Abstract
Cannabinoid receptor 2 (CB2) is a promising therapeutic target for immunological modulation. There is, however, a deficit of knowledge regarding CB2 signaling and function in human primary immunocompetent cells. We applied an experimental paradigm which closely models the in situ state of human primary leukocytes (PBMC; peripheral blood mononuclear cells) to characterize activation of a number of signaling pathways in response to a CB2-selective ligand (HU308). We observed a “lag” phase of unchanged cAMP concentration prior to development of classically expected Gαi-mediated inhibition of cAMP synthesis. Application of G protein inhibitors revealed that this apparent lag was a result of counteraction of Gαi effects by concurrent Gαs activation. Monitoring downstream signaling events showed that activation of p38 was mediated by Gαi, whereas ERK1/2 and Akt phosphorylation were mediated by Gαi-coupled βγ. Activation of CREB integrated multiple components; Gαs and βγ mediated ∼85% of the response, while ∼15% was attributed to Gαi. Responses to HU308 had an important functional outcome—secretion of interleukins 6 (IL-6) and 10 (IL-10). IL-2, IL-4, IL-12, IL-13, IL-17A, MIP-1α, and TNF-α were unaffected. IL-6/IL-10 induction had a similar G protein coupling profile to CREB activation. All response potencies were consistent with that expected for HU308 acting via CB2. Additionally, signaling and functional effects were completely blocked by a CB2-selective inverse agonist, giving additional evidence for CB2 involvement. This work expands the current paradigm regarding cannabinoid immunomodulation and reinforces the potential utility of CB2 ligands as immunomodulatory therapeutics.
Keywords: cannabinoid receptor 2 (CB2), G protein-coupled receptor (GPCR), signaling, leukocytes, interleukin 6, interleukin 10
Cannabinoid Receptor 2 (CB2) is a class A G protein-coupled receptor (GPCR) which is expressed primarily in the immune system1,2 and is a promising therapeutic target for immune modulation in a wide range of disorders.3 CB2 couples to Gαi/o proteins inhibiting adenylyl cyclases,4,5 and limited evidence indicates coupling to Gαq.6 To date, there is no evidence for CB2 coupling to Gαs; although increased cyclic adenosine monophosphate (cAMP) synthesis has been reported, this was coupled to Gαi/o, likely via Gβγ.7,8 Downstream of G protein coupling, and perhaps involving modulation by β-arrestins,9 activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) is a widely studied consequence of CB2 activation reported in cell lines heterologously expressing this receptor10 and in rodent11 and human immunocompetent cell lines,8,12 with limited published evidence for such signaling in human primary leukocytes.8,13 The paucity of studies on human primary leukocytes may in part be due to methodological difficulties such as low cell yields. Mitogenic or antigenic proliferative stimulations are widely used approaches to overcome some of these practical limitations.13,14 However, these methods cause lymphocytes to dedifferentiate and turn into lymphoblasts—mitotically overactive cells—which do not closely model the in situ state of leukocytes.15
The limited literature regarding CB2 signaling via other types of mitogen-activated protein kinases (MAPKs) indicates context-dependence of activation. CB2 can induce p38 phosphorylation (p-p38)12,16,17 which has been linked to growth inhibition of cancer cells, while dendritic cells18 and LPS-stimulated monocytes12 were nonresponsive. Akt kinase (protein kinase B), an important mediator of immunomodulation19 can be activated,20 inhibited,21 or unaffected22 by CB2 activation. A stress-activated protein kinase JNK (c-Jun NH2-terminal kinase), involved in pro-inflammatory signaling,23 has been shown to be activated24,25 and inhibited12 via CB2.
The ultimate outcomes of CB2 activation in immune cells include inhibition of proliferation, induction of apoptosis, influences on cytokine/chemokine networks, and regulation of adhesion and migration.26 In human immunocompetent cells CB2 activation inhibits IL-2,27 IL-17, INF-γ, TNF-α28 secretion, and stimulates IL-429 and TGF-β30 secretion. Murine in vitro and in vivo models indicate that cannabinoids inhibit IL-2,31 IL-12, and IFN-γ,32,33 and induce IL-4 secretion.32,33 However, these studies stimulated leukocytes with mitogens or antibodies. Species differences in cannabinoid signaling notwithstanding,5 cytokine secretion is generally highly dependent on the cellular environment. Indeed, cannabinoid effects on the cytokine network are sensitive to the method of cell activation.34
Therefore, close to the entirety of our current understanding of CB2 signaling and influence on the cytokine secretome has been obtained from models which exhibit considerable limitations in modeling normal human physiology. We endeavored to carry out a comprehensive study of CB2 signaling in unstimulated human primary leukocytes (peripheral blood mononuclear cells, PBMC) under conditions closely preserving their in vivo state. We have observed an unprecedented CB2-mediated signaling and functional profile, including coupling to Gαs.
Results
CB2, but Not CB1, Is Expressed in Unstimulated Human Primary PBMC
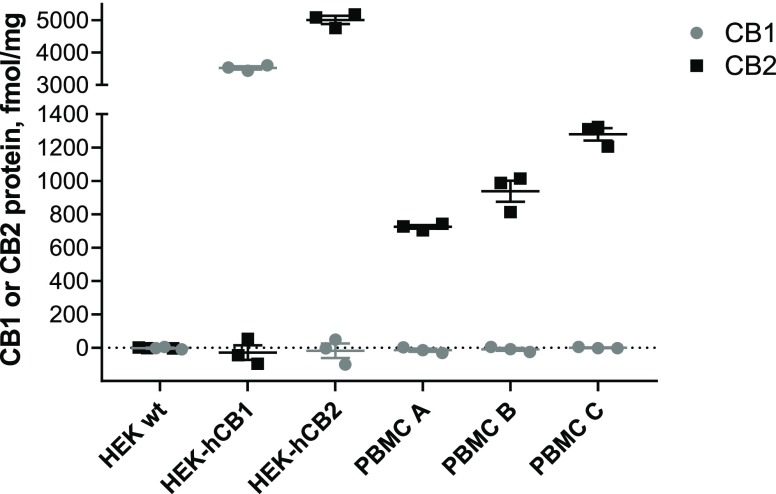
We quantified expression of CB1 and CB2 protein by whole cell radioligand binding with high affinity CB1/CB2 ligand [3H]-CP55940 displaced by CB1- and CB2- selective ligands, ACEA35 and HU30836, respectively. Although CB2 was expected to be the primary cannabinoid-responsive receptor in human PBMC, CB1 transcripts have also been detected in some contexts.1 Whereas CB1 protein could not be detected in PBMC from any of three healthy human donors, CB2 was readily detectable (Figure 1). Intrasubject variability in CB2 expression was low (independent experiments are from three blood draws from the same donor taken over the course of up to 8 months), while intersubject expression was within the same magnitude, ranging from 704 to 1323 fmol/mg. PBMC samples from these same three donors were utilized in subsequent experiments.
Figure 1.
CB1 and CB2 protein quantification by whole cell radioligand binding. Cells were incubated with [3H]-CP55940 (5 nM) in the presence and absence of a CB1-selective displacer ACEA (1 μM), or a CB2-selective displacer HU308 (1 μM). Total binding sites (Bmax) were calculated (see Methods) and converted to fmol/mg of total protein. HEK wt is a negative control (not expressing CB1 or CB2), HEK-hCB1 is a positive control for CB1, HEK-hCB2 is a positive control for CB2. PBMC A, B, and C are from subjects A, B, and C, respectively. The graph shows independent experiment means (from technical triplicate) as well as overall mean ± SEM of these three independent experiments each performed with cells from a separate subject (three subjects in total).
CB2 Activation Induces Simultaneous Gαi and Gαs Coupling, Producing Delayed cAMP Flux
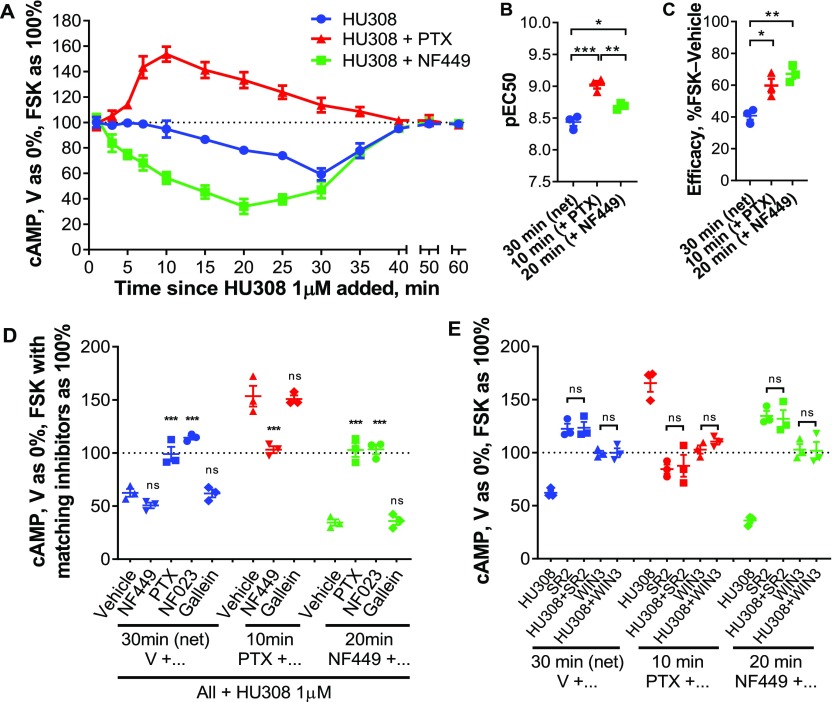
CB2 is widely recognized to inhibit cAMP production via Gαi/o inhibition of adenylyl cyclases. Depending on basal adenylyl cyclase activity in the sample of interest, stimulation of adenylyl cyclase (e.g., with forskolin) is a typical prerequisite for measuring inhibition.4,10 We first studied the time-course of cAMP signaling in PBMC in response to CB2-selective agonist HU308 (1 μM), and observed inhibition of forskolin-stimulated cAMP synthesis in line with our general expectations (Figure 2A). However, there was a lag to the onset of measurable inhibition which lasted at least 10 min, with the 20–35 min time-points being significantly different from time-matched vehicle control (p < 0.0001–0.0005; two-way repeated measures [RM] ANOVA with Holm-Šídák posthoc test). This was followed by a steady reduction in cAMP concentration, reaching a maximum extent by 30 min (to 60.3 ± 2.8% of forskolin-stimulated), with cAMP subsequently returning back to the forskolin-induced level by 60 min. Prior studies in cells overexpressing CB2 describe an immediate onset and earlier peak cAMP inhibition.37 Given that we cannot readily measure inhibition of cAMP production without forskolin being present, we hypothesized that low basal cAMP and/or slow response to forskolin in the primary leukocytes might mask the ability to detect inhibition at early time-points and hence produce the observed delay. However, preincubation with forskolin prior to HU308 addition did not influence the signaling latency, time to peak response, nor efficacy, therefore ruling out this hypothesis (Supporting Information, Figure S1).
