Abstract
The prevalence of diabetes has reached epidemic proportions and is placing a significant burden on healthcare systems globally. Diabetes has a detrimental impact on many organs in the human body, including accelerating the development of micro- and macrovascular complications. Current therapeutic options to treat diabetic complications have their limitations. Importantly, many slow but fail to reverse the progression of diabetic complications. Bone morphogenetic proteins (BMPs) are a highly conserved subgroup of the transforming growth factor β (TGFβ) superfamily, signaling via serine/threonine kinase receptors, that have recently been implicated in glucose homeostasis and insulin resistance in the setting of diabetes. Downstream of the receptors, the signal can be transduced via the canonical Smad-dependent pathway or the noncanonical Smad-independent pathways. BMPs are essential in organ development, tissue homeostasis, and, as expected, disease pathogenesis. In fact, deletion of BMPs can be embryonically lethal or result in severe organ abnormalities. This review outlines the BMP signaling pathway and its relevance to diabetic complications, namely, diabetic nephropathy, diabetes-associated cardiovascular diseases, and diabetic retinopathy. Understanding the complexities of BMP signaling and particularly its tissue-, cellular-, and time-dependent actions will help delineate the underlying pathogenesis of the disease and may ultimately be harnessed in the treatment of diabetes-induced complications. This would replicate progress made in numerous other diseases, including cancer and atherosclerosis.
Keywords: bone morphogenetic proteins, diabetes, diabetic complications, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy
The global prevalence of diabetes is increasing alarmingly; the latest estimates predict that 642 million people will have diabetes by the year 2040.1 The metabolic changes that occur in diabetes perpetuate structural and functional changes in many organs, explaining the increased risk of diabetes patients to develop associated complications including nephropathy, cardiovascular disease, and retinopathy.2,3 Current therapeutic options to treat diabetic complications remain ineffective, at best slowing the progression of diabetic complications, but ultimately failing to reverse the underlying structural and functional anomalies. Therefore, more effective antidiabetic medications are needed. Of interest, several recent studies have reported a role for bone morphogenetic proteins (BMPs) in pancreas development and insulin secretion, as well as glucose homeostasis and insulin resistance in the setting of diabetes.
There are 20 BMPs that are a subgroup of the transforming growth factor β (TGFβ) superfamily, all of which share structural similarities.4 More than 50 years ago, the role of BMPs was first described in the formation and repair of bone.5 More recently, BMPs have shown to be expressed in many tissues, playing a fundamental role in a myriad of cellular processes including cell differentiation, proliferation, and apoptosis.6 In fact, BMP2 is essential for retinal development; BMP2 and BMP4 are crucial in cardiac septation where a conditional knockout of BMP4 causes heart defects in mice. BMP7 in kidney and heart organogenesis.7,8 Moreover, as well as its well-characterized role in bone and cartilage formation, BMP6 has recently been shown to play a novel role in both iron and glucose homeostasis.9,10
The diabetic milieu causes maladaptive changes in several organs leading to remodelling; common changes include hypertrophy, fibrosis, and apoptosis. Several growth factors and cytokines have been implicated in the pathogenesis of these complex disorders, including members of the TGFβ superfamily. The TGFβ/BMP signaling axis regulates important processes, including aiding wound healing by stimulating expression of extracellular matrix (ECM) components, subsequently promoting collagen production and formation, important in the development and progression of diabetic complications.11−13 This review aims to elucidate the potential of harnessing BMP signaling to target key processes underlying diabetic complications.
Bone Morphogenetic Protein Signaling: Canonical and Noncanonical Pathways
The TGFβ superfamily encompasses over 30 members that can be split into several subgroups, including TGFβs, BMPs, growth differentiation factors, glial-derived neurotropic factors, and activins.11 Phylogenetic analysis of nucleotide and amino acid homogeneity have helped further subcategorise BMP proteins.4 BMP proteins are synthesized as large, inactive prepro-polypeptides.14 After cleavage, they become activated and include an N-terminal signal peptide, a prodomain for folding, and a C-terminal signal peptide.15 Signaling via this axis is complex, engaging BMP ligands, cell surface receptors, and various signal transducers via the conserved canonical but also noncanonical signaling pathways, as illustrated in Figure 1. Acting in a tissue-, cell-, and context-dependent manner, BMPs have extensive functions. As expected, BMPs have been implicated in many disease settings, given their critical role in development and essential physiological processes in both mice and humans.6,16,17 In fact, BMP2 and BMP4 knockout mice are embryonically lethal, while knockout of gremlin1, an antagonist of BMPs, yielded a small number of viable mice but with a single enlarged kidney.18 In humans, mutations in BMP7 caused developmental eye abnormalities, deafness, scoliosis, and cleft palate.19
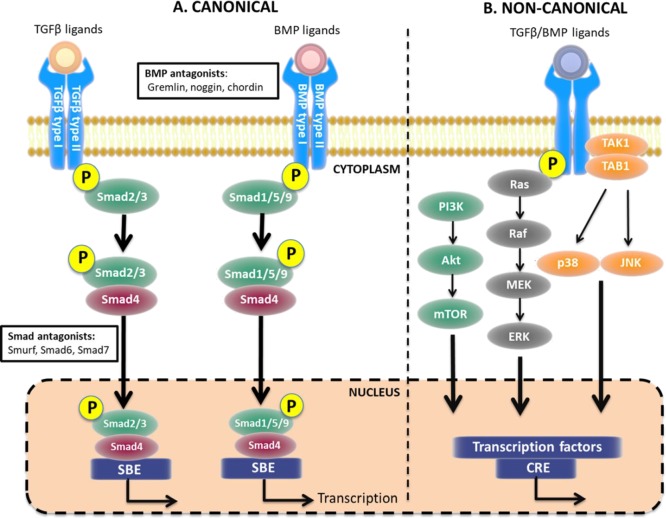
Figure 1.
TGFβ and BMPs signaling pathways. (A) In the canonical pathway, TGFβ and BMPs bind to their respective receptors resulting in phosphorylation of Smad2/3 or Smad1/5/9, respectively. Common Smad4 binds to both Smad complexes and facilitates translocation to the nucleus, thereby activating transcription. (B) Alternatively, after receptor binding, the signal can be transduced via several noncanonical pathways including nitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), and c-Jun N-terminal kinase (JNK), also resulting in gene transcription. Akt, protein kinase B; CRE, cyclic adenosine-monophosphate response element; ERK, extracellular signal-regulated kinases; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; SBE, Smad binding element; TAK1, TGFβ-activated kinase 1; TAB1, TAK-1 binding protein 1. Figure created using artwork provided by Somesault1824, licensed under a Creative Commons License (CC BY-NC-SA 4.0).
In order to initiate signaling, BMPs interact with BMP receptors, forming a heterodimeric complex of type I and type II serine/threonine kinase receptors (Figure 1). BMP type I and type II receptors are structurally analogous and constitute a short extracellular domain, a single membrane-spanning domain, and an intracellular domain containing the active serine/threonine domain.20 BMP type I receptors include activin-receptor-like kinase (ALK)-2, ALK-3, and ALK-6, and BMP type II receptors include BMP receptor II, activin type 2 receptor (ActRII)-A, and ActRII-B.20 BMP members bind to specific receptors with varying affinities depending on structural elements and residues exposed at the binding interface of both the ligand and receptor.21 Binding of the ligand to the receptor causes the active type II receptor to autophosphorylate the type I receptor at the glycine/serine rich domain. Upon receptor activation, instantaneous phosphorylation of the downstream substrate proteins Smad1, Smad5 and Smad8 (Smad1/5/8) occurs.22 In mammals, eight Smad variants have been characterized: Smad1, Smad5, and Smad8 are receptor-regulated Smads (R-Smads) involved in the BMP signaling pathway; Smad2 and Smad3 are R-Smads involved in the TGFβ signaling pathway; Smad4 is a complex stabilizer, required for nuclear translocation; and Smad6 (BMP pathway) and Smad7 (BMP and TGFβ pathways) are inhibitory Smads.23,24 The structure of Smad proteins is highly conserved, possessing regions known as Mad Homology (MH) domains MH1 and MH2. MH1 domains are conserved only in R-Smads and common Smads and are responsible for nuclear translocation.25 MH2 domains are expressed in all eight Smads and are responsible for interaction with BMP receptors, interaction with DNA-binding proteins, and transcriptional activation.25 Many transcription factors have been identified that regulate BMP target genes, although further work is needed to establish if these are universal regulators of BMP signaling or context-dependent in their mode of action, as reviewed in detail by Ampuja et al.26 For example, the transcription factor ATF2 was shown to activate the promoter of β-myosin heavy chain, important in cardiac development.27
Several pathways have also been identified that signal in a Smad-independent manner (Figure 1), known as noncanonical pathways. One example is via mitogen-activated protein kinase (MAPK) cascades: BMP receptor associated molecule 1 (BRAM1) binds to the cytoplasmic tail of the BMP type I receptor, activating TGFβ activated kinase 1 (TAK1) and initiating downstream activation of MAPK pathways including p38-MAPK, Rho-like guanosine triphosphatases (GTPase) and JNK.28−30 Via this initiation, BMPs are able to elicit their effects on cell survival, apoptosis, differentiation, and growth. Rho-like GTPases play a chief role in the TGFβ-induced epithelial to mesenchymal transition (EMT).31 However, more work is required to further understand noncanonical mediators of BMPs, particularly the interplay with the extracellular environment, other cellular processes, and cross-talk between other signaling pathways.
