Abstract
A prospective study of 65 research beagles kept in a rabies-free environment was undertaken to determine the duration of immunity after they received licensed rabies vaccines. The eventual goal was to extend mandated rabies booster intervals to 5 or 7 years and help reduce the risk of vaccine-associated adverse events. Three groups of dogs were vaccinated with 1 of 2 commercial rabies vaccines or saline at 12 and 15 weeks of age. Beginning 5 years 5 months later, vaccinated and unvaccinated dogs were challenged with virulent rabies virus and observed for 90 days over a series of 3 trials. Humoral and cellular immune responses were examined by serology and flow cytometry. Brain tissue from all challenged dogs was tested for rabies virus. Challenge trial 1 was confounded due to insufficiently virulent virus. In trials 2 and 3 virulent challenge provided 100% mortality in controls. Vaccinate survival was 80% (4/5) after 6 years 7 months, 50% (6/12) after 7 years 1 month, and 20% (1/5) after 8years 0 months. Antibody responses 12 days post-challenge correlated strongly with survival. In a separate non-challenge trial, administration of either a recombinant or a killed rabies vaccine demonstrated memory antibody responses 6 years 1 month after initial vaccination compared with unvaccinated controls. Our data demonstrated that i) duration of immunity to rabies in vaccinated dogs extends beyond 3 years; ii) immunologic memory exists even in vaccinated dogs with serum antibody titer < 0.1 IU/mL; and iii) non-adjuvanted recombinant rabies vaccine induces excellent antibody responses in previously vaccinated dogs 14 days after administration.
Résumé
Une étude prospective sur 65 chiens beagle de recherche gardés dans un environnement exempt de rage fut entreprise afin de déterminer la durée de l’immunité après qu’ils reçurent un vaccin homologué contre la rage. Le but éventuel était d’allonger l’intervalle requis du rappel du vaccin contre la rage à 5 ou 7 ans et aider à réduire le risque associé aux réactions adverses au vaccin. Trois groupes de chiens furent vaccinés avec un des deux vaccins commerciaux contre la rage ou de la saline à 12 et 15 semaines d’âge. Débutant 5 ans et 5 mois plus tard, les chiens vaccinés et non-vaccinés furent soumis à une infection défi avec un virus de la rage virulent et observés pendant 90 jours lors d’une série de trois essais. Les réponses immunitaires humorale et cellulaire furent examinées par sérologie et cytométrie de flux. Du tissu cérébral de tous les chiens infectés fut testé pour la présence du virus rabique. L’essai 1 était décevant étant donné la quantité insuffisante de virus virulent. Lors des essais 2 et 3, l’infection défi a entrainé 100 % de mortalité chez les témoins. Le taux de survie des animaux vaccinés était de 80 % (4/5) après 6 ans et 7 mois, 50 % (6/12) après 7 ans et 1 mois et 20 % (1/5) après 8 ans 0 mois. La réponse en anticorps 12 jours post-infection corrélait fortement avec la survie. Dans un essai séparé sans infection défi, l’administration de soit un vaccin recombinant ou un vaccin tué a mis en évidence une réponse anamnestique en anticorps 6 ans et 1 mois après la vaccination initiale comparativement aux témoins non-vaccinés. Nos résultats démontrent que (1) la durée de l’immunité contre la rage chez les chiens vaccinés va au-delà de 3 ans, (2) une mémoire immunologique existe même chez les chiens vaccinés avec des titres d’anticorps sériques < 0,1 IU/mL, et (3) un vaccin antirabique recombinant sans adjuvant induit d’excellentes réponses en anticorps chez des chiens préalablement vaccinés 14 jours après son administration.
(Traduit par Docteur Serge Messier)
Introduction
Rabies is a highly fatal zoonotic disease of mammals caused by viruses of the genus Lyssavirus, family Rhabdoviridae. Widespread vaccination programs have effectively eliminated the canine variant of this virus from North America and have drastically reduced incidence of this disease in companion animals and humans over recent decades. In the United States, rabies vaccination is the only immunization required by law for domestic companion animals and is the one vaccine for which duration of immunity studies are required for licensure in the United States (1,2). While 3-year rabies vaccines are recognized in all 50 States, annual or biannual revaccination for rabies is still required by some State municipalities, even though essentially all United Stated Department of Agriculture (USDA) licensed rabies vaccines have a minimum 3-year duration (3).
Scientific data suggest that commercial rabies vaccines confer durations of immunity well beyond 3 y, and that vaccinating dogs against rabies triennially, as nearly all American States require, is unnecessary. In 1992, Aubert demonstrated in France that dogs remained immune to a rabies virus challenge 5 y after vaccination (4). The serological studies of Schultz (5) and Schultz and Conklin (6) showed that immunity to rabies virus was present 7 y after vaccination.
