Abstract

Allosteric modulation of GPCRs represents an increasingly explored approach in drug development. Due to complex pharmacology, however, the relationship(s) between modulator properties determined in vitro with in vivo concentration-effect phenomena is frequently unclear. We investigated key pharmacological properties of a set of metabotropic glutamate receptor 5 (mGlu5) positive allosteric modulators (PAMs) and their relevance to in vivo concentration–response relationships. These studies identified a significant relationship between in vitro PAM cooperativity (αβ), as well as the maximal response obtained from a simple in vitro PAM concentration–response experiment, with in vivo efficacy for reversal of amphetamine-induced hyperlocomotion. This correlation did not exist with PAM potency or affinity. Data across PAMs were then converged to calculate an in vivo concentration of glutamate putatively relevant to the mGlu5 PAM mechanism of action. This work demonstrates the ability to merge in vitro pharmacology profiles with relevant behavioral outcomes and also provides a novel method to estimate neurotransmitter concentrations in vivo.
Keywords: metabotropic glutamate receptor 5, positive allosteric modulator, antipsychotic, efficacy, cooperativity
Small molecule allosteric modulation has emerged as an attractive strategy for development of novel pharmacotherapies targeting G protein-coupled receptors (GPCRs).1 Modulators act by changing the affinity and/or efficacy of an endogenous orthosteric ligand. Advances in receptor theory have provided detailed mathematical models to describe allosteric ligand–receptor interactions.2−6 The operational model of allosterism (OMA) has proven particularly valuable for quantification of allosteric ligand properties using relatively simple and economical assays common to GPCR research.2,4−8 Beyond incorporation of assay/system-specific parameters, including orthosteric ligand concentration ([A]), affinity (KA), agonism (τA), maximal effect (EM), and slope of the transducer function for occupancy-effect (n), global fitting of the OMA to data from in vitro studies enables determination of modulator-specific parameters: affinity for the unliganded receptor (KB), composite affinity-efficacy cooperativity (αβ) with the orthosteric ligand, and intrinsic agonist activity (τB, if present). Additional data from equilibrium binding assays can also be used to separate composite cooperativity (αβ) into discrete affinity cooperativity (α) and efficacy cooperativity (β) parameters.2 Such modeling approaches represent highly useful and widely recognized strategies for in vitro characterization of novel allosteric modulators.
Many drug discovery programs rely on determinations of modulator potency and efficacy from functional assays involving a modulator concentration–response curve (CRC) in the presence of a fixed concentration of orthosteric agonist.1,3 Chemical optimization efforts then often focus on structure–activity relationships (SAR) using a single potency-based parameter (e.g., pEC50/pIC50 or EC50/IC50). Although this is useful for rank-ordering compounds and may be acceptable in certain contexts, it is generally not ideal for the optimization of allosteric modulators due to the diversity of primary properties (i.e., affinity and cooperativity factors, as well as pharmacological mode switches driven by synthetic chemical changes or metabolism and agonist probe dependence9) that may give rise to similar secondary properties (i.e., EC50 and EMAX) and may mask SAR more relevant to the desired in vivo effect profile. For instance, two modulators with distinct affinity and cooperativity properties (e.g., one with relatively high affinity and low cooperativity, the other with relatively low affinity and high cooperativity) may exhibit similar in vitro potencies yet display substantially different in vivo concentration-effect profiles due to their dissimilar molecular modulation properties. Such observations can preclude construction of reliable in vivo preclinical pharmacokinetic/pharmacodynamic (PK/PD) models, which often serve as the basis for projection of a clinically efficacious human dose level/schedule and must also capably account for frequently present species differences in modulator pharmacology. This approach also fails to account for the potential difference between the in vitro concentration of the orthosteric agonist ligand used in modulator profiling (e.g., fixed EC20 for a PAM) and the in vivo concentration of the agonist in therapeutically relevant tissue(s). Thus, there exists a strong need for improved, holistic in vitro to in vivo translational approaches to GPCR allosteric modulator pharmacology.
Here, we probed data from an internal metabotropic glutamate receptor 5 (mGlu5) positive allosteric modulator (PAM) development program at the Vanderbilt Center for Neuroscience Drug Discovery to determine the relationships between in vitro molecular pharmacological parameter(s), in vivo concentrations, and in vivo efficacy in an acute rodent neurobehavioral paradigm: reversal of amphetamine-induced hyperlocomotion (AHL), a preclinical predictor of antipsychotic efficacy. PAM concentrations in plasma and brain were quantified at the same time point from rat AHL studies employing a discrete dose range of each PAM. Measured and calculated concentrations (plasma total and unbound as well as brain total and unbound) were used to construct individual PAM CRCs. We then attempted to correlate the resulting in vivo EC50 values with relevant in vitro parameters (EC50, KB, and αβ) to determine which modulator properties or profiles may govern in vivo efficacy; this approach was unsuccessful. However, by calculating the in vivo concentration of each PAM needed to reverse AHL to a specific degree (35%), we identified a significant correlation between AHL reversal and modulator cooperativity (composite log αβ) for all examined biophases. Additionally, this method revealed a significant correlation between the concentration of compound needed to reverse AHL and the maximal response (EMAX) elicited in in vitro CRC experiments in the presence of an EC20 concentration of glutamate. Finally, we used these data to predict the in vivo concentration of glutamate potentiated by the PAMs within the AHL paradigm, which converged to a common value. Overall, our data suggest that, at least for the mGlu5 PAMs tested here, specific modulator properties influence efficacy in vivo and point to novel, refined methods that might be exploited during a lead optimization campaign.
Results
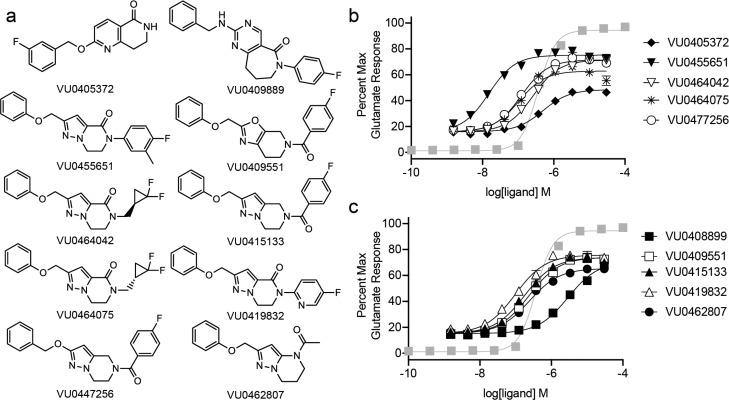
Distinct in Vitro Pharmacology Profiles Are Present within a Set of mGlu5 PAMs
The structures of the 10 mGlu5 PAMs used in these studies are shown in Figure 1a. For each compound, we performed potency determinations in human embryonic kidney (HEK293A) cells expressing the rat mGlu5a receptor (Figure 1b,c, Table 1). In these experiments, the compound was applied to rat mGlu5 cells that had been loaded with the calcium indicator dye, Fluo-4 AM. After 2 min, a concentration of glutamate that elicits an approximately 20% maximal response was added (EC20). The ability of each compound to potentiate the glutamate EC20-induced calcium mobilization response was calculated and normalized to the response elicited by a maximal concentration of glutamate (1 mM). In the absence of glutamate, none of the 10 PAMs exhibited intrinsic agonism in this assay (data not shown).
Figure 1.
Modulator potency curves for enhancement of glutamate stimulation of mGlu5 iCa2+mobilization. (a) Structures of a validation set of 10 mGlu5 PAMs. (b, c) Modulation of glutamate stimulation of iCa2+ mobilization in HEK cells expressing the rat mGlu5a. Increasing concentrations of indicated modulator were added prior to a concentration of glutamate that elicits a 20% maximal response (b, c). The glutamate control curve is shown in gray squares. Data were normalized to the maximal glutamate (1 mM) response and fit with a 4-parameter logistic equation. Data are mean ± s.e.m. of three independent determinations performed in triplicate.
