Abstract
Acute rhinosinusitis in children is a common disorder that is characterized by some or all of the following symptoms: fever, rhinorrhea, nasal congestion, cough, postnasal drainage, and facial pain/headache. It often starts as an upper respiratory tract infection that is complicated by a bacterial infection in which the symptoms worsen, persist, or are particularly severe. The accurate diagnosis of acute rhinosinusitis is challenging because of the overlap of symptoms with other common diseases, heavy reliance on subjective reporting of symptoms by the parents, and difficulties related to the physical examination of the child. Antibiotics are the mainstay of treatment. There is no strong evidence for the use of ancillary therapy. Orbital and intracranial complications may occur and are best treated early and aggressively. This article reviews the diagnosis, pathophysiology, bacteriology, treatment, and complications of acute rhinosinusitis in children.
Keywords: Rhinosinusitis, Acute children, Treatment, Complications, Diagnosis, Symptoms, Pathophysiology, Bacteriology, Prevention, Antibiotics, Intranasal steroids, Complications
Introduction
Rhinosinusitis is a commonly encountered problem in both pediatric and otorhinolaryngologic practices. Acute rhinosinusitis (ARS) in children is diagnosed clinically by symptoms and temporal course, most often in relation to an upper respiratory viral infection. The typical symptoms include two or more of the following symptoms: discolored nasal discharge, nasal blockage/obstruction/congestion, and cough; the duration of the symptoms is usually 12 weeks or less. Recurrent acute rhinosinusitis is defined as frequent episodes of ARS (as defined above) with complete resolution of symptoms between episodes.
Common cold/viral ARS is defined as duration of symptoms for less than 10 days; postviral ARS as increase of symptoms after 5 days or persistent symptoms after 10 days; and suggestive of acute bacterial rhinosinusitis (ABRS) when at least three symptoms/signs among discolored discharge (with unilateral predominance) are present, purulent secretion in cavum nasi, severe local pain (with unilateral predominance), fever (>38 °C), elevated ESR/CRP, and double sickening (i.e., a deterioration after an initial milder phase of illness) [1•].
Diagnosis
The clinical diagnosis of ARS in children is challenging related to the overlap of symptoms with viral upper respiratory tract infections (URI) and allergic rhinitis, common childhood ailments, and the difficulties related to physical examination. The symptoms are often subtle, and the history is usually obtained from the parents and is based on their observation and subjective evaluation. Since many younger children might not tolerate nasal endoscopy, the clinician is often hindered in his/her ability to perform a physical examination and will have to rely primarily on history and a limited exam for appropriate diagnosis.
Symptoms of ARS in children include fever (50–60 %), rhinorrhea (71–80 %), cough (50–80 %), and pain (29–33 %) [2]. In a study involving 69 children between the ages of 3 and 12 years, the investigators used purulent nasal drainage for more than 7 days and abnormal findings in the maxillary sinuses on Water’s projection to establish the diagnosis of ARS. In these children, the most troublesome symptoms were postnasal drip, nasal obstruction, and cough [3]. In a survey of general pediatricians in the USA, the respondents thought that prolonged symptom duration, purulent rhinorrhea, and nasal congestion were very important in the diagnosis of ARS [4].
In children, ARS presents with acute onset of severe symptoms with fever >39 °C, purulent rhinorrhea and facial pain, or, more commonly, as a prolonged URI with chronic cough and nasal discharge. In a study evaluating the relationship between symptoms of acute respiratory infections and objective changes within the sinuses utilizing MRI scans, 60 children (mean age = 5.7 year) who had symptoms for an average of 6 days before scanning were investigated [5]. Close to 60 % of the children had abnormalities in their maxillary and ethmoid sinuses, 35 % in the sphenoid sinuses, and 18 % in the frontal sinuses. A follow-up MRI scan taken 2 weeks later in 26 of the above children with major abnormalities on initial MRI showed a significant reduction in the extent of abnormalities irrespective of resolution of clinical symptoms. Therefore, like in adults, every URI is essentially a self-limited self-resolving episode of rhinosinusitis with common involvement of the paranasal sinuses by the viral process.
Although no good studies are available for support, most clinicians and investigators agree that the diagnosis of bacterial ARS can be made after a viral URI when children have persistent symptoms for ≥10 days without improvement (nasal discharge, daytime cough worsening at night), an abrupt increase in severity of symptoms of a URI after initial improvement, or symptoms that seem more severe than usual (high fever, copious purulent nasal discharge, periorbital edema, and pain) [2, 6, 7].
Distinguishing between ARS and chronic rhinosinusitis (CRS) is based on duration of symptoms. ARS is defined by symptoms lasting <12 weeks with complete resolution. Symptoms lasting >12 weeks without complete resolution are consistent with CRS. A very common clinical scenario in children is that of CRS with URI-induced acute exacerbations.