While rabies vaccines are highly efficacious, they are also among the most reactogenic of vaccines. Redundant rabies vaccination exposes dogs to excipients and other compounds unrelated to the vaccine antigens while increasing the needless risk of adverse events. Despite the “gross under-reporting of vaccine-associated adverse events” cited in the 2007 World Small Animal Veterinary Association (WSAVA) Vaccine Guidelinesa research has shown that potent adjuvanted, killed rabies vaccines elicit the most frequent and severe adverse reactions in animals, which emphasizes the importance of vaccinating at intervals only as necessary to confer or maintain immunity to disease (7–25).
Increasingly, veterinary practitioners and animal owners are concerned about balancing the need to protect animals and the public from rabies while reducing the potential for serious adverse events associated with rabies vaccination. Live rabies virus challenge studies documenting the long-term duration of immunity of rabies vaccines beyond 3 y serve the dual purpose of protecting companion animals and the public from rabies, while eliminating redundant vaccination and decreasing the risk of adverse events associated with vaccine adjuvants, antigens, and excipients (4–6,10,11,17,21–25). The American Animal Hospital Association (AAHA) canine vaccine guidelines from 2003–2011 reflect this evolving perspective, and another set has recently been published (26–29).
The present study was undertaken in puppies that received just 2 doses of 2 different licensed canine rabies vaccines in order to assess vaccine efficacy in dogs after live rabies virus challenge more than 5 y later. A second objective was to establish a database upon which rabies vaccines potentially could be licensed with longer durations of immunity in the future.
Materials and methods
The current study protocol followed the strictly defined federal Animal and Plant Health Inspection Service/United States Department of Agriculture (APHIS/USDA) Title 9 CFR standards for licensing rabies vaccines and was conducted in concurrent 5 to 8 y post-vaccination challenge trials (30).
Animals
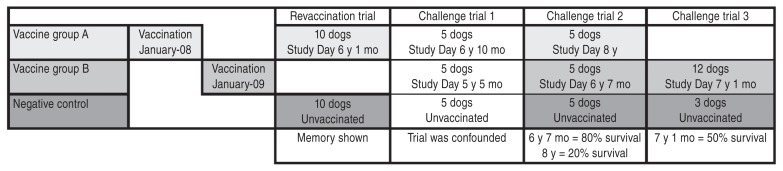
After full approval of the Institutional Animal Care and Use Committee of a USDA-licensed commercial breeding facilityb 100 female beagle puppies were randomly distributed into experimental (rabies vaccinated) and control (rabies unvaccinated) groups. Following assignment to the study, dogs remained in the breeding colony for 5 y and 5 mo to 8 y and 0 mo, under standard husbandry with no further exposure to rabies vaccine or virus. While 100 dogs were originally assigned to the study, only 65 went on to challenge or revaccination trials. The remaining 35 dogs were spayed and adopted as family companions at the end of the study. Dogs were transported to a USDA-licensed biosafety level 3 isolation facilityc for the challenge trials. Due to facility space constraints, challenge trials were not able to contain more than 15 dogs each. In the first and second trials, 10 vaccinated (5 dogs from each vaccine group) and 5 control dogs were challenged. For humane reasons the number of non-vaccinated dogs was decreased, and the older vaccine group was discontinued in the third trial, so that 12 vaccinated dogs and 3 control dogs were challenged. In the non-challenge revaccination trial 10 previously vaccinated and 10 control dogs were used. (see Figure 1, Timeline) The experimental group was a single animal.
Figure 1.
Study Timeline.
Immunization
Vaccinated dogs received 2 doses of rabies vaccine, given 3 wk apart, at 12 and 15 wk of age. The licensed vaccines given were designated Vaccine Ad (which is no longer commercially available), or Vaccine Be (a thimerosol-free product). Both vaccines were labeled for 3-year administration. Study Day 0 was offset between the 2 vaccine groups, with the Vaccine A group starting approximately 1.5 y earlier than Vaccine B. Elapsed time between vaccination and challenge was 5 y 5 mo, 6 y 7 mo, and 7 y 1 mo for Vaccine B, and 6 y 10 mo and 8 y 0 mo for Vaccine A. Trial intervals were scheduled according to the availability of the challenge facility. (see Figure 1, Timeline)
Revaccination trial
Ten dogs that had been previously vaccinated with 2 doses of Vaccine A 6 y and 1 mo earlier, and 10 unvaccinated, age-matched controls, were given a single dose of either Vaccine A or Vaccine Cf (a non-adjuvanted, recombinant feline rabies product). Blood was collected at intervals post-vaccination for detection of anamnestic humoral responses.
