Abstract
Community-acquired pneumonia continues to be an important complication of HIV infection. Rates of pneumonia decrease with the use of antiretroviral therapy but continue to be higher than in HIV uninfected individuals. Risk factors for pneumonia include low blood CD4+ count, unsuppressed plasma HIV load, smoking, injection drug use and renal impairment. Immunization against Streptococcus pneumoniae and smoking cessation can reduce this risk. It is unclear whether newly reported viral respiratory pathogens (such as the Middle East respiratory syndrome coronavirus, will be more of a problem in HIV-infected individuals than the general population.
Keywords: HIV infection, Pneumonia, Pneumococcal disease, Influenza, Smoking, Immunization
Introduction
Community-acquired pneumonia (CAP) remains an important cause of morbidity and mortality in HIV-positive individuals [1]. Although the incidence has decreased with the use of antiretroviral therapy (ART), rates continue to be elevated compared to HIV-uninfected individuals in both developing and developed societies [2]. The clinical presentation, range of typical pathogens and outcomes of CAP are similar to those without HIV infection, but important differential diagnoses and implications for future health merit particular attention in HIV-infected individuals [3].
This review considers the evidence concerning CAP in HIV-infected individuals, with particular emphasis on studies reported in the last year. We consider the role of bacterial and viral causes of pneumonia and other conditions that can mimic pneumonia. The management of hospital-acquired pneumonia and infections caused by opportunistic pathogens such as Pneumocystis jirovecii, CMV, fungal and mycobacterial organisms are covered elsewhere and are not discussed here, beyond stressing that they can all present as an acute pneumonic illness, and hence will often need to be considered as part of the differential diagnosis.
Epidemiology
Prior to the development of effective ART, respiratory tract infection was the most common complication of HIV infection [1]. The advent of ART has been associated with a substantial decline in the rates of opportunistic infections and as the frequency of pulmonary infections such as P. jirovecii pneumonia have fallen, bacterial pneumonias have become increasingly prominent in the care of HIV-infected people [4•, 5•]. Although there has been debate as to the increased rate of pneumonia in HIV-infected persons following ART, recent data from several large observational cohorts have significantly increased our current knowledge on this subject.
The patient cohorts in the Multicentre AIDS Cohort Study (MACS) and the Womens Interagency HIV Study (WIHS) have been followed since 1984 and 1994, respectively, and allow analysis of rates of pneumonia in the ART era. In the MACS cohort the adjusted odds ratio for bacterial pneumonia compared with HIV-uninfected individuals has declined from 21.8 in the pre-ART period to 4.14 following the introduction of ART [6••]. The WIHS cohort of HIV-infected women have also shown an elevated incidence of pneumonia despite ART use with an adjusted odds ratio of 9.55 compared with HIV-uninfected controls. Similarly, in the Veterans Aging Cohort Study (which involved a virtual cohort of 33,420 HIV-infected individuals using data from the US National VA Health Information System from the date of HIV diagnosis until 2007) a rate of 28 per 1,000 person-years was found in HIV-infected individuals compared with 5.8 per 1,000 person-years in HIV-negative controls [7••]. A lower incidence has been seen in the EuroSIDA study of more than 18,000 patients in 34 countries studied between 2006 and 2011, in which an incidence of pneumonia of 5.36/1000 person-years was found [8••]. This may reflect the better immune status of participants in the latter era, who had a mean blood CD4+ count of 457 cells/μL, as very low levels of severe bacterial infections were demonstrated in the EuroSIDA study in those with CD4 counts above 500 cells/μL (a level generally regarded as near normal).
Studies in populations other than in Europe and the US have confirmed the importance of bacterial pneumonia in HIV-infected individuals, with recent work in Taiwan showing this to be the most common respiratory complication of HIV infection in those with CD4 counts above 200 cells/μL [9]. However, the general improvement in incidence rates has not been found in all populations, as surveillance data from Soweto, South Africa, indicate persisting high rates of invasive pneumococcal disease, with no decrease since the introduction of ART [10]. This may be due to the high levels of immunocompromise in this population despite the availability of ART, although an increase in invasive pneumococcal disease was found amongst women in that study, suggesting that general uptake of the childhood pneumococcal conjugate vaccination (PCV; which now forms part of the childhood immunization schedule in South Africa) may be particularly effective at reducing rates of invasive pneumococcal disease amongst HIV-infected adults in this community.
Risk Factors for CAP
Specific risk factors for CAP can be identified in the cohorts studied. Most have studies have shown that pneumonia is more common in HIV-infected people at any level of immunosuppression, and rates of pneumonia increase with increasing immunosuppression [8••]. Cigarette smoking is consistently reported to increase the risk of pneumonia, with a hazard ratio of 0.48 in former smokers compared to current smokers in a cohort study from France [11•]. Injecting drug use increases the risk of pneumonia, and although this may be due to confounding diseases, poor adherence to ART or socioeconomic factors, some recent evidence suggests that direct effects of opiates or their withdrawal may play a role [12].
Ethnic differences in pneumonia rates, which may be reflective of socioeconomic factors, have been found in some cohorts: rates of pneumonia in African-Americans in California are significantly higher than in other ethnic groups [13], Aboriginal ethnicity is a risk factor for invasive pneumococcal disease in Canada [14••], and Asian ethnicity was associated with reduced risk in the Evaluation of Subcutaneous Interleukin-2 (ESPRIT) trial [15]. Being a care-giver to young children (who have high rates of pneumococcal carriage) may predispose to invasive pneumococcal disease, and a recent study from South Africa confirms significant child-to-mother transmission of pneumococcal strains [16•]. The EuroSIDA study showed that a reduced estimated glomerular filtration rate (eGFR) is a significant risk factor for severe bacterial infections including pneumonia, with an incidence rate ratio of 5.07 for those with eGFR <60 ml/min/1.73 m2 compared to those with eGFR >90 ml/min/1.73 m2 [8••]. It is not clear if this is a direct effect on immune function caused by renal impairment, or the result of other comorbid conditions or lifestyle factors.