Table 1. In Vitro Pharmacological Properties of mGlu5 PAMs. Data are Mean ± s.e.m. from Three to Six Independent Experiments.
| compound ID | rat pEC50a (EC50, nM) | %Glu maxb | pKBc (KB, μM) | log αβd (αβ) | log τAe (τA) | nf | basalg | Emh |
|---|---|---|---|---|---|---|---|---|
| VU0419832 | 6.94 ± 0.07 (114) | 76.2 ± 1.7 | 5.93 ± 0.02 (1.2) | 0.99 ± 0.07 (9.7) | 0.31 ± 0.03 (2.0) | 1.7 ± 0.3 | 1.1 ± 0.2 | 96.0 ± 1.1 |
| VU0455651 | 7.78 ± 0.03 (16.8) | 75.9 ± 0.3 | 6.96 ± 0.13 (0.11) | 0.97 ± 0.12 (9.2) | 0.27 ± 0.01 (1.9) | 1.9 ± 0.3 | 1.4 ± 0.4 | 97.1 ± 1.1 |
| VU0464075 | 6.94 ± 0.02 (115) | 61.8 ± 1.8 | 6.40 ± 0.04 (0.40) | 0.58 ± 0.04 (3.8) | 0.32 ± 0.02 (2.1) | 2.1 ± 0.2 | 1.8 ± 0.2 | 97.3 ± 0.5 |
| VU0447256 | 6.91 ± 0.03 (123) | 71.9 ± 2.0 | 6.09 ± 0.03 (0.81) | 0.70 ± 0.03 (5.1) | 0.24 ± 0.11 (1.8) | 2.4 ± 0.3 | 1.9 ± 0.3 | 96.6 ± 2.3 |
| VU0415133 | 6.74 ± 0.01 (181) | 73.3 ± 1.0 | 6.14 ± 0.20 (0.72) | 0.76 ± 0.04 (5.7) | 0.29 ± 0.04 (1.9) | 2.1 ± 0.2 | 2.5 ± 0.5 | 98.7 ± 1.5 |
| VU0409551 | 6.65 ± 0.01 (224) | 74.1 ± 1.0 | 5.13 ± 0.12 (7.4) | 0.97 ± 0.10 (9.4) | 0.33 ± 0.02 (2.2) | 2.2 ± 0.2 | 1.7 ± 0.4 | 97.6 ± 1.9 |
| VU0462807 | 6.63 ± 0.04 (236) | 64.9 ± 0.4 | 5.99 ± 0.05 (1.0) | 0.64 ± 0.07 (4.3) | 0.30 ± 0.02 (2.0) | 2.0 ± 0.2 | 1.6 ± 0.3 | 97.2 ± 1.4 |
| VU0464042 | 6.58 ± 0.07 (264) | 71.5 ± 0.7 | 5.90 ± 0.05 (1.3) | 0.76 ± 0.05 (5.7) | 0.29 ± 0.01 (2.0) | 2.1 ± 0.2 | 2.0 ± 0.3 | 97.9 ± 1.5 |
| VU0405372 | 6.34 ± 0.04 (456) | 48.3 ± 2.9 | 5.70 ± 0.10 (2.0) | 0.32 ± 0.04 (2.1) | 0.40 ± 0.02 (2.5) | 2.1 ± 0.2 | 1.5 ± 0.2 | 95.9 ± 0.7 |
| VU0408899 | 5.37 ± 0.07 (4200) | 79.4 ± 3.0 | 4.94 ± 0.04 (12) | 0.78 ± 0.05 (6.1) | 0.36 ± 0.01 (2.3) | 2.3 ± 0.2 | 1.5 ± 0.3 | 97.2 ± 0.7 |
Negative logarithm of the modulator concentration required to induce half-maximal potentiation of an EC20 glutamate response derived from curve fits in Figure 1.
Maximal level of potentiation of EC20 glutamate in the presence of indicated PAM, expressed as a percentage of the response to 1 mM glutamate.
Negative logarithm of the equilibrium dissociation constant of indicated allosteric modulator, estimated by application of the OMA to functional interaction studies with glutamate (Supplemental Figure 1).
Composite cooperativity factor describing the magnitude and direction of the allosteric interaction between indicated modulator and glutamate for iCa2+ mobilization.
Coupling efficiency of glutamate for iCa2+ mobilization in HEK293A-mGlu5 (rat) cells.
Nonlinear transducer function that links agonist occupancy to response.
Basal response of HEK293A-mGlu5 (rat) cells for iCa2+ mobilization in response to vehicle.
Maximum possible system response, expressed as a percentage of the response to 1 mM glutamate.
PAM potency curves are commonly used within discovery programs to efficiently screen for positive modulator activity, with modulator pEC50’s and EMAX used to interpret SAR. However, both pEC50 and EMAX derived from such curves represent composite values that are influenced by the orthosteric agonist concentration and system coupling efficiency,8 as well as modulator-specific parameters: cooperativity (αβ), affinity (KB) and intrinsic efficacy (τB). To demonstrate this point, we simulated the influence of a pure PAM on an orthosteric agonist functional response (Figure 2a) and modeled the resulting potency and EMAX values in the presence of an EC20 concentration of agonist (Figure 2b,c). As can be seen from the simulations, cooperativity influences both potency and EMAX, whereas modulator affinity influences PAM potency alone. We previously demonstrated that an operational model of allosterism can be directly applied to PAM potency curves to estimate modulator KB and αβ.8 However, given the potential for both system and ligand-dependent factors to influence these estimates as simulated in Figure 2, we further profiled each compound using “progressive fold-shift” experiments. In these studies, an escalating range of compound concentrations was applied two min prior to increasing concentrations of glutamate, generating a full glutamate CRC at each concentration of PAM (Supplemental Figure 1, Table 1). Data were fitted using an operational model of allosterism as described in Supplementary Methods to calculate a composite cooperativity value (log αβ) and PAM affinity (logKB).8 As can be seen in Table 1, these compounds exhibited a range of in vitro potencies, cooperativities, and affinities.
Figure 2.
Simulating allosteric modulation of functional responses and the impact of different operational parameters on modulator potency curves. For all simulations, the orthosteric agonist was defined by pKA = 6, log τA = 1, where basal = 0, Em = 100, and n = 1. In these simulations, the maximum response to orthosteric agonist approaches the system Em. The parameters governing the allosteric modulator are noted in the figure for each simulation; in all instances, affinity modulation was neutral (log α = 0) and allosteric agonism was negligible (log τB = −100). (a) Simulating the effect of a pure PAM with different degrees of efficacy cooperativity on the concentration–response curve to an orthosteric agonist. (b) From the simulations in panel a, we plotted the PAM potency at the EC20 orthosteric agonist response. (c) We performed similar simulations where log β values were held constant (equal to 1), but the modulator affinity (pKB) was changed to show the impact on PAM potency.