Rabies virus challenge trials
The live virulent virus for challenge trials 2 and 3 was feline adapted wild-type rabies (USDA New York strain). Challenge was conducted in a biosafety level 3 facility, under University of Georgia Institutional Animal Care and Use Committee approval.c After sedation and complete anesthesia (oral acepromazine followed by an intramuscular injection of a combination of tiletamine and zolazepamg) 0.5 mL of a 1:1000 dilution of rabies virus was injected into right and left temporalis muscles of each dog. Clinical observation phase extended over 90 d following administration of challenge material. During the first 28 d, all challenged dogs were observed for signs of rabies disease at 8-hour intervals. After the initial 28-day observation, surviving dogs were monitored once daily for the rest of the 90-day period. All dogs that exhibited early signs of rabies virus infection were immediately and humanely euthanized by intravenous administration of euthanasia solution.h Early signs include any of the following: hyper excitability, behavior change, dilation of pupils, photophobia, reduced appetite, inability to drink, vomiting, and/or incoordination.
Sample collection
Serum was collected from all dogs at yearly intervals until time of challenge (data available upon request). For the re-vaccination trial, blood was collected at days 0, 3, 7, and 14 after secondary rabies vaccine administration. During challenge trials, dogs were bled at post-challenge days 0, 4, 12, 26, and 90 (or final) days. All handling procedures during challenge phase were conducted after dogs had been completely anesthetized. Acepromazine was administered orally via “pill pocket” followed by tiletamine/zolazepamg administered intramuscularly via pole syringe. Further procedures to ensure the safety of technicians working with challenged dogs included prior vaccination, proof of protective antibody response against rabies, extensive personal protective gear, and a mandatory “buddy system.” In addition to scheduled blood collection, all dogs were bled at time of euthanasia (final sample), and brain tissue was collected for viral determination.
Assays
Rapid Fluorescent Focus Inhibition Test (RFFIT) of serum samples was completed at an accredited veterinary diagnostic laboratory.i Memory cell response was measured by flow cytometry, and brain tissue was assayed for presence of rabies virus via immunohistochemistry and reverse transcriptase-polymerase chain reaction (RT-PCR) by the challenge facility.c
Results
Challenge trial 1; which included 15 dogs, 5 unvaccinated controls, 5 vaccinates at 5 y 5 mo post-vaccination (Vaccine B), and 5 vaccinates 6 y 10 mo post-vaccination (Vaccine A); was confounded by insufficient virulence of the challenge virus used. Mortality of negative control dogs was 40% (2 of 5 dogs showed signs of rabies virus infection.) Mortality was zero in both groups of vaccinates.
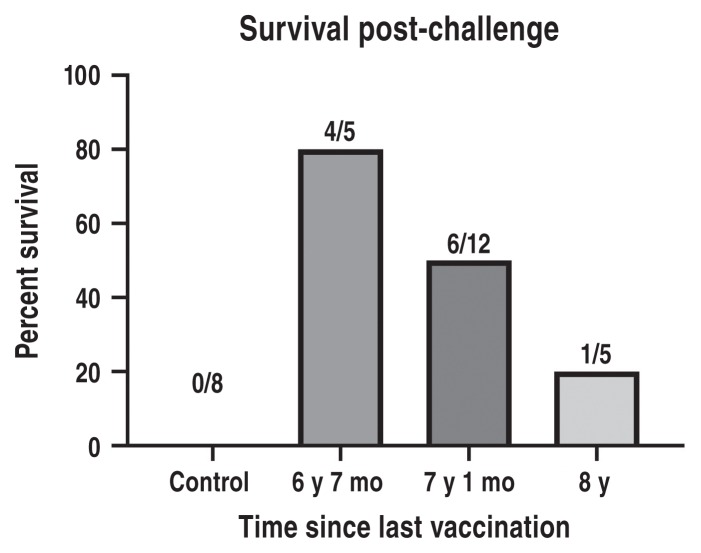
A different rabies challenge virus was obtained for the following challenge trials 2 and 3. All unvaccinated control dogs in both trials showed signs of rabies disease between post-challenge days 13 to 21 and were humanely euthanized, thus proving the virulence of the challenge rabies virus. In trial 2, only 1 of the 5 dogs (20%) [95% confidence interval (CI): 0.5% to 71.6%] vaccinated 8 y previously with Vaccine A showed protection against rabies, while 4 of 5 dogs (80%) (95% CI: 28.4% to 99.5%) vaccinated 6 y 7 mo previously with Vaccine B were protected against the live rabies virus challenge (Figure 2). Memory antibody responses by post-challenge Day 12 correlated strongly with protection (data not shown). As required, the surviving 5 vaccinates were observed for a total of 90 d to detect late development of clinical signs of rabies. No clinical signs occurred, proving successful immunization and protection against rabies in these dogs.