Bone Morphogenetic Proteins and Glucose Homeostasis
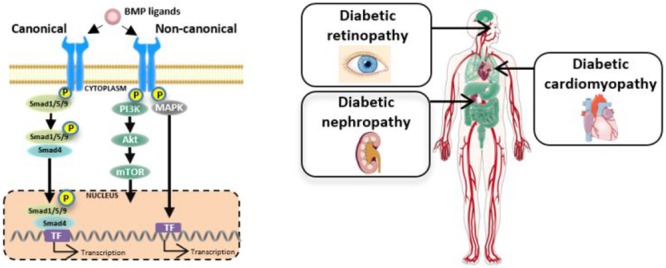
BMPs play an important role in pancreas development and insulin secretion (Figure 2).32 Studies also describe a role for BMPs in glucose homeostasis and insulin resistance in the setting of diabetes.9 Glucose metabolism and insulin regulation involves cross-talk between several systems in the body including skeletal muscle, adipose tissue, and the liver. In the adipose tissue, BMPs have been implicated in determination of adipose cell fate and regulation of adipocyte function.32,33 The distribution of white adipose tissue, the major site of triglyceride storage, in relation to brown adipose tissue, essential for energy expenditure, is associated with an increased risk of developing diabetes. Importantly, studies report that BMP4 promotes differentiation of pluripotent stem cells toward the adipocyte lineage,34,35 while BMP7 induces differentiation of brown preadipocytes.36
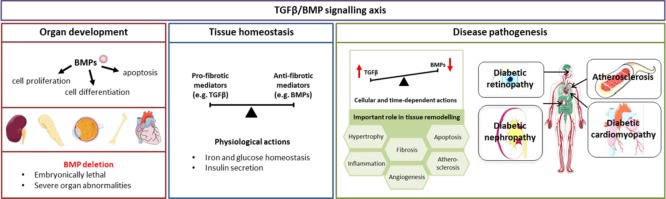
Figure 2.
Overview of BMPs actions in organ development, tissue homeostasis, and disease pathology. BMPs play a role in several cellular processes important in organ development, with BMP deletion leading to severe organ abnormalities. Beyond development, the TGFβ/BMP signaling is finely balanced, maintaining tissue homeostasis. When this balance is lost, aberrant signaling drives tissue remodelling in several organs, including organs commonly affected by diabetes. Figure created using artwork provided by Servier Medical Art by Servier, licensed under a Creative Commons License (CC BY 3.0).
As well as the aforementioned role in adipose balance, BMPs have also been implicated in glucose homeostasis and insulin resistance. BMP2 and BMP6 act as insulin-sensitizing growth factors in adipocytes, via the production of PPARγ. PPARγ upregulates GLUT4 transcription via the noncanonical p38 MAPK pathway.37 In ob/ob mice, administration of BMP6 caused a decrease in plasma glucose concentration and improved glucose tolerance via inhibition of gluconeogenesis,9 actions likely mediated via the ALK-3 and ALK-6 receptors. Serum BMP4 levels are significantly elevated in insulin-resistant db/db mice, a genetic mouse model of type 2 diabetes resultant of a point mutation in the leptin receptor gene.38 Furthermore, addition of BMP4 to adipocytes led to reduced glucose uptake following insulin treatment alone.32 These effects were mediated by activation of the PKC-θ isoform and phosphorylation of IRS-1.32 Serum BMP7 levels are reduced in db/db mice.32 However, following 20 weeks of recombinant BMP7 administration, improvements in glucose tolerance were reported.32 These effects were likely mediated by the assembly of PI3K and consequent phosphorylation of Akt.39 In diabetic mice, BMP9 was reported to be a regulator of blood glucose levels via inhibition of hepatic glucose production, promotion of insulin release, and regulation of key enzymes in fatty acid synthesis.40,41 Given the apparent role of BMPs on pancreas organogenesis, the adipose tissue, and their involvement in glucose homeostasis, harnessing these pathways may have therapeutic potential in the treatment of obesity and type 2 diabetes.
Bone Morphogenetic Proteins and Diabetic Nephropathy
Diabetic nephropathy is a primary cause of end-stage kidney disease worldwide and is characterized by a progressive decline in renal function. Interstitial fibrosis and tubular injury are hallmarks of diabetic nephropathy and correlate well with disease stage.42 One major driver of the pro-fibrotic response and switch in phenotype of the tubular cells is TGFβ, which importantly, is counterbalanced by the actions of BMPs.43 Four BMPs are particularly pertinent in the pathogenesis of diabetic nephropathy and may have therapeutic potential (Table 1).
Table 1. Summary of Actions of BMP Ligands in Diabetic Complications.
| ligand | summary of findings | ref |
|---|---|---|
| Diabetic Nephropathy | ||
| BMP2 | Renal BMP2 expression was increased in db/db mice. | (57) |
| Upregulation of Id-1 via BMP-2 receptors induces reactive oxygen species in human glomerular podocytes. | (52) | |
| BMP2 antagonizes renal interstitial fibrosis by promoting catabolism of TGFβ receptors. | (58) | |
| BMP4 | Renal BMP4 expression increased in streptozotocin (STZ)-induced diabetic nephropathy (Sprague–Dawley rats). | (62) |
| BMP4–Smad1–smooth muscle actin (SMA) signal transduction pathway modulates phenotypic changes in mesangial cells. | (64) | |
| BMP4-overexpressing mice exhibited mesangial matrix expansion and decreased numbers of podocytes in STZ-induced mice | (61) | |
| Activation of BMP4 signaling leads to glomerulosclerosis that mimics diabetic nephropathy. | (63) | |
| BMP6 | BMP6 administration reduced pro-inflammatory cytokine expression in human proximal tubular epithelial cells. | (71) |
| Loss of endogenous BMP6 increased renal fibrosis in BMP6 knockout mice. | (72) | |
| BMP7 | Renal BMP7 expression was reduced in STZ-induced diabetic nephropathy (Sprague–Dawley rats). | (46) |
| Exogenous administration of connective tissue groeth factor (CTGF) rescues BMP7 signal transduction in STZ-induced diabetic nephropathy (C57Bl/6J). | (50) | |
| BMP7 treatment inhibits tubular inflammation and tubulointerstitial fibrosis in STZ-induced CD1 mice. | (44) | |
| Exogenous administration of BMP7 delays onset of glomerulosclerosis and renal hypertrophy in STZ-induced Sprague–Dawley rats. | (49) | |
| BMP-7 enhances SnoN mRNA expression in renal tubular epithelial cells under high-glucose conditions. | (53) | |
| Diabetic Cardiomyopathy | ||
| BMP2 | Plasma BMP2 levels are increased in patients with type 2 diabetes and coronary artery disease. | (75) |
| Cardiac BMP2 and Smad 1/5 protein expression is reduced in high-fat-diet-induced type 2 diabetic C57Bl/6J mice. | (78) | |
| BMP4 | Cardiac BMP4 and Smad 1/5/8 protein expression is increased in STZ-induced ApoE knockout mice. | (81) |
| BMP4 administration in mouse peritoneal macrophages increases uptake of low-density lipoproteins. | (81) | |
| BMP7 | Recombinant BMP7 reduced left ventricular fibrosis in a C57Bl/6J mouse model of pressure-overload. | (84) |
| BMP7 administration is anti-inflammatory and blunts interstitial cardiac fibrosis in STZ-induced diabetic cardiomyopathy (C57Bl/6J mice). | (85) | |
| Diabetic Retinopathy | ||
| BMP2 | Hypoxia and vascular endothelial growth factor (VEGF) upregulates BMP2 mRNA and protein expression in microvascular endothelial cells. | (97) |
| Increase in BMP2 protein expression in human retinal endothelial cells cultured with high glucose. | (98) | |
| Retinal BMP2 levels were increased in a mouse model of STZ-induced diabetic retinopathy. | (99) | |
| ICAM-1 and VEGF protein expression was increased in retinal cells treated with BMP2. | (99) | |
| Retinal BMP2 acts as an inflammatory cytokine to encourage leukocyte adhesion and increase IL-6 and IL-8. | (100) | |