In Vivo EC50 of mGlu5 PAMs To Reverse Amphetamine-Induced Hyperlocomotion in Rodents Does Not Correlate with in Vitro Modulator Properties
mGlu5 PAMs exhibit efficacy in reversing amphetamine-induced hyperlocomotion (AHL) in rodents,10−13 a model that has predictive validity for typical and atypical antipsychotics in schizophrenia, a long-standing proposed indication for mGlu5 PAMs. During the course of our mGlu5 PAM discovery program, we profiled the compound activity using dose–response analysis in the AHL model (Supplementary Figures 2 and 3). At the end of each AHL study (1.5 h post-administration of test compound), brain and plasma samples were collected and subjected to bioanalysis by LC–MS/MS to quantitate compound concentration (Supplemental Figure 4). We plotted the total and unbound (using fraction unbound values determined in vitro) brain and plasma concentrations measured at each dose at the same time point after administration (Figure 3a and Supplemental Figure 5). We then fitted these data using nonlinear regression to determine the in vivo EC50 (black horizontal line) for total brain (black vertical line), unbound brain (blue vertical line), total plasma (green vertical line), and unbound plasma concentrations (red vertical line, Figure 3a, Supplemental Figure 5, Table 2, and Supplemental Tables 1–3). We subsequently used these in vivo EC50 values to perform correlations with the in vitro-derived parameters: potency, affinity, and cooperativity. As shown in Figure 3b–d (total brain) and Supplemental Table 4 (unbound brain, total plasma, and unbound plasma), there was no significant correlation of in vivo EC50 of the compounds to reverse AHL and modulator properties calculated in vitro.
Figure 3.
mGlu5 PAMs exhibit dose-dependent reversal of AHL that correlates with increasing total and unbound exposure in brain and plasma. (a) % AHL reversal was calculated based on the total number of beam breaks (Supplemental Figures 2 and 3) from n = 5–13. Brain and plasma concentrations were determined at the conclusion of each experiment via LC–MS/MS (Supplemental Figure 4). Log [compound] was plotted versus the % AHL reversal, and data (all are mean ± s.e.m.) were fitted using a three-parameter logistical equation. EC50 values (horizontal black dotted line) in each of the four matrices (total brain, black vertical dashed line; unbound brain, blue vertical dashed line; total plasma, green vertical dashed line; and unbound plasma, red vertical dashed line) were calculated (Table 2 and Supplemental Tables 1–3). (b–d) Correlations are shown for the calculated EC50 value versus the in vitro potency (pEC50, b), the unliganded affinity (log KB, c), and cooperativity (log αβ, d). R2 and p values for each regression line are listed in Supplemental Table 4.
Table 2. Total Brain Curve Fits.
| compound ID | AHL pEC50 (EC50, μM) | R2 of curve fit | span/2b (% reversal at pEC50) | pCBr total at 35% reversalc (μM) |
|---|---|---|---|---|
| VU0419832 | 5.80 ± 0.22 (1.6) | 0.575 | 50 | 6.06 (0.9) |
| VU0455651 | 5.91 ± 0.49 (1.2) | 0.466 | 50 | 6.18 (0.67) |
| VU0464075 | 6.20 ± 0.25 (0.63) | 0.501 | 23.1 | 5.71 (1.9) |
| VU0447256 | 5.13 ± 0.32 (7.5) | 0.462 | 35.1 | 5.13 (7.4) |
| VU0415133 | 5.83 ± 0.22 (1.5) | 0.653 | 33.5 | 5.79 (1.6) |
| VU0409551 | 5.64 ± 0.16 (2.4) | 0.705 | 49.1 | 5.89 (1.3) |
| VU0462807 | 6.11 ± 0.28 (0.78) | 0.442 | 31.2 | 6.01 (0.99) |
| VU0464042 | 5.11 ± 0.21 (7.8) | 0.594 | 43.6 | 5.28 (5.2) |
| VU0405372d | 5.00 ± 0.51 (9.9) | 0.272 | 22.7 | 4.48 (33.2) |
| VU0408899 | 6.82 ± 0.26 (0.15) | 0.588 | 36.2 | 6.85 (0.14) |
Curve shown in Figure 3 and Supplemental Figure 3.
The span was calculated as the distance between the bottom and top of the curve fit and divided by 2 to calculate % reversal at the determined pEC50 concentration. Top was constrained between 0 and 100, for which 100% reversal was defined as the locomotor response for rats treated with vehicle/vehicle. Therefore Span/2 values equal to 50 represent curve fits for which the top plateau was not completely defined by the doses used.
Total brain concentration (CBr Total) at a constant 35% AHL reversal.
For VU0405372, the response to 56.6 mg/kg gave a lower % reversal than both 30 and 100 mg/kg doses. To enable curve fitting, these data were excluded from the fit.
The Concentration of mGlu5 PAM Needed to Induce a 35% Reversal of AHL Correlates with in Vitro Cooperativity but Not Potency or Affinity
The EC50 value derived from the in vivo data curve fits results in a distinct level of “percent reversal” at the determined EC50 for each compound (Table 2 and Supplemental Tables 1–3). Indeed, for multiple compounds, there was an incomplete definition of the sigmoidal concentration–response curve fit, requiring extrapolation of the maximal response due to the limited doses administered in the AHL paradigm. The lower confidence observed for some of these curve fits may contribute to the low correlation observed with the various pharmacological parameters. However, rather than relying on individual EC50 values, another way to compare activity of compounds in vivo would be to compare the concentration needed to induce the same level of response in the pharmacodynamic assay (dashed horizontal red line in Figure 3 and Supplemental Figure 5). For this comparison, we chose a reversal level of 35%, as all compounds reversed AHL to at least this magnitude, but this level was also submaximal for all compounds. For example, at the highest dose tested for VU0405372, the maximal degree of reversal observed was 39%. As shown in Figure 3 and Supplemental Figure 5, the horizontal red lines depicting 35% reversal are below the determined EC50 value for some compounds (VU0419832, VU0455651, VU0409551, VU0464042) and equal to, or above, the determined EC50 value for others (VU0464075, VU0447256, VU0415133, VU0462807, VU0405372, and VU0408899). Upon obtaining these “35% reversal concentrations” (Table 2 and Supplemental Tables 1–3), we correlated these concentrations with the in vitro pEC50, pKB, and log αβ values (Table 1). As with the EC50 studies, we did not observe a significant correlation of the PAM concentrations that induce a reversal level of 35% with in vitro potency or unliganded pKB (calculated) in any matrix (Supplemental Figure 6 and Table 4). This was not surprising for in vitro potency values given that this parameter is a composite that is influenced by multiple system and ligand dependent factors as simulated in Figure 2. In contrast, there was a significant correlation with the cooperativity (log αβ) values for this set of 10 mGlu5 PAMs for all matrices (Figure 4a–d, Table 3).
Figure 4.
The concentration needed to reverse AHL by 35% [AHL35] is significantly correlated with the in vitro determined cooperativity (a–d) and% maximal glutamate response (e–h) across all matrices. Values are listed in Table 2 and Supplemental Tables 1–3. In each panel the red data point corresponds to VU0360172. The reported R2 and p values on these graphs exclude VU0360172. When VU0360172 is included in the regression analyses of log αβ, for total brain R2 = 0.447 and p = 0.0244; for unbound brain R2 = 0.360 and p = 0.051; for total plasma R2 = 0.502 and p = 0.0146; for unbound plasma R2 = 0.373 and p = 0.0459. Including VU0360172 for analyses of PAM EMAX values, for total brain R2 = 0.580 and p = 0.0065; for unbound brain R2 = 0.405 and p = 0.035; for total plasma R2 = 0.619 and p = 0.0041; for unbound plasma R2 = 0.372 and p = 0.047.
