Abstract
The porcine reproductive and respiratory syndrome virus (PRRSV) has three major structural proteins which designated as GP5, M, and N. Protein GP5 and M have been considered very important to arouse the humoral and cellular immune responses against PRRSV infection and proposed to be the excellent candidate proteins in the design of PRRS bioengineering vaccine. There were some attempts on expressing GP5 or M in DNA vaccine and adenovirus to arouse humoral and cellular immune responses, but few papers have been reported on that the immune response can be difference because of the expression patterns of GP5 and M proteins in the recombinant virus. In this article, four recombinant viruses that expressed GP5 and M proteins of PRRSV in the modified vaccinia virus ankara (MVA) with different expression patterns were made. In these recombinant virus (rMVAs), GP5 and M proteins were expressed in MVA in the same virus but under the control of two promoters (rMVA-GP5/M), or as a fusion protein under one promoter (rMVA-GP5-M), or separately (rMVA-GP5 and rMVA-M). The humoral and cellular immune responses for the four recombinant viruses were evaluated with mouse model. Every mouse was inoculated with 5 × 105 TCID50 of the different rMVAs and boosted 3 weeks later. Neutralizing antibody titers for each group were detected with virus neutralization test assay weekly after the primary inoculation for 13 weeks to evaluate the humoral immune response. The production of gamma interferon (IFN-γ), interleukin-2 (IL-2), and interleukin-4 (IL-4) was detected in splenocytes of rMVA-inoculated mice at 30, 60, and 90 days post inoculation to evaluate the cellular immune response. Results showed that rMVA-GP5 and rMVA-M cannot induce obvious humoral and cellular immune responses; rMVA-GP5-M inoculated group developed better immune responses than rMVA-GP5 and rMVA-M inoculated groups; however, mice inoculated with rMVA-GP5/M maintained the strongest cellular response against PRRS and consistently enhanced the anti-PRRSV humoral responses. The strategy of co-expressing PRRSV GP5 and M protein in MVA under the control of different promoters might be an attractive method for future PRRSV vaccine design.
Keywords: PRRSV, MVA, GP5, M, Co-expression
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV), a member of the Arteriviridae family, causes an economically devastating of swine production, late-term abortion and stillbirths in sows, and respiratory distress in nursery pigs [1]. The virus was firstly isolated in Netherlands in 1991 and in US in 1992. Today, PRRS has been considered endemic in many swine-producing countries. There are three major structural proteins in PRRSV, glycoprotein 5 (GP5), matrix protein (M), and nuclocapside protein (N), which are encoded by ORF 5, 6, and 7, respectively. The predominant arteriviral envelope proteins are major glycoprotein GP5 and the unglycosylated M, the other membrane proteins occur only in minor quantities in the viral envelope. The 25–27 kDa GP5 protein is the most abundant envelope glycoprotein and a major inducer for neutralizing antibodies. The 18–19 kDa M protein is a membrane protein without N-terminal signal sequence. As demonstrated experimentally by the closely related M protein of coronavirus [2, 3], the arteriviral M protein is likely to play a key role in virus assembly and budding. GP5 and M proteins exist as heterodimeric complexes in the viral surface. The disulfide-linked complexes are formed in the endoplasmic reticulum (ER) of infected cells preceding or during virus assembly [4–7]. GP5/M heterodimers is likely to constitute the basic protein matrix of the virion envelope. The relatively smaller ectodomains of the heterodimers probably constitute the tiny surface projections that can be observed on arterivirus particles under the electron microscopy [8, 9]. These surface structures were presumed to play an important role in the process of the virion attachment to the receptor and entering the host cells. In short, GP5 is the major virus antigen associated with the development of neutralizing antibodies and protection [10–12]. M has been considered associating with the development of strong cellular immunity [13].
Several systems have been used in the antigen expression of PRRSV, including bacteria [13], baculovirus [14, 15], DNA vaccine [16] and adenovirus [17], but nobody has tried the attenuated nonreplicating poxvirus—modified vaccinia virus ankara (MVA) system. MVA has a substantial history as a vaccine agent for the prevention of smallpox, and more recently as a viral vector in both cancer and infectious disease settings [18–21]. There are several characteristics making MVA more outstanding than other viral vaccine vectors. (A) MVA had been attenuated for use as a safe smallpox vaccine by serial passage in chicken embryo fibroblasts (CEF). During these passages, the virus underwent numerous deletions and mutations that result in an incapable replication in most mammalian cells, but it still can replicate to high titers in CEF and BHK-21 cells [22–24]. Consequently, MVA was safe when it be administered to human and was avirulent even for immunosuppressed animals [25–27]. In fact, the safety of MVA has been demonstrated in the most susceptible populations [24, 26]. (B) Due to the defective replication in human cells for MVA only affect virus assembly, while its viral and inserted foreign gene replication, transcription and expression remained unimpaired [28]. (C) MVA has the ability to induce strong humoral and cellular immune responses in both animal models and humans [29], better than or at least as good as that achieved by standard replicating strains of vaccinia virus [30–32]. (D) The immunogenicity of MVA might be enhanced by the spontaneous deletion of many immune evasion genes during the extensive passage history on CEF [22, 33]. (E) MVA has large capacity for inserting foreign DNA. (F) The construction making with MVA is much easier than other viral vaccine vectors.