The cornerstone of the physical exam in children consists of anterior rhinoscopy to examine the middle meatus, inferior turbinates, and mucosal character, and presence or absence of purulent drainage. This is usually accomplished using the largest speculum of an otoscope or, alternatively, a head light and nasal speculum. Topical decongestion may be used to improve visualization. Nasal endoscopy provides for superior visualization of the middle meatus, adenoid bed, and nasopharynx, and is strongly recommended in children who are able to tolerate it. An oral cavity exam may uncover purulent postnasal drainage, cobblestoning of the posterior pharyngeal wall, or tonsillar hypertrophy.
It is usually not necessary to obtain a culture in the context of uncomplicated ARS. However, a culture might be useful in patients who have not responded to conventional medical treatment within 48–72 h, in immune-compromised patients, in the presence of complications, and if the child presents with severe illness and appears toxic [2, 8]. While a maxillary sinus tap will provide the most accurate and reliable results, it is a relatively invasive procedure and is difficult to perform in a child in the office. Alternatively, obtaining cultures from the middle meatus under endoscopic visualization has shown promise in correlating with antral cultures. In children, data regarding the usefulness of this approach are limited and are mostly based on studies in CRS, which is beyond the scope of this review.
The diagnosis of pediatric ARS is generally made on clinical grounds, and imaging should not be used to distinguish ABRS from a viral URI, as the type of clinical symptoms and their duration are sufficient. The most recent recommendations of the American Academy of Pediatrics (AAP) state that contrast-enhanced CT or MRI should be reserved for those patients with suspected complications, especially of the orbit and central nervous system [9•].
Pathophysiology and Bacteriology
ARS is a disorder that involves inflammation of the nasal and paranasal sinus mucosa. The pathophysiology of ARS is multifactorial and involves the interaction between a viral infection, a predisposing condition, and a subsequent inflammatory response in the mucosal lining of the nose and paranasal sinuses. Viruses account for up to 90 % of the causative agents of ARS and include rhinovirus, coronavirus, influenza, parainfluenza, and RSV [10]. Through various mechanisms—such as epithelial damage and cytokine upregulation—viruses activate inflammatory pathways and the parasympathetic nervous system to generate the symptoms of ARS [10]. The inflammatory process leads to edema, engorgement, fluid extravasation, mucus production, and obstruction of the sinus ostium. The ostiomeatal complex (OMC) is the critical anatomic structure in rhinosinusitis in adults and children and is entirely present, though not at full size, in newborns. Mucociliary transport usually moves mucus towards the natural ostia of the sinuses and then into the nasal cavity and the nasopharynx. This motion can be affected and interrupted by mucociliary dysfunction or mucosal inflammation. Ostial obstruction impedes normal ventilation and drainage of the sinuses, which can lead to bacterial infection and ABRS. Conditions that can predispose to, and exacerbate, an inflammatory response include rhinitis (allergic and nonallergic), anatomic variants (septal deviation), coexisting medical conditions (cystic fibrosis, immune deficiency, pregnancy, chronic rhinosinusitis with/without nasal polyps), and environmental factors (smoking, daycare) [11].
Wald et al. recruited children with maxillary sinus opacification documented by Water’s X-ray and performed maxillary sinus taps to obtain cultures from the affected sinuses [12]. The most frequently isolated organisms were Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis [12]. Several studies since then have duplicated these findings and have also shown that the most common organisms responsible for ABRS in children are S. pneumoniae, H. influenzae, M. catarrhalis, Streptococcus pyogenes, and anaerobes [2]. Due to technical constraints, recent estimates of the microbiology of ABRS are based on that of acute otitis media (AOM), a condition with a similar pathogenesis to ABRS. The three most common pathogens recovered from the middle ear fluid of children with AOM from tympanocentesis are the same as those implicated in ABRS: S. pneumoniae, H. influenzae, and M. catarrhalis [9•]. Unlike the visit rate for AOM in children younger than 18 years following the introduction of the heptavalent pneumococcal conjugate vaccine in the USA, the visit rate for ARS has remained stable at 11–14 visits per 1,000 children between 1998 and 2007, based on national hospital and ambulatory care surveys [13]. Hwang et al. performed a retrospective review of pediatric patients requiring surgical intervention for complicated ARS over a 7-year period [14]. They reported Streptococcus viridans as the leading cause of ARS complications. S. viridans was demonstrated in 44 % of the isolates, as opposed to the typical triad of bacteria in only 20 % of the isolates, none of which were found in children with intracranial complications. Anaerobic bacteria have also been isolated in acute infections by Brook and colleagues, but these organisms are seen most frequently in chronic maxillary rhinosinusitis secondary to dental causes. The most common anaerobic bacteria were gram-negative bacilli such as Peptostreptococcus and Fusobacterium [15, 16].
