) Acid rinse |
MgCl2 (50 mM), pH adjusted to 3.5 | EB2 (70 ml) | PEGP (×35) | Pure/river water (1 l) |
E-11 NoV GI AdV-5 JCPyV ØX174 |
Combined i 97/51 (n = 2–4) 90/21 (id.) 88/102 (id.) 25/41 (id.) 6.5/55 (id.) |
485/255 450/106 440/510 123/206 32/275 |
Hamza et al. (2009) |
|
HA (142 mm) Acid rince |
MgCl2(50 mM), pH adjusted to 3.5 | EB2 (70 ml) | PEGP (×35) |
Ground/rain/river water (5 l) |
MNV-1 MS-2 HAV NoV GI NoV GII RV-A |
Combined 22/6/11 (n = 3) 5/6/3 (id.) 32/30/21.5 (id.) 24/13/8 (id.) 27.5/20/14 (id.) 10/1/0 (id.) |
548/145/268 128/140/85 803/750/538 598/330/208 688/495/345 245/33/0 |
Keuckelaere et al. (de Keuckelaere et al. 2013) |
| HA (47 mm) | MgCl2 (25 mM) | EB3 (5 ml) | – | Pure/tap water (500 ml) | PV-1 | 55/23 (n = 2) | 55/23 | Haramoto et al.( 2007a) |
| GS (47 mm) | MgCl2 (50 mM) | EB4 (6 ml) | CU (n.g.) |
Diluted river water (100 ml) |
MS-2/PAdV/ PTV |
Combined h 52/95/1.5 (n = 3) |
– | Jones et al. (2014) |
| SC (47 mm) | pH adjusted to 3.5 | EB5 (4 ml) | CU (×20) or evaporation (×20) | Source water (1 l) | HAdV-41 |
55 (±19) Combined j 19 (CU)/48 |
– | Wu et al. (2011) |
|
HA (47 mm) Acid rinse |
AlCl3 (5 mM) (not for seawater), pH adjusted to 3.5 | EB1 (10 ml) | CU (×50) |
Desalinated seawater (1 l) Seawater (1 l) Secondary effluent (200 ml) |
rAdV-5 |
Combined 66 (±14, n ≥ 3) 49 (±19, n≥3) 28 (±13, n ≥ 3) |
3300 2450 280 |
Li et al. (2010) |
|
HA (47 mm) AlCl3 Acid rinse |
– | EB1 (5 ml) | – |
Pure/tap water 500 ml 1 l 10 l |
PV-1 |
88/82 (n = 5) 98/99 (n = 3) 109 (n = 2) |
88/82 196/198 2180 |
Haramoto et al. (2004) |
aMillipore mixed cellulose ester membranes; pore sizes: 0. 22 μm (GS); 0.45 μm (HA), 8.0 μm (SC). Filter diameters are given in parentheses
bAcid rinse: filter rince with 0.5 mM H2SO4, pH 3.0 before virus elution; AlCl3: a solution of AlCl3 (250 mM) was passed through the filter before sample filtration
cMgCl2 or AlCl3: a solution of MgCl2 or AlCl3 was added to the water sample before filtration. Tap water was dechlorinated by addition of sodium thiosulfate
dEB1: 1 mM NaOH, pH 10.8; EB2, 50 mM KH2PO4, 1.0 M NaCl, 0.1% (v/v) Triton X-100, pH 9.2; EB3, 3% (w/v) beef extract, pH 9.0; EB4, 3% beef extract, 0.5 M NaCl, pH 9.0; EB5, 1.5% beef extract, 0.75% glycerol, pH 9.0
eCU, centrifugal ultrafiltration (using centrifugal filter units); PEGP, polyethylene glycol precipitation (Lewis and Metcalf 1988). Figures in parentheses are volume reduction factors (vol. filter eluate/vol. final viral concentrate)
f(r)AdV-5, (recombinant) adenovirus type 5 vector; E-11, human echovirus (enteric cytopathogenic human orphan virus) type 11; HAdV-40, human adenovirus species F, type 40; HAdV-41, human adenovirus species F, type 41; HAstV-1, human astrovirus type 1; HAV, hepatitis A virus; JCPyV, JC human polyomavirus; MNV-1, murine norovirus type 1; MS-2, F-RNA coliphage; NoV GI, norovirus genogroup I; NoV GII, norovirus genogroup II; PAdV, porcine adenovirus; PV-1, human poliovirus type 1; PTV, porcine teschovirus; RV-A, rotavirus A; ØX174, somatic coliphage
gAverage values are given (±, standard deviation; n, sample size); combined (recovery yields), obtained by combining viradel filtration with a secondary concentration step
hWater samples were spiked with a mixture of 3 viruses that were eluted together
iWater samples were spiked with a mixture of 5 viruses that were eluted together. Median recovery values are given
jObtained by multiplying the recovery yields of the two concentration steps evaluated separately
kCalculated as (recovery yield) × (volume reduction factor)
Table 8.
Viral analysis of environmental water samples following virus concentration by the viradel procedure using Millipore HA mixed cellulose ester membrane filters
| Methoda | Water nature | Samplesd | Detected virusese | References | ||
|---|---|---|---|---|---|---|
| Vol. (l) | No. | >0 | ||||
| MgCl2 | Urban wastewater (raw influent, intermediate and final effluents) | 0.1 | 3 | 3 | NoV GI/GII | Tong et al. (2011) |
| Surface water (freshwater and seawater) | 2.0 | 16 |
13 5/10 |
NoV GI/GII NoV GI/NoV GII |
||
| Wastewater (raw sewage influent) | – | 14 |
9 8 8 5/3 |
PMMoV, klassevirus, NoV, GII/GIV PMMoV Klassevirus NoV GII/NoV GIV |
Han et al. (2014) | |
| MgCl2 (CU) | Seawaterb (receiving water) | 2.0 | 8 | 3/1 | NV GI/GII | Katayama et al. (2002) |
| Seawater (bathing beach) | 2.0 | 6 | 4 | EVs | ||
| Wastewater: raw sewage | 0.1 | 17 | 17 | HAdVs | Haramoto et al. (2007b) | |
|
Secondary-treated sewage Seawaterb |
1.0 |
17 18 |
14 15 |
|||
|
Wastewater: raw influent Secondary-treated effluent |
1.0 0.1 |
72 72 |
72 71 |
HAdVs, EVs, NoV GI/GII | Katayama et al. (2008) | |
| Final effluent | 1.0 | 71 |
68/66 47 71 65/63 41 |
HAdVs NoV GI/GII EVs HAdVs NoV GI/GII EVs |
||
|
Wastewater: raw influent Final effluent |
0.1 1.0 |
12 12 |
12 7 |
SaVs | Haramoto et al. (2008) | |
| Surface (river) water | 2.0 | 52 | 48 | HAV | de Paula et al. (2007) | |
| Surface (river) water | 2.0 | 52 |
31 23 16 8 3 |
RVs, HAdVs, HAstVs, NoVs RVs HAdVs HAstVs NoVs |
Miagostovich et al. (2008) | |
| Wastewater: raw influent | 0.05 | 30 | 30 | HAdVs | Ahmed et al. (2010) | |
| Primary/secondary effluents | 18/16 | 18/2 | HAdVs, BAdVs | |||
| Septic tanks | 10 | 8 | HAdVs | |||
| Surface (river) water |
0.05 4.0–5.0 |
40 |
8 4 4 |
BAdVs | ||
| Seawaterb, brackish water, | 2.0 | 84 | 54/16/7 | HAdVs/RV- | Rigotto et al. (2010) | |
|
wastewater, drinking water Seawater |
36 | 25/13/2 | A/HAV | |||
| Brackish water | 12 | 9/2/2 | ||||
| Wastewater | 12 | 8/1/1 | ||||
| Drinking water | 24 | 12/0/2 | ||||
|
Wastewater: raw influent Treated effluent |
0.1 1.0 |
24 | 24 | Enteric viruses (10 types)f, PMMoV | Kitajima et al. (2014) | |
| MgCl2 (PEGP) | Surface (river) water | 10.0 | 41 |
41 40 40 37 33 13 1 |
HAdVs, HPyVs, RV-A, NoV GII, EVs, somatic coliphages HAdVs HPyVs RV-A Somatic coliphages NoV GII EVs |
Hamza et al. (2009) |
| AlCl3 | Urban surface (river or lake) water | 0.5 | 108 |
58 52 6 5 |
RVs, HAstVs, NoVs RVs HAstVs NoVs |
He et al. (2012) |
| AlCl3 (CU) | Tap water |
303 (100–532) |
98 |
10 4/7 |
NoV GI/GII NoV GI/NoV GII |
Haramoto et al. (2004) |
| Surface (river) water | 0.5 | 64 |
43 34/28 29 6 3 |
NoV GI/GII, HAdVs, EVs, TTVs NoV GI/NoV GII HAdVs EVs TTVs |
Haramoto et al. (2005) | |
| Surface (river) water | 0.5 | 36 | 29 | HAdVs | Haramoto et al. (2007b) | |
| Surface (river) water | 0.5 |
36 60 |
23 12 |
SaVs |
Haramoto et al. (2008) Kitajima et al. (2010) |
|
| Wastewater (raw sewage, primary, secondary and final effluents)/combined sewer overflowsc) | 0.1–2.0 |
46/6 26 |
46/6 10 |
HAdVs | Fong et al. (2010) | |
| Surface (river) water | 0.5–2.0 | |||||
| Groundwater (artesian wells) | 0.5 | 202 | 4/1 | NoV/RV | Chironna et al. (2012) | |
aMgCl2: a solution of MgCl2 was added to the water sample before filtration according to Katayama et al. (2002); AlCl3: a solution of AlCl3 was passed through the filter before water sample filtration, according to Haramoto et al. (2004). Ninety-mm diameter (Ø) filters were used, except in Tong et al. (2011) (Ø = 47 mm), Hamza et al. (2009) and Rigotto et al. (2010) (Ø = 142 mm), and Haramoto et al. (2004) (Ø = 293 mm + Ø = 47 mm). In both methods, filters were rinsed with 0.5 mM H2SO4, pH 3.0 before virus elution using 1 mM NaOH (pH 10.8). In parentheses, secondary concentration step applied: CU, centrifugal ultrafiltration; PEGP, polyethylene glycol precipitation
bNo MgCl2 added
cCombination of storm water and untreated sewage
dThe average volume, total number and number of virus-positive (for at least one virus type in a mixture of viruses or for a given virus type) samples are given
eBAdV, bovine adenovirus; EV (human enterovirus); HAdV, human adenovirus; HPyV, human polyomavirus; HAstV, human astrovirus; HAV, hepatitis A virus; NoV, norovirus; NoV GI/GII/GIV, norovirus genogroup I/II/IV; NV GI/GII, Norwalk virus (norovirus) genogroup I/II; PMMoV, pepper mild mottle virus; RV, rotavirus; RV-A, rotavirus A; SaV, sapovirus (Sapporo-like virus); TTV, torque teno virus
fAiV, (Aichi virus), BKPyV (BK polyomavirus), EVs, HAdVs, JCPyV (JC polyomavirus), NoV GI/GII/GIV, RV-A, SaVs
Despite these promising data, the two viradel methods based on electronegative filters (i.e., addition of MgCl2 to water samples or filter coating with Al3+ ions before filtration) have been essentially implemented for viral analysis of waters containing high amounts of indigenous viruses, requiring limited sample volumes (Table 8)—electropositive filter cartridges being more adapted to virus concentration from large volumes of weakly contaminated water (Table 6). Beside the above-mentioned work by Haramoto et al. (2004), only two studies among those detailed in Table 8 describe virus detection in environmental water samples with low viral content, namely drinking water sources after chlorination or without treatment (Rigotto et al. 2010) and groundwater from artesian wells (Chironna et al. 2012). Both studies used small-volume samples and CU as secondary concentration step, yielding a modest volume reduction factor of 1000. Very few from 202 artesian well water samples were found positive to the tested enteric viruses: none for HAV and enterovirus, 1 for rotavirus and 4 for norovirus (Chironna et al. 2012). A large proportion (50%) of samples from drinking-water supplies was positive to adenovirus, however (Rigotto et al. 2010). These data are compatible with viral analyses of groundwater and water intended for drinking performed using 1 MDS filtration as the first virus concentration step (Table 6). It should be noted, however, that the same remark applies to data collected in Table 8 as to those in Table 6 concerning the numbers of virus-positive samples, i.e., their dependence on the virus detection method. This can be illustrated by the two following examples. Using ICC-PCR (measuring infectious viruses), Katayama et al. (2002) detected enteroviruses in 4 of 6 seawater samples from bathing beach, but no sample was found positive by direct RT-PCR. De Paula et al. (2007) found 48/52 river water samples positive for HAV by quantitative real-time RT-PCR, but only 12/52 by nested RT-PCR.