It has been reported that DNA vaccines co-expressing GP5 and M proteins of PRRSV with two promoters displayed enhanced immunogenicity [16]. Also, another article reported that recombinant adenovirus expressing GP5 and M fusion protein of PRRSV induced both enhanced humoral and cell-mediated immune responses in mice [17]. However, there is no report about the differences on eliciting humoral and cellular immune responses against PRRSV infection between these two expression patterns—co-expressing GP5 and M proteins and expressing GP5 and M as fusion protein. Also, nobody has tried to express GP5 and M proteins in the powerful viral vector—MVA. In this article, four recombinant viruses that expressed GP5 and M proteins of PRRSV in MVA with different expression patterns were constructed. rMVA-GP5/M, expressing GP5 and M proteins under the control of two promoters, rMVA-GP5-M expressing GP5 and M as fusion proteins under one promoter, rMVA-GP5 and rMVA-M, the recombinant virus expressing GP5 or M separately. With these recombinant MVA viruses, the influence of expression pattern for GP5 and M proteins in MVA on their immunogenicity was investigated in this study. Results showed that rMVA-GP5 and rMVA-M cannot induce obvious humoral and cellular immune responses; rMVA-GP5-M inoculated group developed better immune responses than rMVA-GP5 and rMVA-M inoculated groups; however, mice inoculated with rMVA-GP5/M maintained the strongest cellular response against PRRS and consistently enhanced the anti-PRRSV humoral responses. These data indicate the potential usefulness of MVA-based vaccine vector designs co-expressing GP5 and M proteins against PRRS.
Materials and methods
Virus, cells, and plasmids
The PRRSV NJ-a strain (PRRSV NJ-a) was isolated from the lungs of commercial pigs at the acute stage of PRRSV infection in Nanjing, which was identified as a high-virulent North American type isolate. The virus was propagated and titered on MARC-145 cells [34]. Baby hamster kidney cells (BHK-21) were used for transfection and rMVA purification. Both MARC-145 and BHK-21 cells were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 10% heated-inactivated fetal bovine serum (FBS), 100 μg/ml streptomycin and 100 IU/ml penicillin at 37°C and 5% CO2. Plasmid pLR II and MVA were the storage of our laboratory. Plasmid pEFgpt12s, another transfer vector for fowl poxvirus expression system was also kept in our laboratory containing a compound promoter of vaccinia virus. The plasmid pMD-ORF5 and pMD-ORF6, which were made previously by the author, contain the complete cDNA of PRRSV ORF5 and ORF6 gene, respectively.
PCR for amplification of ORF5 and ORF6 gene of PRRSV NJ-a
The primers were designed according to the published sequence of the PRRSV VR2332 strain. (GenBank Accession number is AY686763). Primers used for PCR amplification are listed in Table 1. The amplification was performed in a 50 μl reaction mixture tube containing 1.5 mM MgCl2, 1× PCR buffer, 0.2 mM of each dNTP, 20 pmol of each primer, 0.5 μl of Ex Taq DNA polymerase (TaKaRa) and 1 μl (about 1 ng) of template. The reaction was run in a thermocycler (PTC-150) with the following program: denaturation at 95°C for 5 min, 35 cycles composed of denaturation at 95°C for 40 s, annealing for 40 s (ORF5 at 58°C, ORF6 at 53°C) and extension at 72°C for 1 min, the final extension step was at 72°C for 10 min.
Table 1.
Primers used for PCR amplification
| Primer | Sequence (5′→3′) | Note |
|---|---|---|
| P1 | cacctcgaggtttaaacatgttggagaaatgctt | Upstream primer for ORF5 gene in pLR-ORF5 and pLR-ORF5-ORF6 |
| P2 | tatggcgcgccaagcttctaaggacgaccccatt | Downstream primer for ORF5 gene in pLR-ORF5 and pLR-ORF5-ORF6 |
| P3 | gtcgtttaaacaagcttggtaccatggcttcgtcccttct | Upstream primer for ORF6 gene in pLR-ORF6 and pLR-ORF5-ORF6 |
| P4 | tatgatatcctgcagggcgcgcctcaaattgccaacagaat | Downstream primer for pLR-ORF6 and downstream primer for P7.5+ORF6 gene in pLR-ORF5/ORF6 |
| P5 | cacaagctttttattcactaattccaaaccc | Upstream primer for P7.5+ORF6 gene in pLR-ORF5/ORF6 |
Note: bold and italic letters are restriction enzyme sites for the convenient cloning between plasmids
Construction of the transfer vectors
The PCR product of ORF5 gene was digested with XhoI and HindIII, and cloned into pBluescriptIISK (+), and the plasmid was named as pSK-ORF5. The PCR product of ORF6 gene was digested with HindIII and PstI, and then cloned into the HindIII and PstI sites of pSK-ORF5 to get pSK-ORF5-ORF6. Subsequently, the plasmid pSK-ORF5-ORF6 was treated with PmeI and AscI , and then cloned into the transfer vector pLRII, to get the recombinant transfer plasmid pLR-ORF5-ORF6. The PCR product of ORF6 gene was digested with KpnI and EcoRV, and then cloned into the vector pEFgpt12s to obtain pEF-ORF6. Using primer P4 and P5, gene fragment VP7.5+ORF6 was amplified from pEF-ORF6 and cloned into HindIII—PstI sites of pSK-ORF5 to get pSK-ORF5/ORF6, and then digested with PmeI and AscI, ligated with pLRII to get pLR-ORF5/ORF6. Also, the PCR product of ORF5 and ORF6 gene was digested with PmeI and AscI , ligated with pLRII to get pLR-ORF5 and pLR-ORF6, respectively.
Transfection and purification of rMVAs
rMVA was generated with homologous recombination according to published procedures [35]. Briefly, the four rMVAs were generated by transfecting the corresponding recombinant plasmid on BHK-21 cells in six-well plates which had been infected with wtMVA at m.o.i. 0.01 2 h before transfection. At the same time, wtMVA infected BHK-21 cells was used as infection control. Positive rMVAs were screened for X-galactosidase in the presence of Bluo-gal TM substrate (Sigma–Aldrich, St. Louis, MO). After six rounds of plaque purification, the obtained rMVAs were expanded in BHK-21 cells. Insertion of the target gene into the MVA genome was identified with PCR and the expression of GP5 and M proteins was evaluated by Western-blot and indirect immunofluorescence assay (IFA). The purification of rMVAs was made by ultracentrifugation on a 36% sucrose density gradient. The final obtained rMVAs were suspended in PBS, titrated and stored at −80°C and prepared for the further mouse inoculation and in vitro studies.