ART is an important protective factor against pneumonia. Several cohorts have demonstrated that suppressed HIV loads are associated with a lower incidence of pneumonia, most recently the ICONA study in Italy, which showed an overall incidence of 5.66 per 1,000 person-years with increased risk in people with low nadir CD4+ count, low current CD4 count or high viral load [17•]. This trial followed 4,942 individuals for a median of 63.7 months between 1996 and 2011; 15 % had an AIDS diagnosis prior to ART initiation and 46 % had a nadir CD4 count below 200 cells/μL. A low nadir CD4 count was associated with a hazard ratio for an episode of bacterial pneumonia of 0.86 (per 100 cells/μL higher). Low current blood CD4 was associated with a hazard ratio of 0.88 (per 100 cells/μL higher) and an unsuppressed plasma viral load was associated with a hazard ratio of 1.29.
The Strategies for Management of Antiretroviral Therapy (SMART) trial demonstrated reductions in the incidence of pneumonia in those on continuous compared to those on intermittent ART, as well as a lower incidence in those with an undetectable plasma HIV load [18•]. In this trial (which recruited patients with a CD4 count of >350 cells/μL between 2002 and 2006) intermittent rather than continuous ART was associated with a 1.55-fold increase in pneumonia incidence. This finding is of particular importance as the randomized trial methodology of intermittent versus continuous ART allows confidence that the observed differences in pneumonia incidence are the result of ART rather than unmeasured confounding factors. The ESPRIT trial (which evaluated the effect of subcutaneous IL-2 in HIV-infected patients on or starting ART with a CD4 count >300 cells/μL) also found that a higher HIV plasma viral load was associated with increased risk of pneumonia with a (hazard ratio for a 1 log10 higher viral load of 1.28) [15].
A reduction in pneumonia incidence has also been reported in HIV-infected children in the US, this has been attributed to the use of ART and pneumococcal vaccination. An observational cohort study of 736 HIV-infected children observed between 2000 and 2005 showed lower CD4 counts in those with pneumonia than in those without pneumonia (668 cells/μL, or 23 %. In a multivariate analysis a HIV viral load of >100,000 copies/ml was associated with a hazard ratio for pneumonia of 3.98 [19].
Work in HIV-uninfected populations suggests that the use of statins (HMG coenzyme A reductase inhibitors) may reduce pneumonia-related mortality, possibly by attenuating the associated inflammatory response [20]. However, a recent study in The Netherlands using population-based data found there to be neither a reduction in the frequency of pneumonia nor a worse outcome, when it did occur, in HIV-infected individuals taking regular statin therapy [21].
HIV Testing in Patients with CAP
It should be remembered that the higher incidence of pneumonia in HIV-infected individuals means that presentation with CAP should prompt consideration of HIV testing in patients not known to be HIV-infected. It is estimated that around 24 % of the HIV-infected population in the UK and 18 % of the HIV-infected population in the US are unaware of their serostatus [22, 23]. Surveys from sub-Saharan Africa undertaken by USAID have shown that in many countries the majority of HIV-infected individuals do not know their serostatus [24]. Bacterial pneumonia should be regarded as an indicator condition that must prompt discussion of HIV testing. British HIV Association guidelines recommend that all patients admitted with bacterial pneumonia should be offered an HIV test, and analysis of primary care data confirms that bacterial pneumonia is one of the strongest indicator conditions for HIV infection [25••]. Invasive pneumococcal disease is particularly strongly associated with HIV infection, with recent UK population-based data showing that 2.4 % of patients with invasive pneumococcal disease have undiagnosed HIV infection [26••].
Pathogenesis
Recent studies have contributed to our understanding of the pathogenesis of pneumonia in HIV-infected individuals. Although depletion of the CD4+ T cell is the hallmark of HIV infection, it is recognized that impairment of innate immune responses plays an important role in the increased susceptibility to pneumonia [27].
Reduced CD4+ T-cell responses to respiratory antigens have been demonstrated in bronchoalveolar CD4+ cells when compared with those in HIV-uninfected controls, and a disruption in the T-cell response to pneumococcus appears to precede CD4+ T-cell depletion [28, 29•]. HIV causes chronic activation of dendritic cells of the innate immune system, triggering immune dysregulation and apoptosis of CD4+ and CD8+ T cells [30, 31]. Impairment of CD8+ T-cell responses may also be important, as CD8 levels have been found to be predictive of pneumonia in the HIV Epidemiologic Research Study (HERS) of HIV-infected women in the US after adjustment for age, CD4+ cell count, viral load and antiretroviral use [32•].
Impairment of immune responses within the lung of HIV-infected individuals results in changes to the oral and airway microbiota with increased numbers and diversity of microorganisms, including potentially pathogenic species found in HIV-infected patients with pneumonia compared to HIV-negative controls [33]. HIV-infected individuals appear to be particularly susceptible to pneumococcal disease [34]. Rates of carriage of Streptococcus pneumoniae were found to be higher in HIV-infected individuals in a prospective cohort of HIV-infected and HIV-uninfected mothers in Zambia, although it should be noted that the CD4 counts in these subjects were not known [35]. Smoking appears to increase rates of pneumococcal carriage [36] as does a lower nadir CD4 count and an AIDS diagnoses [37]. There may be an interaction between respiratory viral pathogens and rates of invasive pneumococcal disease, as influenza infection has been reported to be associated with increased blood pneumococcal load [38].