HF ultrafiltration
As attested by the large body of literature data quoted above, virus adsorption–elution methods using electropositive or electronegative filters are routinely applied to the primary concentration of waterborne viruses, each type of filter possessing its own advantages and drawbacks. Ultrafiltration is considered another filtration-based option to concentrate viruses from water samples (Cashdollar and Wymer 2013; Ikner et al. 2012). It has been indicated earlier that CU with microconcentrators based on cellulose ultrafiltration discs is commonly used as a secondary concentration step following the viradel process. Hollow fiber ultrafiltration (HFUF), however, is considered a potential technique for primary concentration of viruses from large-volume water samples, yielding better virus recoveries than the viradel method performed with either electropositive or electronegative filters (Cashdollar and Wymer 2013). Commercial HFUF devices operated in cross-flow mode have been applied to the simultaneous concentration of biological particles, including viruses, spiked in tap (Polaczyk et al. 2008) or reclaimed (Liu et al. 2012) water samples, to virus recovery from seeded tap (Rhodes et al. 2011) and estuarine (Hernandez-Morga et al. 2009) water samples, and also, more rarely, to virus recovery from field water samples (Grassi et al. 2010; Hernandez-Morga et al. 2009). Most of these devices are dialyzers that contain synthetic HF made from polysulfone. Following the early work by Belfort et al. (1976) showing that polysulfone HF membranes were superior to cellulose acetate ones for virus concentration, virus concentration experiments using HFUF dialyzers equipped with HF manufactured from cellulose are scarce. An example is given by Liu et al. (2012). They showed that the Exeltra Plus 210 cellulose triacetate HF dialyzer (Baxter Healthcare Corp., Deerfield, IL, USA) and the Optiflux® F200NR polysulfone dialyzer (Fresenius Medical Care, Walthamm, MA, USA) provided similar recovery efficiencies for MS2 and ΦX174 bacteriophages, Escherichia coli, Clostridium perfringens spores, and Cryptosporidium parvum oocysts from spiked 10-l and 100-l samples of reclaimed water.
Protection against airborne viruses
An array of viral infections can be transmitted by the airborne route, in particular via aerosols (droplet nuclei) (Coia et al. 2013; Tang et al. 2006; Tellier 2006; Verreault et al. 2008). Even though virus inactivation rates in the atmosphere are generally higher than those of bacterial and fungal contaminants, virus-containing aerosols can spread worldwide (Després et al. 2012). The threat of viral outbreaks and pandemics such as those caused by SARS coronavirus (Yu et al. 2004) and highly pathogenic strains of influenza A virus (Tellier 2006), allied with the fear of bioterrorism using viruses (e.g., smallpox and hemorrhagic fever viruses) (Barras and Greub 2014) as biological weapons, has encouraged the search for efficient protection equipment against aerosolized virus-containing particles.
Individual respiratory protective devices consist in respirators, i.e., air-filtering face masks designed to protect the wearer against inhalation of a hazardous atmosphere (here airborne infectious aerosols)—opposing to surgical masks designed to protect the environment from contaminants generated by the wearer’s exhaled breath (i.e., prevention of surgical infections) (Bunyan et al. 2013). Currently, most “filtering facepiece” (FFP) respirators have a multilayer composite structure with a central filtering layer displaying electret properties (Gralton and McLaws 2010). These electret filters (Thakur et al. 2013) are produced by imparting an electrostatic charge to a nonwoven fibrous mat composed of synthetic polymer fibers such as poly(propylene) (PP) (mainly), poly(butylene terephthalate), poly(tetrafluoroethylene) and poly(carbonate) fibers. Electrostatic charging of the filter media is commonly obtained by corona discharge, triboelectrification and electrostatic spinning (Tsai et al. 2002). Electret filters collect particles through the combined action of mechanical and electrostatic forces (Podgórski 2010; Wang 2001). Various FFP respirators are available on the market, classified according to their protection efficiency against particulate aerosols. For instance, a N95 respirator certified under NIOSH 42 CFR 84 US regulations (NIOSH 1995) and the equivalent FFP2 mask meeting the EN 143:2000 European standard (CEN 2000) should retain respectively at least 95 and 94% of influent particles (NaCl particles are used as nonbiological surrogate particles) (Rengasamy et al. 2009). First worn by surgical teams a hundred years ago to prevent bacterial contamination of patient’s open wounds, early surgical masks were constructed from layers of cellulose materials, more particularly cotton cellulose (gauze) (Haller and Colwell 1918) and derivatives (Arnold 1938) (see also Belkin 1997). Much more recently, face masks made from cotton fabrics have been tested as alternative respiratory protective equipment against pandemic outbreaks such as influenza (Dato et al. 2006; Davies et al. 2013; Rengasamy et al. 2010). Like respirators, current commercially available surgical face masks include several layers of non-woven fabrics with, frequently, a cellulose inner layer in contact with the wearer’s face to improve wearer’s comfort. However, the filtering layer, usually made of meltblown fibers (Ghosh 2014), is devoid of electret properties. Since particulate filtration is only mechanical, i.e., less efficient than that of respirators, most surgical masks are not certified for use as respiratory protective devices, standard tests to evaluate their filtration efficacy being less stringent (Oberg and Brosseau 2008).
While a number of studies have confirmed that surgical masks logically offer lower protection than respirators against aerosol particles, the effectiveness of both types of face masks at preventing viral respiratory infection, in particular influenza, is still a matter of controversy (Bin-Reza et al. 2012; Cowling et al. 2010; Gralton and McLaws 2010). The improper facial fit of respirators (Bunyan et al. 2013; Coia et al. 2013; Weiss et al. 2007) may affect their protection efficiency against infectious aerosols. It allows particulate flows outside the filtration area of the mask, resulting in face-seal leaks, i.e., the leakage of infectious particles around the edges of the mask (Grinshpun et al. 2009; Lei et al. 2013). Mask seal leakage can be minimized by applying existing guidelines for correct donning and fit checking of respirators (Coia et al. 2013). Moreover, human face and head form models have been proposed as a tool for designing respirators with improved protection efficiency, simulating interactions between faces and facemasks and describing their fit (Golshahi et al. 2013; Lei et al. 2012). The accumulation of viral particles at the surface and within the filtration media of respirators, where they can remain viable and infectious for extended periods of time (Coulliette et al. 2013; Sakaguchi et al. 2010), is another significant problem with which respirator wearers are confronted in the prevention of viral transmission and spread. Incorrect mask handling by the wearer may lead to accidental self-inoculation, cross-contaminations affecting both other healthcare workers and patients, and contamination of fomites (Casanova et al. 2008). Furthermore, the risk of virus reaerosolization from respirators during extended use, if limited, cannot be ruled out (Fisher et al. 2012). Recommendations for respirator doffing do exist but have proven insufficient to prevent virus transfer from respirator to healthcare employees’ hands and clothing (Casanova et al. 2008). Adding antiviral properties to the filtration process may contribute to limit the risk of viral transmission by improper handling of used respirators. A number of virucidal facemasks have been developed and patented over the past 15 years (Tiliket et al. 2011), some of which are commercially available. In the SpectraShield™ Plus respirator masks designed by Nexera Medical Inc. (Fort Lauderdale, Fla.) (Haas 2008), for instance, two layers of filter media are sandwiched between (inner and outer) antimicrobial layers where a silver–copper zeolite (carrier) antimicrobial agent (Agion® antimicrobial, Sciessent LLC, Wakefield, Mass.) is embedded around the core of synthetic fibers (Fosshield®, Foss Manufacturing, Hampton, NH). The Biofriend™ Biomask™ FFP respirator, manufactured by Filligent Ltd. (Hong Kong) and distributed by Medline Industries, Inc. (Mundelein, Ill.), is another four-layered device in which the melt-blown PP filtration layer is inserted between an inner layer of spunbond PP and two antiviral layers, namely a cellulose/polyester layer containing copper and zinc ions and an outer layer of spunbond PP treated with a low pH (citric acid-acidified) hydrophilic plastic coating (Davison 2012; Stewart et al. 2014). The outer layer absorbs infectious aerosol droplets and viruses are denaturated by exposure to citric acid. In the second layer, inactivation of viruses with damaged structures is completed by the virucidal effect of divalent metal cations. Contributing to the overall antiviral efficiency of the structure, the cellulosic component of this layer is a sulphated or sulfonated rayon fabric (Stewart et al. 2012, 2014) to which a variety of viral human pathogens bind via the cationic sites of viral envelopes/capsids.
Beside the commercial Biomask™ respirator, various composite structures designed for filtration–inactivation of airborne microbial contaminants, where cellulosic materials play an active antimicrobial role, have been patented in recent years (Baney et al. 2012; Bernard 2006; Nakamura and Nakamura 2011; Tsutsumi 2009; Zhang et al. 2012; Zhong 2011). Several of them are based on bacterial cellulose layers/coatings provided with an antimicrobial component, i.e., silver (Zhong 2011) or zeolite-supported silver (Nakamura and Nakamura 2011) nanoparticles, and chitosan (Zhang et al. 2012). The respiratory protective mask described by Zhong (2011) comprises a three-layer bacterial cellulose membrane whose middle layer contains silver nanoparticles. The antimicrobial facemask invented by Nakamura and Nakamura (2011) includes two base cloth elements both of which are made of a woven textile (gauze), a non-woven cellulose fabric (e.g., rayon), or a porous sheet (e.g., a 0.3–0.5 mm sliced sheet of urethane sponge). The first base cloth is filled with bacterial cellulose nanofibers that retain silver zeolite and a humectant (e.g., trehalose or 1,3-butylene glycol) in their network structure. Additional antimicrobial properties are provided to the mask by impregnating the second base cloth with a carboxylic acid (e.g., citric acid). Zhang et al. (2012) also used bacterial cellulose in a composite antimicrobial material suitable for respiratory protective masks. A nonwoven polymer fabric was coated with a film of bacterial cellulose mixed with poly(vinyl alcohol)—to improve the film-forming properties, mechanical strength and air permeability of the coating—and chitosan, a non-sulfated polysaccharide produced commercially by deacetylation of chitin that displays strong antibacterial potency (Kong et al. 2010; Rabea et al. 2003) and, to a lesser extent, antiviral activity (Rabea et al. 2003; Wang et al. 2012).