Identification of the rMVAs
PCR analysis for the rMVAs
The genomic DNA of rMVAs, extracted by the method of SDS-Protease K-Phenol, was used as PCR template and amplifications were performed with TaKaRa Ex Taq DNA polymerase by applying 30 cycles of 94°C for 1 min, 55°C for 30 s, and 72°C for 1 min. The ORF5 and ORF6 gene specific primers are:
PL: 5′-TGTTGGAGAAATGCTTGAC-3′
PR: 5′-TATTTGGCATATTTGACAA-3′
Western-blot
Western-blot was used to identify the expression of GP5 and M proteins in rMVAs. 2 × 105 BHK-21 cells were propagated into the 6-well plate. After 24 h, the cell density reached to around 80% confluence. Then, the cells were infected with different purified rMVAs at MOIs 2.0. 24–48 h after infection (the time depended on whether the cytopathic effect (CPE) appeared or not), the cells were collected and added with equal volume of 2× LSB. SDS-PAGE was running with 12% SDS-PAGE gel. wtMVA infected cells were used as negative control. No infected cell was used as blank control. The first antibody was prepared by immunization of rabbit with PRRSV, secondary antibody was HRP labeled goat anti-rabbit IgG (KPL, Maryland, USA). Transferred membranes were blocked in blocking solution (10% fat-free milk in PBS) at room temperature overnight. First antibody was incubated at 1:1000 dilution for 2 h at room temperature. Second antibody was incubated for 1 h at the dilution of 1/2000. Detections were performed with chemiluminescence luminal reagents (SuperSignal West Pico Trial Kit, PIERCE).
Indirect immunofluorescence assay
The cell preparation and rMVAs infection were same like above. After infection for 24–48 h, the cells were washed with 1× PBS and fixed with cold methanol for 10 min. cell blocking was in 1% bovine serum albumin (BSA) in PBS for 30 min at room temperature. The first antibodies (rabbit anti-PRRSV GP5 or M antibody) were incubated with the cells for 2 h at dilution 1:50. After three times washing with 1× PBS, the cells were incubated with secondary antibody (fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit, KPL, Maryland, USA) at the dilution 1:100 for another 1 h in room temperature. Cells were washed in PBS three times as usual. Observation was taken with the fluorescent microscope (model AX70, Olympus).
Growth characteristics of the rMVAs
The growth characteristics of the rMVAs were evaluated on 96-well plates. The BHK-21 cells were infected with wtMVA, rMVA-GP5, rMVA-M, rMVA-GP5-M, and rMVA-GP5/M at m.o.i. 0.1 (multicycle) and 2.0 (one-step), respectively. At the designated time points (Fig. 5), all contents in the well were taken to determine the virus titer by the method of endpoint titration. Each value at the time point was triplicated.
Fig. 5.
One-step and multicycle growth curves. BHK-21 cells were infected at 2.0 m.o.i (one-step growth curve, A) and 0.1 (multicycle growth, B) with rMVA-GP5-M, rMVA-GP5/M, and wild type MVA. rMVAs growth rate at m.o.i 2.0 almost had the same growth curves with that of at 0.1 m.o.i. The insertion of PRRSV GP5 and M into MVA by the forms of single protein, fusion protein and co-expressed together, did not effect the growth of the recombinant rMVAS. The rMVAs have the similar growth ability with wild type MVA. Experiments were triplicate
The inoculation of mice with rMVAs
For serum neutralization assay (NA), 60 of BALB/c mice (6-week-old) were divided randomly into six groups with 10 of them in each group. Mice in group one were injected with purified MVA-GP5/M. Mice in group two were injected with purified rMVA-GP5-M. Mice in group three were injected with purified MVA-GP5. Mice in group four were injected with purified rMVA-M. Mice in group five, served as negative control, were injected with same amount of wtMVA. Mice in group six were injected with steriled PBS as blank control. All injections were done with the quantity of virus at 5 × 105 TCID50/mouse or equal volume of PBS via intramuscular (i.m.) route. Three weeks post the primary inoculation, the mice were boosted with same dose of correspondence rMVAs, wtMVA, and PBS. Sera for neutralizing antibody detection of PRRSV were collected weekly by the tail bleeding after the first inoculation.
For the cellular immune responses detection, sixty of BALB/c mice (6-week-old) were divided randomly into six groups with ten of them in each group. The inoculation was the same like that for NA. At 30, 60, and 90 days post initial immunization, three mice of each group were sacrificed, and splenocytes were harvested for the cytokine production detection as the description of the Kit’s manual. Experiments were triplicated. Data are presented as the mean ± standard error.
Serum neutralization test assay
All sera samples were inactivated at 56°C for 30 min prior to perform the neutralization test (NT) assay. The serum (50 μl) was two-fold serial diluted with FCS free D-MEM and mixed with equal volume of 200 TCID50 of PRRSV NJ-a strain in 96-well cell cultural plate. The plate was incubated in the incubator for 1 h at 37°C with 5% CO2. After incubation, 100μl of MARC-145 cell suspension containing 5 × 104 cells was aliquoted to each well. The plate was incubated in the incubator for up to 7 days at 37°C in a humidified atmosphere with 5% CO2. The cells were examined every day to observe the appearance of the PRRSV specific CPE. The neutralization titer was expressed as the reciprocal of the highest serum dilution that no CPE was observed.
Cytokine detection
The evaluation of cellular immunity was performed by the detection of IFN-γ, IL-2, and IL-4. Three mice were killed each time at 30, 60, and 90 days post the first inoculation (dpi). The mouse spleen was removed aseptically. The splenocytes were isolated, counted, and diluted to the density of 2 × 107 cells/100 μl cell suspension. The evenly separated cells were aliquoted to 96-well plates according to the amount of 100 μl/well. 100 μl/well of complete 1,640 medium with 200 TCID50 PRRSV (inactivated by UV rays) was added to each well. After incubation for 60 h, the supernatants were collected and the production of IFN-γ, IL-2, and IL-4 were detected with the commercial ELISA Kit according to the manufacturer’s instruction (CytoscreenTM, BioSource International).