Figure 2.
Percentage survival after challenge with virulent rabies virus at time since last rabies vaccination.
In trial 3, the number of unvaccinated control dogs was decreased for humane reasons, as the virulence of the challenge virus had already been established in the previous trial. Vaccine group A was also excluded from further challenge for humane reasons. Thus, 3 negative control dogs and 12 dogs vaccinated with Vaccine B 7 y and 1 mo earlier were challenged with the same lot of rabies virus as trial 2. Similar to trial 2, all negative control dogs succumbed to rabies virus between post-challenge days 14 to 21 and were humanely euthanized. Six of 12 vaccinates (50%) (95% CI: 21.1% to 78.9%) were protected against challenge and were free of signs of rabies for a total of 90 days post-challenge (Figure 2). As was observed in trial 2, post-challenge day 12 antibody responses were strongly linked with survival.
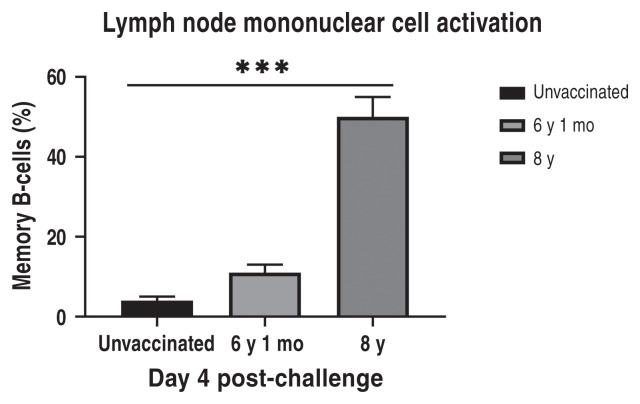
Lymph node mononuclear cells collected at post-challenge days 4 and 12 were tested by flow cytometry to determine the kinetics of immune memory cell responses. A statistically significant increase in memory B-cells was seen in vaccinates at 4 d post-challenge (Figure 3). Rabies virus was detected in brain tissue of all unvaccinated control dogs and vaccinates which showed signs of rabies virus. Rabies virus was not detected in brain tissue from vaccinates which survived until post-challenge day 90 (data not shown).
Figure 3.
Flow cytometry of lymph node mononuclear cells show significant differences in memory B-cells between negative controls and vaccinates at day 4 post-challenge.
*** NO needed.
Memory humoral responses as detected by serology correlated significantly with survival when seen at 12 d post-challenge. Kinetics of antibody response also points to presence of memory B-cell activity and anamnestic response.
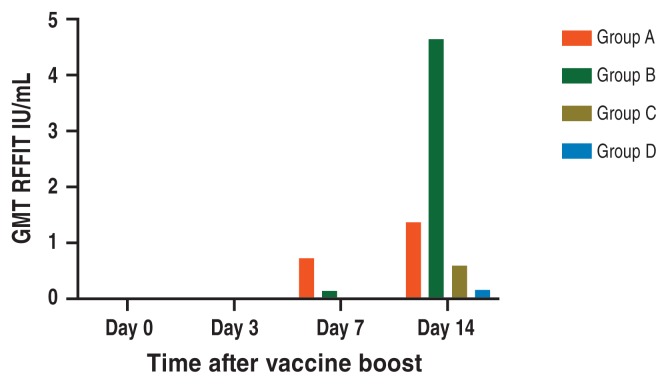
All 20 dogs assigned to the revaccination trial had rabies antibody < 0.5 IU/mL at revaccination day 0, including both those which had been previously vaccinated 6 years and one month earlier and all unvaccinated control dogs. By 14 d after re-vaccination, 90% of the previously vaccinated groups showed antibody responses at or above 0.5 IU/mL, compared to 30% of the naïve dogs. Furthermore, the recombinant rabies productf induced anamnestic responses several fold higher than the killed virus product when given to previously vaccinated dogs. The same recombinant product did not induce any naïve dog to respond above 0.5 IU/mL by study day 14 (Figure 4).
Figure 4.
Average RFFIT titers (IU/mL) after revaccination in dogs previously vaccinated against rabies versus unvaccinated control dogs shows presence of immune memory.
Group A — previously vaccinated, boosted with killed rabies vaccine.
Group B — previously vaccinated, boosted with recombinant rabies vaccine.
Group C — naïve, vaccinated with killed rabies vaccine.
Group D — naïve, vaccinated with recombinant rabies vaccine.