Respiratory Viruses in HIV-Infected individuals
Viral pathogens are responsible for a large proportion of respiratory tract infections in HIV-infected individuals, although there are limited data available on the role of viral pathogens. A prospective study in Montreal found that among 50 HIV-positive patients with fever and symptoms consistent with respiratory tract infection, viruses accounted for 64 % of these illnesses [39••]. In this study, 90 % of patients were on ART and had a median CD4+ T cell count of 325 cells/μL, and a median viral load of <50 copies/mL. Influenza was the most important viral cause of respiratory tract infections with 22 of 34 identified viral infections due to influenza, with equal numbers of influenza A and B. Other viruses isolated were human metapneumovirus types A and B, respiratory syncytial virus, parainfluenza types 2 and 3 and coronavirus. These pathogens also cause respiratory tract infections in HIV-uninfected individuals, and it is not known if the incidence or severity of these infections is different in HIV-infected individuals, although respiratory syncytial virus has been reported to be a cause of severe CAP in HIV-positive individuals [40, 41].
Although it is often suggested that HIV-infected individuals may be more susceptible to viral respiratory tract infections, or suffer more severe disease associated with viral pathogens, the evidence to support this is mixed. Studies prior to the availability of ART suggested that HIV-infected individuals have more severe disease due to influenza infection [42]. Cohen et al. have reported an excess mortality amongst HIV-infected persons during influenza epidemic periods in South Africa and the US, with reductions in this excess mortality since the widespread use of ART in the US [43]. However, it is not clear if this excess mortality arises directly from influenza infection – for example, an observational study in Barcelona found no difference in the severity of influenza in patients with and without HIV infection admitted with influenza H1N1 [44]. The excess deaths in influenza epidemic periods found by Cohen et al. may instead be the result of other conditions which are more prevalent at these times, including pneumococcal pneumonia. In general, data from the H1N1 pandemic of 2009 do not support the suggestion that HIV-infected individuals experience more severe disease [45, 46]. Emerging respiratory pathogens such as the Middle East respiratory syndrome coronavirus (MERS CoV) may pose a threat to everybody. No data are yet available on the relative susceptibility of HIV-infected individuals to this condition [47].
It is possible that antiretroviral medication may modify the presentation of influenza infection as protease inhibitors have been shown to block influenza viral replication, although the clinical impact of this observation remains unclear [48].
Management of CAP in HIV-Infected Individuals
HIV-infected individuals with pneumonia present with typical clinical features of an acute illness characterized by fever, pleuritic chest pain, breathlessness and hypoxaemia [2]. The use of established severity scores has been found to be valid in HIV infection, with the Pneumonia Severity Index (PSI) being the best-studied scoring system to assess severity and predict the likelihood of mortality, as confirmed by a recent study from California [49].
Although the management of CAP in HIV-infected persons is specifically excluded from pneumonia guidelines from the US and Europe [50–52]. treatment should follow similar principles to those in patients without HIV infection. Patients with high CURB-65 or PSI scores have a significant risk of death and should be managed in critical care areas where possible. Typical bacterial pathogens are similar to those in HIV-negative populations, with S. pneumoniae consistently found to be the most common pathogen. Other significant pathogens include Haemophilus influenza, Klebsiella pneumoniae, Staphylococcus aureus, Moraxella catarrhalis and Pseudomonas aeruginosa [3]. “Atypical” organisms such as Legionella pneumophila, Mycoplasma spp. and Chlamydia pneumoniae represent less than 5 % of cases, although the incidence of these infections in HIV-infected people has not been systematically evaluated. It should be remembered that opportunistic pathogens such as P. jirovecii and Cryptococcus neoformans can present with acute pneumonic illnesses and these should be considered in the differential diagnosis of pneumonia in immunocompromised individuals. The incidence of Mycobacterium tuberculosis infection is greatly increased in HIV-infected individuals and can present with clinical and radiological features identical to those of bacterial pneumonias (Fig. 1).
Fig. 1.
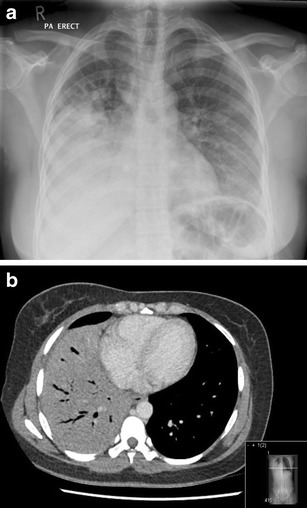
Case example: a 39-year-old Zambian woman presented with a 4-day history of pleuritic chest pain, cough and breathlessness, and a 6-week history of weight loss and night sweats. She was initially treated with amoxicillin and clavulanic acid, which resulted in symptomatic improvement. Tests on admission demonstrated HIV-1 infection, with a CD4 count of 192 cells/μL. a Chest radiograph demonstrates right-sided middle and lower zone consolidation. b CT image of the thorax demonstrates nodular shadowing in both upper lobes, with dense consolidation of the right middle and lower lobes. Sputum microscopy was 3+ positive for acid-fast bacilli and culture confirmed Mycobacterium tuberculosis. This emphasizes the importance of testing for HIV and mycobacterial disease in patients with community-acquired pneumonia
The choice of empirical antibiotic treatment will differ between populations depending on local antibacterial drug resistance. In 2011, rates of penicillin resistance in S. pneumoniae in Europe ranged from <1 % to 61 % [53]. Some data suggest that antibiotic resistance in respiratory pathogens may be higher in HIV-infected individuals, a finding that may be related to the use of cotrimoxazole for Pneumocystis pneumonia prophylaxis, or to differences in serotypes infecting HIV-positive individuals [54, 55].