Statistical analysis
All data analysis was conducted by general SPASS biostatistics software. 0.01 < p < 0.05 means significant difference; p < 0.01 means extreme difference; p > 0.05 means no significant difference.
Results
Construction of rMVAs expressing ORF5 and ORF6 gene of PRRSV
Part of the plasmid structures that used for recombination of MVA are shown in Fig. 1. Plasmid pLR II (A), the basic plasmid that used for making all the four recombinant plasmids, has two deletion II regions. These deletion II regions are the recombinant arms of MVA that will let the recombination happen when the plasmids were co-infected with MVA in BHK-21 cell line. Plasmid pLR-ORF5 (B) was used to produce recombinant virus rMVA-GP5 which can express PRRSV GP5. Plasmid pLR-ORF6(C) was used to produce recombinant virus rMVA-M which can express PRRSV M protein. Plasmid pLR-ORF5-ORF6 (D) was used to generate the recombinant virus rMVA-GP5-M expressing PRRSV GP5 and M as fusion protein. Plasmid-ORF5/ORF6 (E) was used to make the recombinant virus rMVA-GP5/M which can express PRRSV GP5 and M proteins under two vaccinia virus promoters.
Fig. 1.
Design of the recombinant plasmids. (A) The basic structure of plasmid pLRII. (B) ORF5 gene was cloned into pLRII (named as pLR-ORF5) that was used to express GP5 of PRRSV. (C) ORF6 gene was cloned into pLRII (pLR-ORF6) that were used to express M of PRRSV. (D) GP5 and M were expressed as a fusion protein under the control of the same promoter (Psyn). (E) GP5 and M were expressed separately under the control of two different promoters (Psyn and VP7.5). Psyn, the synthesized strong early/late compound promoter; VP7.5, the late/early promoter of vaccine virus; TTTTTAT, the transcription termination signal sequence; ORF5, ORF5 gene of PRRSV; ORF6, ORF6 gene of PRRSV; DeletionII, the deletion II region of MVA genome
All the target genes that cloned into plasmid pLRII were identified by restrictive enzyme double digestion(PmeI and AscI) and the size of the bands were exactly matched with the expected ones (data not shown).
PCR identification for rMVAs
To identify whether ORF5 and/or ORF6 genes were inserted into the genome of MVA, the screening PCR was performed with the primers shown in materials and method, the genomic DNA of rMVAs was extracted and used as PCR template. As shown in Fig. 2, the expected DNA brands of ORF5 and/or ORF6 gene were amplified from the positive rMVAs, no band was detected from wtMVA infected cells. Furthermore, the results were double checked by DNA sequencing (data not shown). Both PCR and sequencing results proved that the target genes had been successfully recombined into the four rMVAs according to previous design.
Fig. 2.
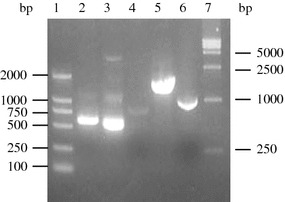
PCR identification of ORF5 and ORF6 in rMVAs. 1, DNA molecular Marker (DL2000); 2, rMVA-GP5; 3, rMVA-M; 4, wtMVA: 5, rMVA-GP5/M; 6, rMVA-GP5-M; 7, DNA molecular Marker (DL15000). Also, the results were double checked by DNA sequencing (data not shown)
The expression of GP5 and M proteins in rMVAs
The results of Western-blot showed that the four expected recombinant MVA viruses were established successfully (Fig. 3). The 25 kDa GP5 only existed in rMVA-GP5 (lane 4). The 19 kDa M protein only existed in rMVA-M (lane 3). The fusion expressed protein of GP5 and M (lane 1) in rMVA-GP5-M had a band around 44 KDa, just as the designed size because both these proteins were expressed in one expression cassette as a fusion protein. Concurrently, there were two specific protein bands reflecting 25 kDa (GP5) and 19 kDa (M) in rMVA-GP5/M infected cells, indicating that the authentic GP5 and M proteins could be co-expressed simultaneously in rMVA-GP5/M, because the expression of GP5 and M proteins was controlled by two promoters. wtMVA infected cells was used as negative control and did not have any PRRSV protein expression (lane 5).
Fig. 3.
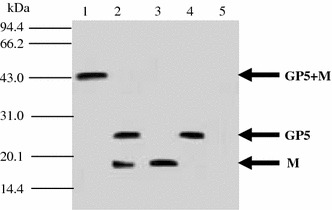
Western-blots identify the expression of GP5 and M proteins in different recombinant rMVAs. 1, rMVA-GP5-M; 2, rMVA-GP5/M; 3, rMVA-M; 4, rMVA-GP; 5, wtMVA. The arrows indicate the expected molecular sizes of GP5+M, GP5, and M
IFA was also done to verify the expression of GP5 and M proteins in BHK-21 cells (Fig. 4). The figures that labeled as 4A, 4B, 4C, and 4D illuminated the IFA results of rMVA-GP5, rMVA-M, rMVA-GP5-M, and rMVA-GP5/M respectively. Figure 4E was the result of negative control that was infected with wtMVA. All the cells that infected with different rMVAs had CPE and could produce fluorescence obviously.
Fig. 4.
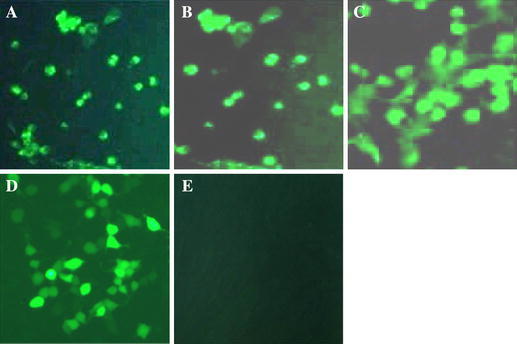
The IFA identification of the GP5 and M expression. BHK-21 cells were infected with rMVA-GP5, rMVA-M, rMVA-GP5-M, and rMVA-GP5/M at 2.0 multiplicities of infection (m.o.i.), respectively. Figure 4A–D represented the IFA identification of the cells infected with rMVA-GP5, rMVA-M, rMVA-GP5-M, and rMVA-GP5/M, respectively. Figure 4E was the picture of negative control that was infected with wtMVA
The Western-blot and IFA results were demonstrated that PRRSV GP5 and M proteins could be expressed correctly in four rMVAs. And all these rMVAs can grow and produce CPE on BHK-21 cells.