| BMP4 | Retinal BMP4 protein expression increased in retinal pigmental epithelial cells exposed to high glucose. | (102) |
| BMP4 treatment inhibited EMT via Smad2/3 signaling pathway in retinal pigmental epithelial cells. | (105) | |
| BMP9 | BMP9 signaling prevents hyperglycaemia-induced vascular permeability in endothelial cells and in STZ-induced diabetic mice. | (112) |
| Activation of BMP9 signaling inhibited neovascularization and reduced vascular lesions in a mouse model of oxygen-induced retinopathy. | (113) | |
BMP7 is primarily expressed in the renal tubules and is the family member most well studied in the setting of diabetic nephropathy due to its established antifibrotic and pro-regenerative properties.44 In fact, BMP7’s antifibrotic properties were first reported in an experimental model of unilateral ureteral obstruction, where loss of BMP7 led to renal dysfunction.45 Restoration of BMP7 expression caused improvements in function, paralleled by changes in the expression of fibrosis markers.45 In the setting of diabetic nephropathy, BMP7 has been reported to be protective. In fact, in a streptozotocin (STZ)-induced model of diabetic nephropathy, renal levels of BMP7 were reduced by over 90% at 30 weeks,46 and this loss of endogenous BMP7 was associated with renal dysfunction.47 Similarly, diabetic nephropathy patients have a reduction in phosphorylated Smad1/5/8 in glomeruli, compared to control kidneys.48 Importantly, studies in STZ-induced rodent models of diabetic nephropathy report that exogenous BMP7 administration delayed the onset of glomerulosclerosis, reversed renal hypertrophy, and restored glomerular filtration rate.44,49 An important factor in this response is the ECM modulator, CTGF. Binding assays indicated that CTGF had high binding affinity for BMP7, with diabetic CTGF knockout mice exhibiting reduced renal Smad1/5/8 pathway activation.48,50 Intraperitoneal administration of CTGF-rescued BMP7 signal transduction.51 BMP7 has also been heavily implicated in EMT, an important process in the pathogenesis of diabetic nephropathy. One study demonstrated that BMP7 could stimulate transdifferentiation of mesenchyme-like tubular epithelial cells to mature epithelial cells, restoring renal function.52 Another study reports that BMP7 upregulates expression of TGFβ inhibitor, Ski-related novel protein N (SnoN), thereby inhibiting the progression of nephropathy.53
Given BMP7’s well-reported antifibrotic actions in experimental models, several clinical studies are now in progress to harness this potential. The BMP7 small-peptide agonists THR-184 and THR-123 have demonstrated favorable antifibrotic results in the setting of human renal disease. THR-123 functions through the ALK3 receptor to suppress both inflammation and the EMT.54 THR-184 has successfully reached Phase II clinical trials in the setting of cardiac-surgery-induced acute kidney injury (clinical trial: NCT01830920), while THR-123 highlights the therapeutic potential of peptide agonists in the setting of diabetic nephropathy.
BMP2 preferentially binds to BMP type II receptors and is typically elevated in models of diabetic nephropathy.52,55,56 In an experimental setting, renal BMP2 mRNA levels were elevated 0.5-fold in db/db mouse, a genetic mouse model of type 2 diabetes due to a point mutation in the leptin receptor gene.57 Furthermore, BMP2 treatment significantly attenuated a TGFβ-induced increase in protein levels of the ECM component, fibronectin, in renal fibroblast cells, suggestive of a protective role for BMP2 in the kidney.58 In terms of mechanism, BMP2 reversed the TGFβ-induced increase in profibrotic signaling complex Smad2/3, while raising levels of inhibitor Smad7; Smad7 is highly conserved in BMP signaling pathways.58,59 It is also known that BMP2 treatment activates ubiquitination regulatory factor Smurf, which controls degradation of TGFβ receptors, reducing the half-life of the TGFβ receptor type I from 90 min to less than 30 min.58,60
By binding with greater affinity to BMP type I than type II receptors, BMP4 plays a central role in podocyte injury and mesangial matrix expansion in diabetic nephropathy.20,61 Immunohistochemical staining revealed significantly elevated levels of renal BMP4 and regulatory Smad1 expression in type 2 diabetic rats.62 Furthermore, overexpression of BMP4 resulted in development of proteinuria and podocyte dysfunction, both hallmarks of diabetic nephropathy.61,63 BMP4 acts to enhance Smad1 activity, leading to increased collagen IV deposition and ECM expansion.64 Numerous studies have reported that in mice with diabetic nephropathy BMP4 secreted from podocytes strongly adheres to collagen IV.61,65,66 A BMP4 neutralizing antibody inhibited Smad1-mediated mesangial matrix expansion.67 Notably, Smad1 is a urinary biomarker of diabetic nephropathy with levels correlating with glomerular hyperfiltration in type 1 and type 2 diabetes patients.50 BMP4 provides an important feedback mechanism that may be targeted therapeutically, as glomerular hyperfiltration activates TGFβ signaling pathways, exacerbating Smad1 production via ALK1.68 BMP4 has also been shown to regulate activation of Smad3 in experimental models.69 Smad3 is a marker of diabetic nephropathy, inversely correlated with the glomerular filtration rate in diabetic patients and activated by TGFβ signaling pathways.70
BMP6 preferentially binds and activates ALK2 and ALK3 receptors, over the BMPR-IIB receptor, and therefore has a distinct mode of action.20 In mice with diabetic nephropathy, BMP6 levels were considerably lower than those in controls, suggestive of a regulatory role in the response to injury.50 BMP6 has been shown to reduce hyperglycaemia in rat models of diabetic nephropathy via MAPK, JNK, and PI3K pathways, independent of insulin pathways. Although further studies are needed, BMP6 is the most effective BMP member in reducing the expression of pro-inflammatory cytokines within proximal tubular epithelial cells, including monocyte chemoattractant protein-1 (MCP-1) and interleukin (IL)-6.71 Furthermore, in BMP6-deficient mice, the degree of renal damage was positively correlated with leukocytes and pro-inflammatory markers MCP-1 and IL-6.72
Bone Morphogenetic Proteins and Diabetes-Associated Cardiovascular Disease
Vascular and renal diseases share common triggers, including diabetes, hypertension, and other hemodynamic irregularities. Moreover, diabetes patients have a 5-fold increased risk of mortality as a result of cardiovascular disease, with coronary artery disease being the primary cause.73 The progression of endothelial dysfunction is exacerbated by diabetes and increases the propensity to develop atherosclerosis, characterized by inflammatory cell infiltration and plaque formation.74 Importantly, recent studies indicate that BMPs can exert antiatherogenic and anti-inflammatory actions (Table 1).