Figure 2.
cAMP signaling in human primary PBMC. (A) PBMC were incubated with 10 μM forskolin (FSK) and HU308 (1 μM) without inhibitors (blue ●), or after pretreatment with NF449 (10 μM for 30 min, green ■), or with PTX (100 ng/mL for 6 h, red ▲). The graph shows mean ± SEM of three independent experiments performed in technical triplicate, each with cells from a separate subject (three subjects in total). (B,C) HU308 concentration–response curve parameters; potency (B) and efficacy (C; span of curves as in Figure S3). Statistical comparisons by one-way ANOVA with Tukey posthoc test. (D,E) Stimulations were with 10 μM forskolin and 1 μM HU308 or vehicle for 30 min for net cAMP flux, 10 min for the stimulatory pathway (revealed by PTX), or 20 min for the inhibitory pathway (revealed by NF449). (D) PBMC were additionally pretreated as indicated with PTX, NF449, NF023, or gallein. Data are normalized to vehicle control as 0% and forskolin controls with matching inhibitors as 100%. Statistical comparisons are to the vehicle plus HU308 control within each set, measured by one-way ANOVA with Tukey posthoc test. (E) PBMC were pretreated as indicated, then coincubated with forskolin (10 μM), SR144528 (SR2, 1 μM), or WIN55212-3 (WIN-3, 5 μM), and HU308 (100 nM) or vehicle for 10, 20, or 30 min. Data are normalized to vehicle control as 0% and forskolin control as 100%. All SR2 and WIN-3 conditions were significantly different from “HU308” control for peak signaling within each set (p < 0.0001–0.004). Within each set, condition “SR2” was compared to “SR2 + HU308”, “WIN3” was compared to “WIN3 + HU308”, and had no significant difference. Statistical comparisons by one-way ANOVA with Tukey posthoc test. Graphs B–E show independent experiment means (from technical triplicate) as well as overall mean ± SEM of these three independent experiments each performed with cells from a separate subject (three subjects in total).
Preincubation with pertussis toxin (PTX), a Gαi38 and Gαi-derived βγ39 inhibitor, followed by its coapplication with HU308 revealed a wave of increased cAMP (Figure 2A). This induction of cAMP accumulation had an earlier onset than the net cAMP inhibition (without PTX), with peak activation at around 10 min, and a slower return to forskolin-stimulated levels than the net cAMP flux, with the 7–25 min time-points being significantly different from time-matched vehicle control (p < 0.0001–0.011; two-way RM ANOVA with Holm-Šídák posthoc test). Given that increased cAMP is associated with Gαs coupling, we tested the effect of Gαs-blocker NF44940 on HU308-stimulated cAMP flux. In comparison with net cAMP flux, not only was the peak of inhibition shifted to an earlier time-point (20 min), but the onset of cAMP inhibition had no apparent delay, with the 5–35 min time-points being significantly different from time-matched vehicle control (p < 0.001–0.035; two-way RM ANOVA with Holm-Šídák posthoc test). The time-courses for onset of reduction and return to forskolin-stimulated cAMP levels for this now apparently “pure” inhibitory signaling had similar timing.
These findings indicate that when no signaling inhibitors are present the opposing influences of Gαi and Gαs coupling produce a net apparent “lag” to the onset cAMP flux. At later time-points, the different time-courses and relative efficacies of Gαi versus Gαs coupling to adenylyl cyclase(s) result in transient net inhibition of cAMP synthesis. Increased cAMP in response to HU308 was detectable even without forskolin stimulation, although inhibition of Gαi was required to reveal this as was the case when forskolin was present (Figure S2).
cAMP fluxes for the three types of responses at their respective peak time-points (net cAMP, 30 min; cAMP accumulation, 10 min; cAMP inhibition, 20 min) were of nanomolar potency, typical for CB2-mediated signaling by HU308,5,41 with subtle differences in potencies between the three cAMP responses with rank order: stimulatory cAMP > “pure” inhibitory > net cAMP (Figure 2B, Figure S3). Not surprisingly from the opposing effects on cAMP concentration, the peak stimulatory and “pure” inhibitory cAMP responses revealed by the Gα inhibitors had greater efficacies than the maximal net cAMP response (Figure 2C, Figure S3). Between-subject variability was small.
We further probed the contribution of G proteins (Figure 2D) and investigated CB2-specificity (Figure 2E). As expected from the time-course data, Gαs inhibitor NF449 had a minimal effect on the net cAMP response peak (30 min), while Gαi/o/βγ inhibitor PTX and Gαi inhibitor NF02342 blocked the HU308-induced inhibition of cAMP synthesis. The “pure” inhibitory response (in the presence of NF449) was also completely blocked by the Gαi/o inhibitors. In contrast, the peak stimulation of cAMP synthesis (measured in the presence of PTX) was completely blocked by Gαs inhibitor NF449. A Gβγ inhibitor gallein43 had no effect on cAMP signaling, indicating lack of Gβγ involvement (Figure 2D). As well as the potencies of HU308-induced cAMP flux being consistent with CB2-specific responses, inverse agonist SR14452844 at 1 μM (a concentration at which it binds to CB2, but not to CB1, Figure S4) completely blocked cAMP signaling induced by HU308 (Figure 2E). Inverse agonism of SR144528 was readily measurable for the inhibitory and net cAMP pathways, in which it increased cAMP above forskolin-induced levels (p < 0.0001, p = 0.0005, respectively; two-way RM ANOVA with Holm-Šídák posthoc test). There was a trend toward inverse agonism in the stimulatory cAMP pathway, but this reduction in cAMP was not significantly different from forskolin alone (p = 0.78; two-way RM ANOVA with Holm-Šídák posthoc test). A CB1/CB2 neutral antagonist WIN55212–345,46 (no significant differences from forskolin controls, p = 0.80–0.98; two-way RM ANOVA with Holm-Šídák posthoc test) also completely abrogated all HU308-induced cAMP signaling (Figure 2E). Given the lack of CB1 protein in PBMC (Figure 1), WIN55212-3 most likely acted via CB2, and taken together with the SR144528 data and response potencies provides strong evidence that the observed signaling is CB2-mediated.
CB2-Stimulated Phosphorylation of ERK1/2 Is Mediated by Gαi- but Not Gαs-Coupled Gβγ
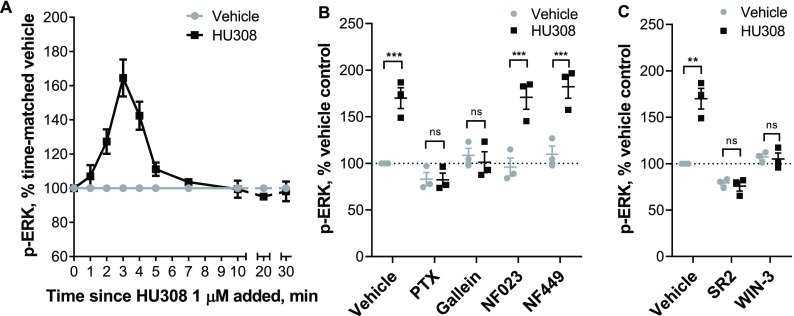
Phosphorylation of ERK1/2 (p-ERK) is a widely observed CB2 response, but the potential pathways to stimulation are many and varied and p-ERK can act as an integrator of responses.47 Time-courses with 1 μM HU308 revealed transient activation of p-ERK, with a peak at around 3 min (Figure 3A). HU308 activated ERK1/2 in a concentration-dependent manner (Figure S5), with approximately one log unit lower potency than observed for cAMP fluxes (pEC50 7.69 ± 0.06). Responses were very consistent between the three donors. Neither Gαi inhibitor NF023 nor Gαs inhibitor NF449 had an effect on the observed ERK1/2 signaling (Figure 3B), whereas Gαi/o/βγ inhibitor PTX and Gβγ inhibitor gallein both completely blocked activation of p-ERK. This indicates that p-ERK1/2 induced by HU308 is mediated by Gαi-linked βγ dimers. We further verified that the observed p-ERK1/2 response was CB2-mediated by coapplying HU308 with SR144528 or WIN55212-3 (Figure 3C). Both antagonists completely blocked p-ERK, supporting the inference that HU308 binding to CB2 receptors is the only initiator of the measured p-ERK signal. Furthermore, we observed no p-ERK response for CB1-selective agonist ACEA at 1 μM (Figure S6), a concentration which binds to CB1 but not CB2 (Figure 1) and can induce CB1-mediated p-ERK.48
Figure 3.
ERK1/2 signaling in human primary PBMC. A. Time-course with 1 μM HU308 (■) or a vehicle control (gray ●). The 2, 3, and 4 min time-points are significantly different from time matched vehicle controls (p < 0.0001; two-way RM ANOVA with Holm-Šídák posthoc test). The graph shows mean ± SEM of three independent experiments performed in technical triplicate, each with cells from a separate subject (three subjects in total). (B) Incubation (3 min) with HU308 (1 μM) or vehicle control in the presence of G protein inhibitors PTX, gallein, NF023, NF449, or vehicle control. (C) Incubation (3 min) with HU308 (100 nM) or vehicle control in the presence of SR144528 (SR2, 1 μM), WIN55212-3 (WIN-3, 5 μM), or vehicle control. Data are normalized to vehicle control (100%). Graphs B and C show independent experiment means (from technical triplicate) as well as overall mean ± SEM of these three independent experiments each performed with cells from a separate subject (three subjects in total). Statistical comparisons by two-way RM ANOVA with Holm-Šídák posthoc test.
CB2 Induces Phosphorylation of p38 via Gαi and Akt via Gβγ, but Does Not Activate JNK
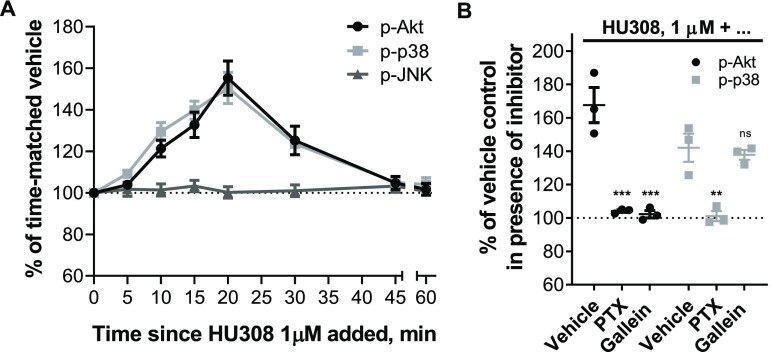
Time-courses stimulating PBMC with 1 μM HU308 revealed phosphorylation of Akt and p38 with signaling peaks at around 20 min, and with similar overall kinetics (Figure 4A). Conversely, there was no phosphorylation of JNK detected. Application of PTX completely abrogated Akt and p38 phosphorylation (Figure 4B), implicating the involvement of Gαi/βγ proteins. Gβγ inhibitor gallein completely blocked p-Akt, but did not influence p-p38 (Figure 4B). HU308 therefore induces phosphorylation of p38 downstream of Gαi subunits, while Akt is activated via Gαi-coupled βγ heterodimers.
Figure 4.
Phosphorylation of Akt, JNK, and p38 MAP kinases in human primary PBMC. (A) Time-courses with HU308 (1 μM) and vehicle control. For p-Akt and p-p38 time-points 10–30 min are significantly different from time-matched vehicle controls (p < 0.0001–0.0002; two-way RM ANOVA with Holm-Šídák posthoc test). For p-JNK, all points are indistinguishable from 0 min (p = 0.988; one-way ANOVA). The graph shows mean ± SEM of three independent experiments performed in technical triplicate, each with cells from a separate subject (three subjects in total). (B) Responses to 20 min 1 μM HU308 after pretreatment with PTX, gallein, or vehicle control. Data are normalized and statistically compared to the corresponding vehicle plus HU308 control by two-way RM ANOVA with Holm-Šídák posthoc test. The graph shows independent experiment means (from technical triplicate) as well as overall mean ± SEM of these three independent experiments each performed with cells from a separate subject (three subjects in total).