Multicycle and one-step growth curves of rMVAs
BHK-21 cells infected with the four recombinant virus at m.o.i 2.0 (one-step, Fig. 5A) almost had the same growth curves with those infected at m.o.i 0.1(multicycle, Fig. 5B). It illuminated that the viruses seeding amount can at least as fewer as 0.1 m.o.i. The growth ability of different rMVAs in BHK-21 cells had no obvious differences. The insertion of PRRSV ORF5 and ORF6 genes into MVA genome in the forms of single protein, fusion protein, and co-expressed together, did not effect the growth characteristics of the recombinant rMVAs. The rMVAs have the similar growth ability compared with wtMVA.
Serum neutralization assay
The humoral immune response was evaluated by detecting the neutralizing antibodies that elicited by the inoculation of different rMVAs with mouse model.
The results are summarized in Fig. 6. The NA titers of the rMVA-GP5/M inoculation group were the highest among the four rMVAs injection groups. The Maxim average NA titer of the mouse vaccinated with rMVA-GP5/M was up to 8.12 (p < 0.01), almost two times than that immunized with single gene expression rMVAs (rMVA-GP5 and rMVA-M). The NA titers of the rMVA-GP5-M inoculated group that expressing GP5 and M as fusion protein were significantly lower (p < 0.05) when compared to mouse group immunized with rMVA-GP5/M, but higher than that evoked by rMVA expressing a single gene, rMVA-GP5, and rMVA-M. Between the two single genes expression rMVAs, the NA titers of rMVA-GP5 were higher than that of rMVA-M. Wild type MVA and PBS inoculation group did not have any neutralizing antibody throughout the experiment. These results suggested that the expression of GP5 and M under two promoters could elicit the highest NA titers of PRRSV; the expression of GP5 and M proteins together as fusion protein under one promoter, better than that elicited by the single gene expression. Between rMVA-GP5 and rMVA-M, rMVA-GP5 evoked higher NA titer. These results should be useful not only in the design of PRRS vaccine, but also in other vaccine designs using bioengineering techniques.
Fig. 6.
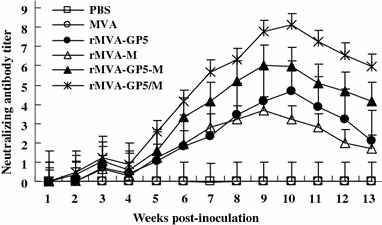
Serum neutralization assay (NA) of the mice immunized with rMVAs of GP5 and M proteins of PRRSV. Each group of mice (n = 5) were immunized (i.m.) with 5 × 105 TCID50 of rMVAs at 0 week and boosted at 3 weeks. Sera were collected weekly after inoculation. The neutralizing antibody titers of each group were measured immediately after bleeding from tail. As shown upon, the neutralizing antibody titers of rMVA-GP5-M inoculation mouse group are significantly less than rMVA-GP5/M inoculation group but higher than that of rMVA-GP5 and rMVA-M inoculation mouse groups (p < 0.05). The efficacy of humoral immunity to PRRSV, co-expressing GP5 and M under the control of different promoters in the same virus is the best. The ranking is rMVA-GP5/M, rMVA-GP5-M, rMVA-GP5, and rMVA according to the order, high to low. No production of PRRSV NA titers was detected in wtMVA and PBS injected mice. Experiments were triplicated. Data are presented as the mean ± standard error
Cellular immunity elicited by rMVAs in mice
Cellular immune response was evaluated by measuring the production of IFN-γ, IL-2, and IL-4 in splenocytes from mice inoculated with rMVAs. The animals were sacrificed and the splenocytes were prepared as described in materials and methods. Splenocytes were stimulated by UV inactivated PRRSV. As shown in Fig. 7, the profiles of the production of IFN-γ and IL-2 were similar to that of NT assays. The rMVA-GP5/M inoculated group had the highest level of IFN-γ and IL-2, which even reached to 72.6 and 76.1 pg/ml, respectively, at 60 dpi. The IFN-γ and IL-2 production of rMVA-GP5-M inoculation mouse group was significantly lower than rMVA-GP5/M inoculation group but higher than that of rMVA-GP5 and rMVA-M inoculation group (p < 0.05). No increase of IL-4 production was detected in all rMVAs inoculated mice compared with the wtMVA and PBS inoculated groups. These results suggested that the expression of GP5 and M proteins of PRRSV in MVA could elicit Th1 type cellular immune response. To the efficacy of cellular immunity of PRRSV, co-expressing GP5 and M under the control of two promoters is the best. The magnitude of the cellular immunity elicited by four rMVAs of GP5 and M proteins of PRRSV consistent with those got from neutralization assays, which the ranking is rMVA-GP5/M, rMVA-GP5-M, rMVA-GP5, and rMVA in the order of high to low. As expected, no significant production increase of IFN-γ, IL-2, and IL-4 were detected in wtMVA compared with PBS injection group. Experiments were triplicated.
Fig. 7.
