Abstract
Circoviruses are small circular DNA viruses causing severe pig and poultry disease, recently identified in various bat species worldwide. We report the detection and full-genome molecular characterization of a novel bat-associated Circovirus identified in faecal samples of Miniopterus schreibersii bats (Schreiber’s bent-winged bats) from Sardinia, Italy. Full-genomic sequencing revealed a new putative member of Circoviridae family, with a genome size of 2063 nt. Sequencing allowed the characterization of the two major ORFs, inversely arranged, encoding replicase and capsid proteins, as well as the finding of a polythymidine tract within the genome, and highlighted phylogenetic relationships of the novel virus. This is the first report of circovirus in European bats. Giving the high level of genetic diversity of bat circoviruses, it is paramount to further investigate the relationships between these viruses and bats.
Keywords: Circovirus, European bats, Genome characterization, Sardinia, Schreiber’s bent-winged bat
Introduction
Viruses of the Circoviridae family are known to infect a wide range of vertebrates and have been recently identified in various wild species, such as bats, foxes, mink and birds [1–4]. They are non-enveloped, single-stranded DNA viruses with a circular genome of approximately 2.0 kb, and typically cause severe pig and poultry disease [5]. Their genome contains two major open-reading frames (ORFs) encoding replicase (Rep) and capsid (Cap) proteins, inversely arranged. Circoviruses are among the most diverse viruses in the world and have recently undergone a rapid expansion, reaching 70 species classified into genera Circovirus and Cyclovirus [6]. Circovirus have been identified as causative agents of many animal diseases, such as swine pathologies caused by porcine circoviruses, psittacine beak and feather diseases in birds, enteritis epidemics in mink and diseases in fish species [7].
Bats, important virus reservoirs, have been identified as hosts contributing to more than one third of member species in the family Circoviridae, especially thanks to virus discovery through high-throughput sequencing techniques [7]. The first bat-associated cyclovirus (BatACyV) and circovirus (BatACV) were reported in 2010 [8], and in 2011 [9], in US and China, respectively. Recently, other Circovirus species were identified in bats from Asia and South America [5]. To date, there is still no evidence of Circoviruses in European bats. As bats may be natural reservoirs for a large variety of emerging viruses, it is essential to identify and characterize the currently unrecognized viruses circulating in bat populations.
In this study, we report the detection and full-genomic characterization of a novel bat-associated Circovirus from Miniopterus schreibersii bats in Sardinia, Italy. Whole-genome sequencing allowed the characterization of the two major ORFs, encoding Rep and Cap proteins, as well as the finding of a polythymidine (poly-T) tract within the genome. Phylogenetic analyses highlighted the relationships of the novel virus with previously identified BatACVs.
Materials and methods
A viral survey was carried out on different bat species in the island of Sardinia [10]. Cutaneous, oral swabs, and faecal samples were collected from 46 bats, belonging to 15 different species, from 8 different localities distributed in northern and central Sardinia, and tested for the presence of Coronavirus, Herpesvirus, Poxvirus, Papillomavirus and Circovirus [10]. Captures were performed by experts within a regular census program of Sardinian bat populations, between 2017 and 2018. All international, national and institutional guidelines for the care of sampled animals were followed. Faecal samples were non-invasively collected and stored at − 80 °C until use. DNA was extracted from faeces using the QiAmp DNA Mini Kit (Qiagen), according to the manufacturer’s instructions, and stored at − 20 °C. A semi-nested PCR based on degenerate primers targeting a conserved Rep sequence in Circoviruses was used to detect and amplify a fragment of about 400 bp of the Rep gene [7, 9]. PCR amplifications were performed in a 50 µl reaction mix containing 0.2 mM of each dNTPs, 0.2 µM each of Forward and Reverse primers, 1X TaqBuffer and GoTaq (Promega) DNA polymerase. PCR conditions were: 5 min at 95 °C followed by 40 cycles of 1 min at 95 °C, 1 min at 50 °C (56 °C in the second PCR), and 1 min at 72 °C, followed by a final extension time of 10 min at 72 °C. PCR products were purified using DNA Clean & Concentrator purification kit (Zymo Research), and directly sequenced in both forward and reverse directions by BMR Genomics Sequencing Service (Padova, Italy). Sequences were checked against the GenBank database using nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
To obtain the whole viral genome sequence, rolling circle amplification (RCA) technique was combined with PCR. RCA was performed using the TempliPhi 100 Amplification Kit (GE Healthcare), according to Lecis et al. [11]. Briefly, extracted DNA samples were mixed with 10 μl of sample buffer and heated for 3 min at 95 °C. Samples were transferred on ice and mixed with 0.4 μl of TempliPhi enzyme mix, TempliPhi reaction buffer (10 μl), 0.4 μl of 10 mM dNTPs, and random hexamers. RCA reactions were incubated for 16 h at 30 °C, and polymerase was inactivated at 65 °C for 10 min.