In the antimicrobial air filtration device proposed by Bernard (2006), cellulose derivatives—more particularly, cellulose acetate phthalate (CAP), a common pharmaceutical excipient for enteric coating of tablets and capsules with antiviral activity (Pirrone et al. 2011)—were used as “biocidal prophylactic compounds” to provide the classical filter media (e.g., a nonwoven PP web, electrostatically charged or not) with antimicrobial properties. Cellulose derivatives could be incorporated as fibers or particles (micronized fibers) into the air filtration device either in a separate layer positioned before or after the filter media, or deposited onto the outer surface of the filter media relative to the air flow direction. To illustrate this invention, handsheets prepared from CAP fibers intermixed or not with CAP particles were successfully tested for virucidal efficacy by exposure to an aerosol challenge of enterobacteria phage ØX174.
By exploiting the antimicrobial properties of dialdehyde polysaccharides such as starch and cellulose dialdehydes, Baney et al. (2012) proposed a new method for producing low-cost virucidal filters suitable for respiratory protective masks. The treatment of a standard cellulose filter paper (Whatman™ Grade 50 filter paper) with sodium periodate improved its antiviral potency due to oxidation of some cellulose to dialdehyde cellulose inside the filter. Challenged with aerosols of MS2 enterobacteria phage at high relative humidity (90% at 23 °C), the treated filter showed higher filtration efficiency than a control, untreated one, i.e., better removal of viable viral particles (Plaque Forming Units) joined to a lower resistance to air flow (pressure drop)—due to increased pore size distribution (Woo et al. 2011). Higher inactivation of MS2 virus by the treated filter was also highlighted, confirming an improvement of its disinfection capability (Woo et al. 2011). However, the filtration and inactivation of airborne viruses were less effective at lower moisture content of the filter, i.e., at air humidity levels commonly encountered in hospital settings, in particular operating rooms (Balaras et al. 2007). Furthermore, the inhalation resistance of the treated filter remained too high for application to respiratory protective masks. The same pros and cons apply to cellulose paper filters treated by immersion into aqueous suspensions of dialdehyde starch (DAS) (Woo et al. 2012). While this treatment did not affect the removal efficiency of viable MS2 viruses from aerosols at high relative humidity, virus survival on the treated filters was reduced (Fig. 5) and the pressure drop decreased as DAS concentration increased. The drop in air flow resistance remained insufficient for practical application to respirators. DAS treatment of a PP filter from a commercial FFP mask did not modify the filtration parameters (virus removal efficiency and pressure drop) of the filter but significantly improved its biocidal efficacy (Fig. 5)—an opening of this treatment towards the development of virucidal facemasks. Another example of cellulose filter material treated with cellulose derivatives to yield antiviral properties is given by Tsurumi (Tsutsumi 2009). This inventor devised a low-cost virucidal mask filter made of a standard cellulose nonwoven fabric impregnated with water extracts of sulfonated or aminated styrene-graft cellulose. To reinforce the water retention properties of the filter media, a superabsorbent resin such as poly(acrylic acid) was added to the graft cellulose extract before impregnation of the fabric or incorporated into a separate layer covering the impregnated fabric. Low (hydrogen ions from sulfonic acid groups) or high (hydroxyl ions generated by aminated groups) pH conditions inside the hydrated material were claimed to make it virucidal against influenza A virus and caliciviruses. The patent gives no illustration of such antiviral properties, however.
Fig. 5.
Relative survivabilitya (RS) of MS2 viruses on filtersb treated with different concentrations of DAS suspension. Error bars (n = 3) are shown. Taken from Woo et al. (2012). aRatio of the virus survival factor in the treated filter to that in the untreated filter, where the virus survival factor in a filter is the number of viruses recovered by elution from the filter divided by the number of viruses removed by the filtration process. b PF PP filter from commercial surgical mask (DuPont™ 01361N), CCF coarse pore cellulose filter paper (Whatman™ Grade 54, 22 μm pore size), FCF fine pore cellulose filter paper (Whatman™ Grade 50, 2.7 μm pore size)
Studies published by Tiliket et al. (2011) and Catel-Ferreira et al. (2015) present another type of cellulose-based material for airborne virus filtration in which a low-cost nonwoven cellulose material, i.e., commercial Kimwipes® (KW) wipes (Kimberly-Clark Worldwide, Inc., Dallas, Tex.), was chemically modified by coating with a synthetic polymer, i.e., poly(ethylenimine) (PEI) (Tiliket et al. 2011) or grafting of an antiviral agent, i.e., catechin (Catel-Ferreira et al. 2015). The filtration efficiency of the modified filter media was first tested on aerosolized T4D viruses (Enterobacteria phage T4, Doermann’s strain T4D). Then the treated filter was inserted inside a commercial medical mask in place of its cellulosic layer (Kolmi M24001 mask, Kolmi-Hopen, Saint-Barthélémy-d’Anjou, France), and the reconstructed mask was challenged with TD4 aerosols to evaluate its virus removal efficiency. Both treatments significantly improved the virus capture factor (ratio of upstream to downstream PFU contents) of KW cellulose wipes and of reconstructed commercial masks compared to original masks and to masks reconstructed with untreated wipes. In these studies, the breathability of reconstructed masks was not quantified, nor was the virus survivability on the modified filter media. However, additional filtration experiments using aerosols of influenza A virus (low pathogenic H5N2 strain) showed that challenged viral particles accumulated in PEI functionalized KW layers with no loss in number and infectivity (Tiliket et al. 2011). Thus these modified masks showed no virucidal effect, the PEI-modified layers behaving like an electret media owing to the polycationic character of PEI. On the other hand, since catechin and catechin-grafted wipes showed antiviral activity against T4D viruses in liquid media (Catel-Ferreira et al. 2015), it may be assumed that part of viruses accumulated in excess in the treated wipes were inactivated by the polyphenol agent, i.e., the reconstructed masks were actually virucidal.
Despite these few examples, the use of cellulosic media in filtration devices aimed at the capture of airborne viral particles remains now very limited compared to filtration of virus-contaminated liquid samples. While patented, several cellulose-based, virucidal filter media designed for insertion in air filtering face masks have not been commercially developed yet.
Besides the wearing of personal respiratory protective equipment, another aspect of the fight against airborne infections lies in the decontamination of indoor air in healthcare facilities, and, more generally, of air processed through heating, ventilating and air conditioning (HVAC) systems in the built environment. High Efficiency Particulate Air (HEPA) filters are the primary technology used for particulate removal in these collective protection devices—allied with UV irradiation, ozonation or air ionization to yield indoor air decontamination (Bolashikov and Melikov 2009; Jacob et al. 2013)—and may be complemented with a photytocatalyst such as TiO2 to improve the inactivation of accumulated microorganisms (Chen et al. 2010; Pigeot-Remy et al. 2014). Since HEPA filters are essentially made of glass fibers, they are beyond the scope of this review and their virus-retentive properties will not be detailed further.
Conclusion
Most frequently associated with other virus reduction (virus clearance of biopharmaceuticals) or concentration (viral analysis of water) steps, size-exclusion and/or adsorptive (charge mediated) filtration is an essential tool to fight against viral contaminants in aqueous media. Owing to inherent properties of cellulose, including mechanical strength and hydrophilicity (that opposes protein adsorption and biofouling), allied with widespread availability and biocompatibility, cellulose-based materials are still widely present in viral filters. Developed commercially over the past thirty years, several cellulosic filter media have been and continue to be used for both routine analyses in laboratories and academic research studies aimed at improving the filtration performance, generating a wealth of data quantifying the efficiency of virus removal or recovery/concentration from biologic or water samples, respectively.
As concerns biopharmaceutical compounds, these data arise from validation studies of the filtration step performed by spiking intermediate biologic solutions (mostly blood proteins) from multistage purification processes with known titers of viral particles. Regenerated cellulose HF Planova filters N15–N35, operated in the dead-end mode as a single filter or two units in series, were shown to provide LRV values ranging between 4 and 7, on the average. Increasingly tested since their launching in 1989, these cellulosic filters compete with filter media made of synthetic polymers such as PVDF (e.g., Viresolve® NFP—Normal Flow Parvovirus—from EMD Millipore; Ultipor® VF Grade DV20/DV50 from Pall Corporation, Port Washington, NY) or PES (e.g., Millipore Viresolve® Pro; Virosart® HS/HC/CPV from Sartorius Stedim Biotech, Aubagne, France). The hydrophilic nature of cellulose fibers is a definite advantage of Planova filters over others for virus removal from protein solutions, minimizing flux decline due to protein-dominated filter plugging (cake formation) at constant pressure (normal-flow) filtration (Rathore et al. 2014). However, they are usually operated at lower flow rate than filters made of synthetic polymers whose operating pressure is higher. Hydrophilic modified PVDF membrane filters designed for virus removal from high-concentration protein solutions at high flow rate are being developed by several manufacturers, including Asahi-Kasei (i.e., Planova™ BioEX filters, launched in 2009).
Many studies have also been reported to assess the virus concentration efficiency of the viradel method using spiked water samples, prior to the detection of waterborne viral contaminants in field water samples. In this method, cellulosic filters, whether positively or negatively charged, are used for adsorptive capture of viruses before virus recovery by elution with a low-volume eluent. Electropositive filters are more particularly suitable for virus capture in low-contaminated, high-volume water samples, e.g., samples of groundwater or source water for drinking water production (Table 6). Though routinely used, these filters are expensive and face competition with nanoalumina fiber filters (e.g., the patented Ahlstrom Disruptor® electroadsorptive filter) (Levi 2011) and glass wool filters, which are cheaper. On the other hand, efficient virus adsorption by electronegative filters—the standard, cost-effective mixed cellulose ester membrane microfilters—requires impractical sample pre-treatment: their use is limited to viral analysis of highly-contaminated waters, for which small-volume samples are sufficient (Table 8).
Cellulose nanomaterials may represent a promising perspective for cellulosic materials in their application to viral filtration of liquid samples, yielding filtration membranes with higher mechanical strength, water permeability, surface hydrophilicicy and resistance to biofouling (Carpenter et al. 2015). Ma et al. (2011) have presented a composite membrane consisting of an electrospun poly(acrylonitrile) nanofibrous scaffold deposited on a non-woven poly(ethylene terephthalate) support. This two-layered membrane was coated by a layer of cellulose nanofibers and tested for retention of MS-2 phages. At acidic pH, phage particles were adsorbed by the negatively charged cellulose nanofiber coating, yielding a LRV value >3.7. Later on, the same nanofibrous composite membrane was doped with cellulose nanofibers functionalized by grafting with poly(vinylamine) (Wang et al. 2013). MS-2 phages were adsorbed at neutral pH onto positively charged amino-modified nanofibers, with a LRV value of 4. Metreveli et al. (2014) and Asper et al. (2015) have presented a size-exclusion-based filter paper made of pure cellulose nanofibers for removal of swine influenza A and murine leukemia viruses, respectively. These non-woven materials could be tailored to ensure efficient virus retention (Gustafsson and Mihranyan 2016a). Self assembled into nanosheets to yield “mille feuille” structures, they showed efficient removal of parvoviruses (Gustafsson et al. 2016b): maybe a new filtration media for viral clearance?
A number of air filtration devices designed for individual protection against airborne viruses also include cellulosic materials. While published data on the filtration performance of cellulosic media for removal of viruses from air are scarce, industrial research is more apparent, in particular in Asian countries (China, Japan) where airborne pollution is a matter of great concern—the main objective being to add virucidal properties to the filtration media so as to inactivate accumulated viral particles. Therefore, some innovative media have been patented over the past few years with, however, no commercial development to date.