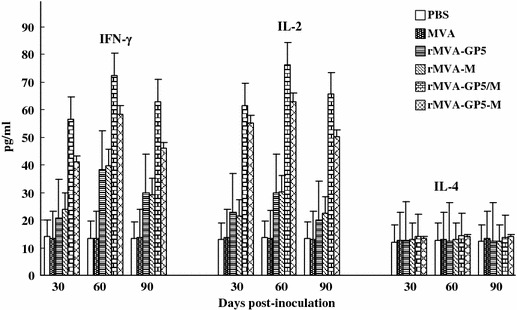
Cellular immune responses in mice immunized with rMVAs. Each group of mice (n = 27) was immunized (i.m.) with 5 × 105 TCID50 of rMVAs at 0 week and boosted at 3 weeks. The IFN-γ and IL-2 level of rMVA-GP5-M inoculation mouse group are significantly less than rMVA-GP5/M inoculation group but higher than that of rMVA-GP5 and rMVA-M inoculation mouse group (p < 0.05). No production of IL-4 was detected in all rMVAs inoculated mice. The efficacy of cellular immunity of PRRSV, co-expressing GP5 and M under the control of different promoters in the same virus is the best ranking in rMVA-GP5/M, rMVA-GP5-M, rMVA-GP5, and rMVA according to the order, high to low. No significant production of IFN-γ, IL-2, and IL-4 were detected in wtMVA and PBS injection mice. Data were triplicated and presented as the mean ± standard error
Discussion
PRRS is a new virus disease almost simultaneously found in US and Europe in the late 1980s and early 1990s, respectively [1, 36]. To date, PRRS has spread worldwide with the characteristics of endemic in those swine-cultivating countries, causing enormous economic losses each year. The research of developing efficient PRRS vaccine has been on the forefront of clinical research in recent years. Although modified live vaccines (MLV) and inactivated vaccines are now commercially available and widely used [37], no ideal vaccines were achieved so far. There has been not a single report suggesting that live vaccine virus might possibly revert to virulence and cause acute PRRS-like symptoms during its persistence in swine herds after vaccination [38]. The killed vaccine cannot always provide solid protective immunity at herd level [39, 40]. There is an urgent requirement to develop vaccines more effective and to alter the strategies against this viral disease. Recombinant virus design is one of the common and useful methods.
To express antigens of PRRSV, different expression systems, including baculovirus [41], DNA vaccine [42] and adenovirus [43], have been tested. However, it has had the problems that the relatively weak and tardy neutralizing antibodies and poorly cellular immunity in the experimental animals [43–45]. The data obtained in this article demonstrated that the rMVA co-expressing PRRSV GP5 and M proteins could elicits higher titer of PRRSV-specific neutralizing antibodies and Th1 type cell-mediated immune responses in mouse model. In comparison of the immunity effect of the four recombinant viruses, rMVA-GP5/M, co-expressing GP5 and M proteins under two promoters, was the best; rMVA-GP5-M, expressing GP5 and M protein as fusion protein, was the second; rMVA-GP5 and rMVA-M, expressing GP5 or M separately in the two recombinant viruses, were the last. Therefore, the rMVA-GP5/M has the potential to be a novel candidate for PRRSV vaccination. The further animal protection experiments executed in pigs were in processing and the original data was consistent with the results obtained in this article (data not shown).
In pig industry, PRRSV usually infected together with other viral organisms, like classic swine fever virus (CSFV), pseudorabbies virus (PRV), porcine circovirus II (PCV-II) and porcine parvovirus (PPV) [46, 47]. These contagious agents, including PRRSV itself, usually have some relationships with the swine immune suppression. Furthermore, to prevent the infectious diseases, farmers have to inoculate various vaccines continuously. These are not only making the vaccination situation of swine complex, but also are part of the reasons that the vaccination of PRRS failed. In present study, we choose MVA as the viral vector for the expression of GP5 and M proteins of PRRSV, besides the reasons mentioned in the introductions, the most important is that the MVA was safe and avirulent even for immunosuppressed animals [25–27, 48]. The recombinant virus developed base on MVA might hopefully deal with the complex situations in pig industry.
In the four recombinant MVA viruses, rMVA-GP5/M was constructed to co-express GP5 and M proteins of PRRSV under the control of two promoters, Psyn and P7.5, respectively. The Psyn and P7.5 promoters, which can efficiently drive high-level expression of recombinant protein both in vitro and in vivo, have been extensively used for foreign gene expression in vaccinia virus system. Psyn is a synthesized early/late strong vaccinia virus promoter and P7.5 is a late/early vaccinia virus promoter. GP5, the most abundant and important viral antigenic protein, has been a leading target for PRRS vaccine design. M protein was considered associating with the development of strong cellular immunity. It has been postulated that the formation of heterodimers of GP5 and M proteins may play a critical role in assembly of virus particle [49] and recognizing of the host cell receptor [50]. Co-expression of GP5 and M proteins under the control of two promoters in MVA may partly mimic the formation of GP5/M heterodimers in PRRSV and the process of antigen recognitions that displayed and treated in vivo, and making the rMVA-GP5/M had much better effect than rMVA-GP5-M expressing GP5 and M proteins as fusion protein on induction of immune responses. The lower neutralizing antibodies and cellular immune responses elicited in mice immunized with rMVA-GP5-M was supposed that fusion expression of GP5 and M proteins might alter the natural conformational structure of these two proteins and led to only partial of the neutralizing epitopes of GP5 and M proteins could be presented to the antigen-presenting cells.
The growth characteristics of recombinant virus are very important on the aspect of whether a virus can be a good vaccine candidate. It should be concerned even early to the time of choosing the viral vector. The virus growth kinetics and virus yields on the susceptible cells usually are useful indicators to evaluate the virus growth ability. To determine whether the insertion of PRRSV genes altered the growth kinetics of MVA, multi-cycle and one-step growth curves were measured. As shown in Fig. 5, the four recombinant viruses have a similar growth kinetics curves compared with wtMVA. The expression of foreign GP5 and M proteins of PRRSV in MVA had hardly any effect on the growth of rMVAs. The growth characters of the recombinant MVA make it possible to be used in the industry.