Table 3. Linear Regression Fits Correlating the PAM Concentration for 35% AHL Reversal with Indicated in Vitro Pharmacological Parameters.
| pEC50 |
pKB |
log αβ |
Glu
max |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| slope | R2 | p | slope | R2 | p | slope | R2 | p | slope | R2 | p | |
| total brain | –0.152 ± 0.322 | 0.0269 | 0.6510 | –0.144 ± 0312 | 0.0261 | 0.6560 | 0.206 ± 0.086 | 0.4210 | *0.0424 | 10.57 ± 3.32 | 0.5595 | *0.0128 |
| unbound brain | –0.193 ± 0.365 | 0.0336 | 0.6123 | –0.355 ± 0.336 | 0.1224 | 0.3217 | 0.236 ± 0.097 | 0.4258 | *0.0408 | 10.85 ± 4.19 | 0.4567 | *0.0319 |
| total plasma | –0.126 ± 0.367 | 0.0145 | 0.7407 | –0.175 ± 0.351 | 0.0301 | 0.6316 | 0.238 ± 0.095 | 0.4376 | *0.0372 | 12.25 ± 3.62 | 0.5882 | *0.0096 |
| unbound plasma | 0.040 ± 0.376 | 0.0014 | 0.9176 | –0.119 ± 0.360 | 0.0134 | 0.7499 | 0.247 ± 0.095 | 0.4554 | *0.0323 | 10.67 ± 4.33 | 0.4318 | *0.0390 |
Notes significant (p<0.05).
The Concentration of mGlu5 PAM Needed To Induce a 35% Reversal of AHL also Correlates with in Vitro Maximal Response for Potentiation
In a chemical optimization structure–activity relationship campaign for PAMs, a compound CRC in the presence of a low orthosteric agonist concentration (e.g., giving 10–20% of maximal response) is commonly generated to initially triage compounds, as generating multiconcentration progressive fold shift data can be time-consuming and cost-prohibitive. Given that differences in αβ can influence the maximal response obtained in a modulator CRC determination with an EC20 of agonist (Figure 2b), we next assessed whether maximal responses for PAMs could be used instead of αβ to predict in vivo efficacy (% glutamate maximum (Glu Max) values are shown in Table 1(8)). Indeed, when we plotted the concentration needed for AHL reversal with the maximal response obtained in our modulator CRC, we found significant correlation for total and unbound levels in both plasma and brain (Figure 4e–h, Table 3). We next sought to ascertain if αβ and EMAX correlated with in vivo efficacy for an earlier, structurally distinct, “pure PAM”, VU0360172. Our previous report demonstrated that VU0360172 reversed AHL by ∼37% when dosed at 10 mg/kg i.p.,12 reaching total brain and plasma concentrations of 5.1 μM and 10.2 μM (with 98.9% plasma protein bound and 97.1% brain homogenate bound), respectively. Inclusion of VU0360172 data in these analyses retained significant correlations with αβ (Figure 4, red symbol), but not EMAX (data not shown). We reasoned that the lack of correlation with EMAX when VU0360172 may be due to the use of different detection methods for iCa2+ mobilization assays, as well as observations that VU0360172 can elicit allosteric agonist activity in some assays.14,15 Therefore, we re-evaluated VU0360172 in the same cell line and assay system used here. We observed no agonism as well as no significant change in potency (pEC50 of 7.47 ± 0.13, ref (12) versus 7.44 ± 0.29, mean ± s.e.m., unpaired t test, p = 0.0016 n = 3) but a significant difference in the maximal response (86.9 ± 2.7, ref (12), versus 61.1 ± 2.1, mean ± s.e.m., unpaired t test, p = 0.002, n = 3). When these new data were included into the correlation, a significant relationship between EMax and AHL efficacy was now observed. This suggests that the correlation of in vitro parameters may require consistent profiling in the same cell background to afford the highest likelihood of developing a relationship between in vivo efficacy and in vitro properties. Overall, these data suggest that, at least for the compounds tested here, efficacy in the AHL model for mGlu5 PAMs is primarily driven by cooperativity, where compounds with the highest cooperativity require lower PAM concentrations to achieve the same amount of efficacy in AHL. Further, for this target and in vivo model, rank-ordering pure PAMs by EMAX responses derived from primary concentration–response data performed in the same cell line and assay can provide valuable information for a chemical optimization campaign without requiring extensive operational modeling analysis.
Employment of a Range of Allosteric Modulators Can Be Used To Predict in Vivo Endogenous Agonist Concentrations
A final question we asked with these data relates to the orthosteric agonist concentration that is present for potentiation in vivo. This can be an important point for multiple reasons, such as validating a true PAM mechanism in vivo, understanding how potential changes in endogenous agonist (i.e., glutamate) levels that occur within a specific disease context might impact allosteric modulator efficacy, and selecting the most relevant fixed agonist concentration for in vitro PAM CRCs to drive chemical optimization. Thus, we returned to the progressive fold shift data performed in vitro (Supplemental Figure 1) and calculated the responses induced by different PAM concentrations in the presence of EC20, EC30, EC40, EC50, EC60, EC70, and EC80 glutamate concentrations. These data were then transformed into concentration–response curves for each compound, and the concentration of each PAM required to induce a 35% response in vivo was interpolated or extrapolated from these curve fits (Figure 5a, Supplemental Figure 7). These data are, in essence, the predicted glutamate-response relationship for the in vivo efficacious concentration of each PAM (Figure 5b, Supplemental Figure 8). We hypothesized that these concentration–response relationships may reflect the relevant concentration of glutamate being potentiated in vivo. When averaged across the four matrices, for 8 of the 10 compounds tested, the glutamate EC50 values derived from these studies (Figure 5c) were not significantly different. For the two PAMs that did not meet this criterion, we note that the glutamate concentrations were derived from fits for which the unbound brain and plasma data were extrapolated, which may contribute to lower estimates. In addition, VU0408899 also showed greater variability with respect to in vivo concentrations across individual animals than the other compounds. These studies predict that an in vivo glutamate EC50 of approximately 100 nM is relevant for the AHL mechanism and suggest that use of a range of allosteric modulators may be useful for establishing a hypothesis to predict in vivo endogenous agonist concentrations.
Figure 5.

Extrapolation of glutamate concentrations from combined in vitro and in vivo data. (a) From progressive fold shift interactions with glutamate (Supplemental Figure 1), the data were transformed to plot the concentration of glutamate versus the PAM concentration. The PAM concentration required to reverse AHL by 35% is indicated by the vertical lines for each of the four matrices (solid black, total brain; dotted black, total plasma; blue, unbound brain; red, unbound plasma). (b) For each matrix, a glutamate concentration–response curve was generated for the 35% reversal concentration (as shown for VU0419832); the EC50 of these curves for each PAM were then averaged for the four matrices and are plotted in panel c. One way ANOVA with a Tukey’s post hoc test on averaged pEC50 values showed VU0408899 was significantly different (p < 0.05) from VU0455651, VU0447256, VU0464042, whereas VU0409551 was significantly different from VU0447256 and VU0464042.
Discussion
Pharmacologists are often faced with the challenges of “which in vitro parameter is the most important for predicting in vivo efficacy, and how do we best screen and optimize for it?” While many drug discovery programs rely on modulator potency in the presence of a fixed, low concentration of agonist to drive modulator structure–activity relationships,1 it is not clear that this experimental design results in a meaningful interpretation of the in vivo situation. In our own experience, it often does not, suggesting that there are components of the allosteric mechanism that warrant further investigation. The molecular and analytical techniques used to characterize allosteric modulators have expanded in scope, allowing quantification of modulator affinity, cooperativity, and intrinsic activity. Consideration of these factors is increasingly being applied in GPCR allosteric modulator discovery campaigns to dissect structure–activity relationships for the individual components that govern efficacy in vitro.14−19 Qualitative understanding of the importance or role of individual modulator parameters might emerge over time from simple “trial and error”. While such broad observations may prove useful and positively impact the direction or focus of a discovery/optimization program, there remains a need for more rigorous assessment of in vitro molecular pharmacological properties that underscores allosteric modulator in vivo activity. Of the 10 mGlu5 PAMs depicted here, we found a significant correlation of modulator cooperativity and maximal potentiation response (with glutamate) with in vivo efficacy in rat AHL, which extended to another structurally distinct PAM, VU0360172.12,20 This correlation did not exist with PAM potency, highlighting the inherent issues with using PAM EC50 values, which are influenced by system, orthosteric agonist, and allosteric modulator properties,1 as a decision point for compound progression.