Based on the detected 400 bp sequence of Sardinian bat circovirus, specific outward-pointing primers Circos1 and Circos2 (5′-GTACCTCTAGCCTTCTCGCAA-3′ and 5′-GTGGAAGAGTGATGTCACTGT-3′) were newly designed, and combined to amplify the missing genome fragment of about 1600 bp, by long template PCR performed on RCA product. Reaction profile was: 3 min at 94 °C, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 2 min, with a final extension step at 72 °C for another 10 min. PCR amplifications were performed in a 50 µl reaction mix as above, and Taq (Qiagen) DNA polymerase was used. The amplicon was purified with the DNA Clean & Concentrator purification kit (Zymo Research), cloned into the pCR4-TOPO Vector (ThermoFisher Scientific), and the sample was sequenced using universal M13 primers by BMR Genomics Sequencing Service (Padova, Italy). Two specific internal primers were newly designed: CircoBatF3 (5′-AAACACTTCCATGTCCGGCT-3′) and CircoBatF4 (5′-CACTCTGTATGTTGTATTCAG -3′), to obtain the complete sequence by primer-walking technique. Putative ORFs were predicted using NCBI ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/), and the circular ssDNA genome’s map was constructed with PlasMapper version 2.0 (https://wishart.biology.ualberta.ca/PlasMapper/index.html). The stem-loop structures were identified using RNAfold web server (https://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi). Phylogenetic analyses were performed using the Maximum Likelihood (ML) and Maximum Parsimony (MP) methods implemented in MEGA 6 [12], after testing the best fitting evolutionary model to the data. Tamura 3-parameter, discrete Gamma distribution (T92 + G), was selected as the best model to analyze data and to infer evolutionary history. A discrete Gamma distribution was used to model evolutionary rate differences among sites. Bootstrap support values and reliability of internal branches were calculated from 1000 replicates and are located at nodes of the tree.
Results
A total of four faecal samples resulted positive for Circovirus, providing a Rep sequence of about 400 bp, which was deposited in GenBank under accession number MN175690. All faecal samples were collected from M. schreibersii bats, originating from the same location in the north of the Sardinian island (Grotta sa Rocca Ulari, Borutta). BLAST analysis conducted on the first detected Rep sequence revealed high similarity of the obtained sequence to Bat Circovirus Acheng 1, 8 and 27 (KX756987, KX756992, KX756991, [7]), found in Vespertilio spp. in China, with a query coverage of 97% and an identity of 84%.
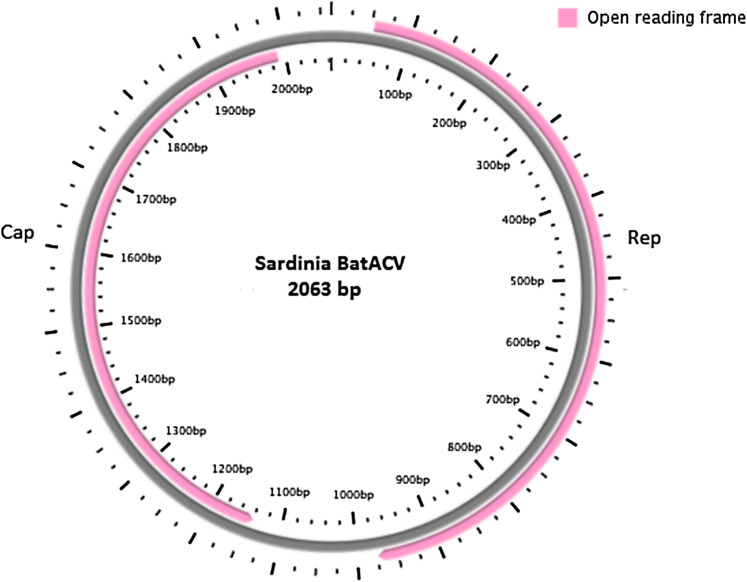
Subsequently, as described above, the complete viral genome sequence was obtained from the positive samples. One full-length circular ssDNA genome of 2063 nt (named Sardinia_BatACV, Fig. 1) was identified. The whole newly characterized genome sequence was deposited in GenBank under accession number MN928506. Predicted ORFs, Rep and Cap (expressing replicase and capsid proteins), were present and inversely arranged, as usual in Circoviruses (Fig. 1). The Rep and Cap nucleotide/protein sequences were 921 nt/306 aa and 849 nt/282 aa long, respectively. Two intergenic regions were also identified: a 5′-IGR of 127 nt and a 3′-IGR of 166 nt. Stem-loop structures were found and considered to initiate the rolling-cycle replication of these circoviruses, as shown in previous studies [5, 9], with a shared conserved nonamer motif (TAGTATTAC) as an open loop located at the 5′ IGR. As reported in a recent study [7], an exceptionally long additional poly-T tract of 58 continuous thymidine was identified in the 3′ IGR of the novel bat Circovirus genome. BLAST analysis conducted on the entire CV genome sequence revealed again high similarity of the obtained sequence to Bat Circovirus Acheng 10, 8 and 27 (KX756989, KX756992, KX756991, [7]), previously identified in Vespertilio spp. in China, with query coverage ranging between 80 and 83% and identity of 82%.