To induce an effective cellular immune response is dependent on the specific cytokines produced during the infection process. IFN-γ and IL-2 are powerful inducers of the differentiation of naive T-helper cells to Th1-type cells that generally produce specific sets of cytokines, such as IFN-α and TNF-h [51, 52]. IL-4, on the other hand, potently induces the Th2 phenotype, which produces IL-4, IL-5, IL-9, IL-10, and IL-13 [51, 53]. IL-4 is critical for Th2 differentiation. IFN-γ and IL-2, generally considered as Th1 cytokine, also play an important role in Th2 induction [54]. In addition, IL-2 is responsible for the proliferation of both T and B cells and increasing cytolytic activity of NK cells [55–57]. In this study, the differences of cellular immune responses which induced by inoculation of rMVAs expressing GP5 and/or M proteins was evaluated by measuring the production of IFN-γ, IL-2, and IL-4 in splenocytes of inoculated mice (Fig. 7). A significant higher production of PRRSV-specific IFN-γ and IL-2 were detected from the mice inoculated with the rMVAs but almost no difference for the detection of IL-4. It can be postulated that the vaccination of rMVAs elicited the PRRSV specific cellular immunity, and this cellular immunity was activated by the proliferation of Th1-type cells [58–60]. The magnitude of the cellular immunity elicited by four rMVAs was consistent with that got from neutralization assays, in which the ranking is rMVA-GP5/M, rMVA-GP5-M, rMVA-GP5, and rMVA-M according to the order, high to low.
In summary, in this article, we demonstrated that the recombinant MVA expressing GP5 and /or M proteins of PRRSV can elicit both humoral and cellular immune responses. The magnitude of the immunity elicited by the rMVAs was remarkably effected by the expression pattern of GP5 and M proteins in the MVA viral vector. Mice inoculated with rMVA-GP5/M maintained the strongest cellular response against PRRS and consistently enhanced the anti-PRRSV humoral responses. This result should be useful to the research on PRRS vaccine design. rMVA-GP5/M should be a good candidate of the PRRSV vaccine for the prevention of PRRS in swine industry.
Footnotes
Co-first authors have same contribution for this article.
Contributor Information
Qisheng Zheng, Phone: +86-25-8439028, FAX: +86-25-84396335, Email: zhengqisheng2004@yahoo.com.cn.
Desheng Chen, Email: desheng.chen@gmail.com.
References
- 1.Wensoort G., Terpstra C., Pol J.M., ter Laak E.A., Bloemraad M., de Kluyver E.P., et al. Vet. Quart. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- 2.de Haan C.A., Roestenberg P., de Wit M., de Vries A.A., Nilsson T., Vennema H., Rottier P.J., et al. J. Biol. Chem. 1998;273:29905–29914. doi: 10.1074/jbc.273.45.29905. [DOI] [PubMed] [Google Scholar]
- 3.Vennema H., Godeke G.J., Rossen J.W., Voorhout W.F., Horzinek M.C., Opstelten D.J., Rottier P.J., et al. EMBO J. 1996;15:2020–2028. doi: 10.1002/j.1460-2075.1996.tb00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vries A.A., Post S.M., Raamsman M.J., Horzinek M.C., Rottier P.J., et al. J. Virol. 1995;69:4668–4674. doi: 10.1128/jvi.69.8.4668-4674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobbe J.C., van der Meer Y., Spaan W.J., et al. Virology. 2001;228:283–294. doi: 10.1006/viro.2001.1074. [DOI] [PubMed] [Google Scholar]
- 6.Faaberg K.S., Even C., Palmer G.A., Plagemann P.G., et al. J. Virol. 1995;69:613–617. doi: 10.1128/jvi.69.1.613-617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mardassi H., Massie B., Dea S. Virology. 1996;221:98–112. doi: 10.1006/viro.1996.0356. [DOI] [PubMed] [Google Scholar]
- 8.Hyllseth B. Arch Gesamte Virusforsch. 1973;40:177–188. doi: 10.1007/BF01242536. [DOI] [PubMed] [Google Scholar]
- 9.Magnusson P., Hyllseth B., Marusyk H. Arch. Gesamte Virusforsch. 1970;30:105–112. doi: 10.1007/BF01250177. [DOI] [PubMed] [Google Scholar]
- 10.Pirzadeh B., Dea S. J. Gen. Virol. 1997;78:1867–1873. doi: 10.1099/0022-1317-78-8-1867. [DOI] [PubMed] [Google Scholar]
- 11.Pirzadeh B., Dea S. J. Gen. Virol. 1998;79:989–999. doi: 10.1099/0022-1317-79-5-989. [DOI] [PubMed] [Google Scholar]
- 12.Weiland E., Wieczorek-Krohmer M., Kohl D., et al. Vet. Microbiol. 1999;66:171–186. doi: 10.1016/S0378-1135(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 13.Bautista E.M., Molitor T.W. Arch. Virol. 1997;144:117–134. doi: 10.1007/s007050050489. [DOI] [PubMed] [Google Scholar]
- 14.Meulenberg J.J.M., Bende R.J., Pol J.M.A., Wensvoort G., Moormann R.J.M. Clin. Diagn. Lab. Immunol. 1995;2:652–656. doi: 10.1128/cdli.2.6.652-656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreutz L.C., Mengeling W.L. Vet. Microbiol. 1997;59:1–13. doi: 10.1016/S0378-1135(97)00142-9. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y.B., Xiao S.B., Fang L.R., et al. Vaccine. 2006;24:2869–2879. doi: 10.1016/j.vaccine.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W.M., Jiang P., Li Y.F., et al. Vet. Immunol. Immunopathol. 2006;113:169–180. doi: 10.1016/j.vetimm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Cosma A., Nagaraj R., Buhler S., et al. Vaccine. 2003;22:21–29. doi: 10.1016/S0264-410X(03)00538-3. [DOI] [PubMed] [Google Scholar]