Outcomes
Mortality rates in CAP differ among published series, but most fall in the range 10–15 % of cases [3]. Although this has been the subject of some controversy, mortality and length of hospital stay in HIV-infected persons with pneumonia do not seem to differ from those in patients without HIV infection [56]. It should be noted that most data arise from the US and Europe, and may not be applicable to resource-limited settings. Recent studies have added to the evidence that blood CD4 counts do not predict mortality and should not be used to guide therapy or decisions regarding admission to intensive care units [57••].
Changes in the demographic composition of HIV-infected populations may lead to changes in outcome as HIV-positive populations age. For example, information derived from hospital claims databases in the US suggest a higher case fatality rate for pneumonia and influenza in HIV-infected elderly individuals compared to those without HIV infection [58••].
Prevention and Immunization
Pneumonia is an important cause of morbidity and mortality in HIV-infected persons and several interventions can reduce this risk. In particular smoking cessation and immunization against pneumococcus and influenza offer the opportunity to modify a patient’s risk of pneumonia.
Smoking Cessation
HIV-infected populations continue to have high rates of exposure to cigarette smoke [59–61]. Rates of pneumonia are higher in smokers than nonsmokers, and smoking cessation can reduce this [62••]. A recent systematic review of the literature by De et al. found that current smokers have an increased risk of bacterial pneumonia compared to nonsmokers (hazard ratio 1.73, 95 % confidence interval 1.44 – 2.06) and that this risk is reduced by smoking cessation. In a prospective study in France, a hazard ratio of 0.48 was found in former smokers compared to current smokers (p = 0.02) [11•]. The use of medication as an adjunct to smoking cessation has been extensively investigated in HIV-uninfected smokers, but there are few data from HIV-infected people. A small study of the safety and tolerability of varenicline tartrate in 36 HIV-positive individuals found a high frequency of adverse events, most commonly nausea, but excellent quit rates with a 42 % abstinence rate at 12 weeks. There were no adverse changes in HIV viral loads detected [63].
Pneumococcal Immunization
Given the high incidence of invasive pneumococcal disease in HIV-infected persons, and the efficacy of pneumococcal vaccination in other at-risk groups, there has been considerable interest in immunization against pneumococcus. Two forms of pneumococcal vaccination, the 23-valent pneumococcal polysaccharide vaccine (PPV-23) and PCV have been developed. PPV-23 has been studied in one randomized trial and 15 observational studies, which are the subject of a recent systematic review [64••]. This concluded that the current evidence base lends only moderate support for the efficacy of the PPV immunization. The only randomized trial was carried out in Africa in a population without widespread access to ART and with a high mortality rate, and found an increased rate of pneumonia in the immunized group. However, these results may not be applicable to a population with access to ART and lower background rates of pneumococcal pneumonia, and most observational studies have shown that PPV-23 immunization is associated with a reduced rate of pneumococcal disease, although in many studies confounding factors that could have influenced this observation were not completely accounted for.
PPV-23 suffers from relatively poor immunogenicity, particularly in those with low CD4 counts, and one study of 103 HIV-infected individuals found that antibody responses declined to pre-immunization levels after 12 months [65]. In an observational cohort study in Canada, 74 % of pneumococcal serotypes isolated were from PPV-23, despite the fact that 73 % of this population had received this immunization, and vaccine failure was associated with low CD4 counts [14••]. PCV has theoretical advantages in that it induces T cell-dependent immune responses and has been demonstrated to be immunogenic in HIV-infected adults in several studies [66]. These have recently been reviewed by Nunes and Madhi who concluded that PCV is effective at reducing rates of invasive pneumococcal disease, and produces more effective and durable antibody responses in individuals on ART than in those who are ART-naive [67]. It should be noted that immune responses to pneumococcal vaccination are significantly better in those with a higher CD4 count [68].
Apart from the possible direct benefits of pneumococcal immunization, vaccination of young children with PCV-7 (which offers protection against seven common serotypes) has been associated with indirect benefits in HIV-infected individuals. In the US, widespread use of the PCV-7 as part of the childhood immunization schedule has been associated with a 91 % reduction in invasive pneumococcal disease caused by vaccine serotypes in HIV-infected adults. These changes in pneumococcal epidemiology following routine childhood immunization have also been observed in other populations, with an 81 % reduction in invasive pneumococcal disease caused by vaccine serotypes in Spain [69]. However, this study also noted an increase in severity and need for mechanical ventilation for pneumococcal pneumonia. Work in South Africa has also demonstrated reduced levels of pneumococcal carriage following the introduction of childhood PCV, including reductions amongst unvaccinated adults. There appeared to be reductions in nonvaccine pneumococcal serotypes which were not restricted to HIV-infected individuals [70]. Analysis of data from England and Wales since the introduction of childhood PCV-7 immunization suggests that there has been a 54 % reduction in invasive pneumococcal disease caused by the serotypes targeted by the vaccine [26••]. This study found that 61 % of invasive pneumococcal disease in HIV-infected patients was caused by the 13 serotypes that would be covered by the new 13-valent PCV (PCV-13).
At present, guidelines from the British HIV association (2008) recommend pneumococcal polysaccharide vaccination in HIV-infected individuals with a CD4 count above 200 cells/μL whilst stating that this should be considered in individuals with lower CD4 counts and noting that efficacy may be poorer in those with low CD4 counts who are not on ART [71]. The Infectious Disease Society of America recommends the combination of PCV-13 and pneumococcal polysaccharide (PPV-23) immunizations with one dose of PCV-13 followed by PPV-23 after 8 weeks, although they suggest that this may be delayed until blood CD4 counts are above 200 cells/μL, when on ART [72].