Correlations were performed with different in vivo matrices: total and unbound plasma levels as well as total and unbound brain levels. Several matrices were examined with the assumption that a principally linear relationship in one or more matrices, with some degree of uniform bias (i.e., systemic over- or underprediction of in vivo potency from in vitro potency by an empirical factor), would be anticipated. This approach can be used to identify the most-relevant matrix (i.e., biophase) that “drives” in vivo activity (e.g., “unbound plasma-driven efficacy”). Our most robust correlations with log αβ were observed with total brain and total plasma levels. We interpret this to be important from several standpoints. For example, allosteric modulators are generally highly lipophilic, which can often result in difficulties in calculating accurate unbound concentrations relevant to engagement of the target (which itself may be present in a deep tissue biophase). Additionally, each modulator has specific molecular pharmacological properties. The correlation with both total and unbound plasma, as well as total and unbound brain concentrations, with cooperativity and/or maximal potentiation is relevant as dose projection is often determined from plasma levels and, for CNS targets, an understanding of a compound’s ability to penetrate the blood brain barrier (i.e., brain:plasma Kp; Kp,uu). Observations of total brain tissue concentrations “driving” in vivo efficacy may also arise from persistent signaling by internalized GPCR-ligand(s) complex (i.e., “endosomal GPCR signaling”)21 or acting via intracellularly located receptors.22 In such scenarios, total concentrations, versus unbound levels, may better approximate the “effective” concentration due to the receptor-bound compound being sequestered within the tissue’s intracellular compartments. However, this possibility would be predicted to be more likely for target receptors with high expression levels and with compounds exhibiting relatively low to moderate brain distribution.
For a given target and allosteric compound mechanism of action it is necessary to deconvolute the critical parameter/s that are predictive of in vivo efficacy. While we found a correlation between parameters measured in an in vitro calcium assay and in vivo activity, this may not be the case for all in vitro assays, between structurally distinct modulators, among different in vivo behavioral assessments, or between compounds with distinct pharmacokinetic properties (note that here exposure was only determined at a single time point). In these studies, we used a calcium mobilization assay to measure activity of mGlu5 PAMs; in this case, this in vitro assay gave a readout that was correlative to in vivo efficacy. However, this may not be the case for other in vitro assays that may be chosen for compound profiling. Indeed, certain mGlu5 PAMs have different degrees of positive cooperativity with the same agonist depending on the measure of mGlu5 activity.23−25 Additionally, even when interacting at the same site,12 distinct modulators can differentially stabilize receptor conformations. More structurally diverse analogues may also engage completely unique sites on the receptor or have distinct residence times.25,26 These differences in receptor engagement can induce signal bias downstream of mGlu5 activation, which can lead to differential efficacy or side effect profiles in vivo.13,27 This is critically important for mGlu5 PAMs as it relates to their pro-convulsive and neurotoxic effects. While it has been argued for mGlu5 that low levels of potentiation (i.e., < 3-fold shift of glutamate CRC) are needed to avoid adverse effects,28 other parameters may be equally important. Indeed, VU0409551 exhibits robust cooperativity and a unique biased agonism profile in vitro, and yet does not induce neurotoxic effects or seizures at very high in vivo exposures in rodents.13 In contrast to other mGlu5 PAMs, VU0409551 does not induce coupling of mGlu5 to NMDA receptor currents,13 and we hypothesize that this property contributes to its safety margin. These results are consistent with the interpretation that NMDA receptor current potentiation is not responsible for efficacy in AHL. However, had potentiation of NMDA receptor activity been a primary screening assay for mGlu5 PAMs, compounds with favorable safety and efficacy profiles, such as VU0409551, would have been eliminated from further consideration. For this reason, building a data set throughout a program to correlate pharmacokinetic and pharmacodynamic relationships for a series of ligands is crucial, and back-translating this information into the primary assay used for compound assessment would be anticipated to allow integration of the most predictive in vitro assays to assist in prioritizing chemistry and driving SAR. Moreover, clear understanding of in vitro to in vivo concentration–response relationships/mechanisms for a given series of compounds in a particular animal model is vital to maximize the probability of accurate translation to higher species for which predictions often require adjustment for species differences in a candidate drug’s pharmacology.
Here, we found that the maximal potentiation response elicited by an mGlu5 PAM in an in vitro potency experiment could be used as a proxy for αβ and correlated with efficacy in rat AHL. There are two important caveats here that are evident from our simulations (Figure 2). PAM Emax values from a titration assay and cooperativity will only correlate in cases (1) for which there is no intrinsic agonist efficacy and (2) when the system max does not impose a limit on the maximal level of potentiation observed. Indeed, for all PAMs tested, the maximal degree of potentiation achieved was lower than the maximal response elicited by glutamate. There are many examples of in vitro assays in which the maximal response induced in the presence of PAM is higher than the response elicited by a maximal concentration of agonist, defined as the EMAX (i.e., the maximal response induced by agonist in the assay), versus the Em (i.e., the maximal system response), particularly when chimeric or promiscuous G proteins are used to couple a Gi/o-coupled GPCR to various signaling pathways (e.g., see ref (29)). Further, for mGlu5 allosteric modulators, cooperativity with glutamate is primarily mediated by efficacy (β) rather than affinity (α) modulation.8,30 Additionally, in the current study employing this cell line and assay, we did not observe allosteric agonist activity. Indeed, mGlu5 PAM-agonist activity and biased agonism has been linked to adverse effect liability,23,28,31 as well as potential to induce acute desensitization,32 all of which may influence apparent EMAX. With increased pharmacological complexity due to the system or ligands under investigation, using EMAX rather than an operationally determined αβ value may not retain the same predictive ability as was observed here.
An additional note relates to the in vivo assay and time points chosen for compound profiling. In the studies here, we have established that cooperativity appears to drive in vivo efficacy in rat AHL (on an in vivo PAM concentration-normalized basis). However, cooperativity alone may NOT underlie adverse effect liability for mGlu5 PAMs, or, potentially, efficacy in a distinct in vivo readout. This divergence in efficacy versus safety margins may be particularly important for the continued development of mGlu5 PAMs, for which recent reports of on-target adverse effect liability have dampened enthusiasm for an otherwise promising target in the treatment of schizophrenia, cognitive impairments, and autism-associated disorders such as Rett syndrome.34−38 We have not determined the elimination half-life of each compound or the kinetics following oral administration at all doses used. Therefore, the concentrations measured at 1.5 h may not represent the maximum concentrations reached during the study, and concentrations of each compound are likely to change in a compound-specific manner over the AHL time-course. However, our goal was to provide a method that could be integrated efficiently into a chemical optimization screening campaign, and the main objective was to correlate efficacy with in vitro parameters. For this reason, these studies were designed to take a “snapshot” approach regarding exposure from the AHL study to determine if such correlations could be made. Additional pharmacokinetic/pharmacodynamic studies, with complete time-course measurements, would provide further information and refinement to our studies.
If a strong, linear relationship is observed between PAM in vitro and in vivo potencies from a given matrix (e.g., total brain concentrations), we predict that these data may conceptually be used to crudely estimate the relevant orthosteric agonist (e.g., endogenous glutamate) concentration present in vivo (i.e., “tone” in the brain regions/circuitry underlying the pharmacodynamic effect(s)). Here we interpolated or extrapolated the concentration of each PAM needed to induce a fixed in vitro response of each modulator and then calculated the predicted glutamate concentration available for potentiation. Predicted glutamate concentrations were remarkably similar across 8 of the 10 PAMs tested. We note that VU0408899 appears to be an outlier in these studies, and exclusion of this compound would tighten the predicted EC50 glutamate concentration even further. Whether this relates to structural differences between VU0408899 versus the other compounds (and/or presence of pharmacologically active metabolites that may be confounding the analysis) remains to be determined, but these data support the idea that it may be possible to predict endogenous agonist tone by using a range of allosteric modulators.