Discussion
At the time of study initiation, Vaccine A was one of the most commonly used canine rabies vaccines available in the United States. However, sometime after study initiation this vaccine was withdrawn from the market, possibly due to corporate merger or other business decision on the part of the manufacturer. Vaccine B continues to be available and was chosen for use in this study because it is free of the preservative thimerosal (mercury salt), which is known to elicit adverse events (2,24).
Challenge trial 1 was confounded when the requisite number of control dogs failed to show clinical signs of infection after challenge as sufficiently virulent rabies challenge virus was not available to the researchers at the 5 y and 5 mo post vaccination mark. In challenge trial 2, 80% survival (4/5 dogs) in animals previously vaccinated with Vaccine B 6 y 7 mo earlier was observed. We believe that had trial 1 been conducted with a fully virulent challenge virus at the 5-year mark, it would have met the USDA 9 CFR ≥ 88% survival requirement.
Results of the revaccination trial indicate presence of immune memory and rapid response to re-exposure to rabies antigen in vaccinates. In the current study, Vaccine Cf, a feline recombinant, non-adjuvanted rabies vaccine, was chosen for extra-label administration to dogs in this trial because only rabies antigen glycoprotein G is expressed by the canarypox-vector virus. This vaccine was therefore unique to both previously vaccinated and unvaccinated groups. It was presumed that after a single dose, Vaccine C would more easily differentiate vaccinates with immune memory than Vaccine A, and this was demonstrated in this trial.
Vaccine C was developed specifically in response to concerns regarding adverse effects following rabies vaccination of cats (31). Furthermore, it has been shown to provide a 3-year duration of immunity in feline vaccinates. Vaccine C was especially promising in our revaccination trial as it was shown to induce very strong antibody responses within 14 days of administration to previously vaccinated dogs (Figure 4), thus warranting further investigation for potential licensing as an adjuvant-free canine rabies booster vaccine containing a lower antigenic mass.
To reduce the risk of adverse events associated with rabies vaccines, and in recognition of the product label instructions that they are for healthy animals, 18 of 50 American States currently have medical exemption clauses in their rabies laws/regulations (2,4,32). Establishing a canine rabies antibody titer standard for protection against rabies would facilitate passage of medical exemptions in more States, enabling veterinarians to write exemptions for seriously ill and immune-compromised dogs. Immunocompromise triggered by disease or corticosteroid therapy in a host animal can result in rabies vaccine failure. Thus, a titer standard would allow practitioners to avoid the dilemma of administering boosters contrary to sound medical practice and vaccine manufacturers’ labeled instructions, while maintaining confidence that exempted animals with a specific antibody level would not pose an increased public health risk.
In cases of potential exposure of dogs and cats to rabies virus, National Association of State Public Health Veterinarians (NASPHV) routinely recommends prospective rabies serology (1,34). A yearly rabies titer would more effectively assess a pet’s level of protection from unknown rabies virus exposures than the current practice of relying on mandated rabies immunization intervals, for which there is no documentation of immune response, or lack thereof in the case of a non-responder or vaccine failure. While the circulating rabies serum virus neutralization (SVN) titer does not last the lifetime of the pet (4,33–35), the data presented here indicate that protective immune memory to rabies virus in previously vaccinated dogs may last 6.5 y.
A review of rabies challenge-studies indicates that there is a positive correlation between rabies SVN titers and the level of protection after virus challenge. Pre-exposure vaccination coupled with an SVN titer at or above 0.5 IU/mL indicates greater assurance of protection than does the animal’s current vaccination status (34). However, a 1988 nationwide study published in the Centers for Disease Control Morbidity and Mortality Weekly Report found that no documented rabies vaccine failures occurred among dogs or cats that had received 2 rabies vaccinations (36). A more recent study by Murray et al (37) found that of the 264 cases of rabies in dogs reported in 21 American States over the years 1997 to 2001, 4.9% (13/264) dogs had some history of rabies vaccination, and 2 of these were considered “currently vaccinated”. However, of the 13 rabid, previously vaccinated dogs, none had received 2 doses of vaccine before rabies exposure, supporting the previous findings reported by the Centers for Disease Controls and Prevention (CDC) (36).
Because the rapid onset of antibody responses after challenge correlated with protection, the current study’s findings support the importance of antibody testing to assure protection of pet dogs instead of relying on revaccination at set intervals as an assumption of protection. Recent importation of rabies-infected dogs into the United States from Egypt (38), gives credence to the consideration of including proof of rabies antibody at or above the World Health Organization (WHO) protective standard as a requirement for import to the continental United States from rabies endemic countries, instead of, or in addition to, a rabies vaccination certificate. Such a requirement is currently in place for importation of dogs to rabies-free areas such as Hawaii.