BMP2 levels were elevated in coronary artery disease patients with type 2 diabetes, with a positive correlation between BMP2 expression and the magnitude of coronary atherosclerotic plaque calcification.75 Smooth muscle cells treated with high glucose exhibited elevated levels of BMP2,76 while in another study, inhibition of BMP2 decreased total BMP activity and restricted vascular calcification in diabetic mice.77 Type 2 diabetic mice with chronic hyperglycaemia and diastolic dysfunction exhibited elevated left ventricular TGFβ and Smad3 protein expression, while Smad1/5 and BMP2 protein levels were reduced.78 Obesity reduction attenuated these signaling changes, improved left ventricular remodelling, and preserved cardiac function.78 These opposing actions highlight the complex nature of growth factor signaling pathways and their context-dependent nature. Here, overexpression of BMP2 in vascular smooth muscle cells promoted a phenotypic switch of vascular smooth muscle cells to osteoblast-like cells, thereby exacerbating vascular calcification in diabetes patients,75,76 whereas in a model of diabetic cardiomyopathy, BMP2 was cardioprotective due to its antifibrotic actions.78
In diabetic patients with atherosclerotic plaques, serum BMP4 levels were inversely correlated with systolic blood pressure, triglycerides, and free fatty acid levels.79 However, other studies describe pro-inflammatory, hypertensive, and atherogenic effects of BMP4 and correlate serum BMP4 levels with early phase atherosclerosis in diabetic subjects.77,80 Furthermore, BMP4 levels were increased in diabetic ApoE knockout mice, activating the Smad1/5/8 signaling cascade.81 In mouse peritoneal macrophages, BMP4 enhanced oxidized low-density lipoprotein uptake, suggesting a role in plaque formation, and may therefore be targeted therapeutically to inhibit plaque progression.81 Endothelial dysfunction and vascular complications are also risk factors of diabetic cardiomyopathy.73,82 Diabetic cardiomyopathy is characterized by myocardial structural abnormalities including cardiac fibrosis, cardiomyocyte hypertrophy, and apoptosis that ultimately lead to cardiac dysfunction.73,82 Although limited, the available evidence suggests that BMPs may have therapeutic potential in this setting. Following myocardial infarction in rats, recombinant BMP7 acted as an antifibrotic cytokine, reducing progression of cardiac fibrosis and improving cardiac function.83 In a model of pressure-overload, collagen staining demonstrated reduced left ventricular fibrosis following administration of BMP7.84 In an experimental model of prediabetes, recombinant BMP7 treatment blunted interstitial cardiac fibrosis.85 Furthermore, differentiation of anti-inflammatory macrophages was stimulated, as well as expression of IL-10, concurrent with improved cardiac function.85 The same researchers also reported that BMP receptor type II expression was significantly enhanced in cultured human apoptotic monocytes compared to that in control monocytes86 and that the anti-inflammatory effects of BMP7 are mediated via the PI3K signaling pathway.86
Several small molecules targeting the TGFβ/Smad pathway have been investigated in the setting of diabetic cardiomyopathy. Matrine, an alkaloid found in plants, inhibits TGFβ ligands and phosphorylation of Smad2/3 in the hearts of diabetic rats, thereby inhibiting collagen production and restoring left ventricular function and cardiac compliance.87 Targeting of the Smad3 signaling axis to attenuate myocardial fibrosis and improving cardiac function is well-documented.88,89 In fact, momordicine, a natural compound found in bitter melon vine, reduced Smad2/3 phosphorylation via inhibition of reactive oxygen species in rat cardiac fibroblasts cultured with high glucose.90 Tranilast, an antiallergic agent typically used in management of respiratory inflammatory conditions, has been shown to reduce cardiac collagen type I and III levels and prevent the onset of diastolic dysfunction in experimental models.91 However, it is believed that tranilast exerts its effects via the noncanonical MAPK pathway of BMP signaling.91
Bone Morphogenetic Proteins and Diabetic Retinopathy
Diabetic retinopathy is a common complication of diabetes and can lead to vision loss. Proliferative diabetic retinopathy is characterized by the growth (angiogenesis) and rupture (hemorrhage) of blood vessels on the retinal surface.92 VEGF primarily secreted from retinal cells acts via the MAPK pathway to stimulate angiogenesis, endothelial cell proliferation and to regulate vascular permeability.93 In diabetic retinopathy, chronic damage to retinal vessels increases expression of TGFβ and VEGF, and initiates chemotaxis of macrophages.94 Together, TGFβ and VEGF induce fibrosis around newly formed vessels, causing retinal detachment and bleeding.95 The role of BMPs in the setting of diabetic retinopathy has been characterized in a number of animal and human studies (Table 1).
BMP2 levels are elevated in the retinas and vitreous of human patients with diabetic retinopathy. Endothelial cells are the major source of BMP2 and can be stimulated by VEGF.96,97 Human retinal endothelial cells cultured under high-glucose conditions showed increased upregulation of both BMP2 and VEGF expression.98 In a mouse model of STZ-induced diabetic retinopathy, expression of BMP2 was elevated, as was intracellular adhesion molecule-1 (ICAM-1) and VEGF in retinal cells.99 In retinal endothelial cells, BMP2 acts as an inflammatory cytokine to encourage leukocyte adhesion and elevate levels of inflammatory markers IL-6 and IL-8.100 Furthermore, Smad knockout in human retinal endothelial cells reduced expression of VEGF and inflammatory markers IL-6 and MCP-1.98 Another study suggests the BMP2/Smad pathway is regulated by nicotinamide adenine dinucleotide phosphate oxidase, attenuating expression of ICAM-1 and VEGF in diabetic retinopathy, to enhance angiogenic and inflammatory pathways.99
In embryonic development, BMP4 promotes capillary apoptosis in the retina.101,102 BMP4 has been associated with not only other physiological processes but also in disease pathogenesis, including in the setting of diabetic retinopathy.103,104 Cytokines including TGFβ and BMPs are regulators in the process of EMT, where epithelial cells acquire mesenchymal properties. Retinal pigment epithelial (RPE) cells exposed to high glucose have increased secretion of BMP4.102 In another study, RPE cells treated with BMP4 appeared to slow EMT by inhibiting the TGFβ/Smad2/3 pathway.105 Furthermore, a BMP4 antagonist, gremlin, induced EMT in the human RPE cell line.106 BMP4 is also expressed in human retinal pericytes that surround the endothelial cells of the capillaries in the eye and help to maintain homeostatic function.107 In diabetes, hyperglycaemia, advanced glycation end product formation, and chronic hypoxia drive apoptosis of pericytes, a key event in the pathogenesis of diabetic retinopathy.107 Importantly, chordin-like 1 and gremlin, both antagonists of BMP4, were able to regulate the balance between TGFβ and BMP4, maintaining angiogenic homeostasis.108,109
BMP9 is important in the development of retinal blood vessels and has recently been implicated in diabetic retinopathy.110,111 In a mouse model of diabetic macular edema, administration of an adenoviral BMP9 vector limited vascular permeability by inhibiting VEGF signaling.112 In cultured endothelial cells, high-glucose incubation impaired BMP9/ALK1 signaling.112 Furthermore, in a mouse model of oxygen-induced retinopathy, activating BMP9/ALK1 signaling inhibited neovascularization and reduced the volume of vascular lesions.113
Summary and Future Perspectives
This review sought to highlight the studies that illustrate the importance of BMPs in the pathophysiology of diabetes-associated complications. BMPs and downstream signaling pathways are essential in the development of many organs, regulating cellular processes including cell proliferation, differentiation, and apoptosis (Figure 2). In fact, deletion of several BMPs is embryonic-lethal or results in severe organ abnormalities. However, BMP signaling is also critical in maintaining adult tissue homeostasis in the disease setting. The balance between TGFβ and BMP signaling, and how they are regulated in response to stress, is particularly relevant. Recent reports have described a role for BMPs in glucose homeostasis and insulin resistance, providing a novel option to treat metabolic syndrome and diabetes. In addition, BMPs also affect the development and progression of diabetes-associated complications. In diabetic kidney disease it is well-established that BMPs (BMP2, BMP4, BMP6, and BMP7) have antifibrotic and pro-regenerative actions, impacting renal function, interstitial fibrosis, and tubular injury. In cardiovascular disease, BMPs (BMP2, BMP4, and BMP7) have been reported to elicit anti-atherogenic and anti-inflammatory effects and, specifically in diabetes-induced cardiomyopathy, to improve left ventricular remodelling and preserve cardiac function. In diabetic retinopathy, BMPs (BMP2, BMP4, and BMP9) have been reported to be regulators of the EMT process and play a role in angiogenesis and inflammation. Given the important role BMPs play in metabolic disease and associated complications, it is likely that inhibition of BMP signaling will be explored in these settings, mirroring similar studies in a wide-range of indications, including chronic diseases such as cancer and atherosclerosis. Likely approaches include repurposing existing inhibitors or employing monoclonal antibodies to inhibit signaling pathways.114,115 Understanding the spatiotemporal context of BMP signaling in metabolic disease and associated complications, as well as the interaction with canonical and noncanonical pathways, will help drive development of more efficacious therapies, mirroring the use of BMP-targeted therapies in other disease settings.
Acknowledgments
R.H.R. (ID 1059960) is supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia. This work was supported by a Research Program grant to M.T. from Diabetes Australia.