Fig. 1.
Genomic organization (circular ssDNA sequence) of the novel bat Circovirus detected from M. schreibersii faecal samples in Sardinia. The two inversely arranged ORFs encoding replication-associated protein (Rep) and capsid protein (Cap) are shown
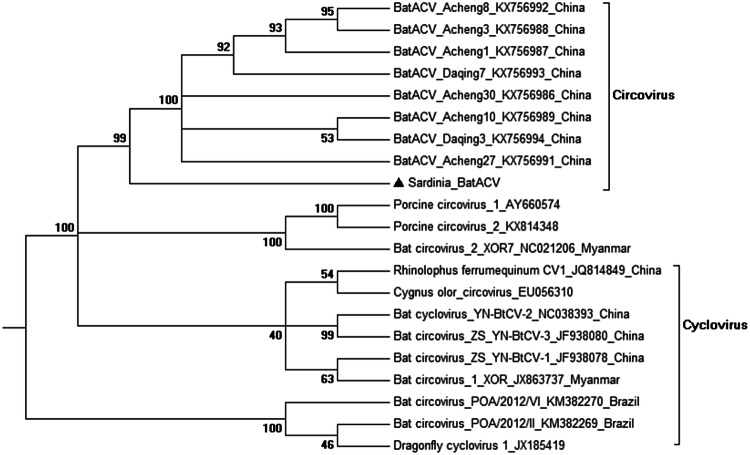
Phylogenetic trees (T92 + G, Fig. 2) clearly showed that the CV detected in Sardinian bats clustered together with these Bat Circoviruses previously identified in China [7], within the genus Circovirus, showing very high bootstrap values. Interestingly, the branch including the BatACV described in this study also appears not phylogenetically distant to porcine CVs (Porcine Circovirus 1 and 2, Fig. 2). On the other hand, bat-associated CVs described in Brazilian bats [5] appear the most distantly related circoviruses.
Fig. 2.
Phylogenetic analysis based on the complete genomes of Circoviruses, including the novel bat Circovirus detected in this study (Sardinia_BatACV) and other 20 sequences from bats and other animal CVs. Representative members within the genera Circovirus and Cyclovirus were included in the analysis (with GenBank accession numbers indicated). Evolutionary analyses were conducted in MEGA6 [12] and bootstrap values are located at nodes of the tree
Discussion
This is the first report of BatACV in Europe, as well as the first identification of these circular ssDNA viruses in European M. schreibersii bat populations (Schreiber’s bent-winged bats). This bat species, listed as Near Threatened by IUCN Red List (https://www.iucnredlist.org/), is found in southern Europe and in Sardinia, typically inhabiting caves and forested habitats, but can also be found in farm environment. Full-genome sequence comparison and phylogenetic analyses clarified the evolutionary relationships of this novel virus within the Circoviridae family, clustering with other previously identified bat-associated circoviruses. Interestingly, a 58 nt polythymidine (poly-T) tract was identified in the 3′ IGR of Sardinian BatACV, which was previously only found, with different nt numbers, in BatACVs from Heilongjiang province, China [7]. These authors tested and showed that poly-T tract did not play any particular role in viral replication, however, the presence of this feature might have implications in the genome post-transcriptional processes, and further research may highlight these mechanisms. As previously discussed [7], it is likely that many CVs or similar circular DNA viruses are circulating in various bat species, and novel viruses thus remain to be identified. BatACVs show an average high level of genetic diversity, as well as a wide distribution. Although it is not known whether BatACVs are associated with disease, however, they are found in a broad bat host range and, as previous works and the present study support, with a wide geographic distribution, spanning from Asia to Europe to South America. Further studies should aim to investigate the possible role of bats in the transmission of circoviruses to other animal species, including porcine or avian hosts, and highlight potential zoonoses risks. Giving the high diversity of bat circoviruses and the high variability and mutation rate of ssDNA circular genomes, it is paramount to further investigate the relationships between CVs and bats, and their relevance in pathogenesis. Future surveillance of BatACVs in Sardinian bat populations should be intensified, as M. schreibersii bats share habitat and caves with many other species (such as Myotis and Rhinolophus spp.), and as cross-species transmission also to terrestrial mammals has been reported [13].