A health-economic analysis by Rozenbaum et al. concluded that the addition of PCV-13 for HIV-infected adults in England would not be cost effective [73•]. Smith et al. have explored the current recommendations in the US and conclude that a single dose of PCV may be more cost-effective than the current dual immunization strategy, whilst Cho et al. have report that the current recommendations would still be cost-saving [74, 75]. It should be noted that these studies of cost-effectiveness are very sensitive to the assumptions made regarding vaccine effectiveness. However, these estimates of the cost-effectiveness of pneumococcal immunization are limited by the poor uptake of the pneumococcal immunization amongst HIV-positive adults which appears to be below that of other immunizations [76].
Influenza Immunization
Clinical guidelines in the US and UK advise immunization of HIV-infected individuals with the influenza vaccine. A meta-analysis by Beck et al. of published studies suggests that influenza vaccination is effective at reducing the incidence of influenza and is well tolerated [77]. It should be noted that respiratory comorbidities such as COPD are common in HIV-infected individuals and immunization of these individuals against influenza may be particularly important. Recent work suggests that the immunogenicity of influenza immunization in HIV-infected persons may be improved by high-dose (four times the standard trivalent dose) vaccination [78]. Whilst the increased dose was well tolerated, it was not clear whether the enhanced antibody response translates into better clinical, cost-effective protection.
Conclusions
CAP is a major cause of morbidity and mortality amongst HIV-infected individuals. Although a wealth of evidence attests to the increased rates of pneumonia in immunocompromised individuals prior to the use of ART, there have been clear reductions over recent years. HIV-infected populations have high rates of smoking and comorbid respiratory pathologies but current data suggest that HIV infection remains an independent risk factor for CAP. At present it is not known whether HIV-infected individuals will have an increased susceptibility to new respiratory pathogens such as MERS-CoV.
The management and outcome of pneumonia in HIV-infected individuals does not appear to differ from those in HIV-uninfected persons. Several interventions can be made that have been shown to reduce this risk; these include: the use of ART and achievement of an undetectable plasma HIV load, smoking cessation, and the uptake of the pneumococcal and influenza immunizations, which international guidelines recommend for HIV-infected individuals.
Despite an increasing knowledge base there continues to be uncertainty regarding the degree of increased risk of pneumonia in HIV-infected individuals and the best option to reduce this. Smoking cessation is of importance, and evidence is required as to the best immunization strategy for this population.
Compliance with Ethics Guidelines
Conflict of Interest
Marc Lipman and James Brown have no conflicts.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Footnotes
This article is part of the Topical Collection on Respiratory Infections
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Morris A, Crothers K, Beck JM, Huang L, Huang L. American Thoracic Society Committee on HIV Pulmonary Disease. An official ATS workshop report: emerging issues and current controversies in HIV-associated pulmonary diseases. Proc Am Thorac Soc. 2011;8:17–26. doi: 10.1513/pats.2009-047WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman C, Anderson R. HIV-associated bacterial pneumonia. Clin Chest Med. 2013;34:205–216. doi: 10.1016/j.ccm.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Benito N, Moreno A, Miro JM, Torres A. Pulmonary infections in HIV-infected patients: an update in the 21st century. Eur Respir J. 2012;39:730–745. doi: 10.1183/09031936.00200210. [DOI] [PubMed] [Google Scholar]
- 4.•.Schwarcz L, Chen MJ, Vittinghoff E, Hsu L, Schwarcz S. Declining incidence of AIDS-defining opportunistic illnesses: results from 16 years of population-based AIDS surveillance. AIDS. 2013;27:597–605. doi: 10.1097/QAD.0b013e32835b0fa2. [DOI] [PubMed] [Google Scholar]
- 5.•.Kim JH, Psevdos G, Jr, Gonzalez E, Singh S, Kilayko MC, Sharp V. All-cause mortality in hospitalized HIV-infected patients at an acute tertiary care hospital with a comprehensive outpatient HIV care program in New York City in the era of highly active antiretroviral therapy (HAART) Infection. 2013;41:545–551. doi: 10.1007/s15010-012-0386-7. [DOI] [PubMed] [Google Scholar]