Prevention
Routine childhood vaccination has affected frequency and bacteriology of ABRS and its complications [17]. The H. influenzae type b (Hib) vaccine has been a routine childhood vaccination since 1999. Before its widespread use, Hib was isolated in about 5–10 % of H. influenzae ABRS cases. Nontypeable H. influenzae is now the responsible pathogen in the vast majority of H. influenzae ABRS cases [17]. Before the vaccine, Hib sinusitis was also the leading cause of periorbital and orbital cellulitis. After its introduction, the annual rate of periorbital cellulitis has decreased by nearly 75 %, and the number of cases with a positive culture for Hib was reduced by 70 % [18].
The heptavalent conjugate pneumococcal vaccine (PCV7) has been available for use in children younger than 2 years of age since 2000. More recently in 2010, a 13-valent conjugate pneumococcal vaccine (PCV13) was introduced for use in children under 5 years to increase coverage for the changing pneumococcal population. Although widespread use of PCV7 has significantly decreased pneumococcal invasive disease, there is no strong evidence of a change in the incidence of ABRS. However, it has produced a change in the bacterial flora of the upper airway. In a study by Brook and Gober [19], nasopharyngeal cultures were obtained from children with acute maxillary rhinosinusitis between 1996 and 2000, and between 2001 and 2005. The proportion of S. pneumoniae declined by 18 %, while the proportion of H. influenzae increased by 8 %.
Medical Treatment of ABRS
Antibiotics
Antibiotics are the most frequently used therapeutic agents in ARS. Published trials in children were reviewed in a recent meta-analysis of randomized controlled trials evaluating antibiotic treatment for ARS [20•]. In total, 435 children were included in the trials that were included in the meta-analysis, with a final number of 382 children after attrition. The diagnosis of ARS was based on clinical criteria in two of the studies [21, 22] and clinical and radiologic criteria in the other two [23, 24]. In two of the studies, inclusion of patients with viral URIs was avoided by enrolling patients whose symptoms were of more than 7–10 days duration [21, 23]. The third study extended their enrollment to also include patients with symptoms of at least 3 days duration if the symptoms were severe [22], and the final study did not specify duration of symptoms for inclusion, only “worsening” of symptoms [24]. The intervention period ranged from 10 to 14 days of antibiotic or placebo. Outcome scores were obtained at multiple timepoints, mostly ranging from 3 to 14 days from onset of treatment. Half of the studies demonstrated a significantly greater likelihood of cure with antibiotics over placebo, while the other half failed to show a difference. However, the pooled odds ratio for symptom improvement at 10–14 days favoring the use of antibiotics was 2.0 [20]. Although the meta-analysis provided modest evidence to support use of antibiotics for ARS in children, it was the authors’ opinion that such efficacy had not been adequately demonstrated in the meta-analysis due to the small number of studies and methodological challenges. In a randomized, controlled study, patients 1–15 years of age with clinical and radiographic signs and symptoms of ARS received either a cephalosporin (cefditoren 8–12 mg/kg daily) or amoxicillin/clavulanate (80–90 mg/kg amoxicillin daily) for 14 days [25]. The results show comparable rates of improvement at 14 days: 78.8 % for cefditoren and 84.7 % for amoxicillin/clavulanate. These rates were not statistically different. The median time to improvement was 3 days in both groups, and the rate of diarrhea was significantly higher in the patients treated with amoxicillin/clavulanate (18 %) compared to those treated with cefditoren (4.5 %).
Many of the studies quoted above could be criticized for potentially including patients with ongoing viral URIs and enrolling patients without verification of the diagnosis of acute rhinosinusitis by radiologic tests. However, the results suggest that most cases of uncomplicated acute sinusitis will improve whether treatment is used or not but will improve faster with a higher chance of improvement if treated with antibiotics. Based on this evidence, physicians could recommend symptomatic treatment alone, without the use of antibiotics, for uncomplicated episodes of ARS in children. The AAP practice guidelines recommend antibiotics for bacterial ARS in children with a severe onset or worsening course but recommend either antibiotics or brief outpatient observation for children with persistent illness [9•]. In many instances, children with purulent nasal drainage are sent home from daycare. This creates significant difficulty for working parents who must consequently miss work to care for their children. Whether improving the symptoms of ARS faster by treatment with antibiotics in these children is worth the increased risk of antimicrobial resistance remains to be seen.