Glossary
Abbreviations
- ActRII
Activin type 2 receptors
- ALK
Activin-like kinase
- BMP
Bone morphogenetic protein
- BRAM1
BMP receptor associated molecule 1
- CTGF
Connective tissue growth factor
- ECM
Extracellular matrix
- EMT
Epithelial-mesenchymal transition
- GTPase
Guanosine triphosphotase
- ICAM-1
Intracellular adhesion molecule-1
- IL
Interleukin
- JNK
c-Jun N terminal kinase
- MAPK
Mitogen activated protein kinase
- MCP-1
Monocyte chemoattractant protein-1
- MH
Mad homology
- PI3K
Phosphoinositide 3-kinase
- RPE
Retinal pigment epithelial
- R-Smads
Receptor regulated Smads
- SnoN
Ski-related novel protein N
- STZ
Streptozotocin
- TAK1
Transforming growth factor β-activated kinase 1
- TGFβ
Transforming growth factor β
- VEGF
Vascular endothelial growth factor
The authors declare no competing financial interest.
This article is made available for a limited time sponsored by ACS under the ACS Free to Read License, which permits copying and redistribution of the article for non-commercial scholarly purposes.
References
- Ogurtsova K.; da Rocha Fernandes J. D.; Huang Y.; Linnenkamp U.; Guariguata L.; Cho N. H.; Cavan D.; Shaw J. E.; Makaroff L. E. (2017) IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50. 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Adler A. I.; Boyko E. J.; Ahroni J. H.; Stensel V.; Forsberg R. C.; Smith D. G. (1997) Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care 20 (7), 1162–7. 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- Semeraro F.; Cancarini A.; Dell’Omo R.; Rezzola S.; Romano M. R.; Costagliola C. (2015) Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 582060. 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T.; Watabe T. (2016) Bone Morphogenetic Proteins. Cold Spring Harbor Perspect. Biol. 8 (6), a021899. 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R. (1965) Bone: formation by autoinduction. Science 150 (3698), 893–9. 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Wang R. N.; Green J.; Wang Z.; Deng Y.; Qiao M.; Peabody M.; Zhang Q.; Ye J.; Yan Z.; Denduluri S.; Idowu O.; Li M.; Shen C.; Hu A.; Haydon R. C.; Kang R.; Mok J.; Lee M. J.; Luu H. L.; Shi L. L. (2014) Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis 1 (1), 87–105. 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes H. P.; Alt A.; Niwa T.; Clausen J. T.; Bretzel R. G.; Brownlee M.; Schleicher E. D. (1999) Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia 42 (6), 728–36. 10.1007/s001250051221. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R.; Craven P. A.; Melhem M. F.; Salah E. M. (2004) Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes 53 (3), 762–8. 10.2337/diabetes.53.3.762. [DOI] [PubMed] [Google Scholar]
- Pauk M.; Bordukalo-Niksic T.; Brkljacic J.; Paralkar V. M.; Brault A. L.; Dumic-Cule I.; Borovecki F.; Grgurevic L.; Vukicevic S. (2019) A novel role of bone morphogenetic protein 6 (BMP6) in glucose homeostasis. Acta Diabetol. 56 (3), 365–371. 10.1007/s00592-018-1265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canali S.; Zumbrennen-Bullough K. B.; Core A. B.; Wang C. Y.; Nairz M.; Bouley R.; Swirski F. K.; Babitt J. L. (2017) Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood 129 (4), 405–414. 10.1182/blood-2016-06-721571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poniatowski L. A.; Wojdasiewicz P.; Gasik R.; Szukiewicz D. (2015) Transforming growth factor Beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediators Inflammation 2015, 137823. 10.1155/2015/137823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozzein W. N.; Badr G.; Al Ghamdi A. A.; Sayed A.; Al-Waili N. S.; Garraud O. (2015) Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/Smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell. Physiol. Biochem. 37 (3), 940–54. 10.1159/000430221. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Li Y.; Li N.; Teng W.; Wang M.; Zhang Y.; Xiao Z. (2016) TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci. Rep. 6, 32231. 10.1038/srep32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. N.; Wharton K. A. (2017) Alternative cleavage of the bone morphogenetic protein (BMP), Gbb, produces ligands with distinct developmental functions and receptor preferences. J. Biol. Chem. 292 (47), 19160–19178. 10.1074/jbc.M117.793513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C. A.; Al-Musawi S. L.; Walton K. L. (2011) Prodomains regulate the synthesis, extracellular localisation and activity of TGF-beta superfamily ligands. Growth Factors 29 (5), 174–86. 10.3109/08977194.2011.608666. [DOI] [PubMed] [Google Scholar]
- Garcia de Vinuesa A.; Abdelilah-Seyfried S.; Knaus P.; Zwijsen A.; Bailly S. (2016) BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 27, 65–79. 10.1016/j.cytogfr.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Winnier G.; Blessing M.; Labosky P. A.; Hogan B. L. (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9 (17), 2105–16. 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Church R. H.; Ali I.; Tate M.; Lavin D.; Krishnakumar A.; Kok H. M.; Hombrebueno J. R.; Dunne P. D.; Bingham V.; Goldschmeding R.; Martin F.; Brazil D. P. (2017) Gremlin1 plays a key role in kidney development and renal fibrosis. Am. J. Physiol Renal Physiol 312 (6), F1141–F1157. 10.1152/ajprenal.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt A. W.; Osborne R. J.; Stewart H.; Ragge N. K. (2010) Bone morphogenetic protein 7 (BMP7) mutations are associated with variable ocular, brain, ear, palate, and skeletal anomalies. Hum. Mutat. 31 (7), 781–7. 10.1002/humu.21280. [DOI] [PubMed] [Google Scholar]
- Miyazono K.; Kamiya Y.; Morikawa M. (2010) Bone morphogenetic protein receptors and signal transduction. J. Biochem. 147 (1), 35–51. 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- Guzman A.; Zelman-Femiak M.; Boergermann J. H.; Paschkowsky S.; Kreuzaler P. A.; Fratzl P.; Harms G. S.; Knaus P. (2012) SMAD versus non-SMAD signaling is determined by lateral mobility of bone morphogenetic protein (BMP) receptors. J. Biol. Chem. 287 (47), 39492–39504. 10.1074/jbc.M112.387639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. S.; Akhtar N.; Jamil H. M.; Banik R. S.; Asaduzzaman S. M. (2015) TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 3, 15005. 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X.; Liao H.; Cheng M.; Shi X.; Lin X.; Feng X. H.; Chen Y. G. (2016) Smad7 Protein Interacts with Receptor-regulated Smads (R-Smads) to Inhibit Transforming Growth Factor-beta (TGF-beta)/Smad Signaling. J. Biol. Chem. 291 (1), 382–92. 10.1074/jbc.M115.694281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K.; Kamiya Y.; Imamura T.; Miyazono K.; Miyazawa K. (2007) Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J. Biol. Chem. 282 (28), 20603–11. 10.1074/jbc.M702100200. [DOI] [PubMed] [Google Scholar]
- Miyazawa K.; Miyazono K. (2017) Regulation of TGF-beta Family Signaling by Inhibitory Smads. Cold Spring Harbor Perspect. Biol. 9 (3), a022095. 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampuja M.; Kallioniemi A. (2018) Transcription factors-Intricate players of the bone morphogenetic protein signaling pathway. Genes, Chromosomes Cancer 57 (1), 3–11. 10.1002/gcc.22502. [DOI] [PubMed] [Google Scholar]