CB2 Induces p-CREB Downstream of Gαs and Gβγ
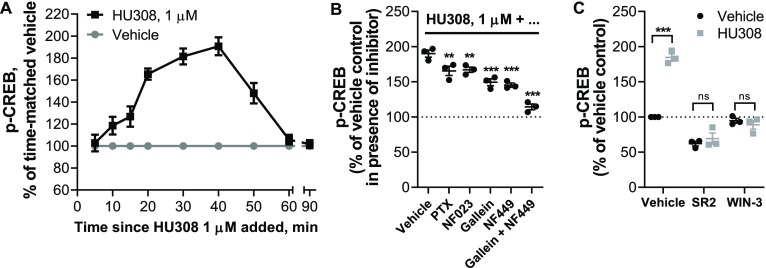
CREB phosphorylation is a classical downstream consequence of Gαs and cAMP-mediated activation of protein kinase A (PKA).49 A time-course with PBMC stimulated by 1 μM HU308 (Figure 5A) revealed a wave of CREB phosphorylation which was maximal around 30–40 min and resolved by 60 min. We applied G protein inhibitors NF023, NF449, gallein, and PTX (Figure 5B), and found that both NF449 and gallein each partially inhibit CREB phosphorylation to a similar degree, but almost completely block the HU308 response when coapplied indicating that Gαs and βγ heterodimers are similarly important in CREB phosphorylation in this context. p-CREB was reduced by PTX and NF023 to a lesser extent, with equivalent efficacy to each other, implying a minor involvement of Gαi but not Gαi-coupled βγ in p-CREB activation. SR144528 and WIN55212-3 completely blocked the peak CREB phosphorylation (Figure 5C), indicating that this pathway activation occurs downstream of CB2. SR144528 reduced p-CREB relative to the vehicle control indicating that this compound acted as an inverse agonist (p = 0.013; two-way RM ANOVA with Holm-Šídák posthoc test).
Figure 5.
Phosphorylation of CREB in human primary PBMC. (A) Time-course with HU308 (1 μM) and vehicle control. 10–50 min time-points are significantly different from time-matched vehicle controls (p < 0.0001–0.0022; two-way RM ANOVA with Holm-Šídák posthoc test). The graph shows mean ± SEM of three independent experiments performed in technical triplicate, each with cells from a separate subject (three subjects in total). (B) p-CREB stimulated by HU308 (1 μM) for 40 min in the presence of G protein inhibitors gallein, NF023, NF449, PTX, or vehicle control; data are normalized to responses in the absence of HU308 (100%); statistical comparisons are to the HU308 response without inhibitors (one-way RM ANOVA with Holm-Šídák posthoc test). (C) p-CREB stimulated by a CB2-selective agonist HU308 (100 nM) or vehicle control at 40 min in the presence of a CB2-selective inverse agonist/antagonist SR144528 (SR2, 1 μM), a CB1/CB2 neutral antagonist WIN55212-3 (WIN-3, 5 μM), or vehicle control; data are normalized to vehicle control (100%). Two-way RM ANOVA with Holm-Šídák posthoc test. Graphs B and C show independent experiment means (from technical triplicate) as well as overall mean ± SEM of these three independent experiments each performed with cells from a separate subject (three subjects in total).
CB2 Activation Induces Secretion of Cytokines IL-6 and IL-10
We stimulated PBMC with HU308 under the same conditions as used for the signaling assays and detected cytokines released into the assay media. After 12 h, interleukin 6 (IL-6) and 10 (IL-10) concentrations were significantly different from vehicle-treated cells (both p < 0.0001; two-way RM ANOVA with Holm-Šídák posthoc test), whereas the remaining seven cytokines in our panel (IL-2, IL-4, IL-12, IL-13, IL-17A, MIP-1α, and TNF-α) were indistinguishable from vehicle-treated cells (all p ≥ 0.999; Figure S7). HU308 was also coapplied with forskolin (10 μM) to mimic conditions of our cAMP signaling assays; forskolin did not induce detectable cytokine secretion by itself (p = 0.733; two-way RM ANOVA; Figure S7) and did not influence the HU308-mediated IL-6 and IL-10 levels (p = 0.834; two-way RM ANOVA; Figure S7). The responses were very similar between PBMC from three subjects and cell numbers were unaffected by the applied ligands (Figure S8). Subsequent interleukin experiments were carried out without forskolin present.
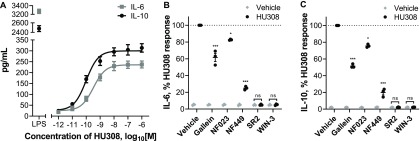
IL-6 and IL-10 accumulation were also detectable at an earlier time-point—after 6 h of HU308 stimulation (Figure 6A). This was concentration-dependent, with HU308 having greater potency for inducing IL-10 (pEC50 10.06 ± 0.08 and 9.55 ± 0.04; p = 0.005, unpaired t test). Application of G protein inhibitors revealed that HU308-induced IL-6 and IL-10 secretion are almost fully blocked by Gαs inhibitor NF449, partially blocked by βγ inhibitor gallein, and to a lesser extent sensitive to Gαi inhibitor NF023 (Figure 6B,C). Responses to HU308 (10 nM) were fully abrogated by SR144528 (1 μM) and WIN55212-3 (5 μM) down to levels below the assays’ limits of quantitation. SR144528 and WIN55212-3 had no measurable effects on IL-6 or IL-10 production when applied alone.
Figure 6.
Induction of cytokine secretion by PBMC. (A) Induction of IL-6 and IL-10 secretion in response to 6 h incubation with HU308. Lipopolysaccharide (LPS, 10 ng/mL) is a positive control. The graph shows mean ± SEM of three independent experiments performed in technical triplicate, each with cells from a separate subject (three subjects in total). (B) IL-6 and (C) IL-10 accumulation in the presence of G protein inhibitors or CB2 antagonists: PBMC were pretreated with gallein, NF023, NF449, SR144528 (SR2, 1 μM), or WIN55212-3 (WIN-3, 5 μM) for 30 min followed by 6 h treatments with HU308 (10 nM) with the inhibitors/antagonists present for the entire duration of incubation; data are normalized to HU308 responses without inhibitors/antagonists. Effects of inhibitors on HU308 responses were statistically compared with the corresponding HU308 response without inhibitor via two-way RM ANOVA with Holm-Šídák posthoc test. In the presence of SR2 and WIN-3, there was no statistical difference between HU308 and the HU308 vehicle control. Graphs B and C show independent experiment means (from technical triplicate) as well as overall mean ± SEM of these three independent experiments each performed with cells from a separate subject (three subjects in total).
Discussion
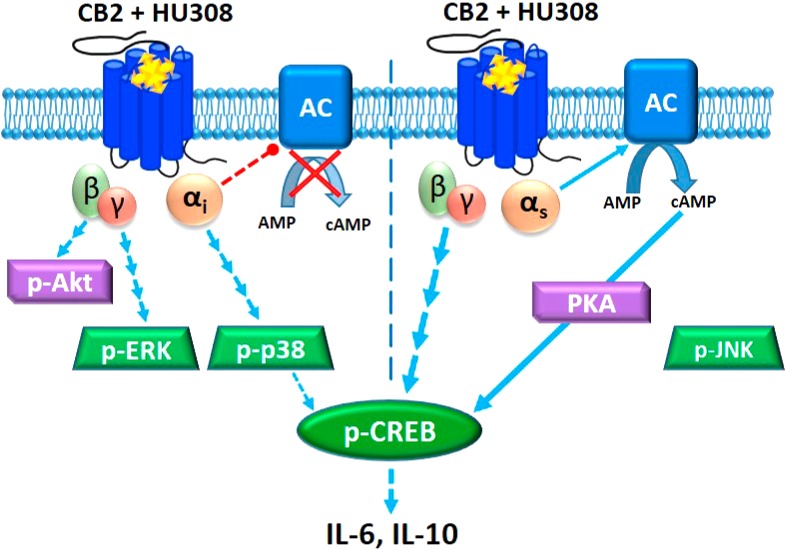
To our knowledge this study represents the first investigation of CB2 signaling and functional implications thereof in unstimulated human primary PBMC. Cells were kept in culture for as little time as practically possible (up to 6 h prior to stimuli of interest) and in 10% serum in order to mimic the state of leukocytes in vivo; a unique experimental paradigm in comparison with the vast majority of other cannabinoid studies to date. Obtaining an accurate understanding of CB2 function in normal cells is critical to meaningful interpretation of CB2 function in abnormal conditions and in assessing therapeutic potential. The signaling profile we observed is summarized in Figure 7.
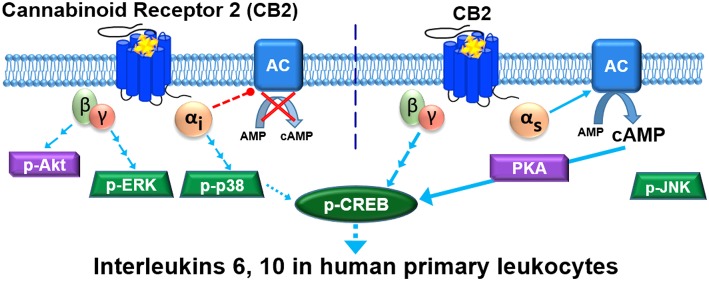
Figure 7.
CB2 signaling network in mixed human primary leukocytes. Binding of HU308 to CB2 leads to activation of Gαiβγ and Gαsβγ heterotrimers. Gαi inhibits adenylyl cyclase(s) (AC) and activates a cascade leading to phosphorylation of p38 and CREB. βγ from Gαiβγ activates Akt and initiates a cascade leading to phosphorylation of ERK. Gαs stimulates AC which leads (likely via protein kinase A, PKA) to p-CREB. βγ from non-Gαiβγ (likely from Gαsβγ) also contributes to p-CREB activation. On the basis of inhibitor effects (Figure 5B), Gαs and non-Gαiβγ are the major mediators of p-CREB, whereas p-p38 is a minor p-CREB mediator. JNK phosphorylation was not observed. Given that Gαi-mediated inhibition of AC does not inhibit p-CREB activation it is likely that Gαi and Gαs modulations of AC are either segregated in separate compartments within one cell, or occur in different cells (represented by vertical dotted line). p-CREB likely mediates IL-6/IL-10 induction, which might be released from one cell or from different cell types.
We found that human primary PBMC respond in a concentration-dependent manner to CB2-selective agonist HU308 with a net inhibition of forskolin-induced cAMP synthesis; however, a surprising feature was a latency of approximately 10 min prior to any measurable change in cAMP concentration. Application of Gα inhibitors revealed that CB2 simultaneously activates stimulatory and inhibitory cAMP pathways, explaining the apparent delay in net cAMP inhibition which arises from these oppositely directed responses balancing each other at early time-points. Response potencies were consistent with CB2 specificity, and signaling was also sensitive to SR144528 and WIN55212-3, indicating that the observed signaling is CB2-mediated. Although the idea that CB2 may couple promiscuously to G proteins is not unprecedented,6,10 to our knowledge CB2 coupling to Gαs has not been reported previously.
Börner and colleagues8 observed stimulatory effects of CB1/CB2 mixed agonists JWH015, THC, and methanandamide on cAMP concentrations in IL-4-stimulated human primary T lymphocytes after prolonged cannabinoid incubations; however, cAMP increases were sensitive to PTX and therefore concluded to be downstream of Gαi, likely via βγ.8 Promiscuous coupling of other receptors to Gαi and Gαs has been observed, including CB1 in our own hands.50 However, we did not observe Gαs coupling of human CB2 in this same model. Studies in other models have also applied PTX and not revealed CB2 signaling consistent with Gαs coupling.10,51,52 We therefore speculate that a critical component of the CB2 immune cell signaling landscape has not been captured in previously studied models.