In summary, we have shown that, for the series of mGlu5 PAMs depicted here, we observe a significant correlation between in vitro cooperativity, as well as maximum potentiation, with in vivo measured concentration levels and in vivo efficacy in an antipsychotic paradigm. Additionally, we propose a mechanism to estimate in vivo concentrations of the endogenous agonist relevant to specific behavioral paradigms. As allosteric modulator programs become more prominent in drug discovery organizations, it is anticipated that building similar data sets for other targets and other pharmacodynamic paradigms (or animal species) will greatly inform efforts to develop safe and effective therapeutics.
Materials and Methods
Compounds
d-Amphetamine hemisulfate was obtained from Sigma-Aldrich. All mGlu5 PAMs were synthesized in house at Vanderbilt University, and detailed methods can be found in the following papers or patents: VU0419372/WO 2012092539; VU0408899,39 VU0409551,10 VU0415133/WO 2012078817; VU0419832/WO 2012083224;40 VU0447256/US 20130345203; VU0455651/WO 2012083224; VU0462807;41 VU0464042/US 20130345204; VU0464075/US 20130345204; VU0360172 (N-cyclobutyl-6-[2-(3-fluorophenyl)ethynyl]-3-pyridinecarboxamide).42
In Vitro Pharmacology
Detailed methods regarding cell culture and in vitro pharmacology/curve fitting are provided in the Supplemental Methods section.
Animal Care and Housing
All in vivo studies were carried out using adult male Sprague–Dawley rats (Harlan, Indianapolis, IN). Animals were group-housed under a 12/12 h light-dark cycle (lights on at 6 AM) with food and water available ad libitum. All animal experiments were approved by the Vanderbilt University Animal Care and Use Committee, and experimental procedures conformed to guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals.
Locomotor Activity Studies in Rats
Open field activity was tested using a SmartFrame Open Field System (Kinder Scientific, San Diego, CA) with a 16 × 16 array of infrared photo beams located 2.5 cm above the floor of the chamber as previously described.9,18 Rats were habituated in an open field for 30 min; vehicle (20% β-cyclodextrin/80% in sterile water), or test compound prepared in vehicle, was administered orally in rats in a volume of 10 mL/kg (doses ranged from 1–56.6 mg/kg). Thirty minutes post-dosing, amphetamine, dissolved in sterile water and administered at a dose of 0.75 mg/kg, was administered subcutaneously at 1 mL/kg. Locomotor activity was recorded for an additional 60 min. Total locomotor activity was calculated as the total number of beam breaks from the time of amphetamine administration [t = 60 min] to the end of the experiment [t = 120 min], where total beam breaks from vehicle treated animals in parallel experiments defined 100% reversal of the amphetamine-induced hyperactivity (defined as 0% reversal).
PK Experiments and Exposure Determinations
Measurement of in vivo total plasma and total brain concentrations of test compounds was performed via quantitative LC–MS/MS bioanalysis of terminal (1.5 h postadministration of each test compound) samples from rat AHL studies essentially according to methods described previously.10,27,43In vivo unbound plasma and unbound brain concentrations for each compound were determined by multiplication of the measured total plasma and total concentrations by each compound’s fraction unbound in plasma (fuplasma) and in brain (fubrain), respectively, which were obtained via in vitro rat plasma protein and rat brain homogenate binding assays using equilibrium dialysis methods described previously.10,27,43
Acknowledgments
We thank the following individuals for contributions throughout the course of this program: Mark Turlington, Chrysa Malosh, Jason Manka, Ya Zhou, Paige Vinson, C. David Weaver, Emily Days, Emma Squire, Atin Lamsal, Kiran Gogi, Sichen Chang, Scott Daniels, Ryan Morrison, Rebecca Lambert, Michael Bubser, Analisa Thompson-Gray, Hilde Lavreysen, José Bartolomé-Nebreda, Susana Conde Ceide, Jesus Alcazar, Carlos Martinez-Viturro, Gregor MacDonald, Thomas Steckler, and Claire Mackie. This work was supported by funding from the NIH (MH062646 and NS031373). K.J.G. is an Australian Research Council Future Fellow [FT170100392].
Glossary
Abbreviations
- AHL
amphetamine-induced hyperlocomotion
- CRC
concentration–response curve
- GPCR
G protein-coupled receptor
- mGlu5
metabotropic glutamate receptor 5
- OMA
operational model of allosterism
- PK/PD
pharmacokinetic/pharmacodynamic
- PAM
positive allosteric modulator
- SAR
structure–activity relationship
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.9b00062.
Detailed methods; unbound brain curve fits; total and unbound plasma curve fits; linear regression fits; additional figures supporting the text; supplemental references (PDF)
Author Contributions
This project arose out of an idea proposed by T.M.B. and C.M.N. K.J.G., R.G.G., M.J.N., and C.M.N. performed/interpreted molecular pharmacology data and simulations using the operational model of allosterism. S.R.R. and C.W.L. supervised chemical synthesis. C.J.K. performed and supervised the AHL experiments and sample collection. T.M.B. analyzed samples for compound exposure. K.J.G. and C.M.N. performed the correlation analyses and glutamate extrapolation with input from T.M.B., M.J.N., and R.G.G. P.J.C. provided discussion of analysis and funding support. K.J.G., T.M.B., R.G.G., and C.M.N. wrote the paper with input from all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Lindsley C. W.; Emmitte K. A.; Hopkins C. R.; Bridges T. M.; Gregory K. J.; Niswender C. M.; Conn P. J. (2016) Practical Strategies and Concepts in GPCR Allosteric Modulator Discovery: Recent Advances with Metabotropic Glutamate Receptors. Chem. Rev. 116, 6707–6741. 10.1021/acs.chemrev.5b00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K.; Sexton P. M.; Christopoulos A. (2007) Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 28, 382–389. 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Christopoulos A. (2014) Advances in G protein-coupled receptor allostery: from function to structure. Mol. Pharmacol. 86, 463–478. 10.1124/mol.114.094342. [DOI] [PubMed] [Google Scholar]
- Hall D. A. (2013) Application of receptor theory to allosteric modulation of receptors. Prog. Mol. Biol. Transl Sci. 115, 217–290. 10.1016/B978-0-12-394587-7.00006-3. [DOI] [PubMed] [Google Scholar]
- Gregory K. J.; Sexton P. M.; Christopoulos A. (2010) Overview of receptor allosterism. Curr. Protoc Pharmacol Chapter 1, Unit 1.21 10.1002/0471141755.ph0121s51. [DOI] [PubMed] [Google Scholar]
- Luttrell L. M.; Kenakin T. P. (2011) Refining efficacy: allosterism and bias in G protein-coupled receptor signaling. Methods Mol. Biol. 756, 3–35. 10.1007/978-1-61779-160-4_1. [DOI] [PubMed] [Google Scholar]