It is essential to note that rabies antibody testing must be completed by an accredited laboratory, such as the laboratory which completed serology for this study.i Various test kits are becoming available, which may not have been validated by challenge studies. Results generated by such kits or laboratories may be useful for screening purposes only, and must be confirmed by a highly reliable, accredited laboratory in order to determine protection (39,40).
While the current study was not able to prove a protective rabies titer standard, our data showed that immune memory B-cell immunity persists even after serum SVN titers drop below 0. 1 IU/mL, which affords additional presumption of protection should a bite from a rabid animal occur. Nevertheless, the risk of contracting rabies increases after a rabid animal challenge once the titer falls below 0.5 IU/mL. At that point, giving a rabies booster vaccination is the prudent, safe decision (1,3,34). Until rabies vaccine manufacturers license a product that demonstrates more than a 3-year duration of immunity, veterinarians are required by law to follow product label instructions. Currently no published data exist which meet Code of Federal Regulations (CFR) Title 9 Part 113.209 standards for a licensed rabies vaccine product which has demonstrated a duration of immunity greater than 3 y.
Conclusions and clinical relevance
The Rabies Challenge Fund trials have confirmed that rabies vaccine may induce a duration of immunity well beyond 3 y in dogs; that antibody is the most important protective factor against rabies virus; and that anamnestic responses to virulent challenge can be seen in the absence of protective titers in previously vaccinated dogs. We have shown that protection persists in the absence of annual or triennial re-vaccination and that antibody testing of individual pets can be an excellent indicator of protection or lack thereof, although further studies are needed to determine a protective antibody threshold for vaccinated dogs.
These data serve as a foundation meriting further studies to: i) license a rabies vaccine with a vaccination interval of 5 to 6 y, which would enable States to incorporate extended booster intervals into their rabies laws/regulations; ii) develop and license a recombinant, non-adjuvanted rabies booster vaccine for dogs; and iii) establish a protective serum rabies titer standard for dogs. In addition, these data support the dual goals of better and safer rabies vaccination of pet dogs as well as improved public health security.
Acknowledgments
Supported by the Rabies Challenge Fund Charitable Trust, a federally registered 501(c)(3) charitable organization [Fed. EIN # 84-6390682]. The authors declare no conflict of interest. This research project was performed by Drs. Ronald Schultz and Laurie Larson of the University of Wisconsin-Madison School of Veterinary Medicine, in collaboration with Ridglan Farms, Drs. Zhen Fu and Clement Gnanadurai of University of Georgia College of Veterinary Medicine, and the Kansas State Veterinary Rabies Diagnostic Laboratory.
Footnotes
Vaccine Guidelines, World Small Animal Veterinary Association 2007, https://wsava.org/global-guidelines/vaccination-guidelines/ Last accessed 27 February 2020.
Ridglan Farms, Mt. Horeb, Wisconsin 53572, USA.
Dr. Zhen Fang Fu, Department of Pathology, University of Georgia, College of Veterinary Medicine, Athens, Georgia, USA.
Continuum® Rabies (Intervet, Merck Animal Health, Millsboro, DE 19966; no longer commercially available).
IMRAB-TF® (Merial, Athens, Duluth, Georgia 30601, USA).
PureVax ® Rabies (Merial, Athens, Duluth Georgia 30601, USA).
Telazol® (Zoetis, 10 Sylvan Way, Parsippany, New Jersey 07054, USA).
Beuthanasia-D, Intervet Inc (d/b/a Merck Animal Health), Madison, New Jersey 07940, USA.
Kansas State University Rabies Diagnostic Laboratory, Manhattan, Kansas 66503, USA.