As we have studied mixed PBMC we cannot provide direct evidence as to whether both cAMP inhibition and stimulation have been induced in the same cell or if our findings arise as a result of assaying a mixture of Gαi-predominant versus Gαs-predominant cell types. Leukocytes are known to express Gαi,53,54 Gαs,39 and Gαi/s-sensitive adenylyl cyclases.55 However, balance between Gα and Gβγ subunit expression may determine overall cAMP responses.56 As well, adenylyl cyclase isoforms can differ in their Gα sensitivities, and expression levels can vary between immune cell types.55 Furthermore, G protein coupling “switches” have been reported for receptors in heterodimers.57,58 The particular profile and stoichiometries of interaction partners can give rise to differential signaling “units” between cell types.59 There are also various explanations for potential dual coupling within the same cell. Signalsome subcellular compartmentalization and/or sequestration of signaling components is one example;60 indeed, subcellular distribution of CB2 can influence CB2 signaling patterns,6 and CB2 seems to have a particularly unique distribution in immune cells.61−63 Homologous signaling feedback upon CB2 activation which results in rapid desensitization of Gαs coupling with little or slower influence on Gαi coupling such as via differential phosphorylation by G Protein-Coupled Receptor Kinases or PKA64−66 might assist in explaining the observed cAMP signaling profile. Gβγ exhaustion/sequestration may also hinder Gαs activation.67,68 It is also important to note and intriguing regarding the downstream functional implications, that subcellular compartmentalization of G proteins and/or adenylyl cyclase subtypes could give rise to localized cAMP fluxes, implying that simultaneous effects of both cAMP increases and decreases are possible even when the net cAMP concentration is unchanged.69
The ERK1/2 signaling cascade regulates cell cycle progression,70 immunological functions, including differentiation of T lymphocytes,14 polarization of CD4+ T lymphocytes,71 and regulation of cytokine network,72 and is dysregulated in a number of disease states.23 We find that human primary PBMC respond to CB2 activation by HU308 with rapid and transient stimulation of p-ERK. As this was completely sensitive to both Gβγ inhibitor gallein and Gαi/o/βγ inhibitor PTX, we conclude that ERK1/2 phosphorylation induced by HU308 is mediated by βγ dimers of Gαiβγ heterotrimeric proteins. Although in some systems gallein may inhibit GRK2 and possibly associated arrestin signaling,73 we do not know of any studies suggesting that PTX blocks arrestin-initiated signaling other than one report of a small potency shift for arrestin recruitment.74 Interestingly NF023, which we confirmed in cAMP assays inhibits Gαi, did not block the p-ERK response, indicating that this blocker specifically inhibits Gα subunits without influencing Gβγ activity as also observed previously.75 HU308 also induced phosphorylation of p38 and Akt. p-p38 was mediated by Gαi/o subunits of G proteins (possibly via Src kinase),76 as it was completely blocked by Gαi/o/βγ inhibitor PTX but not by Gβγ inhibitor gallein, whereas p-Akt was mediated by Gαi/o–coupled βγ heterodimers (fully blocked by PTX and gallein).
In contrast with prior studies of human promyelocytic leukemia HL60 cells25 and CB2-overexpressing HEK cells24 we did not observe JNK phosphorylation. However, we note that activation of this pathway may be cell-context dependent given that in LPS-stimulated human monocytes JNK was inhibited by a CB2 ligand.12 Another explanation may be a different CB2-mediated functional profile (i.e., biased agonism) between HU308 and the previously studied ligands.5,77 There may also be signal-modifying influences of serum as has been shown for CB1.78 The serum factors that circulating immune cells would be exposed to in situ are likely to influence signaling patterns.79 We therefore feel that inclusion of 10% fetal bovine serum is an important methodological improvement upon earlier studies carried out under low serum or serum-free conditions. However, we must acknowledge that this is of course not a perfect model of human serum from the matched donor. Some of the resistance to inclusion of serum in cannabinoid signaling studies is the potential for presence of endocannabinoids and precursor molecules which would complicate the interpretation of reductionist experiments.80 However, careful attention to vehicle controls, the potency of HU308 effects, only subtle inverse agonist effects, and lack of effects of a neutral antagonist when applied alone, indicate that any influence in this regard was minimal to nonexistent in our study.
cAMP/PKA, MAP kinases ERK and p38, and Akt/PKB all have the potential to induce phosphorylation of CREB.49 Immunological functions of p-CREB include antiapoptotic effects on macrophages via p-38,81 proliferative effects on T lymphocytes via p-3882 and p-ERK,83 and activation/proliferation of B lymphocytes via p-ERK.84 CB2-mediated p-CREB activation in primary PBMC is mediated primarily and to a similar degree by Gαs subunits and non-Gαi-derived βγ dimers, and to a limited extent by Gαi subunits. Though Gαs-coupled βγ is not widely appreciated to induce signaling events, at least a few examples exist in the literature.85,86 p-CREB is unlikely to be downstream of pERK or p-Akt in our system given that these pathways were both associated with βγ from Gαiβγ heterotrimers, however Gαi-coupled p-p38 may be involved to a minor degree. One of the primary p-CREB activation pathways is likely via Gαs leading to cAMP-activated PKA. This is interesting given that when measuring cAMP concentrations we did not detect any increase unless a Gαi blocker was present. This implies that the consequences of Gαi versus Gαs activation on cAMP concentration are distinct, supporting theories that either CB2 is signaling differently between cell types or that subcellular compartmentalization can give rise to differential local cAMP concentrations with independent consequences for signaling.
Proceeding to study a physiologically relevant outcome, we detected a CB2-mediated induction of interleukins 6 and 10 at 6 and 12 h. Both were sensitive to NF449 > gallein > NF023, indicating that Gαs is the main contributor to these responses. This pattern of influence of G protein inhibitors was similar to that for p-CREB, and given that p-CREB can induce transcription of IL-6 and IL-10 genes,87 it is very feasible that CREB phosphorylation is an important mediator of HU308-induced IL-6 and IL-10. Although in some instances IL-6 may be secreted from a preformed pool,88 the observed induction of IL-6 by HU308 at 6 and 12 h likely involves its de novo synthesis.89 To our knowledge, PBMC do not have intracellular granules of IL-10, and so it is most likely being synthesized de novo and then secreted. Kinetics of mRNA induction for IL-6 and IL-1090,91 are consistent with a multihour lag in their production after CB2 activation.
Given that p-p38 was blocked by PTX and not gallein, this is a candidate for the NF023-sensitive (Gαi-requiring) component of IL-6/10 induction. p-p38 and p-Akt have also been linked to both IL-692,93 and IL-10 production.94,95 Given the indicated involvement of CREB we were surprised to find that forskolin, a strong activator of signaling pathways downstream of adenylyl cyclases, did not influence cytokine secretion. We postulate that a costimulus enabled by CB2 activation but not by forskolin might be required for IL-6/10 induction.49 It is also plausible that the potential for subcellular compartmentalization and/or temporal integration of cAMP fluxes play critical roles. Application of specific signaling pathway inhibitors would be useful to more directly delineate the exact signaling pathways responsible.
The degree of cytokine induction we observed was small in comparison with the positive control utilized, LPS—a potent mediator of bacterial sepsis,96 which models systemic inflammation in vitro. Given that HU308 was ∼10 times less efficacious than LPS, it is unlikely that CB2 activation would result in dramatic systemic inflammatory reactions in vivo, but might rather elicit immunomodulatory effects with a potential for both pro- and anti-inflammatory functions—likely depending on the state of the immune system.
We did not observe modulation of cytokines IL-2, IL-4, IL-12, IL-13, IL-17A, MIP-1α, or TNF-α, likely because we avoided activation of PBMC with mitogenic/antigenic proliferative or cytokine-inductive stimuli to preserve their in vivo phenotype and hence could not detect any potential inhibitory effects of HU308.
We also noted that HU308 did not influence cell number of PBMC treated for 6 and 12 h, which is prima facie unexpected given prior reports of cannabinoid-induced cell death. However, these prior studies were carried out under completely different experimental paradigms.17,97,98 Furthermore, this response has been found to be cell type- and ligand-dependent,99 and is not always mediated by cannabinoid receptors.100
IL-10 is an anti-inflammatory cytokine expressed by many immunocompetent cell types which regulates T-lymphocytic responses via direct mechanisms101 and by inhibiting effector functions of antigen-presenting cells.102,103 IL-10 can also protect from excessive immunoreactivity by inhibiting overproduction of cytokines, including IL-6 which is widely described as pro-inflammatory. Typical IL-6 effects include induction of acute phase proteins, monocyte recruitment, and macrophage infiltration, activation of plasma cells, and CD4+ lymphocyte differentiation.104 On the basis of these paradigms the combined release of IL-6 and IL-10 seems counterintuitive. However, IL-6 can context-dependently exert anti-inflammatory effects, and indeed simultaneous production of IL-6 and IL-10 from mixed human primary immune cells has been observed previously.105
The HU308-induced production of both IL-6 and IL-10 within the same time frame, and with strikingly similar potencies and patterns of influence by inhibitors, seems to imply that CB2 either stimulates release of one cytokine which induces the second, or that the two cytokines are induced via the same signaling pathways. Considerable literature supports the first possibility; for example, IL-6 in concert with other cytokines can stimulate differentiation of naïve CD4+ T cells into IL-10-producing regulatory type 1 helper T (Tr1) or IL-17-producing T helper (TH-17) cells which have anti-inflammatory effects in vivo.106,107 Low dose recombinant IL-6 can also increase plasma IL-10 and have other anti-inflammatory effects in humans.108 However, given the acute time frame within which we detected both IL-6 and IL-10 (by 6 h), and slightly greater potency of the IL-10 response, it seems unlikely that in our system CB2 activation first induces IL-6 which subsequently leads to IL-10 secretion from a different cell type. It is possible that IL-6 and IL-10 were released independently from different cells; however, the near-identical profile of sensitivity to G protein inhibitors leads us to suspect that the most plausible scenario is that IL-6 and IL-10 are generated from the same cell type(s) in our system. Interestingly, these cytokines have coordinated mRNA regulation via the same nuclear site.109 IL-6 and IL-10 have classically been thought to be produced by T helper 2 (Th2) T lymphocytes,110,111 while concurrent production has been observed in monocytes, tumor-associated macrophages, and dendritic cells.112−114 In future work it will be important to clarify the influence of CB2 activation on different immune subtypes, and this knowledge will also assist in designing follow-on experiments to investigate other CB2-mediated immune cell functions.
There is some precedent for cannabinoid mediation of both IL-6 and IL-10. Endocannabinoids upregulated IL-6 in LPS-stimulated U937 cells80 and in whole blood cultures.115 Cannabinoid-mediated IL-10 induction has been shown in vivo in mice,116 in murine LPS-stimulated macrophages,11 and in murine encephalitogenic T lymphocytes;117 the latter study also shows inhibition of IL-6 by cannabinoids, likely indicating species and/or cell type specificity. To our knowledge, CB2-mediated IL-10 production by human PBMC has never been shown before.
Historically, there is a considerable body of data regarding the general effects of cannabis and THC on human immune function, though somewhat surprisingly, little has been reported on cytokine profiles.118,119 Increased serum IL-10 has been correlated with cannabis consumption,120 while elevated IL-6 and other pro-inflammatory markers were found in individuals with cannabis dependency.121 In a trial of THC in multiple sclerosis, cannabinoid treatments were found not to influence serum concentrations of IL-10 or CRP (a surrogate measure of IL-6), nor other measured cytokines, though sample sizes were small and variability between subjects large.122 Further, THC is a partial agonist at CB2 and so may have only subtle effects in comparison with full agonists. Only a few clinical trials of CB2-selective agonists have been undertaken and to our knowledge none have reported on cytokine profiles. We have also noted serious adverse effects from illicit synthetic cannabinoids, some of which are potent CB2 agonists.123,124 Although these adverse symptoms may be mediated by a noncannabinoid-receptor target,125 we wonder whether a potent and efficacious influence on cytokine balance and/or different cytokine profile arising from biased agonism via CB2 could contribute to deleterious effects.
The results of this study greatly expand our knowledge of CB2 signaling and functional implications in human primary PBMC under conditions closely preserving their in situ state. The discovery of unprecedented CB2 coupling to stimulatory (Gαs) proteins, their simultaneous activation alongside inhibitory (Gαi) proteins in a cAMP pathway, of Gαs-mediated effects on CREB phosphorylation, and of IL-6 and IL-10 induction, offer new perspectives on CB2 signaling and function which may be useful for drug discovery and investigations of CB2 signaling phenomena including functional selectivity. The effects of CB2 agonism that we have observed in this study raise a number of questions and potential challenges but reinforce the potential utility of CB2 ligands as immunomodulatory therapeutics.