Virus species
- AiV
Aichi virus
- BAdV
Bovine adenovirus
- BKPyV
BK polyomavirus
- BVDV
Bovine viral diarrhea virus
- B19V
Human parvovirus B19
- CMV
Cytomegalovirus
- CPV
Canine parvovirus
- CVB3
Coxsackievirus B3
- EMCV
Encephalomyocarditis virus
- EV
Human enterovirus
- E-11
Human echovirus (enteric cytopathogenic human orphan virus) type 11
- ØX174, T2, T4, P22
Enterobacteria phages
- Ø6
Pseudomonas phage
- HAdV
Human adenovirus
- HAstV
Human astrovirus
- HAV
Hepatitis A virus
- HCV
Hepatitis C virus
- HIV-1
Human immunodeficiency virus 1
- HPyV
Human polyomavirus
- HSV
Herpes simplex virus
- HuNV GII.4
Human norovirus genogroup II genotype 4
- JCPyV
JC human polyomavirus
- MNV-1
Murine norovirus type 1
- MS-2
Enterobacteria phages
- NLV GII
Norwalk-like virus (norovirus) genogroup II
- NoV GI/GII/GIV
Norovirus genogroup I/II/IV
- PadV
Porcine adenovirus
- Pan-1
Primate calicivirus
- PMMoV
Pepper mild mottle virus
- PPV
Porcine parvovirus
- PRV
Pseudorabies virus
- PTV
Porcine teschovirus
- PV-1
Poliovirus type 1
- rAdV-5
Recombinant adenovirus type 5 vector
- ReoV
Reovirus (respiratory enteric orphan virus)
- RV-A
Rotavirus A
- SaV
Sapovirus (Sapporo-like virus)
- SiRV-A/SA11
Simian rotavirus A/SA11
- SuHV-1
Suid herpes virus 1
- TTV
Torque teno virus
- WNV
West Nile virus
Other abbreviations
- CAP
Cellulose acetate phtalate
- CCF
Coarse pore cellulose filter paper
- Celite A/E
Celite adsorption/elution
- CU
Centrifugal ultrafiltration
- C1-INH
C1 esterase inhibitor
- DAS
Dialdehyde starch
- DE
Dead end mode
- DsDNA
Double stranded deoxyribonucleic acid
- E
Enveloped
- EB
Elution buffer
- FCF
Fine pore cellulose filter paper
- FFP
Filtering facepiece
- HEPA
High efficiency particulate air
- HF
Hollow fiber
- HFUF
Hollow fiber ultrafiltration
- HVAC
Heating, ventilating and air conditioning
- ICC/PCR
Integrated cell culture/Polymerase chain reaction
- IgG
Immunoglobuline type G
- IVIG
Human intravenous immunoglobulin
- KW
Kimwipes®
- LRV
Virus reduction factor
- MDS
Modularly Designed Software
- MWCO
Molecular-weight cutoff
- N
Nonenveloped
- OF
Organic flocculation
- PEG
Poly(ethylene glycol)
- PEGP
Poly(ethylene glycol) precipitation
- PEI
Poly(ethylenimine)
- PFU
Plaque forming units
- PP
Poly(propylene)
- PVDF
Poly(vinylidene fluoride)
- Qpcr
Quantitative real-time polymerase chain reaction
- RS
Relative survivability
- RT-PCR
Reverse-transcription polymerase chain reaction
- SARS
Severe acute respiratory syndrome
- SsRNA
Single stranded ribonucleic acid
- T
Tangential mode
- TCID50
50% tissue culture infectious dose
- USEPA
United States environmental protection agency
- VIRADEL
Virus adsorption–elution
References
- Ahmed W, Goonetilleke A, Gardner T. Human and bovine adenoviruses for the detection of source-specific fecal pollution in coastal waters in Australia. Water Res. 2010;44:4662–4673. doi: 10.1016/j.watres.2010.05.017. [DOI] [PubMed] [Google Scholar]
- APHA (American Public Health Association), AWWA (American Water Works Association), WEF (Water Environment Federation) Virus concentration from small sample volumes by adsorption to and elution from microporous filters. In: Clesceri LS, Greenberg AE, Eaton AD, editors. Standard methods for the examination of water and wastewater, Part 9510 B. Baltimore: United Book Press; 1998. [Google Scholar]
- Arnold L. A new surgical mask—A bacteriologic air filter. Arch Surg. 1938;37:1008–1016. doi: 10.1001/archsurg.1938.01200060145011. [DOI] [Google Scholar]
- Asper M, Hanrieder T, Quellmalz A, Mihranyan A. Removal of xenotropic murine leukemia virus by nanocellulose based filter paper. Biologicals. 2015;43:452–456. doi: 10.1016/j.biologicals.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Balaras CA, Dascalaki E, Gaglia A. HVAC and indoor thermal conditions in hospital operating rooms. Energy Build. 2007;39:454–470. doi: 10.1016/j.enbuild.2006.09.004. [DOI] [Google Scholar]
- Baney RH, Farrah SR, Song L (2012) Antimicrobial agent, method of preparing an antimicrobial agent and articles comprising the same. U.S. Patent Application Publication no. US 2012/0114724 A1
- Barnette D, Roth NJ, Hotta JA, Cai K, Gall M, Hartwell R, Kent JD, Willis T. Pathogen safety profile of a 10% IgG preparation manufactured using a depth filtration-modified process. Biologicals. 2012;40:247–253. doi: 10.1016/j.biologicals.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Barras V, Greub G. History of biological warfare and bioterrorism. Clin Microbiol Infect. 2014;20:497–502. doi: 10.1111/1469-0691.12706. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Hughes TP. The preparation of the graded collodion membranes of Elford and their use in the study of filterable viruses. J Gener Physiol. 1934;18:143–162. doi: 10.1085/jgp.18.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort G, Rotem Y, Katzenelson E. Virus concentration using hollow fiber membranes–II. Water Res. 1976;10:279–284. doi: 10.1016/0043-1354(76)90166-4. [DOI] [Google Scholar]
- Belkin NL. The evolution of the surgical mask: filtering efficiency versus effectiveness. Infect Control Hosp Epidemiol. 1997;18:49–57. doi: 10.2307/30141964. [DOI] [PubMed] [Google Scholar]
- Bernard BL (2006) Anti-microbial air filter. U. S. Patent Application Publication no. US 2006/0021302 A1
- Bin-Reza F, Lopez Chavarrias V, Nicoll A, Chamberland ME. The use of masks and respirators to prevent transmission of influenza: a systematic review of the scientific evidence. Influenza Other Respir Viruses. 2012;6:257–267. doi: 10.1111/j.1750-2659.2011.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolashikov ZD, Melikov AK. Methods for air cleaning and protection of building occupants from airborne pathogens. Build Environ. 2009;44:1378–1385. doi: 10.1016/j.buildenv.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt MA, Bertz PD, Spencer SK, Battigelli DA. Incidence of enteric viruses in groundwater from household wells in Wisconsin. Appl Environ Microbiol. 2003;69:1172–1180. doi: 10.1128/AEM.69.2.1172-1180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt MA, Haas NL, Hunt RJ. Vulnerability of drinking-water wells in La Crosse, Wisconsin, to enteric-virus contamination from surface water contributions. Appl Environ Microbiol. 2004;70:5937–5946. doi: 10.1128/AEM.70.10.5937-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt MA, Bradbury KR, Gotkowitz MB, Cherry JA, Parker BL. Human enteric viruses in groundwater from a confined bedrock aquifer. Environ Sci Technol. 2007;41:6606–6612. doi: 10.1021/es071110+. [DOI] [PubMed] [Google Scholar]
- Borchardt MA, Kieke BA, Spencer SK. Ranking filter methods for concentrating pathogens in lake water. Appl Environ Microbiol. 2013;79:5418–5419. doi: 10.1128/AEM.01430-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A, Guix S, Sano D, Pinto RM. New tools for the study and direct surveillance of viral pathogens in water. Curr Opin Biotechnol. 2008;19:295–301. doi: 10.1016/j.copbio.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson K. Advances in viral clearance. In: Shukla AA, Etzel MR, Gadam S, editors. Process scale bioseparations for the biopharmaceutical industry. Boca Raton: CRC Press; 2007. pp. 449–462. [Google Scholar]
- Bryant BJ, Klein HG. Pathogen inactivation: the definitive safeguard for the blood supply. Arch Pathol Lab Med. 2007;131:719–733. doi: 10.5858/2007-131-719-PITDSF. [DOI] [PubMed] [Google Scholar]
- Bunyan D, Ritchie L, Jenkins D, Coia JE. Respiratory and facial protection: a critical review of recent literature. J Hosp Infect. 2013;85:165–169. doi: 10.1016/j.jhin.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf T, Radosevich M. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia. 2003;9:24–37. doi: 10.1046/j.1365-2516.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- Burnouf T, Radosevich M, Goubran HA, Willkommen H. Place of nanofiltration for assuring viral safety of biologicals. Curr Nanosci. 2005;1:189–201. doi: 10.2174/157341305774642894. [DOI] [Google Scholar]
- Caballero S, Diez JM, Belda FJ, Otegui M, Herring S, Roth NJ, Lee D, Gajardo R, Jorquera JI. Robustness of nanofiltration for increasing the viral safety margin of biological products. Biologicals. 2014;42:79–85. doi: 10.1016/j.biologicals.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Carpenter AW, de Lannoy CF, Wiesner MR. Cellulose nanomaterials in water treatment technologies. Environ Sci Technol. 2015;49:5277–5287. doi: 10.1021/es506351r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J, Lutz H. An overview of viral filtration in biopharmaceutical manufacturing. Eur J Parenter Sci. 2002;7:72–78. [Google Scholar]
- Casanova L, Alfano-Sobsey E, Rutala WA, Weber DJ, Sobsey M. Virus transfer from personal protective equipment to healthcare employees’ skin and clothing. Emerg Infect Dis. 2008;14:1291–1293. doi: 10.3201/eid1408.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar JL, Wymer L. Methods for primary concentration of viruses from water samples: a review and meta-analysis of recent studies. J Appl Microbiol. 2013;115:1–11. doi: 10.1111/jam.12143. [DOI] [PubMed] [Google Scholar]
- Catel-Ferreira M, Tnani H, Hellio C, Cosette P, Lebrun L. Antiviral effects of polyphenols: development of bio-based cleaning wipes and filters. J Virol Methods. 2015;212:1–7. doi: 10.1016/j.jviromet.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Chang LT, Farrah SR, Bitton G. Positively charged filters for virus recovery from wastewater treatment plant effluents. Appl Environ Microbiol. 1981;42:921–924. doi: 10.1128/aem.42.5.921-924.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapron CD, Ballester NA, Fontaine JH, Frades CN, Margolin AB. Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Appl Environ Microbiol. 2000;66:2520–2525. doi: 10.1128/AEM.66.6.2520-2525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charcosset C. Membrane processes in biotechnology: an overview. Biotechnol Adv. 2006;24:482–492. doi: 10.1016/j.biotechadv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Chen F, Yang X, Mak HK, Chan DW. Photocatalytic oxidation for antimicrobial control in built environment: a brief literature overview. Build Environ. 2010;45:1747–1754. doi: 10.1016/j.buildenv.2010.01.024. [DOI] [Google Scholar]
- Cheong S, Lee C, Song SW, Choi WC, Lee CH, Kim SJ. Enteric viruses in raw vegetables and groundwater used for irrigation in South Korea. Appl Environ Microbiol. 2009;75:7745–7751. doi: 10.1128/AEM.01629-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chironna M, Prato R, Sallustio A, Martinelli D, Tafuri S, Quarto M, Germinario C (2012) Hepatitis A in Puglia (South Italy) after 10 years of universal vaccination: need for strict monitoring and catch-up vaccination. BMC Infect Dis 12:271 (http://www.biomedcentralcom/1471-2334/12/271) [DOI] [PMC free article] [PubMed]