References
- 1.Brown CM, Slavinski S, Ettestad P, Sidwa TJ, Sorhage FE. Public veterinary medicine: Public health. Compendium of animal rabies prevention and control, 2016. Nat Assoc of State Public Health Vet. J Am Vet Med Assoc. 2016;248:505–517. doi: 10.2460/javma.248.5.505. [DOI] [PubMed] [Google Scholar]
- 2.Dodds WJ. Rabies virus protection issues and therapy. Global Vaccines Immunol. 2016;1:51–54. [Google Scholar]
- 3.Frana TS, Clough NE, Gatewood DM, Rupprecht CE. Post-marketing surveillance of rabies vaccines for dogs to evaluate safety and efficacy. J Am Vet Med Assoc. 2008;232:1000–1002. doi: 10.2460/javma.232.7.1000. [DOI] [PubMed] [Google Scholar]
- 4.Aubert MF. The practical significance of rabies antibodies in cats and dogs. Sci and Tech Rev Paris. 1992;11:735. doi: 10.20506/rst.11.3.622. [DOI] [PubMed] [Google Scholar]
- 5.Schultz R. Current and future canine and feline vaccination programs. Vet Med. 1998;93:233–254. [Google Scholar]
- 6.Schultz RD, Conklin S. The immune system and vaccines. Compend Contin Educ Prac Vet. 1998;20:5–18. [Google Scholar]
- 7.Wilcock BP, Yager JA. Focal cutaneous vasculitis and alopecia at sites of rabies vaccination in dogs. J Am Vet Med Assoc. 1986;188:1174–1177. [PubMed] [Google Scholar]
- 8.Scott-Moncrieff JC, Azcona-Olivera J, Glickman NW, Glickman LT, HogenEsch H. Evaluation of antithyroglobulin antibodies after routine vaccination in pet and research dogs. J Am Vet Med Assoc. 2002;221:515–521. doi: 10.2460/javma.2002.221.515. [DOI] [PubMed] [Google Scholar]
- 9.Vascellari M, Melchiotti E, Bozza MA, Mutinelli F. Fibrosarcomas at presumed sites of injection in dogs: Characteristics and comparison with non-vaccination site fibrosarcomas and feline post-vaccinal fibrosarcomas. J Vet Med A Physiol Pathol Clin Med. 2003;50:286–291. doi: 10.1046/j.1439-0442.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- 10.Vadala M, Poddighe D, Laurino C, Palmieri B. Severe side effects of vaccines in the veterinary setting. M J Vetr. 2017;2:008. [Google Scholar]
- 11.Shaw CA, Tomljenovic L. Aluminum in the central nervous system (CNS): Toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. 2013;56:304–316. doi: 10.1007/s12026-013-8403-1. [DOI] [PubMed] [Google Scholar]
- 12.Duval D, Giger U. Vaccine-associated immune-mediated hemolytic anemia in the dog. J Vet Int Med. 1996;10:290–295. doi: 10.1111/j.1939-1676.1996.tb02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HogenEsch H, Azcona-Olivera J, Scott-Moncrieff C, Synder PW, Glickman LT. Vaccine-induced autoimmunity in the dog. Adv Vet Med. 1999;41:733–744. doi: 10.1016/s0065-3519(99)80056-1. [DOI] [PubMed] [Google Scholar]
- 14.Dodds WJ. Vaccination protocols for dogs predisposed to vaccine reactions. J Am Anim Hosp Assoc. 2001;37:211–214. doi: 10.5326/15473317-37-3-211. [DOI] [PubMed] [Google Scholar]
- 15.Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001;19:2666–2672. doi: 10.1016/s0264-410x(00)00498-9. [DOI] [PubMed] [Google Scholar]
- 16.Lawson KF, Crawley JF. The ERA strain of rabies vaccine. Can J Comp Med. 1972;36:339–344. [PMC free article] [PubMed] [Google Scholar]
- 17.Israeli E, Agmon-Levin N, Blank M, Shoenfeld Y. Adjuvants and autoimmunity. Lupus. 2009;18:1217–1225. doi: 10.1177/0961203309345724. [DOI] [PubMed] [Google Scholar]
- 18.Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: Current challenges and future approaches. J Pharm Sci. 2009;98:1278–1316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heegaard PM, Dedieu L, Johnson N, et al. Adjuvants and delivery systems in veterinary vaccinology: Current state and future developments. Arch Virol. 2011;156:183–202. doi: 10.1007/s00705-010-0863-1. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhang S, Zhang F, Hu R. Adjuvant activity of Chinese herbal polysaccharides in inactivated veterinary rabies vaccines. Int J Biol Macromol. 2012;50:598–602. doi: 10.1016/j.ijbiomac.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Luján L, Pérez M, Salazar E, et al. Autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA syndrome) in commercial sheep. Immunol Res. 2013;56:317–324. doi: 10.1007/s12026-013-8404-0. [DOI] [PubMed] [Google Scholar]
- 22.Perricone C, Colafrancesco S, Mazor RD, Soriano A, Agmon-Levin N, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA): Unveiling the pathogenic, clinical and diagnostic aspects. J Autoimmun. 2013;47:1–16. doi: 10.1016/j.jaut.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Stejskal V. Mercury-induced inflammation: Yet another example of ASIA syndrome. Isr Med Assoc J. 2013;15:714–715. [PubMed] [Google Scholar]