- 19.Drexler I., Staib C., Sutter G. Curr. Opin. Biotechnol. 2004;15:506–512. doi: 10.1016/j.copbio.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rochlitz C., Figlin R., Squiban P., et al. J. Gene Med. 2003;5:690–699. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 21.Slifka M.K. Med. Immunol. 2005;4:2. doi: 10.1186/1476-9433-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoine G., Schei-inger F., Dorner F., Falkner F.G. Virology. 1998;244:365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 23.Carroll M.W., Moss B. Biotechniques. 1995;19:4352–4356. [PubMed] [Google Scholar]
- 24.Meyer H., Sutter G., Mayr A. J. Gen. Virol. 1991;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 25.Nam J.H., Wyatt L.S., Chae S.L., et al. Vaccine. 1999;17:261–268. doi: 10.1016/S0264-410X(98)00156-X. [DOI] [PubMed] [Google Scholar]
- 26.Mayr A., Danner K. Dev. Biol. Stand. 1978;41:225–234. [PubMed] [Google Scholar]
- 27.Stittelaar K.J., van A.G., Kondova I., et al. J. Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutter G., Moss B. Proc. Natl. Acad. Sci. USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster D.P., Dunachie S., Vuola J.M., et al. Proc. Natl. Acad. Sci. USA. 2005;102:4836–4841. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belyakov I.M., Earl P., Dzutsev A., Kuznetsov V.A., Lemon M., Wyatt L.S., et al. Proc. Natl. Acad. Sci. USA. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch V.N., Fuerst T.R., Sutter G., et al. J. Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutter G., Wyatt L., Foley P., et al. Vaccine. 1994;12:1032–l040. doi: 10.1016/0264-410X(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 33.Blanchard T.J., Alcami A., Andrea P., Smith G.L. J. Gen. Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 34.Kim T.Su., Benfield D.A., Rowland R.R.R. Virus Res. 2002;85:133–140. doi: 10.1016/S0168-1702(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 35.Moss B. Proc. Natl. Acad. Sci. USA. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keffaber K.K. Am. Assoc. Swine Pract. Newslett. 1989;1:1–10. [Google Scholar]
- 37.Meng X.J. Vet. Microbiol. 2000;74:309–329. doi: 10.1016/S0378-1135(00)00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botner A., Strandbygaard B., Sorensen K.J., Have P., Madsen K.G., Madsen E.S., et al. Vet. Rec. 1997;141:497–499. doi: 10.1136/vr.141.19.497. [DOI] [PubMed] [Google Scholar]
- 39.K.M. Lager, W.L. Mengeling, Current status of vaccines and vaccination for porcine reproductive and respiratory syndrome. In Proceedings of the Annual Meeting of the American Association of Swine Practitioners, pp. 443–446 (1997)
- 40.F.A. Osorio, F. Zuckermann, R. Wills, et al., Comparison of commercial vaccines in their ability to induce protection against current PRRSV strains of high virulence. In Proceedings of the 25th Anniversary Allen D. Leman Swine Conference, College of Veterinary Medicine, University of Minnesota, pp. 176–182 (1998)
- 41.Kreutz L.C., Mengeling W.L. Vet. Microbiol. 1997;59(1):1–13. doi: 10.1016/S0378-1135(97)00142-9. [DOI] [PubMed] [Google Scholar]
- 42.Kwang J., Zuckermann F., Ross G., et al. Res. Vet. Sci. 1999;67:199–201. doi: 10.1053/rvsc.1998.0291. [DOI] [PubMed] [Google Scholar]
- 43.Gagnon C.A., Lachapelle G., Langelier Y., et al. Arch. Virol. 2003;148:951–972. doi: 10.1007/s00705-002-0943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murtaugh M.P., Xiao P.Z., Zuckermann F. Viral Immunol. 2002;15:533–547. doi: 10.1089/088282402320914485. [DOI] [PubMed] [Google Scholar]
- 45.Plana Duran J., Climent I., Sarraseca J., et al. Virus Genes. 1997;14:19–29. doi: 10.1023/A:1007931322271. [DOI] [PubMed] [Google Scholar]
- 46.Albina E., Mesplède A., Chenut G., Potier M.F.L., Bourbao G., Gal S.L., Leforban Y. Vet. Microbiol. 2000;77(1–2):43–57. doi: 10.1016/S0378-1135(00)00255-8. [DOI] [PubMed] [Google Scholar]
- 47.Rose N., Larour G., Diguerher G., Eveno E., Jolly J.P., Blanchard P., et al. Prev. Vet. Med. 2003;61(3):209–225. doi: 10.1016/j.prevetmed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Drexler I., Staib C., Sutter G. Curr. Opin. Biotechnol. 2004;15:506–512. doi: 10.1016/j.copbio.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delputte P.L., Nauwynck H.J. J. Virol. 2004;78:8094–8101. doi: 10.1128/JVI.78.15.8094-8101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansari I.H., Kwon B., Osorio F.A., Pattnaik A.K. J. Virol. 2006;80(8):3994–4004. doi: 10.1128/JVI.80.8.3994-4004.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jankovic D., Liu Z., Gause W.C. Trends Immunol. 2001;22:450–457. doi: 10.1016/S1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi M., Fitz L., Ryan M., et al. J. Exp. Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNeela E.A., Mills K.H. Drug Deliv. Rev. 2001;51:43–54. doi: 10.1016/S0169-409X(01)00169-7. [DOI] [PubMed] [Google Scholar]
- 54.Cote-Sierra J., Foucras G., Guo L., et al. Proc. Natl. Acad. Sci. USA. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jelinek D.F., Splawski J.B., Lipsky P.E. Eur. J. Immunol. 1986;16:925–932. doi: 10.1002/eji.1830160809. [DOI] [PubMed] [Google Scholar]
- 56.Kovanen P.E., Leonard W.J. Immunol. Res. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 57.Splawski J.B., McAnally L.M., Lipsky P.E. J. Immunol. 1990;144:562–569. [PubMed] [Google Scholar]
- 58.Kagi D., Hengartner H. Curr. Opin. Immunol. 1996;8:472–477. doi: 10.1016/S0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Fuertes L., Domenech N., Alvarez B., et al. Virus Res. 1999;64:33–42. doi: 10.1016/S0168-1702(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 60.Rowland R.R., Robinson B., Stefanick J., et al. Arch. Virol. 2000;146:539–555. doi: 10.1007/s007050170161. [DOI] [PMC free article] [PubMed] [Google Scholar]