- Peng Y.; Zhao S.; Song L.; Wang M.; Jiao K. (2013) Sertad1 encodes a novel transcriptional co-activator of SMAD1 in mouse embryonic hearts. Biochem. Biophys. Res. Commun. 441 (4), 751–6. 10.1016/j.bbrc.2013.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J. H.; Xiao C.; Paschal A. E.; Bailey S. T.; Rao P.; Hayden M. S.; Lee K. Y.; Bussey C.; Steckel M.; Tanaka N.; Yamada G.; Akira S.; Matsumoto K.; Ghosh S. (2005) TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19 (22), 2668–81. 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. E. (2017) Non-Smad Signaling Pathways of the TGF-beta Family. Cold Spring Harbor Perspect. Biol. 9 (2), a022129. 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell L. M.; Jonason J. H.; Loiselle A. E.; Kohn A.; Schwarz E. M.; Hilton M. J.; O’Keefe R. J. (2010) TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J. Bone Miner. Res. 25 (8), 1784–97. 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J.; Yoo J. (2007) Rho activation is required for transforming growth factor-beta-induced epithelial-mesenchymal transition in lens epithelial cells. Cell Biol. Int. 31 (10), 1225–30. 10.1016/j.cellbi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay T.; Singh R. R.; Gupta S.; Surolia A. (2017) Bone morphogenetic protein-7 (BMP-7) augments insulin sensitivity in mice with type II diabetes mellitus by potentiating PI3K/AKT pathway. Biofactors 43 (2), 195–209. 10.1002/biof.1334. [DOI] [PubMed] [Google Scholar]
- Schulz T. J.; Tseng Y. H. (2009) Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 20 (5–6), 523–31. 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers R. R.; Lane M. D. (2007) A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle 6 (4), 385–9. 10.4161/cc.6.4.3804. [DOI] [PubMed] [Google Scholar]
- Ahrens M.; Ankenbauer T.; Schroder D.; Hollnagel A.; Mayer H.; Gross G. (1993) Expression of human bone morphogenetic proteins-2 or −4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 12 (10), 871–80. 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- Tseng Y. H.; Kokkotou E.; Schulz T. J.; Huang T. L.; Winnay J. N.; Taniguchi C. M.; Tran T. T.; Suzuki R.; Espinoza D. O.; Yamamoto Y.; Ahrens M. J.; Dudley A. T.; Norris A. W.; Kulkarni R. N.; Kahn C. R. (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454 (7207), 1000–4. 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber I.; Dorpholz G.; Ott C. E.; Kragesteen B.; Schanze N.; Lee C. T.; Kohrle J.; Mundlos S.; Ruschke K.; Knaus P. (2017) BMPs as new insulin sensitizers: enhanced glucose uptake in mature 3T3-L1 adipocytes via PPARgamma and GLUT4 upregulation. Sci. Rep. 7 (1), 17192. 10.1038/s41598-017-17595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulley J.; Dahl U.; Baeza N.; Mishina Y.; Edlund H. (2007) BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 5 (3), 207–19. 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Huang X.; Liu G.; Guo J.; Su Z. (2018) The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14 (11), 1483–1496. 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Grzegorzewski K. J.; Barash S.; Zhao Q.; Schneider H.; Wang Q.; Singh M.; Pukac L.; Bell A. C.; Duan R.; Coleman T.; Duttaroy A.; Cheng S.; Hirsch J.; Zhang L.; Lazard Y.; Fischer C.; Barber M. C.; Ma Z. D.; Zhang Y. Q.; Reavey P.; Zhong L.; Teng B.; Sanyal I.; Ruben S. M.; Blondel O.; Birse C. E. (2003) An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat. Biotechnol. 21 (3), 294–301. 10.1038/nbt795. [DOI] [PubMed] [Google Scholar]
- Kuo M. M.; Kim S.; Tseng C. Y.; Jeon Y. H.; Choe S.; Lee D. K. (2014) BMP-9 as a potent brown adipogenic inducer with anti-obesity capacity. Biomaterials 35 (10), 3172–9. 10.1016/j.biomaterials.2013.12.063. [DOI] [PubMed] [Google Scholar]
- Bader R.; Bader H.; Grund K.; Mackensen-Haen S.; Christ H.; Bohle A. (1980) Structure and function of the kidney in diabetic glomerulosclerosis. Correlations between morphological and functional paramters. Pathol., Res. Pract. 167, 204–216. 10.1016/S0344-0338(80)80051-3. [DOI] [PubMed] [Google Scholar]
- Sharma K.; Ziyadeh F. N. (1995) Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes 44 (10), 1139–1146. 10.2337/diabetes.44.10.1139. [DOI] [PubMed] [Google Scholar]
- Sugimoto H.; Grahovac G.; Zeisberg M.; Kalluri R. (2007) Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 56 (7), 1825–33. 10.2337/db06-1226. [DOI] [PubMed] [Google Scholar]
- Hruska K. A.; Guo G.; Wozniak M.; Martin D.; Miller S.; Liapis H.; Loveday K.; Klahr S.; Sampath T. K.; Morrissey J. (2000) Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am. J. Physiol Renal Physiol 279 (1), F130–43. 10.1152/ajprenal.2000.279.1.F130. [DOI] [PubMed] [Google Scholar]
- Wang S. N.; Lapage J.; Hirschberg R. (2001) Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J. Am. Soc. Nephrol. 12 (11), 2392–2399. [DOI] [PubMed] [Google Scholar]
- Wong M. G.; Perkovic V.; Woodward M.; Chalmers J.; Li Q.; Hillis G. S.; Yaghobian Azari D.; Jun M.; Poulter N.; Hamet P.; Williams B.; Neal B.; Mancia G.; Cooper M.; Pollock C. A. (2013) Circulating bone morphogenetic protein-7 and transforming growth factor-beta1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int. 83 (2), 278–84. 10.1038/ki.2012.383. [DOI] [PubMed] [Google Scholar]
- Turk T.; Leeuwis J. W.; Gray J.; Torti S. V.; Lyons K. M.; Nguyen T. Q.; Goldschmeding R. (2009) BMP signaling and podocyte markers are decreased in human diabetic nephropathy in association with CTGF overexpression. J. Histochem. Cytochem. 57 (7), 623–31. 10.1369/jhc.2009.953224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Chen Q.; Simon T. C.; Strebeck F.; Chaudhary L.; Morrissey J.; Liapis H.; Klahr S.; Hruska K. A. (2003) Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int. 63 (6), 2037–49. 10.1046/j.1523-1755.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- Nguyen T. Q.; Goldschmeding R. (2008) Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis?. Pharm. Res. 25 (10), 2416–26. 10.1007/s11095-008-9548-9. [DOI] [PubMed] [Google Scholar]
- Nguyen T. Q.; Roestenberg P.; van Nieuwenhoven F. A.; Bovenschen N.; Li Z.; Xu L.; Oliver N.; Aten J.; Joles J. A.; Vial C.; Brandan E.; Lyons K. M.; Goldschmeding R. (2008) CTGF inhibits BMP-7 signaling in diabetic nephropathy. J. Am. Soc. Nephrol. 19 (11), 2098–107. 10.1681/ASN.2007111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pache G.; Schafer C.; Wiesemann S.; Springer E.; Liebau M.; Reinhardt H. C.; August C.; Pavenstadt H.; Bek M. J. (2006) Upregulation of Id-1 via BMP-2 receptors induces reactive oxygen species in podocytes. Am. J. Physiol Renal Physiol 291 (3), F654–62. 10.1152/ajprenal.00214.2004. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Xiao Y.; Li S.; Shi L.; Liu L.; Zhang Y.; Shi M.; Guo B. (2017) BMP-7 enhances SnoN mRNA expression in renal tubular epithelial cells under high-glucose conditions. Mol. Med. Rep. 16 (3), 3308–3314. 10.3892/mmr.2017.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H.; LeBleu V. S.; Bosukonda D.; Keck P.; Taduri G.; Bechtel W.; Okada H.; Carlson W. Jr.; Bey P.; Rusckowski M.; Tampe B.; Tampe D.; Kanasaki K.; Zeisberg M.; Kalluri R. (2012) Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat. Med. 18 (3), 396–404. 10.1038/nm.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett P. C.; Ortmann J.; Celeiro J.; Haas E.; Hofmann-Lehmann R.; Tornillo L.; Terraciano L. M.; Barton M. (2006) Transcriptional regulation of vascular bone morphogenetic protein by endothelin receptors in early autoimmune diabetes mellitus. Life Sci. 78 (19), 2213–8. 10.1016/j.lfs.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Qin C. M.; W W.; Liu J.; Fan J. M.; Gong C. P.; Wei X. (2016) Effects of bone morphogenetic protein 2 signal pathway on renal artery calcification in progression of diabetic nephropathy. Chin. J. Nephrol. 32, 173–179. [Google Scholar]