- Gould R. W.; Nedelcovych M. T.; Gong X.; Tsai E.; Bubser M.; Bridges T. M.; Wood M. R.; Duggan M. E.; Brandon N. J.; Dunlop J.; Wood M. W.; Ivarsson M.; Noetzel M. J.; Daniels J. S.; Niswender C. M.; Lindsley C. W.; Conn P. J.; Jones C. K. (2016) State-dependent alterations in sleep/wake architecture elicited by the M4 PAM VU0467154 - Relation to antipsychotic-like drug effects. Neuropharmacology 102, 244–253. 10.1016/j.neuropharm.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory K. J.; Noetzel M. J.; Rook J. M.; Vinson P. N.; Stauffer S. R.; Rodriguez A. L.; Emmitte K. A.; Zhou Y.; Chun A. C.; Felts A. S.; Chauder B. A.; Lindsley C. W.; Niswender C. M.; Conn P. J. (2012) Investigating metabotropic glutamate receptor 5 allosteric modulator cooperativity, affinity, and agonism: enriching structure-function studies and structure-activity relationships. Mol. Pharmacol. 82, 860–875. 10.1124/mol.112.080531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keov P.; Sexton P. M.; Christopoulos A. (2011) Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60, 24–35. 10.1016/j.neuropharm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Conde-Ceide S.; Martinez-Viturro C. M.; Alcazar J.; Garcia-Barrantes P. M.; Lavreysen H.; Mackie C.; Vinson P. N.; Rook J. M.; Bridges T. M.; Daniels J. S.; Megens A.; Langlois X.; Drinkenburg W. H.; Ahnaou A.; Niswender C. M.; Jones C. K.; Macdonald G. J.; Steckler T.; Conn P. J.; Stauffer S. R.; Bartolome-Nebreda J. M.; Lindsley C. W. (2015) Discovery of VU0409551/JNJ-46778212: An mGlu5 Positive Allosteric Modulator Clinical Candidate Targeting Schizophrenia. ACS Med. Chem. Lett. 6, 716–720. 10.1021/acsmedchemlett.5b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory K. J.; et al. (2013) J. Pharmacol. Exp. Ther. 347, 438–457. 10.1124/jpet.113.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook J. M.; Tantawy M. N.; Ansari M. S.; Felts A. S.; Stauffer S. R.; Emmitte K. A.; Kessler R. M.; Niswender C. M.; Daniels J. S.; Jones C. K.; Lindsley C. W.; Conn P. J. (2015) Relationship between in vivo receptor occupancy and efficacy of metabotropic glutamate receptor subtype 5 allosteric modulators with different in vitro binding profiles. Neuropsychopharmacology 40, 755–765. 10.1038/npp.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook J. M.; Xiang Z.; Lv X.; Ghoshal A.; Dickerson J. W.; Bridges T. M.; Johnson K. A.; Foster D. J.; Gregory K. J.; Vinson P. N.; Thompson A. D.; Byun N.; Collier R. L.; Bubser M.; Nedelcovych M. T.; Gould R. W.; Stauffer S. R.; Daniels J. S.; Niswender C. M.; Lavreysen H.; Mackie C.; Conde-Ceide S.; Alcazar J.; Bartolome-Nebreda J. M.; Macdonald G. J.; Talpos J. C.; Steckler T.; Jones C. K.; Lindsley C. W.; Conn P. J. (2015) Biased mGlu5-Positive Allosteric Modulators Provide In Vivo Efficacy without Potentiating mGlu5Modulation of NMDAR Currents. Neuron 86, 1029–1040. 10.1016/j.neuron.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berizzi A. E.; Bender A. M.; Lindsley C. W.; Conn P. J.; Sexton P. M.; Langmead C. J.; Christopoulos A. (2018) Structure-Activity Relationships of Pan-Galphaq/11 Coupled Muscarinic Acetylcholine Receptor Positive Allosteric Modulators. ACS Chem. Neurosci. 9, 1818–1828. 10.1021/acschemneuro.8b00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallagnol J. C. C.; Khajehali E.; van der Westhuizen E. T.; Jorg M.; Valant C.; Goncalves A. G.; Capuano B.; Christopoulos A.; Scammells P. J. (2018) Synthesis and Pharmacological Evaluation of Heterocyclic Carboxamides: Positive Allosteric Modulators of the M1Muscarinic Acetylcholine Receptor with Weak Agonist Activity and Diverse Modulatory Profiles. J. Med. Chem. 61, 2875–2894. 10.1021/acs.jmedchem.7b01812. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Moritz A. E.; Keck T. M.; Bonifazi A.; Ellenberger M. P.; Sibley C. D.; Free R. B.; Shi L.; Lane J. R.; Sibley D. R.; Newman A. H. (2017) Synthesis and Pharmacological Characterization of Novel trans-Cyclopropylmethyl-Linked Bivalent Ligands That Exhibit Selectivity and Allosteric Pharmacology at the Dopamine D3 Receptor (D3R). J. Med. Chem. 60, 1478–1494. 10.1021/acs.jmedchem.6b01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y.; Goldfeld D. A.; Moo E. V.; Sexton P. M.; Christopoulos A.; McCammon J. A.; Valant C. (2016) Accelerated structure-based design of chemically diverse allosteric modulators of a muscarinic G protein-coupled receptor. Proc. Natl. Acad. Sci. U. S. A. 113, E5675–5684. 10.1073/pnas.1612353113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry S. N.; Jorg M.; Lim H.; Vinh N. B.; Sexton P. M.; Capuano B.; Christopoulos A.; Lane J. R.; Scammells P. J. (2016) 4-Phenylpyridin-2-one Derivatives: A Novel Class of Positive Allosteric Modulator of the M1Muscarinic Acetylcholine Receptor. J. Med. Chem. 59, 388–409. 10.1021/acs.jmedchem.5b01562. [DOI] [PubMed] [Google Scholar]
- Shonberg J.; Draper-Joyce C.; Mistry S. N.; Christopoulos A.; Scammells P. J.; Lane J. R.; Capuano B. (2015) Structure-activity study of N-((trans)-4-(2-(7-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)-1H-ind ole-2-carboxamide (SB269652), a bitopic ligand that acts as a negative allosteric modulator of the dopamine D2 receptor. J. Med. Chem. 58, 5287–5307. 10.1021/acs.jmedchem.5b00581. [DOI] [PubMed] [Google Scholar]
- Noetzel M. J.; Rook J. M.; Vinson P. N.; Cho H. P.; Days E.; Zhou Y.; Rodriguez A. L.; Lavreysen H.; Stauffer S. R.; Niswender C. M.; Xiang Z.; Daniels J. S.; Jones C. K.; Lindsley C. W.; Weaver C. D.; Conn P. J. (2012) Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol. Pharmacol. 81, 120–133. 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D.; Nikolaev V. O.; Persani L.; Lohse M. J. (2010) Signaling by internalized G-protein-coupled receptors. Trends Pharmacol. Sci. 31, 221–228. 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Jong Y. J.; Sergin I.; Purgert C. A.; O’Malley K. L. (2014) Location-dependent signaling of the group 1 metabotropic glutamate receptor mGlu5. Mol. Pharmacol. 86, 774–785. 10.1124/mol.114.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengmany K.; Singh J.; Stewart G. D.; Conn P. J.; Christopoulos A.; Gregory K. J. (2017) Biased allosteric agonism and modulation of metabotropic glutamate receptor 5: Implications for optimizing preclinical neuroscience drug discovery. Neuropharmacology 115, 60–72. 10.1016/j.neuropharm.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Rodriguez A. L.; Conn P. J. (2005) Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J. Pharmacol. Exp. Ther. 315, 1212–1219. 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]
- Noetzel M. J.; Gregory K. J.; Vinson P. N.; Manka J. T.; Stauffer S. R.; Lindsley C. W.; Niswender C. M.; Xiang Z.; Conn P. J. (2013) A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. Mol. Pharmacol. 83, 835–847. 10.1124/mol.112.082891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Goudet C.; Pin J. P.; Conn P. J. (2008) N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybe nzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol. Pharmacol. 73, 909–918. 10.1124/mol.107.040097. [DOI] [PubMed] [Google Scholar]