- 6.••.Gingo MR, Balasubramani GK, Kingsley L, Rinaldo CR, Jr, Alden CB, Detels R, et al. The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PLoS One. 2013;8(3):e58812. doi: 10.1371/journal.pone.0058812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.••.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.••.Søgaard OS, Reekie J, Ristola M, Jevtovic D, Karpov I, Beniowski M, et al. Severe bacterial non-AIDS infections in HIV-positive persons: incidence rates and risk factors. J Infect. 2013;66:439–446. doi: 10.1016/j.jinf.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Lee KY, Ho CC, Ji DD, Lee CM, Tsai MS, Cheng AC, et al. Etiology of pulmonary complications of human immunodeficiency virus-1-infected patients in Taiwan in the era of combination antiretroviral therapy: a prospective observational study. J Microbiol Immunol Infect. 2012;46:433–440. doi: 10.1016/j.jmii.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Kuwanda L, Karstaedt AS, et al. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the era of an antiretroviral treatment program. PLoS One. 2011;6:e27929. doi: 10.1371/journal.pone.0027929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.•.Bénard A, Mercié P, Alioum A, Bonnet F, Lazaro E, Dupon M, et al. Groupe d’Epidémiologie Clinique du Sida en Aquitaine. Bacterial pneumonia among HIV-infected patients: decreased risk after tobacco smoking cessation. ANRS CO3 Aquitaine Cohort, 2000–2007. PLoS One. 2010;5:e8896. doi: 10.1371/journal.pone.0008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6:442–465. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuorti JP, Butler JC, Gelling L, Kool JL, Reingold AL, Vugia DJ. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann Intern Med. 2000;132:182–190. doi: 10.7326/0003-4819-132-3-200002010-00003. [DOI] [PubMed] [Google Scholar]
- 14.••.Siemieniuk RA, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis. 2011;11:314. doi: 10.1186/1471-2334-11-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pett SL, Carey C, Lin E, Wentworth D, Lazovski J, Miró JM, et al. INSIGHT-ESPRIT Study Group. Predictors of bacterial pneumonia in Evaluation of Subcutaneous Interleukin-2 in a Randomized International Trial (ESPRIT) HIV Med. 2011;12:219–227. doi: 10.1111/j.1468-1293.2010.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.•.Shiri T, Auranen K, Nunes MC, Adrian PV, van Niekerk N, de Gouveia L, et al. Dynamics of pneumococcal transmission in vaccine-naive children and their HIV-infected or HIV-uninfected mothers during the first 2 years of life. Am J Epidemiol. 2013;178:1629–1637. doi: 10.1093/aje/kwt200. [DOI] [PubMed] [Google Scholar]
- 17.•.Mussini C, Galli L, Lepri AC, De Luca A, Antinori A, Libertone R, et al. ICONA Foundation Study Group. Incidence, timing, and determinants of bacterial pneumonia among HIV-infected patients: data from the ICONA Foundation Cohort. J Acquir Immune Defic Syndr. 2013;63:339–345. doi: 10.1097/QAI.0b013e318295ab85. [DOI] [PubMed] [Google Scholar]
- 18.•.Gordin FM, Roediger MP, Girard PM, Lundgren JD, Miro JM, Palfreeman A, et al. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. Am J Respir Crit Care Med. 2008;178:640–646. doi: 10.1164/rccm.200804-617OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steenhoff AP, Josephs JS, Rutstein RM, Gebo KA, Siberry GK, Gaur AH, et al. Incidence of and risk factors for community acquired pneumonia in US HIV-infected children, 2000-2005. AIDS. 2011;25:717–720. doi: 10.1097/QAD.0b013e3283440583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troeman DP, Postma DF, van Werkoven CH, Oosterheert JJ. The immunomodulatory effects of statins in community-acquired pneumonia: a systematic review. J Infect. 2013;67:93–101. doi: 10.1016/j.jinf.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Janssen NE, van Lelyveld SF, Hoepelman AI, Gras L, Groenwold RH, Oosterheert JJ. The effect of statin therapy on pneumonia in an HIV-infected population in the Netherlands. J Infect. 2013;67:238–241. doi: 10.1016/j.jinf.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Health Protection Agency . HIV in the United Kingdom: 2012 report. London: Health Protection Services, Colindale; 2012. [Google Scholar]
- 23.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data – United States and 6 U.S. dependent areas – 2010. HIV Surveillance Supplemental Report, vol 17, number 3 (part A).
- 24.Staveteig S, Wang S, Head SK, Bradley SE, Nybro E. Demographic patterns of HIV testing uptake in sub-Saharan Africa. DHS Comparative Reports No. 30. Calverton: ICF International; 2013. [Google Scholar]
- 25.••.Damery S, Nichols L, Holder R, Ryan R, Wilson S, Warmington S, et al. Assessing the predictive value of HIV indicator conditions in general practice: a case-control study using the THIN database. Br J Gen Pract. 2013;63:e370–e377. doi: 10.3399/bjgp13X668159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.••.Yin Z, Rice BD, Waight P, Miller E, George R, Brown AE, et al. Invasive pneumococcal disease among HIV-positive individuals, 2000-2009. AIDS. 2012;26:87–94. doi: 10.1097/QAD.0b013e32834dcf27. [DOI] [PubMed] [Google Scholar]
- 27.Collini P, Noursadeghi M, Sabroe I, Miller RF, Dockrell DH. Monocyte and macrophage dysfunction as a cause of HIV-1 induced dysfunction of innate immunity. Curr Mol Med. 2010;10:727–740. doi: 10.2174/156652410793384141. [DOI] [PubMed] [Google Scholar]