When considering antibiotic choices, the AAP recommends amoxicillin at 45 mg/kg/day for uncomplicated ARS in a child who does not attend daycare and who has not received antibiotic therapy within the last 4 weeks [9•]. High-dose amoxicillin therapy (80 to 90 mg/kg/day) is recommended in communities with a high prevalence of nonsusceptible S. pneumoniae. In children with moderate to severe illness, younger than 2 years, attending daycare, or recently treated with antibiotics, high-dose amoxicillin-clavulanate (80 to 90 mg/kg/day of the amoxicillin component) is recommended. Low-dose amoxicillin-clavulanate (45 mg/kg/day) is appropriate for children suspected of harboring β-lactamase-producing M. catarrhalis and H. influenzae, increased isolates of which may be seen within the next several years since the licensure of PCV13. Cephalosporins (cefdinir, cefuroxime, or cefpodoxime) also provide good coverage of typical organisms. Patients with a true type 1 reaction to amoxicillin can also be treated with the abovementioned cephalosporins as recent publications have indicated that the risk of a serious allergic reaction to second- and third-generation cephalosporins in patients with penicillin allergy appears minimal [26–28]. Other options include clindamycin and linezolid (good activity against S. pneumonia but lacks activity against H. influenzae and M. catarrhalis). Recent resistance surveillance data suggest resistance of pneumococcus and H. influenzae to trimethoprim-sulfamethoxazole and azithromycin, to a degree that precludes recommending these agents for the treatment of ABRS in patients with penicillin hypersensitivity [9•].
Intranasal Steroids
In a randomized, placebo-controlled trial, 89 children with ARS were treated with amoxicillin-clavulanate and, additionally, received either budesonide or placebo nasal sprays for 3 weeks [29]. While the children in both groups showed improvement, there were significant additional reductions in the scores of cough and nasal discharge after the second week in the budesonide group compared to placebo. This data suggests an added benefit of intranasal steroids to antibiotics in the treatment of ARS. Many other trials, performed in mixed adult and pediatric populations (usually 12–14 years and older), have shown similar improvements after using an intranasal steroid along with an antibiotic for the treatment of ARS [30, 31]. Therefore, although not overwhelming, there is good evidence to support adding an intranasal steroid to antibiotics in patients being treated for ARS. Finally, in a randomized, placebo-controlled trial in patients older than 12 years with ARS, mometasone 200 mcg twice daily was more effective in controlling symptoms than placebo and amoxicillin [32]. Therefore, there is also some evidence that a high dose of intranasal steroids in older children might be effective as a stand-alone treatment for ARS. Because the appropriate studies have not been performed in that age group, generalizing the use of intranasal steroids as monotherapy for ARS in younger children is not justified. It is important to note, however, that many of these trials suffer from methodological issues.
Ancillary Therapy
Shaikh and colleagues undertook a systematic review of the literature to evaluate the efficacy of decongestants (oral or intranasal), antihistamines, and nasal irrigation in children with clinically diagnosed acute rhinosinusitis [33]. Randomized controlled trials (RCTs) or quasi-RCTs that evaluated children 0–18 years of age with ARS defined as 10–30 days of rhinorrhea, congestion, or daytime cough were included. There were no articles that satisfied the set criteria. The authors concluded that there is no evidence to determine whether the use of the abovementioned agents is efficacious in children with ARS. However, in one randomized, placebo-controlled trial, children treated with standard therapy (antibiotics, mucolytics, and decongestants) and nasal irrigation showed greater improvement in nasal airflow, quality of life, and rate of improvement in total symptom score when compared to children taking standard therapy with placebo [34]. Erdosteine, a mucolytic agent, was investigated in a randomized, placebo-controlled trial [35]. Eighty-one patients with an average age of 8.5 years completed the study. They all had symptoms consistent with ARS. Both treatment groups had an improvement in symptoms on day 14, but there were no statistically significant differences between the active and placebo groups. As with intranasal steroids, there are no formal recommendations about the use of mucolytics, decongestants, and antihistamines. In fact, the AAP specifically states that antihistamines should not be used for the primary indication of ABRS in any child, except in children with typical allergic symptoms with atopy who also have ABRS [9•].
Complications
The complications of rhinosinusitis, which may include orbital and intracranial extension of infection, are quite rare but can be serious medical emergencies. The proximity of the paranasal sinuses to the orbit and the brain allows for spread of infection via a direct route. Extension to the orbit can occur when there is a dehiscent lamina papyracea, through the neurovascular foramina, and via thrombophlebitis of the ophthalmic veins. Hematogenous spread is mainly responsible for the development of intracranial complications, though direct extension can occur due to the proximity of the frontal, ethmoid, and sphenoid sinuses to the cranial vault. Bacteria may spread through the diploic veins of the skull and ethmoid sinuses and seed the adjacent meninges. It is paramount to recognize the incipient stages of these complications and initiate appropriate treatment in a timely fashion. This is especially challenging and critical in the case of intracranial complications in which symptoms may be nonspecific in the early stages but can progress rapidly and have significant ramifications if not recognized and treated promptly. The diagnosis of orbital complications is best achieved by physical exam, supplemented by a contrast-enhanced CT scan (Fig. 1). Magnetic resonance imaging with or without a venogram is preferred for intracranial evaluation.
Fig. 1.