- Gregory K. J.; Herman E. J.; Ramsey A. J.; Hammond A. S.; Byun N. E.; Stauffer S. R.; Manka J. T.; Jadhav S.; Bridges T. M.; Weaver C. D.; Niswender C. M.; Steckler T.; Drinkenburg W. H.; Ahnaou A.; Lavreysen H.; Macdonald G. J.; Bartolome J. M.; Mackie C.; Hrupka B. J.; Caron M. G.; Daigle T. L.; Lindsley C. W.; Conn P. J.; Jones C. K. (2013) N-aryl piperazine metabotropic glutamate receptor 5 positive allosteric modulators possess efficacy in preclinical models of NMDA hypofunction and cognitive enhancement. J. Pharmacol. Exp. Ther. 347, 438–457. 10.1124/jpet.113.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier-Batteur S.; Hutson P. H.; Menzel K.; Uslaner J. M.; Mattson B. A.; O’Brien J. A.; Magliaro B. C.; Forest T.; Stump C. A.; Tynebor R. M.; Anthony N. J.; Tucker T. J.; Zhang X. F.; Gomez R.; Huszar S. L.; Lambeng N.; Faure H.; Le Poul E.; Poli S.; Rosahl T. W.; Rocher J. P.; Hargreaves R.; Williams T. M. (2014) Mechanism based neurotoxicity of mGlu5 positive allosteric modulators--development challenges for a promising novel antipsychotic target. Neuropharmacology 82, 161–173. 10.1016/j.neuropharm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Niswender C. M.; Johnson K. A.; Weaver C. D.; Jones C. K.; Xiang Z.; Luo Q.; Rodriguez A. L.; Marlo J. E.; de Paulis T.; Thompson A. D.; Days E. L.; Nalywajko T.; Austin C. A.; Williams M. B.; Ayala J. E.; Williams R.; Lindsley C. W.; Conn P. J. (2008) Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol. Pharmacol. 74, 1345–1358. 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengmany K.; Hellyer S. D.; Albold S.; Wang T.; Conn P. J.; May L. T.; Christopoulos A.; Leach K.; Gregory K. J. (2019) Kinetic and system bias as drivers of metabotropic glutamate receptor 5 allosteric modulator pharmacology. Neuropharmacology 149, 83–96. 10.1016/j.neuropharm.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook J. M.; Noetzel M. J.; Pouliot W. A.; Bridges T. M.; Vinson P. N.; Cho H. P.; Zhou Y.; Gogliotti R. D.; Manka J. T.; Gregory K. J.; Stauffer S. R.; Dudek F. E.; Xiang Z.; Niswender C. M.; Daniels J. S.; Jones C. K.; Lindsley C. W.; Conn P. J. (2013) Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol. Psychiatry 73, 501–509. 10.1016/j.biopsych.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer S. D.; Albold S.; Sengmany K.; Singh J.; Leach K.; Gregory K. J. (2019) Metabotropic glutamate receptor 5 (mGlu5)-positive allosteric modulators differentially induce or potentiate desensitization of mGlu5 signaling in recombinant cells and neurons. J. Neurochem 151, 301. 10.1111/jnc.14844. [DOI] [PubMed] [Google Scholar]
- Stansley B. J.; Conn P. J. (2018) The therapeutic potential of metabotropic glutamate receptor modulation for schizophrenia. Curr. Opin. Pharmacol. 38, 31–36. 10.1016/j.coph.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti R. G.; Senter R. K.; Rook J. M.; Ghoshal A.; Zamorano R.; Malosh C.; Stauffer S. R.; Bridges T. M.; Bartolome J. M.; Daniels J. S.; Jones C. K.; Lindsley C. W.; Conn P. J.; Niswender C. M. (2016) mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Hum. Mol. Genet. 25, 1990–2004. 10.1093/hmg/ddw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosin N.; Fernandez-Enright F.; Lum J. S.; Newell K. A. (2017) Shifting towards a model of mGluR5 dysregulation in schizophrenia: Consequences for future schizophrenia treatment. Neuropharmacology 115, 73–91. 10.1016/j.neuropharm.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Millan M. J.; Rivet J. M.; Gobert A. (2016) The frontal cortex as a network hub controlling mood and cognition: Probing its neurochemical substrates for improved therapy of psychiatric and neurological disorders. J. Psychopharmacol. 30, 1099–1128. 10.1177/0269881116672342. [DOI] [PubMed] [Google Scholar]
- Homayoun H.; Moghaddam B. (2010) Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur. J. Pharmacol. 639, 33–39. 10.1016/j.ejphar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Bartolome-Nebreda J. M.; Conde-Ceide S.; Delgado F.; Iturrino L.; Pastor J.; Pena M. A.; Trabanco A. A.; Tresadern G.; Wassvik C. M.; Stauffer S. R.; Jadhav S.; Gogi K.; Vinson P. N.; Noetzel M. J.; Days E.; Weaver C. D.; Lindsley C. W.; Niswender C. M.; Jones C. K.; Conn P. J.; Rombouts F.; Lavreysen H.; Macdonald G. J.; Mackie C.; Steckler T. (2013) Dihydrothiazolopyridone derivatives as a novel family of positive allosteric modulators of the metabotropic glutamate 5 (mGlu5) receptor. J. Med. Chem. 56, 7243–7259. 10.1021/jm400650w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Ceide S.; Alcazar J.; Alonso de Diego S. A.; Lopez S.; Martin-Martin M. L.; Martinez-Viturro C. M.; Pena M. A.; Tong H. M.; Lavreysen H.; Mackie C.; Bridges T. M.; Daniels J. S.; Niswender C. M.; Jones C. K.; Macdonald G. J.; Steckler T.; Conn P. J.; Stauffer S. R.; Lindsley C. W.; Bartolome-Nebreda J. M. (2016) Preliminary investigation of 6,7-dihydropyrazolo[1,5-a]pyrazin-4-one derivatives as a novel series of mGlu5 receptor positive allosteric modulators with efficacy in preclinical models of schizophrenia. Bioorg. Med. Chem. Lett. 26, 429–434. 10.1016/j.bmcl.2015.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malosh C.; Turlington M.; Bridges T. M.; Rook J. M.; Noetzel M. J.; Vinson P. N.; Steckler T.; Lavreysen H.; Mackie C.; Bartolome-Nebreda J. M.; Conde-Ceide S.; Martinez-Viturro C. M.; Piedrafita M.; Sanchez-Casado M. R.; Macdonald G. J.; Daniels J. S.; Jones C. K.; Niswender C. M.; Conn P. J.; Lindsley C. W.; Stauffer S. R. (2015) Acyl dihydropyrazolo[1,5-a]pyrimidinones as metabotropic glutamate receptor 5 positive allosteric modulators. Bioorg. Med. Chem. Lett. 25, 5115–5120. 10.1016/j.bmcl.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A. L.; Grier M. D.; Jones C. K.; Herman E. J.; Kane A. S.; Smith R. L.; Williams R.; Zhou Y.; Marlo J. E.; Days E. L.; Blatt T. N.; Jadhav S.; Menon U. N.; Vinson P. N.; Rook J. M.; Stauffer S. R.; Niswender C. M.; Lindsley C. W.; Weaver C. D.; Conn P. J. (2010) Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol. Pharmacol. 78, 1105–1123. 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges T. M.; Rook J. M.; Noetzel M. J.; Morrison R. D.; Zhou Y.; Gogliotti R. D.; Vinson P. N.; Xiang Z.; Jones C. K.; Niswender C. M.; Lindsley C. W.; Stauffer S. R.; Conn P. J.; Daniels J. S. (2013) Biotransformation of a novel positive allosteric modulator of metabotropic glutamate receptor subtype 5 contributes to seizure-like adverse events in rats involving a receptor agonism-dependent mechanism. Drug Metab. Dispos. 41, 1703–1714. 10.1124/dmd.113.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.