- Chtourou S, Porte P, Nogré M, Bihoreau N, Cheesman E, Samor B, Sauger A, Raut S, Mazurier C. A solvent/detergent-treated and 15-nm filtered factor VIII: a new safety standard for plasma-derived coagulation factor concentrates. Vox Sang. 2007;92:327–337. doi: 10.1111/j.1423-0410.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- Cliver DO. Virus interactions with membrane filters. Biotechnol Bioeng. 1968;10:877–889. doi: 10.1002/bit.260100612. [DOI] [Google Scholar]
- Cohen HJ, Birkner JS. Respiratory protection. Clin Chest Med. 2012;33:783–793. doi: 10.1016/j.ccm.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Coia JE, Ritchie L, Adisesh A, Makison Booth C, Bradley C, Bunyan D, Carson G, Fry C, Hoffman P, Jenkins D, Phin N, Taylor B, Nguyen-Van-Tam JS, Zuckerman M. The healthcare infection society working group on respiratory and facial protection, guidance on the use of respiratory and facial protection equipment. J Hosp Infect. 2013;85:170–182. doi: 10.1016/j.jhin.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulliette AD, Perry KA, Edwards JR, Noble-Wang JA. Persistence of the 2009 pandemic influenza A (H1N1) virus on N95 respirators. Appl Environ Microbiol. 2013;79:2148–2155. doi: 10.1128/AEM.03850-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling BJ, Zhou Y, Ip DKM, Leung GM, Aiello AE. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138:449–456. doi: 10.1017/S0950268809991658. [DOI] [PubMed] [Google Scholar]
- Dahling DR, Wright BA. Recovery of viruses from water by a modified flocculation procedure for second-step concentration. Appl Environ Microbiol. 1986;51:1326–1331. doi: 10.1128/aem.51.6.1326-1331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dato VM, Hostler D, Hahn ME. Simple respiratory mask. Emerg Infect Dis. 2006;12:1033–1034. doi: 10.3201/eid1206.051468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Thompson KA, Giri K, Kafatos G, Walker J, Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AM. Pathogen inactivation and filtration efficacy of a new anti-microbial and anti-viral surgical facemask and N95 against dentistry-associated microorganisms. Int Dent Austral Ed. 2012;7:36–42. [Google Scholar]
- de Keuckelaere A, Baert L, Duarte A, Stals A, Uyttendaele M. Evaluation of viral concentration methods from irrigation and processing water. J Virol Methods. 2013;187:294–303. doi: 10.1016/j.jviromet.2012.11.028. [DOI] [PubMed] [Google Scholar]
- de Mendoza C, Altisent C, Aznar JA, Batlle J, Soriano V. Emerging viral infections—a potential threat for blood supply in the 21st century. AIDS Rev. 2012;14:279–289. [PubMed] [Google Scholar]
- de Paula VS, Diniz-Mendes L, Villar LM, Luz SL, Silva LA, Jesus MS, da Silva NM, Gaspar AM. Hepatitis a virus in environmental water samples from the Amazon Basin. Water Res. 2007;41:1169–1176. doi: 10.1016/j.watres.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Deboosere N, Horm SV, Pinon A, Gachet J, Coldefy C, Buchy P, Vialette M. Development and validation of a concentration method for the detection of influenza A viruses from large volumes of surface water. Appl Environ Microbiol. 2011;77:3802–3808. doi: 10.1128/AEM.02484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després VR, Huffman JA, Burrows SM, Hoose C, Safatov AS, Buryak G, Frohlich-Nowoisky J, Elbert W, Andreae MO, Poschl U, Jaenicke R. Primary biological aerosol particles in the atmosphere: a review. Tellus B. 2012;64:15598. doi: 10.3402/tellusb.v64i0.15598. [DOI] [Google Scholar]
- Dichtelmüller HO, Flechsig E, Sananes F, Kretschmar M, Dougherty CJ. Effective virus inactivation and removal by steps of Biotest Pharmaceuticals IGIV production process. Results Immunol. 2012;2:19–24. doi: 10.1016/j.rinim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford WJ. A new series of graded collodion membranes suitable for general bacteriological use, especially in filterable virus studies. J Pathol Bact. 1931;34:505–521. doi: 10.1002/path.1700340414. [DOI] [Google Scholar]
- Elford WJ, Andrewes CH. Filtration of vaccinia virus through gradocol membranes. Br J Exp Pathol. 1932;13:36–42. [Google Scholar]
- Elford WJ, Perdrau JR, Smith W. The filtration of herpes virus through graded collodion membranes. J Pathol Bact. 1933;36:49–54. doi: 10.1002/path.1700360107. [DOI] [Google Scholar]
- Elford WJ, Galloway IA, Perdrau JR. The size of the virus of poliomyelitis as determined by ultrafiltration analysis. J Pathol. 1935;40:135–141. doi: 10.1002/path.1700400111. [DOI] [Google Scholar]
- Elford WJ, Andrewes CH, Tang FF. The sizes of the viruses of human and swine influenza, as determined by ultra-filtration. Br J Exp Pathol. 1936;17:51–53. [Google Scholar]
- European Committee for Standardization (CEN) Respiratory protective devices—particle filters—requirements, testing, marking, EN 143:2000 standard. Brussels: CEN/TC 79 Committee; 2000. [Google Scholar]
- Farrah SR, Shah DO, Ingram LO. Effect of chaotropic and antichaotropic agents on elution of poliovirus adsorbed on membrane filters. Proc Natl Acad Sci USA. 1981;78:1229–1232. doi: 10.1073/pnas.78.2.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry JD. Ultrafilter membranes and ultrafiltration. Chem Rev. 1936;18:373–455. doi: 10.1021/cr60061a001. [DOI] [Google Scholar]
- Fisher EM, Richardson AW, Harpest SD, Hofacre KC, Shaffer RE. Reaerosolization of MS2 bacteriophage from an N95 filtering facepiece respirator by simulated coughing. Ann Occup Hyg. 2012;56:315–325. doi: 10.1093/annhyg/mer101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TT, Lipp EK. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol Mol Biol Rev. 2005;69:357–371. doi: 10.1128/MMBR.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TT, Phanikumar MS, Xagoraraki I, Rose JB. Quantitative detection of human adenoviruses in wastewater and combined sewer overflows influencing a Michigan river. Appl Environ Microbiol. 2010;76:715–723. doi: 10.1128/AEM.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fout GS, Dahling DR, Safferman RS (2001) Concentration and processing of waterborne viruses by positive charge 1MDS cartridge filters and organic flocculation. In: USEPA manual of methods for virology, Chapter 14, EPA 600/4–84/013 (N14). United States Environmental Protection Agency, Office of Research and Development, Washington
- Fröhlich H, Villian L, Melzner D, Strube J. Membrane technology in bioprocess science. Chem Ing Tech. 2012;84:905–917. [Google Scholar]
- Fumian TM, Leite JPG, Castello AA, Gaggero A, de Caillou MSL, Miagostovich MP. Detection of rotavirus a in sewage samples using multiplex qPCR and an evaluation of the ultracentrifugation and adsorption–elution methods for virus concentration. J Virol Methods. 2010;170:42–46. doi: 10.1016/j.jviromet.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Furuya K, Murai K, Yokoyama T, Maeno H, Takeda Y, Murozuka T, Wakisaka A, Tanifuji M, Tomono T. Implementation of a 20-nm pore-size filter in the plasma-derived factor VIII manufacturing process. Vox Sang. 2006;91:119–125. doi: 10.1111/j.1423-0410.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- Galloway IA, Elford WJ. Filtration of the virus of foot-and-mouth disease through a new series of graded collodion membranes. Br J Exp Pathol. 1931;12:407–425. [Google Scholar]
- Ghosh S. Composite nonwovens in medical applications. In: Das D, Pourdeyhimi B, editors. Composite nonwoven materials: structure, properties and applications. Cambridge: Woodhead Publishing Ltd.; 2014. pp. 211–233. [Google Scholar]
- Gibbons CD, Rodriguez RA, Tallon L, Sobsey MD. Evaluation of positively charged alumina nanofiber cartridge filters for the primary concentration of noroviruses, adenoviruses and male-specific coliphages from seawater. J Appl Microbiol. 2010;109:635–641. doi: 10.1111/j.1365-2672.2010.04691.x. [DOI] [PubMed] [Google Scholar]
- Gibson KE. Viral pathogens in water: occurrence, public health impact, and available control strategies. Curr Opin Virol. 2014;4:50–57. doi: 10.1016/j.coviro.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshahi L, Telidetzki K, King B, Shaw D, Finlay WH. A pilot study on the use of geometrically accurate face models to replicate ex vivo N95 mask fit. Am J Infect Control. 2013;41:77–79. doi: 10.1016/j.ajic.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Goodridge LD, Steiner T. Phage detection as an indication of faecal contamination. In: Hyman P, Abedon ST, editors. Bacteriophages in health and disease, advances in molecular and cellular microbiology no. 24. Wallingford: CAB International; 2012. pp. 153–167. [Google Scholar]
- Gottschalk U. Bioseparation in antibody manufacturing: the good, the bad and the ugly. Biotechnol Prog. 2008;24:496–503. doi: 10.1021/bp070452g. [DOI] [PubMed] [Google Scholar]
- Goyal SM, Zerda KS, Gerba CP. Concentration of coliphages from large volumes of water and wastewater. Appl Environ Microbiol. 1980;39:85–91. doi: 10.1128/aem.39.1.85-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralton J, McLaws ML. Protecting healthcare workers from pandemic influenza: n95 or surgical masks? Crit Care Med. 2010;38:657–667. doi: 10.1097/CCM.0b013e3181b9e8b3. [DOI] [PubMed] [Google Scholar]
- Grassi T, Bagordo F, Idolo A, Lugoli F, Gabutti G, De Donno A. Rotavirus detection in environmental water samples by tangential flow ultrafiltration and RT-nested PCR. Environ Monit Assess. 2010;164:199–205. doi: 10.1007/s10661-009-0885-x. [DOI] [PubMed] [Google Scholar]
- Grein TA, Kovacs Z, Ebrahimi M, Michalsky R, Czermak P. Membrane supported virus separation from biological solutions. Chem Ing Tech. 2013;85:1–11. doi: 10.1002/cite.201200241. [DOI] [Google Scholar]
- Grinshpun SA, Haruta H, Eninger RM, Reponen T, McKay RT, Lee SA. Performance of an N95 filtering facepiece particulate respirator and a surgical mask during human breathing: two pathways for particle penetration. J Occup Environ Hyg. 2009;6:593–603. doi: 10.1080/15459620903120086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröner A. Pathogen safety of Beriate®. Thromb Res. 2014;134:S10–S15. doi: 10.1016/j.thromres.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Gröner A, Nowak T, Schäfer W. Pathogen safety of human C1 esterase inhibitor concentrate. Transfusion. 2012;52:2104–2112. doi: 10.1111/j.1537-2995.2012.03590.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson S, Mihranyan A. Strategies for tailoring the pore-size distribution of virus retention filter papers. ACS Appl Mater Interfaces. 2016;8:13759–13767. doi: 10.1021/acsami.6b03093. [DOI] [PubMed] [Google Scholar]