Methods
Preparation of Human PBMC
Human primary PBMC were isolated from blood samples collected from three healthy volunteers (male and female, aged 22–26) after obtaining written informed consents in accordance with ethical approval from the University of Auckland Human Participants Ethics Committee (#014035).
Whole venous blood samples, 50 mL per draw, 3–6 draws per donor (with each set of experiments performed on one draw from each donor) were collected into K2EDTA-coated BD Vacutainer Blood Collection Tubes (Becton, Dickinson and Company), transferred to polypropylene tubes (Corning), and diluted 1.25 volumes of blood with 1 volume RPMI 1640 medium (HyClone) supplemented with 2 mM l-Glutamine (Thermo Fisher Scientific).
PBMC were isolated by gradient centrifugation in Histopaque-1077 (Sigma-Aldrich) in Greiner Leucosep 50 mL tubes (Greiner Bio-One) for 15 min at 800g at room temperature. The collected PBMC were washed twice with RPMI by centrifugation for 10 min at 250g, resuspended in a culture medium—RPMI supplemented with 10% v/v fetal bovine serum (FBS; New Zealand origin, Moregate Biotech) and 2 mM l-glutamine. Following isolation, PBMC were either used in assays immediately or cryopreserved in 50% RPMI with 40% FBS and 10% DMSO and stored at −80 °C for up to 12 months until later use. Cryopreservation did not affect CB2 expression (Figure S9) or HU308 signaling responses of PBMC (Figure S10).
Cell Lines and Cell Culture
Human embryonic kidney (HEK) cell lines stably transfected with human CB1 (HEK-hCB1126) or human CB2 (HEK-hCB250), or an untransfected wild type HEK Flp-in cell line (HEK wt, Invitrogen, R75007) were cultured in high-glucose DMEM (HyClone) with 10% v/v FBS, in the presence of Zeocin (InvivoGen) at 250 μg/mL, Hygromycin B (Thermo Fisher Scientific) at 50 μg/mL, or Zeocin at 100 μg/mL, respectively.
Whole Cell Radioligand Binding
Human primary PBMC or HEK cells were pelleted by centrifugation for 10 min at 250g or 5 min at 125g, respectively, and resuspended in binding medium (RPMI with 1 mg/mL BSA, 2 mM l-Glutamine, 25 mM HEPES). PBMC (3 × 105 cells per assay point), HEK-hCB1 or HEK-hCB2 (0.4 × 105 diluted in 1.6 × 105 HEK wt to avoid ligand depletion), or HEK wt (2 × 105) cells were dispensed in deep well 96-well plates (Gene Era Biotech) with reaction mix already present to produce a final 200 μL reaction volume. The reaction mix included 5 nM (final concentration) [3H]-CP55940 (PerkinElmer), and an appropriate displacer to measure nonspecific displacement of the radioligand or a vehicle (DMSO) control to measure total binding of the radioligand. The displacer was either ACEA at 1 μM (arachidonyl-2-chloroethylamide, synthesized from arachidonic acid as described in ref (127)), or HU308 at 1 μM (Tocris Bioscience).
Plates were incubated on the surface of a 15 °C thermostatic bath for 3 h to reach steady-state, then placed on ice. Reactions were then rapidly filtered under vacuum through GF/C glass fiber filter-bottom 96-well microplates (PerkinElmer, MA, USA) pretreated with 0.1% polyethylenimine (PEI, Sigma-Aldrich). After three washes with ice-cold wash buffer (50 mM HEPES pH 7.4, 500 mM NaCl, 1 mg/mL BSA) filter plates were dried overnight, then sealed, and 50 μL per well of Irgasafe Plus scintillation fluid (PerkinElmer) was dispensed. After 30 min, plates were counted in a Wallac MicroBeta TriLux liquid scintillation counter (PerkinElmer). Each independent experiment was verified to have undergone <10% ligand depletion. CB1 and CB2 protein quantification was based on the maximum binding parameter Bmax, the concentration of binding sites for the radioligand,128 using the following formula based on eq 7.5.21 from ref (129):
Bmax values were then converted to moles using the radioligand specific activity (as stated by the manufacturer), and then converted to units of fmol/mg of total protein added to an assay point (as measured by Bio-Rad DC Protein Assay Kit). Equilibrium dissociation constants (Kd) for [3H]-CP55940 were measured by carrying out homologous competition binding under the same experimental conditions. pKd values were 7.94 ± 0.04 at CB1, 7.80 ± 0.05 at CB2 (mean ± SEM, n = 3).
cAMP Assay
PBMC were resuspended in assay medium (RPMI with 2 mM l-glutamine, 25 mM HEPES, 10% v/v FBS) and seeded at 1 × 105 cells per well in 10 μL into Falcon 384-well plates (Corning). All ligand and vehicle solutions were prepared at 2× concentrations in the assay medium, and dispensed 10 μL per well in 384-well plates at requisite time points. After equilibration in a humidified atmosphere at 37 °C and 5% CO2 for 1 h, cells were incubated with 10 μM forskolin (canonical adenylyl cyclase activator, Tocris Bioscience) with or without stimulations of interest and/or vehicle (DMSO) controls. At the end of incubation, plates were rapidly cooled by placing on ice, and cells were lysed by adding 10 μL per well of 3× concentrated ice-cold lysis buffer (formulation as per LANCE cAMP kit described below). The plates were placed on shakers at 500 rpm for 10 min at 4 °C, and frozen at −80 °C. The assays were performed without any phosphodiesterase inhibitors to allow for assessment of signaling kinetics.
cAMP detection was performed in 1/2-area white 96-well plates (PerkinElmer), using the LANCE cAMP Kit (PerkinElmer) with a high sensitivity method with 12 μL per point lysate and total detection volume of 24 μL using 2× concentrated detection mix. The signal was detected on a CLARIOstar plate reader (BMG Labtech) using recommended TR-FRET settings. Assay sample cAMP concentrations were interpolated from standard curves. Data were normalized to vehicle (set as 0%) and forskolin (set as 100%) controls to allow compilation of data from independent experiments.
ERK1/2 Phosphorylation Assay (p-ERK)
PBMC were pelleted by centrifugation for 10 min at 250g, resuspended in assay medium, and seeded at 1 × 105 cells in 10 μL per well into Falcon 384-well plates. Plates were incubated in a humidified atmosphere at 37 °C and 5% CO2 for 55 min, and then transferred to the surface of a water bath at 37 °C and incubated for 5 min prior to adding ligands or vehicle controls. Treatments were prepared at 2× concentrations in assay medium, and dispensed at 10 μL per well at requisite time points. At the end of incubation, plates were placed on ice, and cells were lysed by the addition of 10 μL per well of 3× concentrated ice-cold lysis buffer (formulation as per AlphaLISA kit described below). The plates were placed on shakers (500 rpm, 10 min at 4 °C) and frozen at −80 °C. p-ERK detection was performed using the AlphaLISA SureFire Ultra p-ERK 1/2 (Thr202/Tyr204) Assay Kit (PerkinElmer) per the manufacturer’s specifications in 1/2-area white 96-well plates. The signal was detected on a CLARIOstar plate reader with AlphaLISA-compatible filters. Counts were normalized to vehicle controls (100%) to allow compilation of data from independent experiments.
Akt, p38, JNK, CREB Phosphorylation Assays
PBMC were treated and lysed under the same conditions as described for the p-ERK assay. Analytes were detected using AlphaLISA SureFire Ultra p-Akt 1/2/3 (Ser473), p-JNK 1/2/3 (Thr183/Tyr185), p-p38 MAPK (Thr180/Tyr182), and p-CREB (Ser133) assay kits as described above.
Application of G Protein Inhibitors
In experiments with PTX (Sigma-Aldrich), cells were pelleted, resuspended in the assay medium containing PTX 100 ng/mL or vehicle, and incubated for 5 h in a humidified atmosphere at 37 °C and 5% CO2. After the incubation, the cells were counted and resuspended in the same assay media with PTX or vehicle and equilibrated for 1 h as described for the signaling assays (bringing the total PTX incubation to 6 h). Then ligands/vehicles were added for signaling experiments. In gallein (Santa Cruz Biotechnology) or suramin derivatives NF023 (Abcam) and NF449 (Abcam) experiments, the inhibitors or their vehicles were added to cells at 10 μM 30 min prior to adding ligands or vehicles. All ligand and vehicle solutions used in assays with inhibitors were prepared in assay media containing the inhibitors or vehicles, so that inhibitors or vehicle controls at 1× concentration were present for the entire duration of stimulations.
Analysis of Cytokine Secretion
PBMC were seeded at 4.5 × 105 cells per well in assay medium into 96 Well V-Bottom plates (Interlab) and incubated for 30 min. If applicable, cells were pretreated for 30 min with 1 μM SR144528 (a kind gift from Roche Pharmaceuticals, Basel, Switzerland), 5 μM WIN55212-3 (Tocris), 10 μM G protein inhibitors (NF023, NF449, gallein), or vehicle control (DMSO), and subsequently treated with HU308 or vehicle, with or without forskolin and G protein inhibitors or antagonists if applicable, in 150 μL final volume. After 12 h (multiplex screen) or 6 h (quantitative IL-6/IL-10 analysis) incubation in a humidified atmosphere at 37 °C and 5% CO2, plates were centrifuged for 10 min at 250g, and the supernatants were collected and frozen at −80 °C. The remaining cell pellets were resuspended, and aliquots (in technical duplicate) were diluted in Trypan blue for hemocytometer counting. Interleukins 2, 4, 6, 10, 12, 13, and 17A, macrophage inflammatory protein (MIP-1α), and tumor necrosis factor (TNF-α) were detected using Cytometric Bead Array (CBA) technology (BD Biosciences) and assayed on an Accuri C6 flow cytometer (BD Biosciences) as described previously.130 For the multiplex screen, two concentrations of standard in the lower and middle range of standard curves (78 and 625 pg/mL) were utilized as internal controls, and the mean fluorescent intensity (MFI) of each cytokine was calculated. Concentrations of IL-6 and IL-10 were interpolated using FCAP Array software (v. 3.1, BD Biosciences) from standard curves. Manufacturer stated limits of detection (LOD) were 11.2, 1.4, 1.6, 0.13, 7.9, 0.6, 0.3, 0.2, and 1.2 pg/mL for interleukins 2, 4, 6, 10, 12, 13, 17A, MIP-1α, and TNF-α, respectively.
Data Analysis
All analysis was performed in GraphPad Prism Software (v. 8.0; GraphPad Software Inc.). Data were presented as mean ± standard error of the mean (SEM) from three independent experiments performed in triplicate, each with samples from a separate subject (three subjects in total). Concentration–response curves were obtained by fitting three-parameter nonlinear regression curves. Normalized data were utilized for statistical analysis of selected experiments (Figures 2D,E, 4B, and 5B, as described in figure legends). Otherwise, statistical analyses were performed on raw data. Repeated measures (RM) designs were utilized to reflect that each independent experiment was carried out on a separate subject. Normal distribution and equality of variance were verified with Shapiro-Wilk and Brown-Forsythe tests. If these tests did not pass, a decimal logarithm transformation was performed which enabled adherence to parametric test assumptions. If a statistically significant difference (p < 0.05) was detected in one- or two-way ANOVA, data were further analyzed using either Holm-Šídák (for comparisons to control) or Tukey (when comparisons between multiple conditions were of interest) posthoc tests with significance levels indicated graphically as p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), nonsignificant (ns).
Acknowledgments
We thank John McGowan (University of Auckland, NZ) for synthesizing the ACEA. This research was supported by funding from the Auckland Medical Research Foundation and the Health Research Council NZ (to N.G.). Y.S. was supported by Dean’s International Doctoral Scholarship, Faculty of Medical and Health Sciences, The University of Auckland. The Accuri C6 flow cytometer was purchased with funding from the NZ Lottery Health Board (to E.S.G.).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsptsci.9b00049.