- Thomsen L. H.; Fog-Tonnesen M.; Nielsen Fink L.; Norlin J.; Garcia de Vinuesa A.; Hansen T. K.; de Heer E.; Ten Dijke P.; Rosendahl A. (2017) Disparate phospho-Smad2 levels in advanced type 2 diabetes patients with diabetic nephropathy and early experimental db/db mouse model. Renal Failure 39 (1), 629–642. 10.1080/0886022X.2017.1361837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. L.; Liu Y. S.; Chuang L. Y.; Guh J. Y.; Lee T. C.; Liao T. N.; Hung M. Y.; Chiang T. A. (2009) Bone morphogenetic protein-2 antagonizes renal interstitial fibrosis by promoting catabolism of type I transforming growth factor-beta receptors. Endocrinology 150 (2), 727–40. 10.1210/en.2008-0090. [DOI] [PubMed] [Google Scholar]
- Liu F. Y.; Li X. Z.; Peng Y. M.; Liu H.; Liu Y. H. (2007) Arkadia-Smad7-mediated positive regulation of TGF-beta signaling in a rat model of tubulointerstitial fibrosis. Am. J. Nephrol. 27 (2), 176–83. 10.1159/000100518. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.; Kurisaki A.; Yamakawa N.; Minakuchi K.; Sugino H. (2006) FKBP12 functions as an adaptor of the Smad7-Smurf1 complex on activin type I receptor. J. Mol. Endocrinol. 36 (3), 569–79. 10.1677/jme.1.01966. [DOI] [PubMed] [Google Scholar]
- Fujita Y.; Tominaga T.; Abe H.; Kangawa Y.; Fukushima N.; Ueda O.; Jishage K. I.; Kishi S.; Murakami T.; Saga Y.; et al. (2018) An adjustment in BMP4 function represents a treatment for diabetic nephropathy and podocyte injury. Sci. Rep. 8 (1), 13011. 10.1038/s41598-018-31464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Lin J.; Li L.; Zhu T.; Gao L.; Wu W.; Liu Q.; Ou S. (2019) The role of the BMP4/Smad1 signaling pathway in mesangial cell proliferation: A possible mechanism of diabetic nephropathy. Life Sci. 220, 106–116. 10.1016/j.lfs.2019.01.049. [DOI] [PubMed] [Google Scholar]
- Tominaga T.; Abe H.; Ueda O.; Goto C.; Nakahara K.; Murakami T.; Matsubara T.; Mima A.; Nagai K.; Araoka T.; Kishi S.; Fukushima N.; Jishage K.; Doi T. (2011) Activation of bone morphogenetic protein 4 signaling leads to glomerulosclerosis that mimics diabetic nephropathy. J. Biol. Chem. 286 (22), 20109–16. 10.1074/jbc.M110.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H.; Matsubara T.; Iehara N.; Nagai K.; Takahashi T.; Arai H.; Kita T.; Doi T. (2004) Type IV collagen is transcriptionally regulated by Smad1 under advanced glycation end product (AGE) stimulation. J. Biol. Chem. 279 (14), 14201–6. 10.1074/jbc.M310427200. [DOI] [PubMed] [Google Scholar]
- Wang X.; Harris R. E.; Bayston L. J.; Ashe H. L. (2008) Type IV collagens regulate BMP signalling in Drosophila. Nature 455 (7209), 72–7. 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Abe H.; Tominaga T.; Matsubara T.; Abe N.; Kishi S.; Nagai K.; Murakami T.; Araoka T.; Doi T. (2012) Scleraxis modulates bone morphogenetic protein 4 (BMP4)-Smad1 protein-smooth muscle alpha-actin (SMA) signal transduction in diabetic nephropathy. J. Biol. Chem. 287 (24), 20430–42. 10.1074/jbc.M111.275610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T.; Araki M.; Abe H.; Ueda O.; Jishage K.; Mima A.; Goto C.; Tominaga T.; Kinosaki M.; Kishi S.; Nagai K.; Iehara N.; Fukushima N.; Kita T.; Arai H.; Doi T. (2015) Bone Morphogenetic Protein 4 and Smad1Mediate Extracellular Matrix Production in the Development of Diabetic Nephropathy. Diabetes 64 (8), 2978–90. 10.2337/db14-0893. [DOI] [PubMed] [Google Scholar]
- Fu W. J.; Fang Y. G.; Deng R. T.; Wen S.; Chen M. L.; Huang Z. H.; Tang H. H.; Xiong S. L.; Huang X. Z.; Wang Q. (2013) Correlation of high urinary Smad1 level with glomerular hyperfiltration in type 2 diabetes mellitus. Endocrine 43 (2), 346–50. 10.1007/s12020-012-9741-9. [DOI] [PubMed] [Google Scholar]
- Sakurai A.; Ono H.; Ochi A.; Matsuura M.; Yoshimoto S.; Kishi S.; Murakami T.; Tominaga T.; Nagai K.; Abe H.; Doi T. (2019) Involvement of Elf3 on Smad3 activation-dependent injuries in podocytes and excretion of urinary exosome in diabetic nephropathy. PLoS One 14 (5), e0216788 10.1371/journal.pone.0216788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K.; Lu J.; Kou J.; Wu M.; Zhang L.; Yu H.; Zhang M.; Bao Y.; Chen H.; Jia W. (2015) Increased urinary Smad3 is significantly correlated with glomerular hyperfiltration and a reduced glomerular filtration rate and is a new urinary biomarker for diabetic nephropathy. BMC Nephrol. 16, 159. 10.1186/s12882-015-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. E.; Day M.; Jones S. S.; Dorai H. (2002) BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 61 (1), 51–60. 10.1046/j.1523-1755.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- Dendooven A.; van Oostrom O.; van der Giezen D. M.; Leeuwis J. W.; Snijckers C.; Joles J. A.; Robertson E. J.; Verhaar M. C.; Nguyen T. Q.; Goldschmeding R. (2011) Loss of endogenous bone morphogenetic protein-6 aggravates renal fibrosis. Am. J. Pathol. 178 (3), 1069–79. 10.1016/j.ajpath.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M.; Grieve D. J.; Ritchie R. H. (2017) Are targeted therapies for diabetic cardiomyopathy on the horizon?. Clin. Sci. 131 (10), 897–915. 10.1042/CS20160491. [DOI] [PubMed] [Google Scholar]
- Van Gaal L. F.; Mertens I. L.; De Block C. E. (2006) Mechanisms linking obesity with cardiovascular disease. Nature 444 (7121), 875–80. 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Sara J. D.; Wang F. L.; Liu L. P.; Su L. X.; Zhe J.; Wu X.; Liu J. H. (2015) Increased plasma BMP-2 levels are associated with atherosclerosis burden and coronary calcification in type 2 diabetic patients. Cardiovasc. Diabetol. 14, 64. 10.1186/s12933-015-0214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. X.; Duan D.; O’Neill K. D.; Moe S. M. (2006) High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol., Dial., Transplant. 21 (12), 3435–42. 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]
- Bostrom K. I.; Jumabay M.; Matveyenko A.; Nicholas S. B.; Yao Y. (2011) Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ. Res. 108 (4), 446–57. 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. T.; Liu C. F.; Tsai T. H.; Chen Y. L.; Chang H. W.; Tsai C. Y.; Leu S.; Zhen Y. Y.; Chai H. T.; Chung S. Y.; Chua S.; Yen C. H.; Yip H. K. (2012) Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. J. Transl. Med. 10, 145. 10.1186/1479-5876-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J. W.; Jang E. H.; Kim M. K.; Baek K. H.; Song K. H.; Yoon K. H.; Cha B. Y.; Son H. Y.; Lee K. W.; Jo H.; Kwon H. S. (2011) Serum BMP-4 levels in relation to arterial stiffness and carotid atherosclerosis in patients with Type 2 diabetes. Biomarkers Med. 5 (6), 827–35. 10.2217/bmm.11.81. [DOI] [PubMed] [Google Scholar]
- Miriyala S.; Gongora Nieto M. C.; Mingone C.; Smith D.; Dikalov S.; Harrison D. G.; Jo H. (2006) Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation 113 (24), 2818–25. 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- Koga M.; Yamauchi A.; Kanaoka Y.; Jige R.; Tsukamoto A.; Teshima N.; Nishioku T.; Kataoka Y. (2013) BMP4 is increased in the aortas of diabetic ApoE knockout mice and enhances uptake of oxidized low density lipoprotein into peritoneal macrophages. J. Inflammation (London, U. K.) 10 (1), 32. 10.1186/1476-9255-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G.; Hill M. A.; Sowers J. R. (2018) Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 122 (4), 624–638. 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.; Cheng X.; Lu J.; Li X. (2018) Exogenous BMP-7 Facilitates the Recovery of Cardiac Function after Acute Myocardial Infarction through Counteracting TGF-beta1 Signaling Pathway. Tohoku J. Exp. Med. 244 (1), 1–6. 10.1620/tjem.244.1. [DOI] [PubMed] [Google Scholar]
- Merino D.; Villar A. V.; Garcia R.; Tramullas M.; Ruiz L.; Ribas C.; Cabezudo S.; Nistal J. F.; Hurle M. A. (2016) BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovasc. Res. 110 (3), 331–45. 10.1093/cvr/cvw076. [DOI] [PubMed] [Google Scholar]