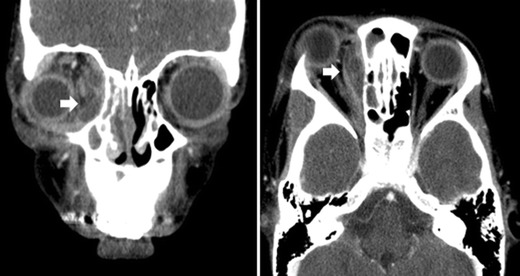
CT of the paranasal sinuses with contrast depicting a subperiosteal abscess. Left panel shows a soft tissue coronal view that shows proptotic right eye and rim-enhancing subperiosteal medial orbital abscess with central lucency (arrow). Right panel shows axial view of the same
Orbital complications, as categorized by the Chandler staging, begin with preseptal cellulitis (stage I) commonly seen with ethmoid rhinosinusitis and presents as edema, erythema, and tenderness of the upper eyelid. Extraocular movements and visual acuity remain intact. Orbital cellulitis (stage II) can have a similar symptom profile but can rapidly progress to subperiosteal (stage III, Fig. 1) or orbital abscesses (stage IV). Mass effect of an abscess can lead to chemosis, exophthalmos, visual impairment, and ophthalmoplegia. Thrombophlebitis of the orbital vessels can lead to cavernous sinus thrombosis (stage V) which is considered both an orbital and intracranial complication. Severe retroorbital pain, high fever, meningitis, ophthalmoplegia, and blindness can all occur if this complication is not treated early and aggressively.
In general, most clinicians treat early preseptal and orbital cellulitis with oral antibiotics targeted to the common pathogens of rhinosinusitis. Advanced preseptal cellulitis (severe lid edema, eye pain, or copious discharge) or lack of improvement with oral antibiotics should be treated with intravenous antibiotics. The transition from intravenous therapy to oral therapy has not been well studied but ranges between 24 and 48 h and hinges on improvement on physical exam. Israele and colleagues published their findings of successful treatment of postseptal cellulitis with targeted intravenous therapy alone [36]. They noted in their findings that orbital cellulitis seems to present more commonly in very young children (under 5 years of age). Similar reports have been published since then in the ophthalmology and otorhinolaryngology head and neck surgery literatures suggesting that medical therapy was very effective in this young age group [37, 38].
Historically, the presence of a subperiosteal or orbital abscess was an indication for surgical drainage in addition to IV therapy. However, studies have shown that empiric IV antibiotic therapy (ampicillin-sulbactam) for 24–48 h followed by oral therapy is curative in many cases [39]. In communities where there is a high prevalence of methicillin-resistant Staphylococcus aureus, antibiotic coverage may be modified accordingly [38]. A retrospective review of children who were either managed medically or surgically proposed the following criteria for medical management in select cases: children with normal vision, pupil, and retina; no ophthalmoplegia; an intraocular pressure of less than 20 mmHg; proptosis not greater than 5 mm; and an abscess width of 4 mm or less [40]. A review of the literature that included one prospective case series and four retrospective studies demonstrated a variable cure rate for medical treatment alone, ranging from 26 to 93 %. Surgical intervention was recommended for the following conditions: decrease of visual acuity, nonmedial abscess, clinical deterioration, and failure to improve within 48 h of antibiotic treatment [41].
In the case of a subperiosteal orbital abscess, intravenous broad-spectrum antibiotics (clindamycin and ceftriaxone) should be initiated and an ophthalmology consultation obtained. If the eye findings are benign, IV antibiotics can be continued and the patient followed daily with serial ophthalmology exams. If the clinical exam does not improve, or eye findings deteriorate, surgical drainage should be pursued, which is usually possible endoscopically. In these cases, an ethmoidectomy is performed with opening of the lamina papyracea and drainage of the subperiosteal abscess. The orbital periosteum is usually not violated. In the rare cases where drainage is not feasible endoscopically, an external ethmoidectomy with drainage is performed.
Intracranial complications include meningitis (most common); cavernous sinus thrombosis; and subdural, intracerebral, and epidural abscesses. The incidence of these complications is between 3 and 10 % [42]. Altman et al. have noted that adolescent males with frontal rhinosinusitis are at an increased risk of rapidly developing intracranial complications and should be aggressively managed. In their series of seven patients, surgical intervention was required with a combined approach with neurosurgery [43]. A review of hospital discharge data across the USA demonstrated that roughly half of all admissions for sinogenic intracranial abscesses were in children ages 10 to 15 [44]. In a large review of cases at two institutions (including adult patients), Clayman et al. found that intracerebral abscesses localized to the frontal lobe were the most common manifestation [45]. Headache and eye pain are the most frequently reported symptoms. Prompt neurological evaluation and imaging should be obtained if any suspicion exists, as intracranial abscesses can be preceded by a quiescent course. Though CT scanning offers excellent visualization of the orbit and bony limits of the brain, MRI is superior in delineating intracranial suppuration [42]. Antibiotic therapy for intracranial complications is appropriate in the setting of meningitis and cavernous sinus thrombosis, but any evidence of abscess by MRI evaluation is an indication for neurosurgical consultation for possible craniotomy in combination with endoscopic sinus surgery [46].