- 28.Jambo KC, Sepako E, Fullerton DG, Mzinza D, Glennie S, Wright AK, et al. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax. 2011;66:375–382. doi: 10.1136/thx.2010.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.•.Glennie SJ, Sepako E, Mzinza D, Harawa V, Miles DJ, Jambo KC, et al. Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PLoS One. 2011;6:e25610. doi: 10.1371/journal.pone.0025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller E, Bhardwaj N. Dendritic cell dysregulation during HIV-1 infection. Immunol Rev. 2013;254:170–189. doi: 10.1111/imr.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pritschet K, Donhauser N, Schuster P, Ries M, Haupt S, Kittan NA, et al. CD4- and dynamin-dependent endocytosis of HIV-1 into plasmacytoid dendritic cells. Virology. 2012;423:152–164. doi: 10.1016/j.virol.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 32.•.Gohil SK, Heo M, Schoenbaum EE, Celentano D, Pirofski LA. CD8+ T cells and risk for bacterial pneumonia and all-cause mortality among HIV-infected women. J Acquir Immune Defic Syndr. 2012;60:191–198. doi: 10.1097/QAI.0b013e31824d90fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwai S, Fei M, Huang D, Fong S, Subramanian A, Grieco K, et al. Oral and airway microbiota in HIV-infected pneumonia patients. J Clin Microbiol. 2012;50:2995–3002. doi: 10.1128/JCM.00278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordano Q, Falcó V, Almirante B, Planes AM, del Valle O, Ribera E, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38(11):1623–1628. doi: 10.1086/420933. [DOI] [PubMed] [Google Scholar]
- 35.Gill CJ, Mwanakasale V, Fox MP, Chilengi R, Tembo M, Nsofwa M, et al. Impact of human immunodeficiency virus infection on Streptococcus pneumoniae colonization and seroepidemiology among Zambian women. J Infect Dis. 2008;197:1000–1005. doi: 10.1086/528806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo YC, Lauderdale TL, Chang SY, Hsiao CF, Hung CC, Chang SC. Streptococcus pneumoniae colonization among patients with human immunodeficiency virus-1 who had received 23-valent polysaccharide pneumococcal vaccine. J Microbiol Immunol Infect. 2009;42:234–242. [PubMed] [Google Scholar]
- 37.Öbrink-Hansen K, Søgaard OS, Harboe ZB, Schønheyder HC. Risk factors for pneumococcal nasopharyngeal colonization before and after pneumococcal conjugate vaccination in persons with HIV: brief report. Curr HIV Res. 2012;10:252–255. doi: 10.2174/157016212800618101. [DOI] [PubMed] [Google Scholar]
- 38.Wolter N, Cohen C, Tempia S, Madhi SA, Venter M, Moyes J, et al. HIV and influenza virus infections are associated with increased blood pneumococcal load: a prospective, hospital-based observational study in South Africa, 2009-2011. J Infect Dis. 2013;209:46–65. doi: 10.1093/infdis/jit427. [DOI] [PubMed] [Google Scholar]
- 39.••.Klein MB, Yang H, DelBalso L, Carbonneau J, Frost E, Boivin G. Viral pathogens including human metapneumovirus are the primary cause of febrile respiratory illness in HIV-infected adults receiving antiretroviral therapy. J Infect Dis. 2010;201:297–301. doi: 10.1086/649587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunha BA, Syed U, Hage JE. Respiratory syncytial virus (RSV) community-acquired pneumonia (CAP) in a hospitalized adult with human immunodeficiency virus (HIV) mimicking influenza A and Pneumocystis (carinii) jiroveci pneumonia (PCP) Heart Lung. 2012;41:76–82. doi: 10.1016/j.hrtlng.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A, Mody P, Gupta S. A case of respiratory syncytial virus infection in an HIV-positive adult. Case Rep Infect Dis. 2012;2012:267028. doi: 10.1155/2012/267028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheth AN, Althoff KN, Brooks JT. Influenza susceptibility, severity, and shedding in HIV-infected adults: a review of the literature. Clin Infect Dis. 2011;52:219–227. doi: 10.1093/cid/ciq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen C, Simonsen L, Sample J, Kang JW, Miller M, Madhi SA, et al. Influenza-related mortality among adults aged 25–54 years with AIDS in South Africa and the United States of America. Clin Infect Dis. 2012;55:996–1003. doi: 10.1093/cid/cis549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez E, Marcos MA, Hoyo-Ulloa I, Antón A, Sánchez M, Vilella A, et al. Influenza A H1N1 in HIV-infected adults. HIV Med. 2011;12:236–245. doi: 10.1111/j.1468-1293.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- 45.Sheth AN, Patel P, Peters PJ. Influenza and HIV: lessons from the 2009 H1N1 influenza pandemic. Curr HIV/AIDS Rep. 2011;8:181–191. doi: 10.1007/s11904-011-0086-4. [DOI] [PubMed] [Google Scholar]
- 46.Cooper CL. Pandemic H1N12009 influenza and HIV: a review of natural history, management and vaccine immunogenicity. Curr Opin Infect Dis. 2012;25:26–35. doi: 10.1097/QCO.0b013e32834ef56c. [DOI] [PubMed] [Google Scholar]
- 47.Assiri A, AL-Tawfiq JA, AL−Rabeeah AA, Al Rabiah FA, AlHajjar S, Al-Barrak A, et al. Epidemiological, demographic and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Federico M. HIV-protease inhibitors block the replication of both vesicular stomatitis and influenza viruses at an early post-entry replication step. Virology. 2011;417:37–49. doi: 10.1016/j.virol.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chew KW, Yen IH, Li JZ, Winston LG. Predictors of pneumonia severity in HIV-infected adults admitted to an urban public hospital. AIDS Patient Care STDS. 2011;25(5):273–277. doi: 10.1089/apc.2010.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. Pneumonia Guidelines Committee of the BTS Standards of Care Committee. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 51.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society Consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases. Guidelines for the management of adult lower respiratory tract infections. Clin Microbiol Infect. 2011;17(Suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.http://onlinelibrary.wiley.com/doi/10.1111/j.1469-0691.2011.03672.x/pdf.