Author contributions
RL and AA conceived the study; MM and EP provided bat samples; RL conducted field and lab study, analyzed sequence data and wrote the manuscript; RZ contributed to lab work; MP and AA reviewed the ms. All authors revised and approved the manuscript.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All international, national and institutional guidelines for the care of sampled animals were followed. Samples were collected under ethical and capture permits: “Autorizzazione Ministero Ambiente e Tutela del Territorio e del Mare 0007134 PNM (08/04/2016, DIV II)”, and “Autorizzazione Regione Autonoma della Sardegna, Assessorato Difesa dell’Ambiente, Det. 7325 Rep. N. 171 (13/04/2016)”.
Informed consent
All the authors consent to publish.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hu D, Zhu C, Wang Y, Ai L, Yang L, Ye F, et al. Virome analysis for identification of novel mammalian viruses in bats from Southeast China. Sci Rep. 2017;7:10917. doi: 10.1038/s41598-017-11384-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bexton S, Wiersma LC, Getu S, van Run PR, Verjans GM, Schipper D, et al. Detection of circovirus in foxes with meningoencephalitis, United Kingdom 2009–2013. Emerg Infect Dis. 2015;21:1205–1208. doi: 10.3201/eid2107.150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lian H, Liu Y, Li N, Wang Y, Zhang S, Hu R. Novel circovirus from mink, China. Emerg Infect Dis. 2014;20:1548–1550. doi: 10.3201/eid2009.140015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinder M, Schmitz A, Peschel A, Korbel R. Complete genome sequence of a novel circovirus from zebra finch. Genome Announc. 2015 doi: 10.1128/genomeA.00560-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lima FE, Cibulski SP, Dos Santos HF, Teixeira TF, Varela AP, Roehe PM, et al. Genomic characterization of novel circular ssDNA viruses from insectivorous bats in Southern Brazil. PLoS ONE. 2015;10:e0118070. doi: 10.1371/journal.pone.0118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ICTV (2017) ICTV Online Report. https://talk.ictvonline.org/taxonomy. Accessed 15 Sep 2018
- 7.Zhu A, Jiang T, Hu T, Mi S, Zhao Z, Zhang F, et al. Molecular characterization of a novel bat-associated circovirus with a poly-T tract in the 3’ intergenic region. Virus Res. 2018;250:95–103. doi: 10.1016/j.virusres.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Victoria JG, Wang C, Jones M, Fellers GM, Kunz TH, et al. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J Virol. 2010;84:6955–6965. doi: 10.1128/JVI.00501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge X, Li J, Peng C, Wu L, Yang X, Wu Y, et al. Genetic diversity of novel circular ssDNA viruses in bats in China. J Gen Virol. 2011;92:2646–2653. doi: 10.1099/vir.0.034108-0. [DOI] [PubMed] [Google Scholar]
- 10.Lecis R, Mucedda M, Pidinchedda E, Pittau M, Alberti A. Molecular identification of Betacoronavirus in bats from Sardinia (Italy): first detection and phylogeny. Virus Genes. 2019;55:60–67. doi: 10.1007/s11262-018-1614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecis R, Tore G, Scagliarini A, Antuofermo E, Dedola C, Cacciotto C, et al. Equus asinus papillomavirus (EaPV1) provides new insights into equine papillomavirus diversity. Vet Microbiol. 2014;170:213–223. doi: 10.1016/j.vetmic.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delwart E, Li L. Rapidly expanding genetic diversity and host range of the Circoviridae viral family and other Rep encoding small circular ssDNA genomes. Virus Res. 2012;164:114–121. doi: 10.1016/j.virusres.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]