Overall, intraorbital and intracranial complications have a good long-term prognosis, though morbidity does increase with lengthened hospital stays. It is important to keep the age predilection of more severe complications in mind when evaluating children with rhinosinusitis. Early diagnosis and appropriate intervention for these complications can limit management to intravenous antibiotic therapy and circumvent the need for more aggressive treatment modalities.
Conclusions
ARS in children is a common entity and most commonly occurs in the context of a URI. When illness extends beyond 7–10 days, many agree that a bacterial infection is likely. History and type of symptoms as well as their duration are the cornerstone of the clinical diagnosis (Fig. 2). This is supported by characteristic findings on physical examination. In most cases, this is a self-limited process, and treatment with antibiotics has been shown to accelerate resolution. Whether more speedy recovery provides enough benefit as compared to the disadvantage of the risks associated with frequent antibiotic prescriptions remains to be clarified. Intranasal steroids, nasal irrigations, antihistamines, decongestants, and mucolytics are all utilized for ancillary therapy and are supported by variable degrees of evidence. Because the evidence supporting these ancillary therapies is scanty in children, there is no strong formal recommendation for their use. Early recognition and diagnosis of orbital and intracranial complications can limit management to intravenous antibiotic therapy, with surgical intervention reserved for more aggressive cases.
Fig. 2.
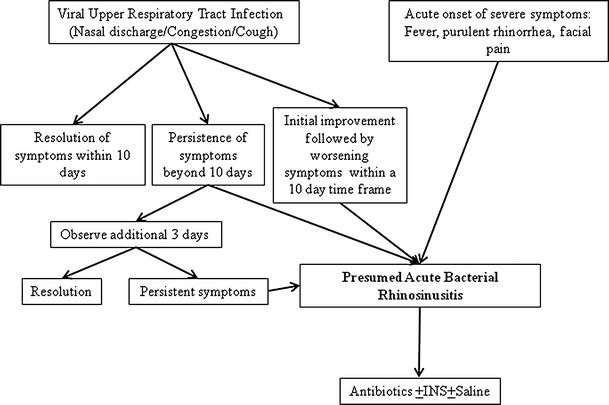
Acute rhinosinusitis. The diagnosis of acute bacterial rhinosinusitis in children is usually made on clinical grounds based on nasal symptoms (nasal drainage, congestion, and cough) and their duration. In the context of an upper respiratory tract infection, most would agree that ABRS can be diagnosed if the symptoms do not resolve within 10 days or worsen after an initial improvement. Some children also present with ABRS without an antecedent URI, and their symptoms tend to be more severe (fever, purulent rhinorrhea, facial pain)
Compliance with Ethics Guidelines
Conflict of Interest
Fuad Baroody has served as a consultant for Acclarent and has received honoraria from Merck & Co. Cheryl C. Nocon declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Rhinosinusitis
Contributor Information
Cheryl C. Nocon, Phone: +1-773-7020080, FAX: +1-773-7026809, Email: Cheryl.Nocon@uchospitals.edu
Fuad M. Baroody, Phone: +1-773-7024790, FAX: +1-773-7026809, Email: fbaroody@surgery.bsd.uchicago.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.•.Fokkens W, Lund V, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps. Rhinol Suppl. 2012;50:48–54. [PubMed] [Google Scholar]
- 2.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps. Rhinol Suppl. 2007;20:1–136. [PubMed] [Google Scholar]
- 3.Lin SW, Wang YH, Lee MY, et al. Clinical spectrum of acute rhinosinusitis among atopic and nonatopic children in Taiwan. In T Pediatr Otorhinolaryngol. 2012;76:70–75. doi: 10.1016/j.ijporl.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 4.McQuillan L, Crane LA, Kempe A. Diagnosis and management of acute sinusitis by pediatricians. Pediatrics. 2009;123:e193–e198. doi: 10.1542/peds.2008-2479. [DOI] [PubMed] [Google Scholar]
- 5.Kristo A, Uhari M, Luotonen J, et al. Paranasal sinus findings in children during respiratory infection evaluated with magnetic resonance imaging. Pediatrics. 2003;111:e586–e589. doi: 10.1542/peds.111.5.e586. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108:798–808. doi: 10.1542/peds.108.3.798. [DOI] [PubMed] [Google Scholar]
- 7.Wald ER. Beginning antibiotics for acute rhinosinusitis and choosing the right treatment. Clin Rev Allergy Immunol. 2006;30:143–151. doi: 10.1385/CRIAI:30:3:143. [DOI] [PubMed] [Google Scholar]
- 8.Clement PA, Bluestone CD, Gordts F, et al. Management of rhinosinusitis in children. Int J Pediatr Otorhinolaryngol. 1999;49(Suppl 1):S95–S100. doi: 10.1016/s0165-5876(99)00141-x. [DOI] [PubMed] [Google Scholar]
- 9.•.Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262–e280. doi: 10.1542/peds.2013-1071. [DOI] [PubMed] [Google Scholar]