- Gustafsson S, Lordat P, Hanrieder T, Asper M, Schaefer O, Mihranyan A. Mille-feuille paper: a novel type of filter architecture for advanced virus separation applications. Mater Horiz. 2016;3:320–327. doi: 10.1039/C6MH00090H. [DOI] [Google Scholar]
- Guttman-Bass N, Armon R. Concentration of simian rotavirus SA-11 from tap water by membrane filtration and organic flocculation. Appl Environ Microbiol. 1983;45:850–855. doi: 10.1128/aem.45.3.850-855.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman-Bass N, Tchorsh Y, Marva E. Comparison of methods for rotavirus detection in water and results of a survey of Jerusalem wastewater. Appl Environ Microbiol. 1987;53:761–767. doi: 10.1128/aem.53.4.761-767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas MB (2008) Self sanitizing face masks and method of manufacture. U. S. Patent Application Publication no. US 2008/0295843 A1
- Haller DA, Colwell RC. The protective qualities of the gauze face mask. J Am Med Assoc. 1918;71:1213. doi: 10.1001/jama.1918.26020410008008a. [DOI] [Google Scholar]
- Hamamoto Y, Harada S, Kobayashi S, Yamaguchi K, Iijima H, Manabe SI, Tsurumi T, Aizawa H, Yamamoto N. A novel method for removal of human immunodeficiency virus: filtration with porous polymeric membranes. Vox Sang. 1989;56:230–236. doi: 10.1111/j.1423-0410.1989.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Hamza IA, Jurzik L, Stang A, Sure K, Überla K, Wilhelm M. Detection of human viruses in rivers of a densely populated area in Germany using a virus adsorption elution method optimized for PCR analyses. Water Res. 2009;43:2657–2668. doi: 10.1016/j.watres.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Hamza IA, Jurzik L, Überla K, Wilhelm M. Methods to detect infectious human enteric viruses in environmental water samples. Int J Hyg Environ Health. 2011;214:424–436. doi: 10.1016/j.ijheh.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TH, Kim SC, Kim ST, Chung CH, Chung JY. Detection of norovirus genogroup IV, klassevirus, and pepper mild mottle virus in sewage samples in South Korea. Arch Virol. 2014;159:457–463. doi: 10.1007/s00705-013-1848-7. [DOI] [PubMed] [Google Scholar]
- Haramoto E, Katayama H, Ohgaki S. Detection of noroviruses in tap water in Japan by means of a new method for concentrating enteric viruses in large volumes of freshwater. Appl Environ Microbiol. 2004;70:2154–2160. doi: 10.1128/AEM.70.4.2154-2160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E, Katayama H, Oguma K, Ohgaki S. Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque teno viruses in the Tamagawa River in Japan. Appl Environ Microbiol. 2005;71:2403–2411. doi: 10.1128/AEM.71.5.2403-2411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E, Katayama H, Oguma K, Ohgaki S. Recovery of naked viral genomes in water by virus concentration methods. J Virol Methods. 2007;142:169–173. doi: 10.1016/j.jviromet.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Haramoto E, Katayama H, Oguma K, Ohgaki S. Quantitative analysis of human enteric adenoviruses in aquatic environments. J Appl Microbiol. 2007;103:2153–2159. doi: 10.1111/j.1365-2672.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- Haramoto E, Katayama H, Phanuwan C, Ohgaki S. Quantitative detection of sapoviruses in wastewater and river water in Japan. Lett Appl Microbiol. 2008;46:408–413. doi: 10.1111/j.1472-765X.2008.02330.x. [DOI] [PubMed] [Google Scholar]
- He X, Wei Y, Cheng L, Zhang D, Wang Z. Molecular detection of three gastroenteritis viruses in urban surface waters in Beijing and correlation with levels of fecal indicator bacteria. Environ Monit Assess. 2012;184:5563–5570. doi: 10.1007/s10661-011-2362-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Morga J, Leon-Felix J, Peraza-Garay F, Gil-Salas BG, Chaidez C. Detection and characterization of hepatitis a virus and norovirus in estuarine water samples using ultrafiltration—RT-PCR integrated methods. J Appl Microbiol. 2009;106:1579–1590. doi: 10.1111/j.1365-2672.2008.04125.x. [DOI] [PubMed] [Google Scholar]
- Hirani ZM, Bukhari Z, Oppenheimer J, Jjemba P, LeChevallier MW, Jacangelo JG. Characterization of effluent water qualities from satellite membrane bioreactor facilities. Water Res. 2013;47:5065–5075. doi: 10.1016/j.watres.2013.05.048. [DOI] [PubMed] [Google Scholar]
- Hongo-Hirasaki T, Yamaguchi K, Yanagida K, Okuyama K. Removal of small viruses (parvovirus) from IgG solution by virus removal filter Planova®20N. J Membr Sci. 2006;278:3–9. doi: 10.1016/j.memsci.2005.10.057. [DOI] [Google Scholar]
- Hsiung GD. Use of ultrafiltration for animal virus grouping. Bact Rev. 1965;29:477–486. doi: 10.1128/br.29.4.477-486.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PW, Laborde D, Land VR, Matson DO, Smith AW, Jiang X. Concentration and detection of caliciviruses in water samples by reverse transcription-PCR. Appl Environ Microbiol. 2000;66:4383–4388. doi: 10.1128/AEM.66.10.4383-4388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttinen M, Rautio A, Pasanen P, Reponen T, Earnest GS, Streifel A, Kalliokoski P. Airborne infection isolation rooms—a review of experimental studies. Indoor Built Environ. 2011;20:584–594. doi: 10.1177/1420326X11409452. [DOI] [Google Scholar]
- ICH (1999) International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. In: viral safety evaluation of biotechnology products derived from cell lines of animal or human origin, ICH Harmonised Tripartite Guideline Q5A (R1). ICH, Geneva
- Ikner LA, Soto-Beltran M, Bright KR. New method using a positively charged microporous filter and ultrafiltration for concentration of viruses from tap water. Appl Environ Microbiol. 2011;77:3500–3506. doi: 10.1128/AEM.02705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikner LA, Gerba CP, Bright KR. Concentration and recovery of viruses from water: a comprehensive review. Food Environ Virol. 2012;4:41–67. doi: 10.1007/s12560-012-9080-2. [DOI] [PubMed] [Google Scholar]
- Jacob JT, Kasali A, Steinberg JP, Zimring C, Denham ME. The role of the hospital environment in preventing healthcare-associated infections caused by pathogens transmitted through the air. Health Environ Res Des J. 2013;7(suppl 1):74–98. [Google Scholar]
- Johnson TB, McKay LD, Layton AC, Jones SW, Johnson GC, Cashdollar JL, Dahling DR, Villegas LF, Fout GS, Williams DE, Sayler G. Viruses and bacteria in karst and fractured rock aquifers in East Tennessee, USA. Ground Water. 2011;49:98–110. doi: 10.1111/j.1745-6584.2010.00698.x. [DOI] [PubMed] [Google Scholar]
- Jones TH, Muehlhauser V, Thériault G. Comparison of ZetaPlus 60S and nitrocellulose membrane filters for the simultaneous concentration of F-RNA coliphages, porcine teschovirus and porcine adenovirus from river water. J Virol Methods. 2014;206:5–11. doi: 10.1016/j.jviromet.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Julian TR, Schwab KJ. Challenges in environmental detection of human viral pathogens. Curr Opin Virol. 2012;2:78–83. doi: 10.1016/j.coviro.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Karim MR, Rhodes ER, Brinkman N, Wymer L, Fout GS. New electropositive filter for concentrating enteroviruses and noroviruses from large volumes of water. Appl Environ Microbiol. 2009;75:2393–2399. doi: 10.1128/AEM.00922-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Shimasaki A, Ohgaki S. Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl Environ Microbiol. 2002;68:1033–1039. doi: 10.1128/AEM.68.3.1033-1039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Haramoto E, Oguma K, Yamashita H, Tajima A, Nakajima H, Ohgaki S. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42:1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Katzenelson E, Fattal B, Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976;32:638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessick MA, Wagner RA. Electrophoretic mobilities of virus adsorbing filter materials. Water Res. 1978;12:263–268. doi: 10.1016/0043-1354(78)90096-9. [DOI] [Google Scholar]
- Kitajima M, Oka T, Haramoto E, Katayama H, Takeda N, Katayama K, Ohgaki S. Detection and genetic analysis of human sapoviruses in river water in Japan. Appl Environ Microbiol. 2010;76:2461–2467. doi: 10.1128/AEM.02739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M, Iker BC, Pepper IL, Gerba CP. Relative abundance and treatment reduction of viruses during wastewater treatment processes—identification of potential viral indicators. Sci Total Environ. 2014;488–489:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- Klamroth R, Gröner A, Simon TL. Pathogen inactivation and removal methods for plasma-derived clotting factor concentrates. Transfusion. 2014;54:1406–1417. doi: 10.1111/trf.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein HG, Bryant BJ. Pathogen-reduction methods: advantages and limits. ISBT Sci Ser. 2009;4:154–160. doi: 10.1111/j.1751-2824.2009.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderman AHL, ter Hart HGJ, Prins-de Nijs IMM, Bloem J, Stoffers S, Kempers A, Derksen GJ, Al B, Dekker L, Over J. Virus safety of plasma products using 20 nm instead of 15 nm filtration as virus removing step. Biologicals. 2012;40:473–481. doi: 10.1016/j.biologicals.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Kundu A, Reindel K. Evaluation of viral clearance in purification processes. In: Shukla AA, Etzel MR, Gadam S, editors. Process scale bioseparations for the biopharmaceutical industry. Boca Raton: CRC Press; 2007. pp. 419–448. [Google Scholar]
- Kuo D, Simmons FJ, Blair S, Hart E, Rose JB, Xagoraraki I. Assessment of human adenovirus removal in a full-scale membrane bioreactor treating municipal wastewater. Water Res. 2010;44:1520–1530. doi: 10.1016/j.watres.2009.10.039. [DOI] [PubMed] [Google Scholar]
- Lambertini E, Spencer SK, Bertz PD, Loge FJ, Kieke BA, Borchardt MA. Concentration of enteroviruses, adenoviruses, and noroviruses from drinking water by use of glass wool filters. Appl Environ Microbiol. 2008;74:2990–2996. doi: 10.1128/AEM.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim M, Paik SY, Lee CH, Jheong WH, Kim J, Ko G. Evaluation of electropositive filtration for recovering norovirus in water. J Water Health . 2011;9:27–36. doi: 10.2166/wh.2010.190. [DOI] [PubMed] [Google Scholar]
- Lee GC, Kim MJ, Nam S, Lee CH. Incidence and molecular characterization of hepatitis a viruses in Korean surface water between 2007 and 2010. Microbiol Immunol. 2014;58:342–351. doi: 10.1111/1348-0421.12151. [DOI] [PubMed] [Google Scholar]
- Lei Z, Yang J, Zhuang Z. Headform and N95 filtering facepiece respirator interaction: contact pressure simulation and validation. J Occup Environ Hyg. 2012;9:46–58. doi: 10.1080/15459624.2011.635130. [DOI] [PubMed] [Google Scholar]