Preincubation with forskolin has no effect on net cAMP kinetics in human primary PBMC; stimulatory cAMP signaling is detectable without forskolin; effects of HU308 on cAMP and p-ERK responses in PBMC are concentration-dependent; SR144528 is selective for CB2 over CB1; PBMC do not respond to a CB1-selective agonist ACEA in p-ERK assay; HU308 induces interleukins 6 and 10 but not IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, IL-17A, MIP-1α or TNF-α in PBMC; HU308 has no effect on cell concentrations after 6 or 12 h of incubation; cryopreservation of PBMC has no effect on CB2 expression, nor p-ERK response (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Galiegue S.; Mary S.; Marchand J.; Dussossoy D.; Carriere D.; Carayon P.; Bouaboula M.; Shire D.; Le Fur G.; Casellas P. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232, 54–61. 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W.; Hesk D.; Chen S.-C.; Kinsley D.; Fine J. S.; Jackson J. V.; Bober L. A.; Deno G.; Bian H.; Fossetta J.; Lunn C. A.; Kozlowski J. A.; Lavey B.; Piwinski J.; Narula S. K.; Lundell D. J.; Hipkin R. W. (2006) Characterization of peripheral human cannabinoid receptor (hCB2) expression and pharmacology using a novel radioligand, [35S]Sch225336. J. Biol. Chem. 281, 28143–51. 10.1074/jbc.M602364200. [DOI] [PubMed] [Google Scholar]
- Pacher P.; Kunos G. (2013) Modulating the endocannabinoid system in human health and disease--successes and failures. FEBS J. 280, 1918–43. 10.1111/febs.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M.; Poinot-Chazel C.; Marchand J.; Canat X.; Bourrié B.; Rinaldi-Carmona M.; Calandra B.; Le Fur G.; Casellas P. (1996) Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur. J. Biochem. 237, 704–11. 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Soethoudt M.; Grether U.; Fingerle J.; Grim T. W.; Fezza F.; de Petrocellis L.; Ullmer C.; Rothenhäusler B.; Perret C.; van Gils N.; Finlay D.; MacDonald C.; Chicca A.; Gens M. D.; Stuart J.; de Vries H.; Mastrangelo N.; Xia L.; Alachouzos G.; Baggelaar M. P.; Martella A.; Mock E. D.; Deng H.; Heitman L. H.; Connor M.; Di Marzo V.; Gertsch J.; Lichtman A. H.; Maccarrone M.; Pacher P.; Glass M.; van der Stelt M. (2017) Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun. 8, 13958. 10.1038/ncomms13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu G. C.; Deliu E.; Marcu J.; Hoffman N. E.; Console-Bram L.; Zhao P.; Madesh M.; Abood M. E.; Brailoiu E. (2014) Differential activation of intracellular versus plasmalemmal CB2 Cannabinoid receptors. Biochemistry 53, 4990–4999. 10.1021/bi500632a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee M. H.; Bayewitch M.; Avidor-Reiss T.; Levy R.; Vogel Z. (1998) Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J. Neurochem. 71, 1525–1534. 10.1046/j.1471-4159.1998.71041525.x. [DOI] [PubMed] [Google Scholar]
- Börner C.; Smida M.; Höllt V.; Schraven B.; Kraus J. (2009) Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J. Biol. Chem. 284, 35450–35460. 10.1074/jbc.M109.006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueras-Ortiz C.; Roman-Vendrell C.; Mateo-Semidey G. E.; Liao Y.-H.; Kendall D. A.; Yudowski G. A. (2017) Retromer stops beta-arrestin 1–mediated signaling from internalized cannabinoid 2 receptors. Mol. Biol. Cell 28, 3554–3561. 10.1091/mbc.e17-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker J. L.; Ruckle M. B.; Mayeux P. R.; Prather P. L. (2005) Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J. Pharmacol. Exp. Ther. 315, 828–38. 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- Correa F.; Mestre L.; Docagne F.; Guaza C. (2005) Activation of cannabinoid CB 2 receptor negatively regulates IL-12p40 production in murine macrophages: Role of IL-10 and ERK1/2 kinase signaling. Br. J. Pharmacol. 145, 441–448. 10.1038/sj.bjp.0706215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J.; Leonti M.; Raduner S.; Racz I.; Chen J.-Z.; Xie X.-Q.; Altmann K.-H.; Karsak M.; Zimmer A. (2008) Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U. S. A. 105, 9099–9104. 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coopman K.; Smith L. D.; Wright K. L.; Ward S. G. (2007) Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int. Immunopharmacol. 7, 360–71. 10.1016/j.intimp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Yamashita M.; Kimura M.; Kubo M.; Shimizu C.; Tada T.; Perlmutter R. M.; Nakayama T. (1999) T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 96, 1024–1029. 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. (1960) Phytohemagglutinin: An Initiator of Mitosis in Cultures of Normal Human Leukocytes. Cancer Res. 20, 462–466. [PubMed] [Google Scholar]
- Herrera B.; Carracedo A.; Diez-Zaera M.; Guzman M.; Velasco G. (2005) p38 MAPK is involved in CB2 receptor-induced apoptosis of human leukaemia cells. FEBS Lett. 579, 5084–5088. 10.1016/j.febslet.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Gustafsson K.; Christensson B.; Sander B.; Flygare J. (2006) Cannabinoid receptor-mediated apoptosis induced by R(+)-methanandamide and Win55,212–2 is associated with ceramide accumulation and p38 activation in mantle cell lymphoma. Mol. Pharmacol. 70, 1612–20. 10.1124/mol.106.025981. [DOI] [PubMed] [Google Scholar]
- Do Y.; McKallip R. J.; Nagarkatti M.; Nagarkatti P. S. (2004) Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 173, 2373–2382. 10.4049/jimmunol.173.4.2373. [DOI] [PubMed] [Google Scholar]; [pii].
- Zhang Y.; Wang X.; Yang H.; Liu H.; Lu Y.; Han L.; Liu G. (2013) Kinase AKT controls innate immune cell development and function. Immunology 140, 143–152. 10.1111/imm.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez O.; Sanchez-Rodriguez A.; Le M. Q. U.; Sanchez-Caro C.; Molina-Holgado F.; Molina-Holgado E. (2011) Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Br. J. Pharmacol. 163, 1520–1532. 10.1111/j.1476-5381.2011.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo W. K.; Peppelenbosch M. P.; Braat H.; Parikh K.; de Vries M.; Fuhler G. M.; Comalada M.; Bruno M. J.; van Goor H. (2017) Modulation of Human Peripheral Blood Mononuclear Cell Signaling by Medicinal Cannabinoids. Front. Mol. Neurosci. 10, 1–11. 10.3389/fnmol.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez del Pulgar T.; Velasco G.; Guzmán M. (2000) The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem. J. 347, 369–73. 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. K.; Choi E.-J. (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta, Mol. Basis Dis. 1802, 396–405. 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Li A.-L.; Lin X.; Dhopeshwarkar A. S.; Thomaz A. C.; Carey L. M.; Liu Y.; Nikas S. P.; Makriyannis A.; Mackie K.; Hohmann A. G. (2019) Cannabinoid CB2 Agonist AM1710 Differentially Suppresses Distinct Pathological Pain States and Attenuates Morphine Tolerance and Withdrawal. Mol. Pharmacol. 95, 155–168. 10.1124/mol.118.113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T., Kishimoto S., Oka S., Gokoh M., and Waku K. (2004) Metabolism and physiological significance of anandamide and 2-arachidonoylglycerol, endogenous cannabinoid receptor ligands, in Arachidonate Remodeling and Inflammation (Fonteh A. N., and Wykle R. L., Eds.), pp 211–237. Birkhäuser Basel, Basel. DOI: 10.1007/978-3-0348-7848-7_11. [DOI] [Google Scholar]
- Rom S.; Persidsky Y. (2013) Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. J. Neuroimmune Pharmacol. 8, 608–20. 10.1007/s11481-013-9445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihenetu K.; Molleman A.; Parsons M.; Whelan C. (2003) Pharmacological characterisation of cannabinoid receptors inhibiting interleukin 2 release from human peripheral blood mononuclear cells. Eur. J. Pharmacol. 464, 207–15. 10.1016/S0014-2999(03)01379-7. [DOI] [PubMed] [Google Scholar]
- Cencioni M. T.; Chiurchiù V.; Catanzaro G.; Borsellino G.; Bernardi G.; Battistini L.; Maccarrone M. (2010) Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS One 5, e8688. 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M.; Kiertscher S. M.; Cheng Q.; Zoumalan R.; Tashkin D. P.; Roth M. D. (2002) Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 133, 124–31. 10.1016/S0165-5728(02)00370-3. [DOI] [PubMed] [Google Scholar]
- Gardner B.; Zu L. X.; Sharma S.; Liu Q.; Makriyannis A.; Tashkin D. P.; Dubinett S. M. (2002) Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-beta. Biochem. Biophys. Res. Commun. 290, 91–6. 10.1006/bbrc.2001.6179. [DOI] [PubMed] [Google Scholar]
- Massi P.; Sacerdote P.; Ponti W.; Fuzio D.; Manfredi B.; Viganó D.; Rubino T.; Bardotti M.; Parolaro D. (1998) Immune function alterations in mice tolerant to Δ9- tetrahydrocannabinol: Functional and biochemical parameters. J. Neuroimmunol. 92, 60–66. 10.1016/S0165-5728(98)00177-5. [DOI] [PubMed] [Google Scholar]
- Newton C. A.; Klein T. W.; Friedman H. (1994) Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect. Immun. 62, 4015–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. W.; Newton C. A.; Nakachi N.; Friedman H. (2000) 9-Tetrahydrocannabinol Treatment Suppresses Immunity and Early IFN-gamma, IL-12, and IL-12 Receptor 2 Responses to Legionella pneumophila Infection. J. Immunol. 164, 6461–6466. 10.4049/jimmunol.164.12.6461. [DOI] [PubMed] [Google Scholar]
- Nakano Y.; Pross S.; Friedman H. (1993) Contrasting effect of delta-9-tetrahydrocannabinol on IL-2 activity in spleen and lymph node cells of mice of different ages. Life Sci. 52, 41–51. 10.1016/0024-3205(93)90287-D. [DOI] [PubMed] [Google Scholar]
- Hillard C. J.; Manna S.; Greenberg M. J.; DiCamelli R.; Ross R. A.; Stevenson L. A.; Murphy V.; Pertwee R. G.; Campbell W. B. (1999) Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J. Pharmacol. Exp. Ther. 289, 1427–33. [PubMed] [Google Scholar]
- Hanus L.; Breuer A.; Tchilibon S.; Shiloah S.; Goldenberg D.; Horowitz M.; Pertwee R. G.; Ross R. A.; Mechoulam R.; Fride E. (1999) HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. U. S. A. 96, 14228–33. 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyagawa C. R. M.; de la Harpe S. M.; Saroz Y.; Glass M.; Vernall A. J.; Grimsey N. L. (2018) Cannabinoid Receptor 2 Signalling Bias Elicited by 2,4,6-Trisubstituted 1,3,5-Triazines. Front. Pharmacol. 9, 1202. 10.3389/fphar.2018.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C.; Antoine R. (1995) A proposed mechanism of ADP-ribosylation catalyzed by the pertussis toxin S1 subunit. Biochimie 77, 333–340. 10.1016/0300-9084(96)88143-0. [DOI] [PubMed] [Google Scholar]
- Milligan G.; Kostenis E. (2006) Heterotrimeric G-proteins: A short history. Br. J. Pharmacol. 147 (147), S46. 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenegger M.; Waldhoer M.; Beindl W.; Bing B.; Kreimeyer A.; Nickel P.; Nanoff C.; Freissmuth A. M.; Gilman A. G. (1998) Gs alpha-selective G protein antagonists. Proc. Natl. Acad. Sci. U. S. A. 95, 346–351. 10.1073/pnas.95.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhopeshwarkar A.; Mackie K. (2016) Functional selectivity of CB2 cannabinoid receptor ligands at a canonical and non-canonical pathway. J. Pharmacol. Exp. Ther. 358, 342–351. 10.1124/jpet.116.232561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M.; Boehm S.; Beindl W.; Nickel P.; Ijzerman A. P.; Hohenegger M.; Nanoff C. (1996) Suramin analogues as subtype-selective G protein inhibitors. Mol. Pharmacol. 49, 602–11. [PubMed] [Google Scholar]