- 24.Cerpa-Cruz S, Paredes-Casillas P, Landeros Navarro E, Bernard-Medina AG, Martinez-Boilla G, Gutiérrez-Ureña S. Adverse events following immunization with vaccines containing adjuvants. Immunol Res. 2013;56:299–303. doi: 10.1007/s12026-013-8400-4. [DOI] [PubMed] [Google Scholar]
- 25.Shaw CA, Li D, Tomljenovic L. Are there negative CNS impacts of aluminum adjuvants used in vaccines and immunotherapy? Immunotherapy. 2014;6:1055–1071. doi: 10.2217/imt.14.81. [DOI] [PubMed] [Google Scholar]
- 26.American Animal Hospital Association (AAHA) Report of the AAHA Canine Vaccine Task Force: 2003 canine vaccine guidelines, recommendations, and supporting literature. Paul MA, chair, Appel M, Barrett R, et al. J Am Anim Hosp Assoc. 2003 Apr;:28. [PubMed] [Google Scholar]
- 27.American Animal Hospital Association Report of the AAHA Canine Vaccine Task Force: 2006 AAHA canine vaccine guidelines. Paul MA, chair, Carmichael LE, Childers H, et al. J Am Anim Hosp Assoc. 2006 Mar;:28. [Google Scholar]
- 28.American Animal Hospital Association (AAHA) Canine Vaccination Task Force. Welborn LV, DeVries JG, Ford R, et al. 2011 AAHA canine vaccination guidelines. J Am Anim Hosp Assoc. 2011;47:1–42. doi: 10.5326/jaaha-ms-4000. [DOI] [PubMed] [Google Scholar]
- 29.American Animal Hospital Association (AAHA) Canine Vaccination Task Force. Ford R, Larson LJ, Schultz RD, McClure KD, et al. 2017 AAHA canine vaccination guidelines. J Am Anim Hosp Assoc September/October. 2017;53:243–251. doi: 10.5326/JAAHA-MS-6741. [DOI] [PubMed] [Google Scholar]
- 30.Code of Federal Regulations (annual edition). 9 CFR 113.209 — Rabies Vaccine, Killed Virus. Jan 1, 2001. [Last accessed February 25, 2020]. Available from: https://www.govinfo.gov/app/details/CFR-2001-title9-vol1/CFR-2001-title9-vol1-sec113-209.
- 31.Jas D, Coupier C, Toulemonde CE, Guigal PM, Poulet H. Three-year duration of immunity in cats vaccinated with a canarypox-vectored recombinant rabies virus vaccine. Vaccine. 2012;30:6991–6996. doi: 10.1016/j.vaccine.2012.09.068. [DOI] [PubMed] [Google Scholar]
- 32.Rabies Challenge Fund. States with Medical Exemptions in Lieu of Rabies Vaccination. [Last accessed February 27, 2020]. Available from: https://www.rabieschallengefund.org/states-with-medical-exemptions.
- 33.AVMA Council on Biologic and Therapeutic Agents’ report on cat and dog vaccines. Klingborg DJ, Hustead DR, Curry-Galvin E, et al. J Am Vet Med Assoc. 2002;221:1401–1407. doi: 10.2460/javma.2002.221.1401. [DOI] [PubMed] [Google Scholar]
- 34.Moore MC, Davis RD, Kang Q, et al. Comparison of anamnestic responses to rabies vaccination in dogs and cats with current and out-of-date vaccination status. J Am Vet Med Assoc. 2015;246:205–211. doi: 10.2460/javma.246.2.205. [DOI] [PubMed] [Google Scholar]
- 35.Lawson KF, Chiu H, Crosgrey SJ, Matson M, Casey GA, Campbell JB. Duration of immunity in foxes vaccinated orally with ERA vaccine in a bait. Can J Vet Res. 1997;61:39–42. [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control. Morbidity and Mortality Weekly Report. Rabies Prevention — United States, 1991 Recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR. Mar 22, [Last accessed February 25, 2020]. pp. 1–19. 1991/40(RR03) Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/00041987.htm. [PubMed]
- 37.Murray KO, Holmes KC, Hanlon CA. Rabies in vaccinated dogs and cats in the United States, 1997–2001. J Am Vet Med Assoc. 2009;235:691–695. doi: 10.2460/javma.235.6.691. [DOI] [PubMed] [Google Scholar]
- 38.Hercules Y, Bryant NJ, Wallace RM, et al. Rabies in a dog imported from Egypt — Connecticut, 2017. MMWR. 2018;67:1388–1391. doi: 10.15585/mmwr.mm6750a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Servat A, Cliquet F. OIE Reference Laboratory for Rabies, WHO. Collaborative study to evaluate a new ELISA test to monitor the effectiveness of rabies vaccination in domestic carnivores. Virus Res. 2006;120:17–27. doi: 10.1016/j.virusres.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Moore SM. Rabies. Current preventive strategies. [Last accessed February 27, 2020];Vet Clin North Am Small Anim Pract. 2019 Apr 5; doi: 10.1016/j.cvsm.2019.02.014. pii: S0195-5616(19)30044. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0195561619300440?via%3Dihub. [DOI] [PubMed] [Google Scholar]