- Lei Z, Yang J, Zhuang Z, Roberge R. Simulation and evaluation of respirator faceseal leaks using computational fluid dynamics and infrared imaging. Ann Occup Hyg. 2013;57:493–506. doi: 10.1093/annhyg/mes085. [DOI] [PubMed] [Google Scholar]
- Levi E (2011) Fluid purification media and systems and methods using same. U. S. Patent Application No. 2011/0278243 A1
- Lewis GD, Metcalf TG. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol. 1988;54:1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shi HC, Jiang SC. Concentration of viruses from environmental waters using nanoalumina fiber filters. J Microbiol Methods. 2010;81:33–38. doi: 10.1016/j.mimet.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. mAbs. 2010;2:480–499. doi: 10.4161/mabs.2.5.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Hill VR, Hahn D, Johnson TB, Pan Y, Jothikumar N, Moe CL. Hollow-fiber ultrafiltration for simultaneous recovery of viruses, bacteria and parasites from reclaimed water. J Microbiol Methods. 2012;88:155–161. doi: 10.1016/j.mimet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Logan KB, Rees GE, Seeley ND, Primrose SB. Rapid concentration of bacteriophages from large volumes of freshwater: evaluation of positively charged, microporous filters. J Virol Methods. 1980;1:87–97. doi: 10.1016/0166-0934(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Lukasik J, Scott TM, Andryshak D, Farrah SR. Influence of salts on virus adsorption to microporous filters. Appl Environ Microbiol. 2000;66:2914–2920. doi: 10.1128/AEM.66.7.2914-2920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HY, Burger C, Hsiao BS, Chu B. Ultra-fine polysaccharide nanofibrous membranes for water purification. Biomacromol. 2011;12:970–976. doi: 10.1021/bm1013316. [DOI] [PubMed] [Google Scholar]
- Makino M, Ishikawa G, Yamaguchi K, Okada Y, Watanabe K, Sasaki-Iwaki Y, Manabe S, Honda M, Komuro K. Concentration of live retrovirus with a regenerated cellulose hollow fiber. BMM Arch Virol. 1994;139:87–96. doi: 10.1007/BF01309456. [DOI] [PubMed] [Google Scholar]
- Manabe S, Tsurumi T, Ishikawa G, Satani M, Yamashiki T, Hamamoto Y, Yamaguchi K, Kobayashi S, Yamamoto N. Mechanism of human immunodeficiency virus (HIV) removal by regenerated cellulose hollow fiber (BMM) Membrane. 1989;14:77–83. doi: 10.5360/membrane.14.77. [DOI] [Google Scholar]
- Mattison K, Bidawid S. Analytical methods for food and environmental viruses. Food Environ Virol. 2009;1:107–122. doi: 10.1007/s12560-009-9017-6. [DOI] [Google Scholar]
- McMinn BR. Optimization of adenovirus 40 and 41 recovery from tap water using small disk filters. J Virol Methods. 2013;193:284–290. doi: 10.1016/j.jviromet.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Metreveli G, Wågberg L, Emmoth E, Belák S, Strømme M, Mihranyan A. A size-exclusion nanocellulose filter paper for virus removal. Adv Healthc Mater. 2014;3:1546–1550. doi: 10.1002/adhm.201300641. [DOI] [PubMed] [Google Scholar]
- Miagostovich MP, Ferreira FFM, Guimarães FR, Fumian TM, Diniz-Mendes L, Luz SLB, Silva LA, Leite JPG. Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of manaus, central Amazonia, Brazil. Appl Environ Microbiol. 2008;74:375–382. doi: 10.1128/AEM.00944-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michen B, Graule T. Isoelectric points of viruses. J Appl Microbiol. 2010;109:388–397. doi: 10.1111/j.1365-2672.2010.04663.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Nakamura K (2011) Antibacterial mask, antibacterial filter for the mask, and antibacterial method using the mask or the filter. Japanese Patent No. JP 2011167226 A
- National Institute for Occupational Safety and Health (NIOSH) (1995) 42 CFR Part 84: Respiratory protective devices; final rules and notice. In: Office of the Federal Register, National Archives and Records Administration, Federal Register, vol 60. U.S. Government Printing Office, Washington 60(110), pp 30336–30398
- Oberg T, Brosseau LM. Surgical mask filter and fit performance. Am J Infect Control. 2008;36:276–282. doi: 10.1016/j.ajic.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr V, Zhong L, Moo-Young M, Chou CP. Recent advances in bioprocessing application of membrane chromatography. Biotechnol Adv. 2013;31:450–465. doi: 10.1016/j.biotechadv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Ostreicher, EA (1991) Cationic charge modified filter media. U. S. Patent No. 4,981,591
- Pang XL, Lee BE, Pabbarajua K, Gabosd S, Craike S, Payment P, Neumann N. Pre-analytical and analytical procedures for the detection of enteric viruses and enterovirus in water samples. J Virol Methods. 2012;184:77–83. doi: 10.1016/j.jviromet.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Pelletier JPR, Transue S, Snyder EL. Pathogen inactivation techniques. Best Pract Res Clin Haematol. 2006;19:205–242. doi: 10.1016/j.beha.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MW, Bolton G, Krishnan M, Lewnard JJ, Raghunath B. Virus filtration process design and implementation. In: Shukla A, Etzel M, Gadam S, editors. Process scale bioseparations for the biopharmaceutical industry, biotechnology and bioprocessing series 31. Boca Raton: CRC Press; 2007. pp. 333–365. [Google Scholar]
- Pigeot-Remy S, Lazzaroni JC, Simonet F, Petinga P, Vallet C, Petit P, Vialle PJ, Guillard C. Survival of bioaerosols in HVAC system photocatalytic filters. Appl Catal B Environ. 2014;144:654–664. doi: 10.1016/j.apcatb.2013.07.036. [DOI] [Google Scholar]
- Pirrone V, Wigdahl B, Krebs FC. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 2011;90:168–182. doi: 10.1016/j.antiviral.2011.03.176. [DOI] [PubMed] [Google Scholar]
- Podgórski A. Protection of the respiratory system against nanoparticles inhalation. In: Marijnissen JCM, Gradoń L, editors. Nanoparticles in medicine and environment. Dordrecht: Springer; 2010. pp. 251–275. [Google Scholar]
- Polaczyk AL, Roberts JM, Hill VR. Evaluation of 1MDS electropositive microfilters for simultaneous recovery of multiple microbe classes from tap water. J Microbiol Methods. 2007;68:260–266. doi: 10.1016/j.mimet.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Polaczyk AL, Narayanan J, Cromeans TL, Hahn D, Roberts JM, Amburgey JE, Hill VR. Ultrafiltration-based techniques for rapid and simultaneous concentration of multiple microbe classes from 100-L tap water samples. J Microbiol Methods. 2008;73:92–99. doi: 10.1016/j.mimet.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Prowse CV. Component pathogen inactivation: a critical review. Vox Sang. 2013;104:183–199. doi: 10.1111/j.1423-0410.2012.01662.x. [DOI] [PubMed] [Google Scholar]
- Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromol. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox Sang. 2010;98:12–28. doi: 10.1111/j.1423-0410.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- Ramia S, Sattar SA. Second-step concentration of viruses in drinking and surface waters using polyethylene glycol hydroextraction. Can J Microbiol. 1979;25:587–592. doi: 10.1139/m79-084. [DOI] [PubMed] [Google Scholar]
- Raphael RA, Sattar SA, Springthorpe VS. Rotavirus concentration from raw water using positively charged filters. J Virol Methods. 1985;11:131–140. doi: 10.1016/0166-0934(85)90036-9. [DOI] [PubMed] [Google Scholar]
- Rathore AS, Kumar V, Arora A, Lute S, Brorson K, Shukla A. Mechanistic modeling of viral filtration. J Membr Sci. 2014;458:96–103. doi: 10.1016/j.memsci.2014.01.037. [DOI] [Google Scholar]
- Rengasamy A, Zhuang Z, BerryAnn R. Respiratory protection against bioaerosols: literature review and research needs. Am J Infect Control. 2004;32:345–354. doi: 10.1016/j.ajic.2004.04.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy S, Eimer BC, Shaffer RE. Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Ann Occup Hyg. 2009;53:117–128. doi: 10.1093/annhyg/men086. [DOI] [PubMed] [Google Scholar]
- Rengasamy S, Eimer B, Shaffer RE. Simple respiratory protection–evaluation of the filtration performance of cloth masks and common fabric materials against 20–1000 nm size particles. Ann Occup Hyg. 2010;54:789–798. doi: 10.1093/annhyg/meq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ER, Hamilton DW, See MJ, Wymer L. Evaluation of hollow-fiber ultrafiltration primary concentration of pathogens and secondary concentration of viruses from water. J Virol Methods. 2011;176:38–45. doi: 10.1016/j.jviromet.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Rigotto C, Victoria M, Moresco V, Kolesnikovas CK, Corrêa AA, Souza DSM, Miagostovich MP, Simões CMO, Barardi CRM. Assessment of adenovirus, hepatitis A virus and rotavirus presence in environmental samples in Florianopolis, South Brazil. J Appl Microbiol. 2010;109:1979–1987. doi: 10.1111/j.1365-2672.2010.04827.x. [DOI] [PubMed] [Google Scholar]
- Roberts PL, Feldman P, Crombie D, Walker C, Lowery K. Virus removal from factor IX by filtration: validation of the integrity test and effect of manufacturing process conditions. Biologicals. 2010;38:303–310. doi: 10.1016/j.biologicals.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Rodrigues T, Carrondo MJT, Alves PM, Cruz PE. Purification of retroviral vectors for clinical application: biological implications and technological challenges. J Biotechnol. 2007;127:520–541. doi: 10.1016/j.jbiotec.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Rodríguez RA, Gundy PM, Rijal GK, Gerba CP. The impact of combined sewage overflows on the viral contamination of receiving waters. Food Environ Virol. 2012;4:34–40. doi: 10.1007/s12560-011-9076-3. [DOI] [PubMed] [Google Scholar]
- Rose JB, Singh SN, Gerba CP, Kelley LM. Comparison of microporous filters for concentration of viruses from wastewater. Appl Environ Microbiol. 1984;47:989–992. doi: 10.1128/aem.47.5.989-992.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H, Wada K, Kajioka J, Watanabe M, Nakano R, Hirose T, Ohta H, Aizawa Y. Maintenance of influenza virus infectivity on the surfaces of personal protective equipment and clothing used in healthcare settings. Environ Health Prev Med. 2010;15:344–349. doi: 10.1007/s12199-010-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak G, Bina D, MacDonald J, Couillard L. Nine-year study of the occurrence of culturable viruses in source water for two drinking water treatment plants and the influent and effluent of a wastewater treatment plant in Milwaukee, Wisconsin (August 1994 through July 2003) Appl Environ Microbiol. 2005;71:1042–1050. doi: 10.1128/AEM.71.2.1042-1050.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura MM, Kamen A, Garnier A. Downstream processing of oncoretroviral and lentiviral gene therapy vectors. Biotechnol Adv. 2006;24:321–337. doi: 10.1016/j.biotechadv.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Segura MM, Kamen AA, Garnier A. Overview of current scalable methods for purification of viral vectors. In: Merten OW, Al-Rubeai M, editors. Viral vectors for gene therapy: methods and protocols, methods in molecular biology. New York: Humana Press; 2011. pp. 89–116. [DOI] [PubMed] [Google Scholar]