- Lehmann D. M.; Seneviratne A. M. P. B.; Smrcka A. V. (2008) Small Molecule Disruption of G Protein Subunit Signaling Inhibits Neutrophil Chemotaxis and Inflammation. Mol. Pharmacol. 73, 410–418. 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M.; Barth F.; Millan J.; Derocq J. M.; Casellas P.; Congy C.; Oustric D.; Sarran M.; Bouaboula M.; Calandra B.; Portier M.; Shire D.; Breliere J. C.; Le Fur G. L. (1998) SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 284, 644–650. [PubMed] [Google Scholar]
- Savinainen J. R.; Kokkola T.; Salo O. M. H.; Poso A.; Järvinen T.; Laitinen J. T. (2005) Identification of WIN55212–3 as a competitive neutral antagonist of the human cannabinoid CB 2 receptor. Br. J. Pharmacol. 145, 636–645. 10.1038/sj.bjp.0706230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T.; Kondo S.; Miyashita T.; Kodaka T.; Suhara Y.; Takayama H.; Waku K. (2000) Evidence That 2-Arachidonoylglycerol but Not N -Palmitoylethanolamine or Anandamide Is the Physiological Ligand for the Cannabinoid CB2 Receptor. J. Biol. Chem. 275, 605–12. 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- Arkun Y.; Yasemi M.; Thattai M. (2018) Dynamics and control of the ERK signaling pathway: Sensitivity, bistability, and oscillations. PLoS One 13, e0195513. 10.1371/journal.pone.0195513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnucci C.; Di Siena S.; Bustamante M. B.; Di Giacomo D.; Di Tommaso M.; Maccarrone M.; Grimaldi P.; Sette C.; Androutsellis-Theotokis A. (2013) Type-1 (CB1) Cannabinoid Receptor Promotes Neuronal Differentiation and Maturation of Neural Stem Cells. PLoS One 8, e54271. 10.1371/journal.pone.0054271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M.; Delghandi M. P.; Moens U. (2004) What turns CREB on?. Cell. Signalling 16, 1211–1227. 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Finlay D. B.; Cawston E. E.; Grimsey N. L.; Hunter M. R.; Korde A.; Vemuri V. K.; Makriyannis A.; Glass M. (2017) Gαs signalling of the CB1 receptor and the influence of receptor number. Br. J. Pharmacol. 174, 2545–2562. 10.1111/bph.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C.; Joyce K. E.; Briley E. M.; Mansouri J.; Mackie K.; Blond O.; Lai Y.; Ma A. L.; Mitchell R. L. (1995) Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 48, 443–50. [PubMed] [Google Scholar]
- Slipetz D. M.; O’Neill G. P.; Favreau L.; Dufresne C.; Gallant M.; Gareau Y.; Guay D.; Labelle M.; Metters K. M. (1995) Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Mol. Pharmacol. 48, 352–361. [PubMed] [Google Scholar]
- Scherzer J. A.; Lin Y.; McLeish K. R.; Klein J. B. (1997) TNF translationally modulates the expression of G1 protein alpha(i2) subunits in human polymorphonuclear leukocytes. J. Immunol 158, 913–918. [PubMed] [Google Scholar]
- Kuwano Y.; Adler M.; Zhang H.; Groisman A.; Ley K. (2016) Gαi2 and Gαi3 Differentially Regulate Arrest from Flow and Chemotaxis in Mouse Neutrophils. J. Immunol. 196, 3828–3833. 10.4049/jimmunol.1500532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B.; Davis R.; Sadat E. L.; Collins J.; Sternweis P. C.; Yuan D.; Jiang L. I. (2010) Distinct Roles of Adenylyl Cyclase VII in Regulating the Immune Responses in Mice. J. Immunol. 185, 335–344. 10.4049/jimmunol.0903474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander R.; Friedman A. (2014) Modulation of the cAMP Response by G alpha i and G beta gamma: A Computational Study of G Protein Signaling in Immune Cells. Bull. Math. Biol. 76, 1352–1375. 10.1007/s11538-014-9964-4. [DOI] [PubMed] [Google Scholar]
- Glass M.; Felder C. C. (1997) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 17, 5327–33. 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G.; Reyes-Resina I.; Rivas-Santisteban R.; Sánchez de Medina V.; Morales P.; Casano S.; Ferreiro-Vera C.; Lillo A.; Aguinaga D.; Jagerovic N.; Nadal X.; Franco R. (2018) Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem. Pharmacol. 157, 148–158. 10.1016/j.bcp.2018.08.046. [DOI] [PubMed] [Google Scholar]
- Ferré S. (2015) The GPCR heterotetramer: challenging classical pharmacology. Trends Pharmacol. Sci. 36, 145–52. 10.1016/j.tips.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisdon A. M.; Halls M. L. (2016) Compartmentalization of GPCR signalling controls unique cellular responses. Biochem. Soc. Trans. 44, 562–7. 10.1042/BST20150236. [DOI] [PubMed] [Google Scholar]
- Kleyer J.; Nicolussi S.; Taylor P.; Simonelli D.; Furger E.; Anderle P.; Gertsch J. (2012) Cannabinoid receptor trafficking in peripheral cells is dynamically regulated by a binary biochemical switch. Biochem. Pharmacol. 83, 1393–1412. 10.1016/j.bcp.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Castaneda J. T.; Harui A.; Kiertscher S. M.; Roth J. D.; Roth M. D. (2013) Differential Expression of Intracellular and Extracellular CB 2 Cannabinoid Receptor Protein by Human Peripheral Blood Leukocytes. J. Neuroimmune. Pharmacol. 8, 323–332. 10.1007/s11481-012-9430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda J. T.; Harui A.; Roth M. D. (2017) Regulation of Cell Surface CB2 Receptor during Human B Cell Activation and Differentiation. J. Neuroimmune Pharmacol. 12, 544–554. 10.1007/s11481-017-9744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y.; Luttrell L. M.; Lefkowitz R. J. (1997) Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91. 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Tesmer V. M.; Kawano T.; Shankaranarayanan A.; Kozasa T.; Tesmer J. J. G. (2005) Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science 310, 1686–90. 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Petrashevskaya N.; Ren S.; Zhao A.; Chakir K.; Gao E.; Chuprun J. K.; Wang Y.; Talan M.; Dorn G. W.; Lakatta E. G.; Koch W. J.; Feldman A. M.; Xiao R.-P. (2012) Gi-biased β2AR signaling links GRK2 upregulation to heart failure. Circ. Res. 110, 265–74. 10.1161/CIRCRESAHA.111.253260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olianas M. C.; Ingianni A.; Onali P. (1998) Role of G protein betagamma subunits in muscarinic receptor-induced stimulation and inhibition of adenylyl cyclase activity in rat olfactory bulb. J. Neurochem. 70, 2620–7. 10.1046/j.1471-4159.1998.70062620.x. [DOI] [PubMed] [Google Scholar]
- Uezono Y.; Kaibara M.; Murasaki O.; Taniyama K. (2004) Involvement of G protein betagamma-subunits in diverse signaling induced by G(i/o)-coupled receptors: study using the Xenopus oocyte expression system. Am. J. Physiol. Cell Physiol. 287, C885–94. 10.1152/ajpcell.00125.2004. [DOI] [PubMed] [Google Scholar]
- Conti M.; Mika D.; Richter W. (2014) Cyclic AMP compartments and signaling specificity: role of cyclic nucleotide phosphodiesterases. J. Gen. Physiol. 143, 29–38. 10.1085/jgp.201311083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R. (2012) ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 66, 105–143. 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Agrawal A.; Dillon S.; Denning T. L.; Pulendran B. (2006) ERK1–/– Mice Exhibit Th1 Cell Polarization and Increased Susceptibility to Experimental Autoimmune Encephalomyelitis. J. Immunol. 176, 5788–5796. 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]; [pii].
- Dumitru C. D.; Ceci J. D.; Tsatsanis C.; Kontoyiannis D.; Stamatakis K.; Lin J. H.; Patriotis C.; Jenkins N. A.; Copeland N. G.; Kollias G.; Tsichlis P. N. (2000) TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103, 1071–1083. 10.1016/S0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Kamal F. A.; Travers J. G.; Schafer A. E.; Ma Q.; Devarajan P.; Blaxall B. C. (2017) G Protein–Coupled Receptor-G–Protein βγ – Subunit Signaling Mediates Renal Dysfunction and Fibrosis in Heart Failure. J. Am. Soc. Nephrol. 28, 197–208. 10.1681/ASN.2015080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Der Lee M. M. C.; Bras M.; van Koppen C. J.; Zaman G. J. R. (2008) beta-Arrestin recruitment assay for the identification of agonists of the sphingosine 1-phosphate receptor EDG1. J. Biomol. Screening 13, 986–98. 10.1177/1087057108326144. [DOI] [PubMed] [Google Scholar]
- Saitoh F.; Wakatsuki S.; Tokunaga S.; Fujieda H.; Araki T. (2016) Glutamate signals through mGluR2 to control Schwann cell differentiation and proliferation. Sci. Rep. 6, 1–14. 10.1038/srep29856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo-Cheyou E. R.; Vardatsikos G.; Srivastava A. K. (2016) Src tyrosine kinase mediates endothelin-1-induced early growth response protein-1 expression via MAP kinase-dependent pathways in vascular smooth muscle cells. Int. J. Mol. Med. 38, 1879–1886. 10.3892/ijmm.2016.2767. [DOI] [PubMed] [Google Scholar]
- Dhopeshwarkar A.; Mackie K. (2014) CB2 Cannabinoid receptors as a therapeutic target-what does the future hold?. Mol. Pharmacol. 86, 430–7. 10.1124/mol.114.094649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E. S.; Ball N.; Scotter E. L.; Narayan P.; Dragunow M.; Glass M. (2006) Induction of Krox-24 by Endogenous Cannabinoid Type 1 Receptors in Neuro2A Cells Is Mediated by the MEK-ERK MAPK Pathway and Is Suppressed by the Phosphatidylinositol 3-Kinase Pathway. J. Biol. Chem. 281, 29085–29095. 10.1074/jbc.M602516200. [DOI] [PubMed] [Google Scholar]
- Gurevich V. V.; Gurevich E. V. (2018) Arrestins and G proteins in cellular signaling: The coin has two sides. Sci. Signaling 11, eaav1646. 10.1126/scisignal.aav1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazzi J.; Kleyer J.; Paredes J. M. V.; Gertsch J. (2011) Endocannabinoid content in fetal bovine sera — Unexpected effects on mononuclear cells and osteoclastogenesis. J. Immunol. Methods 373, 219–228. 10.1016/j.jim.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Park J. M.; Greten F. R.; Wong A.; Westrick R. J.; Arthur J. S. C.; Otsu K.; Hoffmann A.; Montminy M.; Karin M. (2005) Signaling Pathways and Genes that Inhibit Pathogen-Induced Macrophage Apoptosis— CREB and NF-κB as Key Regulators. Immunity 23, 319–329. 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Yu C.-T.; Shih H. -m.; Lai M.-Z. (2001) Multiple Signals Required for Cyclic AMP-Responsive Element Binding Protein (CREB) Binding Protein Interaction Induced by CD3/CD28 Costimulation. J. Immunol. 166, 284–292. 10.4049/jimmunol.166.1.284. [DOI] [PubMed] [Google Scholar]
- Grady G. C.; Mason S. M.; Stephen J.; Zuniga-Pflucker J. C.; Michie A. M. (2004) Cyclic Adenosine 5′-Monophosphate Response Element Binding Protein Plays a Central Role in Mediating Proliferation and Differentiation Downstream of the Pre-TCR Complex in Developing Thymocytes. J. Immunol. 173, 1802–1810. 10.4049/jimmunol.173.3.1802. [DOI] [PubMed] [Google Scholar]