- 10.Anon JB, Jacobs MR, Poole MD, et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(Suppl 1):1–45. doi: 10.1016/j.otohns.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eloy P, Poirrier AL, De Dorlodot C, et al. Actual concepts in rhinosinusitis: a review of clinical presentations, inflammatory pathways, cytokine profiles, remodeling, and management. Curr Allergy Asthma Rep. 2011;11:146–162. doi: 10.1007/s11882-011-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wald ER, Milmoe GJ, Bowen A, et al. Acute maxillary sinusitis in children. N Engl J Med. 1981;304:749–754. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro DJ, Gonzales R, Cabana MD, et al. National trends in visit rates and antibiotic prescribing for children with acute sinusitis. Pediatrics. 2011;127:28–34. doi: 10.1542/peds.2010-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang SY, Tan KK. Streptococcus viridians has a leading role in rhinosinusitis complications. Ann Otol Rhinol Laryngol. 2007;116:381–385. doi: 10.1177/000348940711600511. [DOI] [PubMed] [Google Scholar]
- 15.Brook I. Bacteriology of acute and chronic ethmoid sinusitis. J Clin Microbiol. 2005;43:3479–3480. doi: 10.1128/JCM.43.7.3479-3480.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brook I. Microbiology of acute and chronic maxillary sinusitis associated with an odontogenic origin. Laryngoscope. 2005;115:823–825. doi: 10.1097/01.MLG.0000157332.17291.FC. [DOI] [PubMed] [Google Scholar]
- 17.Benninger MS, Manz R. The impact of vaccination on rhinosinusitis and otitis media. Curr Allergy Asthma Rep. 2010;10:411–418. doi: 10.1007/s11882-010-0139-6. [DOI] [PubMed] [Google Scholar]
- 18.Ambati BK, Ambati J, Azar N, et al. Periorbital and orbital cellulitis before and after the advent of Haemophilus influenza type B vaccine. Ophthalmology. 2000;107:1450–1453. doi: 10.1016/s0161-6420(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 19.Brook I, Gober AE. Frequency of recovery of pathogens from the nasopharynx of children with acute maxillary sinusitis before and after the introduction of vaccination with the 7-valent pneumococcal vaccine. Int J Pediatr Otorhinolaryngol. 2007;71:575–579. doi: 10.1016/j.ijporl.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 20.•.Cronin MJ, Khan S, Saeed S. The role of antibiotics in the treatment of acute rhinosinusitis in children: a systematic review. Arch Dis Child. 2013;98:299–303. doi: 10.1136/archdischild-2012-302983. [DOI] [PubMed] [Google Scholar]
- 21.Garbutt JM, Goldstein M, Cellman E, et al. A randomized, placebo-controlled trial of antimicrobial treatment of children with clinically diagnosed acute sinusitis. Pediatrics. 2001;107:619–625. doi: 10.1542/peds.107.4.619. [DOI] [PubMed] [Google Scholar]
- 22.Wald ER, Nash D, Eickhoff J. Effectiveness of amoxicillin/clavulanate potassium in the treatment of acute bacterial sinusitis in children. Pediatrics. 2009;124:9–15. doi: 10.1542/peds.2008-2902. [DOI] [PubMed] [Google Scholar]
- 23.Wald ER, Chiponis D, Ledesma-Medina J. Comparative effectiveness of amoxicillin and amoxicillin-clavulanate potassium in acute paranasal sinus infections in children: a double-blind, placebo-controlled trial. Pediatrics. 1986;77:795–800. [PubMed] [Google Scholar]
- 24.Kristo A, Uhari M, Luotonen J, et al. Cefuroxime axetil versus placebo for children with acute respiratory infection and imaging evidence of sinusitis: a randomized, controlled trial. Acta Paediatr. 2005;94:1208–1213. doi: 10.1111/j.1651-2227.2005.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 25.Poachanukoon O, Kitcharoensakkul M. Efficacy of cefditoren pivoxil and amoxicillin/clavulanate in the treatment of pediatric patients with acute bacterial rhinosinusitis in Thailand: a randomized, investigator-blinded, controlled trial. Clin Ther. 2008;30:1870–1879. doi: 10.1016/j.clinthera.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.DePestel DD, Benninger MS, Danziger L, et al. Cephalosporin use in treatment of patients with penicillin allergies. J Am Pharm Assoc. 2008;48:530–540. doi: 10.1331/JAPhA.2008.07006. [DOI] [PubMed] [Google Scholar]
- 27.Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2005;115:1048–1057. doi: 10.1542/peds.2004-1276. [DOI] [PubMed] [Google Scholar]