- Urbina P.; Singla D. K. (2014) BMP-7 attenuates adverse cardiac remodeling mediated through M2 macrophages in prediabetic cardiomyopathy. American journal of physiology. Heart and circulatory physiology 307 (5), H762–72. 10.1152/ajpheart.00367.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher C.; Singla D. K. (2013) SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One 8 (12), e84009 10.1371/journal.pone.0084009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Cui L.; Guan G.; Wang J.; Qiu C.; Yang T.; Guo Y.; Liu Z. (2017) Matrine suppresses cardiac fibrosis by inhibiting the TGFbeta/Smad pathway in experimental diabetic cardiomyopathy. Mol. Med. Rep. 17 (1), 1775–1781. 10.3892/mmr.2017.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernacka A.; Cavalera M.; Wang J.; Russo I.; Shinde A.; Kong P.; Gonzalez-Quesada C.; Rai V.; Dobaczewski M.; Lee D. W.; Wang X. F.; Frangogiannis N. G. (2015) Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice. Circ.: Heart Failure 8 (4), 788–98. 10.1161/CIRCHEARTFAILURE.114.001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talior-Volodarsky I.; Connelly K. A.; Arora P. D.; Gullberg D.; McCulloch C. A. (2012) alpha11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc. Res. 96 (2), 265–75. 10.1093/cvr/cvs259. [DOI] [PubMed] [Google Scholar]
- Chen P. Y.; Shih N. L.; Hao W. R.; Chen C. C.; Liu J. C.; Sung L. C. (2018) Inhibitory Effects of Momordicine I on High-Glucose-Induced Cell Proliferation and Collagen Synthesis in Rat Cardiac Fibroblasts. Oxid. Med. Cell. Longevity 2018, 3939714. 10.1155/2018/3939714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. J.; Zhang Y.; Connelly K.; Cox A. J.; Martin J.; Krum H.; Gilbert R. E. (2007) Tranilast attenuates diastolic dysfunction and structural injury in experimental diabetic cardiomyopathy. American journal of physiology. Heart and circulatory physiology 293 (5), H2860–9. 10.1152/ajpheart.01167.2006. [DOI] [PubMed] [Google Scholar]
- Kusuhara S.; Fukushima Y.; Ogura S.; Inoue N.; Uemura A. (2018) Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab J. 42 (5), 364–376. 10.4093/dmj.2018.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Singh R. P. (2018) The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. DIC 7, 212532. 10.7573/dic.212532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorena K.; Raczynska D.; Raczynska K. (2013) Biomarkers in diabetic retinopathy and the therapeutic implications. Mediators Inflammation 2013, 193604. 10.1155/2013/193604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S. (2006) TGFbeta pathobiology in the eye. Lab. Invest. 86 (2), 106–15. 10.1038/labinvest.3700375. [DOI] [PubMed] [Google Scholar]
- Matsubara H.; Hogan D. E.; Morgan E. F.; Mortlock D. P.; Einhorn T. A.; Gerstenfeld L. C. (2012) Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 51 (1), 168–80. 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouletreau P. J.; Warren S. M.; Spector J. A.; Peled Z. M.; Gerrets R. P.; Greenwald J. A.; Longaker M. T. (2002) Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg 109 (7), 2384–97. 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Wan G.; Liang S.; Qian C. (2019) Effect of bone morphogenetic protein-2 on diabetic retinopathy and its mechanism of action. Trop. J. Pharm. Res. 18 (5), 1069–1076. 10.4314/tjpr.v18i5.22. [DOI] [Google Scholar]
- Megyerdi S.; Akeel S.; Choksi K.; Ahmad S.; Othman A.; Liou G.; Shabrawey M. (2012) Interrelation between NADPH oxidase and BMP2/Smad Pathway in Diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 53 (14), 5413. [Google Scholar]
- Akeel S.; El-Awady A.; Hussein K.; El-Refaey M.; Elsalanty M.; Sharawy M.; Al-Shabrawey M. (2012) Recombinant bone morphogenetic protein-2 induces up-regulation of vascular endothelial growth factor and interleukin 6 in human pre-osteoblasts: role of reactive oxygen species. Arch. Oral Biol. 57 (5), 445–52. 10.1016/j.archoralbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Trousse F.; Esteve P.; Bovolenta P. (2001) Bmp4 mediates apoptotic cell death in the developing chick eye. J. Neurosci. 21 (4), 1292–301. 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt R. R.; Unda R.; Yeh L. C.; Vidro E. K.; Lee J. C.; Tsin A. T. (2006) Bone morphogenetic protein-4 enhances vascular endothelial growth factor secretion by human retinal pigment epithelial cells. J. Cell. Biochem. 98 (5), 1196–202. 10.1002/jcb.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi H.; Shinohara M.; Mao M.; Phaosawasdi P.; El-Zaatari M.; Zhang M.; Ji T.; Eaton K. A.; Dang D.; Kao J.; Todisco A. (2014) Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology 147 (2), 396–406. 10.1053/j.gastro.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Wang J.; Wang Y.; Jiang H.; Xu X.; Zhang C.; Li D.; Xu C.; Zhang K.; Qi Y.; Gong X.; Tang C.; Zhong N.; Lu W. (2014) Bone morphogenetic protein 4 inhibits liposaccharide-induced inflammation in the airway. Eur. J. Immunol. 44 (11), 3283–94. 10.1002/eji.201344287. [DOI] [PubMed] [Google Scholar]
- Yao H.; Li H.; Yang S.; Li M.; Zhao C.; Zhang J.; Xu G.; Wang F. (2016) Inhibitory Effect of Bone Morphogenetic Protein 4 in Retinal Pigment Epithelial-Mesenchymal Transition. Sci. Rep. 6, 32182. 10.1038/srep32182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; O’Meara S. J.; O’Brien C.; Kane R. (2007) The role of gremlin, a BMP antagonist, and epithelial-to-mesenchymal transition in proliferative vitreoretinopathy. Invest. Ophthalmol. Visual Sci. 48 (9), 4291–9. 10.1167/iovs.07-0086. [DOI] [PubMed] [Google Scholar]
- Beltramo E.; Porta M. (2013) Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr. Med. Chem. 20 (26), 3218–25. 10.2174/09298673113209990022. [DOI] [PubMed] [Google Scholar]
- Kane R.; Stevenson L.; Godson C.; Stitt A. W.; O’Brien C. (2005) Gremlin gene expression in bovine retinal pericytes exposed to elevated glucose. Br. J. Ophthalmol. 89 (12), 1638–42. 10.1136/bjo.2005.069591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane R.; Godson C.; O’Brien C. (2008) Chordin-like 1, a bone morphogenetic protein-4 antagonist, is upregulated by hypoxia in human retinal pericytes and plays a role in regulating angiogenesis. Mol. Vis. 14, 1138–1148. [PMC free article] [PubMed] [Google Scholar]
- Scharpfenecker M.; van Dinther M.; Liu Z.; van Bezooijen R. L.; Zhao Q.; Pukac L.; Lowik C. W.; ten Dijke P. (2007) BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J. Cell Sci. 120, 964–972. 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- Suzuki Y.; Ohga N.; Morishita Y.; Hida K.; Miyazono K.; Watabe T. (2010) BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J. Cell Sci. 123, 1684–1692. 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- Akla N.; Viallard C.; Popovic N.; Lora Gil C.; Sapieha P.; Larrivee B. (2018) BMP9 (Bone Morphogenetic Protein-9)/Alk1 (Activin-Like Kinase Receptor Type I) Signaling Prevents Hyperglycemia-Induced Vascular Permeability. Arterioscler., Thromb., Vasc. Biol. 38 (8), 1821–1836. 10.1161/ATVBAHA.118.310733. [DOI] [PubMed] [Google Scholar]
- Ntumba K.; Akla N.; Oh S. P.; Eichmann A.; Larrivee B. (2016) BMP9/ALK1 inhibits neovascularization in mouse models of age-related macular degeneration. Oncotarget 7 (35), 55957–55969. 10.18632/oncotarget.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin J. F.; Celeste A. J. (2006) Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discovery Today 11 (9–10), 405–11. 10.1016/j.drudis.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R. (2016) Inhibitors of the bone morphogenetic protein (BMP) signaling pathway: a patent review (2008–2015). Expert Opin. Ther. Pat. 26 (10), 1115–1128. 10.1080/13543776.2016.1217330. [DOI] [PubMed] [Google Scholar]