- Yasuda T.; Sanjo H.; Pagès G.; Kawano Y.; Karasuyama H.; Pouysségur J.; Ogata M.; Kurosaki T. (2008) Erk Kinases Link Pre-B Cell Receptor Signaling to Transcriptional Events Required for Early B Cell Expansion. Immunity 28, 499–508. 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Bomsel M.; Mostov K. E. (1993) Possible role of both the alpha and beta gamma subunits of the heterotrimeric G protein, Gs, in transcytosis of the polymeric immunoglobulin receptor. J. Biol. Chem. 268, 25824–35. [PubMed] [Google Scholar]
- McIntire W. E.; MacCleery G.; Garrison J. C. (2001) The G protein beta subunit is a determinant in the coupling of Gs to the beta 1-adrenergic and A2a adenosine receptors. J. Biol. Chem. 276, 15801–9. 10.1074/jbc.M011233200. [DOI] [PubMed] [Google Scholar]
- Wen A. Y.; Sakamoto K. M.; Miller L. S. (2010) The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 185, 6413–6419. 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baló-Banga J. M.; Schweitzer K.; Lakatos S.; Sipka S. (2015) A novel rapid (20-minute) IL-6 release assay using blood mononuclear cells of patients with various clinical forms of drug induced skin injuries. World Allergy Organ. J. 8, 1. 10.1186/1939-4551-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh S. M.; Wilson A. B.; Deighton J.; Lachmann P. J.; Ewan P. W. (1994) The profiles of interleukin (IL)-2, IL-6, and interferon-gamma production by peripheral blood mononuclear cells from house-dust-mite-allergic patients: a role for IL-6 in allergic disease. Allergy 49, 751–9. 10.1111/j.1398-9995.1994.tb02098.x. [DOI] [PubMed] [Google Scholar]
- Fan J.; Nishanian P.; Breen E. C.; McDonald M.; Fahey J. L. (1998) Cytokine gene expression in normal human lymphocytes in response to stimulation. Clin. Diagn. Lab. Immunol. 5, 335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen E. C.; McDonald M.; Fan J.; Boscardin J.; Fahey J. L. (2000) Cytokine gene expression occurs more rapidly in stimulated peripheral blood mononuclear cells from human immunodeficiency virus-infected persons. Clin. Diagn. Lab. Immunol. 7, 769–73. 10.1128/CDLI.7.5.769-773.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysk M.; Yang D. D.; Lu H. T.; Flavell R. A.; Davis R. J. (1999) Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc. Natl. Acad. Sci. U. S. A. 96, 3763–8. 10.1073/pnas.96.7.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E.; Lazar Z.; Koenderman L.; Kraneveld A. D.; Nijkamp F. P.; Folkerts G. (2009) Cigarette smoke attenuates the production of cytokines by human plasmacytoid dendritic cells and enhances the release of IL-8 in response to TLR-9 stimulation. Respir. Res. 10, 47. 10.1186/1465-9921-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K.; Ohashi M.; Satoh Y.; Sairenji T. (2007) The role of p38 mitogen-activated protein kinase in regulating interleukin-10 gene expression in Burkitt’s lymphoma cell lines. Microbiol. Immunol. 51, 149–161. 10.1111/j.1348-0421.2007.tb03885.x. [DOI] [PubMed] [Google Scholar]
- Pengal R. A.; Ganesan L. P.; Wei G.; Fang H.; Ostrowski M. C.; Tridandapani S. (2006) Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol. Immunol. 43, 1557–1564. 10.1016/j.molimm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Morrison D. C.; Ulevitch R. J. (1978) The effects of bacterial endotoxins on host mediation systems. A review. Am. J. Pathol. 93, 526–618. [PMC free article] [PubMed] [Google Scholar]
- Galve-Roperh I.; Sánchez C.; Cortés M. L.; Gómez del Pulgar T.; Izquierdo M.; Guzmán M. (2000) Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 6, 313–9. 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Sánchez C.; de Ceballos M. L.; Gomez del Pulgar T.; Rueda D.; Corbacho C.; Velasco G.; Galve-Roperh I.; Huffman J. W.; Ramón y Cajal S.; Guzmán M. (2001) Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 61, 5784–9. [PubMed] [Google Scholar]
- Cridge B. J.; Rosengren R. J. (2013) Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manage. Res. 5, 301–13. 10.2147/CMAR.S36105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M.; Lorenzon T.; Bari M.; Melino G.; Finazzi-Agro A. (2000) Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 275, 31938–31945. 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- Joss A.; Akdis M.; Faith A.; Blaser K.; Akdis C. A. (2000) IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur. J. Immunol. 30, 1683–90. . [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F.; Zlotnik A.; Mosmann T. R.; Howard M.; O’Garra A. (1991) IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147, 3815–22. [PubMed] [Google Scholar]
- Corinti S.; Albanesi C.; la Sala A.; Pastore S.; Girolomoni G. (2001) Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 166, 4312–8. 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- Schett G. (2018) Physiological effects of modulating the interleukin-6 axis. Rheumatology 57, ii43–ii50. 10.1093/rheumatology/kex513. [DOI] [PubMed] [Google Scholar]
- Janský L.; Reymanová P.; Kopecký J. (2003) Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol. Res. 52, 593–8. [PubMed] [Google Scholar]
- McGeachy M. J.; Bak-Jensen K. S.; Chen Y.; Tato C. M.; Blumenschein W.; McClanahan T.; Cua D. J. (2007) TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat. Immunol. 8, 1390–1397. 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Jin J.-O.; Han X.; Yu Q. (2013) Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J. Autoimmun. 40, 28–44. 10.1016/j.jaut.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A.; Fischer C. P.; Keller C.; Møller K.; Pedersen B. K. (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. - Endocrinol. Metab. 285, E433–E437. 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- Lee S.; Lee T. A.; Lee E.; Kang S.; Park A.; Kim S. W.; Park H. J.; Yoon J. H.; Ha S. J.; Park T.; Lee J. S.; Cheon J. H.; Park B. (2015) Identification of a subnuclear body involved in sequence-specific cytokine RNA processing. Nat. Commun. 6, 1–14. 10.1038/ncomms6791. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F.; Bond M. W.; Mosmann T. R. (1989) Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170, 2081–95. 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R.; Sad S. (1996) The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17, 138–46. 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Schuerwegh A. J.; De Clerck L. S.; Bridts C. H.; Stevens W. J. (2003) Comparison of intracellular cytokine production with extracellular cytokine levels using two flow cytometric techniques. Cytometry 55B, 52–58. 10.1002/cyto.b.10041. [DOI] [PubMed] [Google Scholar]
- Jang S.; Uematsu S.; Akira S.; Salgame P. (2004) IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J. Immunol. 173, 3392–7. 10.4049/jimmunol.173.5.3392. [DOI] [PubMed] [Google Scholar]
- Qu Q.-X.; Xie F.; Huang Q.; Zhang X.-G. (2018) Membranous and Cytoplasmic Expression of PD-L1 in Ovarian Cancer Cells. Cell. Physiol. Biochem. 43, 1893–1906. 10.1159/000484109. [DOI] [PubMed] [Google Scholar]
- Raduner S.; Majewska A.; Chen J.-Z.; Xie X.-Q.; Hamon J.; Faller B.; Altmann K.-H.; Gertsch J. (2006) Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J. Biol. Chem. 281, 14192–206. 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- Zhu L. X.; Sharma S.; Stolina M.; Gardner B.; Roth M. D.; Tashkin D. P.; Dubinett S. M. (2000) Delta-9-Tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-Dependent Pathway. J. Immunol. 165, 373–380. 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]
- Kozela E.; Juknat A.; Kaushansky N.; Rimmerman N.; Ben-Nun A.; Vogel Z. (2013) Cannabinoids Decrease the Th17 Inflammatory Autoimmune Phenotype. J. Neuroimmune Pharmacol. 8, 1265–1276. 10.1007/s11481-013-9493-1. [DOI] [PubMed] [Google Scholar]
- Massi P.; Vaccani A.; Parolaro D. (2006) Cannabinoids, immune system and cytokine network. Curr. Pharm. Des. 12, 3135–46. 10.2174/138161206777947425. [DOI] [PubMed] [Google Scholar]
- Eisenstein T. K.; Meissler J. J. (2015) Effects of Cannabinoids on T-cell Function and Resistance to Infection. J. Neuroimmune Pharmacol. 10, 204–16. 10.1007/s11481-015-9603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R.; Zuccaro P.; Pichini S.; Roset P. N.; Poudevida S.; Farré M.; Segura J.; De la Torre R. (2003) Modulation of the immune system in cannabis users. J. Am. Med. Assoc. 289, 1929–31. 10.1001/jama.289.15.1929-a. [DOI] [PubMed] [Google Scholar]
- Bayazit H.; Selek S.; Karababa I. F.; Cicek E.; Aksoy N. (2017) Evaluation of oxidant/antioxidant status and cytokine levels in patients with cannabis use disorder. Clin. Psychopharmacol. Neurosci. 15, 237–242. 10.9758/cpn.2017.15.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona S.; Kaminski E.; Sanders H.; Zajicek J. (2005) Cannabinoid influence on cytokine profile in multiple sclerosis. Clin. Exp. Immunol. 140, 580–5. 10.1111/j.1365-2249.2005.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannaert A.; Storme J.; Franz F.; Auwärter V.; Stove C. P. (2016) Detection and Activity Profiling of Synthetic Cannabinoids and Their Metabolites with a Newly Developed Bioassay. Anal. Chem. 88, 11476–11485. 10.1021/acs.analchem.6b02600. [DOI] [PubMed] [Google Scholar]
- Doi T.; Tagami T.; Takeda A.; Asada A.; Sawabe Y. (2018) Evaluation of carboxamide-type synthetic cannabinoids as CB1/CB2 receptor agonists: difference between the enantiomers. Forensic Toxicol. 36, 51–60. 10.1007/s11419-017-0378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley S. B.; Sochat M.; Moser K.; Lynch K. L.; Tochtrop R.; Isbell T. S.; Scalzo A. (2019) Case of brodifacoum-contaminated synthetic cannabinoid. Clin. Toxicol. 57, 143–144. 10.1080/15563650.2018.1502444. [DOI] [PubMed] [Google Scholar]
- Cawston E. E.; Redmond W. J.; Breen C. M.; Grimsey N. L.; Connor M.; Glass M. (2013) Real-time characterization of cannabinoid receptor 1 (CB1) allosteric modulators reveals novel mechanism of action. Br. J. Pharmacol. 170, 893–907. 10.1111/bph.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.; Khanolkar A. D.; Fan P.; Goutopoulos A.; Qin C.; Papahadjis D.; Makriyannis A. (1998) Novel analogues of arachidonylethanolamide (anandamide): Affinities for the CB1 and CB2 cannabinoid receptors and metabolic stability. J. Med. Chem. 41, 5353–5361. 10.1021/jm970257g. [DOI] [PubMed] [Google Scholar]
- Hulme E. C.; Trevethick M. A. (2010) Ligand binding assays at equilibrium: Validation and interpretation. Br. J. Pharmacol. 161, 1219–1237. 10.1111/j.1476-5381.2009.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J.; Neubig R. R. (2010) Analyzing binding data. Curr. Protoc. Neurosci. 52, 7.5.1. 10.1002/0471142301.ns0705s52. [DOI] [PubMed] [Google Scholar]
- O’Carroll S. J.; Kho D. T.; Wiltshire R.; Nelson V.; Rotimi O.; Johnson R.; Angel C. E.; Graham E. S. (2015) Pro-inflammatory TNFα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J. Neuroinflammation 12, 1–18. 10.1186/s12974-015-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.