- 28.Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: a meta-analysis. Otolaryngol Head Neck Surg. 2007;136:340–347. doi: 10.1016/j.otohns.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Barlan IB, Erkan E, Bakir M, et al. Intranasal budesonide spray as an adjunct to oral antibiotic therapy for acute sinusitis in children. Ann Allergy Asthma Immunol. 1997;78:598–601. doi: 10.1016/S1081-1206(10)63223-1. [DOI] [PubMed] [Google Scholar]
- 30.Meltzer EO, Orgel HA, Backhaus JW, Busse WW, Druce HM, Metzger WJ, et al. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. J Allergy Clin Immunol. 1993;92:812–823. doi: 10.1016/0091-6749(93)90058-n. [DOI] [PubMed] [Google Scholar]
- 31.Meltzer EO, Charous BL, Busse WW, et al. Added relief in the treatment of acute recurrent sinusitis with adjunctive mometasone furoate nasal spray. J Allergy Clin Immunol. 2000;106:630–637. doi: 10.1067/mai.2000.109056. [DOI] [PubMed] [Google Scholar]
- 32.Meltzer EO, Bachert C, Staudinger H. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol. 2005;116:1289–1295. doi: 10.1016/j.jaci.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 33.Shaikh N, Wald ER, Pi M. Decongestants, antihistamines and nasal irrigation for acute sinusitis in children. Cochrane Database Syst Rev. 2010;12:CD007909. doi: 10.1002/14651858.CD007909.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Wang YH, Yang CP, Ku MS, et al. Efficacy of nasal irrigation in the treatment of acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 2009;73:1696–1701. doi: 10.1016/j.ijporl.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Unuvar E, Tamay Z, Yildiz I, et al. Effectiveness of erdosteine, a second generation mucolytic agent, in children with acute rhinosinusitis: a randomized, placebo controlled, double-blinded clinical study. Acta Paediatr. 2010;99:585–589. doi: 10.1111/j.1651-2227.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- 36.Israele V, Nelson JD. Periorbital and orbital cellulitis. Pediatr Infect Dis J. 1987;6:404–410. doi: 10.1097/00006454-198704000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Hermann BW, Forsen JW., Jr Simultaneous intracranial and orbital complications of acute rhinosinusitis in children. Int J Pediatr Otorhinolaryngol. 2004;68:619–625. doi: 10.1016/j.ijporl.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Wald ER. Periorbital and orbital infection. In: Long SS, Pickering LD, Prober CG, editors. Principles and practice of infectious diseases. 2. New York: Churchill Livingstone; 2003. pp. 508–513. [Google Scholar]
- 39.Brown CL, Graham SM, Griffin MC, et al. Pediatric medical subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18:321–327. [PubMed] [Google Scholar]
- 40.Oxford LE, McClay J. Medical and surgical management of subperiosteal orbital abscess secondary to acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 2006;70:1853–1861. doi: 10.1016/j.ijporl.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Coenraad S, Buwalda J. Surgical or medical management of subperiosteal orbital abscess in children: a critical appraisal of the literature. Rhinol. 2009;47:18–23. [PubMed] [Google Scholar]
- 42.Lerner DH, Choi SS, Zalzal GH, et al. Intracranial complications of sinusitis in childhood. Ann Otol Rhinol Laryngol. 1995;104:288–294. doi: 10.1177/000348949510400406. [DOI] [PubMed] [Google Scholar]
- 43.Altman KW, Austin MB, Tom LW, et al. Complications of frontal sinusitis in adolescents: case presentations and treatment options. Int J Pediatr Otorhinolaryngol. 1997;41:9–20. doi: 10.1016/s0165-5876(97)00047-5. [DOI] [PubMed] [Google Scholar]
- 44.Piatt JH., Jr Intracranial suppuration complicating sinusitis among children: an epidemiological and clinical study. J Neurosurg Pediatr. 2011;7:567–574. doi: 10.3171/2011.3.PEDS10504. [DOI] [PubMed] [Google Scholar]
- 45.Clayman GL, Adams GL, Paugh DR, et al. Intracranial complications of paranasal sinusitis: a combined institutional review. Laryngoscope. 1991;101:234–239. doi: 10.1288/00005537-199103000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Lieser JD, Derkay CS. Pediatric sinusitis: when do we operate? Curr Opin Otolaryngol Head Neck Surg. 2005;13:60–66. doi: 10.1097/00020840-200502000-00014. [DOI] [PubMed] [Google Scholar]