- Sekiguchi S, Ito K, Kobayashi M, Ikeda H, Tsurumi T, Ishikawa G, Manabe S, Satani M, Yamashiki T. Possibility of hepatitis B virus (HBV) removal from human plasma using regenerated cellulose hollow fiber (BMM) Membrane. 1989;14:253–261. doi: 10.5360/membrane.14.253. [DOI] [Google Scholar]
- Sekiguchi S, Ito K, Kobayashi M, Ikeda H, Manabe S, Tsurumi T, Ishikawa G, Satani M, Yamaguchi K. An attempt to prepare Hepatitis B Virus (HBV)-free plasma by ultrafiltration using microporous regenerated cellulose hollow fiber. Transfus Sci. 1990;11:211–216. doi: 10.1016/0955-3886(90)90097-3. [DOI] [PubMed] [Google Scholar]
- Shields PA, Farrah SR. Influence of salts on electrostatic interactions between poliovirus and membrane filters. Appl Environ Microbiol. 1983;45:526–531. doi: 10.1128/aem.45.2.526-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AA, Hubbard B, Tressel T, Guhan S, Low D. Downstream processing of monoclonal antibodies—application of platform approaches. J Chromatogr B. 2007;848:28–39. doi: 10.1016/j.jchromb.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Simmons FJ, Kuo DHW, Xagoraraki I. Removal of human enteric viruses by a full-scale membrane bioreactor during municipal wastewater processing. Water Res. 2011;45:2739–2750. doi: 10.1016/j.watres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Sobsey MD, Glass JS. Poliovirus concentration from tap water with electropositive adsorbent filters. Appl Environ Microbiol. 1980;40:201–210. doi: 10.1128/aem.40.2.201-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey MD, Wallis C, Henderson M, Melnick JL. Concentration of enteroviruses from large volumes of water. Appl Microbiol. 1973;26:529–534. doi: 10.1128/am.26.4.529-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim BG. Pathogen reduction of blood components. Transfus Apher Sci. 2008;39:75–82. doi: 10.1016/j.transci.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Stewart N., Lau FCN, Kung TW, Lo LY, Ryan DJ, von Borstel RW (2012) Composition for use in decreasing the transmission of human pathogens. U. S. Patent Application Publication No. US 2012/0060258 A1
- Stewart NG, Lo LY, Lau FCN, Ryan DJ, von Borstel RW (2014) Devices and methods for decreasing human pathogen transmission. U. S. Patent No. US 8,678,002 B2
- Tang JW, Li Y, Eames I, Chan PKS, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper F, Kaledin L (2005) Nanosize electropositive fibrous adsorbent. U.S. Patent No. 6,838,005 B2
- Tepper F, Kaledin L. A high-performance electropositive filter. Bioprocess Int. 2006;4:64–68. [Google Scholar]
- Terpstra FG, Parkkinen J, Tölö H, Koenderman AHL, Ter Hart HGJ, von Bonsdorff L, Törmä E, van Engelenburg FAC. Viral safety of Nanogam®, a new 15 nm-filtered liquid immunoglobulin product. Vox Sang. 2006;90:21–32. doi: 10.1111/j.1423-0410.2005.00710.x. [DOI] [PubMed] [Google Scholar]
- Terpstra FG, Kleijn M, Koenderman AHL, Over J, van Engelenburg FAC, Schuitemaker H, Van’t Wout AB. Viral safety of C1-inhibitor NF. Biologicals. 2007;35:173–181. doi: 10.1016/j.biologicals.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Thakur R, Das D, Das A. Electret air filters. Sep Purif Rev. 2013;42:87–129. doi: 10.1080/15422119.2012.681094. [DOI] [Google Scholar]
- Tiliket G, Le Sage D, Moules V, Rosa-Calatrava M, Lina B, Valleton JM, Nguyen QT, Lebrun L. A new material for airborne virus filtration. Chem Eng J. 2011;173:341–351. doi: 10.1016/j.cej.2011.07.059. [DOI] [Google Scholar]
- Tong HI, Connell C, Boehm AB, Lu Y. Effective detection of human noroviruses in Hawaiian waters using enhanced RT-PCR methods. Water Res. 2011;45:5837–5848. doi: 10.1016/j.watres.2011.08.030. [DOI] [PubMed] [Google Scholar]
- Tsai PP, Schreuder-Gibson H, Gibson P. Different electrostatic methods for making electret filters. J Electrostat. 2002;54:333–341. doi: 10.1016/S0304-3886(01)00160-7. [DOI] [Google Scholar]
- Tsurumi T, Osawa N, Hirasaki T, Yamaguchi K, Manabe SI, Yamashiki T. Mechanism of removing monodisperse gold particles from a suspension using cuprammonium regenerated cellulose hollow fiber (BMM hollow fiber) Polym J. 1990;22:304–311. doi: 10.1295/polymj.22.304. [DOI] [Google Scholar]
- Tsurumi T, Osawa N, Hitaka H, Hirasaki T, Yamaguchi K, Manabe S, Yamashiki T. Structure of cuprammonium regenerated cellulose hollow fiber (BMM hollow fiber) for virus removal. Polym J. 1990;22:751–758. doi: 10.1295/polymj.22.751. [DOI] [Google Scholar]
- Tsurumi T, Sato T, Osawa N, Hitaka H, Hirasaki T, Yamaguchi K, Hamamoto Y, Manabe S, Yamashiki T, Yamamoto N. Structure and filtration performances of improved cuprammonium regenerated cellulose hollow fiber (improved BMM hollow fiber) for virus removal. Polym J. 1990;22:1085–1100. doi: 10.1295/polymj.22.1085. [DOI] [Google Scholar]
- Tsutsumi K (2009) Mask and graft polymer extract. Japanese Patent No. JP 2009225930 A
- van Reis R, Zydney AL. Review: bioprocess membrane technology. J Membr Sci. 2007;297:16–50. doi: 10.1016/j.memsci.2007.02.045. [DOI] [Google Scholar]
- Verheyen J, Timmen-Wego M, Laudien R, Boussaad I, Sen S, Koc A, Uesbeck A, Mazou F, Pfister H. Detection of adenoviruses and rotaviruses in drinking water sources used in rural areas of Benin, West Africa. Appl Environ Microbiol. 2009;75:2798–2801. doi: 10.1128/AEM.01807-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreault D, Moineau S, Duchaine C. Methods for sampling of airborne viruses. Microbiol Mol Biol Rev. 2008;72:413–444. doi: 10.1128/MMBR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente T, Roldão A, Peixoto C, Carrondo MJT, Alves PM. Large-scale production and purification of VLP-based vaccines. J Invertebr Pathol. 2011;107:S42–S48. doi: 10.1016/j.jip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente T, Mota JPB, Peixoto C, Alves PM, Carrondo MJT. Rational design and optimization of downstream processes of virus particles for biopharmaceutical applications: current advances. Biotechnol Adv. 2011;29:869–878. doi: 10.1016/j.biotechadv.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Victoria M, Guimarães F, Fumian T, Ferreira F, Vieira C, Leite JP, Miagostovich M. Evaluation of an adsorption–elution method for detection of astrovirus and norovirus in environmental waters. J Virol Methods. 2009;156:73–76. doi: 10.1016/j.jviromet.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Vilaginès P, Sarrette B, Husson G, Vilaginès R. Glass wool for virus concentration at ambient water pH level. Water Sci Technol. 1993;27:299–306. doi: 10.1021/es00039a009. [DOI] [Google Scholar]
- Wallis C, Melnick JL. Virus concentration on aluminum and calcium salts. Amer J Epidemiol. 1967;85:459–468. doi: 10.1093/oxfordjournals.aje.a120708. [DOI] [PubMed] [Google Scholar]
- Wallis C, Henderson M, Melnick JL. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972;23:476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SE, Denyer SP. Filtration sterilization. In: Fraise AP, Maillard JY, Sattar SA, editors. Russell, Hugo & Ayliffe’s principles and practice of disinfection, preservation and sterilization. 5. Oxford: Blackwell Publishing; 2012. pp. 343–370. [Google Scholar]
- Wang CS. Electrostatic forces in fibrous filters—a review. Powder Technol. 2001;118:166–170. doi: 10.1016/S0032-5910(01)00307-2. [DOI] [Google Scholar]
- Wang W, Wang SX, Guan HS. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Guan S, Sato A, Wang X, Wang Z, Yang R, Hsiao BS, Chu B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J Membr Sci. 2013;446:376–382. doi: 10.1016/j.memsci.2013.06.020. [DOI] [Google Scholar]
- Watzinger F, Ebner K, Lion T. Detection and monitoring of virus infections by real-time PCR. Mol Asp Med. 2006;27:254–298. doi: 10.1016/j.mam.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MM, Weiss PD, Weiss DE, Weiss JB. Disrupting the transmission of Influenza a: face masks and ultraviolet light as control measures. Am J Public Health. 2007;97:S32–S37. doi: 10.2105/AJPH.2006.096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Fong TT, Bibby K, Molina M. Application of enteric viruses for fecal pollution source tracking in environmental waters. Environ Intl. 2012;45:151–164. doi: 10.1016/j.envint.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Woo MH, Lee JH, Rho SG, Ulmer K, Welch JC, Wu CY, Song L, Baney RH. Evaluation of the performance of dialdehyde cellulose filters against airborne and waterborne bacteria and viruses. Ind Eng Chem Res. 2011;50:11636–11643. doi: 10.1021/ie201502p. [DOI] [Google Scholar]
- Woo MH, Grippin A, Wu CY, Baney RH. Use of dialdehyde starch treated filters for protection against airborne viruses. J Aerosol Sci. 2012;46:77–82. doi: 10.1016/j.jaerosci.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rodriguez RA, Stewart JR, Sobsey MD. A simple and novel method for recovering adenovirus 41 in small volumes of source water. J Appl Microbiol. 2011;110:1332–1340. doi: 10.1111/j.1365-2672.2011.04987.x. [DOI] [PubMed] [Google Scholar]
- Wyn-Jones AP, Carducci A, Cook N, D’Agostino M, Divizia M, Fleischer J, Gantzer C, Gawler A, Girones R, Holler C, Husman AMD, Kay D, Kozyra I, Lopez-Pila J, Muscillo M, Nascimento MS, Papageorgiou G, Rutjes S, Sellwood J, Szewzyk R, Wyer M. Surveillance of adenoviruses and noroviruses in European recreational waters. Water Res. 2011;45:1025–1038. doi: 10.1016/j.watres.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xagoraraki I, Kuo DHW, Wong K, Wong M, Rose JB. Occurrence of human adenoviruses at two recreational beaches of the Great Lakes. Appl Environ Microbiol. 2007;73:7874–7881. doi: 10.1128/AEM.01239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ITS, Li Y, Wong TW, Tam W, Chan AT, Lee JHW, Leung DYC, Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Yuasa T, Ishikawa G, Manabe SI, Sekiguchi S, Takeuchi K, Miyamura T. The particle size of hepatitis C virus estimated by filtration through microporous regenerated cellulose fibre. J Gen Virol. 1991;72:2021–2024. doi: 10.1099/0022-1317-72-8-2021. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang HP, Chen SY, Zhou BH, Zhang ZR, Li Z, Zhang WL (2012) Antivirus bacterial cellulose protective material and its preparation method. Chinese Patent No. CN 102321261 A
- Zhong CY (2011) Air filter type bacterial cellulose mask and production method thereof. Chinese Patent No. CN 101589854 A
- Zhou JX, Solamo F, Hong T, Shearer M, Tressel T. Viral clearance using disposable systems in monoclonal antibody commercial downstream processing. Biotechnol Bioeng. 2008;100:488–496. doi: 10.1002/bit.21781. [DOI] [PubMed] [Google Scholar]