- 54.Crowther-Gibson P, Cohen C, Klugman KP, de Gouveia L, von Gottberg A. Group for Enteric, Respiratory, and Meningeal Disease Surveillance in South Africa (GERMS-SA). Risk factors for multidrug-resistant invasive pneumococcal disease in South Africa, a setting with high HIV prevalence, in the prevaccine era from 2003 to 2008. Antimicrob Agents Chemother. 2012;56:5088–5095. doi: 10.1128/AAC.06463-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munier AL, de Lastours V, Varon E, Donay JL, Porcher R, Molina JM. Invasive pneumococcal disease in HIV-infected adults in France from 2000 to 2011: antimicrobial susceptibility and implication of serotypes for vaccination. Infection. 2013;41:663–668. doi: 10.1007/s15010-013-0419-x. [DOI] [PubMed] [Google Scholar]
- 56.Christensen D, Feldman C, Rossi P, Marrie T, Blasi F, Luna C, et al. Community-Acquired Pneumonia Organization Investigators. HIV infection does not influence clinical outcomes in hospitalized patients with bacterial community-acquired pneumonia: results from the CAPO international cohort study. Clin Infect Dis. 2005;41:554–556. doi: 10.1086/432063. [DOI] [PubMed] [Google Scholar]
- 57.••.Bordon J, Kapoor R, Martinez C, Portela D, Duvvuri P, Klochko A, et al. CD4+ cell counts and HIV-RNA levels do not predict outcomes of community-acquired pneumonia in hospitalized HIV-infected patients. Int J Infect Dis. 2011;15:e822–e827. doi: 10.1016/j.ijid.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 58.••.Mor SM, Aminawung JA, Demaria A, Jr, Naumova EN. Pneumonia and influenza hospitalization in HIV-positive seniors. Epidemiol Infect. 2011;139:1317–1325. doi: 10.1017/S0950268810002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shirley DK, Kesari RK, Glesby MJ. Factors associated with smoking in HIV-infected patients and potential barriers to cessation. AIDS Patient Care STDS. 2013;27:603–612. doi: 10.1089/apc.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vijayaraghavan M, Penko J, Vittinghoff E, Bangsberg DR, Miaskowski C, Kushel MB. Smoking behaviors in a community-based cohort of hiv-infected indigent adults. AIDS Behav. 2013 doi: 10.1007/s10461-013-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waweru P, Anderson R, Steel H, Venter WD, Murdoch D, Feldman C. The prevalence of smoking and the knowledge of smoking hazards and smoking cessation strategies among HIV-positive patients in Johannesburg, South Africa. S Afr Med J. 2013;103:858–860. doi: 10.7196/samj.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.••.De P, Farley A, Lindson N, Aveyard P. Systematic review and meta-analysis: influence of smoking cessation on incidence of pneumonia in HIV. BMC Med. 2013;11:15. doi: 10.1186/1741-7015-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, et al. Safety and tolerability of varenicline tartrate (Champix(®)/Chantix(®)) for smoking cessation in HIV-infected subjects: a pilot open-label study. AIDS Patient Care STDS. 2012;26:12–19. doi: 10.1089/apc.2011.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.••.Pedersen RH, Lohse N, Østergaard L, Søgaard OS. The effectiveness of pneumococcal polysaccharide vaccination in HIV-infected adults: a systematic review. HIV Med. 2011;12:323–333. doi: 10.1111/j.1468-1293.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen H, Kvinesdal B, Benfield TL, Lundgren JD, Konradsen HB. Rapid loss of specific antibodies after pneumococcal vaccination in patients with human immunodeficiency virus-1 infection. Scand J Infect Dis. 1998;30:597–601. doi: 10.1080/00365549850161160. [DOI] [PubMed] [Google Scholar]
- 66.Lu CL, Hung CC, Chuang YC, Liu WC, Su CT, Su YC, et al. Serologic response to primary vaccination with 7-valent pneumococcal conjugate vaccine is better than with 23-valent pneumococcal polysaccharide vaccine in HIV-infected patients in the era of combination antiretroviral therapy. Hum Vaccin Immunother. 2013;9(2):398–404. doi: 10.4161/hv.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nunes MC, Madhi SA. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum Vaccin Immunother. 2012;8:161–173. doi: 10.4161/hv.18432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slayter KL, Singer J, Lee TC, Kayhty H, Schlech WF. Immunization against pneumococcal disease in HIV-infected patients: conjugate versus polysaccharide vaccine before or after reconstitution of the immune system (CTN-147) Int J STD AIDS. 2013;24:227–231. doi: 10.1177/0956462412472450. [DOI] [PubMed] [Google Scholar]
- 69.Burgos J, Peñaranda M, Payeras A, Villoslada A, Curran A, Garau M, et al. Invasive pneumococcal disease in HIV-infected adults: clinical changes after the introduction of the pneumococcal conjugate vaccine in children. J Acquir Immune Defic Syndr. 2012;59:31–38. doi: 10.1097/QAI.0b013e31823d0f5f. [DOI] [PubMed] [Google Scholar]
- 70.Nzenze SA, Shiri T, Nunes MC, Klugman KP, Kahn K, Twine R, et al. Temporal changes in pneumococcal colonization in a rural African community with high HIV prevalence following routine infant pneumococcal immunization. Pediatr Infect Dis J. 2013;32:1270–1278. doi: 10.1097/01.inf.0000435805.25366.64. [DOI] [PubMed] [Google Scholar]
- 71.Geretti AM, BHIVA Immunization Writing Committee. Brook G, Cameron C, Chadwick D, Heyderman RS, et al. British HIV Association guidelines for immunization of HIV-infected adults 2008. HIV Med. 2008;9:795–848. doi: 10.1111/j.1468-1293.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 72.AIDSinfo. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Rockville, MD: US Department Health and Human Services. http://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/0.
- 73.•.Rozenbaum MH, van Hoek AJ, Fleming D, Trotter CL, Miller E, Edmunds WJ. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ. 2012;345:e6879. doi: 10.1136/bmj.e6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31:3950–3956. doi: 10.1016/j.vaccine.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31:6011–6031. doi: 10.1016/j.vaccine.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 76.Hunter MG, Post JJ. An audit of pneumococcal and hepatitis vaccination in an outpatient HIV clinic. Sex Health. 2012;9:495–496. doi: 10.1071/SH11192. [DOI] [PubMed] [Google Scholar]
- 77.Beck CR, McKenzie BC, Hashim AB, Harris RC. University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis. 2012;206:1250–1259. doi: 10.1093/infdis/jis487. [DOI] [PubMed] [Google Scholar]
- 78.McKittrick N, Frank I, Jacobson JM, White CJ, Kim D, Kappes R, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med. 2013;158:19–26. doi: 10.7326/0003-4819-158-1-201301010-00005. [DOI] [PubMed] [Google Scholar]


